Summary
A role for calcium in epithelial growth control is well-established in the colon and other tissues. In the colon, Ca2+ “drives” the differentiation process. This results in sequestration of ß-catenin in the cell surface / cytoskeletal complex, leaving ß-catenin unavailable to serve as a growth-promoting transcription enhancer in the nucleus. The signaling events that lead from Ca2+ stimulation to differentiation are not fully understood. A critical role for the extracellular calcium-sensing receptor (CaSR) is assumed, based on CaSR localization to the differentiating epithelial cells in the normal colonic mucosa (upper half of the crypt and crypt surface), decreased CaSR expression in colon carcinoma, and the results from in vitro studies with colonic epithelial cell lines.
While Ca2+ is well-accepted as a growth-regulating agent in the colon, suppression of cell proliferation is not complete. At least part of the reason for this is the inherent variability in Ca2+ responsiveness among individual epithelial cells. Of interest, colon epithelial cells that are resistant to the growth-regulating activity of Ca2+ alone are still responsive to Ca2+ in conjunction with other transition metals. Whether a multi-mineral approach will, ultimately, prove to be more effective than Ca2+ alone as a colon cancer chemopreventive agent remains to be seen, but certainly worth investigating.
Keywords: ß-catenin, Calcium, Colon cancer, Extracellular calcium-sensing receptor, E-cadherin
Introduction
Colon cancer is the third most common cause of cancer mortality in the United States for both men and women and a leading cause of cancer deaths worldwide (Tamima et al., 2004; American Cancer Society, 2009). Colon cancer develops over years or decades. Areas of hyperplasia in the colonic mucosa (aberrant crypts) are the earliest histologically-identifiable lesions. Aberrant crypts are believed to be the precursor lesions for most adenomatous polyps. Adenomatous polyps are raised neoplastic growths in which glandular structures are still evident, but increased in size and disorganized. Some colonic polyps are identified when they are still small, but on occasion, large polyps can be seen. Most polyps are non-malignant, but some contain malignant cells, in which case the lesion may be referred to as carcinoma in situ. All of these precursor lesions can be distinguished from invasive colon cancer by their confinement to the mucosal space - i.e., by a lack of invasion into the submucosa (Lipkin, 1974; Fearon and Vogelstein, 1990).
There are well-defined genetic alterations in the majority of colon cancers (Marian, 2004). The best known genetic alterations are the functional-inactivating mutations in the adenomatous polyposis coli (APC) gene, associated with defective ß-catenin degradation. In APC-mutant colon epithelial cells, cytoplasmic ß-catenin is not degraded in the cytoplasm and becomes over-abundant. Mutations in the ß-catenin gene, itself, can stabilize the protein and prevent cytoplasmic degradation. When ß-catenin is over-abundant in the cytoplasm, excess translocation to the nucleus occurs. Nuclear ß-catenin functions as a transcription enhancer for the TCF/LEF family of transcription factors and Wnt-pathway signaling (Korinek et al., 1997; Tetsu and McCormick, 1999). In addition to its role as a transcription enhancer, ß-catenin also functions as a component of the cell surface - cytoskeletal complex in epithelial cells. When ß-catenin is locked up in the cell surface - cytoskeletal complex, it is not available for translocation to the nucleus (Behrens et al., 1992; Mareel et al., 1996; Hugh et al., 1999; Van Aken et al., 2001 Conacci-Sorrel et al., 2003; Brembeck et al., 2004; Gottardi et al., 2001). Epithelial differentiation and growth control in the colonic mucosa are, therefore, closely linked. Knowing how differentiation is regulated in the colonic mucosa is essential for understanding growth control in the colon.
Ca2+ and growth control in the colon
Compelling epidemiological evidence indicates that Ca2+ has colon cancer chemopreventive activity. Case-controlled studies have demonstrated an inverse association between dietary intake of calcium and the risk of colorectal cancer among individuals with no previous history of colonic abnormalities and in individuals with at least one previous polyp (Bostick et al., 1993; Kampman et al., 1994, 2000; McCullough et al., 2003; Wakai et al., 2006). A calcium-supplementation study in a group of individuals with already-diagnosed colon polyps found that supplementation with 1200 mg of calcium twice per day for four years resulted in a significant reduction in polyp recurrence (Baron et al., 1999; Grau et al., 2003). The protective effects of Ca2+ - supplementation were still evident 5 years after cessation of treatment (Grau et al., 2007). Short-term studies have also demonstrated potential efficacy. In one study, subjects received 900 mg/day of calcium in two 4-week treatment periods. At the end of the second treatment period, cell proliferation based on labeling index of biopsy material was decreased by approximately 25% (Holt et al., 2001). These studies in human are backed by animal research showing reduced abnormalities in colonic epithelial growth (including a reduction in colon cancer) by dietary Ca2+ supplementation (Sitrin et al., 1991; Beaty et al., 1993; Comer et al., 1993; Mokady et al., 2000; Lamprecht and Lopkin, 2003; Newmark et al., 2009). It should be noted, that not all epidemiological studies have generated supportive data. At least three published studies concluded with a lack of correlation between calcium intake and risk of colon cancer (Kampman et al., 1994).
Cell culture studies provide insight into how Ca2+ may exert its epithelial cell growth-regulating activity. In cell culture, Ca2+ is known to reduce epithelial cell proliferation and to induce differentiation (Tu et al., 1999, 2004). In the presence of Ca2+ there is a reduction in several genes that are associated with the proliferation response. Among these are cyclin D1, c-fos, c-myc, cjun and members of the TGF-ß family. At the same time, Ca2+ induces expression of genes associated with onset of differentiation including P27(Kip1), P21(WAF1), cytokeratins recognized by the AE1/AE3 anti-keratin antibody combination, and especially, E-cadherin (Tu et al., 1999, 2004; Lamprecht and Lopkin, 2003). Several studies have shown that in the colon, Ca2+ promotes E-cadherin synthesis and a dramatic increase in cell surface expression. When E-cadherin is present in the cell membrane, cytosolic ß-catenin binds to the cytoplasmic tail of E-cadherin and connects the cell surface to the actin cytoskeleton. Sequestration of ß-catenin in the cell surface-cytoskeletal complex reduces ß-catenin translocation to the nucleus and TCF4 / Wnt signaling (growth-promoting) (Behrens et al., 1992; Hugh et al., 1999; Mareel et al., 1996; Korinek et al., 1997; Gottardi et al., 2001; Van Aken et al., 2001; Conacci-Sorrell et al., 2003; Brembeck et al., 2004).
Modulation of cell proliferation and differentiation provides a mechanism for the observed chemopreventive effects of Ca2+, but it must be kept in mind that other mechanisms are also possible. For example, a diet rich in Ca2+ may be chemopreventive, in part, because excess calcium in the bowel can complex with fatty acids and precipitate factors that would otherwise be carcinogenic. Equally important, Ca2+ is a critical regulator of stromal cell function, and may influence the epithelium of the colon indirectly through an effect on the stroma. These potential mechanisms are beyond the scope of the present review.
In summary, a role for ß-catenin in colonic epithelial cell growth regulation is well-established. Mutations leading to defects in the ß-catenin protein itself or mutations that interfere with its intracellular degradation maintain a high level of cytoplasmic protein. Under such conditions, ß-catenin levels in the nucleus are high, and growth-promoting pathways are constantly stimulated. Because ß-catenin also has a role in colonic epithelial cell differentiation, growth regulation and differentiation are closely linked. To the extent that ß-catenin is engaged with E-cadherin in the cell surface - cytoskeletal complex, it is not available to participate in nuclear transcription. Given this reality, factors that promote differentiation might be expected to regulate cell growth concomitantly. There is a large amount of information from clinical studies in humans and from experimental studies in animals and/or cell culture to indicate that Ca2+ plays a critical, if not unique, role in regulating epithelial cell differentiation in the colonic mucosa (as it does in other epithelia). Although other possibilities exist, it is not unreasonable to suggest that the colon polyp chemopreventive activity of Ca2+ is related to its ability to promote differentiation in the colonic mucosa.
The mechanistic events through which Ca2+ exerts its pro-differentiating effects in the colon are not fully understood. Studies conducted over the past decade support the notion that an epithelial cell protein known as the extracellular calcium-sensing receptor (CaSR) is a critical mediator of Ca2+ - generated pro-differentiating and growth-regulating signals in the human colonic mucosa. The remainder of this review is focused on CaSR expression and function in the colon.
Cell surface CaSR: Role in Ca2+ - mediated growth control in the colon
The release of Ca2+ from intracellular stores in response to a variety of extracellular signals has long been regarded as a “second messenger” in triggering a diversity of cellular responses. The characterization and cloning of the cell-surface CaSR from the bovine and human parathyroid gland (Brown et al., 1993; Garrett et al., 1995). has demonstrated that CaSR and extracellular Ca2+ can function as a first messenger system in controlling physiologic function. It has been proposed that the function of the CaSR, a member of the superfamily of G protein-coupled receptors, is to sense minute changes in extracellular Ca2+ concentration and coordinate the secretion of endocrine factors (most notably, parathyroid hormone) to regulate systemic Ca2+ homeostasis. The role of CaSR in control of plasma Ca2+ is a separate field, and beyond the scope of this review.
In addition to a role in systemic Ca2+ control, CaSR appears to be a key cell surface moiety determining the ability of epithelial cells to respond to growth-regulating signals generated via interaction with extracellular Ca2+. Solid evidence supports the notion that CaSR and extracellular Ca2+ participate as a first-messenger system in the regulation of growth and differentiation in the colon. This is based on the relationship between CaSR expression and differentiation in normal and abnormal areas of human colonic mucosa in vivo; and the relationship between CaSR expression on colonic epithelial cells in vitro and capacity of Ca2+ to induce differentiation in these cells.
CaSR expression in the colon
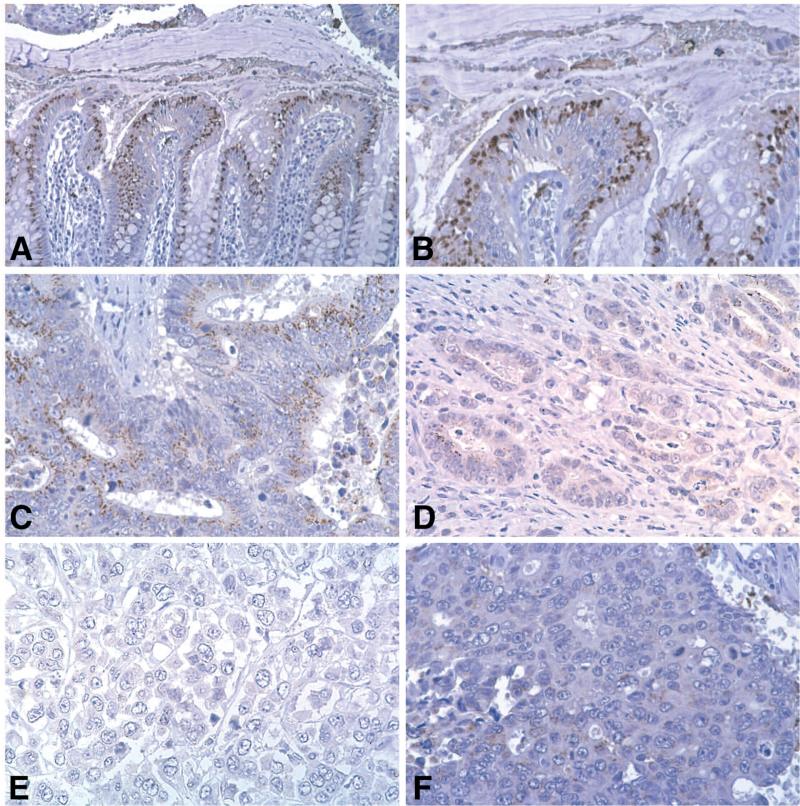
CaSR expression in the epithelial cells of the large intestine (as well as throughout the entire gastrointestinal tract) is well documented (Rutten et al., 1999; Kallay et al., 2000; Sheinin et al., 2000; Cheng et al., 2002; Chakrabarty et al., 2003, 2005; Justinich et al., 2008). In our own initial studies (Chakrabarty et al., 2003, 2005). a total of 10 human colon cancer specimens were examined for CaSR expression. The data from this small group of tissue specimens are summarized in Table 1. Figure 1 presents typical immuno-histochemical staining results from several of the specimens. It can be seen from Table 1 and Figure 1 that CaSR expression is strong in normal colonic crypts. In the normal crypt, cells in the upper half of the crypt and at the crypt surface (most differentiated) are strongly positive while cells at the base of the crypt stain much more weakly or not at all. In contrast, CaSR staining is weak and variable throughout the malignant tissue. In some areas, CaSR is faintly detectable, while in other areas it is completely absent. It is clear from Table 1 and Figure 1 that there is a relationship between histological features of differentiation and CaSR expression. Where there is detectable glandular structure, CaSR can be seen. In contrast, CaSR expression is almost completely missing in the most undifferentiated tumor sections where only “sheets” of anaplastic cells are seen. In addition, it can be seen in Figure 1 that CaSR expression is absent in the individual malignant epithelial cells and small groups of cells at the invasion front.
Table 1.
Quantitative assessment of CaSR immunoreactivity in normal colonic crypt epithelium and colon carcinoma.
| Histological features | % positive cells | Staining intensity |
|---|---|---|
| Normal colonic crypt epithelium (n=9) | ||
| Basal zone | 6±1 | 1.1±0.1 |
| Mid-crypt | 90±5** | 2.3±1.5* |
| Surface | 96±2** | 2.9±1.0** |
| Well- to moderately-differentiated tumor (n=7) | 63±13 | 1.7±0.7 |
| ***Poorly- to undifferentiated tumor (n=4) | 3±1** | 1.1±0.1** |
Normal colon: Basal zone - base of the crypt containing small, densely-packed cells without evidence of goblet cell structure. Mid-crypt - area from the edge of the basal zone to an area 1/3 of the way from the top of the crypt. Surface zone - upper 1/3 of the crypt and the cells at the apex of the crypt. Values are means and standard errors based on four separate areas in each specimen.
indicates statistically-significant difference from basal zone at p<0.05 level.
indicates statistically-significant difference from basal zone at p<0.01 level.
Colon carcinoma: Well- to moderately-differentiated tumor - evidence of glandular structure. Poorly- to undifferentiated tumor - areas in which only cell sheets were detected (i.e., no glandular structure). Values are means and standard errors based on four separate areas in each specimen.
indicates statistically-significant difference from well- to moderately-differentiated tumor at p<0.01 level.
***includes small clusters of tumor cells (1-3 cells) at the invasive front; imbedded in the stroma with no evidence of glandular structure.
Fig. 1.
CaSR expression in normal colonic epithelium and in colon carcinoma. A and B. In the normal crypt, there is intense staining of the differentiated cells in the upper part of the crypt. C. In an area of moderately-differentiated tumor, weak and variable staining is observed. D. There is essentially no staining of isolated cells at the invasive front. Likewise, there is little or no staining of invasive cells even where there is rudimentary glandular structure. E and F. In areas of histologically undifferentiated tumor (no glandular structure evident; only sheets of undifferentiated tumor cells), there is no CaSR staining (See Chakrabarty et al. 2003; 2005 for details).
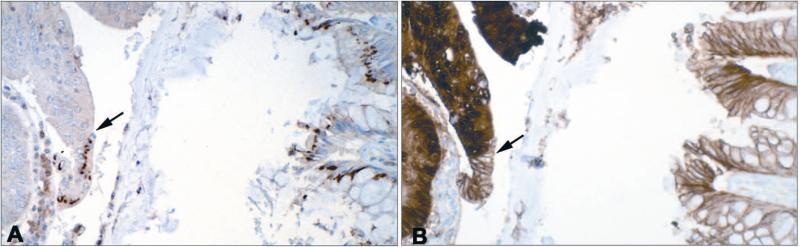
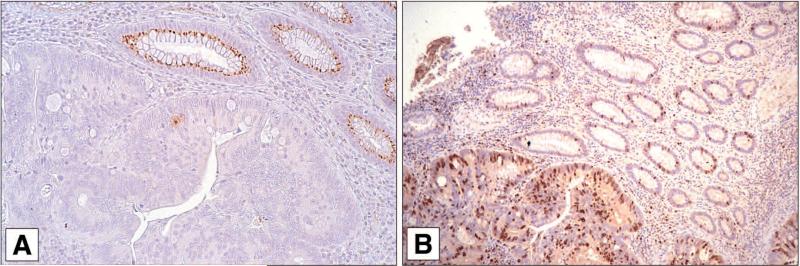
One of the questions raised by these data is whether differences in CaSR expression correlate with altered expression of other markers associated with colon cancer growth and differentiation. Figure 2 shows staining for CaSR and for ß-catenin in a section from a colon cancer specimen. One can see a direct correlation between CaSR expression and surface ß-catenin, and an inverse correlation between CaSR expression and cytoplasmic/nuclear ß-catenin. The arrow in the left side of Panel A shows a sharp transition between CaSR-positive and CaSR-negative regions of histologically-abnormal tissue. The same sharp transition between surface ß-catenin and cytoplasmic/nuclear ß-catenin can be seen in Panel B. There is a similar relationship between CaSR expression and expression of the proliferation marker Ki67 (Fig. 3). Where CaSR is observed in this figure (i.e., in normal colon crypts), few cells are positive for Ki67. In contrast, where CaSR is reduced or absent (in areas of tumor), Ki67-positive cells are present throughout the epithelium.
Fig. 2.
Relationship between CaSR staining and ß-catenin staining. A: CaSR and B: ß-catenin: Where CaSR is expressed in the tissue, ß-catenin staining is strongly associated with the cell surface. Where CaSR is not expressed, intense cytoplasmic/nuclear ß-catenin staining is seen. Arrows in each panel demonstrate area of marked transition. (See Bhagavathula 2007 for details).
Fig. 3.
Relationship between CaSR staining and Ki67 reactivity. A: CaSR and B: Ki67: Where CaSR is expressed (normal glandular structure), Ki67 staining is sporadic. Where CaSR is not expressed (tumor), a high percentage of cells are Ki67-positive.
In summary, a number of past studies have established that CaSR expression is reduced or missing in malignant areas in human colon tissue specimens. In the malignant tissue, the more abnormal the histological presentation of the tumor, the lower the CaSR expression level appears to be. Reduced or absent CaSR expression is associated with increased expression of moieties associated with proliferation (nuclear ß-catenin and Ki67) while the inverse is true for high CaSR expression and markers of differentiation.
CaSR expression and regulation of colonic epithelial cell proliferation in vitro
Epithelial cells from various tissues including the colon proliferate optimally when exposed to a concentration of Ca2+ that is below the plasma level of 1.4-1.6 mM. When the extracellular Ca2+ concentration is raised to approximately 1 mM or above, differentiation occurs. Proliferation concomitantly slows (Tu et al., 1999, 2004). The low Ca2+ requirement may have physiological relevance since the basement membrane, which separates the epithelium from the underlying stroma, contains large amounts of anionic substances. The anionic components could be expected to sequester much of the Ca2+ before it reached the epithelial cells.
CaSR expression is low in colon epithelial cells maintained under conditions optimized for proliferation (i.e., in low-Ca2+). When the Ca2+ concentration is raised to levels that promote differentiation, CaSR expression is, concomitantly, increased (Kallay et al., 1997; Bhagavathula et al., 2005, 2007). Presumably, in the colon itself, the lack of CaSR expression in cells at the base of the crypts reflects inability of extracellular Ca2+ in the colon fluid to penetrate to that level. In contrast, cells in the upper part of the crypt are exposed to Ca2+ in the colonic fluid. At the molecular level, CaSR has promoter regions responsive to Ca2+ (Canaff and Hendy, 2002; Chakrabarty et al., 2005). which accounts for the response to Ca2+. The question is this - does it matter? Is there a relationship between CaSR up-regulation by Ca2+ and the subsequent biological responses to Ca2+? We have attempted to address this question using two distinct but similar approaches. One involves selecting colon epithelial cells that are biologically non-responsive to Ca2+ and determining how Ca2+ affects CaSR expression in these cells, and down-stream signaling events. The other involves using a small interfering RNA (siRNA) to reduce CaSR expression, and then determining how this alters the same signaling events and the subsequent biological responses.
For these studies, we initially utilized a human colon epithelial cell line, referred to as CBS. This line was originally derived from a moderately-differentiated tumor. These cells proliferate in monolayer culture at Ca2+ levels as low as 0.035 mM and form colonies in soft agar under low-Ca2+ conditions. When the extracellular Ca2+ level is raised to above 1 mM, monolayer growth slows, and the cells take on a flattened (differentiated) appearance. Colony formation in soft agar is suppressed. However, in either monolayer culture or soft agar, a few colonies typically grow out even when the Ca2+ concentration is such that most of the cells are growth-suppressed. We took advantage of this to select for cells that continued to proliferate in the presence of Ca2+. When these cells were examined for CaSR expression under low-Ca2+ conditions, the expression level was similar to that of unselected parental cells. However, while CaSR expression increased in the parental cells upon exposure to Ca2+, up-regulation was not seen with the Ca2+ - non-responsive cells.
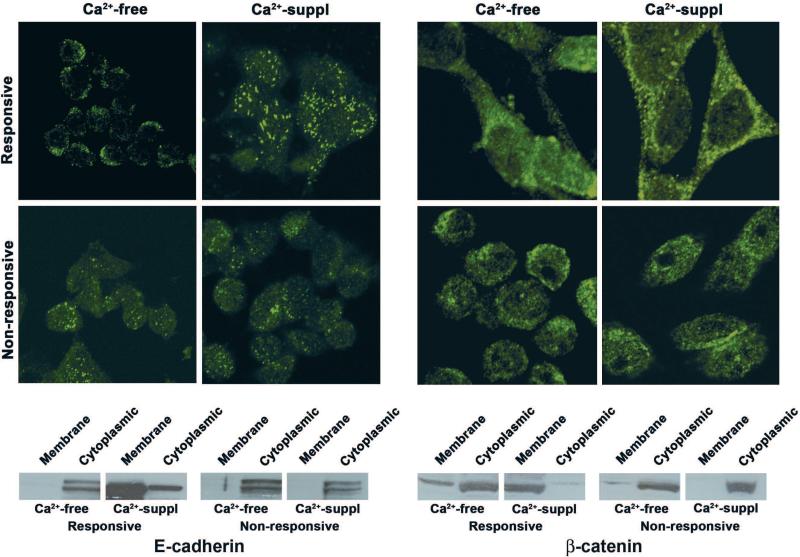
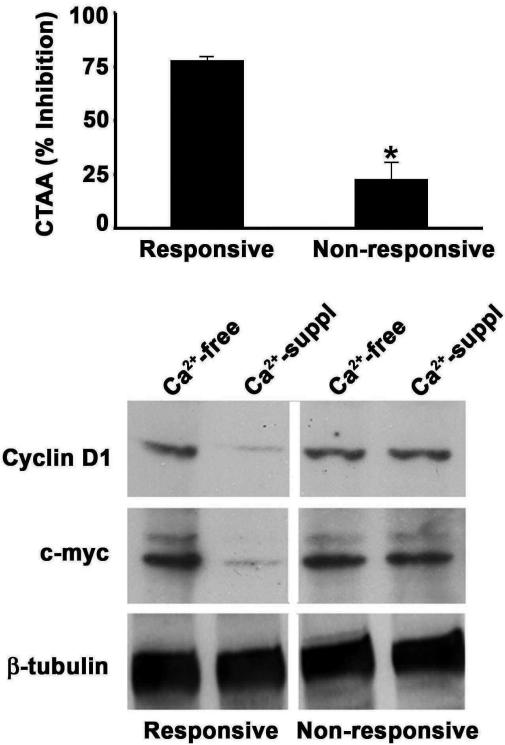
Figure 4 (left) demonstrates E-cadherin expression in Ca2+ - responsive and Ca2+ - non-responsive CBS cells. Under low-Ca2+ conditions, the two populations are nearly indistinguishable, with E-cadherin expression seen as diffuse cytoplasmic and diffuse membrane staining. In the presence of 1.4 mM Ca2+, the responsive cells undergo differentiation as indicated by morphological change (not shown) and by a shift in E-cadherin expression from the cytoplasm to the cell surface. In contrast, in the Ca2+-non-responsive cells, such a shift does not take place. A parallel response is seen with ß-catenin (Fig. 4 right). In the low-Ca2+ environment, ß-catenin is predominantly located in the cytoplasm/nucleus (both populations). In response to Ca2+ stimulation, there is translocation to the cell surface in the Ca2+-responsive cells but not in the Ca2+-non-responsive cells. In parallel with these results, it can be seen from Figure 5 that extracellular Ca2+ reduces TCF-4 transcriptional activity and, concomitantly, reduces TCF-4-mediated gene transcription (ß-catenin-dependent) in the responsive cells more effectively than in the non-responsive cells.
Fig. 4.
E-cadherin and ß-catenin expression in Ca2+-responsive and non-responsive CBS cells: Upper panels: Confocal fluorescence microscopy showing E-cadherin and ß-catenin staining patterns in Ca2+-responsive and non-responsive CBS cells. In the absence of extracellular Ca2+, diffuse cytoplasmic staining is observed with both proteins in both populations. In response to Ca2+, peripheral staining is observed in the Ca2+-responsive cells but the staining pattern in the non-responsive cells for both proteins is similar to what is observed in the absence of Ca2+. Lower panels: Cell fractionation studies showing relative distribution of the two proteins between cytosolic/nuclear fraction and membrane fraction in the absence and presence of Ca2+ (See Bhagavathula 2007 for details).
Fig. 5.
Effect of extracellular Ca2+ on TCF-4 transcription in Ca2+-responsive and non-responsive CBS cells. Upper panel: TCF-4 transcription was measured using the dual luciferase reporter assay, and results were expressed as percent inhibition of CTAA in the presence of extracellular Ca2+ relative to control cells cultured in the absence of Ca2+. Lower panel: Cells were grown under Ca2+-free or Ca2+-containing conditions for 2 days. Lysates from these cells were probed for c-myc and cyclin D1 expression by Western blot analysis. (See Bhagavathula 2007 for details).
In order to provide direct support for a CaSR role in this process, we used an siRNA to down-regulate CaSR in the same cells (Bhagavathula et al., 2007). When CaSR protein expression was reduced to below detectable limits in the Ca2+-responsive CBS cells, they behaved in a similar manner to the intrinsically non-responsive CBS cells. That is, they failed to up-regulate E-cadherin and neither E-cadherin nor ß-catenin localized at the cell surface in response to Ca2+. In parallel, Ca2+ did not reduce TCF-4 transcription or lower the levels of c-myc and cyclin-D1. Based on these findings with two different approaches in cell culture, we conclude that, at least in the CBS colon carcinoma cells, CaSR plays a critical role in transducing growth-regulating Ca2+ signals. Taken together, these observations provide a molecular link between Ca2+/CaSR signaling and events that lead to differentiation and growth reduction in colon carcinoma cells.
The existence of subpopulations of cells that are inherently unresponsive to growth-regulating Ca2+ signals has implications in regard to chemoprevention strategies. The presence of Ca2+-resistant cells in the colonic mucosa could explain why some polyps develop in the face of Ca2+ chemoprevention. While chemoprevention with Ca2+ has been unequivocally shown to reduce polyp formation (Kampman et al., 1994; Baron et al., 1999; Holt et al., 2001; Grau et al., 2003, 2007). inhibition is far from complete. More discouraging, since CaSR expression and anaplastic histological features in colon carcinoma cells appear to be inversely correlated (Kallay et al., 2000; Sheinin et al., 2000; Chakrabarty et al., 2003, 2005). it may, ultimately, prove to be that those cells that resist Ca2+ chemoprevention are the cells most likely to progress to invasive colon cancers. It would be of interest to assess CaSR expression in colon polyps picked up during colonoscopy and compare expression levels in polyps from untreated control subjects versus those taking supplemental Ca2+ as part of a chemopreventive strategy. Finally, it would be of interest to know if colon polyps inherently resistant to Ca2+ will also prove to be resistant to other interventions.
Colon cell growth control by Ca2+ in conjunction with other trace minerals
One can pick up any general nutrition book and find chapters devoted to the trace elements and their known or suspected functions (Strain and Cashman, 2002; Stipanuk, 2006). Suffice to state here is that while Ca2+ is critical to growth control, a number of other monovalent, divalent and trivalent cationic metals are also important. In some cases, the functions are independent of Ca2+. For example, certain transition metals including copper, zinc, manganese and selenium are components of anti-oxidant enzymes (Harris, 1992). An adequate supply of these metals is considered essential to preventing the formation of oxidant - driven mutations that, ultimately, cause cell growth dis-regulation. In other cases, different metals modulate cellular responses to Ca2+. Recent studies have shown that an adequate supply of magnesium (Mg2+) in the diet is critical to effective colon chemoprevention by Ca2+. While Mg2+ supplementation by itself has little chemopreventive activity, the ratio of Ca2+ to Mg2+ appears to be as important as the level of Ca2+ per se (Dai et al., 2007). As a group, the lanthanide metals may be particularly important in modulating the growth-regulating activity of Ca2+. The lanthanide elements are similar in size and orbital configuration to Ca2+ but with a higher overall charge density. Several of the lanthanide metals bind to Ca2+-binding sites on proteins (including CaSR). In some cases, the affinity is higher than that of Ca2+ itself (Huang et al., 2009). Lanthanide metals trigger events normally mediated by Ca2+, and modulate the effects of Ca2+ on these same events.
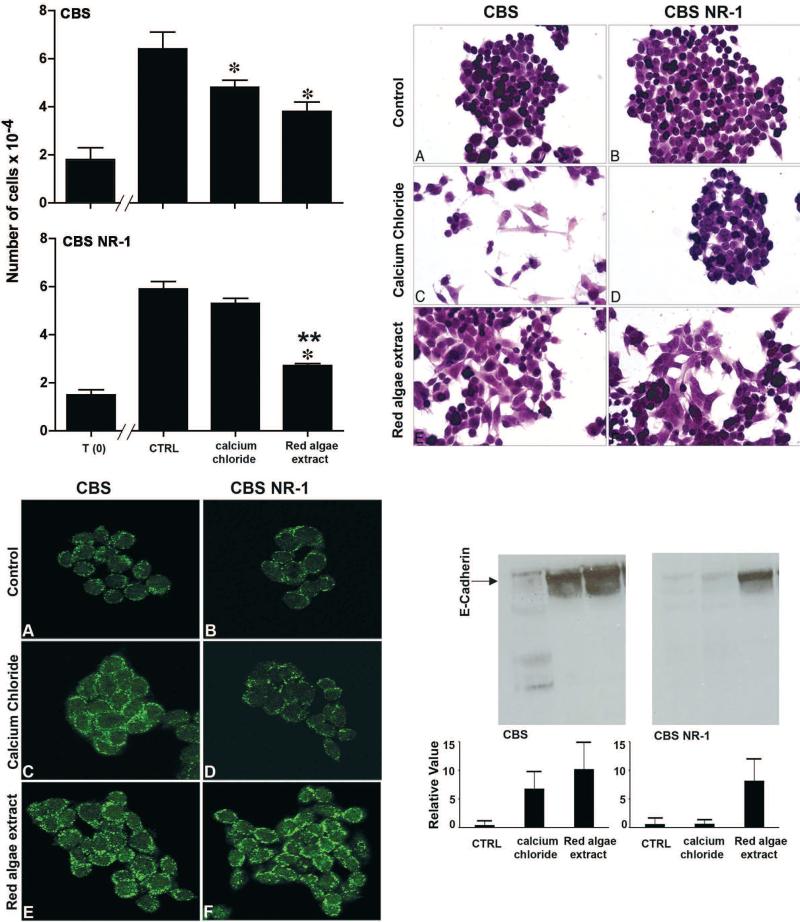
We carried out a series of studies comparing the colon epithelial cell growth-regulating properties of Ca2+ alone versus Ca2+ as part of a multi-mineral-rich natural product derived from the skeletal remains of red marine algae, Lithothamnion calcareum. The algae thrives in the shallow Atlantic waters off the southwest coast of Ireland and northwest coast of Iceland, and accumulates minerals from the ocean water over its lifespan. Eventually, the mineral-rich fronds break off of the living algae and fall to the ocean floor. The fronds are harvested from the ocean floor, separated from extraneous materials, and sterilized, dried, and milled under ISO and HACCP certification. The natural product contains high levels of Ca2+ and Mg2+, but in addition, contains measurable levels of 72 different trace minerals. The underlying assumption for these studies was that individual metals (or combinations) in the mineral-rich natural product might provoke a response under conditions in which Ca2+ alone was ineffective. To test this hypothesis, we compared Ca2+ alone with the mineral-rich product for effects on growth and differentiation in parental CBS cells and in cells selected for resistance to Ca2+ (Aslam et al., 2009). As seen in Figure 6, the mineral-rich algae extract was similar to Ca2+ alone in its effects on the Ca2+-responsive (parental) cells. However, when cells selected for non-responsiveness to Ca2+ were examined in place of the parental cells, the combination of Ca2+ and the other minerals was still effective in stimulating differentiation and reducing proliferation. Similar results were observed with three other human colon epithelial cell lines. Our interpretation of these findings is that resistance to Ca2+-mediated growth regulation does not imply, a priori, resistance to other interventions.
Fig. 6.
Effects of a multi-mineral-rich, red marine algae extract on proliferation and differentiation in Ca2+-responsive and non-responsive CBS cells. Upper-left panel: Cells were treated with the mineral-rich extract or with Ca2+ alone (calcium chloride) and cell numbers were determined after 2 days of incubation. Upper-right panel: Morphology: Cells were stained with hematoxylin and eosin. Lower-left panel: Confocal immunofluorescence microscopy - E-cadherin. Lower-right panel: Western blot for E-cadherin. Parent cells demonstrated reduced growth and differentiation in response to either treatment. The Ca2+-non-responsive cells demonstrated growth reduction and differentiation in response to the multi-mineral product, but did not respond to Ca2+ alone (see Aslam 2009 for details).
Given the capacity of the multi-mineral rich product to suppress in vitro proliferation of epithelial cells that are resistant to Ca2+ alone, a long-term dietary intervention study with the same mineral-rich product was conducted. Healthy (C57Bl/6) mice were maintained for 15 months on either normal (low-fat) rodent chow or on a high-fat modification of the same rodent chow. The high-fat diet was initially developed by Newmark et al., 2001). to mimic the diet typically consumed by many individuals in Western countries. In addition to the increased content of saturated fat, the high-fat diet has several other features. Among these are replacement of methionine with cysteine, and reductions in choline, folate, total fiber, and elemental Ca2+. In the initial studies (Newmark et al., 2001). healthy mice on the high-fat diet developed polyps in approximately 25% of the animals by 15-18 months while there were virtually no polyps in control-diet animals. Subsequent “add-back” experiments showed that only Ca2+ made a difference. When the missing Ca2+ was replaced, polyp formation fell significantly - though not back to the level in the low-fat diet (Yang et al., 2008). In our pilot study (Aslam et al., 2010). polyps were detected in 4 of 20 animals on the high-fat diet and in 0 of 20 animals that were fed the multi-mineral extract in the high-fat diet. Of interest, while mice on the high-fat diet with or without the mineral supplement gained excess weight as compared to mice on the low-fat control diet, the mice without the supplement had changes in several organs/organ systems consistent with chronic inflammation (Aslam et al., 2010a,b). Such changes were mitigated in mice receiving the high-fat diet with the mineral supplement. Thus, the reduction in polyp formation may be a direct consequence of a mineral effect on epithelial cell proliferation, but could also reflect a reduction in inflammation and the down-stream consequences of chronic inflammation.
Conclusions
A role for Ca2+ in epithelial growth control is well-established in the colon and other tissues. This is based on both in vitro and in vivo findings. In the colon, Ca2+ “drives” the differentiation process, and leads, ultimately, to sequestration of ß-catenin in the cell surface - cytoskeletal complex. As a consequence, there is less ß-catenin available to serve as a growth-promoting transcription enhancer in the nucleus. Thus, we have a direct link between increased differentiation and reduced growth.
The signaling events that lead from Ca2+ stimulation to differentiation are not fully understood. A critical role for CaSR is assumed, based on CaSR localization to the differentiating epithelial cells in the normal colonic mucosa, a lack of expression in the proliferating cells in the base of the normal crypt as well as in malignant colon epithelial cells, and, finally, the results from in vitro studies with colonic epithelial cell lines.
While Ca2+ is well-accepted as a regulator of colonic epithelial cell growth, its ability to suppress colon polyp formation in vivo is far from complete. At least part of the reason for this is the inherent variability in Ca2+ responsiveness among individual colonic epithelial cells. If there are cells in a developing adenomatous lesion that are resistant to Ca2+ - mediated growth regulation, one might assume that these would “grow out” in the face of a Ca2+ concentration that inhibits more sensitive cells. A high concentration of Ca2+ might even provide a selective pressure to enhance the outgrowth of the most resistant populations. Of interest, we have found that colon epithelial cells resistant to the growth-regulating activity of Ca2+ alone may still respond to a preparation containing a combination of Ca2+ and a wide range of other transition metals. Whether such a preparation will, ultimately, prove to be more effective than Ca2+ as a colon cancer chemopreventive agent remains to be seen. Only the relevant clinical studies can address that question. Such studies are currently in progress, but it will be some time before the data are available.
Acknowledgements
This study was supported in part by grant CA140760 from the USPHS.
References
- American Cancer Society Health Policy Research Leading sites of new cancer cases and deaths - 2009 estimates [Google Scholar]
- Aslam MN, Bhagavathula N, Chakrabarty S, Varani J. Growth-inhibitory effects of Aquamin, a mineralized extract from the red algae, Lithothamnion calcerum, on Ca2+-sensitive and Ca2+-resistant human colon carcinoma cells. Cancer Lett. 2009;283(2):186–192. doi: 10.1016/j.canlet.2009.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslam MN, Paruchuri T, Bhagavathula N, Varani J. A mineralized extract from the red algae, Lithothamnion calcerum, inhibits polyp formation and inflammation in the gastrointestinal tract of normal mice on a high-fat diet. Integr. Cancer Ther. 2010a;9:93–99. doi: 10.1177/1534735409360360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslam MN, Paruchuri T, Bhagavathula N, DaSilva M, Goldstein SA, Varani J. A mineralized extract from the red algae, Lithothamnion calcerum, preserves bone structure and function in normal mice on a high-fat diet. Calc. Tissue Int. 2010b;86:313–324. doi: 10.1007/s00223-010-9340-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron JA, Beach M, Mandel JS. A randomized trial of calcium supplementation to prevent colorectal adenomas. N. Engl. J. Med. 1999;340:101–107. doi: 10.1056/NEJM199901143400204. [DOI] [PubMed] [Google Scholar]
- Beaty MM, Lee EY, Giauert HP. Influence of dietary calcium on colon epithelial proliferation and 1,2-dimethyhydrazine-induced colonic cancer in rats fed high fat diets. J. Nutr. 1993;123:144–152. doi: 10.1093/jn/123.1.144. [DOI] [PubMed] [Google Scholar]
- Behrens J, Vakaet L, Friis R, Winterchger E, Van roy F, Mareel MM, Birchmeier W. Loss of epithelial differentiation and gain of invasiveness correlates with tryrosine phosphorylation of the E-cadherin/ß-catenin complex in cells transformed with a temperature-sensitive v-SRC gene. J. Cell Biol. 1992;120:757–766. doi: 10.1083/jcb.120.3.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhagavathula N, Kelley EA, Reddy M, Nerusu KC, Leonard C, Fay K, Chakrabarty S, Varani J. Upregulation of calcium-sensing receptor and mitogen-activated protein kinase signaling in the regulation of growth and differentiation in colon carcinoma. Brit . J. Cancer. 2005;93:1364–1371. doi: 10.1038/sj.bjc.6602852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhagavathula N, Hanosh AW, Nerusu KC, Appelman H, Chakrabarty S, Varani J. Regulation of E-cadherin and? ß-catenin by Ca2+ in colon carcinoma is dependent on calcium-sensing receptor expression and function. Int. J. Cancer. 2007;121:1455–1462. doi: 10.1002/ijc.22858. [DOI] [PubMed] [Google Scholar]
- Bostick RM, Potter JD, Sellers TA, McKenzie DR, Kushi LH, Folsom AR. Calcium, vitamin D, dairy foods intake to incidence of colon cancer among older women. The Iowa women's health study. Am. J. Epidermal. 1993;137:1302–1317. doi: 10.1093/oxfordjournals.aje.a116640. [DOI] [PubMed] [Google Scholar]
- Brembeck FH, Schwarz-Romond T, Bakkers J, Wilhelm S, Hammerschmidt M, Birchmeier W. Essential role of BCL9-2 in the switch between ß-catenin's adhesive function and transcriptional functions. Genes Dev. 2004;18:2225–2230. doi: 10.1101/gad.317604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown EM, Gamba G, Riccardi D. Cloning and characterization of an extracellular Ca2+-sensing receptor from bovine parathyroid. Nature (London) 1993;636:575–580. doi: 10.1038/366575a0. [DOI] [PubMed] [Google Scholar]
- Canaff L, Hendy GN. Human calcium-sensing receptor gene. Vitamin D response elements in promoters P1 and P2 confer transcriptional responsiveness to 1,25-dihydroxyvitamin D. J. Biol. Chem. 2002;277:30337–30350. doi: 10.1074/jbc.M201804200. [DOI] [PubMed] [Google Scholar]
- Conacci-Sorrell M, Simcha I, Ben-Yedidia T, Blechman J, Savagner P, Ben-Ze'ev Autoregulation of E-cadherin expression by cadherin-cadherin interactions: the roles of ß-catenin signaling, Slug, and MAPK. J. Cell Biol. 2003;163:847–857. doi: 10.1083/jcb.200308162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer PF, Clark TD, Glauert HP. Effect of dietary vitamin D3 on colon carcinogenesis induced by 1,2-dimethylhydrazine in male Fisher 344 rats. Nutr. Cancer. 1993;19:113–124. doi: 10.1080/01635589309514242. [DOI] [PubMed] [Google Scholar]
- Chakrabarty S, Radjendirane V, Appelman H, Varani J. Extracellular calcium and calcium sensing receptor function in human colon carcinomas: Promotion of E-cadherin expression and suppression of ß-catenin/TCF activation. Cancer Res. 2003;63:67–71. [PubMed] [Google Scholar]
- Chakrabarty S, Wang H, Canaff L, Hendy GN, Appelman H, Varani J. Calcium sensing receptor in human colon carcinoma: Interaction with Ca2+ and 1,25-Dihydroxyvitamin D3. Cancer Res. 2005;65:493–498. [PubMed] [Google Scholar]
- Cheng SX, Okuda M, Hall AE, Geibel JP, Hebert SC. Expression of calcium-sensing receptor in rat colonic epithelium: evidence of modulation by fluid secretion. Am. J. Physiol. 2002;283:G240–G250. doi: 10.1152/ajpgi.00500.2001. [DOI] [PubMed] [Google Scholar]
- Dai Q, Shrubsole MJ, Ness RM, Schlundt D, Cai Q, Smalley WE, Li M, Shyr Y, Zheng W. The relation of magnesium and calcium intakes and a genetic polymorphism in the magnesium transporter to colorectal neoplasia risk. Am. J. Clin. Nutr. 2007;86:743–751. doi: 10.1093/ajcn/86.3.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- Garrett JE, Capuano IV, Hammerland LG, Hung BCP, Brown EM, Hebert SC, Nemeth EF, Fuller F. Molecular cloning and functional expression of human parathyroid calcium receptor cDNAs. J. Biol. Chem. 1995;270:12919–12925. doi: 10.1074/jbc.270.21.12919. [DOI] [PubMed] [Google Scholar]
- Gottardi CJ, Wong E, Gumbiner BM. E-cadherin suppresses cellular transformation by inhibiting ß-catenin signaling in an adhesion-independent manner. J. Cell Biol. 2001;153:1049–1059. doi: 10.1083/jcb.153.5.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grau MV, Baron JA, Sandler RS, Haile RW, Beach ML, Church TR, Heber D. Vitamin D, calcium supplementation and colorectal adenomas: results of a randomized trial. J. Natl. Cancer Inst. 2003;95:1765–1771. doi: 10.1093/jnci/djg110. [DOI] [PubMed] [Google Scholar]
- Grau MV, Baron JA, Sandler RS, Wallace K, Haile RW, Church TR, Beck GJ, Summers RW, Barry EL, Cole BF, Snover DC, Rothstein R, Mandel JS. Prolonged effect of calcium supplementation on risk of colorectal adenomas in a randomized trial. J. Natl. Cancer Inst. 2007;99:129–136. doi: 10.1093/jnci/djk016. [DOI] [PubMed] [Google Scholar]
- Harris ED. Regulation of antioxidant enzymes. FASEB J. 1992;6:2675–2683. doi: 10.1096/fasebj.6.9.1612291. [DOI] [PubMed] [Google Scholar]
- Holt PR, Wolper C, Moss SF, Yang K, Lipkin M. Comparison of calcium supplementation or low-fat dairy foods on epithelial cell proliferation and differentiation. Nutr. Cancer. 2001;41:150–155. doi: 10.1080/01635581.2001.9680626. [DOI] [PubMed] [Google Scholar]
- Huang Y, Zhou Y, Castiblanco A, Yang W, Brown EM, Yang JJ. Multiple Ca2+ binding sites in the extracellular domain of the Ca2+-sensing receptor corresponding to cooperative Ca2+ response. Biochemistry. 2009;48:388–398. doi: 10.1021/bi8014604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugh TJ, Dillion SA, Taylor BA, Pignatelli M, Poston G, Kinsella AR. Cadherin-catenin expression in primary colorectal cancer: a survival analysis. Brit. J. Cancer. 1999;80:1046–1051. doi: 10.1038/sj.bjc.6690461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justinich CJ, Mak N, Pacheco I, Mulder D, Wells RW, Blennerhassett MG, MacLeod R. The extracellular calcium-sensing receptor (CaSR) on human esophagus and evidence of expression of CaSR on the esophageal epithelial cell line (HET-1A). Am. J. Physiol - Gastrointest. Liver Physiol. 2008:G120–G129. doi: 10.1152/ajpgi.00226.2006. [DOI] [PubMed] [Google Scholar]
- Kallay E, Kifor O, Chattopadhyay N, Brown EM, Bischof MG, Peterlik M, Cross HS. Calcium-dependent c-myc protooncogene expression and proliferation of CACO-2 cells: A role for luminal extracellular calcium-sensing receptor. Biochem. Biophys. Res. Commun. 1997;232:80–83. doi: 10.1006/bbrc.1997.6225. [DOI] [PubMed] [Google Scholar]
- Kallay E, Bajna E, Wrba F, Kriwanek S, Peterlik M, Cross HS. Dietary calcium and growth modulation of human colon cancer cells: Role of the extracellular calcium-sensing receptor. Cancer Detect. Prevent. 2000;24:127–136. [PubMed] [Google Scholar]
- Kampman E, Giovannucci E, van `t Veer P, Rimm E, Stampfer MJ, Colditz GA, Kok FJ, Willett WC. Calcium, vitamin D, diary foods and the occurrence of colorectal adenomas in men and women in two prospective studies. Am. J. Epidemiol. 1994;139:16–29. doi: 10.1093/oxfordjournals.aje.a116931. [DOI] [PubMed] [Google Scholar]
- Kampman E, Slattery ML, Caan B, Potter JD. Calcium, vitamin D, sunshine exposure, dairy products and colon cancer risk. Cancer Causes Control. 2000;11:459–465. doi: 10.1023/a:1008914108739. [DOI] [PubMed] [Google Scholar]
- Korinek V, Barker N, Morin PJ, van Wichen D, de Weger R, Kinzler KW, Vogelstein B, Clevers H. Constitutive transcriptional activation by ß-catenin - TCF complex in APC−/− in colon carcinoma. Science. 1997;275:1784–1787. doi: 10.1126/science.275.5307.1784. [DOI] [PubMed] [Google Scholar]
- Lamprecht SA, Lopkin M. Chemoprevention of colon cancer by calcium, vitamin D and folate: molecular mechanisms. Natl. Rev. Cancer. 2003;3:601–614. doi: 10.1038/nrc1144. [DOI] [PubMed] [Google Scholar]
- Lipkin M. Phase 1 and phase 2 proliferative lesion of colonic epithelial cells in disease leading to colonic cancer. Cancer. 1974;34:878–888. doi: 10.1002/1097-0142(197409)34:3+<878::aid-cncr2820340715>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Mareel M, Berx G, Van Roy F, Bracke M. Cadherin/catenin complex: a target for antiinvasive therapy? J. Cell Biochem. 1996;61:524–530. doi: 10.1002/(SICI)1097-4644(19960616)61:4%3C524::AID-JCB5%3E3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Marian B. Colorectal cancer: modeling causes, prevention and therapy. Drug Discovery Today: Disease Models. 2004;1:1–7. [Google Scholar]
- McCullough ML, Robertson AS, Rodriguez C, Jacobs EJ, Chao A, Jonas C, Calle EE, Willett WC, Thun MJ. Calcium, vitamin D, dairy products, and risk of colorectal cancer in the Cancer Prevention Study II Nutrition Cohort (United States). Cancer Causes Control. 2003;14:1–12. doi: 10.1023/a:1022591007673. [DOI] [PubMed] [Google Scholar]
- Mokady E, Schwartz B, Shany S, Lamprecht SA. A protective role of dietary vitamin D3 in rat colon carcinogeneeis. Nutr. Cancer. 2000;38:66–73. doi: 10.1207/S15327914NC381_10. [DOI] [PubMed] [Google Scholar]
- Newmark HL, Yang K, Lipkin M, Kopelovich L, Liu Y, Fan K, Shinozaki H. A Western-style diet induces benign and malignant neoplasms in the colon of normal C57B1/6 mice. Carcinogenesis. 2001;22:1871–1875. doi: 10.1093/carcin/22.11.1871. [DOI] [PubMed] [Google Scholar]
- Newmark H, Yang K, Kurihara N, Fan K, Augenlicht L, Lipkin M. Western-style diet-induced colonic tumors and their modulation by calcium and vitamin D in C57bl/6 mice: A preclinical model for human sporadic colon cancer. Carcinogenesis. 2009;30:88–92. doi: 10.1093/carcin/bgn229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutten MJ, Bacon KD, Marling KL, Stoney M, Meichsner CL, Lee FP, Hobson SA, Rodland KD, Sheppard BC, Trunkey DD, Deveney KE, Deveney CW. Identification of Ca2+ sensing receptor in normal human gastric mucous epithelial cells. Am. J. Physiol. 1999;277:G662–670. doi: 10.1152/ajpgi.1999.277.3.G662. [DOI] [PubMed] [Google Scholar]
- Sheinin Y, Kallay E, Wrba F, Kriwanek S, Peterlik M, Cross HS. Immunocytochemical localization of the extracellular calcium-sensing receptor in normal and malignant human large intestinal mucosa. J. Histochem. Cytochem. 2000;48:595–602. doi: 10.1177/002215540004800503. [DOI] [PubMed] [Google Scholar]
- Sitrin MD, Haline AG, Abrahams C, Brasitus TA. Dietary calcium and vitamin D modulates 1,2-dimethylhydrazine - induced colon cancer in the rat. Cancer Res. 1991;51:5608–5613. [PubMed] [Google Scholar]
- Stipanuk MH. Biochemical, physiological, molecular aspects of human nutrition. 2nd ed. Elsevier; St. Louis, MO.: 2006. [Google Scholar]
- Strain JJ, Cashman KD. Minerals and trace elements. In: Gibney MJ, Vorster HH, Kok FJ, editors. Introduction to human nutrition. Blackwell; 2002. pp. 177–224. [Google Scholar]
- Tamima K, Kuroishi T, Oshima A. Cancer mortality and morbidity statistics. Japan Scientific Societies Press; Tokyo: 2004. The Research Group for Population-based Cancer Registration in Japan. pp. 95–130. [Google Scholar]
- Tetsu O, McCormick F. ß-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398:422–426. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- Tu C-L, Oda Y, Bikle DD. Effects of a calcium receptor activator on the cellular response to calcium in human keratinocytes. J. Invest. Dermatol. 1999;113:340–345. doi: 10.1046/j.1523-1747.1999.00698.x. [DOI] [PubMed] [Google Scholar]
- Tu C-L, Oda Y, Komuves L, Bikle DD. The role of the calcium-sensing receptor in epidermal differentiation. Cell Calcium. 2004;35:265–273. doi: 10.1016/j.ceca.2003.10.019. [DOI] [PubMed] [Google Scholar]
- Van Aken E, De Wever O, Correia da Rocha AS, Mareel M. Defective E-cadherin/ß-catenin complexes in human cancer. Virchows Arch. 2001;439:725–751. doi: 10.1007/s004280100516. [DOI] [PubMed] [Google Scholar]
- Wakai K, Hirose K, Matsuo K, Ito H, Kuriki K, Suzuk T, Kato T, Hirai T, Kanemitsu Y, Tajima K. Dietary risk factors for colon and rectal cancer: A comparative case-controlled study. J. Epidemiol. 2006;16:125–135. doi: 10.2188/jea.16.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K, Kurihara N, Fan K, Newmark H, Rigas B, Bancroft L, Corner G, Livote E, Lesser M, Edelmann W, Velcich A, Lipkin M, Augenlicht L. Dietary induction of colonic tumors in a mouse model of sporadic colon cancer. Cancer Res. 2008;68:7803–7810. doi: 10.1158/0008-5472.CAN-08-1209. [DOI] [PubMed] [Google Scholar]