Abstract
Background
Recent evidence suggests that postpartum psychiatric episodes may share similar etiological mechanisms with immune-related disorders. Pre-eclampsia is one of the most prevalent immune-related disorders of pregnancy. Multiple clinical features are shared between pre-eclampsia and postpartum psychiatric disorders, most prominently a strong link to first pregnancies. Therefore, we aimed to study if pre-eclampsia is a risk factor for first-onset postpartum psychiatric episodes.
Method
We conducted a cohort study using the Danish population registry, with a total of 400 717 primiparous women with a singleton delivery between 1995 and 2011. First-lifetime childbirth was the main exposure variable and the outcome of interest was first-onset postpartum psychiatric episodes. The main outcome measures were monthly incidence rate ratios (IRRs), with the period 11–12 months after birth as the reference category. Adjustments were made for age, calendar period, reproductive history, and perinatal maternal health including somatic and obstetric co-morbidity.
Results
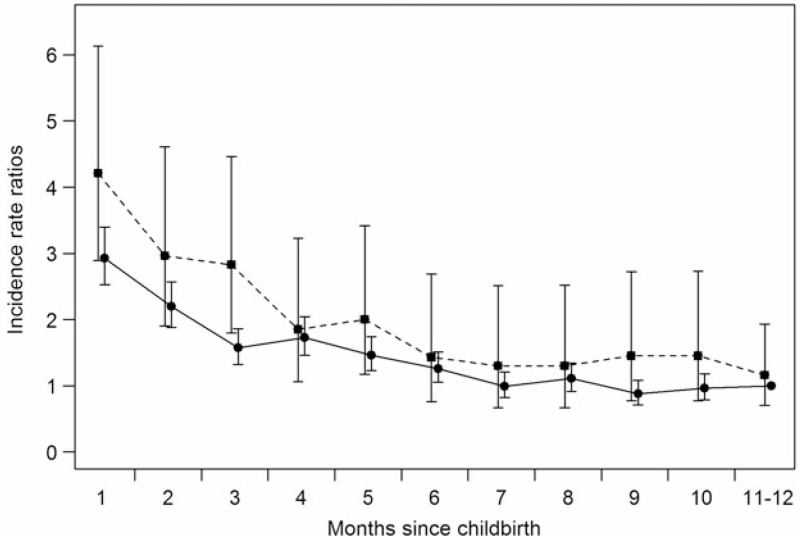
Primiparous women were at particularly high risk of first-onset psychiatric episodes during the first month postpartum [IRR 2.93, 95% confidence interval (CI) 2.53–3.40] and pre-eclampsia added to that risk (IRR 4.21, 95% CI 2.89–6.13). Having both pre-eclampsia and a somatic co-morbidity resulted in the highest risk of psychiatric episodes during the 3-month period after childbirth (IRR 4.81, 95% CI 2.72–8.50).
Conclusions
We confirmed an association between pre-eclampsia and postpartum psychiatric episodes. The possible explanations for this association, which are not mutually exclusive, include the psychological impact of a serious medical condition such as pre-eclampsia and the neurobiological impact of pre-eclampsia-related vascular pathology and inflammation.
Keywords: Epidemiology, immunology, postpartum depression, postpartum psychosis, pre-eclampsia
Introduction
Childbirth has the highest relative risk of any vulnerability factor for the onset of serious psychiatric episodes such as depression, mania and psychosis (Jones et al. 2014). Furthermore, childbirth has also been associated with the substantial risk of additional psychiatric morbidity including personality, anxiety, adjustment and post-traumatic stress disorders (Howard et al. 2014). Several studies have demonstrated an increased risk during the first months after delivery for the onset of these episodes, compared with the risk of occurrence outside the postpartum period (Kendell et al. 1987; Munk-Olsen et al. 2006, 2009). The acute onset of postpartum psychiatric episodes is a serious event for which both the mother and the child are at risk of infanticide and/or maternal suicide, as well as long-term effects on social, emotional and cognitive outcomes (Grace et al. 2003; Stein et al. 2014). Therefore, a deeper understanding of the underlying etiologies would greatly facilitate both the prevention and treatment of postpartum psychiatric episodes.
The pathogenesis of postpartum psychiatric disorders is considered complex and heterogeneous. Childbirth is a major life event with profound and often stressful changes in every aspect of daily life, which could make women vulnerable for psychiatric episodes. In addition, several neurobiological pathways are highly sensitized in the immediate postpartum period and could function as potential triggers (Bloch et al. 2003). Major alterations occur in steroid and stress hormones following delivery. However, evidence of a causual influence of steroid hormones on the onset of postpartum psychiatric episodes remains limited (Klier et al. 2007) and findings in the stress hormone axis in postpartum mood disorders have been inconclusive, as both increased and decreased activity have been reported (Harris et al. 1996; Parry et al. 2003; Groer & Morgan, 2007). Alternatively, the risk of postpartum psychiatric episodes might be more sensitive to the rapid change of hormone levels, rather than simply to the absolute levels of the hormones themselves (Bloch et al. 2003). In addition, it is likely that genetic factors influence the maternal response to these peripartum triggers. Accordingly, there is evidence for the familiarity of postpartum episodes but specific genes have not yet been identified (Jones et al. 2007; Byrne et al. 2014).
Despite extensive efforts, a definitive pathophysiology for postpartum psychiatric disorders has remained elusive. Recently, based on a convergence of both immunological and epidemiological studies, there has been increasing support for the hypothesis that first-onset postpartum disorders may share similar etiological mechanisms with immune-related disorders (Gleicher, 2007; Bergink et al. 2013; Osborne & Monk, 2013). In our recent work, we showed that patients with severe first-onset postpartum episodes had abnormalities in monocyte activation and T cell function during the acute phase (Bergink et al. 2013) and a small subgroup of patients suffered from undiagnosed autoimmune encephalitis (Bergink et al. 2015a). In support of this hypothesis is the consistent finding that medical conditions with a postpartum flare pattern – including postpartum thyroiditis, multiple sclerosis and rheumatoid arthritis – have an etiology originating from immune system dysfunction (Buyon, 1998; Confavreux et al. 1998; Gleicher, 2007; Haupl et al. 2008; Weetman, 2010).
Pre-eclampsia is a severe immune-related disorder of pregnancy. Interestingly, multiple clinical features are shared between pre-eclampsia and first-onset postpartum psychiatric disorders (Di Florio et al. 2014). The initial onset of both disorders is strongly associated with first pregnancy, compared with subsequent pregnancies. Furthermore, a history of a prior episode is the strongest known risk factor for each disorder in subsequent pregnancies (Steegers et al. 2010; Robillard et al. 2011; Munk-Olsen et al. 2014). Finally, several pre-eclampsia studies have demonstrated increasing risk with longer intervals between subsequent pregnancies, a relationship that has recently been confirmed for first-onset postpartum psychiatric episodes (Munk-Olsen et al. 2014). Together, the shared pattern of risk between pre-eclampsia and postpartum psychiatric disorders may be evidence of a more fundamental relationship in their underlying pathophysiology (Steegers et al. 2010; Robillard et al. 2011; Di Florio et al. 2014; Munk-Olsen et al. 2014).
To date, the association between pre-eclampsia and severe postpartum psychiatric disorders has been examined only within disease-specific clinical cohorts (Brockington, 1996; Bergink et al. 2011). There is evidence linking pre-eclampsia and general psychiatric symptomatology such as anxiety and depressive symptoms (Delahaije et al. 2013), but conclusions were limited by difficulty in controlling for pre-pregnancy mental health. Therefore, the present study was designed to investigate the risk of a first-onset psychiatric episode after pre-eclampsia in primiparous women without psychiatric history. This large population-based Danish cohort study is the first epidemiological study in a general population on this topic.
Method
Study design and study population
We conducted an epidemiological population-based cohort study to investigate the association between pre-eclampsia (exposure) and the risk of a first-onset postpartum psychiatric episode (outcome). We identified all singleton births to primiparous women between 1 January 1995 and 31 December 2011. Each woman was followed within the cohort from the date of delivery until a postpartum psychiatric episode, migration, death or 31 December 2012, whichever occurred first. As an additional restriction for uniformity, we considered only women born in Denmark from 1 January 1960 to 31 December 1995. In total, we identified 400 717 women who contributed 372 067 person-years, of which 2723 mothers were recorded as having received either in-patient or out-patient treatment at a psychiatric facility within the initial 360 days postpartum.
Data resources and study variables
Information from three nationwide registers was linked for the present study. Registers were combined based on the unique Danish identification number assigned to each person at birth. We included information from the Civil Registration System on date of birth, parents’ personal identification numbers, and daily updated information on vital status and migration (Pedersen, 2011).
Our main exposure variable was pre-eclampsia during primiparous pregnancies, identified using The Danish National Patient/Hospital Register (Andersen et al. 1999; Lynge et al. 2011). This register contains information on all in-patient and out-patient treatment administered at medical hospitals in Denmark since 1995. Pre-eclampsia was defined by International Classification of Diseases, tenth version (ICD-10) code O14 (including moderate and severe pre-eclampsia). Importantly, pregnancy-induced hypertension (ICD-10 code O13) and eclampsia (ICD-10 code O15) were not considered as exposure variables for the present study.
The outcome of interest was first-onset psychiatric episodes during the postpartum period. These data were derived from The Danish Psychiatric Central Research Register (Mors et al. 2011), which holds information on all contacts with a psychiatric treatment facility in Denmark as in-patient and out-patient treatment during the entire study period. The diagnostic classification system used in Denmark is the ICD-10 (World Health Organization, 1994), from which we specifically used all diagnoses of mental and behavioral disorders in chapter V (F00–F99). A postpartum psychiatric episode was defined as any recorded contact with a psychiatric in-patient or out-patient treatment facility within the first 360 days postpartum, divided into monthly intervals (0–12 months). In addition, we conducted subanalyses for a more narrowly defined postpartum period within the first 90 days following delivery (0–3 months) compared with 4–12 months postpartum, as the first 3 months following childbirth have been associated with the highest lifetime risk of first-onset psychiatric episodes (Munk-Olsen et al. 2006). More detailed analyses were conducted on three diagnostic postpartum groups: unipolar depression (ICD-10 codes: F32, F33, F34, F38, F39), adjustment disorders (ICD-10 code: F43), and remaining diagnoses (remaining chapter V ICD-10 codes). Note that the subdivision into unipolar depression and adjustment disorders was made to provide additional clarification, since these episodes are the most frequently recorded diagnoses during the postpartum period. Further diagnostic subdivisions (e.g. bipolar disorder or a distinct diagnosis of postpartum psychosis (Bergink et al. 2015b) were not possible due to the reduction in statistical power that would result from the limited number of cases in many of the diagnostic subtypes. The analyses were restricted to include only first-onset episodes of mental disorders and not chronic disorders. Therefore, women with a registered diagnosis of any mental disorder occurring prior to the date of delivery were excluded from this study.
A range of potential confounders were considered as covariates: gestational diabetes (ICD-10 codes O24.4 and O24.9) and birth complications (an overall measure of number of recorded birth-related complications divided into three groups: 0–8 recorded complications, 9–14 recorded complications and 15+ complications). We furthermore adjusted for reproductive histories, defined as records of induced abortions (ICD-10 codes O04, O05, O06), spontaneous abortions (ICD-10 codes O021, O03) and stillbirths (ICD-10 codes Z371, Z374, Z377, P95). Additionally, we adjusted the analyses for family history of psychiatric disorders (grandparents of index child having records of any treatment at a psychiatric facility) and somatic co-morbidities using the Charlson co-morbidity index (Charlson et al. 1987). The Charlson co-morbidity index is an indicator of medical disease burden and consists of 19 severe chronic diseases. Each disease is assigned a weight from one to six corresponding to the severity of the disease, and the score is subsequently calculated as the sum of all weights. All of the mentioned covariates were handled as time-dependent variables.
Statistical analysis
For the present study, analyses included calculation of incidence rates of psychiatric hospital contacts per 1000 person-years under risk. Furthermore, we conducted a survival analysis (Poisson regression) and calculated incidence rate ratios (IRRs). We calculated the IRRs of psychiatric contacts at monthly intervals for the first year postpartum, while the incidence rate 11–12 months after the birth was defined as the reference category. In the interaction analysis of pre-eclampsia with the Charlson co-morbidity index and gestational diabetes, we examined the risks of overall psychiatric episodes from 0–3 months (0–90 days) and 4–12 months (91–360 days) postpartum, due to the limited number of cases. We adjusted the IRRs for age, calendar period, reproductive history, gestational diabetes, maternal well-being around childbirth (number of diagnoses related to the specific childbirth), somatic co-morbidity (Charlson co-morbidity index) and family history of psychiatric disorders.
Results
A total of 400 717 women were included in our cohort study, of whom 17 149 had a diagnosis of pre-eclampsia (4.28%). A total of 2723 women received psychiatric treatment for any type of psychiatric diagnosis within the first 360 days postpartum, including 435 women via in-patient treatment and 2297 receiving treatment at out-patient mental health services. Out of the 2723 women with psychiatric episodes during the first 360 days postpartum, 162 had pre-eclampsia during pregnancy (5.93%). Among the 39.8% (n = 1087) of women who received their psychiatric diagnosis during the first 3 months postpartum, 71 had pre-eclampsia during pregnancy (6.53%).
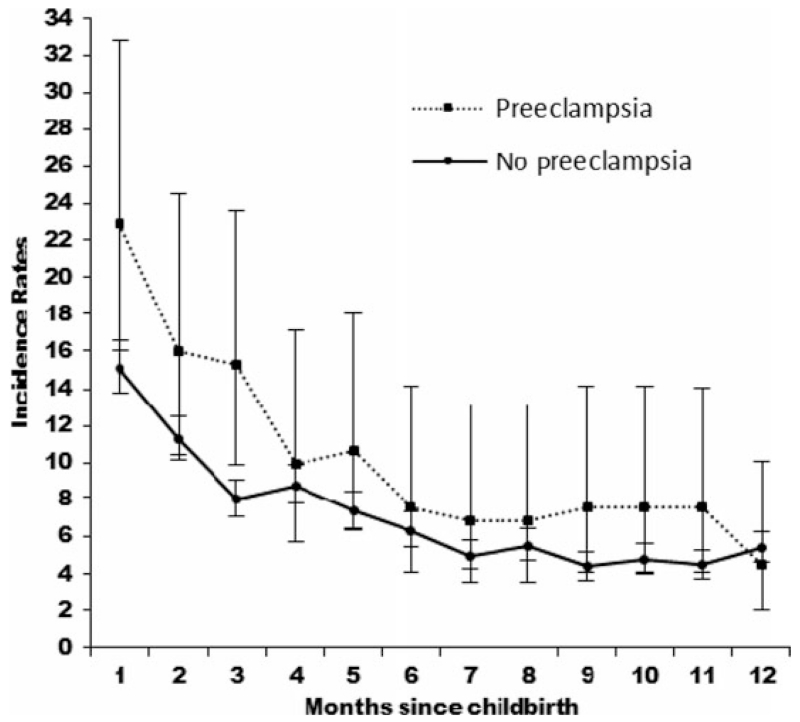
Incidence rates of psychiatric contacts during the postpartum period are shown in Fig. 1, with the highest incidence of first-onset psychiatric episodes within the first month postpartum observed in women with or without pre-eclampsia. The corresponding IRRs are shown in Fig. 2. The first month postpartum was associated with the highest risk of psychiatric episodes for mothers with pre-eclampsia [IRR 4.21, 95% confidence interval (CI) 2.89–6.13] and without pre-eclampsia (IRR 2.93, 95% CI 2.53–3.40), compared with the reference category of women who gave birth 11–12 months prior with no pre-eclampsia (Fig. 2). The overall effect of pre-eclampsia on the risk of psychiatric episodes from 0 to 12 months after birth was IRR 1.43 (95% CI 1.22–1.68; results not shown).
Fig. 1.
Incidence rates per 1000 person-years for psychiatric contacts in primiparous mothers with and without pre-eclampsia 0–12 months postpartum. Values are incidence rates, with 95% confidence intervals represented by vertical bars.
Fig. 2.
Incidence rate ratios for psychiatric contacts in primiparous mothers with (--■--) and without (–●–) pre-eclampsia 0–12 months postpartum. Adjusted for age, calendar period, birth complications (number of diagnoses related to childbirth), reproductive history, gestational diabetes, somatic co-morbidities and family history of psychiatric disorders. Values are incidence rate ratios, with 95% confidence intervals represented by vertical bars.
For subanalyses, we defined three diagnostic groups [unipolar depression (ICD-10 codes: F32, F33, F34, F38, F39), adjustment disorder (F43) and other psychiatric diagnoses (any other chapter V code)] to identify possible changes in the risk of occurrence of an episode (Table 1). For all three groups, the highest risks were observed 0–3 months postpartum in women with pre-eclampsia [unipolar depression (IRR 2.85, 95% CI 1.84–4.42), adjustment disorder (IRR 4.04, 95% CI 2.85–5.72), other disorders (IRR 1.90, 95% CI 1.17–3.07)]. As CIs were overlapping for the above-mentioned results, there was no statistical difference in risk of the studied diagnostic subgroups. For this reason the results presented below focus on the single overall diagnostic group containing all psychiatric disorders as the outcome variable.
Table 1. Incidence rate ratios of first-onset depression, adjustment and remaining diagnoses 0–3 months and 4–12 months postpartum in primiparous mothersa.
| Pre-eclampsia |
No pre-eclampsia |
|||
|---|---|---|---|---|
| Postpartum diagnostic groups |
0–3 months postpartum |
4–12 months postpartum |
0–3 months postpartum |
4–12 months postpartum |
| Unipolar depression | 2.85 (1.84–4.42) | 1.25 (0.84–1.84) | 2.11 (1.83–2.43) | 1 (reference category) |
| n | 21 | 27 | 325 | 456 |
| Adjustment disorder | 4.04 (2.85–5.72) | 1.54 (1.10–2.14) | 2.29 (2.02–2.61) | 1 (reference category) |
| n | 34 | 38 | 420 | 537 |
| Other disorders | 1.90 (1.17–3.07) | 0.99 (0.67–1.47) | 1.49 (1.29–1.72) | 1 (reference category) |
| n | 17 | 26 | 289 | 570 |
Data are given as incidence rate ratio (95% confidence interval).
Adjusted for age, calendar period, birth complications (number of diagnoses related to childbirth), reproductive history, somatic co-morbidities (Charlson co-morbidity index) and family history of psychiatric disorders. If a woman had records of more than one of the diagnoses of interest (dual diagnoses), she contributed with information in more than one category.
Stratified analyses were performed for somatic co-morbidity and gestational diabetes to investigate potentially moderating influences in the relationship between postpartum psychiatric episodes and pre-eclampsia. Having both pre-eclampsia and a somatic co-morbidity defined by the Charlson co-morbidity index yielded the highest risk of first-onset psychiatric episodes during the interval of 0–3 months postpartum, compared with women without somatic co-morbidities in the reference category (IRR 4.81, 95% CI 2.72–8.50) (Table 2). In particular, women with both gestational diabetes and pre-eclampsia had a significantly elevated risk of psychiatric episodes 0–3 months postpartum compared with mothers without these two disorders (IRR 3.86, 95% CI 1.24–12.00) (Table 3).
Table 2. Somatic co-morbidity, pre-eclampsia and incidence rate ratios of first-onset psychiatric episodes 0–3 months and 4–12 months postpartum in primiparous mothersa.
| Pre-eclampsia |
No pre-eclampsia |
|||
|---|---|---|---|---|
| Charlson co-morbidity index |
0–3 months postpartum |
4–12 months postpartum |
0–3 months postpartum |
4–12 months postpartum |
| Yes (1+ score) | 4.81 (2.72–8.50) | 1.22 (0.63–2.35) | 2.18 (1.73–2.76) | 1.43 (1.20–1.69) |
| n | 12 | 9 | 75 | 143 |
| No (0 score) | 2.78 (2.14–3.61) | 1.32 (1.06–1.65) | 1.97 (1.81–2.14) | 1 (reference category) |
| n | 59 | 82 | 941 | 1402 |
Data are given as incidence rate ratio (95% confidence interval).
Adjusted for age, calendar period, birth complications (number of diagnoses related to childbirth), reproductive history, gestational diabetes and family history of psychiatric disorders.
Table 3. Gestational diabetes, pre-eclampsia and incidence rate ratios of first-onset psychiatric episodes 0–3 and 4–12 months postpartum in primiparous mothersa.
| Pre-eclampsia |
No pre-eclampsia |
|||
|---|---|---|---|---|
| Gestational diabetes | 0–3 months postpartum | 4–12 months postpartum | 0–3 months postpartum | 4–12 months postpartum |
| Yes | 3.86 (1.24–12.00) | 1.32 (0.43–4.12) | 2.35 (1.49–3.70) | 1.60 (1.15–2.23) |
| n | 3 | 3 | 19 | 37 |
| No | 2.89 (2.27–3.69) | 1.28 (1.03–1.59) | 1.94 (1.79–2.10) | 1 (reference category) |
| n | 68 | 88 | 997 | 1508 |
Data are given as incidence rate ratio (95% confidence interval).
Adjusted for age, calendar period, birth complications (number of diagnoses related to childbirth), reproductive history, somatic co-morbidities (Charlson co-morbidity index) and family history of psychiatric disorders.
Discussion
This population-based cohort study demonstrates that all primiparous women are at risk of first-onset psychiatric episodes during the early postpartum period, but that pre-eclampsia adds considerably to that risk. This is observed broadly across psychiatric diagnoses in general, as well as independent for each of the following diagnostic groups: unipolar depression, adjustment disorders, and other psychiatric disorders. Further, our study confirms the well-described association between somatic co-morbidities and psychiatric episodes, as an increasing burden of somatic diseases or pregnancy complications such as gestational diabetes significantly increases the risk for postpartum psychiatric episodes, especially in women with pre-eclampsia. Explanations for the association between pre-eclampsia and first-onset psychiatric episodes could be sought in both psychological and neurobiological models, which are not mutually exclusive.
Effects of maternal and neonatal adverse events
Pre-eclampsia is associated with perinatal complications such as preterm delivery, poor neonatal outcome and death. It is plausible that these adverse events lead to feelings of stress and grief, making women vulnerable for psychiatric episodes after delivery. Indeed, unfavorable outcomes after pre-eclampsia have been described as a risk factor for postpartum depression (Hoedjes et al. 2011). In the present studies, cases of eclampsia or neonatal death were excluded and therefore by adjusting the results for gestational age and birth complications, we aimed to overcome this potential bias. Nevertheless, the psychological impact of a serious medical condition such as pre-eclampsia might have contributed to the vulnerability for psychiatric episodes postpartum.
Maternal–fetal incompatibility
There are three clinical characteristics of serious postpartum psychiatric episodes that show a remarkable overlap with pre-eclampsia: primiparity, a high recurrence risk after a subsequent pregnancy, and increased risk with prolonged birth interval. Accordingly, these associations raise the possibility of a shared biological mechanism. Pre-eclampsia is considered to be a disease of immunological maternal–fetal incompatibility. An immunological maternal–fetal conflict is thought to result in pathophysiological changes in the placenta early in pregnancy, preceding the clinical symptoms of pre-eclampsia (Sargent et al. 2006; Steegers et al. 2010; Roberts & Bell, 2013). During a first pregnancy, paternally inherited antigens expressed by the fetus are viewed by the maternal immune system as foreign. In contrast, during subsequent pregnancies the maternal immune system has already had the opportunity to develop tolerance to paternally inherited antigens expressed by the fetus. This pathophysiological model elegantly accounts for the well-replicated findings of decreased risk of pre-eclampsia during subsequent pregnancies, compared with a woman’s first pregnancy in which the risk is highest (Sargent et al. 2006; Steegers et al. 2010). Consistent with this epidemiological finding, increasing intervals and/or a new partner have been shown to reduce maternal tolerance and thereby increase pre-eclampsia risk (Tandberg et al. 2015).
In theory, maternal–fetal incompatibility could potentially explain many of the similarly enigmatic clinical characteristics of acute postpartum episodes, including the substantially increased risk of first pregnancies and an increasing risk with longer intervals between subsequent pregnancies. However, one important difference between postpartum disorders and pre-eclampsia is the timing of onset of clinical symptoms. Pre-eclampsia is a disease of pregnancy, in which delivery resolves the pathophysiological process, whereas the highest known risk factor for the onset of psychiatric disorders falls within the early postpartum interval following delivery. Accordingly, maternal immune-related pathophysiological changes early in pregnancy could only lead to acute postpartum episodes several months later if there is a second trigger around the time of delivery.
Vascular damage
When considering pathophysiological changes later in pregnancy and closer to the postpartum onset of psychiatric episodes, the second pathophysiological stage of pre-eclampsia might be of interest. This stage is characterized by excessive endothelial activation and a generalized hyperinflammatory state compared with normal pregnancy (Redman & Sargent, 2001; Sargent et al. 2006; Steegers et al. 2010). Widespread endothelial dysfunction establishes a high vulnerability to disruption of the blood–brain barrier (Amburgey et al. 2010). An increased permeability of the blood–brain barrier, in the setting of a hyperinflammatory state, is known to broadly influence brain function, including neurotransmitter metabolism, neuroendocrine function and synaptic plasticity (Capuron & Miller, 2011; Bergink et al. 2014). Together, vascular inflammation as observed in pre-eclampsia could be seen as a candidate neurobiological vulnerability mechanism for psychiatric disorders. The systemic consequences of this vascular inflammation, such as renal and liver dysfunction (including insulin resistance), could further influence brain function. Under this model, psychiatric episodes could be seen as the direct consequence of the later-stage pathophysiological changes of pre-eclampsia.
Methodological considerations
The present study is based on data from Danish population registers covering the entire nation, thereby providing information on a large, unselected group of women during pregnancy. However, there are certain limitations to the data, including the definition of our main outcome of interest: any type of psychiatric disorder treated at an in-patient or out-patient psychiatric treatment facility. The present study included primiparous pregnancies and first-onset psychiatric episodes as exposure and outcome variables. It is likely that women in our cohort are less likely to continue reproducing (Laursen & Munk-Olsen, 2010), and hence generalizability to multiparous women can be questioned. As this information is obtained from women who actively sought care, the rates of psychiatric episodes presented in the present study may be an underestimation of the true population incidence. Furthermore, our results do not provide information on less severe mental health problems treated through primary care providers. In an effort to avoid bias by clinical heterogeneity, the present study focused exclusively on primiparous mothers with no previous evidence of psychiatric history. Importantly, we acknowledge that this decision also potentially limits the generalizability of our findings. Residual confounding factors cannot be excluded since unmeasured patient characteristics may have influenced our results, including the lack of information regarding diseases of milder severity not captured by the Charlson co-morbidity index, lifestyle factors such as obesity and smoking, as well as aspects regarding infant health.
Conclusion
This population-based cohort study demonstrates that primiparous women are at high risk of first-onset psychiatric episodes particularly during the early postpartum period. The present study confirms a link between pre-eclampsia and postpartum psychiatric episodes, mainly representing unipolar depression and adjustment disorders. Additionally, our findings suggest a particularly elevated risk of postpartum episodes in women with somatic and obstetric co-morbidities, compared with women without these co-morbidities. Explanatory models linking pre-eclampsia and postpartum episodes could include exogenous and/or endogenous factors:
-
(1)
The psychological impact of a serious medical condition such as pre-eclampsia might have contributed to the vulnerability for psychiatric episodes.
-
(2)
An underlying immune-mediated pathophysiological mechanism could lead to both pre-eclampsia and postpartum psychiatric episodes. In particular, vascular pathology and hyperinflammation are important candidate vulnerability triggers for postpartum psychiatric episodes.
The clinical relevance of our findings is most importantly found among pregnant primiparous patients with somatic co-morbidities including pre-eclampsia, in whom clinicians should be aware of the increased risk of first-onset postpartum psychiatric episodes.
Acknowledgements
This study was funded by the Lundbeck Foundation Initiative for Integrative Psychiatric Research, iPSYCH, Denmark. V.B. is supported by an Erasmus University fellowship and has received funding from the Netherlands Organization for Scientific Research (NWO, Rubicon incentive). S.M.-B. and T.M.-O. have received funding from the National Institute of Mental Health (R01MH104468).
References
- Amburgey OA, Chapman AC, May V, Bernstein IM, Cipolla MJ. Plasma from preeclamptic women increases blood–brain barrier permeability: role of vascular endothelial growth factor signaling. Hypertension. 2010;56:1003–1008. doi: 10.1161/HYPERTENSIONAHA.110.158931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen TF, Madsen M, Jorgensen J, Mellemkjoer L, Olsen JH. The Danish National Hospital Register. A valuable source of data for modern health sciences. Danish Medical Bulletin. 1999;46:263–268. [PubMed] [Google Scholar]
- Bergink V, Armangue T, Titulaer MJ, Markx S, Dalmau J, Kushner SA. Autoimmune encephalitis in postpartum psychosis. American Journal of Psychiatry. 2015a doi: 10.1176/appi.ajp.2015.14101332. Published online 17 July 2015. doi:10.1176/appi.ajp.2015.14101332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergink V, Boyce P, Munk-Olsen T. Postpartum psychosis: a valuable misnomer. Australian and New Zealand Journal of Psychiatry. 2015b;49:102–103. doi: 10.1177/0004867414564698. [DOI] [PubMed] [Google Scholar]
- Bergink V, Burgerhout KM, Weigelt K, Pop VJ, de Wit H, Drexhage RC, Kushner SA, Drexhage HA. Immune system dysregulation in first-onset postpartum psychosis. Biological Psychiatry. 2013;73:1000–1007. doi: 10.1016/j.biopsych.2012.11.006. [DOI] [PubMed] [Google Scholar]
- Bergink V, Gibney SM, Drexhage HA. Autoimmunity, inflammation and psychosis: a search for peripheral markers. Biological Psychiatry. 2014;75:324–331. doi: 10.1016/j.biopsych.2013.09.037. [DOI] [PubMed] [Google Scholar]
- Bergink V, Lambregtse-van den Berg MP, Koorengevel KM, Kupka R, Kushner SA. First-onset psychosis occurring in the postpartum period: a prospective cohort study. Journal of Clinical Psychiatry. 2011;72:1531–1537. doi: 10.4088/JCP.10m06648. [DOI] [PubMed] [Google Scholar]
- Bloch M, Daly RC, Rubinow DR. Endocrine factors in the etiology of postpartum depression. Comprehensive Psychiatry. 2003;44:234–246. doi: 10.1016/S0010-440X(03)00034-8. [DOI] [PubMed] [Google Scholar]
- Brockington I. Motherhood and Mental Health. Oxford University Press; Oxford: 1996. [Google Scholar]
- Buyon JP. The effects of pregnancy on autoimmune diseases. Journal of Leukocyte Biology. 1998;63:281–287. doi: 10.1002/jlb.63.3.281. [DOI] [PubMed] [Google Scholar]
- Byrne EM, Carrillo-Roa T, Penninx BW, Sallis HM, Viktorin A, Chapman B, Henders AK, Psychiatric Genomic Consortium Major Depressive Disorder Working Group. Pergadia ML, Heath AC, Madden PA, Sullivan PF, Boschloo L, van Grootheest G, McMahon G, Lawlor DA, Landen M, Lichtenstein P, Magnusson PK, Evans DM, Montgomery GW, Boomsma DI, Martin NG, Meltzer-Brody S, Wray NR. Applying polygenic risk scores to postpartum depression. Archives of Women’s Mental Health. 2014;17:519–528. doi: 10.1007/s00737-014-0428-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuron L, Miller AH. Immune system to brain signaling: neuropsychopharmacological implications. Pharmacology and Therapeutics. 2011;130:226–238. doi: 10.1016/j.pharmthera.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. Journal of Chronic Diseases. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- Confavreux C, Hutchinson M, Hours MM, Cortinovis-Tourniaire P, Moreau T. Rate of pregnancy-related relapse in multiple sclerosis. Pregnancy in Multiple Sclerosis Group. New England Journal of Medicine. 1998;339:285–291. doi: 10.1056/NEJM199807303390501. [DOI] [PubMed] [Google Scholar]
- Delahaije DH, Dirksen CD, Peeters LL, Smits LJ. Anxiety and depression following preeclampsia or hemolysis, elevated liver enzymes, and low platelets syndrome. A systematic review. Acta Obstetricia et Gynecologica Scandinavica. 2013;92:746–761. doi: 10.1111/aogs.12175. [DOI] [PubMed] [Google Scholar]
- Di Florio A, Jones L, Forty L, Gordon-Smith K, Blackmore ER, Heron J, Craddock N, Jones I. Mood disorders and parity – a clue to the aetiology of the postpartum trigger. Journal of Affective Disorders. 2014;152–154:334–339. doi: 10.1016/j.jad.2013.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleicher N. Postpartum depression, an autoimmune disease? Autoimmune Reviews. 2007;6:572–576. doi: 10.1016/j.autrev.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Grace SL, Evindar A, Stewart DE. The effect of postpartum depression on child cognitive development and behavior: a review and critical analysis of the literature. Archives of Women’s Mental Health. 2003;6:263–274. doi: 10.1007/s00737-003-0024-6. [DOI] [PubMed] [Google Scholar]
- Groer MW, Morgan K. Immune, health and endocrine characteristics of depressed postpartum mothers. Psychoneuroendocrinology. 2007;32:133–139. doi: 10.1016/j.psyneuen.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Harris B, Lovett L, Smith J, Read G, Walker R, Newcombe R. Cardiff puerperal mood and hormone study. III. Postnatal depression at 5 to 6 weeks postpartum, and its hormonal correlates across the peripartum period. British Journal of Psychiatry. 1996;168:739–744. doi: 10.1192/bjp.168.6.739. [DOI] [PubMed] [Google Scholar]
- Haupl T, Ostensen M, Grutzkau A, Burmester GR, Villiger PM. Interaction between rheumatoid arthritis and pregnancy: correlation of molecular data with clinical disease activity measures. Rheumatology. 2008;47(Suppl. 3):iii19–iii22. doi: 10.1093/rheumatology/ken157. [DOI] [PubMed] [Google Scholar]
- Hoedjes M, Berks D, Vogel I, Franx A, Bangma M, Darlington AS, Visser W, Duvekot JJ, Habbema JD, Steegers EA, Raat H. Postpartum depression after mild and severe preeclampsia. Journal of Women’s Health. 2011;20:1535–1542. doi: 10.1089/jwh.2010.2584. [DOI] [PubMed] [Google Scholar]
- Howard LM, Molyneaux E, Dennis CL, Rochat T, Stein A, Milgrom J. Non-psychotic mental disorders in the perinatal period. Lancet. 2014;384:1775–1788. doi: 10.1016/S0140-6736(14)61276-9. [DOI] [PubMed] [Google Scholar]
- Jones I, Chandra PS, Dazzan P, Howard LM. Bipolar disorder, affective psychosis, and schizophrenia in pregnancy and the post-partum period. Lancet. 2014;384:1789–1799. doi: 10.1016/S0140-6736(14)61278-2. [DOI] [PubMed] [Google Scholar]
- Jones I, Hamshere M, Nangle JM, Bennett P, Green E, Heron J, Segurado R, Lambert D, Holmans P, Corvin A, Owen M, Jones L, Gill M, Craddock N. Bipolar affective puerperal psychosis: genome-wide significant evidence for linkage to chromosome 16. American Journal of Psychiatry. 2007;164:1099–1104. doi: 10.1176/ajp.2007.164.7.1099. [DOI] [PubMed] [Google Scholar]
- Kendell RE, Chalmers JC, Platz C. Epidemiology of puerperal psychoses. British Journal of Psychiatry. 1987;150:662–673. doi: 10.1192/bjp.150.5.662. [DOI] [PubMed] [Google Scholar]
- Klier CM, Muzik M, Dervic K, Mossaheb N, Benesch T, Ulm B, Zeller M. The role of estrogen and progesterone in depression after birth. Journal of Psychiatric Research. 2007;41:273–279. doi: 10.1016/j.jpsychires.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Laursen TM, Munk-Olsen T. Reproductive patterns in psychotic patients. Schizophrenia Research. 2010;121:234–240. doi: 10.1016/j.schres.2010.05.018. [DOI] [PubMed] [Google Scholar]
- Lynge E, Sandegaard JL, Rebolj M. The Danish National Patient Register. Scandinavian Journal of Public Health. 2011;39:30–33. doi: 10.1177/1403494811401482. [DOI] [PubMed] [Google Scholar]
- Mors O, Perto GP, Mortensen PB. The Danish Psychiatric Central Research Register. Scandinavian Journal of Public Health. 2011;39:54–57. doi: 10.1177/1403494810395825. [DOI] [PubMed] [Google Scholar]
- Munk-Olsen T, Jones I, Laursen TM. Birth order and postpartum psychiatric disorders. Bipolar Disorders. 2014;16:300–307. doi: 10.1111/bdi.12145. [DOI] [PubMed] [Google Scholar]
- Munk-Olsen T, Laursen TM, Mendelson T, Pedersen CB, Mors O, Mortensen PB. Risks and predictors of readmission for a mental disorder during the postpartum period. Archives of General Psychiatry. 2009;66:189–195. doi: 10.1001/archgenpsychiatry.2008.528. [DOI] [PubMed] [Google Scholar]
- Munk-Olsen T, Laursen TM, Pedersen CB, Mors O, Mortensen PB. New parents and mental disorders: a population-based register study. Journal of the American Medical Association. 2006;296:2582–2589. doi: 10.1001/jama.296.21.2582. [DOI] [PubMed] [Google Scholar]
- Osborne LM, Monk C. Perinatal depression – the fourth inflammatory morbidity of pregnancy?: Theory and literature review. Psychoneuroendocrinology. 2013;38:1929–1952. doi: 10.1016/j.psyneuen.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry BL, Sorenson DL, Meliska CJ, Basavaraj N, Zirpoli GG, Gamst A, Hauger R. Hormonal basis of mood and postpartum disorders. Current Women’s Health Reports Journal. 2003;3:230–235. [PubMed] [Google Scholar]
- Pedersen CB. The Danish Civil Registration System. Scandinavian Journal of Public Health. 2011;39:22–25. doi: 10.1177/1403494810387965. [DOI] [PubMed] [Google Scholar]
- Redman CW, Sargent IL. The pathogenesis of pre-eclampsia. Gynécologie Obstétrique et Fertilité. 2001;29:518–522. doi: 10.1016/s1297-9589(01)00180-1. [DOI] [PubMed] [Google Scholar]
- Roberts JM, Bell MJ. If we know so much about preeclampsia, why haven’t we cured the disease? Journal of Reproductive Immunology. 2013;99:1–9. doi: 10.1016/j.jri.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robillard PY, Dekker G, Chaouat G, Hulsey TC, Saftlas A. Epidemiological studies on primipaternity and immunology in preeclampsia – a statement after twelve years of workshops. Journal of Reproductive Immunology. 2011;89:104–117. doi: 10.1016/j.jri.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Sargent IL, Borzychowski AM, Redman CW. NK cells and human pregnancy – an inflammatory view. Trends in Immunology. 2006;27:399–404. doi: 10.1016/j.it.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Steegers EA, von Dadelszen P, Duvekot JJ, Pijnenborg R. Pre-eclampsia. Lancet. 2010;376:631–644. doi: 10.1016/S0140-6736(10)60279-6. [DOI] [PubMed] [Google Scholar]
- Stein A, Pearson RM, Goodman SH, Rapa E, Rahman A, McCallum M, Howard LM, Pariante CM. Effects of perinatal mental disorders on the fetus and child. Lancet. 2014;384:1800–1819. doi: 10.1016/S0140-6736(14)61277-0. [DOI] [PubMed] [Google Scholar]
- Tandberg A, Klungsoyr K, Romundstad L, Skjaerven R. Pre-eclampsia and assisted reproductive technologies: consequences of advanced maternal age, interbirth intervals, new partner and smoking habits. BJOG: An International Journal of Obstetrics and Gynaecology. 2015;122:915–922. doi: 10.1111/1471-0528.13051. [DOI] [PubMed] [Google Scholar]
- Weetman AP. Immunity, thyroid function and pregnancy: molecular mechanisms. Nature Reviews Endocrinology. 2010;6:311–318. doi: 10.1038/nrendo.2010.46. [DOI] [PubMed] [Google Scholar]
- World Health Organization . WHO ICD-10: Psykiske lidelser og adfærdsmæssige forstyrrelser. Klassifikation og diagnostiske kriterier (WHO ICD-10: Mental and Behavioural Disorders. Classification and Diagnostic Criteria) Munksgaard; Copenhagen: 1994. [Google Scholar]