Abstract
Purpose
Nivolumab is a fully human immunoglobulin G4 programmed death–1 immune checkpoint inhibitor antibody that restores T-cell immune activity. This phase II trial assessed the antitumor activity, dose-response relationship, and safety of nivolumab in patients with metastatic renal cell carcinoma (mRCC).
Patients and Methods
Patients with clear-cell mRCC previously treated with agents targeting the vascular endothelial growth factor pathway were randomly assigned (blinded ratio of 1:1:1) to nivolumab 0.3, 2, or 10 mg/kg intravenously once every 3 weeks. The primary objective was to evaluate the dose-response relationship as measured by progression-free survival (PFS); secondary end points included objective response rate (ORR), overall survival (OS), and safety.
Results
A total of 168 patients were randomly assigned to the nivolumab 0.3- (n = 60), 2- (n = 54), and 10-mg/kg (n = 54) cohorts. One hundred eighteen patients (70%) had received more than one prior systemic regimen. Median PFS was 2.7, 4.0, and 4.2 months, respectively (P = .9). Respective ORRs were 20%, 22%, and 20%. Median OS was 18.2 months (80% CI, 16.2 to 24.0 months), 25.5 months (80% CI, 19.8 to 28.8 months), and 24.7 months (80% CI, 15.3 to 26.0 months), respectively. The most common treatment-related adverse event (AE) was fatigue (24%, 22%, and 35%, respectively). Nineteen patients (11%) experienced grade 3 to 4 treatment-related AEs.
Conclusion
Nivolumab demonstrated antitumor activity with a manageable safety profile across the three doses studied in mRCC. No dose-response relationship was detected as measured by PFS. These efficacy and safety results in mRCC support study in the phase III setting.
INTRODUCTION
An understanding of the mechanisms involved in the pathogenesis of renal cell carcinoma (RCC) led to development of treatment options that inhibit vascular endothelial growth factor (VEGF)–mediated signaling or the mammalian target of rapamycin pathway.1,2 Although these treatment options have demonstrated progression-free survival (PFS) benefit, most patients with metastatic RCC (mRCC) eventually experience progression,1–3 underscoring the need for treatment options with novel mechanisms of action that could potentially result in improved efficacy and a survival advantage.
Multiple resistance mechanisms, including systemic dysfunction in T-cell signaling4–7 and exploitation of immune checkpoints,8 evolve in tumors, helping them evade specific immune responses despite the presentation of tumor antigens to the immune system.8 Recent understanding of these host-tumor immune interactions has given rise to novel antibodies directed against immune checkpoint proteins.9,10
Nivolumab is a fully human immunoglobulin (Ig) G4 programmed death (PD) –1 immune checkpoint inhibitor antibody that selectively blocks the interaction between PD-1 and its ligands PD-L1 and PD-L2—a mechanism that normally leads to downregulation of cellular immune response.11–13 By inhibiting this interaction, nivolumab can enhance T-cell function in vitro, which may result in antitumor activity.14 In a phase I study that included patients with mRCC, nivolumab demonstrated objective responses and a manageable safety profile; no maximum-tolerated dose was identified (0.1 to 10 mg/kg every 3 weeks).15 Herein, we report the results of a randomized phase II trial that evaluated three doses of nivolumab to identify a potential dose-response relationship and assess the activity and safety of nivolumab in patients with mRCC.
PATIENTS AND METHODS
Study Design and Treatment
This was a blinded, randomized, multicenter phase II trial. Previously treated patients were randomly assigned at a ratio of 1:1:1 to receive nivolumab 0.3, 2, or 10 mg/kg administered intravenously every 3 weeks. Randomization was stratified by Memorial Sloan-Kettering Cancer Center (MSKCC) risk group16 (favorable v intermediate v poor) and number of prior treatment regimens (one v more than one) in the metastatic setting.
Nivolumab was provided by the sponsor (Bristol-Myers Squibb, Lawrenceville, NJ; Ono Pharmaceutical Company, Osaka City, Japan) and administered as a 60-minute intravenous infusion on day 1 of each treatment cycle. No dose escalations or reductions were allowed. Dose delay of up to 3 weeks was permitted for management of adverse events (AEs). Treatment was continued until disease progression or intolerance or until stopped for other protocol-defined reasons. Treatment beyond first progression was allowed in patients continuing to tolerate nivolumab and exhibiting investigator-assessed clinical benefit at the time of progression.
The study was conducted in accordance with the International Conference on Harmonisation Good Clinical Practice guidelines17 and approved by the institutional review board or independent ethics committee of each center. Each institutional review board or independent ethics committee comprised a review panel that was responsible for ensuring protection of the rights, safety, and well-being of human participants involved in the study and was adequately constituted to provide assurance of that protection. All patients provided written informed consent before enrollment, based on ethical principles outlined in the Declaration of Helsinki.18
Patients
Patients eligible for study inclusion had histologic confirmation of RCC with a clear-cell component and measurable disease defined by RECIST (version 1.1) and had received prior treatment with at least one antiangiogenic therapy (eg, VEGF tyrosine kinase inhibitors, monoclonal antibodies) in the metastatic setting. Previous treatment with cytokines, cytotoxic drugs, or other targeted agents was permitted but not required. Other key inclusion criteria included disease progression during or after last therapy received and within 6 months of enrollment, Karnofsky performance status ≥ 70%, available tumor tissue for correlative studies, and adequate bone marrow, renal, and hepatic function.
Exclusion criteria included active CNS metastases, autoimmune disease, previous therapy with a T-cell costimulation or checkpoint inhibitor, or treatment with more than three prior treatment regimens in the metastatic setting.
End Points and Assessments
The primary end point was comparison of PFS across each of the three dose arms (0.3, 2, and 10 mg/kg) to assess whether a dose-response relationship exists. Secondary end points included assessment of PFS, objective response rate (ORR), time to response, duration of response, overall survival (OS), and AE rate. Exploratory end points included evaluation of immune-related PFS (based on immune-related RECIST [version 1.1]19; Appendix Table A1, online only) and ORR (definitions provided in Appendix, online only), and tumor PD-L1 expression to explore associations between expression in tumors and clinical outcome.
Tumor assessments were performed at baseline and every 6 weeks from random assignment for the first 12 months and every 12 weeks thereafter, until disease progression or treatment discontinuation (whichever occurred later). Tumor response was based on investigator assessment using RECIST (version 1.1). After treatment discontinuation, patients were observed every 3 months for survival.
Safety was assessed at every clinic visit. AEs were graded for severity according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.0).20
PD-L1 protein expression was measured in archival tumor tissue (or fresh, pretreatment tissue if archival material was not available) by immunohistochemistry using a rabbit antihuman PD-L1 monoclonal antibody (clone 28-8; subsequently developed as part of an automated PD-L1 assay by Dako Denmark A/S, Glostrup, Denmark).21 Blinded scoring was completed by two independent pathologists. PD-L1 positivity was defined by membrane staining of ≥ 5% of tumor cells. A cutoff of ≥ 1% for positivity was also assessed. Patients with multiple specimens were considered PD-L1 positive if any specimen met this criterion.22,23
Statistical Analyses
PFS was defined as time from random assignment to date of investigator-assessed clinical or radiographic progression or death. With a target number of PFS events set at 116, it was calculated that the study objective could be met with ≥ 150 patients to provide ≥ 90% power to detect a dose-response relationship across the three treatment arms (assuming median PFS was 4.0, 5.7, and 8.1 months for arms 0.3, 2, and 10 mg/kg, respectively, derived using exponential distribution assumption, where treatment difference of hazard ratio [HR] of 0.7 was assumed between two consecutive doses and 4 months assumed for smallest dose based on historical data). With approximately 150 patients, it was expected that accrual would be completed after 10 months, and final analysis of PFS could be conducted 19 months from the start of the study.
Evaluation of a dose-response relationship as measured by PFS was performed using a two-sided 20%-level log-rank trend test stratified by MSKCC risk group and number of prior treatment regimens in the metastatic setting. The HRs and two-sided 80% CIs of the nivolumab 0.3, 2, and 10 mg/kg doses relative to each other dose were estimated using the Cox proportional hazards model,24 stratified by MSKCC risk group and number of prior therapies, with randomized treatment arm as the single covariate.
Analysis of ORR was performed based on best overall response (RECIST [version 1.1]; investigator assessed). For each treatment group, ORR was estimated along with exact 80% CI using the Clopper-Pearson method.25 The dose-response relationship was evaluated using a two-sided 20%-level Cochran-Armitage test.26,27
Median OS and 80% CI for each treatment group were estimated using Kaplan-Meier methodology.28 OS was defined as the time from random assignment to date of death. P values for secondary or exploratory end point analyses were not controlled for multiplicity and were conducted for descriptive purposes only. Data cutoffs were May 15, 2013, for the primary PFS and ORR analyses and March 5, 2014, for OS and response duration.
RESULTS
Patient Population
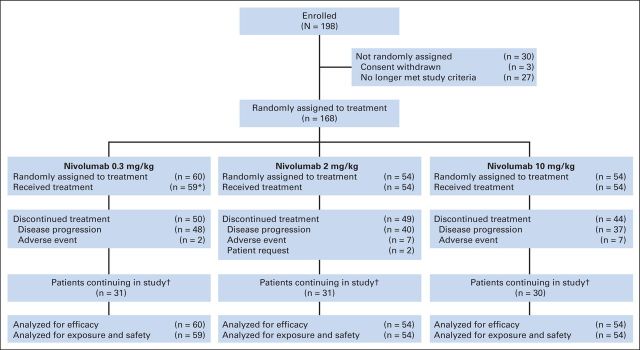
Between May 2011 and January 2012, 168 patients from 39 participating sites in the United States, Canada, Finland, and Italy were randomly assigned: 60 to the nivolumab 0.3-mg/kg arm and 54 patients each to the nivolumab 2- and 10-mg/kg arms. The efficacy population (N = 168) included all randomly assigned patients, and the safety population (n = 167) included all patients who received at least one dose of nivolumab (Fig 1).
Fig 1.
Patient disposition (as of May 15, 2013, data cutoff). (*) One patient not treated; no longer met study criteria. (†) Includes patients continuing in treatment period and patients in follow-up period.
Baseline characteristics were balanced among treatment groups (Table 1). In total, 70% (n = 118) had received more than one prior systemic regimen for mRCC, and 25% (n = 42) met MSKCC poor-risk criteria.
Table 1.
Baseline Demographic and Clinical Characteristics (randomly assigned patients)
| Characteristic | Nivolumab Arm (mg/kg) |
Total (N = 168) |
||||||
|---|---|---|---|---|---|---|---|---|
| 0.3 (n = 60) |
2 (n = 54) |
10 (n = 54) |
||||||
| No. | % | No. | % | No. | % | No. | % | |
| Age, years | ||||||||
| Mean | 61 | 61 | 61 | 61 | ||||
| SD | 9 | 8 | 10 | 9 | ||||
| Sex | ||||||||
| Male | 41 | 68 | 40 | 74 | 40 | 74 | 121 | 72 |
| Female | 19 | 32 | 14 | 26 | 14 | 26 | 47 | 28 |
| MSKCC risk group* | ||||||||
| Favorable | 20 | 33 | 18 | 33 | 18 | 33 | 56 | 33 |
| Intermediate | 26 | 43 | 22 | 41 | 22 | 41 | 70 | 42 |
| Poor | 14 | 23 | 14 | 26 | 14 | 26 | 42 | 25 |
| Karnofsky performance status, %† | ||||||||
| 70 or 80 | 22 | 37 | 30 | 56 | 25 | 46 | 77 | 46 |
| 90 or 100 | 38 | 63 | 24 | 44 | 28 | 52 | 90 | 54 |
| No. of evaluable sites‡ | ||||||||
| 1 | 13 | 22 | 5 | 9 | 12 | 22 | 30 | 18 |
| ≥ 2 | 47 | 78 | 49 | 91 | 42 | 78 | 138 | 82 |
| Site of lesion (> 20% in any group)ठ| ||||||||
| Lung | 46 | 77 | 39 | 72 | 39 | 72 | 124 | 74 |
| Lymph node | 29 | 48 | 35 | 65 | 34 | 63 | 98 | 58 |
| Liver | 15 | 25 | 13 | 24 | 19 | 35 | 47 | 28 |
| Skin/soft tissue | 18 | 30 | 11 | 20 | 11 | 20 | 40 | 24 |
| Adrenal | 8 | 13 | 19 | 35 | 10 | 19 | 37 | 22 |
| Prior radiotherapy | 18 | 30 | 21 | 39 | 22 | 41 | 61 | 36 |
| Prior surgery | 58 | 97 | 53 | 98 | 54 | 100 | 165 | 98 |
| No. of prior systemic regimens in metastatic setting | ||||||||
| 1 | 16 | 27 | 16 | 30 | 18 | 33 | 50 | 30 |
| 2 | 20 | 33 | 19 | 35 | 23 | 43 | 62 | 37 |
| ≥ 3 | 24 | 40 | 19 | 35 | 13 | 24 | 56 | 33 |
| No. of prior systemic antiangiogenic regimens in metastatic setting | ||||||||
| 1 | 34 | 57 | 35 | 65 | 35 | 65 | 104 | 62 |
| 2 | 22 | 37 | 16 | 30 | 18 | 33 | 56 | 33 |
| 3 | 4 | 7 | 3 | 6 | 1 | 2 | 8 | 5 |
| Common prior systemic therapies in metastatic setting‖ | ||||||||
| Sunitinib | 46 | 77 | 42 | 78 | 37 | 69 | 125 | 74 |
| Everolimus | 21 | 35 | 18 | 33 | 18 | 33 | 57 | 34 |
| Pazopanib | 15 | 25 | 18 | 33 | 13 | 24 | 46 | 27 |
| Interleukin-2 | 15 | 25 | 11 | 20 | 12 | 22 | 38 | 23 |
| Sorafenib | 13 | 22 | 8 | 15 | 10 | 19 | 31 | 19 |
Abbreviations: MSKCC, Memorial Sloan-Kettering Cancer Center; SD, standard deviation.
Interactive voice response system source.
One patient (1.9%) in 10-mg/kg group had deviation to Karnofsky performance status < 70%.
Including target and nontarget lesions.
Patients could have lesions at more than one site.
> 20% of patients in any group.
Efficacy
Median PFS was 2.7 months (80% CI, 1.9 to 3.0 months), 4.0 months (80% CI, 2.8 to 4.2 months), and 4.2 months (80% CI, 2.8 to 5.5 months) for the 0.3-, 2-, and 10-mg/kg groups, respectively (Table 2; Fig 2A), and no dose-response relationship for PFS was detected (stratified trend test P = .9). When immune-response PFS was assessed as an exploratory end point, median immune-response PFS was 4.3 months (80% CI, 2.8 to 6.9 months), 5.4 months (80% CI, 4.2 to 7.1 months), and 6.9 months (80% CI, 4.4 to 8.5 months) in the 0.3-, 2-, and 10-mg/kg treatment groups, respectively (test for trend P = .6; Appendix Table A2, online only).
Table 2.
Summary of Efficacy Results (randomly assigned patients)
| Parameter | Nivolumab Arm (mg/kg) |
|||||
|---|---|---|---|---|---|---|
| 0.3 (n = 60) |
2 (n = 54) |
10 (n = 54) |
||||
| No. | % | No. | % | No. | % | |
| Primary End Point | ||||||
| PFS | ||||||
| Median, months | 2.7 | 4.0 | 4.2 | |||
| 80% CI | 1.9 to 3.0 | 2.8 to 4.2 | 2.8 to 5.5 | |||
| 6-month rate, % | 0.3 | 0.3 | 0.4 | |||
| 80% CI | 0.2 to 0.4 | 0.2 to 0.4 | 0.3 to 0.4 | |||
| HRa | ||||||
| 2 v 0.3 mg/kg | 1.0 | |||||
| 80% CI | 0.7 to 1.3 | |||||
| 10 v 0.3 mg/kg | 1.0 | |||||
| 80% CI | 0.8 to 1.3 | |||||
| 10 v 2 mg/kg | 1.0 | |||||
| 80% CI | 0.8 to 1.3 | |||||
| Trend test Pb | .9 | |||||
| Secondary End Points | ||||||
| Best objective responsec | ||||||
| CR | 1 | 2 | 1 | 2 | 0 | 0 |
| PR | 11 | 18 | 11 | 20 | 11 | 20 |
| SD | 22 | 37 | 23 | 43 | 24 | 44 |
| PD | 24 | 40 | 18 | 33 | 17 | 32 |
| Not evaluable | 2d | 3 | 1e | 2 | 2f | 4 |
| ORRg | 12 | 20 | 12 | 22 | 11 | 20 |
| Exact 80% CI | 13.4 to 28.2 | 15.0 to 31.1 | 13.4 to 29.1 | |||
| Stratified odds ratio | ||||||
| 2 v 0.3 mg/kg | 1.2 | |||||
| 80% CI | 0.6 to 2.4 | |||||
| 10 v 0.3 mg/kg | 0.9 | |||||
| 80% CI | 0.4 to 1.8 | |||||
| 10 v 2 mg/kg | 0.9 | |||||
| 80% CI | 0.4 to 1.8 | |||||
| Trend test Ph | 1.0 | |||||
Abbreviations: CR, complete response; HR, hazard ratio; ORR, objective response rate; PD, progressive disease; PFS, progression-free survival; PR, partial response; SD, stable disease.
Stratified Cox proportional hazards model.
Stratified log-rank trend test with 20% significance level (two sided).
Per RECIST (version 1.1) criteria (investigator assessment).
Never treated (n = 1), and death before disease assessment (n = 1).
Early discontinuation because of toxicity.
Death before disease assessment.
CR plus PR per RECIST (version 1.1) criteria (investigator assessment).
Exact Cochran-Armitage trend test with 20% significance level (two sided).
Fig 2.
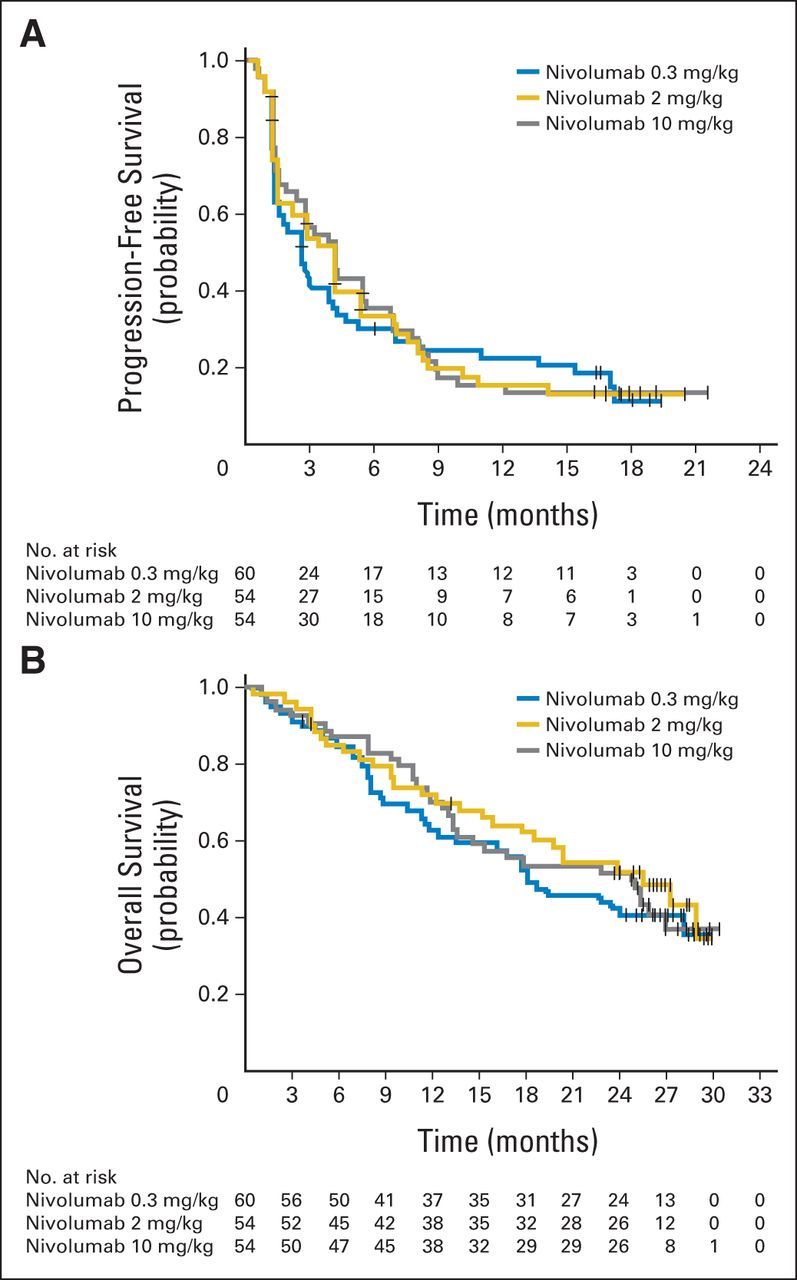
(A) Progression-free and (B) overall survival by treatment arm (randomly assigned patients). Tick marks represent censored observations.
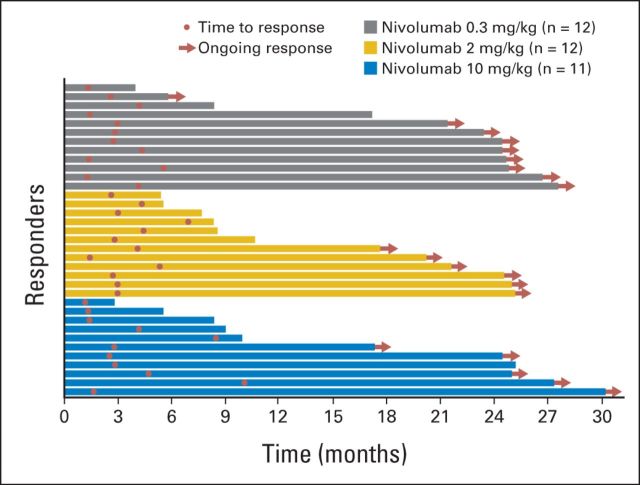
ORR was 20% (n = 12), 22% (n = 12), and 20% (n = 11) in the 0.3-, 2-, and 10-mg/kg groups, respectively (exact Cochran-Armitage trend test P = 1.0; Table 2). Median time to achieving an objective response was 2.8 months (range, 1.3 to 5.6 months) in the 0.3-mg/kg group (n = 12), 3.0 months (range, 1.4 to 6.9 months) in the 2-mg/kg group (n = 12), and 2.8 months (range, 1.2 to 10 months) in the 10-mg/kg group (n = 11). Median duration of response was not reached (NR) in the 0.3-mg/kg (80% CI, NR to NR) and 2-mg/kg groups (80% CI, 4.2 months to NR) and 22.3 months (80% CI, 4.8 months to NR) in the 10-mg/kg group. Of patients who responded to treatment, 75% (nine of 12) in the 0.3-mg/kg group, 50% (six of 12) in the 2-mg/kg group, and 45% (five of 11) in the 10-mg/kg group were ongoing responders (Fig 3). Forty percent (14 of 35) were responding at 24 months from start of study therapy (of the remainder, 14 had stopped responding, and seven were ongoing responders who had not yet reached the 24-month mark).
Fig 3.
Duration of response in patients who achieved objective response by dose treatment arm. Based on data cutoff date of March 5, 2014.
Median OS was 18.2 months (80% CI, 16.2 to 24.0 months), 25.5 months (80% CI, 19.8 to 28.8 months), and 24.7 months (80% CI, 15.3 to 26.0 months) in the 0.3-, 2-, and 10-mg/kg groups, respectively (Fig 2B), with a minimum follow-up of 24 months. HRs for OS in the 2- and 10-mg/kg groups compared with the 0.3-mg/kg group were 0.8 (80% CI, 0.6 to 1.1) and 0.9 (80% CI, 0.6 to 1.2), respectively. OS analyses by MSKCC risk group and by number of prior therapies are shown in Figures 4A and 4B.
Fig 4.
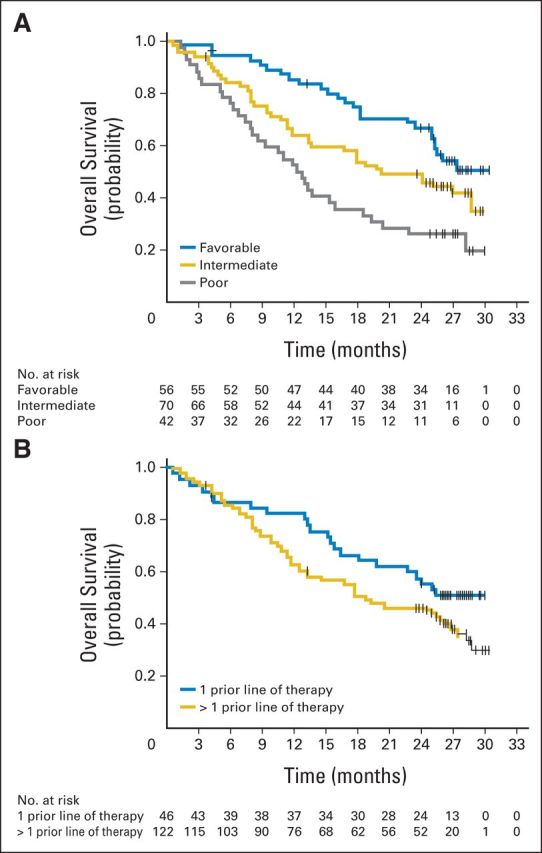
Overall survival (randomly assigned patients) by (A) Memorial Sloan-Kettering Cancer Center risk group and (B) number of prior therapies in advanced or metastatic setting. Tick marks represent censored observations.
Treatment Administered and Safety
Median number of doses received was 6.0 (range, one to 29), 7.5 (range, one to 32), and 8.0 (range, one to 31) in the 0.3-, 2-, and 10-mg/kg groups, respectively. Dose delay occurred in 41% (n = 24), 43% (n = 23), and 39% (n = 21) of patients, respectively.
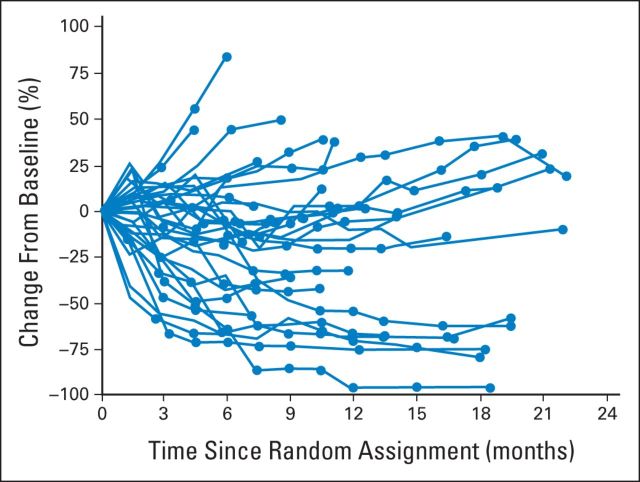
The percentage of patients treated beyond progression (patients with at least one nivolumab dose received > 6 weeks after date of RECIST [version 1.1] progression) was 17% (n = 10) in the 0.3-mg/kg group, 22% (n = 12) in the 2-mg/kg group, and 26% (n = 14) in the 10-mg/kg group. Median number of doses received after progression was 4.5, 7.5, and 8.5 in the 0.3-, 2-, and 10-mg/kg treatment groups, respectively. In some patients who continued treatment beyond initial progression, sustained reductions and/or stabilization in the size of target lesions were observed (Appendix Fig A1, online only).
Most of the 167 patients (n = 122; 73%) experienced treatment-related AEs (any grade); 19 (11%) experienced a grade 3 to 4 event (Table 3). Incidence of treatment-related AEs of any grade was similar across dose arms: 75%, 67%, and 78% in the 0.3-, 2-, and 10-mg/kg groups, respectively. Grade 3 to 4 events occurred in 5%, 17%, and 13% of patients in the 0.3-, 2-, and 10-mg/kg groups, respectively. Fatigue was the most common treatment-related AE in each group (24%, 22%, and 35% of patients, respectively). Incidence of hypersensitivity was higher in the nivolumab 10-mg/kg group than in the lower-dose groups; none of these events were grade 3 to 4. No grade 3 to 4 pneumonitis events were reported (Table 3). Systemic corticosteroids for the management of AEs (regardless of causality) were administered to nine (15%), 10 (19%), and 18 (33%) patients in the 0.3-, 2-, and 10-mg/kg groups, respectively.
Table 3.
Treatment-Related AEs
| AE | Nivolumab Arm (mg/kg) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.3 (n = 59) |
2 (n = 54) |
10 (n = 54) |
||||||||||
| Any Grade |
Grade 3 to 4 |
Any Grade |
Grade 3 to 4 |
Any Grade |
Grade 3 to 4 |
|||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
| Any treatment-related AE | 44 | 75 | 3 | 5 | 36 | 67 | 9 | 17 | 42 | 78 | 7 | 13 |
| Treatment-related AEs occurring in ≥ 10% of patients in any group | ||||||||||||
| Fatigue | 14 | 24 | 0 | 0 | 12 | 22 | 0 | 0 | 19 | 35 | 0 | 0 |
| Nausea | 6 | 10 | 1 | 2 | 7 | 13 | 1 | 2 | 7 | 13 | 0 | 0 |
| Pruritus | 6 | 10 | 0 | 0 | 5 | 9 | 1 | 2 | 6 | 11 | 0 | 0 |
| Rash | 5 | 9 | 0 | 0 | 4 | 7 | 0 | 0 | 7 | 13 | 0 | 0 |
| Appetite decreased | 2 | 3 | 0 | 0 | 7 | 13 | 0 | 0 | 2 | 4 | 0 | 0 |
| Diarrhea | 2 | 3 | 0 | 0 | 6 | 11 | 0 | 0 | 8 | 15 | 0 | 0 |
| Dry mouth | 2 | 3 | 0 | 0 | 3 | 6 | 0 | 0 | 6 | 11 | 0 | 0 |
| Arthralgia | 1 | 2 | 0 | 0 | 4 | 7 | 0 | 0 | 8 | 15 | 1 | 2 |
| Dry skin | 1 | 2 | 0 | 0 | 3 | 6 | 0 | 0 | 7 | 13 | 0 | 0 |
| Hypersensitivity | 1 | 2 | 0 | 0 | 1 | 2 | 0 | 0 | 9 | 17 | 0 | 0 |
| Treatment-related select AEs* | ||||||||||||
| Skin | 13 | 22 | 0 | 0 | 12 | 22 | 2 | 4 | 15 | 28 | 0 | 0 |
| Pruritus | 6 | 10 | 0 | 0 | 5 | 9 | 1 | 2 | 6 | 11 | 0 | 0 |
| Rash | 5 | 9 | 0 | 0 | 4 | 7 | 0 | 0 | 7 | 13 | 0 | 0 |
| Endocrine | 4 | 7 | 0 | 0 | 6 | 11 | 2 | 4 | 8 | 15 | 0 | 0 |
| Hypothyroidism | 2 | 3 | 0 | 0 | 4 | 7 | 1 | 2 | 4 | 7 | 0 | 0 |
| GI | 3 | 5 | 0 | 0 | 6 | 11 | 1 | 2 | 8 | 15 | 0 | 0 |
| Diarrhea | 2 | 3 | 0 | 0 | 6 | 11 | 0 | 0 | 8 | 15 | 0 | 0 |
| Pulmonary | 3 | 5 | 0 | 0 | 2 | 4 | 0 | 0 | 4 | 7 | 0 | 0 |
| Pneumonitis | 3 | 5 | 0 | 0 | 2 | 4 | 0 | 0 | 3 | 6 | 0 | 0 |
| Hepatic | 2 | 3 | 1 | 2 | 4 | 7 | 2 | 4 | 3 | 6 | 0 | 0 |
| AST increased | 2 | 3 | 1 | 2 | 4 | 7 | 1 | 2 | 2 | 4 | 0 | 0 |
| ALT increased | 2 | 3 | 1 | 2 | 2 | 4 | 1 | 2 | 3 | 6 | 0 | 0 |
| Renal | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 0 | 0 |
| Blood creatinine increased | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 0 | 0 |
Abbreviation: AE, adverse event.
Defined as AE with potential immune-mediated etiology, which may require special monitoring and specific unique interventions. Full listing of preferred terms for each select AE category is provided in online-only Appendix Table A4.
Treatment-related AEs leading to discontinuation of study drug occurred in 7% (n = 11) of patients (2% [n = 1], 11% [n = 6], and 7% [n = 4] in 0.3-, 2-, and 10-mg/kg groups, respectively). The most common reason for treatment-related discontinuation was an elevated level of serum AST, occurring in two patients. Types of treatment-related AEs leading to discontinuation in each group included cardiac disorders (0.3-mg/kg group, n = 1 patient), endocrine disorders (2-mg/kg group, n = 2 patients), and nervous system and respiratory or thoracic disorders (10-mg/kg group, n = 2 patients each). No treatment-related deaths were reported.
PD-L1 Expression
As an exploratory end point, efficacy parameters were assessed according to PD-L1–expression status at a 5% cutoff. In total, 107 of 168 patients (64%) were PD-L1 quantifiable. Of these, 29 (27%) had PD-L1 expression ≥ 5%, and 78 (73%) had expression < 5%. Median PFS was 4.9 months in the PD-L1 ≥ 5% subgroup versus 2.9 months in the PD-L1 < 5% subgroup (Appendix Table A3, online only); ORR was 31% in the PD-L1 ≥ 5% subgroup and 18% in the PD-L1 < 5% subgroup (Appendix Table A3, online only); median OS was NR in the PD-L1 ≥ 5% subgroup and 18.2 months in the PD-L1 < 5% subgroup (Appendix Table A3, online only). When a cutoff ≥ 1% for PD-L1 expression was used to define PD-L1 positivity, median PFS, ORR, and OS were similar in PD-L1–positive (n = 43) and PD-L1–negative patients (n = 64; data not shown).
DISCUSSION
Nivolumab demonstrated antitumor activity in this randomized, dose-ranging phase II trial. ORR was similar by treatment arm, ranging from 20% to 22% and including patients with ongoing, durable objective responses. At data cutoff, 40% of the 35 objective responders were still responding ≥ 24 months from start of nivolumab therapy.
No dose-response relationship was observed. Seventy percent of patients had received more than one prior systemic regimen, including 40% who had received two or three prior antiangiogenic drugs and approximately one third who had received prior everolimus. In our study, nivolumab treatment resulted in a median PFS of up to 4.2 months (10-mg/kg dose arm) when assessed by conventional RECIST criteria. Median OS values observed in our study were numerically higher than those reported in pivotal phase III trials in mRCC.29–32 Median OS was 15.2 months (95% CI, 12.8 to 18.3 months) with axitinib and 16.5 months (95% CI, 13.7 to 19.2 months) with sorafenib as second-line treatment (both in patients who experienced progression on sunitinib) in the AXIS (Axitinib Versus Sorafenib) trial31 and 11 months (95% CI, 8.6 to 13.5 months) with sorafenib as third-line therapy in the GOLD (Global Oncologic Learnings for Dovitinib) trial.32 In RECORD-1 (Renal Cell Cancer Treatment With Oral RAD001 Given Daily), median OS for everolimus was 14.8 months (95% CI not stated).30 Cross-study comparisons should be interpreted cautiously, because differences in trial design influence the results. Nevertheless, they can be used to generate hypotheses. Our data suggest that nivolumab may produce a greater improvement in OS than that observed in previous trials.29–32 Also, comparison of OS results among arms suggests that a higher dose could be an important factor in achieving a longer OS. Although longer OS was observed in patients with MSKCC favorable-risk score and in those who had received only one line of prior therapy, robust results were seen across all risk groups and lines of therapy.
The OS benefit observed in our study was of a greater degree than would have been predicted from the PFS results and may be related to the immunostimulatory mechanism of action of nivolumab. Tumor kinetics could initially outpace the time required for immune-cell activation to occur. In addition, immune-cell infiltration of the tumor might mimic progression. Together, these phenomena may lead to the detection of transient progression that could negatively affect the assessment of PFS, but not OS. Similar findings have been observed with ipilimumab and nivolumab in patients with malignant melanoma.33 Results from the phase III trial (Clinicaltrials.gov identifier NCT01668784) might help us to further understand this relationship.
A modified version of RECIST, the immune-related response criteria, was proposed to more adequately address the delayed and mixed responses observed with immunotherapy.19 Using these immune-related criteria, median PFS values for the three study arms were longer, compared with those based on standard RECIST assessment. Treatment beyond RECIST-defined progression was allowed in this study and may be an important strategy for extending OS. Also, our data show that although response to treatment was higher in patients with greater PD-L1 expression (≥ 5%), those with lower PD-L1 expression (< 5%) also had meaningful responses. These PD-L1 outcome data were assessed as an exploratory end point but are consistent with earlier published observations.15,34 New strategies to assess response and improve outcomes are important to maximize benefit for patients treated with novel immunotherapy agents such as nivolumab.
The safety profile of nivolumab was manageable in all treatment groups (Appendix Table A4, online only) and consistent with that reported in the phase I trial.15 The frequency of treatment-related AEs was similar across groups, and treatment-related AEs were primarily low grade in severity. Cases of drug-related pneumonitis associated with fatal outcome were observed in the phase I trial in other tumor types,15 but no high-grade pneumonitis was observed in our trial.
Findings from our dose-ranging study, coupled with analyses of safety and efficacy across tumor types from a large phase I study,15 support the selection of nivolumab 3 mg/kg intravenously every 2 weeks as the monotherapy dosing regimen for further study. Our results add to a growing body of evidence supporting the efficacy of nivolumab immunotherapy in mRCC.15,34,35 There are a number of ongoing studies that will further elucidate this evidence, including a phase III trial comparing nivolumab versus everolimus using an OS primary end point in patients with mRCC pretreated with antiangiogenic therapy (Clinicaltrials.gov identifier NCT01668784). Encouraging antitumor activity was observed in a phase IB trial evaluating the combination of nivolumab and ipilimumab in patients with mRCC.36 A phase III trial using OS as a primary end point is under way to evaluate this combination in the first-line setting (Clinicaltrials.gov identifier NCT02231749).
Supplementary Material
Acknowledgment
We thank the patients and their families; research colleagues and clinical teams; Bristol-Myers Squibb (Lawrenceville, NJ) and Ono Pharmaceutical Company (Osaka City, Japan) for supporting the work; and Sola Neunie and Shala Thomas, PhD, of PPSI, who provided writing and editorial assistance, funded by Bristol-Myers Squibb.
Appendix
Immune-Related Response Criteria
The immune-related response criteria are based on the conventional RECIST (version 1.1; Appendix Table A1), with the following major modifications: requirement to confirm progression ≥ 4 weeks after scan indicating initial progression and not scoring new small nontarget lesions as evidence of progression (instead, net tumor burden is used to gauge progression).19 Immune-related progression-free survival (PFS) was defined in the same way as PFS, and analysis was conducted similar to the analysis of PFS. Median immune-related PFS and hazard ratios along with 80% CIs were estimated.
Immune-Related Responses
Immune-related response assessment criteria were applied by the sponsor to investigator-assessed tumor measurements. Median immune-related PFS was 4.3 (80% CI, 2.8 to 6.9), 5.4 (80% CI, 4.2 to 7.1), and 6.9 months (80% CI, 4.4 to 8.5) in the nivolumab 0.3-, 2-, and 10-mg/kg groups, respectively (Appendix Table A2). Immune-related objective response rate was 20%, 22%, and 26% in the nivolumab 0.3-, 2-, and 10-mg/kg groups, respectively (Appendix Table A2), similar to the corresponding objective response rate (Table 2).
Table A1.
Immune-Related RECIST (version 1.1) Definitions
| Target Lesion Response | Nontarget Lesion Response | New Measurable Lesions | New Nonmeasurable Lesions | Change in Tumor Burden (%)* | Overall Immune-Related Response |
|---|---|---|---|---|---|
| CR | CR | Any | Any | −100 | CR |
| PR | Any | Any | Any | ≤ −30 | PR |
| > −30 to < 20 | SD | ||||
| ≥ 20 | PD | ||||
| SD | Any | Any | Any | > −30 to < 20 | SD |
| ≥ 20 | PD | ||||
| PD | Any | Any | Any | ≥ 20 | PD |
Abbreviations: CR, complete response; PD, progressive disease; PR, partial response; SD, stable disease.
Including measurable new lesions when present.
Table A2.
Exploratory End Points Based on Immune-Related RECIST (version 1.1) Criteria
| Parameter | Nivolumab Arm (mg/kg) |
|||||
|---|---|---|---|---|---|---|
| 0.3 (n = 60) |
2 (n = 54) |
10 (n = 54) |
||||
| No. | % | No. | % | No. | % | |
| Immune-related PFS | ||||||
| Events | 35 | 58 | 33 | 61 | 31 | 57 |
| Median, months | 4.3 | 5.4 | 6.9 | |||
| 80% CI | 2.8 to 6.9 | 4.2 to 7.1 | 4.4 to 8.5 | |||
| 6-month rate, % | 0.4 | 0.5 | 0.5 | |||
| 80% CI | 0.3 to 0.5 | 0.4 to 0.6 | 0.5 to 0.6 | |||
| HR* | ||||||
| 2 v 0.3 mg/kg | 0.9 | |||||
| 80% CI | 0.7 to 1.2 | |||||
| 10 v 0.3 mg/kg | 0.9 | |||||
| 80% CI | 0.6 to 1.2 | |||||
| 10 v 2 mg/kg | 1.0 | |||||
| 80% CI | 0.7 to 1.4 | |||||
| Trend test P† | .6 | |||||
| Immune-related ORR | ||||||
| Events‡ | 12 | 20 | 12 | 22 | 14 | 26 |
| Exact 80% CI | 13.4 to 28.2 | 15.0 to 31.1 | 18.2 to 35.1 | |||
| Stratified odds ratio | ||||||
| 2 v 0.3 mg/kg | 1.2 | |||||
| 80% CI | 0.6 to 2.3 | |||||
| 10 v 0.3 mg/kg | 1.3 | |||||
| 80% CI | 0.7 to 2.6 | |||||
| 10 v 2 mg/kg | 1.3 | |||||
| 80% CI | 0.7 to 2.6 | |||||
| Trend test P§ | .5 | |||||
Abbreviations: HR, hazard ratio; ORR, objective response rate; PFS, progression-free survival.
Stratified Cox proportional hazards model.
Stratified log-rank trend test with 20% significance level (two sided).
Using same definition of PFS as for primary end point but accounting for assessment that occurred after initiation of subsequent anticancer therapy.
Complete plus partial responses per immune-related RECIST (version 1.1) criteria (sponsor assessment).
Table A3.
Summary of Efficacy Results According to PD-L1 Expression Status (prototype assay) at 5% Cutoff (randomly assigned patients*)
| Parameter | PD-L1 Expression |
|||
|---|---|---|---|---|
| < 5% (n = 78) |
≥ 5% (n = 29) |
|||
| No. | % | No. | % | |
| PFS | 64 | 82 | 22 | 76 |
| Median, months | 2.9 | 4.9 | ||
| 95% CI | 2.1 to 4.2 | 1.4 to 7.8 | ||
| ORR | 14 | 18 | 9 | 31 |
| 95% CI | 10.2 to 28.3 | 15.3 to 50.8 | ||
| OS | 47 | 60 | 13 | 45 |
| Median, months | 18.2 | NR | ||
| 95% CI | 12.7 to 26.0 | 13.4 to NR | ||
Abbreviations: NR, not reached; ORR, objective response rate; OS, overall survival; PD-L1, programmed death ligand 1; PFS, progression-free survival.
Data for 61 patients were not available or missing from analysis.
Table A4.
Nivolumab Select AEs
| Category | Preferred Term |
|---|---|
| Endocrine events | Adrenal insufficiency |
| Adrenal suppression | |
| Blood corticotrophin decreased | |
| Blood corticotrophin increased | |
| Secondary adrenocortical insufficiency | |
| Diabetes mellitus | |
| Latent autoimmune diabetes in adults | |
| Hypophysitis | |
| Autoimmune thyroiditis | |
| Blood thyroid-stimulating hormone decreased | |
| Blood thyroid-stimulating hormone increased | |
| Hyperthyroidism | |
| Hypothyroidism | |
| Thyroid function test abnormal | |
| Thyroiditis | |
| Thyroxine decreased | |
| Thyroxine free decreased | |
| Thyroxine free increased | |
| Thyroxine increased | |
| Tri-iodothyronine uptake increased | |
| GI events | Colitis |
| Diarrhea | |
| Enteritis | |
| Enterocolitis | |
| Frequent bowel movements | |
| GI perforation | |
| Hepatic events | Acute hepatic failure |
| ALT increased | |
| AST increased | |
| Bilirubin conjugated increased | |
| Blood bilirubin increased | |
| Hepatic enzyme increased | |
| Hepatic failure | |
| Hepatitis | |
| Hyperbilirubinemia | |
| Liver disorder | |
| Liver function test abnormal | |
| Transaminases increased | |
| Infusion reactions events | Anaphylactic reaction |
| Hypersensitivity | |
| Infusion-related reaction | |
| Pulmonary events | Acute respiratory distress syndrome |
| Acute respiratory failure | |
| Interstitial lung disease | |
| Lung infiltration | |
| Pneumonitis | |
| Renal events | Blood creatinine increased |
| Creatinine renal clearance decreased | |
| Hypercreatininemia | |
| Nephritis | |
| Nephritis allergic | |
| Renal failure | |
| Renal failure acute | |
| Renal tubular necrosis | |
| Tubulointerstitial nephritis | |
| Skin events | Blister |
| Dermatitis | |
| Dermatitis exfoliative | |
| Drug eruption | |
| Eczema | |
| Erythema | |
| Exfoliative rash | |
| Palmar-plantar erythrodysesthesia syndrome | |
| Photosensitivity reaction | |
| Pruritus | |
| Pruritus allergic | |
| Pruritus generalized | |
| Psoriasis | |
| Rash | |
| Rash erythematous | |
| Rash generalized | |
| Rash macular | |
| Rash maculopapular | |
| Rash papular | |
| Rash pruritic | |
| Skin exfoliation | |
| Skin irritation | |
| Urticaria |
Abbreviation: AE, adverse event.
Fig A1.
Changes in measurable lesions from study baseline in patients treated beyond progression (n = 36 [0.3 mg/kg, n = 10; 2 mg/kg, n = 12; 10 mg/kg, n = 14]). Circles represent assessments that occurred after initial progression.
Footnotes
Processed as a Rapid Communication manuscript.
Supported by Bristol-Myers Squibb (Lawrenceville, NJ; which also funded writing and editorial assistance) and Ono Pharmaceutical Company (Osaka City, Japan).
Presented at the 50th Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, May 30-June 3, 2014, and the European Society for Medical Oncology Congress, Madrid, Spain, September 26-30, 2014.
Clinical trial information: NCT01354431.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at www.jco.org.
AUTHOR CONTRIBUTIONS
Conception and design: Robert J. Motzer, Saby George, Alexandre M. Lambert, Ian M. Waxman
Provision of study materials or patients: Robert J. Motzer, Brian I. Rini, David F. McDermott, Bruce G. Redman, Ulka N. Vaishampayan, Saby George, Elizabeth R. Plimack
Collection and assembly of data: Robert J. Motzer, Brian I. Rini, David F. McDermott, Bruce G. Redman, Timothy M. Kuzel, Michael R. Harrison, Ulka N. Vaishampayan, Harry A. Drabkin, Saby George, Theodore F. Logan, Elizabeth R. Plimack, Ian M. Waxman, Hans J. Hammers
Data analysis and interpretation: Robert J. Motzer, Brian I. Rini, David F. McDermott, Bruce G. Redman, Timothy M. Kuzel, Michael R. Harrison, Ulka N. Vaishampayan, Saby George, Kim A. Margolin, Elizabeth R. Plimack, Alexandre M. Lambert, Ian M. Waxman, Hans J. Hammers
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Nivolumab for Metastatic Renal Cell Carcinoma: Results of a Randomized Phase II Trial
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Robert J. Motzer
Consulting or Advisory Role: Pfizer
Research Funding: Bristol-Myers Squibb (Inst), Pfizer (Inst), Genentech (Inst), Novartis (Inst), Merck (Inst), GlaxoSmithKline (Inst), Eisai (Inst)
Brian I. Rini
Consulting or Advisory Role: Pfizer, Bristol-Myers Squibb, Merck
Research Funding: Pfizer (Inst), Bristol-Myers Squibb (Inst), Roche/Genentech (Inst), GlaxoSmithKline (Inst), Immatics Biotechnologies (Inst), Millennium Pharmaceutials (Inst)
David F. McDermott
Consulting or Advisory Role: Bristol-Myers Squibb, Merck, Genentech, Pfizer
Bruce G. Redman
No relationship to disclose
Timothy M. Kuzel
Honoraria: Bayer/Onyx, Genentech/Roche, Janssen Pharmaceuticals, Celgene, Bionomics, Eisai, Argos Therapeutics, Amgen, Astellas Pharma
Speakers' Bureau: Celgene, Janssen Oncology, Genentech/Roche, Astellas Pharma
Research Funding: Millennium Takeda (Inst), Genentech/Roche (Inst), Eisai (Inst), Bayer/Onyx (Inst), Merck/Schering Plough (Inst), CureTech (Inst), MedImmune (Inst), Bristol-Myers Squibb (Inst)
Travel, Accommodations, Expenses: Genentech, Celgene, Astellas Pharma, Bayer/Onyx, Janssen Oncology, Elorac, Argos Therapeutics
Michael R. Harrison
Honoraria: Novartis, Dendreon, Prometheus
Consulting or Advisory Role: Novartis, Pfizer, Exelis, Dendreon, Bayer, AVEO Pharmaceuticals, Prometheus
Research Funding: Argos, Bristol-Myers Squibb, Dendreon, Exelixis, Janssen, Pfizer
Travel, Accommodations, Expenses: Novartis, Dendreon, Prometheus, Bristol-Myers Squibb
Ulka N. Vaishampayan
No relationship to disclose
Harry A. Drabkin
No relationship to disclose
Saby George
Employment: Amgen (I)
Consulting or Advisory Role: Bayer, Novartis, Astellas, sanofi-aventis
Research Funding: GlaxoSmithKline (Inst)
Travel, Accommodations, Expenses: Bayer, sanofi-aventis, Astellas
Theodore F. Logan
Consulting or Advisory Role: Argos, AVEO Pharmaceuticals, Bristol-Myers Squibb, Celgene, Genentech, GlaxoSmithKline, Novartis, Pfizer, Prometheus, Wyeth
Speakers' Bureau: Bristol-Myers Squibb, Novartis, Pfizer, Prometheus, Wyeth, GlaxoSmithKline
Research Funding: Bristol-Myers Squibb (Inst), Abbott Laboratories (Inst), Abraxis (Inst), Acceleron Pharma (Inst), Amgen (Inst), Argos (Inst), AstraZeneca (Inst), AVEO Pharmaceuticals (Inst), Biovex (Inst), Bristol-Myers Squibb (Inst), Eisai (Inst), Eli Lilly (Inst), GlaxoSmithKline (Inst), Hoffman-LaRoche (Inst), Immatics Biotechnologies (Inst), Merck (Inst), Novartis (Inst), Pfizer (Inst), Prometheus (Inst), Roche (Inst), Synta (Inst), Threshold (Inst)
Kim A. Margolin
Consulting or Advisory Role: Oncosec, Nektar, NeoStem, Prothena
Elizabeth R. Plimack
Consulting or Advisory Role: Merck, Dendreon, GlaxoSmithKline, Astellas
Research Funding: Merck (Inst), Bristol-Myers Squibb (Inst), GlaxoSmithKline (Inst), Acceleron Pharma (Inst), Dendreon (Inst), Eli Lilly (Inst), AstraZeneca (Inst)
Alexandre M. Lambert
Employment: Bristol-Myers Squibb
Stock or Other Ownership: Bristol-Myers Squibb
Ian M. Waxman
Employment: Bristol-Myers Squibb
Stock or Other Ownership: Bristol-Myers Squibb
Hans J. Hammers
Consulting or Advisory Role: AVEO Pharmaceuticals, Bristol-Myers Squibb
Research Funding: Bristol-Myers Squibb (Inst), GlaxoSmithKline (Inst), Pfizer (Inst), Exelixis (Inst)
Travel, Accommodations, Expenses: Bristol-Myers Squibb
REFERENCES
- 1.Escudier B, Eisen T, Porta C, et al. Renal cell carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(suppl 7):vii65–vii71. doi: 10.1093/annonc/mds227. [DOI] [PubMed] [Google Scholar]
- 2.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Kidney cancer. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp. [DOI] [PubMed]
- 3.Finley DS, Pantuck AJ, Belldegrun AS. Tumor biology and prognostic factors in renal cell carcinoma. Oncologist. 2011;16(suppl 2):4–13. doi: 10.1634/theoncologist.2011-S2-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drake CG, Jaffee E, Pardoll DM. Mechanisms of immune evasion by tumors. Adv Immunol. 2006;90:51–81. doi: 10.1016/S0065-2776(06)90002-9. [DOI] [PubMed] [Google Scholar]
- 5.Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480:480–489. doi: 10.1038/nature10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mizoguchi H, O'Shea JJ, Longo DL, et al. Alterations in signal transduction molecules in T lymphocytes from tumor-bearing mice. Science. 1992;258:1795–1798. doi: 10.1126/science.1465616. [DOI] [PubMed] [Google Scholar]
- 7.Topalian SL, Weiner GJ, Pardoll DM. Cancer immunotherapy comes of age. J Clin Oncol. 2011;29:4828–4836. doi: 10.1200/JCO.2011.38.0899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zitvogel L, Tesniere A, Kroemer G. Cancer despite immunosurveillance: Immunoselection and immunosubversion. Nat Rev Immunol. 2006;6:715–727. doi: 10.1038/nri1936. [DOI] [PubMed] [Google Scholar]
- 9.Bedke J, Gouttefangeas C, Singh-Jasuja H, et al. Targeted therapy in renal cell carcinoma: Moving from molecular agents to specific immunotherapy. World J Urol. 2014;32:31–38. doi: 10.1007/s00345-013-1033-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inman BA, Harrison MR, George DJ. Novel immunotherapeutic strategies in development for renal cell carcinoma. Eur Urol. 2013;63:881–889. doi: 10.1016/j.eururo.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 11.Hamanishi J, Mandai M, Iwasaki M, et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci U S A. 2007;104:3360–3365. doi: 10.1073/pnas.0611533104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamid O, Carvajal RD. Anti-programmed death-1 and anti-programmed death-ligand 1 antibodies in cancer therapy. Expert Opin Biol Ther. 2013;13:847–861. doi: 10.1517/14712598.2013.770836. [DOI] [PubMed] [Google Scholar]
- 13.Nurieva RI, Liu X, Dong C. Molecular mechanisms of T-cell tolerance. Immunol Rev. 2011;241:133–144. doi: 10.1111/j.1600-065X.2011.01012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brahmer JR, Drake CG, Wollner I, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: Safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28:3167–3175. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Motzer RJ, Bacik J, Schwartz LH, et al. Prognostic factors for survival in previously treated patients with metastatic renal cell carcinoma. J Clin Oncol. 2004;22:454–463. doi: 10.1200/JCO.2004.06.132. [DOI] [PubMed] [Google Scholar]
- 17.International Conference on Harmonisation Expert Working Group. Guidance for Industry: E6 Good Clinical Practice—Consolidated Guidance. http://www.fda.gov/downloads/Drugs/Guidances/ucm073122.pdf.
- 18.World Medical Association. World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. http://www.wma.net/en/30publications/10policies/b3/17c.pdf. [DOI] [PubMed]
- 19.Wolchok JD, Hoos A, O'Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: Immune-related response criteria. Clin Cancer Res. 2009;15:7412–7420. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 20.National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_40.
- 21.Cogswell JP, Goldberg SM, Gupta AK, et al. Cancer immunotherapy by disrupting pd-1/pd-l1 signaling. http://www.freepatentsonline.com/20130309250.pdf.
- 22.Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: A potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 23.Taube JM, Anders RA, Young GD, et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med. 2012;4:127ra37. doi: 10.1126/scitranslmed.3003689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cox DR. Regression models and life tables. J R Stat Soc B. 1972;34:187–220. [Google Scholar]
- 25.Clopper CJ, Pearson ES. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika. 1934;26:404–413. [Google Scholar]
- 26.Armitage P. Tests for linear trends in proportions and frequencies. Biometrics (International Biometric Society) 1955;11:375–386. [Google Scholar]
- 27.Cochran WG. Some methods for strengthening the common chi-squared tests. Biometrics (International Biometric Society) 1954;10:417–451. [Google Scholar]
- 28.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 29.Hutson TE, Escudier B, Esteban E, et al. Randomized phase III trial of temsirolimus versus sorafenib as second-line therapy after sunitinib in patients with metastatic renal cell carcinoma. J Clin Oncol. 2014;32:760–767. doi: 10.1200/JCO.2013.50.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Motzer RJ, Escudier B, Oudard S, et al. Phase 3 trial of everolimus for metastatic renal cell carcinoma: Final results and analysis of prognostic factors. Cancer. 2010;116:4256–4265. doi: 10.1002/cncr.25219. [DOI] [PubMed] [Google Scholar]
- 31.Motzer RJ, Escudier B, Tomczak P, et al. Axitinib versus sorafenib as second-line treatment for advanced renal cell carcinoma: Overall survival analysis and updated results from a randomised phase 3 trial. Lancet Oncol. 2013;14:552–562. doi: 10.1016/S1470-2045(13)70093-7. [DOI] [PubMed] [Google Scholar]
- 32.Motzer RJ, Porta C, Vogelzang NJ, et al. Dovitinib versus sorafenib for third-line targeted treatment of patients with metastatic renal cell carcinoma: An open-label, randomised phase 3 trial. Lancet Oncol. 2014;15:286–296. doi: 10.1016/S1470-2045(14)70030-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Choueiri TK, Fishman MN, Escudier BJ, et al. Immunomodulatory activity of nivolumab in previously treated and untreated metastatic renal cell carcinoma (mRCC): Biomarker-based results from a randomized clinical trial. J Clin Oncol. 2014;(suppl 15s):32. abstr 5012. [Google Scholar]
- 35.McDermott DF, Drake CG, Sznol M, et al. Clinical activity and safety of antiprogrammed death-1 (PD-1) (BMS-936558/MDX-1106/ONO-4538) in patients (pts) with previously treated, metastatic renal cell carcinoma (mRCC): An updated analysis. J Clin Oncol. 2013;(suppl):31. abstr 351. [Google Scholar]
- 36.Hammers HJ, Plimack ER, Infante JR, et al. Phase I study of nivolumab in combination with ipilimumab in metastatic renal cell carcinoma (mRCC) J Clin Oncol. 2014;32(suppl 15s):297s. doi: 10.1200/JCO.2016.72.1985. abstr 4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.