Abstract
Hematopoietic stem cell transplantation is a curative therapy for hematological malignancies. T cell deficiency following transplantation is a major cause of morbidity and mortality. In this review, we discuss adoptive transfer of committed precursor cells to enhance T cell reconstitution and improve overall prognosis after transplantation.
Keywords: T cell, Bone marrow transplantation, Immune reconstitution, Adoptive immunotherapy
Hematopoietic stem cell transplantation (HSCT) is a potentially curative therapy for a variety of hematological malignancies, solid tumors, and nonmalignant hematological diseases. One of its major complications is a prolonged posttransplant immune deficiency, which is most evident in the T cell compartment and leads to an increase in morbidity and mortality predominantly due to opportunistic infections [1]. In addition, in some studies, enhanced early lymphocyte reconstitution has been associated with less malignant relapse and can be used as a prognostic indicator of disease outcome in both autologous and allogeneic HSCT [2–5]. Following HSCT, T cell reconstitution occurs via (1) clonal expansion of radio/chemoresistant host T cells, (2) clonal expansion of mature donor T cells transferred with the graft, and (3) thymus-dependent and possibly thymus-independent de novo generation of donor lymphocytes. T cell recovery is inversely proportional to age [5]. In younger patients, T cell recovery can take up to 12 months, while in older patients, T cells may never reach pretransplant levels [6]. The significant delays in T cell reconstitution in older patients can be attributed to age-related thymic involution and the coincident decrease in export of naïve T cells from the thymus [6]. In general, the thymi of young recipients are capable of supporting the production of a new repertoire of selected, functional T cells. This does, however, depend on several factors: thymic damage due to radio/chemotherapy, engraftment of donor-derived hematopoietic precursors, and graft-versus-host disease (GVHD) occurrence and treatment.
In this review, we will discuss the use of adoptive precursor cell therapies as a means to enhance T cell reconstitution and decrease morbidity and mortality following HSCT.
Mouse T cell development
T cells develop in the thymus, unlike other lymphoid lineages, which develop in the bone marrow from resident hematopoietic stem cells [7, 8]. Although the thymus is essential for T cell differentiation, it does not contain self-renewing T cell progenitors. Instead, bone marrow-derived precursors enter the thymus via the bloodstream [9–12]. Although T cell precursors in the early stages of T cell development maintain their potential to develop into non-T lineages [13, 14], under normal circumstances, the majority will follow a well-characterized differentiation pathway. Thymic entry is a gated phenomenon in which progenitors are able to engraft only when space is available in thymic niches and microvascular entry sites or gates are open [15–18]. Engraftment in the thymus is further regulated by a variety of adhesion interactions and chemotactic events [19]. P-selectin glycoprotein ligand 1 (PSGL-1) and CD44 [20], as well as the C–C chemokine receptor 9 (CCR9) [21–23] facilitate progenitor entry into the thymus.
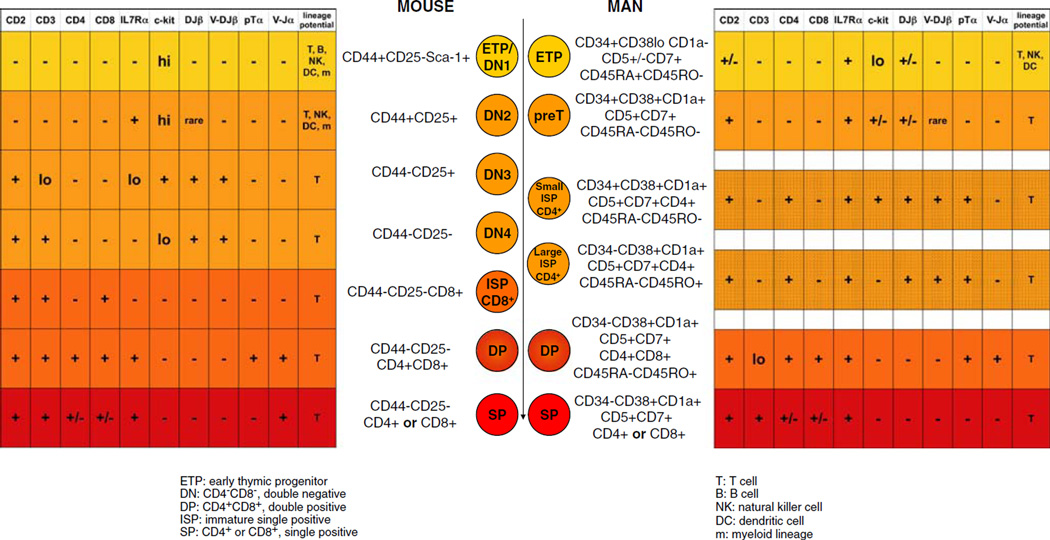
The identity of the thymus-seeding progenitor remains unknown; however, the phenotype of the early T lineage progenitor (ETP), the earliest thymocyte subset, is defined as lineage marker-negative (lin)−CD44+CD25−c-kit+ [24]. As shown in Fig. 1, ETPs are, therefore, contained within the most immature thymocyte subset, CD4−CD8− (double negative [DN]) cells. DN thymocytes later develop into CD4+CD8+ (double positive [DP]) cells, then pass through a CD8 intermediate single positive phase followed by differentiation into either CD4+ or CD8+ (single positive [SP]) cells. The DN compartment is further divided on the basis of CD44 and CD25 expression [25–27]. In order to mature, thymocytes traverse the thymus and receive differentiation signals from thymic stromal (especially epithelial) and dendritic cells. DN1 (CD44+CD25−) cells are found in the cortex near the corticomedullary junction, the site at which bloodborne precursors enter the thymus [28–30]. Accordingly, the subsequent developmental stages of thymocytes are observed in distinct regions of the thymus. DN2 (CD44+CD25+) cells are located in the cortex, DN3 (CD44−CD25+) near the subcapsular region, and DN4 (CD44−CD25−) and DP cells begin to travel from the subcapsular region back toward the medulla [28–30]. Subsequent thymocyte subsets are primarily located in the medulla where they finish the process of T cell receptor (TCR) rearrangement and undergo positive and negative selection, all of which require interaction with the thymic microenvironment.
Fig. 1.
Comparison of known stages of mouse and human T cell development in the thymus [24, 36, 40, 77–83]
During the DN2 stage, thymocytes upregulate the recombination-activating gene (Rag) 1 and 2 genes and begin TCR β chain (TCRβ) rearrangement [25, 31–33]. Thymocytes express TCRβ at the cell surface with the pre-TCR α chain (pTα) during the DN3 stage. Signaling through the pre-TCR indicates a productive β chain rearrangement, in the process of β selection, which entails allelic exclusion, and induction of α chain rearrangement [34]. Additionally, pre-TCR signaling promotes CD25 downregulation and progression to DN4 and DP stages [32]. The vast majority of thymocytes undergo apoptosis before differentiating into naive T cells, which is prevented via pre-TCR signaling [35]. Thymocytes then undergo positive selection, during which they are rescued from death by neglect by TCR/major histocompatibility complex (MHC) interactions mediated by medullary thymic epithelial cells [36]. Thymocytes that react too strongly to the peripheral self-antigens induced by Aire in the thymus are deleted in the process known as negative selection, which results in a pool of T cells that are tolerant to self [37–39]. Throughout T cell development in the thymus, thymic epithelial cells provide crucial signals to developing thymocytes, including TCR ligation via MHC/peptide complexes, Wnt and Hedgehog pathway activation, and chemokine and cytokine signals [32]. Of particular interest are signals activating the Notch pathway, as discussed below. The correlation of developmental stages with specific microenvironments within the thymus indicates a highly specialized function for thymic stromal cells in instructing progenitors to adopt the T cell fate.
Human T cell development
As in the mouse, human T cells develop primarily in the thymus. The stages of T cell development in the human thymus have yet to be definitively characterized; however, the current information about human T cell development within the thymus is represented in Fig. 1. It is known that hematopoietic precursors in man express CD34+, and precursors with a bias toward T/NK cell fate found in the bone marrow and cord blood are additionally CD45RA+CD7+CD10−IL-7Rα− [40]. The phenotype of the thymus-seeding progenitor is unknown, but precursors must enter from the bloodstream because the human thymus, like the mouse, does not contain self-renewing precursors [41]. Human ETPs, the most immature thymocyte subset, are CD34+CD45RA+CD7+CD10+IL-7Rα+, but they do not express CD1a; downstream of the ETP, the first cell type committed to T cell fate shares the ETP cell surface phenotype but additionally expresses CD1a [40]. Subsequently, human thymocytes pass through a CD4 intermediate single positive stage before reaching the CD4+CD8+ double positive stage, undergoing positive and negative selection, and eventually becoming single positive cells [41]. The program of β selection does not appear to be as tightly regulated in man as in mouse and does not correlate well with CD4 or CD8 expression [40, 41]. However, the human, like the murine, thymic microenvironment provides critical T cell developmental cues such as Notch signaling [40].
In vitro T cell development systems
In vivo, thymic stromal cells are necessary for the production of T cells that are both functional and self-tolerant. To study T cell development in vitro, it has, therefore, been necessary to culture precursor cells with supporting cells. Early studies were undertaken using dispersed culture methods, analyzing single cell suspensions of thymocytes and/or stromal cells (reviewed in [42]). Prior to the advent of the OP9–DL1 system, fetal thymus organ culture and reaggregate thymus organ culture were the most common techniques used to study in vitro T cell development. Both provide a three-dimensional environment for T cell development allowing for the complex array of thymocyte–stromal interactions to be studied [43, 44]. While these culture systems made it possible to study stromal–thymocyte interaction as well as thymic selection in vitro, they cannot be used as a means to generate large numbers of cells for adoptive immunotherapy.
Adoptive transfer of committed progenitors isolated from bone marrow
Due to technical limitations in generating sufficient numbers of committed T cell precursors in vitro for adoptive therapy, there have been few attempts to employ committed lymphoid precursors for adoptive transfer. Arber et al. [45] developed an adoptive transfer strategy for improving lymphoid reconstitution after HSCT utilizing CLPs, the lin−IL-7Rα+Thy-1−c-kitloSca-1lo bone marrow cell population with restricted lymphoid potential. The addition of small numbers of CLPs to HSC grafts after lethal irradiation led to greater lymphoid reconstitution in the early stages posttransplant and overall improved survival. CLPs were safe to use in syngeneic and allogeneic models of transplantation, and they never caused GVHD. The inclusion of CLPs in the graft also improved resistance to murine cytomegalovirus, in both euthymic and thymectomized recipients. The early improvement in lymphoid reconstitution included T, NK, and B cells; however, in the later stages posttransplant, the immune reconstitution benefit was restricted primarily to the B cell lineage. Despite its early T cell reconstitution benefit, this strategy has its limitations, given the difficulty of isolating sufficient numbers of CLPs from the bone marrow and the limited T lineage potential of CLPs.
The role of Notch signaling in T cell development
Among the known critical signaling events in the thymus, Notch activation has been identified as a master regulator of lymphocyte differentiation [46, 47]. The highly conserved Notch proteins were originally identified in Drosophila melanogaster where the name derives from a “notched” wing phenotype in mutant fly strains [46]. Mammals express four Notch receptors, Notch1–4, on cells of hematopoietic origin [47]. The ligands responsible for activating Notch signaling in mammals are Delta-like 1, 3, and 4 and Jagged 1 and 2 [47]. Both receptors and ligands are transmembrane proteins expressed at the cell surface. After engaging its ligand, a Notch receptor undergoes a series of proteolytic cleavages, which release the intracellular portion of Notch. The cleaved portion translocates to the nucleus where it binds RBP-J, recruits coactivators, and initiates transcription of target genes, which include the Hes family transcription factors and cell cycle proteins [47]. Notch signaling is highly conserved between species and mediates numerous cellular developmental processes, among them T cell development [46].
Radtke et al. demonstrated that inactivation of Notch1 leads to a block in T cell development and consequent ectopic development of B cells in the thymus [48]. Complementary studies demonstrated that overexpression of Notch in extrathymic sites, such as bone marrow, is sufficient to drive T cell differentiation outside the thymus [49]. The importance of Notch signaling for instructing T cell over B cell fate has been corroborated with loss- and gain-of-function studies using other members of the Notch signaling pathway, such as the transcription factor CSL [47]. Maeda et al. demonstrated that extrathymic T cell development in the bone marrow is prevented by LRF, which represses Notch signaling [50]. Additional studies have indicated that Notch signaling mediates numerous steps along the T cell differentiation pathway, both in the thymus and in the periphery [46, 47, 51]. Among the posited roles for Notch signaling in T cell development are progression through the DN stages, mediating β selection, αβ versus γδ lineage commitment, CD4 versus CD8 expression, and T helper fate [47].
Off-the-shelf adoptive immunotherapy across MHC barriers using T cell precursors generated in a Notch-based culture system
Once the essential T cell developmental role of Notch signaling was appreciated, several groups aimed to utilize the pathway for in vitro T cell development systems. It has long been possible to drive the differentiation of many hematopoietic lineages in vitro. T cells, however, were extremely difficult to generate in a culture dish and it was assumed that the complex architecture of the thymus was required for T cell development. Several recent studies demonstrate that T lymphocyte precursors can be generated in vitro with Notch-based culture and used for adoptive cell therapy [52–55]. Schmitt et al. reported that T cell precursors can be generated by coculture of murine embryonic stem cells and OP9–DL1, the OP9 mouse bone marrow stromal cell line transduced to express the Notch ligand DL1 [56] and that these precursor cells can reconstitute immunodeficient mice [53]. Dallas et al. demonstrated that mouse BM-derived HSCs that were cultured with a Delta-like 1-human IgG fusion protein (Delta1(ext-IgG)) in the presence of murine stem cell factor, human Flt-3 ligand, human IL-6, and human IL-11 could be developed into early T cell precursors (DN1 and DN2 cells, but not into further stages of T cell development) [52]. Adoptive transfer of these cells to syngeneic HSCT recipients resulted in enhanced thymic and peripheral T cell reconstitution.
We have used the OP9–DL1 system to generate DN2/3 T cell precursors for adoptive therapy in T cell-depleted allogeneic HSCT recipients (using either purified HSCs or lineage marker-negative BM as allograft) and for tumor immunotherapy across MHC barriers. In order to be able to generate large numbers of T cell precursors for adoptive therapy, we used culture conditions that result in a partial block of T cell differentiation at the DN3 stage (by using BM instead of fetal liver-derived HSCs, frequent passaging, and maintaining the IL-7 dose at 5 ng/mL), resulting in consistent and reproducible generation and expansion of up to 95%pure DN2/3 T cell precursors by 2 to 3 weeks of OP9–DL1 coculture (Zuniga-Pflucker, personal communication) [54, 57].
Critical for the success of this form of adoptive therapy is the choice of an appropriate conditioning regimen to create tolerance to allogeneic cells while clearing thymic niches to allow engraftment of adoptively transferred cells. As discussed, thymic engraftment is a gated phenomenon [15, 16, 18] and clearance of thymic niches alone does not ensure effective engraftment unless thymic gates are open at the same time, and this can be effectively achieved by irradiation (either as targeted thymic/mediastinal irradiation or as total body irradiation). Importantly, effective thymic engraftment of transferred cells was only observed on the day of irradiation [54], most likely due to radioresistant residual host thymocytes that start to proliferate within hours after irradiation and quickly occupy empty thymic niches [58]. Alternative regimens based on chemotherapeutic agents, high-dose steroids, or depleting antibodies are under investigation.
We found that large numbers (1,000-fold expansion during 3 weeks of culture) of T cell precursors can be generated from adult murine HSCs by OP9–DL1 coculture and can be used for adoptive therapy in irradiated recipients: in vitro-generated T cell precursors efficiently engrafted in the thymus, as well as extrathymic sites such as the spleen and small intestine, and gave rise to functional, mature T cells in vivo [54]. OP9–DL1-derived T cell precursors express a variety of molecules that are implicated in lymphocyte adhesion and homing, including CCR9, PSGL-1, and CD103 (unpublished data and [54]), which are required for thymic homing (PSGL-1 and CCR9) [21–23, 59] and gut homing (CCR9 and CD103) [60–63] of bone marrow-derived hematopoietic progenitor cells. Furthermore, CD44 has been suggested to be involved in thymic homing [20]. Interestingly, OP9–DL1-derived DN2 cells (CD25+CD44+) displayed better thymus as well as gut repopulating capabilities than OP9–DL1-derived DN3 cells (CD25+CD44−), indicating that CD44 may be required for efficient T cell precursor homing (unpublished data and [54]).
After allogeneic HSCT with purified HSCs, recipients of T cell precursors had earlier and enhanced T and NK cell reconstitution compared with recipients of HSCs alone, resulting in better T cell function, including increased resistance to infection with Listeria monocytogenes and increased graft-versus-tumor activity [54].
The supplementation of an allograft (purified HSCs) with T cell precursors lead to a significantly improved donor chimerism, which translated into a decreased incidence of graft failure and improved survival (unpublished data). This important finding may be attributed to a variety of mechanisms: (a) a quantitative increase in donor T cell numbers due to the additional cells derived from adoptively transferred T cell precursors, (b) a qualitative advantage due to an overall increase in donor T cell function, which will be helpful to overcome host-versus-graft activity, and (c) T cell precursors may serve as facilitating cells that improve engraftment of donor HSCs [64–67].
Interestingly, we observed that HSCT recipients, which also received T cell precursors, had improved thymopoiesis even after T cell precursor-derived cells had left the thymus (unpublished data and [54, 55]), suggesting a sustained beneficial effect of T cell precursor administration on thymic recovery, possibly due to lymphostromal interactions (thymic crosstalk) of adoptively transferred T cell precursors and regenerating host-derived thymic stroma [10, 68].
We did not observe GVHD, a major complication of allogeneic HSCT, which is mediated by donor T cells that attack host tissues such as skin, liver, or gut, indicating that thymic education of adoptively transferred allogeneic T cell precursors resulted in host tolerance [54, 55].
Histoincompatibility (especially MHC disparity) between donor and recipient is an important limiting factor of adoptive T cell therapy and can result in the elimination of the transferred T cells by the host immune system or, conversely, in GVHD. We, therefore, evaluated the TCR restriction, positive and negative selection of mature T cells derived from adoptively transferred allogeneic T cell precursors that were transferred with or without syngeneic HSCs to lethally or sublethally irradiated MHC-disparate recipients (BALB/c). We found that the progeny of adoptively transferred allogeneic T cell precursors were tolerant to both allogeneic (donor) and syngeneic (host) MHC antigens while mounting a strong proliferative response to a third party [55], which is consistent with studies in allogeneic BM chimeras [69–71]. We found tolerance to host and donor even after adoptive transfer of allogeneic T cell precursors without HSCT support in sublethally irradiated recipients. On further analysis, we found dendritic cells, which were derived from the transferred T cell precursors. These findings suggest that negative selection of adoptively transferred T cell precursors can occur by both host dendritic cells and dendritic cells that are progeny of the T cell precursors. We found that positive selection of adoptively transferred allogeneic T cell precursors is dependent on host-MHC molecules on nonhematopoietic cells (presumably thymic epithelial cells), resulting in the selection of a host-MHC-restricted TCR. Based on these findings on positive and negative selection of adoptively transferred allogeneic T cell precursors, one would predict these cells will give rise to host-tolerant and functional T cells.
We found that adoptive transfer of allogeneic precursor T cells improved survival after radiation-induced injury and enhanced antitumor activity against immunogenic tumors (lymphoma and renal cell carcinoma). This means that T cell precursors from any donor can indeed be used universally for immunotherapy in any immunosuppressed individual regardless of MHC disparities, since they will give rise to functional, host-MHC-restricted, and host-tolerant T cells.
Gene transfer is feasible with T cell precursors and we were able to generate antigen-specific T cells with a chimeric antigen receptor (CAR) for targeted immunotherapy, which is particularly relevant since most human malignancies are not very immunogenic. CARs consist of an antigen-binding domain derived from an antibody and a signal transduction domain, often derived from the ζ-chain of CD3. CARs are ideal for “off-the-shelf” therapies because they are able to activate T cells in an MHC-independent fashion, and their clinical efficacy is currently being tested in clinical trials. We adoptively transferred allogeneic T cell precursors that had been lentivirally transduced to express CAR 19z1 that targets human CD19 [72] and analyzed their progeny at days 27 and 40 after transplantation [54]. We found both CD3+CD4+ and CD3+CD8+ 19z1-expressing peripheral T cells in these animals, and the percentage of transduced T cells of allogeneic origin corresponded with the percentage of transduced DN2 cells in the cultures that had been used for adoptive transfer, suggesting normal positive and negative selection of transduced T cell precursors. TCR repertoire analysis revealed no difference between transduced and untransduced cells. In vitro stimulation with target T cells expressing hCD19 or an irrelevant antigen (hPSMA) revealed a strong response (production of IFN-γ as well as increased Lck recruitment) to hCD19 in 19z1-transduced T cells compared to WT C57BL/6 T cells. Survival in HSCT recipients that had been challenged with a 19z1-sensitive tumor (A20 lymphoma transduced to express hCD19) was significantly improved in recipients of transduced T cell precursors as opposed to recipients of nontransduced T cell precursors or of HSCs only. Moreover, when mice were challenged with a bioluminescent mouse lymphoma cell line expressing human CD19 to determine tumor growth by in vivo bioluminescence imaging, we found significantly increased antitumor activity in recipients of 19z1-expressing T cell precursors compared with recipients of nontransduced T cell precursors. These results suggest the feasibility of nonpatient-specific T cell therapy with universal “off-the shelf” T cell precursors. While immunogenic tumors may respond to therapy with nonmodified allogeneic T cell precursors, less immunogenic tumors can be targeted with genetically enhanced tumor antigen-specific T cell precursors.
The OP9–DL1 system has been used to generate T cells from human cord blood and human adult BM-derived CD34+ progenitor cells, indicating that this method could be adapted for clinical use [73, 74]. OP9–DL1 cells can be cultured in serum-free conditions (unpublished data) and may be qualified for phase I/II studies after FDA-required biosafety testing. Bernstein and colleagues have established a fully humanized Notch-based culture system [52, 75, 76] and are currently using it to expand cord blood-derived CD34+ cells from a single cord blood unit before double cord blood unit transplantation. Other possibilities for Notch-based culture systems with clinical potential include adenovirally transduced autologous BM-derived stromal cells or retrovirally transduced GMP-grade human BM-derived stromal cell lines.
T cell precursor therapy is particularly attractive as a strategy to enhance immune reconstitution after TCD-HSCT in the absence of immunosuppressive medication as GVHD prophylaxis or therapy. Its supporting effects on engraftment, donor chimerism, and long-term thymopoiesis could help to decrease the incidence of graft failure and mixed chimerism. The enhancement of overall T cell function should result in decreased morbidity and mortality from opportunistic infections as well as decreased rates of malignant relapse as a result of improved tumor immunosurveillance. It would also allow earlier (re)immunization against common pathogens and earlier applications of experimental tumor vaccination protocols after HSCT.
Since adoptively transferred T cell precursors need to be able to develop into mature T cells in vivo, thymic function of the recipients will be an important factor for the efficacy of this strategy. However, T cell precursor therapy resulted in enhanced thymic reconstitution in aging recipients with reduced thymic function (unpublished data) [55], and extrathymic T cell development was also shown to contribute to T cell reconstitution by adoptively transferred T cell precursors (unpublished data) [54]. Furthermore, immunotherapy with T cell precursors can be combined with other strategies to enhance T cell reconstitution including treatment with keratinocyte growth factor [54], IL-7, or sex steroid inhibition.
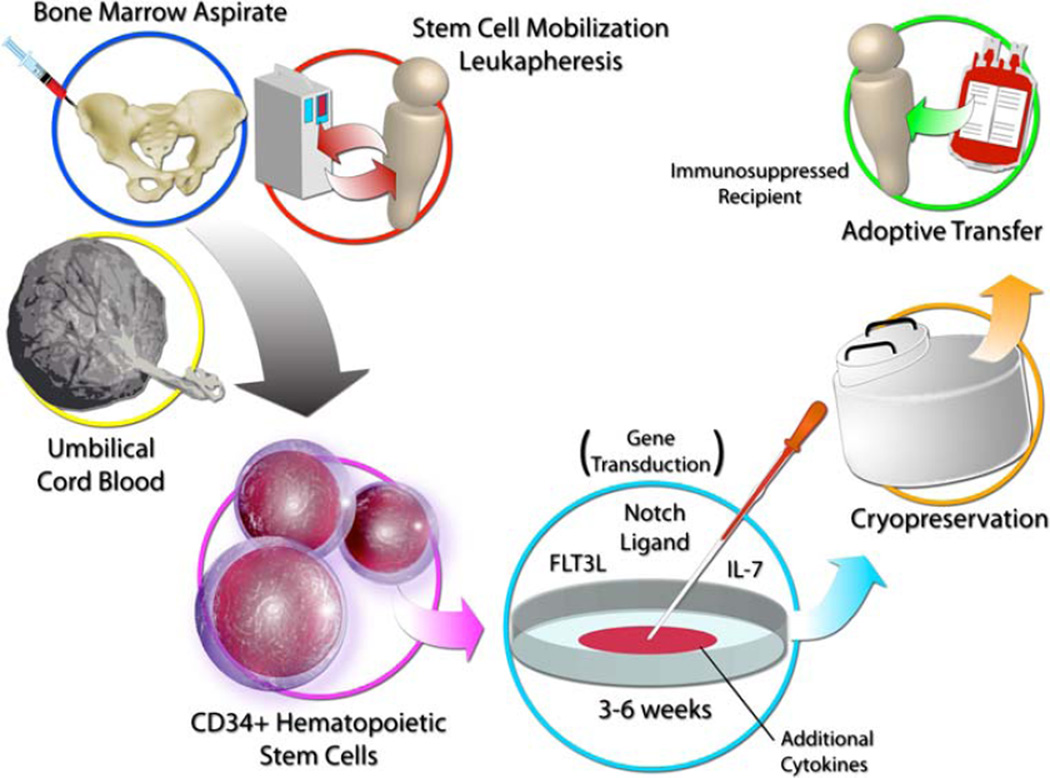
Based on the findings of these preclinical studies, adoptive transfer of allogeneic and genetically enhanced T cell precursors represents a promising novel strategy for immunotherapy in HSCT recipients, cancer patients, and possibly other conditions associated with T cell deficiency, as outlined in Fig. 2. This strategy allows, for the first time, the use of non-MHC-matched cells that can be generated in vitro and stored for immediate “off-the-shelf” use.
Fig. 2.
Proposed design for clinical use of “off-the-shelf” in vitro-derived T cell precursors for adoptive immunotherapy
Acknowledgments
This research was supported by the National Institutes of Health grants RO1-HL069929 (MvdB), RO1-CA107096 (MvdB), RO1-AI080455 (MvdB), and PO1-CA33049 (MvdB). Support was also received from the Ryan Gibson Foundation, the Elsa U. Pardee Foundation, the Byrne Fund, the Emerald Foundation, and The Experimental Therapeutics Center of Memorial Sloan-Kettering Cancer Center funded by Mr. William H. Goodwin and Mrs. Alice Goodwin and the Commonwealth Foundation for Cancer Research (MvdB).
Contributor Information
Amanda M. Holland, Department of Immunology, Memorial Sloan-Kettering Cancer Center, New York, NY 10065, USA Department of Immunology and Microbial Pathogenesis, Weill Cornell Medical College, New York, NY 10065, USA.
Johannes L. Zakrzewski, Department of Immunology, Memorial Sloan-Kettering Cancer Center, New York, NY 10065, USA
Gabrielle L. Goldberg, Department of Immunology, Memorial Sloan-Kettering Cancer Center, New York, NY 10065, USA
Arnab Ghosh, Department of Immunology, Memorial Sloan-Kettering Cancer Center, New York, NY 10065, USA.
Marcel R. M. van den Brink, Email: m-van-den-brink@ski.mskcc.org, Department of Immunology, Memorial Sloan-Kettering Cancer Center, New York, NY 10065, USA; Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY 10065, USA.
References
- 1.Parkman R, Cohen G, Carter SL, Weinberg KI, Masinsin B, Guinan E, Kurtzberg J, Wagner JE, Kernan NA. Successful immune reconstitution decreases leukemic relapse and improves survival in recipients of unrelated cord blood transplantation. Biol Blood Marrow Transplant. 2006;12:919–927. doi: 10.1016/j.bbmt.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 2.Joao C, Porrata LF, Inwards DJ, Ansell SM, Micallef IN, Johnston PB, Gastineau DA, Markovic SN. Early lymphocyte recovery after autologous stem cell transplantation predicts superior survival in mantle-cell lymphoma. Bone Marrow Transplant. 2006;37:865–871. doi: 10.1038/sj.bmt.1705342. [DOI] [PubMed] [Google Scholar]
- 3.Porrata LF, Litzow MR, Tefferi A, Letendre L, Kumar S, Geyer SM, Markovic SN. Early lymphocyte recovery is a predictive factor for prolonged survival after autologous hematopoietic stem cell transplantation for acute myelogenous leukemia. Leukemia. 2002;16:1311–1318. doi: 10.1038/sj.leu.2402503. [DOI] [PubMed] [Google Scholar]
- 4.Savani BN, Rezvani K, Mielke S, Montero A, Kurlander R, Carter CS, Leitman S, Read EJ, Childs R, Barrett AJ. Factors associated with early molecular remission after T cell-depleted allogeneic stem cell transplantation for chronic myelogenous leukemia. Blood. 2006;107:1688–1695. doi: 10.1182/blood-2005-05-1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Small TN, Papadopoulos EB, Boulad F, Black P, Castro-Malaspina H, Childs BH, Collins N, Gillio A, George D, Jakubowski A, Heller G, Fazzari M, Kernan N, MacKinnon S, Szabolcs P, Young JW, O’Reilly RJ. Comparison of immune reconstitution after unrelated and related T-cell-depleted bone marrow transplantation: effect of patient age and donor leukocyte infusions. Blood. 1999;93:467–480. [PubMed] [Google Scholar]
- 6.Parkman R, Weinberg KI. Immunological reconstitution following bone marrow transplantation. Immunol Rev. 1997;157:73–78. doi: 10.1111/j.1600-065x.1997.tb00975.x. [DOI] [PubMed] [Google Scholar]
- 7.Hirose J, Kouro T, Igarashi H, Yokota T, Sakaguchi N, Kincade PW. A developing picture of lymphopoiesis in bone marrow. Immunol Rev. 2002;189:28–40. doi: 10.1034/j.1600-065x.2002.18904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang H, Spangrude GJ. Aspects of early lymphoid commitment. Curr Opin Hematol. 2003;10:203–207. doi: 10.1097/00062752-200305000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Donskoy E, Goldschneider I. Thymocytopoiesis is maintained by blood-borne precursors throughout postnatal life. A study in parabiotic mice. J Immunol. 1992;148:1604–1612. [PubMed] [Google Scholar]
- 10.Anderson G, Jenkinson EJ. Lymphostromal interactions in thymic development and function. Nat Rev Immunol. 2001;1:31–40. doi: 10.1038/35095500. [DOI] [PubMed] [Google Scholar]
- 11.Miller JF. The discovery of thymus function and of thymus-derived lymphocytes. Immunol Rev. 2002;185:7–14. doi: 10.1034/j.1600-065x.2002.18502.x. [DOI] [PubMed] [Google Scholar]
- 12.Petrie HT. Role of thymic organ structure and stromal composition in steady-state postnatal T-cell production. Immunol Rev. 2002;189:8–19. doi: 10.1034/j.1600-065x.2002.18902.x. [DOI] [PubMed] [Google Scholar]
- 13.Balciunaite G, Ceredig R, Rolink AG. The earliest subpopulation of mouse thymocytes contains potent T, significant macrophage, and natural killer cell but no B-lymphocyte potential. Blood. 2005;105:1930–1936. doi: 10.1182/blood-2004-08-3087. [DOI] [PubMed] [Google Scholar]
- 14.Bell JJ, Bhandoola A. The earliest thymic progenitors for T cells possess myeloid lineage potential. Nature. 2008;452:764–767. doi: 10.1038/nature06840. [DOI] [PubMed] [Google Scholar]
- 15.Donskoy E, Foss D, Goldschneider I. Gated importation of prothymocytes by adult mouse thymus is coordinated with their periodic mobilization from bone marrow. J Immunol. 2003;171:3568–3575. doi: 10.4049/jimmunol.171.7.3568. [DOI] [PubMed] [Google Scholar]
- 16.Foss DL, Donskoy E, Goldschneider I. The importation of hematogenous precursors by the thymus is a gated phenomenon in normal adultmice. J Exp Med. 2001;193:365–374. doi: 10.1084/jem.193.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Foss DL, Donskoy E, Goldschneider I. Functional demonstration of intrathymic binding sites and microvascular gates for prothymocytes in irradiated mice. Int Immunol. 2002;14:331–338. doi: 10.1093/intimm/14.3.331. [DOI] [PubMed] [Google Scholar]
- 18.Goldschneider I. Cyclical mobilization and gated importation of thymocyte progenitors in the adult mouse: evidence for a thymus-bone marrow feedback loop. Immunol Rev. 2006;209:58–75. doi: 10.1111/j.0105-2896.2006.00354.x. [DOI] [PubMed] [Google Scholar]
- 19.Bhandoola A, Sambandam A. From stem cell to T cell: one route or many? Nat Rev Immunol. 2006;6:117–126. doi: 10.1038/nri1778. [DOI] [PubMed] [Google Scholar]
- 20.Wu L, Kincade PW, Shortman K. The CD44 expressed on the earliest intrathymic precursor population functions as a thymus homing molecule but does not bind to hyaluronate. Immunol Lett. 1993;38:69–75. doi: 10.1016/0165-2478(93)90121-h. [DOI] [PubMed] [Google Scholar]
- 21.Schwarz BA, Sambandam A, Maillard I, Harman BC, Love PE, Bhandoola A. Selective thymus settling regulated by cytokine and chemokine receptors. J Immunol. 2007;178:2008–2017. doi: 10.4049/jimmunol.178.4.2008. [DOI] [PubMed] [Google Scholar]
- 22.Scimone ML, Aifantis I, Apostolou I, von Boehmer H, von Andrian UH. A multistep adhesion cascade for lymphoid progenitor cell homing to the thymus. Proc Natl Acad Sci USA. 2006;103:7006–7011. doi: 10.1073/pnas.0602024103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uehara S, Grinberg A, Farber JM, Love PE. A role for CCR9 in T lymphocyte development and migration. J Immunol. 2002;168:2811–2819. doi: 10.4049/jimmunol.168.6.2811. [DOI] [PubMed] [Google Scholar]
- 24.Allman D, Sambandam A, Kim S, Miller JP, Pagan A, Well D, Meraz A, Bhandoola A. Thymopoiesis independent of common lymphoid progenitors. Nat Immunol. 2003;4:168–174. doi: 10.1038/ni878. [DOI] [PubMed] [Google Scholar]
- 25.Godfrey DI, Kennedy J, Mombaerts P, Tonegawa S, Zlotnik A. Onset of TCR-beta gene rearrangement and role of TCR-beta expression during CD3−CD4−CD8− thymocyte differentiation. J Immunol. 1994;152:4783–4792. [PubMed] [Google Scholar]
- 26.Godfrey DI, Kennedy J, Suda T, Zlotnik A. A developmental pathway involving four phenotypically and functionally distinct subsets of CD3−CD4−CD8− triple-negative adult mouse thymocytes defined by CD44 and CD25 expression. J Immunol. 1993;150:4244–4252. [PubMed] [Google Scholar]
- 27.Pearse M, Wu L, Egerton M, Wilson A, Shortman K, Scollay R. A murine early thymocyte developmental sequence is marked by transient expression of the interleukin 2 receptor. Proc Natl Acad Sci USA. 1989;86:1614–1618. doi: 10.1073/pnas.86.5.1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lind EF, Prockop SE, Porritt HE, Petrie HT. Mapping precursor movement through the postnatal thymus reveals specific microenvironments supporting defined stages of early lymphoid development. J Exp Med. 2001;194:127–134. doi: 10.1084/jem.194.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petrie HT. Cell migration and the control of post-natal T-cell lymphopoiesis in the thymus. Nat Rev Immunol. 2003;3:859–866. doi: 10.1038/nri1223. [DOI] [PubMed] [Google Scholar]
- 30.Porritt HE, Gordon K, Petrie HT. Kinetics of steady-state differentiation and mapping of intrathymic-signaling environments by stem cell transplantation in nonirradiated mice. J Exp Med. 2003;198:957–962. doi: 10.1084/jem.20030837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Capone M, Hockett RD, Jr, Zlotnik A. Kinetics of T cell receptor beta, gamma, and delta rearrangements during adult thymic development: T cell receptor rearrangements are present in CD44(+)CD25(+) Pro-T thymocytes. Proc Natl Acad Sci USA. 1998;95:12522–12527. doi: 10.1073/pnas.95.21.12522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ciofani M, Zuniga-Pflucker JC. The thymus as an inductive site for T lymphopoiesis. Annu Rev Cell Dev Biol. 2007;23:463–493. doi: 10.1146/annurev.cellbio.23.090506.123547. [DOI] [PubMed] [Google Scholar]
- 33.Livak F, Tourigny M, Schatz DG, Petrie HT. Characterization of TCR gene rearrangements during adult murine T cell development. J Immunol. 1999;162:2575–2580. [PubMed] [Google Scholar]
- 34.Dudley EC, Petrie HT, Shah LM, Owen MJ, Hayday AC. T cell receptor beta chain gene rearrangement and selection during thymocyte development in adult mice. Immunity. 1994;1:83–93. doi: 10.1016/1074-7613(94)90102-3. [DOI] [PubMed] [Google Scholar]
- 35.Michie AM, Zuniga-Pflucker JC. Regulation of thymocyte differentiation: pre-TCR signals and beta-selection. Semin Immunol. 2002;14:311–323. doi: 10.1016/s1044-5323(02)00064-7. [DOI] [PubMed] [Google Scholar]
- 36.Rothenberg EV, Dionne CJ. Lineage plasticity and commitment in T-cell development. Immunol Rev. 2002;187:96–115. doi: 10.1034/j.1600-065x.2002.18709.x. [DOI] [PubMed] [Google Scholar]
- 37.Liston A, Lesage S, Wilson J, Peltonen L, Goodnow CC. Aire regulates negative selection of organ-specific T cells. Nat Immunol. 2003;4:350–354. doi: 10.1038/ni906. [DOI] [PubMed] [Google Scholar]
- 38.Scott HS, Heino M, Peterson P, Mittaz L, Lalioti MD, Betterle C, Cohen A, Seri M, Lerone M, Romeo G, Collin P, Salo M, Metcalfe R, Weetman A, Papasavvas MP, Rossier C, Nagamine K, Kudoh J, Shimizu N, Krohn KJ, Antonarakis SE. Common mutations in autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy patients of different origins. Mol Endocrinol. 1998;12:1112–1119. doi: 10.1210/mend.12.8.0143. [DOI] [PubMed] [Google Scholar]
- 39.Zuklys S, Balciunaite G, Agarwal A, Fasler-Kan E, Palmer E, Hollander GA. Normal thymic architecture and negative selection are associated with Aire expression, the gene defective in the autoimmune-polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED) J Immunol. 2000;165:1976–1983. doi: 10.4049/jimmunol.165.4.1976. [DOI] [PubMed] [Google Scholar]
- 40.Blom B, Spits H. Development of human lymphoid cells. Annu Rev Immunol. 2006;24:287–320. doi: 10.1146/annurev.immunol.24.021605.090612. [DOI] [PubMed] [Google Scholar]
- 41.Res P, Spits H. Developmental stages in the human thymus. Semin Immunol. 1999;11:39–46. doi: 10.1006/smim.1998.0152. [DOI] [PubMed] [Google Scholar]
- 42.Hare KJ, Jenkinson EJ, Anderson G. In vitro models of T cell development. Semin Immunol. 1999;11:3–12. doi: 10.1006/smim.1998.0151. [DOI] [PubMed] [Google Scholar]
- 43.Jenkinson EJ, Franchi LL, Kingston R, Owen JJ. Effect of deoxyguanosine on lymphopoiesis in the developing thymus rudiment in vitro: application in the production of chimeric thymus rudiments. Eur J Immunol. 1982;12:583–587. doi: 10.1002/eji.1830120710. [DOI] [PubMed] [Google Scholar]
- 44.Anderson G, Jenkinson EJ. Use of explant technology in the study of in vitro immune responses. J Immunol Methods. 1998;216:155–163. doi: 10.1016/s0022-1759(98)00076-3. [DOI] [PubMed] [Google Scholar]
- 45.Arber C, BitMansour A, Sparer TE, Higgins JP, Mocarski ES, Weissman IL, Shizuru JA, Brown JM. Common lymphoid progenitors rapidly engraft and protect against lethal murine cytomegalovirus infection after hematopoietic stem cell transplantation. Blood. 2003;102:421–428. doi: 10.1182/blood-2002-12-3834. [DOI] [PubMed] [Google Scholar]
- 46.Allman D, Punt JA, Izon DJ, Aster JC, Pear WS. An invitation to T and more: notch signaling in lymphopoiesis. Cell. 2002;109(Suppl):S1–S11. doi: 10.1016/s0092-8674(02)00689-x. [DOI] [PubMed] [Google Scholar]
- 47.Radtke F, Wilson A, Mancini SJ, MacDonald HR. Notch regulation of lymphocyte development and function. Nat Immunol. 2004;5:247–253. doi: 10.1038/ni1045. [DOI] [PubMed] [Google Scholar]
- 48.Radtke F, Wilson A, Stark G, Bauer M, van Meerwijk J, MacDonald HR, Aguet M. Deficient T cell fate specification in mice with an induced inactivation of Notch1. Immunity. 1999;10:547–558. doi: 10.1016/s1074-7613(00)80054-0. [DOI] [PubMed] [Google Scholar]
- 49.Pui JC, Allman D, Xu L, DeRocco S, Karnell FG, Bakkour S, Lee JY, Kadesch T, Hardy RR, Aster JC, Pear WS. Notch1 expression in early lymphopoiesis influences B versus T lineage determination. Immunity. 1999;11:299–308. doi: 10.1016/s1074-7613(00)80105-3. [DOI] [PubMed] [Google Scholar]
- 50.Maeda T, Merghoub T, Hobbs RM, Dong L, Maeda M, Zakrzewski J, van den Brink MR, Zelent A, Shigematsu H, Akashi K, Teruya-Feldstein J, Cattoretti G, Pandolfi PP. Regulation of B versus T lymphoid lineage fate decision by the protooncogene LRF. Science. 2007;316:860–866. doi: 10.1126/science.1140881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Osborne BA, Minter LM. Notch signalling during peripheral T-cell activation and differentiation. Nat Rev Immunol. 2007;7:64–75. doi: 10.1038/nri1998. [DOI] [PubMed] [Google Scholar]
- 52.Dallas MH, Varnum-Finney B, Martin PJ, Bernstein ID. Enhanced T-cell reconstitution by hematopoietic progenitors expanded ex vivo using the Notch ligand Delta1. Blood. 2007;109:3579–3587. doi: 10.1182/blood-2006-08-039842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schmitt TM, de Pooter RF, Gronski MA, Cho SK, Ohashi PS, Zuniga-Pflucker JC. Induction of T cell development and establishment of T cell competence from embryonic stem cells differentiated in vitro. Nat Immunol. 2004;5:410–417. doi: 10.1038/ni1055. [DOI] [PubMed] [Google Scholar]
- 54.Zakrzewski JL, Kochman AA, Lu SX, Terwey TH, Kim TD, Hubbard VM, Muriglan SJ, Suh D, Smith OM, Grubin J, Patel N, Chow A, Cabrera-Perez J, Radhakrishnan R, Diab A, Perales MA, Rizzuto G, Menet E, Pamer EG, Heller G, Zuniga-Pflucker JC, Alpdogan O, van den Brink MR. Adoptive transfer of T-cell precursors enhances T-cell reconstitution after allogeneic hematopoietic stem cell transplantation. Nat Med. 2006;12:1039–1047. doi: 10.1038/nm1463. [DOI] [PubMed] [Google Scholar]
- 55.Zakrzewski JL, Suh D, Markley JC, Smith OM, King C, Goldberg GL, Jenq R, Holland AM, Grubin J, Cabrera-Perez J, Brentjens RJ, Lu SX, Rizzuto G, Sant’Angelo DB, Riviere I, Sadelain M, Heller G, Zuniga-Pflucker JC, Lu C, van den Brink MR. Tumor immunotherapy across MHC barriers using allogeneic T-cell precursors. Nat Biotechnol. 2008;26:453–461. doi: 10.1038/nbt1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schmitt TM, Zuniga-Pflucker JC. Induction of T cell development from hematopoietic progenitor cells by delta-like-1 in vitro. Immunity. 2002;17:749–756. doi: 10.1016/s1074-7613(02)00474-0. [DOI] [PubMed] [Google Scholar]
- 57.Balciunaite G, Ceredig R, Fehling HJ, Zuniga-Pflucker JC, Rolink AG. The role of Notch and IL-7 signaling in early thymocyte proliferation and differentiation. Eur J Immunol. 2005;35:1292–1300. doi: 10.1002/eji.200425822. [DOI] [PubMed] [Google Scholar]
- 58.Mehr R, Fridkis-Hareli M, Abel L, Segel L, Globerson A. Lymphocyte development in irradiated thymuses: dynamics of colonization by progenitor cells and regeneration of resident cells. J Theor Biol. 1995;177:181–192. doi: 10.1006/jtbi.1995.0237. [DOI] [PubMed] [Google Scholar]
- 59.Rossi FM, Corbel SY, Merzaban JS, Carlow DA, Gossens K, Duenas J, So L, Yi L, Ziltener HJ. Recruitment of adult thymic progenitors is regulated by P-selectin and its ligand PSGL-1. Nat Immunol. 2005;6:626–634. doi: 10.1038/ni1203. [DOI] [PubMed] [Google Scholar]
- 60.Kunkel EJ, Campbell JJ, Haraldsen G, Pan J, Boisvert J, Roberts AI, Ebert EC, Vierra MA, Goodman SB, Genovese MC, Wardlaw AJ, Greenberg HB, Parker CM, Butcher EC, Andrew DP, Agace WW. Lymphocyte CC chemokine receptor 9 and epithelial thymus-expressed chemokine (TECK) expression distinguish the small intestinal immune compartment: epithelial expression of tissue-specific chemokines as an organizing principle in regional immunity. J Exp Med. 2000;192:761–768. doi: 10.1084/jem.192.5.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Papadakis KA, Prehn J, Nelson V, Cheng L, Binder SW, Ponath PD, Andrew DP, Targan SR. The role of thymus-expressed chemokine and its receptor CCR9 on lymphocytes in the regional specialization of the mucosal immune system. J Immunol. 2000;165:5069–5076. doi: 10.4049/jimmunol.165.9.5069. [DOI] [PubMed] [Google Scholar]
- 62.Wurbel MA, Philippe JM, Nguyen C, Victorero G, Freeman T, Wooding P, Miazek A, Mattei MG, Malissen M, Jordan BR, Malissen B, Carrier A, Naquet P. The chemokine TECK is expressed by thymic and intestinal epithelial cells and attracts double- and single-positive thymocytes expressing the TECK receptor CCR9. Eur J Immunol. 2000;30:262–271. doi: 10.1002/1521-4141(200001)30:1<262::AID-IMMU262>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 63.Schon MP, Arya A, Murphy EA, Adams CM, Strauch UG, Agace WW, Marsal J, Donohue JP, Her H, Beier DR, Olson S, Lefrancois L, Brenner MB, Grusby MJ, Parker CM. Mucosal T lymphocyte numbers are selectively reduced in integrin alpha E (CD103)-deficient mice. J Immunol. 1999;162:6641–6649. [PubMed] [Google Scholar]
- 64.Colson YL, Shinde Patil VR, Ildstad ST. Facilitating cells: novel promoters of stem cell alloengraftment and donor-specific transplantation tolerance in the absence of GVHD. Crit Rev Oncol Hematol. 2007;61:26–43. doi: 10.1016/j.critrevonc.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 65.Grimes HL, Schanie CL, Huang Y, Cramer D, Rezzoug F, Fugier-Vivier I, Ildstad ST. Graft facilitating cells are derived from hematopoietic stem cells and functionally require CD3, but are distinct from T lymphocytes. Exp Hematol. 2004;32:946–954. doi: 10.1016/j.exphem.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 66.Rezzoug F, Huang Y, Tanner MK, Wysoczynski M, Schanie CL, Chilton PM, Ratajczak MZ, Fugier-Vivier IJ, Ildstad ST. TNF-alpha is critical to facilitate hemopoietic stem cell engraftment and function. J Immunol. 2008;180:49–57. doi: 10.4049/jimmunol.180.1.49. [DOI] [PubMed] [Google Scholar]
- 67.Yaroslavskiy B, Colson Y, Ildstad S, Parrish D, Boggs SS. Addition of a bone marrow “facilitating cell” population increases stem cell-derived cobblestone area formation in impaired long-term bone marrow culture stroma. Exp Hematol. 1998;26:604–611. [PubMed] [Google Scholar]
- 68.Gray DH, Ueno T, Chidgey AP, Malin M, Goldberg GL, Takahama Y, Boyd RL. Controlling the thymic microenvironment. Curr Opin Immunol. 2005;17:137–143. doi: 10.1016/j.coi.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 69.Ishida Y, Onoe K, Aizawa M. Autologous or syngeneic mixed lymphocyte reaction in bone marrow and thymic chimera mice. Cell Immunol. 1982;73:141–150. doi: 10.1016/0008-8749(82)90442-7. [DOI] [PubMed] [Google Scholar]
- 70.Glimcher LH, Sr, Longo DL, Singer A. The specificity of the syngeneic mixed leukocyte response, a primary anti-I region T cell proliferative response, is determined intrathymically. J Immunol. 1982;129:987–991. [PubMed] [Google Scholar]
- 71.Iwabuchi K, Ogasawara K, Ogasawara M, Yasumizu R, Noguchi M, Geng L, Fujita M, Good RA, Onoe K. A study on proliferative responses to host Ia antigens in allogeneic bone marrow chimera in mice: sequential analysis of the reactivity and characterization of the cells involved in the responses. J Immunol. 1987;138:18–25. [PubMed] [Google Scholar]
- 72.Brentjens RJ, Latouche JB, Santos E, Marti F, Gong MC, Lyddane C, King PD, Larson S, Weiss M, Riviere I, Sadelain M. Eradication of systemic B-cell tumors by genetically targeted human T lymphocytes co-stimulated by CD80 and interleukin-15. Nat Med. 2003;9:279–286. doi: 10.1038/nm827. [DOI] [PubMed] [Google Scholar]
- 73.De Smedt M, Hoebeke I, Plum J. Human bone marrow CD34+ progenitor cells mature to T cells on OP9–DL1 stromal cell line without thymus microenvironment. Blood Cells Mol Dis. 2004;33:227–232. doi: 10.1016/j.bcmd.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 74.La Motte-Mohs RN, Herer E, Zuniga-Pflucker JC. Induction of T-cell development from human cord blood hematopoietic stem cells by Delta-like 1 in vitro. Blood. 2005;105:1431–1439. doi: 10.1182/blood-2004-04-1293. [DOI] [PubMed] [Google Scholar]
- 75.Dallas MH, Varnum-Finney B, Delaney C, Kato K, Bernstein ID. Density of the Notch ligand Delta1 determines generation of B and T cell precursors from hematopoietic stem cells. J Exp Med. 2005;201:1361–1366. doi: 10.1084/jem.20042450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Varnum-Finney B, Wu L, Yu M, Brashem-Stein C, Staats S, Flowers D, Griffin JD, Bernstein ID. Immobilization of Notch ligand, Delta-1, is required for induction of notch signaling. J Cell Sci. 2000;113(Pt 23):4313–4318. doi: 10.1242/jcs.113.23.4313. [DOI] [PubMed] [Google Scholar]
- 77.Benz C, Martins VC, Radtke F, Bleul CC. The stream of precursors that colonizes the thymus proceeds selectively through the early T lineage precursor stage of T cell development. J Exp Med. 2008;205:1187–1199. doi: 10.1084/jem.20072168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bhandoola A, von Boehmer H, Petrie HT, Zuniga-Pflucker JC. Commitment and developmental potential of extrathymic and intrathymic T cell precursors: plenty to choose from. Immunity. 2007;26:678–689. doi: 10.1016/j.immuni.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 79.Ceredig R, Rolink T. A positive look at double-negative thymocytes. Nat Rev Immunol. 2002;2:888–897. doi: 10.1038/nri937. [DOI] [PubMed] [Google Scholar]
- 80.Di Santo JP, Rodewald HR. In vivo roles of receptor tyrosine kinases and cytokine receptors in early thymocyte development. Curr Opin Immunol. 1998;10:196–207. doi: 10.1016/s0952-7915(98)80249-5. [DOI] [PubMed] [Google Scholar]
- 81.Ktorza S, Sarun S, Rieux-Laucat F, de Villartay JP, Debre P, Schmitt C. CD34-positive early human thymocytes: T cell receptor and cytokine receptor gene expression. Eur J Immunol. 1995;25:2471–2478. doi: 10.1002/eji.1830250910. [DOI] [PubMed] [Google Scholar]
- 82.Rodewald HR, Awad K, Moingeon P, D’Adamio L, Rabinowitz D, Shinkai Y, Alt FW, Reinherz EL. Fc gamma RII/III and CD2 expression mark distinct subpopulations of immature CD4−CD8− murine thymocytes: in vivo developmental kinetics and T cell receptor beta chain rearrangement status. J Exp Med. 1993;177:1079–1092. doi: 10.1084/jem.177.4.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rothenberg EV, Moore JE, Yui MA. Launching the T-cell-lineage developmental programme. Nat Rev Immunol. 2008;8:9–21. doi: 10.1038/nri2232. [DOI] [PMC free article] [PubMed] [Google Scholar]