Abstract
Introduction
For lung squamous cell carcinomas, there are no pathological findings that have been universally accepted as prognostic factors, with the exception of pathological stage. Tumor budding and nuclear grade have been recognized as a poor prognostic factor in other carcinomas. In this study, we investigated whether pathological findings could determine prognosis in lung squamous cell carcinomas.
Methods
All available tumor slides from patients with surgically resected, solitary lung squamous cell carcinomas (1999–2009) were reviewed (n = 485; stage I/II/III, 281/136/68). Tumors were evaluated for differentiation, subtypes (keratinizing, non-keratinizing, basaloid pattern, papillary growth, and clear cell feature), tumor nest size (tumor budding and single cell invasion), and nuclear grade (nuclear diameter and mitosis). Overall survival (OS) was estimated using the Kaplan-Meier method (stratified by pathological stage) and group differences were investigated using the stratified log-rank test and the Cox proportional hazards model.
Results
OS was significantly decreased in patients with vs. without single cell invasion (p = 0.002 for the entire tumor and p = 0.001 for tumor edge), with large vs. small nuclei (p = 0.011), and with high vs. low grade tumor budding (p < 0.001 for maximum and p = 0.007 for total). In multivariate analyses, single cell invasion (hazard ratio [HR], 1.47–1.49), nuclear diameter (HR, 1.09–1.33) and tumor budding (HR, 1.04) were independent prognostic factors of OS. However, histologic subtyping including keratinizing, nonkeratinizing, basaloid, and clear cell subtypes did not show prognostic significance.
Conclusions
Pathological factors can help stratify prognosis in patients with lung squamous cell carcinomas.
Keywords: squamous cell carcinoma, lung, pathology, prognosis
INTRODUCTION
Currently, the tumor-node-metastasis (TNM) stage rather than any specific histologic feature is the most reliable prognostic predictor of non-small cell lung cancers (NSCLC).1 However, recently for lung adenocarcinoma, in addition to the TNM staging, the new international multidisciplinary histologic classification proposed by the International Association for the Study of Lung Cancer (IASLC), American Thoracic Society (ATS), and European Respiratory Society (ERS) in 20112 has led to identification of the prognostic significance of the predominant histological patterns, and this has been validated in separate, large cohort studies (>400 patients) across multiple countries.3–6 Traditionally, lung squamous cell carcinomas have been graded by the degree of keratinization (well, moderately, and poorly differentiated tumors). In the current World Health Organization (WHO) classification of lung carcinomas, squamous cell carcinomas are classified into papillary, clear cell, small cell, and basaloid subtypes, however, these have not been shown to have prognostic or other clinical significance.7 Furthermore, no alternative histologic features or grading system have been identified that clinicians could use to predict patient clinical outcome.
Since histologic subtyping of lung squamous cell carcinoma has not proven to be associated with survival, we considered evaluating two approaches to assessment of patterns of tumor invasion: single cell invasion and tumor budding, that have been demonstrated to have prognostic significance in several types of cancers. One initial study identified single cell invasion as an unfavorable prognostic indicator in patients with lung squamous cell carcinomas.8 Tumor budding is defined as the presence of isolated small tumor nests composed of less than 5 tumor cells in the stroma of the invasive tumor edge and it corresponds to significant tumor invasiveness.9, 10 It has been shown to correlate with an unfavorable clinical outcome (i.e. patient survival and disease recurrence) in colorectal cancer.9, 10 Interestingly enough, tumor budding may also exhibit the process of epithelial mesenchymal transition, which regulates the epithelial tumor cells transformation into the mesenchymal phenotype, thus increasing the capacity of migration and invasion.11–13 In addition to tumor budding, the size of the tumor nests (tumor clusters composed of ≤15 tumor cells) was determined to be a poor prognostic factor for the histological risk grading system of head and neck squamous cell carcinomas.14, 15 Despite the aforementioned correlations, comprehensive analyses on the prognostic value of tumor budding and tumor nest size have not been performed using a large cohort of resected lung squamous cell carcinomas.
A universally recognized histologic grading system for lung cancer has not been established. The clinical utility of using a nuclear grading system (e.g. mitotic count and nuclear atypia) has already been established in other major cancers such as breast carcinoma.16, 17 For lung adenocarcinoma, data are emerging for architectural and nuclear grading approaches that hopefully will lead to a uniform grading system in the near future.18–20 After evaluating all of the nuclear features in stage I lung adenocarcinomas, our group has recently determined that a higher mitotic count is an independent predictor of a higher risk of recurrence. We then proposed a new grading system that combined architectural features (2011 IASLC/ATS/ERS classification) and nuclear grade (mitotic count).21 However, for lung squamous cell carcinoma, a grading system for the prediction of patient’s outcomes has not been rigorously investigated.
In this study of a large series of patients with resected lung squamous cell carcinomas, we performed comprehensive analyses of pathological factors (tumor differentiation, histologic subtype, tumor budding, tumor nest size, and nuclear grade). We investigated whether or not any of the pathological factors correlated with clinical outcomes (overall survival and disease recurrence), independent of pathological stage.
MATERIALS AND METHODS
Patients
This retrospective study was approved by the Institutional Review Board of Memorial Sloan-Kettering Cancer Center (MSKCC). We reviewed all patients with solitary lung squamous cell carcinoma who underwent surgical resection at MSKCC between 1999 and 2009; tumor slides were available for histologic evaluation from 485 of those patients. Clinical data were collected from the prospectively maintained Thoracic Surgery Service lung carcinoma database and disease stage was assigned on the basis of the 7th edition of the American Joint Committee on Cancer TNM Staging Manual.22 On chest computed tomography (CT), the tumor locations were divided into two categories: “peripheral lesion” when located within the outer third ellipse, and “non-peripheral lesion” when located within the middle third (intermediate lesion) or within the inner third (central lesion).23, 24
Histologic Evaluation
All available hematoxylin and eosin (H&E) stained slides were reviewed by 2 pathologists (K.K. and W.D.T.) using an Olympus BX51 microscope (Olympus, Tokyo, Japan) with a standard 22-mm diameter eyepiece. Both pathologists had no knowledge of those patients’ clinical outcomes.
Tumors were graded by a degree of squamous differentiation into well, moderately, and poorly differentiated, in accordance with the 2004 WHO classification of lung carcinomas.7 In the well differentiated tumors, there were tumor nests composed of differentiated keratinocyte-like tumor cells with prominent keratinization (layered and cytoplasmic keratin) and intercellular bridges. In the poorly differentiated tumors, squamous morphology was only noticeable in a small area of the tumor. The moderately differentiated tumors showed an intermediate degree of squamous differentiation that was between well and poorly differentiated tumors.
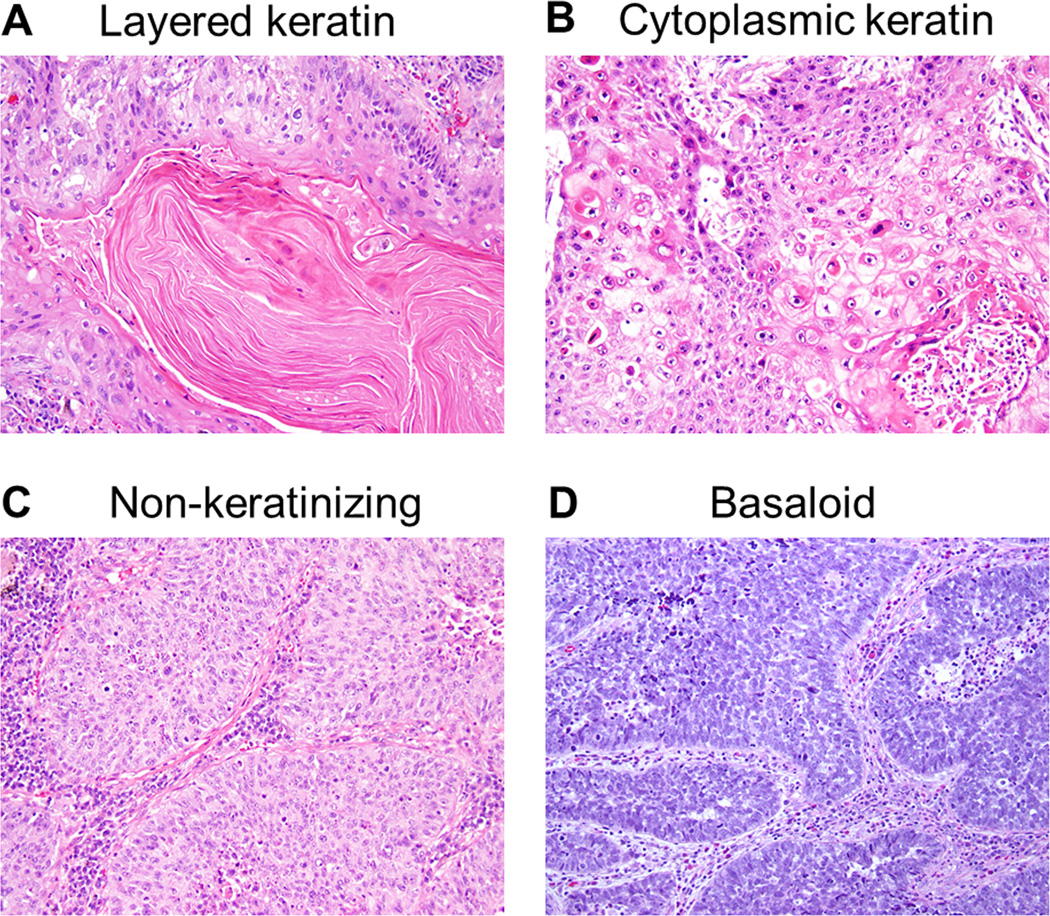
Histologic subtyping was performed in a similar fashion to nasopharyngeal carcinomas in the 2005 WHO Classification, Pathology and Genetics of Head and Neck Tumours; they were classified as non-keratinizing, keratinizing, and basaloid squamous cell carcinomas.25 The percentage of keratinizing pattern, including layered (Fig. 1A) and cytoplasmic keratinization (Fig. 1B), was recorded and then tumors were classified as having a keratinizing subtype when there was ≥5% keratinizing pattern of the entire tumor while non-keratinizing subtypes were defined as having <5% keratinizing pattern (Fig. 1C). The basaloid pattern was defined as tumor nests showing prominent peripheral palisading of tumor cells with scanty cytoplasm (high nuclear/cytoplasmic ratio) and a greater amount of hyperchromatic nuclei (Fig. 1D).7 The percentage of basaloid pattern was recorded and then the tumors were classified as having a basaloid subtype if there was >50% basaloid pattern as previously recommended.26, 27 The percentage of papillary growth was recorded in 5% increments. Clear cell features were defined as tumor cells with clear cytoplasm and were recorded in 5% increments; it was considered present when ≥5% of the tumor cells had a clear cell pattern. No cases were classified as the small cell variant of squamous cell carcinoma, although occasional basaloid carcinomas had tumor cells that resembled small cell carcinoma.
FIGURE 1.
Histologic subtypes (hematoxylin and eosin-stain; original magnification, ×200: A–D). (A) Keratinizing subtype with layered keratin. (B) Keratinizing subtype with cytoplasmic keratinization. (C) Non-keratinizing subtype. (D) Basaloid subtype.
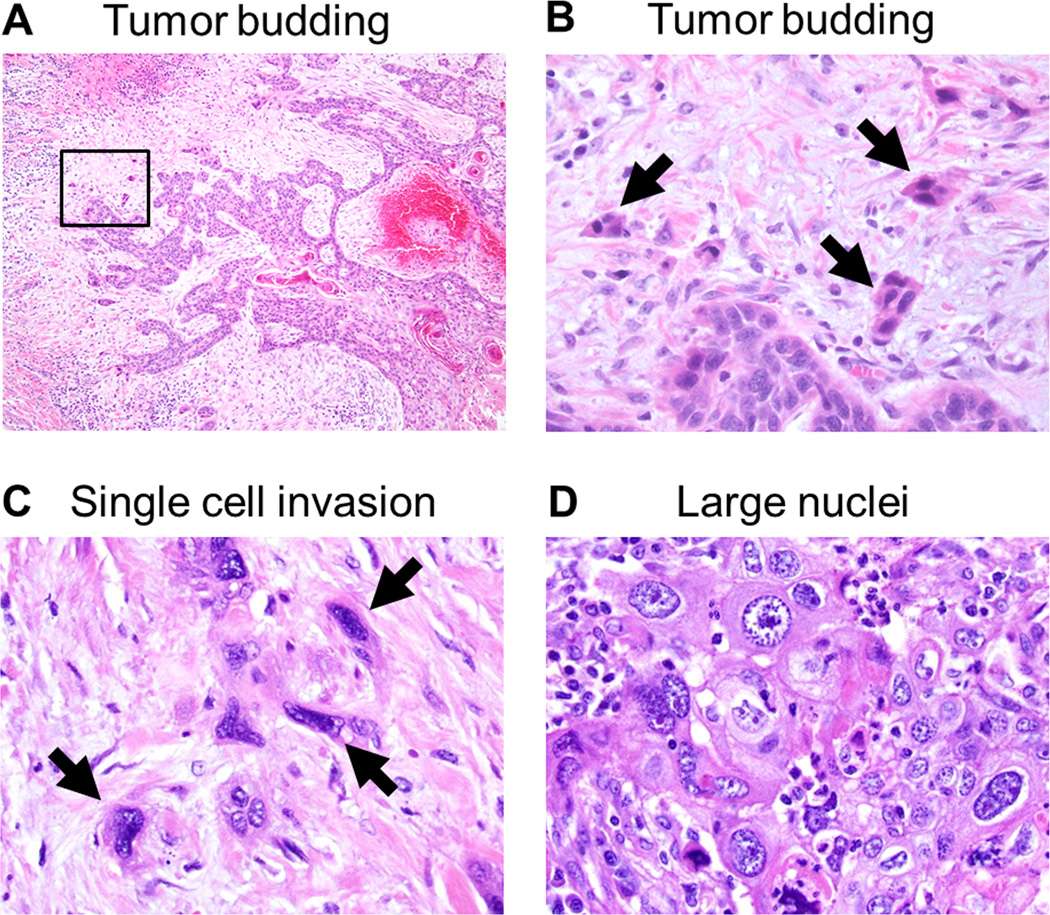
After scanning through the entire set of tumor slides at intermediate-power fields at ×100 magnification, tumor budding and the size of the smallest tumor nest were assessed at the most invasive area with the maximal number of the smallest tumor nests. Tumor budding was defined as small tumor nests composed of less than 5 tumor cells (Fig.2A and 2B) and they were counted in 10 high-power fields (HPFs) at ×200 magnification.9 According to the number of tumor budding counted in 10 HPFs, tumor budding was assessed 2 ways: 1) the maximum number of tumor budding per HPF among the 10 HPFs (maximum budding /1 HPF) and 2) the total number of tumor budding of 10 HPFs (total budding /10 HPFs). Based on the approach of previously published studies analyzing the prognostic significance of the tumor nest size assessed by the number of tumor cells,8, 10, 14, 15 the size of the smallest invasive tumor nest was classified into large nest (composed of >15 tumor cells), intermediate nest (5–15 tumor cells), small nest (2–4 tumor cells), and single cell invasion (Fig. 2C). The size of the smallest tumor nest was assessed 2 ways: 1) the tumor nests in entire tumor area and 2) the tumor nests infiltrating the tumor edge on the outside of the tumor.
FIGURE 2.
Tumor budding and single cell invasion (hematoxylin and eosin-stain; original magnification, ×40: A, ×400: C–D).
(A) Tumor budding identified in invasive tumor edge. (B) Higher magnification of a square box in the Figure 3A showing tumor budding composed of less than 5 tumor cells (arrows). (C) Single cell invasion of tumor cells in stroma (arrows). (D) Large nuclei defined as >4 small lymphocytes in diameter.
The percentages of tumor necrosis and fibrosis were recorded. Tumor necrosis was considered present when there was ≥10% necrosis in the entire tumor.28 When there was ≥50% fibrosis in the entire tumor, it was considered severe.29 In addition, pleural invasion, which was classified as absent (PL0) or present (PL1, PL2 and PL3)22, and lymphovascular invasion were investigated.
The nuclear features were evaluated according to the methodologies used in our previous publications.21, 30 They were assessed using a HPF at ×400 magnification (0.237mm2 field of view) at the region of the tumor with the greatest abnormal nuclear features. This was done after scanning through the entire set of tumor slides at intermediate-power fields at ×100 magnification. For nuclear diameter, we selected at least 3 HPFs with the largest nuclei and then calculated the average nuclear diameter of at least 100 tumor cells using nearby small lymphocytes (≈4.0 µm) as reference.19 Nuclear atypia was recorded in the area of the tumor with the highest degree of atypia; at least 5% of the entire tumor area needed to be affected. The degree of atypia was assessed using the following gradation: mild atypia - uniform nuclei in size and shape; moderate atypia - nuclei in intermediate size with slight irregularity in shape; and severe atypia - enlarged nuclei of varied sizes and irregular contours with some nuclei at least twice as large as others. The nuclear/cytoplasmic (N/C) ratio was broken down into the following three categories: low N/C ratio (<1/3 nucleus to cytoplasm area), intermediate N/C ratio (1/3–2/3), and high N/C ratio (>2/3). Chromatin pattern was differentiated using two distinctions, finely granular and coarsely granular. The prominence of nucleoli was also broken down into 2 distinct categories: indistinct - inconspicuous at intermediate-power fields at ×100 magnifications, and distinct - conspicuous at intermediate-power fields. Intranuclear inclusions were determined as present or absent in an examination of 50 HPFs. Mitoses were evaluated in the 50 HPF areas that contained the highest mitotic activity and then were calculated as an average of mitotic figures per 10 HPFs (2.37mm2 area).21, 30 Atypical mitoses were considered present if any were observed after examination of 50 HPF.21, 30
Tissue Microarray
Formalin-fixed, paraffin-embedded tumor specimens were used for tissue microarray construction. We marked 3 representative tumor areas on H&E-stained slides and, using an automated tissue arrayer ATA-27 (Beecher Instruments, Sun Prairie, WI, USA), we arrayed cylindrical 0.6-mm tissue cores from the corresponding paraffin blocks into a recipient block; this resulted in 5 tissue microarray blocks. In total, there were 447 available cases with adequate cores for immunohistochemical analysis.
Immunohistochemistry and Scoring of Ki-67
We took 4-mm sections from the tissue microarray blocks and briefly deparaffinized them in xylene and dehydrated them in graded alcohols. The standard avidin–biotin complex peroxidase technique was used for immunohistochemical stains of anti-Ki-67 antibodies (clone MIB-1, Immunotech, Westbrook, ME, USA; diluted at 1:100). Sections were stained using a Ventana Discovery XT Automated Immunohistochemical Stainer (Ventana, Tucson, AZ, USA) according to the manufacturer’s guidelines. Diaminobenzidine was used as the chromogen and hematoxylin was used as the nuclear counterstain. Positive control tissues were stained in parallel with the study cases. The Ki-67 proliferation index was recorded as the percentage of tumor cells with nuclear positive immunostaining in each tissue microarray core. The average percentage of the tumor cores was used as the Ki-67 proliferation index for each patient.21, 30 Immunohistochemical studies performed to confirm squamous differentiation are being reported elsewhere.31
Statistical Analysis
Associations between variables were analyzed using the chi-squared test (used for categorical variables) and the two-sample t-test (used for continuous variables). In order to dichotomize the continuous pathological variables (tumor budding, tumor nest size, mitotic count and Ki-67 index) into high/low categories, we applied the method of optimal cut-point estimation, which uses a maximally selected log-rank statistic to choose the cut-point that best stratifies the cohort.32
Two endpoints were investigated; overall survival (OS) in patients with all stages and cumulative incidence of recurrence (CIR) in patients with stage I diseases. OS was estimated by the Kaplan-Meier method, and associations between factors and survival were analyzed using the log-rank test, stratified by pathologic stage. For those variables dichotomized by using the optimal cut-point method, p-values were adjusted for optimal search.32 OS was defined as the time from surgery to death or the last follow-up. Multivariate analyses were performed using the Cox proportional hazards regression model.
The associations between factors and the risk of recurrence were evaluated with competing risks analyses.33, 34 The risk of recurrence (or CIR) was estimated using a cumulative incidence function that accounted for death without recurrence as a competing event. Patients were censored if they were alive and without a documented recurrence at the time of their most recent follow-up. The differences in CIR between groups were assessed using the methods of Gray (univariate nonparametric analyses) and the Fine and Gray model for competing risks (multivariate analysis).35
For each of the two outcomes, multivariate models were built to include all significant factors in the univariate analyses. Any associations between pathologic factors were checked, and if there were any strong associations discovered, only one factor was included into the model at any given time.
All statistical tests were two-sided and used a 5% significance level. Statistical analyses were performed using R (version 3.0.1; R Development Core Team) with the “maxstat,” “survival,” and “cmprsk” packages.
RESULTS
Patient Clinical Characteristics and Their Associations with OS and CIR
The median age in this 485 patient cohort was 71 years (range, 39–88 years). Most patients had pathological stage I disease (58%) while a smaller percentage of patients presented with stage II (28%) and stage III (14%) pathological disease. With regard to surgical procedure, 83% underwent lobectomies and 17% underwent limited resections (segmentectomy [n = 35] and wedge resection [n = 47]). Among patients undergoing limited resections, most patients were classified as stage I (n=68) while a smaller number of patients were classified as stage II (n=7) and stage III (n=7). Among limited resection group, most patients (n=60) underwent lymph node sampling or dissection; the remaining patients (n=22) were staged by chest CT and/or PET scans only, and all of them were classified as stage I disease. Almost one-fifth (19%) of patients received adjuvant therapy. Among the patients whose chest CT scans were available (n=423), 233 (55%) were classified as peripheral lesion. Perhaps since the time period of cases included in this study only spanned the most recent decade, we did not have the opportunity to see a significant shift from central to peripheral predominant location. We also did not find significant clinical associations with central vs. peripheral location. During the study period, 29% of patients (n = 139) experienced a recurrence and 58% (n = 281) died from both related and unrelated causes. The median follow-up period for all patients was 4.1 years (range, 0.01 – 13 years) and the 5-year patient OS was 59%.
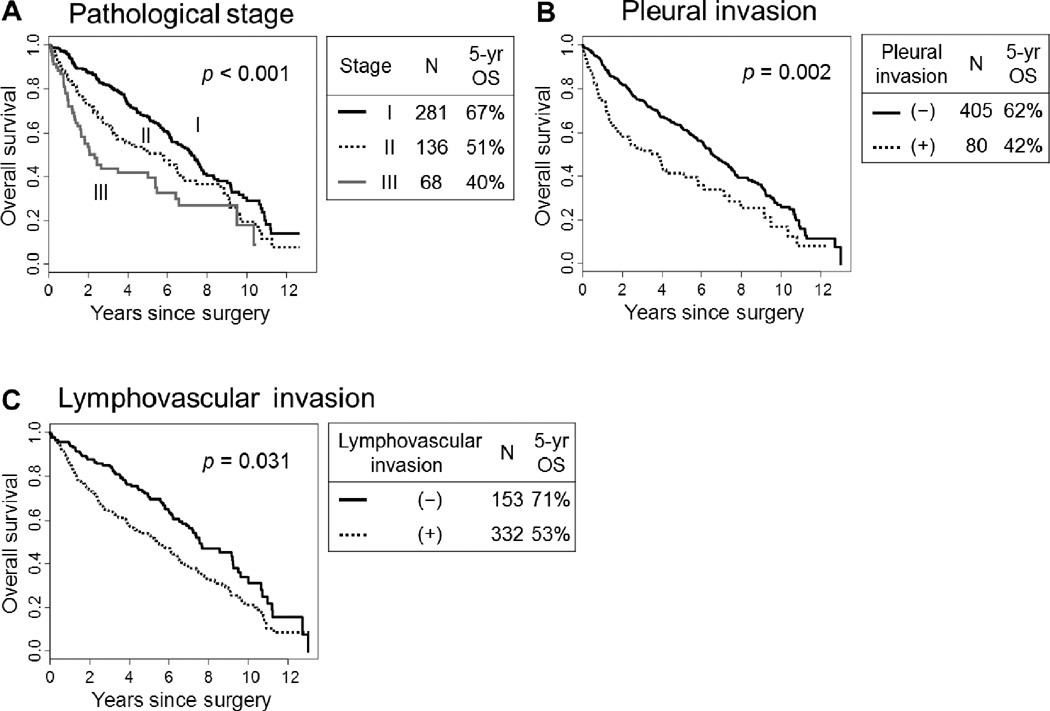
The associations between the clinical characteristics of patients and OS/CIR were summarized in Table 1. Regarding pathological stage, the 5-year OS was the worst for patients with stage III disease, followed by patients with stage II disease, and finally those with stage I disease (40%, 51%, and 67%, respectively; p < 0.001) (Fig. 3). In the analysis of OS stratified by pathological stage, older age (>65 years old; p < 0.001), the male sex (p = 0.049), a history of heavy smoking (>90 smoking pack-year; p = 0.011), limited resection (p = 0.016), and undergoing adjuvant therapy (p = 0.029) were all associated with a worse OS (Table 1A).
TABLE 1.
Patient characteristics and their associations with patient's outcomes
| A. Overall survival in all stages | B. Risk of recurrence in stage I | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | N | (%) | 5-year OS† |
p | Variables | N | (%) | 5-year CIR‡ |
p |
| Age, years | <0.001* | Age, years | 0.19 | ||||||
| ≤65 | 119 | (25) | 68% | ≤65 | 61 | (22) | 16% | ||
| >65 | 366 | (75) | 56% | >65 | 220 | (78) | 24% | ||
| Sex | 0.049* | Sex | 0.008 | ||||||
| Female | 200 | (41) | 65% | Female | 124 | (44) | 16% | ||
| Male | 285 | (59) | 55% | Male | 157 | (56) | 26% | ||
| Smoking pack-year | 0.011* | Smoking pack-year | 0.071 | ||||||
| ≤90 | 397 | (82) | 61% | ≤90 | 225 | (80) | 20% | ||
| >90 | 88 | (18) | 48% | >90 | 56 | (20) | 29% | ||
| Surgery | 0.016* | Surgery | 0.44 | ||||||
| Lobectomy | 403 | (83) | 60% | Lobectomy | 213 | (76) | 23% | ||
| Limited resection | 82 | (17) | 54% | Limited resection | 68 | (24) | 17% | ||
| Adjuvant therapy | 0.029* | Adjuvant therapy | |||||||
| No | 391 | (81) | 59% | No | 266 | (95) | 21% | 0.55 | |
| Yes | 94 | (19) | 57% | Yes | 15 | (5) | 27% | ||
| T classification | <0.001 | T classification | 0.029 | ||||||
| T1 | 213 | (44) | 67% | T1 | 169 | (60) | 18% | ||
| T2 | 211 | (44) | 56% | T2 | 112 | (40) | 27% | ||
| T3 | 52 | (11) | 38% | T3 | Not applicable | ||||
| T4 | 9 | (2) | 22% | T4 | |||||
| N classification | 0.001 | N classification | Not applicable | ||||||
| N0 | 349 | (72) | 63% | N0 | |||||
| N1 | 87 | (18) | 50% | N1 | |||||
| N2 | 49 | (10) | 44% | N2 | |||||
| Pathological stage | <0.001 | Pathological stage | Not applicable | ||||||
| Stage I | 281 | (58) | 67% | Stage I | |||||
| Stage II | 136 | (28) | 51% | Stage II | |||||
| Stage III | 68 | (14) | 40% | Stage III | |||||
P-values stratified by pathologic stage. Significant P-values are shown in bold.
OS, overall survival represents percentage of patients who did not die
CIR, cumulative incidence of recurrence represents percentage of patients who had a recurrence
FIGURE 3.
Overall survival (OS) by pathological stage, pleural invasion and lymphovascular invasion
(A) The 5-year OS was the worst for patients with stage III disease, followed by patients with stage II and stage I disease (40%, 51% and 67%, respectively; p < 0.001). (B) The 5-year OS of patients with pleural invasion was significantly worse (n=80; 42%) than those without pleural invasion (n=405; 62%; p = 0.002). (C) The 5-year OS of patients with lymphovascular invasion was significantly worse (n=332; 53%) than those without lymphovascular invasion (n=153; 71%; p = 0.031).
In the CIR analysis of stage I disease, the male sex (p = 0.008) and a higher T classification (T2 vs. T1; p = 0.029) were associated with an increased risk of recurrence (Table 1B).
Histological and Nuclear Features and Their Associations with OS
The associations between the histological and nuclear features and OS were summarized in Tables 2A and 3A. With regard to histologic subtyping, a major finding was the lack of prognostic significance for presence of keratinization (p = 0.97, keratinizing vs. nonkeratinizing) and clear cell (p = 0.23) features. Patients with a basaloid subtype tumor had a slightly better 5-year OS than those with non-basaloid tumors (69% vs. 58%). However, this finding was not statistically significant after we stratified the patients by pathological stage (p = 0.071). Clear cell and papillary features were infrequent. While clear cell features were identified in 61 (13%) cases, there were only 3 cases that showed predominant clear cell features (>50%). While focal papillary growth (usually <10% of the tumor) was identified in 41 cases, there was only 1 case that showed predominant papillary growth (>50%) and this patient has been alive for more than 5 years since surgical resection and without disease recurrence.
TABLE 2.
Histologic features and their associations with patient's outcomes
| A. Overall survival in all stages | B. Risk of recurrence in stage I | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | N | (%) | 5-year OS† |
p | Variables | N | (%) | 5-year CIR‡ |
p |
| Tumor differentiation | 0.98* | Tumor differentiation | 0.60 | ||||||
| Well | 56 | (12) | 58% | Well | 34 | (12) | 27% | ||
| Moderately | 196 | (40) | 58% | Moderately | 120 | (43) | 21% | ||
| Poorly | 233 | (48) | 60% | Poorly | 127 | (45) | 21% | ||
| Keratinization | 0.97* | Keratinization | 0.19 | ||||||
| Non-keratinizing | 363 | (75) | 59% | Non-keratinizing | 211 | (75) | 22% | ||
| Keratinizing | 122 | (25) | 58% | Keratinizing | 70 | (25) | 32% | ||
| Basaloid pattern | 0.071* | Basaloid pattern | 0.73 | ||||||
| Non-basaloid | 452 | (93) | 58% | Non-basaloid | 257 | (91) | 22% | ||
| Basaloid | 33 | (7) | 69% | Basaloid | 24 | (9) | 17% | ||
| Clear cell feature | 0.23* | Clear cell feature | 0.73 | ||||||
| Absence | 424 | (87) | 58% | Absence | 253 | (90) | 22% | ||
| Presence | 61 | (13) | 63% | Presence | 28 | (10) | 20% | ||
| Tumor budding (max) | <0.001* | Tumor budding (max) | 0.75 | ||||||
| Low (<10/1 HPF) | 409 | (84) | 62% | Low (<10/1 HPF) | 252 | (90) | 22% | ||
| High (≥10/1 HPF) | 76 | (16) | 39% | High (≥10/1 HPF) | 29 | (10) | 22% | ||
| Tumor budding (total) | 0.007* | Tumor budding (total) | 0.12 | ||||||
| Low (<8/10 HPFs) | 304 | (63) | 67% | Low (<8/10 HPFs) | 206 | (73) | 22% | ||
| High (≥ 8/10 HPFs) | 181 | (37) | 46% | High (≥ 8/10 HPFs) | 75 | (27) | 31% | ||
| Single cell inv. (entire) | 0.002* | Single cell inv. (entire) | 0.84 | ||||||
| Absent | 288 | (59) | 67% | Absent | 190 | (68) | 21% | ||
| Present | 197 | (41) | 47% | Present | 91 | (32) | 23% | ||
| Single cell inv. (edge) | 0.001* | Single cell inv. (edge) | 0.95 | ||||||
| Absent | 351 | (72) | 65% | Absent | 226 | (80) | 22% | ||
| Present | 134 | (28) | 42% | Present | 55 | (20) | 21% | ||
| Pleural inv. | 0.002* | Pleural inv. | 0.13 | ||||||
| Absent (PL0) | 405 | (84) | 62% | Absent (PL0) | 254 | (90) | 20% | ||
| Present (PL1–3) | 80 | (16) | 42% | Present (PL1–3) | 27 | (10) | 35% | ||
| Lymphovascular inv. | 0.031* | Lymphovascular inv. | 0.041 | ||||||
| Absent | 153 | (32) | 71% | Absent | 134 | (48) | 17% | ||
| Present | 332 | (68) | 53% | Present | 147 | (52) | 26% | ||
| Necrosis | 0.72* | Necrosis | 0.65 | ||||||
| Absent | 190 | (39) | 63% | Absent | 120 | (43) | 21% | ||
| Present | 295 | (61) | 56% | Present | 161 | (57) | 23% | ||
| Fibrosis | 0.85* | Fibrosis | 0.019 | ||||||
| Mild | 403 | (83) | 60% | Mild | 244 | (87) | 24% | ||
| Severe | 82 | (17) | 53% | Severe | 37 | (13) | 8% | ||
P-values stratified by pathologic stage. Significant P-values are shown in bold.
OS, overall survival represents percentage of patients who did not die.
CIR, cumulative incidence of recurrence represents percentage of patients who had a recurrence.
HPF, high-power field
TABLE 3.
Nuclear features and their associations with patient's outcomes
| A. Overall survival in all stages | B. Risk of recurrence in stage I | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | N | (%) | 5-year OS† |
p | Variables | N | (%) | 5-year CIR‡ |
p |
| Nuclear diameter | 0.011* | Nuclear diameter | 0.26 | ||||||
| Small | 332 | (68) | 63% | Small | 198 | (70) | 21% | ||
| Large | 153 | (32) | 50% | Large | 83 | (30) | 24% | ||
| Nuclear atypia | 0.050* | Nuclear atypia | 0.71 | ||||||
| Mild | 78 | (16) | 67% | Mild | 59 | (21) | 25% | ||
| Moderate | 275 | (57) | 61% | Moderate | 154 | (55) | 21% | ||
| Severe | 132 | (27) | 49% | Severe | 68 | (24) | 21% | ||
| Nuclear/cytoplasmic ratio | 0.20* | Nuclear/cytoplasmic ratio | 0.24 | ||||||
| Low | 61 | (13) | 65% | Low | 38 | (14) | 12% | ||
| Intermediate | 310 | (64) | 56% | Intermediate | 177 | (63) | 24% | ||
| High | 114 | (24) | 63% | High | 66 | (23) | 22% | ||
| Chromatin pattern | 0.73* | Chromatin pattern | 0.64 | ||||||
| Fine | 198 | (41) | 65% | Fine | 145 | (52) | 21% | ||
| Coarse | 287 | (59) | 54% | Coarse | 136 | (48) | 23% | ||
| Prominence of nucleoli | 0.46* | Prominence of nucleoli | 0.69 | ||||||
| Indistinct | 340 | (70) | 62% | Indistinct | 202 | (72) | 21% | ||
| Distinct | 145 | (30) | 52% | Distinct | 79 | (28) | 23% | ||
| Nuclear inclusion | 0.89* | Nuclear inclusion | |||||||
| Absent | 475 | (98) | 59% | Absent | 276 | (98) | 22% | 0.20 | |
| Present | 10 | (2) | 40% | Present | 5 | (2) | 0% | ||
| Mitotic count | 0.070* | Mitotic count | 0.84 | ||||||
| Low (<15/10 HPFs) | 147 | (30) | 56% | <15/10 HPFs | 98 | (35) | 24% | ||
| High (≥15/10 HPFs) | 338 | (70) | 60% | ≥15/10 HPFs | 183 | (65) | 21% | ||
| Atypical mitosis | 0.50* | Atypical mitosis | 0.18 | ||||||
| Absent | 149 | (31) | 60% | Absent | 95 | (34) | 19% | ||
| Present | 336 | (69) | 58% | Present | 186 | (66) | 23% | ||
| Ki-67 labeling index | 0.26* | Ki-67 labeling index | 0.39 | ||||||
| Low (<20%) | 47 | (11) | 66% | Low (<20%) | 31 | (12) | 17% | ||
| High (≥20%) | 400 | (89) | 57% | High (≥20%) | 220 | (88) | 23% | ||
P-values stratified by pathologic stage. Significant P-values are shown in bold.
OS, overall survival represents percentage of patients who did not die.
CIR, cumulative incidence of recurrence represents percentage of patients who had a recurrence.
HPF, high-power field
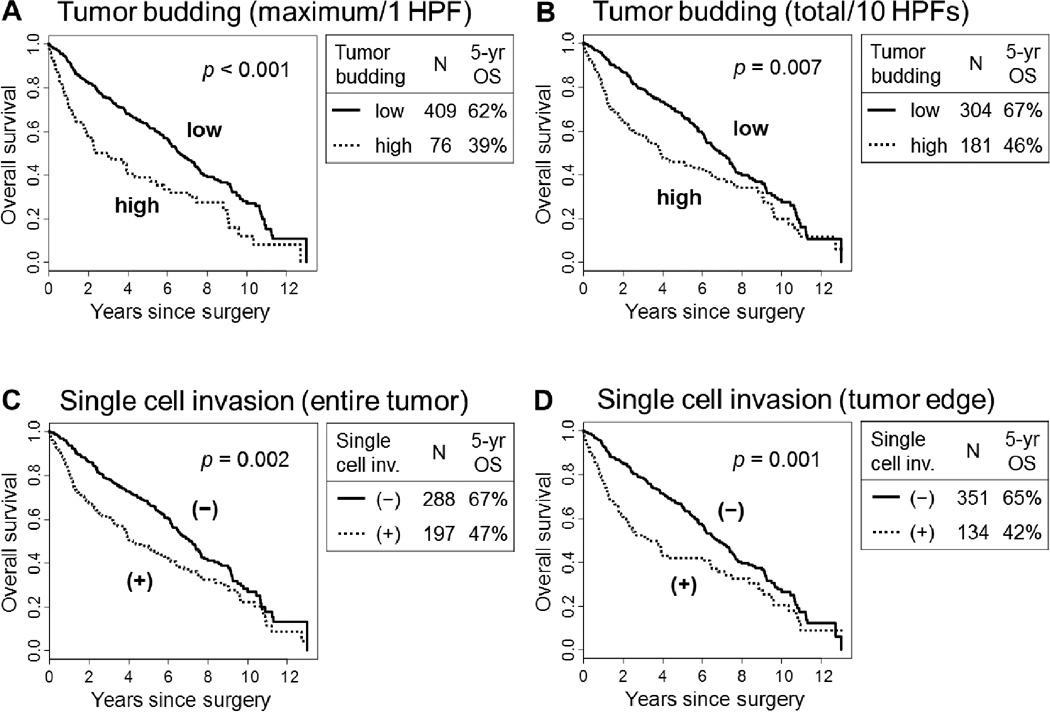
Due to this lack of clear prognostic significance for histologic subtyping, we focused our attention to patterns of invasion and nuclear grading. Using an optimal cut-point of 10 buds/1 HPF, the 5-year OS of patients with a high grade (≥10 buds /1 HPF) for maximum tumor budding was significantly worse (n = 76; 39%) than those with a low grade (<10 buds /1 HPF; n = 409; 62%; p < 0.001) (Fig. 4A). Using an optimal cut-point of 8 buds/10 HPFs, the 5-year OS of patients with high grade (≥8 buds /10 HPFs) for total tumor budding was significantly worse (n = 181; 46%) than those with a low grade (<8 buds /10 HPFs; n = 304; 67%; p = 0.007) (Fig. 4B). The 5-year OS of patients with single cell invasion in the entire tumor was significantly worse (n = 197; 47%) than those without single cell invasion (n = 288; 67%; p = 0.002) (Fig. 4C). Similarly, the 5-year OS of patients with single cell invasion in the tumor edge was significantly worse (n = 134; 42%) than those without single cell invasion (n = 351; 65%; p = 0.001) (Fig. 4D).
FIGURE 4.
Overall survival (OS) by tumor budding and single cell invasion.
(A) The 5-year OS of patients with high grade (≥10 buds /1 HPF) for maximum budding was significantly worse (n = 76; 39%) than those with low grade (<10 buds /1 HPF; n = 409; 62%; p<0.001). (B) The 5-year OS of patients with high grade (≥8 buds /10 HPFs) for total tumor budding was significantly worse (n = 181; 46%) than those with low grade (<8 buds /10 HPFs; n = 304; 67%; p = 0.007). (C) The 5-year OS of patients with single cell invasion in entire tumor was significantly worse (n = 197; 47%) than those without single cell invasion (n = 288; 67%; p = 0.002). (D) The 5-year OS of patients with single cell invasion in tumor edge was significantly worse (n = 134; 42%) than those without single cell invasion (n = 351; 65%; p = 0.001).
The presence of pleural invasion (p = 0.002) (Fig. 3B) and lymphovascular invasion (p = 0.031) (Fig. 3C) were also associated with a worse OS on univariate analysis. However, tumor differentiation (p = 0.98), tumor necrosis (p = 0.72), and fibrosis (p = 0.85) did not correlate with OS.
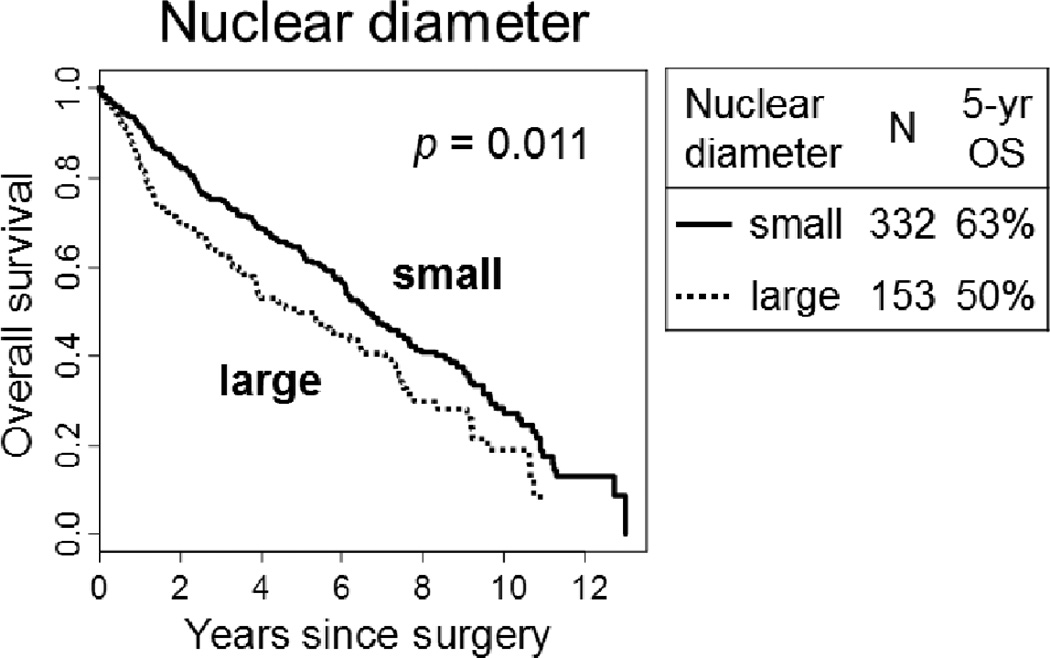
Using an optimal cut-point of 4 small lymphocytes (Fig. 2D) for nuclear diameter, the 5-year OS of patients with large nuclei (>4 small lymphocytes) was significantly worse (n = 153; 50%) than those with small nuclei (n = 332; 63%; p = 0.011) (Fig. 5). A higher degree of nuclear atypia and low mitotic count (using an optimal cut-point of 15/10 HPF) often resulted in a worse OS. However, these findings were not statistically significant (p = 0.050 and p = 0.070, respectively). Features that were not associated with OS in this cohort were N/C ratio (p = 0.20), chromatin pattern (p = 0.73), prominence of nucleoli (p = 0.46), nuclear inclusions (p = 0.89), atypical mitoses (p = 0.50), and Ki-67 labeling index (using an optimal cut-point of 20%, p = 0.26).
FIGURE 5.
Overall survival (OS) by nuclear diameter.
The 5-year OS of patients with large nuclei (>4 small lymphocytes) was significantly worse (n = 153; 50%) than those with small nuclei (n = 332; 63%; p = 0.011).
Histological and Nuclear Features and Their Associations with Recurrence
Tables 2B and 3B summarize the associations between histological and nuclear features and the risk of recurrence. In the CIR analysis of stage I disease subgroup, while lymphovascular invasion was associated with an increased risk of recurrence (p = 0.041), severe fibrosis was associated with a reduced risk of recurrence (p = 0.019).
Multivariate Analysis of OS and Recurrence
Since there was a strong association between tumor budding (maximum and total) and single cell invasion (entire tumor and tumor edge), we built four multivariate OS models in which each of these variables were included separately. In each multivariate model, high grade for maximum budding (≥10 buds /1 HPF /1 HPF; Hazard ratio [HR] = 1.04; p = 0.014), high grade for total tumor budding (≥8 buds /1 HPF /10 HPFs; HR = 1.04; p = 0.029), single cell invasion in the entire tumor (HR = 1.47; p = 0.003), and single cell invasion at the tumor edge (HR = 1.49; p = 0.004) were independent prognostic factors of worse OS. In addition, having large nuclei was an independent prognostic factor of OS in all the multivariate models (HR between 1.09 – 1.33, p = 0.028 – 0.039 in the four models). Table 4 presents two models that include maximum tumor budding (Table 4A) and single cell invasion in entire tumor (Table 4B), respectively.
TABLE 4.
Multivariate analysis of overall survival in all stages (n=485)
| A. Model for tumor budding (maximum /1 HPF) | HR | 95% CI | p | |
| Age | > 65 vs. ≤ 65 | 1.72 | 1.26–2.36 | <0.001 |
| Sex | male vs. female | 1.24 | 0.96–1.59 | 0.096 |
| Smoking pack-year | >90 vs. ≤90 | 1.25 | 0.92–1.70 | 0.15 |
| Surgery | lobectomy vs. limited resection | 1.30 | 0.93–1.80 | 0.12 |
| Pathological stage | II vs. I | 1.38 | 1.02–1.87 | 0.037 |
| III vs. I | 2.18 | 1.51–3.15 | <0.001 | |
| Lymphovascular inv. | present vs. absent | 1.25 | 0.92–1.70 | 0.15 |
| Tumor budding (maximum) | high (≥10/HPF) vs. low (<10/HPF) | 1.04 | 1.01–1.07 | 0.014 |
| Nuclear diameter | large vs. small | 1.33 | 1.03–1.70 | 0.028 |
| B. Model for single cell invasion (entire tumor) | HR | 95% CI | p | |
| Age | > 65 vs. ≤ 65 | 1.89 | 1.37–2.59 | <0.001 |
| Sex | male vs. female | 1.22 | 0.95–1.57 | 0.11 |
| Smoking pack-year | >90 vs. ≤90 | 1.26 | 0.93–1.70 | 0.14 |
| Surgery | lobectomy vs. limited resection | 1.35 | 0.97–1.88 | 0.074 |
| Pathological stage | II vs. I | 1.43 | 1.05–1.93 | 0.021 |
| III vs. I | 2.20 | 1.52–3.18 | <0.001 | |
| Lymphovascular inv. | present vs. absent | 1.21 | 0.89–1.64 | 0.23 |
| Single cell inv. (entire tumor) | present vs. absent | 1.47 | 1.14–1.88 | 0.003 |
| Nuclear diameter | large vs. small | 1.30 | 1.01–1.68 | 0.039 |
Significant P-values are shown in bold.
HR, hazard ratio; CI, confidence interval; HPF, high-power field
In the subgroup analysis of stage I disease, severe fibrosis within the tumor stroma was an independent predictor of a reduced risk of recurrence (severe vs. mild; HR = 0.98; 95% confidence interval (CI) = 0.95–0.99; p = 0.030) after adjusting for sex (male vs. female; HR = 1.80; 95% CI = 1.04–3.13; p = 0.037) and tumor classification (T2 vs. T1; HR = 1.55; 95% CI = 0.95–2.54; p = 0.082). However, the magnitude of the effect of fibrosis was small.
Associations between Prognostic Histological or Nuclear Features and Other Clinicopathological Factors
Table 5 provides a summarization of all of the associations between the prognostic histological/nuclear features and the other clinicopathological factors. Tumor budding (maximum and total) and single cell invasion (entire tumor and tumor edge) were associated with larger tumor size, higher tumor stage, presence of nodal metastasis, higher pathological stage, lymphovascular invasion, and pleural invasion (p < 0.05 for each analysis). The presence of large nuclei were associated with larger tumor size (p = 0.027) and lymphovascular invasion (p = 0.040).
TABLE 5.
Associations between clinicopathologic characteristics and prognostic pathological factors
| Variables | Tumor budding (maximum, /1 HPF) |
Tumor budding (total, /10 HPFs) |
Single cell invasion (entire tumor) |
Single cell invasion (tumor edge) |
Nuclear diameter | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low | High | p | Low | High | p | Absent | Present | p | Absent | Present | p | Small | Large | p | |
| Age, years | 0.098 | 0.059 | 0.033 | 0.16 | 0.14 | ||||||||||
| Median | 71 | 74 | 72 | 70 | 72 | 70 | 71 | 71 | 71 | 73 | |||||
| Range | 42–88 | 39–87 | 42–88 | 39–87 | 44–88 | 38–87 | 42–88 | 39–87 | 39–88 | 42–88 | |||||
| Sex | 0.77 | 0.081 | 0.15 | 0.015 | 0.75 | ||||||||||
| Female | 41% | 43% | 44% | 36% | 44% | 37% | 45% | 32% | 42% | 40% | |||||
| Male | 59% | 57% | 56% | 64% | 56% | 63% | 55% | 68% | 58% | 60% | |||||
| Smoking pack-year | 0.74 | 0.39 | 0.51 | 0.79 | 0.092 | ||||||||||
| Median | 53 | 52 | 51 | 53 | 50 | 54 | 54 | 51 | 50 | 59 | |||||
| Range | 0–180 | 0–252 | 0–180 | 0–252 | 0–180 | 0–252 | 0–180 | 0–252 | 0–180 | 5–252 | |||||
| Tumor size | 0.006 | <0.001 | <0.001 | <0.001 | 0.027 | ||||||||||
| Median | 3 | 3.8 | 2.6 | 4 | 2.6 | 3.8 | 2.8 | 4 | 3 | 3.5 | |||||
| Range | 0.5–14 | 1.3–17 | 0.5–14 | 1.2–17 | 0.5–14 | 1.2–17 | 0.5–14 | 1.4–17 | 0.5–17 | 0.9–14 | |||||
| Tumor stage | <0.001 | <0.001 | <0.001 | <0.001 | 0.51 | ||||||||||
| T1 + T2 | 90% | 71% | 93% | 78% | 92% | 80% | 93% | 74% | 88% | 86% | |||||
| T3 + T4 | 10% | 29% | 7% | 22% | 8% | 20% | 7% | 26% | 12% | 14% | |||||
| Nodal stage | 0.046 | <0.001 | 0.020 | <0.001 | 0.93 | ||||||||||
| N0 | 74% | 62% | 77% | 63% | 76% | 66% | 75% | 63% | 72% | 73% | |||||
| N1 + N2 | 26% | 38% | 23% | 37% | 24% | 34% | 25% | 37% | 28% | 27% | |||||
| Pathologic stage | <0.001 | <0.001 | <0.001 | 0.007 | 0.31 | ||||||||||
| stage I | 62% | 38% | 68% | 41% | 66% | 46% | 64% | 41% | 60% | 54% | |||||
| stage II + III | 38% | 62% | 32% | 59% | 34% | 54% | 36% | 59% | 40% | 46% | |||||
| Lymphovascular inv. | <0.001 | <0.001 | <0.001 | <0.001 | 0.040 | ||||||||||
| Absent | 36% | 5% | 43% | 12% | 42% | 16% | 39% | 12% | 35% | 25% | |||||
| Present | 64% | 95% | 57% | 88% | 58% | 84% | 61% | 88% | 65% | 75% | |||||
| Pleural inv. | <0.001 | <0.001 | <0.001 | <0.001 | 0.056 | ||||||||||
| Absent (PL0) | 88% | 62% | 91% | 71% | 90% | 74% | 89% | 69% | 86% | 78% | |||||
| Present (PL123) | 12% | 38% | 9% | 29% | 10% | 26% | 11% | 31% | 14% | 22% | |||||
Significant P-values are shown in bold.
HPF, high-power field
DISCUSSION
We have performed a series of comprehensive pathological analyses in an effort to identify prognostic indicators of death and recurrence in patients with resected lung squamous cell carcinomas. We have demonstrated that single cell invasion (entire tumor and tumor edge), large nuclear size, and tumor budding (maximum/1 HPF and total /10 HPFs) were independently associated with an unfavorable OS. This contrasts with histologic subtyping which did not show prognostic significance according to keratinizing, nonkeratinizing, clear cell and basaloid patterns. In the absence of prognostic significance for histologic subtyping, we found it of interest to explore why the patterns of tumor invasion and nuclear grading correlated with clinical outcome.
Tumor budding is a relatively recently recognized morphologic pattern of tumor invasion that has been shown to be a poor prognostic factor in colorectal cancer.9, 10 Moreover, tumor budding has also been identified as an unfavorable prognostic indicator in lung squamous cell carcinomas and adenocarcinomas.12, 13, 36 In a previous study on tumor budding in lung squamous cell carcinomas, the total number of tumor budding was counted using 1 HPF at ×200 magnification.12 Using a maximal budding intensity, the presence of tumor budding (≥1 buds /1 HPF) was identified as an independent prognostic factor.12 In our study, we used the same method to count tumor budding and that tumors with ≥10 buds /1 HPF was an independent prognostic factors for worse survival. However, the magnitude of the effect was very small (HR = 1.04), which suggests that, despite statistical significance, the clinical value might be limited. A generally agreed cutoff for tumor budding has not yet been established for lung squamous cell carcinomas and this awaits further validation studies. When counting the number of tumor budding using the 1 HPF approach, interobserver agreement could be problematic because of the improbability of different pathologists selecting the field with maximal intensity of tumor budding. In our study, the total number of tumor budding counted when using the 10 HPFs scoring method was also an independent prognostic factor. We propose that the 10 HPFs scoring system for tumor budding is a reliable and reproducible method, similar to a recent proposal in colon carcinoma.9 In addition, this method could easily be used by pathologists in their clinical practice.
With regard to the size of the smallest tumor nest, our study demonstrated that single cell invasion (in both the entire tumor and the tumor edge) was an independent prognostic factor. This data was also supported by comparable findings in a previous study from Japan.8 The presence of small tumor clusters composed of ≤15 tumor cells has previously been used as a histologic risk model in head and neck squamous cell carcinomas,14, 15 but this approach did not prove to be a prognostic indicator in our study on lung squamous cell carcinoma.
Published data on the prognosis of basaloid carcinomas is conflicting. Basaloid carcinomas are a rare subtype of lung cancer with a reported incidence of 6% of NSCLCs.27 In the 1999 and 2004 WHO classifications, basaloid carcinomas were included as a variant of large cell carcinomas and squamous cell carcinomas.7 In the past classifications, basaloid variant was described as the tumor having prominent peripheral palisading of tumor cells with scanty cytoplasm and hyperchromatic nuclei; however, the percentage cutoff for basaloid patter was not defined in order to classify the tumors as basaloid subtype.7, 26 In the upcoming revision of the WHO classification, all squamous cell carcinomas with a basaloid component >50% will be considered as basaloid subtype of squamous cell carcinoma and pure tumors will no longer be variants of large cell carcinoma. Moreover, non-basaloid squamous cell carcinomas (tumors with no or less than 50% basaloid pattern) will be classified into keratinizing or nonkeratinizing subtype using a similar definition to nasopharyngeal carcinomas in the 2005 WHO Classification, Pathology and Genetics of Head and Neck Tumours.25 Several studies have reported that basaloid carcinomas (including basaloid squamous cell carcinomas and basaloid large cell carcinomas) had a shorter patient survival than non-basaloid squamous cell carcinomas.27, 37 Conversely, there are studies from East Asian countries that have reported no statistical difference in survival between patients with basaloid squamous cell carcinomas and those with poorly differentiated, non-basaloid squamous cell carcinomas.38, 39 In our study of patients from the United States, patients with basaloid squamous cell carcinomas had a trend for better prognosis than those with non-basaloid tumors although the finding was not statistically significant. These differences in the prognosis of basaloid squamous cell carcinomas between the studies may be due to variations in disease stage, the histologic type of basaloid tumors (including basaloid NSCLCs, basaloid squamous cell carcinomas, or basaloid large cell carcinomas), treatment modalities, ethnic differences, genetic differences, or interobserver agreement in identifying basaloid patterns. The prognostic significance and reproducibility of the basaloid subtype in lung squamous cell carcinomas is still controversial and further investigation is required.
Our data has potential implications for the upcoming revision of the WHO classification. Similar to previous studies, the degree of keratinization, including the presence of keratinization and tumor differentiation, was not associated with prognosis.8, 40. However, keratinizing and nonkeratinizing subtypes of squamous cell carcinoma are well established subtypes in sites like the nasopharynx where they are part of the WHO Classification, despite the lack of clear prognostic importance.25. Recognizing a nonkeratinizing subtype of squamous cell carcinoma in the lung is also important because these tumors can have morphologic overlap with pseudosquamous adenocarcinomas. This distinction has molecular and therapeutic implications so accurate diagnosis requires immunohistochemistry for adenocarcinoma and squamous markers such as TTF-1 and p40, respectively.41 To the best of our knowledge, the prognostic significance of clear cell features in lung squamous cell carcinomas is unknown and there was no prognostic value in our study. This combined with the infrequency of clear cell predominant features is against maintaining clear cell as a major subtype of squamous cell carcinoma.
The papillary variant of lung squamous cell carcinomas can show exophytic and endobronchial growth but most cases show submucosal or bronchial wall invasion.42 Even though it has been reported that tumors were almost always recognized in early stages (mainly T1N0) and that the prognosis is not any better than other stage I lung cancers, the prognostic significance of the papillary subtype is still unknown.42 The papillary subtype of lung squamous cell carcinoma is extremely rare and there was only 1 case in our cohort of 485 patients that exhibited predominant papillary growth. The rarity of papillary predominant squamous cell carcinomas as well as the lack of clear prognostic importance provides good reasons to question whether it should be a major subtype of squamous cell carcinoma in the WHO classification. The “small cell” subtype of squamous cell carcinoma included in the 1999 and 2004 WHO classifications is no longer accepted as these tumors most likely represented basaloid carcinomas with very small tumor cell size and because the term small cell could lead to clinical confusion with diagnosis of small cell carcinoma.7, 26, 43 Given these considerations the upcoming revision of the WHO classification for subclassification of squamous cell carcinoma includes keratinizing, nonkeratinizing and basaloid subtypes.
The clinical utility of a nuclear grading system that includes nuclear atypia and mitotic count has already been established in other carcinomas, such as breast, kidney, and bladder.16, 17, 44–46 Although our group and others have demonstrated the prognostic value of nuclear grades in lung adenocarcinomas,18, 21, 47, 48 the prognostic utility of nuclear grade still remains unknown in lung squamous cell carcinomas. In our study, large nuclei, which is defined as >4 small lymphocytes in nuclear diameter, was independently associated with a worse OS; this was done after stratifying by pathologic stage. Although there was a trend of worse prognosis in those with severe atypia, nuclear atypia was not statistically significant for predicting prognosis. Therefore, we believe that nuclear diameter might be a more reliable factor associated with clinical outcome. It could provide greater reproducibility by use of a semi-quantitative method that measures nuclear diameter with a small lymphocyte. In contrast to the unfavorable prognostic value of a higher mitotic count in lung adenocarcinomas,21, 47, 48 our study of lung squamous cell carcinomas showed that patients with a higher mitotic count often had better prognoses. This similar finding was also reached in a previous study.49 A higher mitotic count may have a different prognostic and biological relevance in lung squamous cell carcinomas than it does in lung adenocarcinomas. In addition to mitotic count, a high Ki-67 proliferation index was also reported to be a poor prognostic marker in lung adenocarcinomas and NSCLCs by our group and others.21, 50, 51 However, we did not identify any prognostic value in the Ki-67 proliferation index in our lung squamous cell carcinoma cohort.
In multivariate analysis of OS adjusting for clinical and pathological factors (including pathologic stage and lymphovascular invasion) which were significantly prognostic in univariate analyses, maximum budding (HR = 1.04), total tumor budding (HR = 1.04), single cell invasion in the entire tumor (HR = 1.47), and single cell invasion in the tumor edge (HR = 1.49) were found to be independent prognostic factors for worse outcomes. However, these aggressive invasive patterns (tumor budding and single cell invasion) were significantly associated with other strong prognostic factors (such as pathologic stage and lymphovascular invasion), and the HRs close to one for these invasive patterns suggest limited clinical utility (especially for tumor budding variables). Therefore, further validation studies are warranted to confirm independent prognostic impact in these factors.
Tumor necrosis has been proposed as a prognostic factor that should be included in the grading system for renal cell carcinomas.52, 53 In a previous study, our group demonstrated that the presence of tumor necrosis was an independent predictor of a higher risk of recurrence in stage I lung adenocarcinomas.21 In our current study on lung squamous cell carcinoma, however, tumor necrosis was not associated with patient’s outcomes although the 5-year OS of patients with tumor necrosis was slightly worse (56%) than those without necrosis (63%). Prominent fibrous stroma in tumors has been reported as a poor prognostic factor in squamous cell carcinomas of the lung and esophagus.29, 40 However, in our study of lung squamous cell carcinomas, severe intratumoral stromal fibrosis was not associated with OS. Conversely, severe intratumoral stromal fibrosis did independently correlate with a reduced risk of recurrence in patients with stage I disease. These differences in the prognostic value of tumor fibrosis between the studies might be due to variations in tumor locations (lung vs. esophagus), definitions for severe fibrosis, endpoints for prognostic analyses (survival vs. recurrence), or the ethnic groups of patients. In addition, HR for fibrosis in predicting disease recurrence is close to 1.00 (actual HR = 0.98) and does not have strong impact even if it is statistically significant (p = 0.030). Therefore, this finding warrants further investigation using more uniform cohorts and methods.
One of the criticisms of microscopic analysis is that there can be interobserver variability in evaluating histological and nuclear features. Therefore, standardized definitions are necessary for a pathologic factor especially if it has a prognostic impact. However, in the current WHO classification for lung squamous cell carcinoma, there are no standardized definitions (e.g. % cutoffs of each histologic pattern) for classifying tumors into histologic subtypes, such as basaloid pattern and clear cell feature.7 In lung squamous cell carcinoma, furthermore, tumor grade based on degree of squamous differentiation (keratinization) has no standardized cutoff for distribution (%) of differentiated area in each category (well, moderately and poorly differentiated). In this study, we recorded the distribution (%) of each histologic feature in 5% increments (such as keratinization, basaloid pattern, clear cell feature, tumor necrosis and fibrosis), and used small lymphocytes to evaluate nuclear diameter. Similarly, in the 2011 IASLC/ATS/ERS classification, histologic subtyping is recommended to be recorded in in 5% increments.2 According to this recommendation, prognostic impact of predominant histologic subtypes has been validated in independent large cohorts.3–6 Additionally, this method provided good reproducibility (kappa score: 0.77±0.07) in identifying predominant histologic subtypes based on an international interobserver study.54 As used in our previous study for lung adenocarcinoma,18 we measured nuclear diameter using small lymphocytes in this study for lung squamous cell carcinoma. This method was originally described by Nakazato et al., with prognostic significance of nuclear diameter and moderate to good reproducibility (kappa score: 0.58 ± 0.09) in measuring it by small lymphocytes in lung adenocarcinoma.19, 55
In conclusion, we have addressed the prognostic importance of histologic subtyping as well as single cell invasion, nuclear diameter and tumor budding. These histological features were analyzed using a large patient cohort who underwent resection of squamous cell carcinomas of the lung and with respect to OS and CIR. We discovered that the traditional 2004 WHO histologic subtyping and grading system that was based on the degree of keratinization were not prognostically significant factors. Since morphological assessment using H&E-stained slides is utilized in routine clinical practice for resected lung carcinomas, our findings can be validated in different datasets. Therefore, in order to establish a prognostic grading system for patients with lung squamous cell carcinomas based on the prognostic pathological factors we reported here, our results should be validated in a series of independent cohorts and consistent interobserver agreement should be assured by using uniform methods. If our findings can be confirmed, they may help clinical management of patients with lung squamous cell carcinomas and stratify patients for adjuvant therapy.
ACKNOWLEDGMENTS
This work was supported, in part, by William H. Goodwin and Alice Goodwin, the Commonwealth Foundation for Cancer Research and the Experimental Therapeutics Center; the National Cancer Institute (R21-CA164568 and R21-CA164585); and the U.S. Department of Defense (LC110202).
We thank Dr. Ronald A. Ghossein, our Head and Neck pathology expert at MSKCC, for his advice on the evaluation of histological features and for reviewing many of the cases in this study, Joe Dycoco for his help with the lung carcinoma database in the Thoracic Service of the Department of Surgery at MSKCC, and Alex Torres and K. Alexandra Macdonald for their editorial assistance.
Footnotes
DISCLOSURES: All authors affirm no actual or potential conflicts of interest, including any financial, personal, or other relationships with other people or organizations.
REFERENCES
- 1.Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol. 2007;2(8):706–714. doi: 10.1097/JTO.0b013e31812f3c1a. [DOI] [PubMed] [Google Scholar]
- 2.Travis WD, Brambilla E, Noguchi M, et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol. 2011;6(2):244–285. doi: 10.1097/JTO.0b013e318206a221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yoshizawa A, Motoi N, Riely GJ, et al. Impact of proposed IASLC/ATS/ERS classification of lung adenocarcinoma: prognostic subgroups and implications for further revision of staging based on analysis of 514 stage I cases. Mod Pathol. 2011;24(5):653–664. doi: 10.1038/modpathol.2010.232. [DOI] [PubMed] [Google Scholar]
- 4.Tsuta K, Kawago M, Inoue E, et al. The utility of the proposed IASLC/ATS/ERS lung adenocarcinoma subtypes for disease prognosis and correlation of driver gene alterations. Lung Cancer. 2013;81(3):371–376. doi: 10.1016/j.lungcan.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 5.Yoshizawa A, Sumiyoshi S, Sonobe M, et al. Validation of the IASLC/ATS/ERS lung adenocarcinoma classification for prognosis and association with EGFR and KRAS gene mutations: analysis of 440 Japanese patients. J Thorac Oncol. 2013;8(1):52–61. doi: 10.1097/JTO.0b013e3182769aa8. [DOI] [PubMed] [Google Scholar]
- 6.Warth A, Muley T, Meister M, et al. The novel histologic International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society classification system of lung adenocarcinoma is a stage-independent predictor of survival. J Clin Oncol. 2012;30(13):1438–1446. doi: 10.1200/JCO.2011.37.2185. [DOI] [PubMed] [Google Scholar]
- 7.Travis W, Brambilla E, Muller-Hermelink H, Harris C World Health Organization Classification of Tumours. Tumours of the Lung, Pleura, Thymus, and Heart. IARC Press: Lyon, France; 2004. Pathology and Genetics. [Google Scholar]
- 8.Maeshima AM, Maeshima A, Asamura H, Matsuno Y. Histologic prognostic factors for small-sized squamous cell carcinomas of the peripheral lung. Lung Cancer. 2006;52(1):53–58. doi: 10.1016/j.lungcan.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 9.Karamitopoulou E, Zlobec I, Kolzer V, et al. Proposal for a 10-high-power-fields scoring method for the assessment of tumor budding in colorectal cancer. Mod Pathol. 2013;26(2):295–301. doi: 10.1038/modpathol.2012.155. [DOI] [PubMed] [Google Scholar]
- 10.Mitrovic B, Schaeffer DF, Riddell RH, Kirsch R. Tumor budding in colorectal carcinoma: time to take notice. Mod Pathol. 2012;25(10):1315–1325. doi: 10.1038/modpathol.2012.94. [DOI] [PubMed] [Google Scholar]
- 11.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119(6):1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taira T, Ishii G, Nagai K, et al. Characterization of the immunophenotype of the tumor budding and its prognostic implications in squamous cell carcinoma of the lung. Lung Cancer. 2012;76(3):423–430. doi: 10.1016/j.lungcan.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 13.Yamaguchi Y, Ishii G, Kojima M, et al. Histopathologic features of the tumor budding in adenocarcinoma of the lung: tumor budding as an index to predict the potential aggressiveness. J Thorac Oncol. 2010;5(9):1361–1368. doi: 10.1097/JTO.0b013e3181eaf2f3. [DOI] [PubMed] [Google Scholar]
- 14.Brandwein-Gensler M, Smith RV, Wang B, et al. Validation of the histologic risk model in a new cohort of patients with head and neck squamous cell carcinoma. Am J Surg Pathol. 2010;34(5):676–688. doi: 10.1097/PAS.0b013e3181d95c37. [DOI] [PubMed] [Google Scholar]
- 15.Brandwein-Gensler M, Teixeira MS, Lewis CM, et al. Oral squamous cell carcinoma: histologic risk assessment, but not margin status, is strongly predictive of local disease-free and overall survival. Am J Surg Pathol. 2005;29(2):167–178. doi: 10.1097/01.pas.0000149687.90710.21. [DOI] [PubMed] [Google Scholar]
- 16.Meyer JS, Alvarez C, Milikowski C, et al. Breast carcinoma malignancy grading by Bloom-Richardson system vs proliferation index: reproducibility of grade and advantages of proliferation index. Mod Pathol. 2005;18:1067–1078. doi: 10.1038/modpathol.3800388. [DOI] [PubMed] [Google Scholar]
- 17.Thomas JS, Kerr GR, Jack WJ, et al. Histological grading of invasive breast carcinoma - a simplification of existing methods in a large conservation series with long-term follow-up. Histopathology. 2009;55(6):724–731. doi: 10.1111/j.1365-2559.2009.03429.x. [DOI] [PubMed] [Google Scholar]
- 18.Barletta JA, Yeap BY, Chirieac LR. Prognostic significance of grading in lung adenocarcinoma. Cancer. 2010;116(3):659–669. doi: 10.1002/cncr.24831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakazato Y, Minami Y, Kobayashi H, et al. Nuclear grading of primary pulmonary adenocarcinomas: correlation between nuclear size and prognosis. Cancer. 2010;116:2011–2019. doi: 10.1002/cncr.24948. [DOI] [PubMed] [Google Scholar]
- 20.Sica G, Yoshizawa A, Sima CS, et al. A grading system of lung adenocarcinomas based on histologic pattern is predictive of disease recurrence in stage I tumors. Am J Surg Pathol. 2010;34(8):1155–1162. doi: 10.1097/PAS.0b013e3181e4ee32. [DOI] [PubMed] [Google Scholar]
- 21.Kadota K, Suzuki K, Kachala SS, et al. A grading system combining architectural features and mitotic count predicts recurrence in stage I lung adenocarcinoma. Mod Pathol. 2012;25(8):1117–1127. doi: 10.1038/modpathol.2012.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Edge SB, Byrd DR, Compton CC, et al. American Joint Committee on Cancer Cancer Staging Manual. 7th. New York, NY: Springer; 2009. pp. 253–270. [Google Scholar]
- 23.Baaklini WA, Reinoso MA, Gorin AB, Sharafkaneh A, Manian P. Diagnostic yield of fiberoptic bronchoscopy in evaluating solitary pulmonary nodules. Chest. 2000;117(4):1049–1054. doi: 10.1378/chest.117.4.1049. [DOI] [PubMed] [Google Scholar]
- 24.Shentu Y, Zhang L, Gu H, et al. A new technique combining virtual simulation and methylene blue staining for the localization of small peripheral pulmonary lesions. BMC Cancer. 2014;14:79. doi: 10.1186/1471-2407-14-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barnes L, Eveson J, Reichart P, Sidransky D World Health Organization Classification of Tumours. Tumours of the Head and Neck. IARC Press: Lyon, France; 2005. Pathology and Genetics. [Google Scholar]
- 26.Travis W, Colby T, Corrin B, Shimosato Y, Brambilla E World Health Organization Classification of Tumours. Histological Subtyping of Lung and Pleural Tumours. Berlin: Springer; 1999. [Google Scholar]
- 27.Moro-Sibilot D, Lantuejoul S, Diab S, et al. Lung carcinomas with a basaloid pattern: a study of 90 cases focusing on their poor prognosis. Eur Respir J. 2008;31(4):854–859. doi: 10.1183/09031936.00058507. [DOI] [PubMed] [Google Scholar]
- 28.Suzuki K, Kadota K, Sima CS, et al. Chronic inflammation in tumor stroma is an independent predictor of prolonged survival in epithelioid malignant pleural mesothelioma patients. Cancer Immunol Immunother. 2011;60(12):1721–1728. doi: 10.1007/s00262-011-1073-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang K, Ma W, Wang J, et al. Tumor-stroma ratio is an independent predictor for survival in esophageal squamous cell carcinoma. J Thorac Oncol. 2012;7(9):1457–1461. doi: 10.1097/JTO.0b013e318260dfe8. [DOI] [PubMed] [Google Scholar]
- 30.Kadota K, Suzuki K, Colovos C, et al. A nuclear grading system is a strong predictor of survival in epitheloid diffuse malignant pleural mesothelioma. Mod Pathol. 2012;25(2):260–271. doi: 10.1038/modpathol.2011.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kadota K, Nitadori J, Rusch VW, et al. Reclassification of resected nonsmall cell lung carcinomas originally diagnosed as squamous cell carcinoma, after reevaluation using immunohistochemical analysis (P40, P63, TTF-1, and napsin A): Memorial Sloan-Kettering Cancer Center experience. J Thorac Oncol. 2013;8:S415–S416. [Google Scholar]
- 32.Mazumdar M, Glassman JR. Categorizing a prognostic variable: review of methods, code for easy implementation and applications to decision-making about cancer treatments. Stat Med. 2000;19(1):113–132. doi: 10.1002/(sici)1097-0258(20000115)19:1<113::aid-sim245>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 33.Chappell R. Competing Risk Analyses: How Are They Different and Why Should You Care? Clin Cancer Res. 2012;18(8):2127–2129. doi: 10.1158/1078-0432.CCR-12-0455. [DOI] [PubMed] [Google Scholar]
- 34.Dignam JJ, Zhang Q, Kocherginsky M. The Use and Interpretation of Competing Risks Regression Models. Clin Cancer Res. 2012;18(8):2301–2308. doi: 10.1158/1078-0432.CCR-11-2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gray RJ. A Class of K-Sample Tests for Comparing the Cumulative Incidence of a Competing Risk. Ann Stat. 1988;16(3):1141–1154. [Google Scholar]
- 36.Kawakami T, Nabeshima K, Hamasaki M, et al. Small cluster invasion: a possible link between micropapillary pattern and lymph node metastasis in pT1 lung adenocarcinomas. Virchows Arch. 2009;454(1):61–70. doi: 10.1007/s00428-008-0695-5. [DOI] [PubMed] [Google Scholar]
- 37.Moro D, Brichon P-Y, Brambilla E, et al. Basaloid bronchial carcinoma. A histologic group with a poor prognosis. Cancer. 1994;73(11):2734–2739. doi: 10.1002/1097-0142(19940601)73:11<2734::aid-cncr2820731114>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 38.Kim DJ, Kim KD, Shin DH, Ro JY, Chung KY. Basaloid carcinoma of the lung: a really dismal histologic variant? Ann Thorac Surg. 2003;76(6):1833–1837. doi: 10.1016/s0003-4975(03)01296-7. [DOI] [PubMed] [Google Scholar]
- 39.Wang L, Wang L, Kwauk S, et al. Analysis on the clinical features of 22 basaloid squamous cell carcinoma of the lung. J Cardiothorac Surg. 2011;6(1):10. doi: 10.1186/1749-8090-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takahashi Y, Ishii G, Taira T, et al. Fibrous Stroma Is Associated with Poorer Prognosis in Lung Squamous Cell Carcinoma Patients. J Thorac Oncol. 2011;6(9):1460–1467. doi: 10.1097/JTO.0b013e318229189d. [DOI] [PubMed] [Google Scholar]
- 41.Travis WD, Brambilla E, Noguchi M, et al. Diagnosis of lung cancer in small biopsies and cytology: implications of the 2011 International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society classification. Arch Pathol Lab Med. 2013;137(5):668–684. doi: 10.5858/arpa.2012-0263-RA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dulmet-Brender E, Jaubert F, Huchon G. Exophytic endobronchial epidermoid carcinoma. Cancer. 1986;57(7):1358–1364. doi: 10.1002/1097-0142(19860401)57:7<1358::aid-cncr2820570719>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 43.Travis WD. Pathology of lung cancer. Clin Chest Med. 2011;32(4):669–692. doi: 10.1016/j.ccm.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 44.Rioux-Leclercq N, Karakiewicz PI, Trinh QD, et al. Prognostic ability of simplified nuclear grading of renal cell carcinoma. Cancer. 2007;109:868–874. doi: 10.1002/cncr.22463. [DOI] [PubMed] [Google Scholar]
- 45.Epstein JI. The new World Health Organization/International Society of Urological Pathology (WHO/ISUP) classification for TA, T1 bladder tumors: is it an improvement? Crit Rev Oncol Hematol. 2003;47:83–89. doi: 10.1016/s1040-8428(03)00073-8. [DOI] [PubMed] [Google Scholar]
- 46.Lipponen PK, Eskelinen MJ, Jauhiainen K, et al. Independent clinical, histological and quantitative prognostic factors in transitional-cell bladder tumours, with special reference to mitotic frequency. Int J Cancer. 1992;51:396–403. doi: 10.1002/ijc.2910510311. [DOI] [PubMed] [Google Scholar]
- 47.Asamura H, Ando M, Matsuno Y, Shimosato Y. Histopathologic prognostic factors in resected adenocarcinomas: is nuclear DNA content prognostic? Chest. 1999;115(4):1018–1024. doi: 10.1378/chest.115.4.1018. [DOI] [PubMed] [Google Scholar]
- 48.Kurokawa T, Matsuno Y, Noguchi M, Mizuno S, Shimosato Y. Surgically curable"early" adenocarcinoma in the periphery of the lung. Am J Surg Pathol. 1994;18(5):431–438. doi: 10.1097/00000478-199405000-00001. [DOI] [PubMed] [Google Scholar]
- 49.Komaki R, Fujii T, Perkins P, et al. Apoptosis and mitosis as prognostic factors in pathologically staged N1 nonsmall cell lung cancer. Int J Radiat Oncol Biol Phys. 1996;36(3):601–605. doi: 10.1016/s0360-3016(96)00351-3. [DOI] [PubMed] [Google Scholar]
- 50.Poleri C, Morero JL, Nieva B, et al. Risk of recurrence in patients with surgically resected stage I non-small cell lung carcinoma: histopathologic and immunohistochemical analysis. Chest. 2003;123:1858–1867. doi: 10.1378/chest.123.6.1858. [DOI] [PubMed] [Google Scholar]
- 51.Demarchi LM, Reis MM, Palomino SA, et al. Prognostic values of stromal proportion and PCNA, Ki-67, and p53 proteins in patients with resected adenocarcinoma of the lung. Mod Pathol. 2000;13:511–520. doi: 10.1038/modpathol.3880089. [DOI] [PubMed] [Google Scholar]
- 52.Delahunt B, Cheville JC, Martignoni G, et al. The International Society of Urological Pathology (ISUP) grading system for renal cell carcinoma and other prognostic parameters. Am J Surg Pathol. 2013;37(10):1490–1504. doi: 10.1097/PAS.0b013e318299f0fb. [DOI] [PubMed] [Google Scholar]
- 53.Delahunt B, McKenney JK, Lohse CM, et al. A novel grading system for clear cell renal cell carcinoma incorporating tumor necrosis. Am J Surg Pathol. 2013;37(3):311–322. doi: 10.1097/PAS.0b013e318270f71c. [DOI] [PubMed] [Google Scholar]
- 54.Thunnissen E, Beasley MB, Borczuk AC, et al. Reproducibility of histopathological subtypes and invasion in pulmonary adenocarcinoma. An international interobserver study. Mod Pathol. 2012;25(12):1574–1583. doi: 10.1038/modpathol.2012.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nakazato Y, Maeshima AM, Ishikawa Y, et al. Interobserver agreement in the nuclear grading of primary pulmonary adenocarcinoma. J Thorac Oncol. 2013;8(6):736–743. doi: 10.1097/JTO.0b013e318288dbd8. [DOI] [PubMed] [Google Scholar]