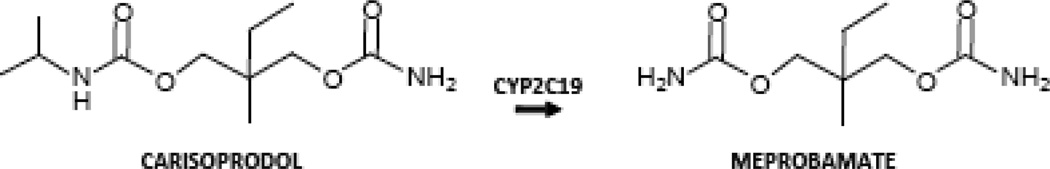Abstract
Meprobamate is a schedule II anxiolytic and the primary metabolite of the muscle relaxant carisoprodol. Meprobamate modulates GABAA (γ-aminobutyric acid type A) receptors, and has barbiturate-like activity. To gain insight into its actions, we have conducted a series of studies using recombinant GABAA receptors. In αxβzγ GABAA receptors (where x = 1–6 and z = 1–3), the ability to enhance GABA-mediated current was evident for all α subunit isoforms, with the largest effect observed in α5-expressing receptors. Direct gating was present with all α subunits, although attenuated in α3-expressing receptors. Allosteric and direct effects were comparable in α1β1γ2 and α1β2γ2 receptors, whereas allosteric effects were enhanced in α1β2 compared to α1β2γ2 receptors. In “extrasynaptic” (α1β3δ and α4β3δ) receptors, meprobamate enhanced EC20 and saturating GABA currents, and directly activated these receptors. The barbiturate antagonist bemegride attenuated direct effects of meprobamate. Whereas pentobarbital directly gated homomeric β3 receptors, meprobamate did not, and instead blocked the spontaneously open current present in these receptors. In wild type homomeric ρ1 receptors, pentobarbital and meprobamate were ineffective in direct gating; a mutation known to confer sensitivity to pentobarbital did not confer sensitivity to meprobamate. Our results provide insight into the actions of meprobamate and parent therapeutic agents such as carisoprodol. Whereas in general actions of meprobamate were comparable to those of carisoprodol, differential effects of meprobamate at some receptor subtypes suggest potential advantages of meprobamate may be exploited. A re-assessment of previously synthesized meprobamate-related carbamate molecules for myorelaxant and other therapeutic indications is warranted.
Keywords: GABAA receptor, meprobamate, carisoprodol, muscle relaxant, drug abuse
1. Introduction
Meprobamate, a propanediol carbamate, was the first drug to be used as an anxiolytic agent, and was also prescribed as an anticonvulsant and sedative/hypnotic (Hendley et al., 1954). By the late 1950s, meprobamate was the most widely prescribed drug in the United States and in many other countries. It retained this position until diazepam the benzodiazepine diazepam was introduced into clinical use in late 1960 (Berger, 1964; Greenblatt and Shader, 1974). Within two years of introduction of meprobamate, cases of its abuse and withdrawal after long term use were reported (Ewing and Fullilove, 1957), and it was relatively soon thereafter listed as a controlled substance.
While meprobamate itself is no longer widely used, drugs metabolized to meprobamate are widely available, and misuse of these drugs is associated with serious side effects. One such drug is the centrally acting skeletal muscle relaxant carisoprodol, which remains highly prescribed for low back pain (Sullivan, 2015; Toth and Urtis, 2004). Use of carisoprodol is associated with abuse and dependence, and related dangers such as psychomotor impairment (Elder, 1991; Rust et al., 1993; Zacny et al., 2011, 2012). Dependence and severe withdrawal may lead to seizures and death (Adams et al., 1975; Littrell et al., 1993; Reeves and Parker, 2003; Rust et al., 1993). Although carisoprodol has its own independent effects (Gonzalez et al., 2009b), many of its therapeutic and abuse-dependent effects are likely due to its metabolite meprobamate, which differs from carisoprodol by the absence of an isopropyl group (Fig. 1). Indeed, the half-life of meprobamate far exceeds that of carisoprodol ( t ½ for carisoprodol of 1–2 h (Olsen et al., 1994) and t ½ for meprobamate between 6.4 h and 16.6 h (Hollister and Levy, 1964; Maddock and Bloomer, 1967)).
Figure 1. Structure of meprobamate and a prominent parent molecule, carisoprodol.
The molecules differ by an isopropyl present on one of carisoprodol’s carbamyl nitrogens; the isopropyl is removed by CYP2C19 to form meprobamate.
Meprobamate has been shown to have barbiturate-like activity at neuronal GABAA receptors (Rho et al., 1997), the predominant inhibitory neurotransmitter receptor in the central nervous system and the target of many therapeutics. Carisoprodol has similarly been suggested to have barbiturate-like actions (Gonzalez et al., 2009a). A detailed understanding of meprobamate’s interaction with GABAA receptors is lacking, however. For instance, the extent to which its interaction with GABAA receptors may be subunit-dependent is unknown. As the extensive array of GABAA receptor configurations that exist throughout the CNS contribute to specific physiological and pharmacological responses of GABA and modulatory agents (Olsen and Sieghart, 2008), an understanding of meprobamate’s subunit-dependent actions could prove enlightening regarding therapeutic and adverse effects of both it and parent therapeutics that are metabolized to meprobamate, such as carisoprodol. We have thus assessed the potential subunit-dependent interaction of meprobamate at these receptors and have further explored potential commonality and differences of action with barbiturates.
2. Materials and Methods
2.1 Cell Culture and Transfection
Human embryonic kidney 293 (HEK293) cells, stably or transiently expressing varying GABAA receptor subunits, were used in the present study. For transient expression, cells were transfected with human GABAA α1-α6; human β1-2; and human γ2s (short isoform) cDNA in a 1:1:5 ratio (2 μg total cDNA) using PolyJet™ in vitro transfection reagent (SignaGen Laboratories, Jamesville, MD). The γ2s subunit will be referred to as γ2 from here forward. For studies assessing meprobamate effects in a model of extrasynaptic receptors (rat α1β3δ and α4β3δ subunits), a total of 3 μg of cDNA in a transfection ratio of 2:1:0.25 for α:β:δ plasmids was used (Wagoner and Czajkowski, 2010). For studies on wild type β3 homomeric receptors, wild type ρ1 subunits, and barbiturate-sensitive ρ1 (W328M) subunits (generated previously in our lab, Gonzalez et al, 2009b), 2 μg of cDNA was transfected. The rat GABAA α4 subunit cDNA was purchased from Genescript (Piscataway, New Jersey). Human GABAA α1 subunit cDNA was generously provided by Neil Harrison (Columbia University Medical Center, New York). The wild-type human GABA ρ1 subunit was generously provided by David Weiss (University of Texas Health Science Center at San Antonio). HEK293 cells stably expressing human α2β2γ2 receptors were also used. A complete description of the preparation and maintenance of these stable cell lines has been published previously (Hawkinson et al., 1996). Cells were plated on glass coverslips coated with poly-L-lysine in 35-mm culture dishes, and maintained at 37°C in a humidified incubator with an atmosphere of 5% CO2. In all cases, cells were used for recording 24–72 hr after transfection.
2.2 Electrophysiology
Whole-cell patch clamp electrophysiology was used to assess GABA-, meprobamate–, or pentobarbital-activated Cl− currents. All electrophysiology experiments were conducted at room temperature (22 – 25 °C) with the membrane potential clamped at −60 mV. Patch pipettes of borosilicate glass (1B150F; World Precision Instruments, Inc., Sarasota, FL) were pulled (Flaming/Brown, P-87/PC; Sutter Instrument Company, Novato, CA) to a tip resistance of 4–6MΩ. Patch pipettes were filled with a solution consisting of 140 mM CsCl, 10 mM EGTA-Na+, 10 mM HEPES-Na+, and 4 mM Mg2+-ATP, pH 7.2. Coverslips containing cultured cells were placed in the recording chamber on the stage of an inverted light microscope and superfused continuously with an external solution consisting of 125 mM NaCl, 20 mM HEPES, 3 mM CaCl2, 5.5 mM KCl, 0.8 mM MgCl2, and 10 mM glucose, pH 7.3. Agonist-induced Cl− currents were obtained with an Axopatch 200B amplifier (Molecular Devices, Sunnyvale, CA) equipped with a CV-203BU headstage. Currents were low-pass filtered at 5 kHz, monitored simultaneously on an oscilloscope and a chart recorder (Gould TA240; Gould Instrument Systems Inc., Cleveland, OH), and stored on a computer using an on-line data acquisition system (pCLAMP 6.0; Axon Instruments) for subsequent off-line analysis.
2.3 Chemicals and solutions
Meprobamate, carisoprodol, pentobarbital, diazepam, THIP (4,5,6,7-tetrahydroisoxazolo(5,4-c)pyridin-3-ol), salts and buffers were purchased from Sigma Aldrich (St. Louis, MO). GABA was obtained from Acros Organics (New Jersey, US). Bemegride was obtained from Pfaltz & Bauer, Inc. (Waterbury, CT). Stock solutions of meprobamate, pentobarbital and carisoprodol were made using dimethylsulfoxide (DMSO). Drugs were diluted in normal saline, so that the final DMSO concentration (vol/vol) of the test solutions was ≤0.3%. GABA, diazepam and bemegride stock solutions were prepared using de-ionized H2O. All stock solutions were stored at −20 °C. On experimental days, drug-containing solutions were prepared from stock by serial dilution into external solution.
2.4 Experimental Protocol
GABA (with or without modulatory ligand), meprobamate, carisoprodol or pentobarbital were prepared in external solution on the day of use and applied via a 16-barrel rapid perfusion system in which all barrels (200 μm outer diameter quartz tubes; ALA Scientific, Folsom, CA) emptied via a common tip positioned adjacent to the cell under study. Flow through each barrel was pressure fed and regulated by solenoid or pinch valves operated by a programmable microprocessor-based controller. Only one valve was open at a time, and the buffer solution was applied continuously between drug applications via gravity or positive pressure. As per the manufacturer specifications, solution flow rate as configured in the present studies is approximately 3 μl/second, and exchange rate is approximately 5 msec. For some experiments, ligands were applied via gravity flow using a Y-tube placed adjacent to the cell.
The modulatory effects of meprobamate on GABA-gated currents were assessed using an EC20 gating concentration of GABA as the control. This gating concentration was selected to ensure there was a sufficient range to observe the full allosteric potential of meprobamate. To ensure the gating concentration was approximately an EC20, control responses were compared to the maximal GABA-gated current for each individual cell. On a cell by cell basis, control GABA currents were deemed acceptable for assessment of allosteric modulatory effects of meprobamate if they were within a 15–25 % range of maximal current for that particular cell. Control responses were established by observing two consecutive agonist-activated currents that varied in amplitude by no more than ± 10%. In our analyses of the modulatory effects of meprobamate, peak current amplitude was defined as the maximum current elicited by meprobamate. For studies investigating meprobamate-mediated currents, meprobamate was dissolved in external solution and applied in the manner described above. In all studies assessing direct gating effects of meprobamate, the magnitude of the response was expressed relative to the maximal effect of GABA (designated 100%). In studies on β3 homomeric receptors, the magnitude of meprobamatemediated block of spontaneously open channels was measured relative to the maximal blocking effect of picrotoxin (designated 100%). For αβγ configurations, GABA-gated control currents were recorded in the presence of diazepam to confirm incorporation of the γ2 subunit. Presence of the δ subunit in α1β3δ and α4β3δ receptors was confirmed by loss of inhibition to 1 μM Zn2+.
2.5 Data Analysis
To ensure equipotent concentrations were used for gating, GABA concentration-response data were collected for all synaptic and extrasynaptic GABAA receptors tested, (Table 1). From these data, EC20 and saturating GABA concentrations were calculated for each configuration and used in subsequent investigations of the allosteric and direct effects of meprobamate, respectively. For studies assessing allosteric actions of meprobamate, a correction was applied to subtract underlying direct gating effects, which were present at higher meprobamate concentrations. All data are presented as mean ± S.E.M. For experiments in which three or more datasets were statistically analyzed, one-way analysis of variance was conducted, followed by a Tukey-Kramer post hoc test as indicated. For those experiments in which two datasets were analyzed, Student’s t-tests (paired or unpaired) were conducted. In all cases, statistical significance was designated at a P value of 0.05 or less.
Table 1.
GABA sensitivity of the GABAA receptor subunit configurations studied. EC50 values for each receptor are expressed as mean ± S.E.M.
| Receptor Configuration |
EC50 (µM) | n |
|---|---|---|
| α1β2 | 16.4 ± 2.39 | 5 |
| α1β2γ2 | 28.6 ± 2.75 | 8 |
| α2β2γ2 | 32.8 ± 1.81 | 5 |
| α3β2γ2 | 34.8 ± 2.09 | 8 |
| α4β2γ2 | 5.9 ± 0.16 | 5 |
| α5β2γ2 | 0.8 ± 0.23 | 4 |
| α6β2γ2 | 1.7 ± 0.24 | 4 |
| α1β1γ2 | 15.7 ± 0.15 | 7 |
| α4β3δ | 0.04 ± 0.02 | 4 |
3. Results
3.1 α subunit isoform influence on direct and allosteric actions of meprobamate
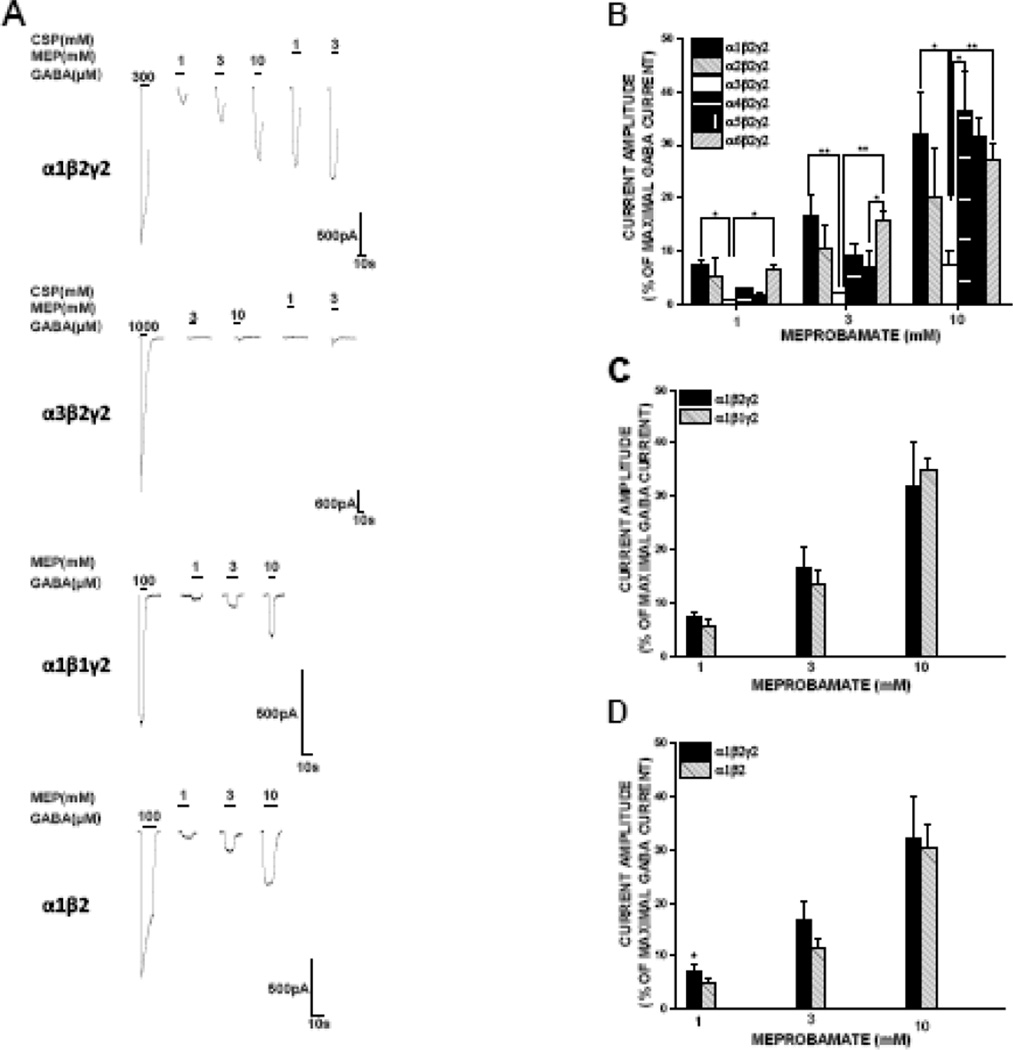
Direct effects of meprobamate were assessed in αxβ2γ2 GABAA receptors, where x = α subunit 1-6. We initiated direct gating studies using concentrations of meprobamate as low as 100 μM; as direct gating effects were not generally observed below 1 mM, we report here direct gating effects of 1, 3 and 10 mM meprobamate. Meprobamate directly gated each of the configurations tested at high concentrations, evoking inward currents in the absence of GABA (Fig. 2). The relatively low apparent affinity of meprobamate combined with limits on its solubility precluded definitive calculation of maximal meprobamate-gated current. At 10 mM, meprobamate-gated current was of similar magnitude in α1-, α2-, α4-, α5- and α6β2γ2s GABAA receptors (20–36% of maximal GABA current), and significantly less (7%) in α3-expressing receptors (Fig. 2).
Figure 2. Assessment of subunit-dependent effects of meprobamate on direct activation of GABAA receptors.
A, Representative traces demonstrating direct activation by meprobamate in α1β2γ2, α3β2γ2, α1β1γ2 and α1β2 receptors. B, C and D, summary data of meprobamate mediated direct-gating effects associated with varying α β and γ subunits, respectively. Direct activation capability was diminished in α3β2γ2 compared to the other configurations, whereas varying the β subunit or deleting the γ2 subunit had negligible effects. Each data point represents the mean ± S.E.M. of a minimum of 4 cells. *, P < 0.05; **, P < 0.01.
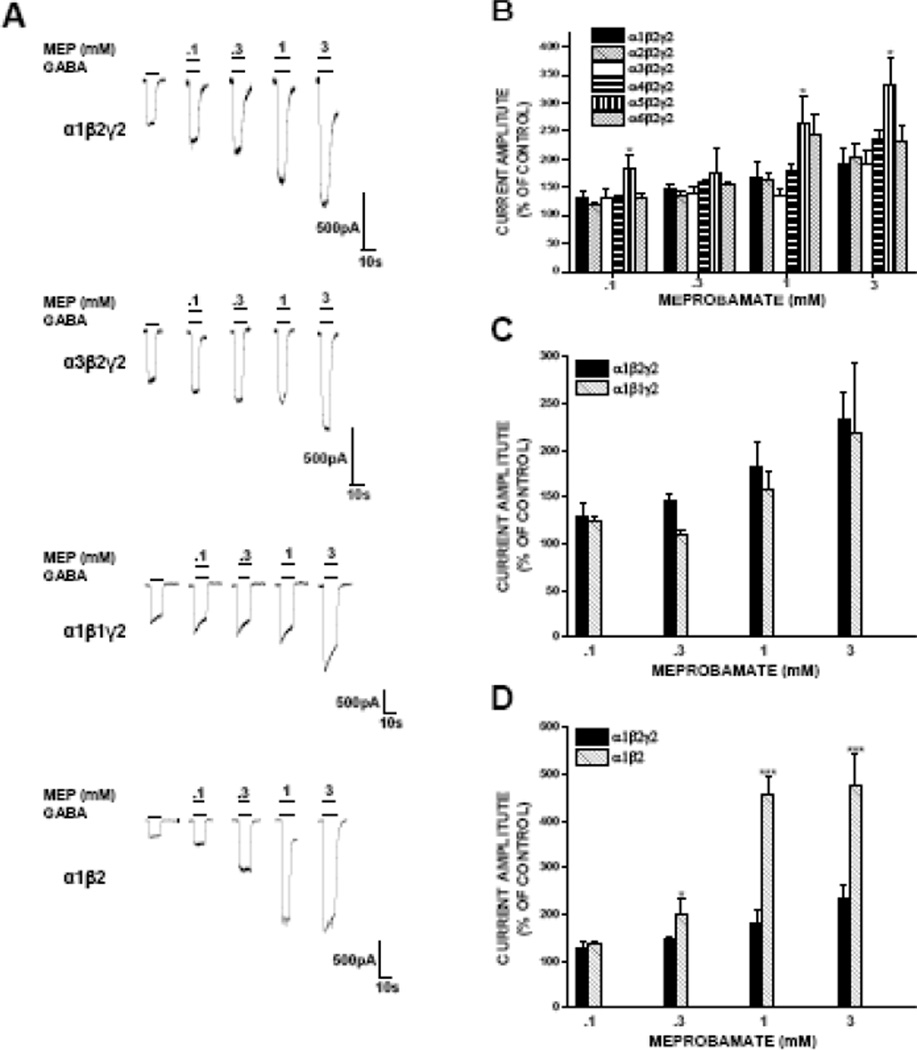
We also assessed α subunit isoform effects on allosteric modulatory actions of meprobamate. Meprobamate positively modulated the effects of GABA EC20 concentration in all configurations tested (Fig. 3). 3 mM meprobamate on average roughly doubled the amplitude of the GABA EC20 current for most α subunit isoforms. We observed a somewhat enhanced effect in receptors expressing the α5 subunit, as 3 mM meprobamate elicited a potentiating effect of more than 3-fold (Fig. 3).
Figure 3. Assessment of subunit-dependent effects of allosteric modulatory effects of meprobamate on GABAA receptors.
A, Representative traces demonstrating direct allosteric potentiation by meprobamate of GABA-gated (EC20) currents in α1β2γ2, α3β2γ2, α1β1γ2 and α1β2 receptors. B, C and D, summary data of allosteric effects of meprobamate associated with varying α β and γ subunits, respectively. Allosteric potentiation was present in all αβγ receptors, but greatest in those expressing the α5 subunit; absence of the γ subunit also resulted in enhanced potentiation. Note for this and other figures assessing allosteric modulation, underlying direct gating effects were subtracted out for calculation of summary data. Each data point represents the mean ± S.E.M. of a minimum of 4 cells. *, P < 0.05; ***, P < 0.001.
3.2 β subunit isoform influence on direct and allosteric actions of meprobamate
To assess whether the β subunit isoform affected the actions of meprobamate, we assessed both direct and allosteric effects of it in α1β2γ2 and α1β1γ2 receptors. We observed no significant difference in the magnitude of current elicited by meprobamate alone in these receptors, as 10 mM meprobamate elicited currents roughly 1/3 the amplitude of peak GABA current in both α1β2γ2 and α1β1γ2 receptors (Fig. 2C).
With regard to β subunit effects on allosteric actions of meprobamate, we found receptors expressing either β1 or β2 subunits (in combination with α1 and γ2) showed similar allosteric modulation by meprobamate. In both receptors, 3 mM meprobamate effectively doubled the amplitude of GABAgated current (Fig. 3C).
3.3 γ subunit isoform influence on direct and allosteric actions of meprobamate
Presence of a γ subunit is critical for the allosteric actions of benzodiazepines (Pritchett et al., 1989), but not for direct or allosteric effects of other ligands such as barbiturates (Horne et al., 1993) or neurosteroids (Hosie et al., 2006). To assess the extent to which the γ subunit impacts actions of meprobamate, we tested effects of it in receptors lacking the γ2 subunit. In comparison to α1β2γ2 receptor, direct gating in α1β2 receptor was generally comparable. There was a statistically smaller direct gating response at 1 mM meprobamate in α1β2 receptor compared to α1β2γ2 receptor, but this difference was modest and not present at other concentrations tested (Fig. 2D).
The γ2 subunit had a more prominent effect on allosteric modulation by meprobamate. Its presence significantly attenuated the ability of meprobamate to allosterically an EC20 concentration of GABA at all concentrations of MEP above 100 μM. At 1 and 3 mM MEP, the magnitude of enhancement in α1β2 receptors was more than twice that observed in α1β2γ2 receptors (Fig 3D).
3.4 Effects of meprobamate on δ-subunit containing “extrasynaptic” receptors
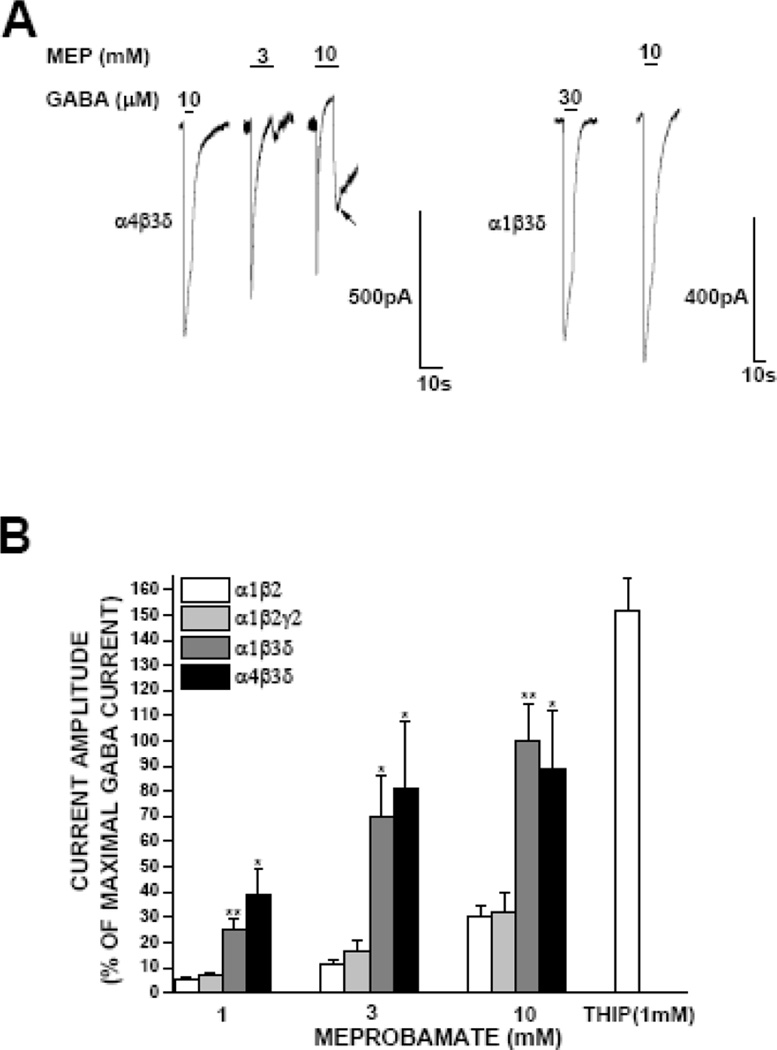
GABA and ligands that regulate its receptors may act at synaptic or extrasynaptic sites. To assess the influence of meprobamate extrasynaptically, we studied its effect in recombinant α4β3δ and α1β3δ receptors, models of extrasynaptic GABAA receptors. As reported by others (Brown et al., 2002), sensitivity of α4β3δ receptors to GABA was high, with an EC50 of 0.04 ± 0.02 μM. As shown in Figure 4, meprobamate directly gated α4β3δ receptors with an efficacy comparable to that of GABA itself. This direct gating effect was concentration-dependent, and saturated at 3 mM meprobamate. We found very similar effects for the ability of meprobamate to directly gate α1β3δ receptors. Sensitivity to the direct gating effects in extrasynaptic receptors was comparable to that observed in synaptic receptors. Current gated by meprobamate rapidly decayed following activation, and we noted a prominent rebound effect following termination of ligand application (Fig 4A, arrow). GABA is not a full agonist in δ expressing receptors (Brown et al., 2002); we observed the same effect, as the agonist THIP elicited a current 151 ± 12% of that elicited by saturating GABA.
Figure 4. Assessment of direct gating effects of meprobamate in extrasynaptic GABAA receptors.
A, representative traces demonstrating meprobamate direct gating action on α4β3δ and α1β3δ GABAA receptors, in comparison to a saturating concentration of GABA. The maximal efficacy of meprobamate was comparable to that of GABA. B, Summary data illustrating the efficacy of meprobamate compared to GABA in α1β2, α1β2γ2, α1β3δ and α4β3δ GABAA receptor. Data for α1β2 and α1β2γ2 receptors are replotted from Figure 1 for comparison. Efficacy of the full agonist THIP was also assessed; it was significantly more efficacious than either GABA or meprobamate on δ-containing GABAA receptors. Each bar represents the mean ± S.E.M. of a minimum of 3 cells for δ-containing receptors, and mean ± S.E.M. of a minimum of 4 for all others. *, P < 0.05; **, P < 0.01.
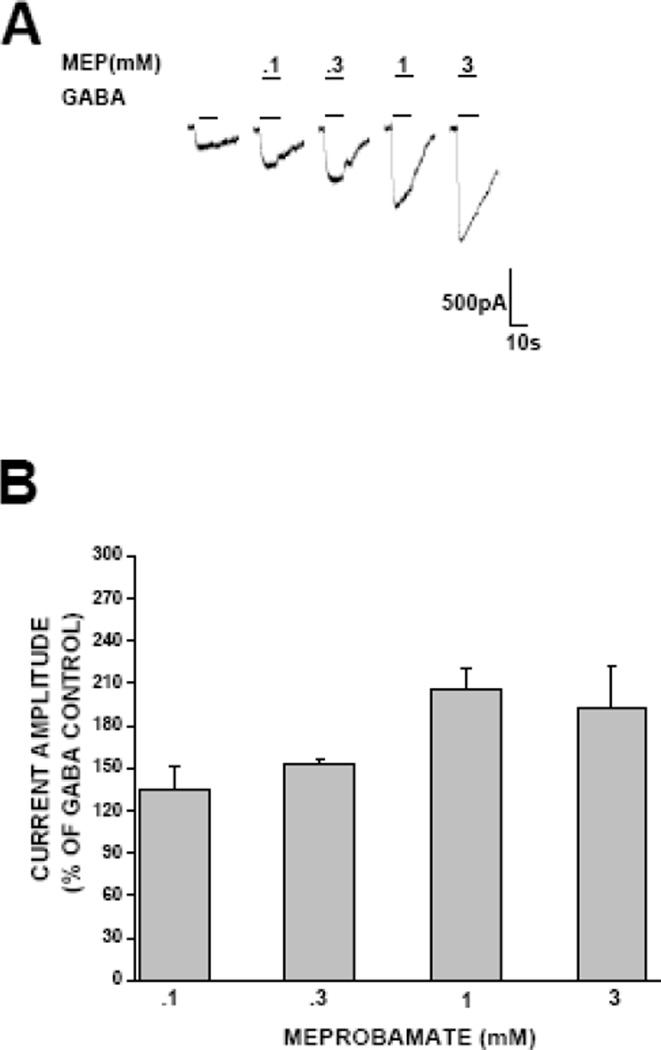
We also assessed the extent to which meprobamate might allosterically potentiate extrasynaptic GABAA receptors. As illustrated in Fig. 5, meprobamate enhanced GABA-gated (EC20) current, with a threshold effect at 100 μM, and a maximal effect observed at 1 mM. The sensitivity and magnitude of the potentiating effect is comparable to that observed in synaptic receptors.
Figure 5. Assessment of allosteric effects of meprobamate in extrasynaptic receptors.
A, representative traces demonstrating the potentiation by meprobamate of GABA-gated (EC20) currents in α4β3δ GABAA receptors. B, Bar graph summarizing the concentration-response profile for the allosteric modulatory effects of meprobamate on extrasynaptic α4β3δ GABAA receptors. Each bar represents the mean ± S.E.M. of a minimum of 3 cells.
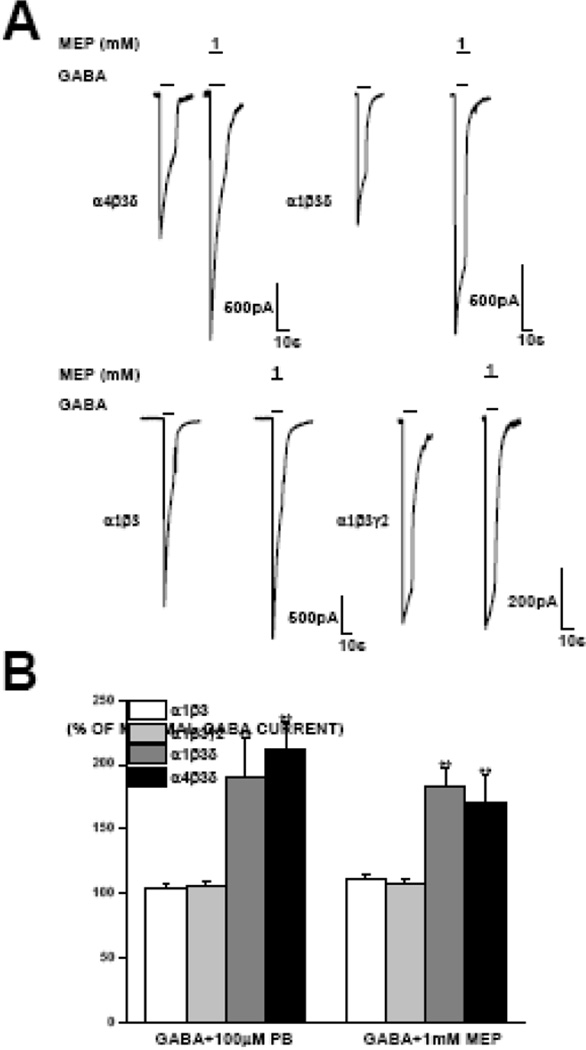
Pentobarbital and carisoprodol, the parent molecule to meprobamate, have been reported to enhance maximal GABA-gated current in δ-subunit containing receptor (Feng et al., 2004; Kumar et al., 2015). We thus assessed for this capability with meprobamate. Co-application of 1 mM meprobamate with 1 mM GABA (EC95+) to α4β3δ receptors resulted in a current 183 ± 17% of that seen with maximal GABA alone. As anticipated, 100 μM pentobarbital, which was used as a positive control, showed enhancement of maximal GABA-gated current (to 223 ± 38% of maximal GABA, Fig. 6B). No enhancement was observed in α1β3γ or α1β3 subunit containing receptors with meprobamate or pentobarbital (Fig. 6B).
Figure 6. Potentiation of maximal GABA by meprobamate in extrasynaptic receptors.
A, Illustrative traces showing that in both α4β3δ and α1β3δ extrasynaptic GABAA receptors, 1 mM meprobamate significantly enhanced the magnitude of a saturating concentration of GABA alone, whereas this potentiation was not observed in α1β3 or α1β2γ2 receptors. B, Summary data illustrating potentiation of maximal GABA current by both pentobarbital and meprobamate in δ-subunit expressing receptors, compared to those not expressing the δ subunit. Data are expressed relative to the peak current amplitude elicited by saturating concentration of GABA. Each bar represents the mean ± S.E.M. of a minimum of 3 cells for δ-containing receptors, and mean ± S.E.M. of a minimum of 4 for all others. **, P < 0.01.
3.5 Further assessment of the barbiturate-like effects of meprobamate
Based on a mechanistic assessment of its effects in GABAA receptors recorded from cultured rat hippocampal neurons, others have concluded meprobamate has “barbiturate-like” activity (Rho et al., 1997). Our results are broadly consistent with that suggestion. We thus conducted additional studies to test further commonality of action between meprobamate and pentobarbital.
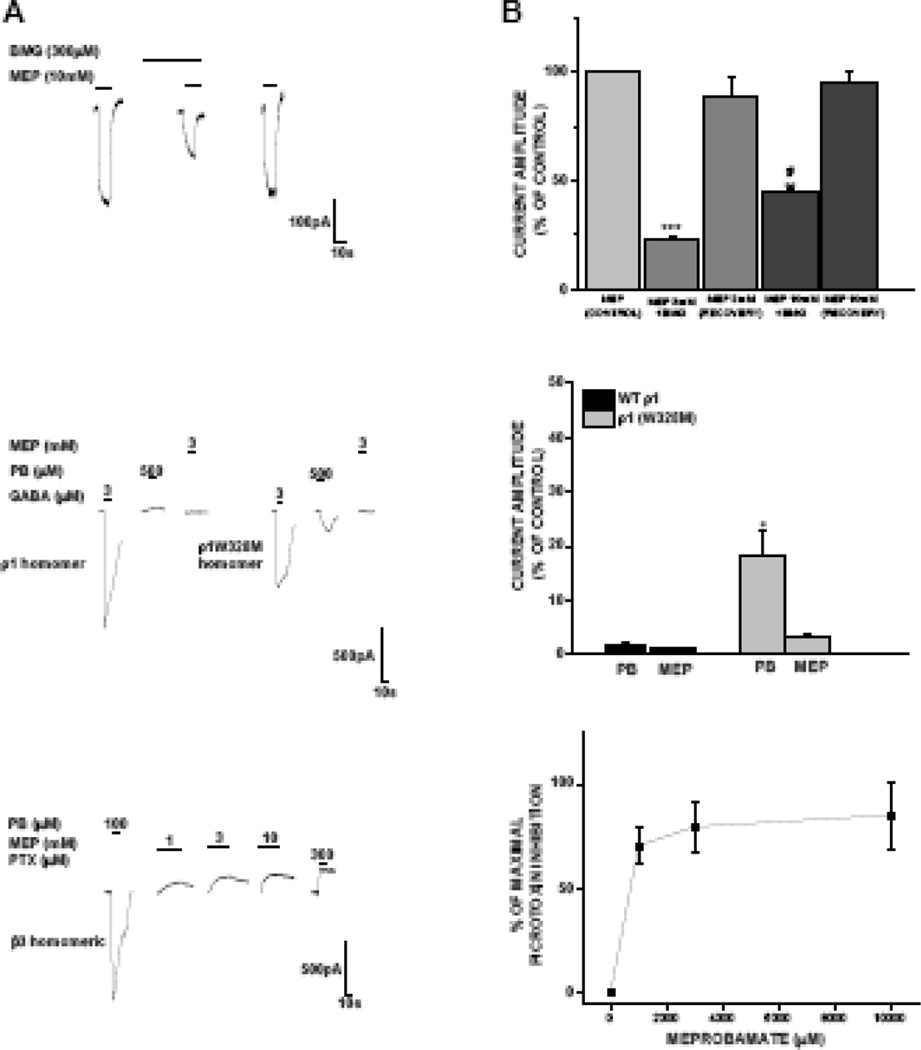
Bemegride is known to antagonize pentobarbital stimulus effect in animal models (Schechter, 1984) and phenobarbital direct gating effects in mouse spinal neurons (Mistry and Cottrell, 1990). We thus assessed the influence of bemegride on the direct gating effects of meprobamate. As shown in Figure 7, meprobamate-mediated direct gating current was significantly and reversibly attenuated by bemegride. The ability of bemegride to antagonize meprobamate-activate current was greater when tested against a lower concentration of meprobamate, demonstrating a concentration-dependent effect.
Figure 7. Assessment of “barbiturate-like” actions of meprobamate.
A, top, Representative traces demonstrating meprobamate-activated currents are reversibly reduced in the presence of the barbiturate antagonist bemegride in stably expressed human α1β2γ2 receptors. A, middle, Neither pentobarbital nor meprobamate could directly gate wild type homomeric ρ1 receptors; the W328M mutation conferred sensitivity to pentobarbital, but not meprobamate. A, bottom, Pentobarbital directly gated homomeric β3 receptors, whereas meprobamate blocked the spontaneously open current present in these channels. B, Summary results for experiments presented in panel A. Each data point represents the mean ± S.E.M. of a minimum of 4 cells for all data sets. *, P < 0.05; **, P < 0.01; ***, P < 0.001; #, P < 0.05 compared to the 3 mM MEP effect, illustrating a concentration-dependent effect of bemegride.
ρ1 homomeric GABA receptors are anion-selective cys loop ligand-gated channels, with a pharmacology distinct from that of heteromeric GABAA receptors (Amin and Weiss, 1994). They are found in very high concentration in retina and other visual areas (Boue-Grabot et al., 1998; Enz et al., 1995). Barbiturates cannot directly activate wild type ρ1 homomeric GABA receptors, but can when tryptophan at position 328 is mutated to methionine (W328M) (Amin, 1999). We assessed whether meprobamate can gate wild type or W328M ρ1 homomeric GABA receptors. In wild type homomeric ρ1 receptors, both pentobarbital and meprobamate were ineffective in direct gating (Fig. 7A, 7B). As originally reported (Amin, 1999), we observed that the W328M mutation in the ρ1 subunit conferred sensitivity to direct gating effects of pentobarbital. In contrast, the W328M mutation did not confer on meprobamate the ability to directly gate the channel (Fig. 7A, 7B).
Homomeric β3 receptors do not appear to form natively, but they can form a functional receptor in vitro, and thus are a useful tool for studying drug action (Chen et al., 2012; Davies et al., 1997). Homomeric β3 receptors are spontaneously open, and they can be directly activated by barbiturates (Davies et al., 1997). We evaluated whether meprobamate had direct gating effects in these receptors. Meprobamate, in contrast to pentobarbital, could not directly gate homomeric β3 receptors. Instead, meprobamate, blocked the spontaneously open current present in homomeric β3 receptors (Fig. 7A, 7B). This effect was concentration-dependent, and approached the efficacy of the GABA receptor antagonist picrotoxin in blocking the spontaneously open channel (Fig. 7B).
4. Discussion
This is the first study to assess subunit-dependent effects of meprobamate at its major target, GABAA receptors. Drugs metabolized to meprobamate, such as carisoprodol, have remained widely utilized. Carisoprodol was actually synthesized as an alternative to meprobamate, and early studies suggested it had a profile that was preferable to meprobamate (Berger et al., 1960). Early metabolism studies in dogs indicated the primary metabolite of carisoprodol was hydroxyl-carisoprodol (Douglas et al., 1962). Toxicology studies some years later demonstrated that in humans, carisoprodol was in fact metabolized primarily to meprobamate, which has a half-life that is several-fold longer than that of carisoprodol (Bramness et al., 2004; Olsen et al., 1994). Whereas the recent scheduling of carisoprodol will likely result in a reduction in its use (Bramness et al., 2012), it remains a key therapeutic for treatment of acute low back pain (Witenko et al., 2014). Even with its recent scheduling, a quick internet search reveals a number of pharmacies noting the ready availability of carisoprodol without a prescription. Thus the potential for continuing abuse of carisoprodol remains. Considering a significant proportion of its effect are likely attributable to its metabolite meprobamate, the focus of the present study was thus to gain greater insight into meprobamate modulation of its major therapeutic target, the GABAA receptor.
Our assessment of meprobamate’s ability to directly gate and allosterically modulate the GABAA receptor is comparable to that reported in neurons (Rho et al., 1997), in that both effects are of relatively low potency. Rho et al. (1997) further categorized its actions as “barbiturate-like”, and carisoprodol also displays barbiturate-like effects both in vivo and in vitro (Gonzalez et al., 2009b). We conducted a number of experiments to better understand the extent to which effects of meprobamate mimic those of the barbiturate pentobarbital. The results demonstrate some commonality of effect between the two ligands, but in general do not support a common site of action for meprobamate and barbiturates. The barbiturate antagonist bemegride reversibly and significantly inhibited meprobamate-mediated direct gating current in a concentration-dependent manner, consistent with a competitive nature of inhibition. However, in homomeric ρ1 receptors, a mutation (W328M) that conferred sensitivity to pentobarbital (Amin, 1999) did not confer sensitivity to meprobamate. In addition, whereas pentobarbital could directly gate homomeric β3 receptors, this effect was not observed with meprobamate. Thus, whereas a number of in vivo and in vitro actions of meprobamate are in fact similar to those observed with pentobarbital, the comparison cannot be extended to indicate a common binding domain for these ligands. In this regard, meprobamate is comparable to carisoprodol (Gonzalez et al., 2009b). Recent photoaffinity labeling studies have provided considerable evidence that barbiturates bind to inter-subunit interfaces in GABAA (Chiara et al., 2013) and nicotinic (Hamouda et al., 2014) receptors. With regard to GABAA receptors, a photoreactive barbiturate binds to a number of residues at both the α–β and γ–β interfaces in the transmembrane domain (Chiara et al., 2013). Our current results suggest it unlikely that meprobamate would interact with those residues.
It has become increasingly clear in recent years that many therapeutic and adverse actions of GABAergic drugs associate predominantly with particular subunits of the GABAA receptor (Crestani et al., 2002; Crestani et al., 2001; Licata and Rowlett, 2008; Low et al., 2000; Rudolph and Mohler, 2014; Tan et al., 2010; van Rijnsoever et al., 2004). Of particular relevance to the current study is the fact that α2- and α3 expressing receptors are involved in both anxiolytic (Crestani et al., 2001; Licata and Rowlett, 2008; Low et al., 2000) and muscle-relaxing (Crestani et al., 2001; Griebel et al., 2003) effects of GABAergic ligands, while abuse potential associates with the α1 subunit (Licata and Rowlett, 2008; Tan et al., 2010). Our recent report (Kumar et al., 2015) shed some light on the subunit-dependent effects of carisoprodol itself and how they may relate to its therapeutic and advserse effects. To fully understand actions of carisoprodol, it is necessary to also understand potential subunit-dependent effects of the primary and comparatively long-lived metabolite, meprobamate. At the maximal concentration assessed, meprobamate-gated current was of similar magnitude in α1-, α2-, α4-, α5- and α6β2γ2s GABAARs, and significantly less in α3-expressing receptors. The parent drug carisoprodol has a similar profile, with roughly three-fold higher potency. With regard to allosteric modulation, carisoprodol displayed considerably greater efficacy in α1-expressing receptors (Kumar et al., 2015), whereas meprobamate displayed generally similar effects regardless of α subunit, with some enhanced efficacy in α5-expressing receptors.
The lack of effect of β subunit isoform on direct gating or allosteric effects of meprobamate contrasts with carisoprodol, in which direct gating was most efficacious in β1-expressing receptors, and allosteric modulation was greatest in β2-expressing receptors (Kumar et al., 2015). In addition, whereas the γ subunit did not have an effect on direct gating or allosteric modulation of carisoprodol (Kumar et al., 2015), its presence quite markedly attenuated the modulatory capacity of meprobamate. This effect may not be therapeutically significant as few native receptors express only α and β subunits, but it may prove useful in identifying critical domains for effects of meprobamate.
Ligands that influence the actions of GABA may also act at extrasynaptic GABAA receptors, which include α4β3δ and α1β3δ subtypes; these receptors have been implicated in diseases such as schizophrenia, depression and epilepsy (Brickley and Mody, 2012). Meprobamate directly gated these receptors with an efficacy comparable to that of GABA, which is a partial agonist in these receptors (Bianchi and Macdonald, 2003). We also observed that GABA’s efficacy was about 2/3 that of the full agonist THIP in δ subunit-expressing receptors. Barbiturates (Feng et al., 2004), neurosteroids (Wohlfarth et al., 2002) and the parent drug carisoprodol (Kumar et al., 2015) all have the ability to enhance the actions of a saturating concentration of GABA in extrasynaptic receptors. We observed a similar effect of meprobamate in the present investigation. Whereas a portion of the peak GABA current enhancement could be attributable to some direct action of meprobamate, the majority of the enhancement may relate to shifting of GABA from a low efficacy to high efficacy ligand (Bianchi and Macdonald, 2003).
In considering where on the receptor meprobamate acts, key questions include: 1) is the site(s) where meprobamate acts the same as that for carisoprodol?; and 2) does meprobamate acts at distinct sites for gating and allosteric effects? When initiating these studies, we hypothesized that the two ligands act at the same site for both effects. As noted, experimental evidence has shown that both carisoprodol and meprobamate allosterically modulate, directly gate and inhibit the GABAA receptor. Both ligands also display similar actions at δ-expressing receptors. Specifically with regard to question 1 above, we consider here both direct and allosteric effects. Results for direct gating studies demonstrate the subunit-dependent profile for the two ligands are quite similar, and the main difference between the two ligands is potency. These data are consistent with a single overlapping site of action. The isopropyl group of carisoprodol may enhance hydrophobic interactions that result in its enhanced potency. In considering allosteric modulatory effects of the two ligands, the subunit-dependent profile showed significantly more variability than that observed for direct gating effects. The influence of α β and γ subunits was different for meprobamate compared to carisoprodol. Although a definitive conclusion is not warranted, the data are consistent with only modestly overlapping or possibly distinct sites for allosteric effects of meprobamate, compared to carisoprodol. Further studies are required to more definitively address this issue.
In considering whether the direct and allosteric effects of meprobamate are mediated via one or two sites, the possibility of two sites is likely. With regard to carisoprodol, separate sites for direct gating and allosteric modulatory effects have been proposed (Kumar and Dillon, 2015). As with carisoprodol, the subunit-dependent profiles for direct and allosteric effects of meprobamate are distinct. Additional evidence suggestive of distinct sites comes from recent mutagenesis studies. We have reported in abstract form (Kumar et al., 2013) the fact that amino acid mutations in transmembrane domain IV of the α subunit impact direct gating of carisoprodol, but have no significant effect on its allosteric actions. Unpublished results of effects of these mutations on the actions of meprobamate suggest a similar effect. Whether the amino acid residues in question form part of the binding site for these ligands or are critical for transduction is not known. Nevertheless, the fact that the mutations had effects only on direct gating is consistent with the conclusion that distinct sites exist for direct and allosteric modulatory effects for both meprobamate and carisoprodol.
Our results provide additional insight regarding the therapeutic and abuse-facilitating effects of carisoprodol. Both carisoprodol and meprobamate display direct gating and allosteric modulatory effects on GABAA receptors across the spectrum of α expressing receptors, which supports physical dependence and abuse potential (Licata and Rowlett, 2008). Both ligands effectively modulate α2-, α3- and α5- expressing receptors, which provides an effective muscle relaxant profile (Crestani et al., 2001; Griebel et al., 2003. Drawing further from an understanding of benzodiazepines, the considerably higher efficacy (and potency) of carisoprodol for allosteric enhancement of α1-expressing receptors would seem to be a key contributor for its abuse and addictive potential (Licata and Rowlett, 2008; Tan et al., 2010). In addition, in a study of impaired and unimpaired drivers, blood concentration of carisoprodol, but not meprobamate, correlated with driver impairment (Bramness et al., 2004). The authors concluded that carisoprodol itself has impairing effects. As sedative effects are strongly associated with activity at α1- expressing receptors, our finding that meprobamate has considerably reduced effects at these receptors, compared to carisoprodol, provides a molecular basis in support of the conclusion of Bramness et al. (2004). Meprobamate itself has important limitations as a therapeutic; hence its classification as a scheduled drug decades ago. However, as meprobamate strongly modulates α5-expressing receptors (consistent with muscle relaxant effects), and has comparatively inefficient ability to modulate α1- expressing receptors (associated with dependence and abuse potential), a re-evaluation of meprobamate for possible modification is warranted.
During the time period both meprobamate and carisoprodol were being widely prescribed, an extensive series of derivatives of them was generated (Ludwig et al., 1969). Many of these displayed enhanced potency for muscle relaxation (as measured via paralyzing effects that leads to loss of righting reflex) compared to both carisoprodol and meprobamate, as well as an enhanced safety margin. It appears none of these molecules made it to market. Whether they failed for safety or other reasons, or whether the decision to abandon this series of compounds was made in light of the subsequent scheduling of meprobamate is not known. Considering the availability of improved assays and our current understanding of molecular pharmacology associated with therapeutic effects, it might prove worthwhile to re-assess a number of these meprobamate-related carbamate molecules for myorelaxant and other indications.
Acknowledgments
This work was supported by the National Institutes of Health National Institute of Drug Abuse [Grant R01 DA022370 to GHD] and by National Institutes of General Medical Sciences Grant U54GM104942. We appreciate the technical assistance of Mr. John Freund and Ms. Cathy Bell-Horner.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams HR, Kerzee T, Morehead CD. Carisoprodol-related death in a child. J. Forensic Sci. 1975;20:200–202. [PubMed] [Google Scholar]
- Amin J. A single hydrophobic residue confers barbiturate sensitivity to gamma-aminobutyric acid type C receptor. Mol. Pharmacol. 1999;55:411–423. [PubMed] [Google Scholar]
- Amin J, Weiss DS. Homomeric rho 1 GABA channels: activation properties and domains. Recep. Chan. 1994;2:227–236. [PubMed] [Google Scholar]
- Berger FM. Symposium on Anxiety and a Decade of Tranquilizer Therapy. The Tranquilizer Decade. J. Neuropsychiatr. 1964;5:403–410. [PubMed] [Google Scholar]
- Berger FM, Kletzkin M, Ludwig BJ, Margolin S. The history, chemistry, and pharmacology of carisoprodol. Ann. N. Y. Acad. Sci. 1960;86:90–107. doi: 10.1111/j.1749-6632.1960.tb42792.x. [DOI] [PubMed] [Google Scholar]
- Bianchi MT, Macdonald RL. Neurosteroids shift partial agonist activation of GABA(A) receptor channels from low- to high-efficacy gating patterns. J. Neurosci. 2003;23:10934–10943. doi: 10.1523/JNEUROSCI.23-34-10934.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boue-Grabot E, Roudbaraki M, Bascles L, Tramu G, Bloch B, Garret M. Expression of GABA receptor rho subunits in rat brain. J. Neurochem. 1998;70:899–907. doi: 10.1046/j.1471-4159.1998.70030899.x. [DOI] [PubMed] [Google Scholar]
- Bramness JG, Furu K, Skurtveit S, Engeland A. Effect of the market withdrawal of carisoprodol on use of other prescribed drugs with abuse potential. Clin. Pharmacol. Ther. 2012;91:438–441. doi: 10.1038/clpt.2011.250. [DOI] [PubMed] [Google Scholar]
- Bramness JG, Skurtveit S, Morland J. Impairment due to intake of carisoprodol. Drug Alcohol Depend. 2004;74:311–318. doi: 10.1016/j.drugalcdep.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Brickley SG, Mody I. Extrasynaptic GABA(A) receptors: their function in the CNS and implications for disease. Neuron. 2012;73:23–34. doi: 10.1016/j.neuron.2011.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown N, Kerby J, Bonnert TP, Whiting PH, Wafford KA. Pharmacological characterization of a novel cell line expressing human alpha(4)beta(3)delta GABA(A) receptors. Br. J. Pharmacol. 2002;136:965–974. doi: 10.1038/sj.bjp.0704795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZW, Manion B, Townsend RR, Reichert DE, Covey DF, Steinbach JH, Sieghart W, Fuchs K, Evers AS. Neurosteroid analog photolabeling of a site in the third transmembrane domain of the beta3 subunit of the GABA(A) receptor. Mol. Pharmacol. 2012;82:408–419. doi: 10.1124/mol.112.078410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiara DC, Jayakar SS, Zhou X, Zhang X, Savechenkov PY, Bruzik KS, Miller KW, Cohen JB. Specificity of intersubunit general anesthetic-binding sites in the transmembrane domain of the human alpha1beta3gamma2 gamma-aminobutyric acid type A (GABAA) receptor. J. Biol. Chem. 2013;288:19343–19357. doi: 10.1074/jbc.M113.479725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crestani F, Keist R, Fritschy JM, Benke D, Vogt K, Prut L, Bluthmann H, Mohler H, Rudolph U. Trace fear conditioning involves hippocampal alpha5 GABA(A) receptors. Proc. Natl. Acad. Sci. U. S. A. 2002;99:8980–8985. doi: 10.1073/pnas.142288699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crestani F, Low K, Keist R, Mandelli M, Mohler H, Rudolph U. Molecular targets for the myorelaxant action of diazepam. Mol. Pharmacol. 2001;59:442–445. doi: 10.1124/mol.59.3.442. [DOI] [PubMed] [Google Scholar]
- Davies PA, Kirkness EF, Hales TG. Modulation by general anaesthetics of rat GABAA receptors comprised of alpha 1 beta 3 and beta 3 subunits expressed in human embryonic kidney 293 cells. Br. J. Pharmacol. 1997;120:899–909. doi: 10.1038/sj.bjp.0700987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas JF, Ludwig BJ, Schlosser A. The metabolic fate of carisoprodol in dog. J. Pharmacol. Exp. Ther. 1962;138:21–27. [Google Scholar]
- Elder NC. Abuse of skeletal muscle relaxants. Am. Fam. Physician. 1991;44:1223–1226. [PubMed] [Google Scholar]
- Enz R, Brandstatter JH, Hartveit E, Wassle H, Bormann J. Expression of GABA receptor rho 1 and rho 2 subunits in the retina and brain of the rat. Eur. J. Neurosci. 1995;7:1495–1501. doi: 10.1111/j.1460-9568.1995.tb01144.x. [DOI] [PubMed] [Google Scholar]
- Ewing JA, Fullilove RE. Addiction to meprobamate. N. Engl. J. Med. 1957;257:76–77. doi: 10.1056/NEJM195707112570207. [DOI] [PubMed] [Google Scholar]
- Feng HJ, Bianchi MT, Macdonald RL. Pentobarbital differentially modulates alpha1beta3delta and alpha1beta3gamma2L GABAA receptor currents. Mol. Pharmacol. 2004;66:988–1003. doi: 10.1124/mol.104.002543. [DOI] [PubMed] [Google Scholar]
- Gonzalez LA, Gatch MB, Forster MJ, Dillon GH. Abuse Potential of Soma: the GABA(A) Receptor as a Target. Mol. Cell. Pharmacol. 2009a;1:180–186. [PMC free article] [PubMed] [Google Scholar]
- Gonzalez LA, Gatch MB, Taylor CM, Bell-Horner CL, Forster MJ, Dillon GH. Carisoprodol-mediated modulation of GABAA receptors: in vitro and in vivo studies. J. Pharmacol. Exp. Ther. 2009b;329:827–837. doi: 10.1124/jpet.109.151142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenblatt DJ, Shader RI. Drug therapy. Benzodiazepines (second of two parts) N. Engl. J. Med. 1974;291:1239–1243. doi: 10.1056/NEJM197412052912308. [DOI] [PubMed] [Google Scholar]
- Griebel G, Perrault G, Simiand J, Cohen C, Granger P, Depoortere H, Francon D, Avenet P, Schoemaker H, Evanno Y, Sevrin M, George P, Scatton B. SL651498, a GABAA receptor agonist with subtype-selective efficacy, as a potential treatment for generalized anxiety disorder and muscle spasms. CNS Drug Rev. 2003;9:3–20. doi: 10.1111/j.1527-3458.2003.tb00241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamouda AK, Stewart DS, Chiara DC, Savechenkov PY, Bruzik KS, Cohen JB. Identifying barbiturate binding sites in a nicotinic acetylcholine receptor with [3H]allyl m-trifluoromethyldiazirine mephobarbital, a photoreactive barbiturate. Mol. Pharmacol. 2014;85:735–746. doi: 10.1124/mol.113.090985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkinson JE, Drewe JA, Kimbrough CL, Chen JS, Hogenkamp DJ, Lan NC, Gee KW, Shen KZ, Whittemore ER, Woodward RM. 3 alpha-Hydroxy-3 beta-trifluoromethyl-5 alpha-pregnan-20-one (Co 2-1970): a partial agonist at the neuroactive steroid site of the gamma-aminobutyric acidA receptor. Mol. Pharmacol. 1996;49:897–906. [PubMed] [Google Scholar]
- Hendley CD, Lynes TE, Berger FM. Effect of 2-methyl, 2-n-propyl-1,3-propanediol dicarbamate (Miltown) on central nervous system. Proc. Soc. Exp. Biol. Med. 1954;87:608–610. doi: 10.3181/00379727-87-21459. [DOI] [PubMed] [Google Scholar]
- Hollister LE, Levy G. Kinetics of Meprobamate Elimination in Humans. Chemotherapy. 1964;9:20–24. doi: 10.1159/000220338. [DOI] [PubMed] [Google Scholar]
- Horne AL, Harkness PC, Hadingham HL, Whiting P, Kemp JA. The influence of the gamma2L subunit of the modulation of responses to GABAA receptor activation. Br. J. Pharmacol. 1993;108:711–716. doi: 10.1111/j.1476-5381.1993.tb12866.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosie AM, Wilkins ME, da Silva HMA, Smart TG. Endogenous steroids regulate GABAA receptors through two distinct transmembrane sites. Nature. 2006;444:486–489. doi: 10.1038/nature05324. [DOI] [PubMed] [Google Scholar]
- Kumar M, Freund J, Dillon GH. GABAA receptor subunit domains that confer carisoprodol sensitivity. Soc. Neurosci. Abst. 2013 319.37/D27. [Google Scholar]
- Kumar M, Gonzalez LA, Dillon GH. Assessment of subunit-dependent direct gating and allosteric modulatory effects of carisoprodol at GABAA receptors. Neuropharmacology. 2015;97:414–425. doi: 10.1016/j.neuropharm.2015.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licata SC, Rowlett JK. Abuse and dependence liability of benzodiazepine-type drugs: GABA(A) receptor modulation and beyond. Pharmacol. Biochem. Behav. 2008;90:74–89. doi: 10.1016/j.pbb.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littrell RA, Hayes LR, Stillner V. Carisoprodol (Soma): a new and cautious perspective on an old agent. South Med. J. 1993;86:753–756. doi: 10.1097/00007611-199307000-00006. [DOI] [PubMed] [Google Scholar]
- Ludwig BJ, Powell LS, Berger FM. Carbamate derivatives related to meprobamate. J. Med. Chem. 1969;12:462–474. doi: 10.1021/jm00303a029. [DOI] [PubMed] [Google Scholar]
- Low K, Crestani F, Keist R, Benke D, Brunig I, Benson JA, Fritschy JM, Rulicke T, Bluethmann H, Mohler H, Rudolph U. Molecular and neuronal substrate for the selective attenuation of anxiety. Science. 2000;290:131–134. doi: 10.1126/science.290.5489.131. [DOI] [PubMed] [Google Scholar]
- Maddock RK, Jr, Bloomer HA. Meprobamate overdosage. Evaluation of its severity and methods of treatment. J. A. M. A. 1967;201:999–1003. doi: 10.1001/jama.201.13.999. [DOI] [PubMed] [Google Scholar]
- Mistry DK, Cottrell GA. Actions of steroids and bemegride on the GABAA receptor of mouse spinal neurones in culture. Exp. Physiol. 1990;75:199–209. doi: 10.1113/expphysiol.1990.sp003394. [DOI] [PubMed] [Google Scholar]
- Olsen H, Koppang E, Alvan G, Morland J. Carisoprodol elimination in humans. Ther. Drug Monit. 1994;16:337–340. doi: 10.1097/00007691-199408000-00001. [DOI] [PubMed] [Google Scholar]
- Olsen RW, Sieghart W. International Union of Pharmacology. LXX. Subtypes of gamma aminobutyric acid(A) receptors: classification on the basis of subunit composition, pharmacology, and function. Update. Pharmacol. Rev. 2008;60:243–260. doi: 10.1124/pr.108.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchett DB, Sontheimer H, Shivers BD, Ymer S, Kettenmann H, Schofield PR, Seeburg PH. Importance of a novel GABAA receptor subunit for benzodiazepine pharmacology. Nature. 1989;338:582–585. doi: 10.1038/338582a0. [DOI] [PubMed] [Google Scholar]
- Reeves RR, Parker JD. Somatic dysfunction during carisoprodol cessation: evidence for a carisoprodol withdrawal syndrome. J. Am. Osteopath. Assoc. 2003;103:75–80. [PubMed] [Google Scholar]
- Rho JM, Donevan SD, Rogawski MA. Barbiturate-like actions of the propanediol dicarbamates felbamate and meprobamate. J. Pharmacol. Exp. Ther. 1997;280:1383–1391. [PubMed] [Google Scholar]
- Rudolph U, Mohler H. GABAA receptor subtypes: Therapeutic potential in Down syndrome, affective disorders, schizophrenia, and autism. Annu. Rev. Pharmacol. Toxicol. 2014;54:483–507. doi: 10.1146/annurev-pharmtox-011613-135947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rust GS, Hatch R, Gums JG. Carisoprodol as a drug of abuse. Arch Fam Med. 1993;2:429–432. doi: 10.1001/archfami.2.4.429. [DOI] [PubMed] [Google Scholar]
- Schechter MD. Specific antagonism of the behavioral effects of chlordiazepoxide and pentobarbital in the rat. Prog Neuropsychopharmacol. Biol. Psych. 1984;8:359–364. [PubMed] [Google Scholar]
- Sullivan MD. What are we treating with opioid and sedative-hypnotic combination therapy? Pharmacoepidemiol. Drug Saf. 2015;24:893–895. doi: 10.1002/pds.3821. [DOI] [PubMed] [Google Scholar]
- Tan KR, Brown M, Labouebe G, Yvon C, Creton C, Fritschy JM, Rudolph U, Luscher C. Neural bases for addictive properties of benzodiazepines. Nature. 2010;463:769–774. doi: 10.1038/nature08758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth PP, Urtis J. Commonly used muscle relaxant therapies for acute low back pain: a review of carisoprodol, cyclobenzaprine hydrochloride, and metaxalone. Clin. Ther. 2004;26:1355–1367. doi: 10.1016/j.clinthera.2004.09.008. [DOI] [PubMed] [Google Scholar]
- van Rijnsoever C, Tauber M, Choulli MK, Keist R, Rudolph U, Mohler H, Fritschy JM, Crestani F. Requirement of alpha5-GABAA receptors for the development of tolerance to the sedative action of diazepam in mice. J. Neurosci. 2004;24:6785–6790. doi: 10.1523/JNEUROSCI.1067-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagoner KR, Czajkowski C. Stoichiometry of expressed alpha(4)beta(2)delta gamma aminobutyric acid type A receptors depends on the ratio of subunit cDNA transfected. J. Biol. Chem. 2010;285:14187–14194. doi: 10.1074/jbc.M110.104257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witenko C, Moorman-Li R, Motycka C, Duane K, Hincapie-Castillo J, Leonard P, Valaer C. Considerations for the appropriate use of skeletal muscle relaxants for the management of acute low back pain. P. T. 2014;39:427–435. [PMC free article] [PubMed] [Google Scholar]
- Wohlfarth KM, Bianchi MT, Macdonald RL. Enhanced neurosteroid potentiation of ternary GABA(A) receptors containing the delta subunit. J. Neurosci. 2002;22:1541–1549. doi: 10.1523/JNEUROSCI.22-05-01541.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacny JP, Paice JA, Coalson DW. Characterizing the subjective and psychomotor effects of carisoprodol in healthy volunteers. Pharmacol. Biochem. Behav. 2011;100:138–143. doi: 10.1016/j.pbb.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacny JP, Paice JA, Coalson DW. Subjective and psychomotor effects of carisoprodol in combination with oxycodone in healthy volunteers. Drug. Alcohol Depend. 2012;120:229–232. doi: 10.1016/j.drugalcdep.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]