Abstract
Objective
The role of genetic factors in the aetiology of tinnitus is not yet settled. Objective of the present study was to estimate the heritability of tinnitus.
Design
Self report questionnaire data from The Nord-Trøndelag hearing loss study (an integrated part of the HUNT study) was used. This is a health screening in Nord-Trøndelag county, Norway, including all inhabitants aged 18 and older (N=51,574) with overall response rate 62.8%. The study also included information on first-degree family relationships. Age corrected polychoric correlations between relatives’ tinnitus status were calculated. A structural equation model was fit to the data, and the relative contributions of genes and unique environmental effects were estimated. Models including sex-specific effects were also tested.
Subjects
Frequency and intensity of tinnitus including information from a population based sample of 12,940 spouses, 27,607 parent-offspring and 11,498 siblings, was used. 27,792 respondents were tested twice yielding a test – retest correlation of 0.65 for the report of tinnitus.
Results
Correlations for parent – offspring ranged form 0.01–0.07 for the various sex combinations, sibling correlation ranged from 0.06–0.14 and the spouse correlation was 0.04. This family correlation pattern implies an upper limit for heritability of 0.11 with no sex differences in the heritability estimates.
Conclusions
This is the first large, population based family study to report on the heritability of tinnitus. In contrast to previous speculations in the literature this low heritability indicates that additive genetic effects explain only a small proportion of the variance of tinnitus in the population.
Keywords: heritability, family study, genetic epidemiology, tinnitus, HUNT
Introduction
Tinnitus, or the perception of sound without an external acoustic stimulus, is a common but poorly understood symptom. Although the list of factors associated with tinnitus is long, the causes of tinnitus onset and maintenance are far from fully understood, and attempts to develop evidence-based therapies have been thwarted by a poor understanding of the pathophysiology 1.
The close relation to hearing impairment 2, 3 has suggested that tinnitus is caused by cochlear damage, but observation of tinnitus in persons where the auditory nerve has been severed imply that tinnitus can occur without involvement of peripheral auditory system. The neurophysiological model of tinnitus 4 postulates that other systems in the brain, in addition to the auditory system, have to be involved in tinnitus. Buzzing or ringing in the ear itself is not the only source of tinnitus related complaints; individuals who find tinnitus troublesome evaluate and perceive it as a threat or annoyance, rather than as a sound of little or no consequence 5.
A significant familial aggregation of tinnitus has recently been reported 6, and based on same sex siblings from the present data set a significant familial association in tinnitus risk that could not be attributed to known risk factors for tinnitus was found 7. Except from a report based on a small cohort of elderly (>70 years old) Danish twins 8 reporting a significant heritability of tinnitus for females, little is known about the relative importance of genetic effects in tinnitus liability.
Heritability needs to be estimated through quantitative genetic studies, such as twin and family studies 9. Candidate gene studies of tinnitus may also be warranted 10, but only if quantitative studies can demonstrate a substantial heritability for tinnitus. The aim of the present study is to estimate the relative contribution of genetic effects on the liability to tinnitus in a large population based sample of nuclear families. The correlation structure between relatives is observed and, based on these correlations, the heritability is estimated.
Materials and methods
Sample
From August 1995 to June 1997, the adult populations of the 24 municipalities of Nord-Trøndelag County, Norway, were invited to take part in a health screening survey, the Nord-Trøndelag Health Study (HUNT 2). The survey included as an integrated project the Nord-Trøndelag Hearing Loss Study (NTHLS) 11, and the populations of 17 of the 24 municipalities in the county were invited to participate in the hearing loss study. The invitation list was based on population files stored and continuously updated by the Statistics Norway. Age ranged from 20 to 101 years (mean, 50; SD, 17). In one municipality, Levanger, the subjects were re-invited to participate in the hearing examination after the ordinary HUNT 2 was finished. The participation rate for all municipalities together (except Levanger) was 66.7%; for Levanger the overall participation rate was 41.1%. Altogether 51,574 people, including 5,114 from Levanger, attended the hearing examination and signed an informed consent. Information on first-degree relationships was obtained from registries administered by the governmental Statistics Norway, identifying mother-offspring pairs with absolute certainty but with a slight chance that the father registered at birth is not the biological father. The number of pairings is listed in Table 1. One person may be included in more than one pairing, for instance a woman being mother in one family, and a sister in another.
Table 1.
Age corrected polychoric correlations for tinnitus between members of nuclear families
| Family relation | Polychoric correlation (95% CI) | Number of observations* |
|---|---|---|
| Mother – son | 0.052 (0.010, 0.094) | 7970 |
| Mother – daughter | 0.068 (0.023, 0.112) | 7855 |
| Father – son | 0.060 (0.013, 0.106) | 5907 |
| Father – daughter | 0.012 (−0.038, 0.061) | 5875 |
| Sisters | 0.074 (−0.007, 0.148) | 2739 |
| Brothers | 0.141 (0.077, 0.207) | 3137 |
| Different-sex siblings | 0.062 (0.010, 0.144) | 5622 |
| Spouses | 0.044 (0.011, 0.075) | 12940 |
The same person can be included in more than one family relation.
Questionnaire
A one-page questionnaire (Q1) was distributed to all participants and completed immediately before the hearing examination took place. A second questionnaire (Q2) was distributed, usually a few months after the hearing examination, to cases with a certain degree of hearing loss (n = 16,186) and to a control group (n = 17,785). Altogether 28,066 persons (71.8%) returned Q2. Q1 included questions about bothersome tinnitus (response categories: yes, no, don’t know/may be), tinnitus frequency (response categories: monthly, weekly, daily and almost always) and typical duration of tinnitus attacks (response categories: a few minutes, 10 minutes - 1 hour and longer than 1 hour). Q2 included a slightly differently phrased question about the degree to which the respondent is bothered by tinnitus (response categories: not bothered, a little bothered and strongly bothered). In the present study data from Q1 were used in the estimation of the heritability while both Q1 and Q2 were used in the estimation of the test-retest correlation.
Records with missing values on all three items in Q1 were treated as not bothered by tinnitus. In cases with any information indicating the presence of tinnitus, that is, endorsing any of the three items, missing values for ‘frequency’ and ‘duration’ were imputed, using these two items as predictors for each other together with age and sex. Missing Values Analysis, option EM, in the SPSS statistical software (SPSS Inc., Chicago, IL) was chosen as a tool for imputation. Responses on all items were z-scaled and summed to create an index. When including information from all three items, 79.1% of the respondents reported no signs of tinnitus. The remaining 20.9% reporting tinnitus symptoms were split in four groups each containing approximately 5% yielding an index with five response categories. This index was used as input in the heritability analyses.
Model and estimation
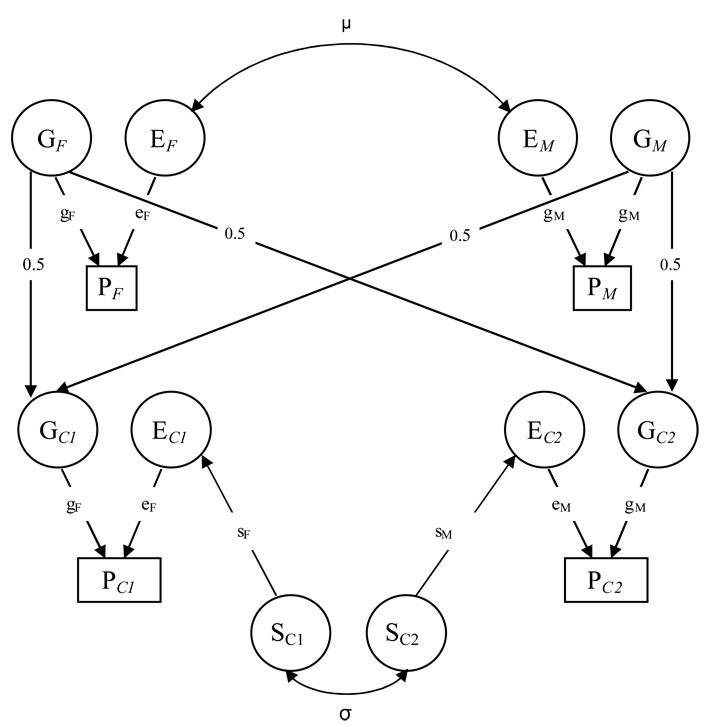
Structural equation modelling is well established and widely used for the analysis of family data 9 to disentangle the relative contribution from genetic and environmental effects on complex traits. Information about genetic relation (siblings, parent-child) combined with information on family membership without genetic relation (spouses) can be used to quantify the relative importance of genetic and environmental effects in disease liability. Sex specific genetic effect is indicated by a lower correlation between different sexed relatives (i.e. mother-son and brother-sister) than between same sexed relatives. We observe statistical association between the phenotypes in relatives with different degree of relatedness in the population. The correlations between various kinds of relatives are fit to a structural equation model based on the rules of path analysis. A path diagram of the model is illustrated in Figure 1.
Figure 1.
Model including genetic and environmental effects, sibling effects, and social homogamy on tinnitus for a nuclear family including parents with two children. Capital letters G, E in circles denote the latent variables for genetic and environmental effects, respectively. S denotes environmental factors common to siblings. P denotes phenotype (tinnitus). Parameters (small letters): g = genetic effect, e = environmental effect, s = environmental effects shared by siblings (sibling effect). Subscript F=female, M=male, C1=child number 1 (female in this diagram), C2=child number 2 (male in this diagram). σ = correlation between male and female sibling environmental effect, µ = correlation between spouses’ environment (social homogamy).
In line with convention, the observed phenotype (tinnitus) in each relative is drawn as rectangles, and latent variables are drawn as circles. Genetic effects influencing tinnitus are transmitted from the latent paternal (GF) and maternal (GM) genotypes to the latent genotypes of their children (Gc1 and Gc2). The phenotype is also influenced by environmental effects in the parents (EM and EF) and children (EC1 and EC2). Environmental sibling effects (SC1 and SC2) are shared fully by same-sex and partly by opposite-sexed siblings.
Effect of environmental transmission from parents to offspring and genetic effect (heritability) can not be separated using data from nuclear families only. The heritability estimates presented in the following text are the upper limit of the heritability estimates and may also include environmental parent-offspring transmission.
Assortative mating is a tendency for individuals who mate to be similar for the trait under study, generating a correlation between spouses. A significant spouse correlation may be classified as phenotypic homogamy where spouses choose each other based on the trait under study or as social homogamy where spouse similarity results from phenotypic similarity within social groups, where mating tends to take place. These two forms of assortative mating are not mutually exclusive, but both cannot be specified in the same model. We chose a model with social homogamy in the analyses of tinnitus implying that a correlation is specified between the environments of the spouses.
Age corrected correlations for tinnitus between various sets of relatives were calculated, and the model shown in Figure 1 was fit to these correlations by weighted least squares using the statistical package R 12. First we fit a full model including as many parameters as possible given the number of statistics. Then the full model was reduced in a stepwise manner comparing the fit of the more constrained sub-models to the full model. The difference in fit between two nested models is approximately chi-squared distributed with degrees of freedom equal to the number of parameters dropped. The goodness-of-fit for the different models were evaluated according to Akaike´s Information Criterion (AIC=χ2−2df) 13, addressing both likelihood and simplicity of models and allowing non-nested models to be compared. The function to be minimized during estimation was the squared difference between the Fisher transformation of the observed and expected correlations, multiplied by 1/(the variance of the observed correlation). Traditional tests of significance assume independence across observations. This is violated in our data set, as a given individual can be part of many pair wise observations (for example, all permutations of siblings in a large family). The weighted least squares method gives estimates which are usually very close to the maximum likelihood estimates in kinship studies, but the significance levels will be slightly overestimated, implying a small risk of falsely rejecting a true model. Confidence intervals were constructed by means of bootstrap sampling.
Results
Correlations and prevalence
Test – retest polychoric correlation for 28,066 persons tested twice was 0.65 (95% CI 0.63 – 0.66) indicating relatively high reliability for our tinnitus measure. The prevalence of tinnitus was 15.1% using only respondents with a positive report in the one item on bothersome tinnitus (“Are you bothered by ringing in your ears?”) as cases. The index used for the heritability estimation includes information from records with missing data or ‘may be’ report of bothersome tinnitus that also had valid data on either tinnitus duration or frequency. Using this index, 20.9% of the sample reported symptoms of tinnitus. Polychoric correlations for tinnitus (Table 1) are small yet all, except for the father-daughter and sister correlations, are statistically significant.
Model fitting
We used the age corrected correlations as input in the structural equation model. The fit of each model was evaluated by use of the goodness-of-fit index AIC; goodness-of-fit is expressed by low values. The results of the model fitting procedure, including model parameter estimates and model fit statistics, are listed in Table 2 starting with the least constrained model, subsequently adding constraints and comparing more parsimonious models to the full model. The parameter estimates correspond to the model illustrated in Figure 1.
Table 2.
Parameter estimates for the full model and reduced sub models fit to family polychoric correlations for tinnitus
| Model | Constraints | sm | sf | gm | gf | σ | µ | Df | Δχ2 | p | AIC |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Full | 0.33 | 0.15 | 0.30 | 0.33 | 0.30 | 0.05 | 0 | |||
| 2 | gm=gf | 0.32 | 0.17 | 0.32 | 0.32 | 0.27 | 0.05 | 1 | 0.09 | 0.76 | −1.91 |
| 3 | sm=sf | 0.27 | 0.27 | 0.34 | 0.29 | 0.21 | 0.05 | 1 | 1.62 | 0.20 | −0.38 |
| 4 | sm=sf, σ=1 | 0.21 | 0.21 | 0.35 | 0.28 | 1 | 0.05 | 2 | 3.50 | 0.17 | −0.50 |
| 5 | Drop sf and σ | 0.33 | 0 | 0.30 | 0.35 | 0 | 0.05 | 2 | 0.35 | 0.84 | −3.65 |
| 6 | Drop s from full | 0 | 0 | 0.39 | 0.31 | 0 | 0.05 | 3 | 6.86 | 0.08 | 0.86 |
| 7 | gm=gf, sm=sf | 0.27 | 0.27 | 0.32 | 0.32 | 0.19 | 0.05 | 2 | 1.84 | 0.40 | −2.17 |
| 8 | gm=gf, sm=sf, σ=1 | 0.21 | 0.21 | 0.32 | 0.32 | 1 | 0.05 | 3 | 3.98 | 0.26 | −2.02 |
| 9 | gm=gf, drop σ and sf | 0.32 | 0 | 0.33 | 0.33 | 0 | 0.05 | 3 | 0.60 | 0.90 | −5.40 |
| 10 | Drop g fom full | 0.38 | 0.27 | 0 | 0 | 0.61 | 0.04 | 2 | 18.66 | <0.01 | 14.66 |
Small letter g denotes the genetic effects. Small letter s denotes the effect of environmental factors common to same sex siblings. Subscript f=female, m=male. σ = correlation between male and female sibling effect, µ = correlation between spouses’ environment (social homogamy). Df= degrees of freedom, Δχ2 = difference in χ2 value compared to the full model, AIC= Akaike’s information criterion.
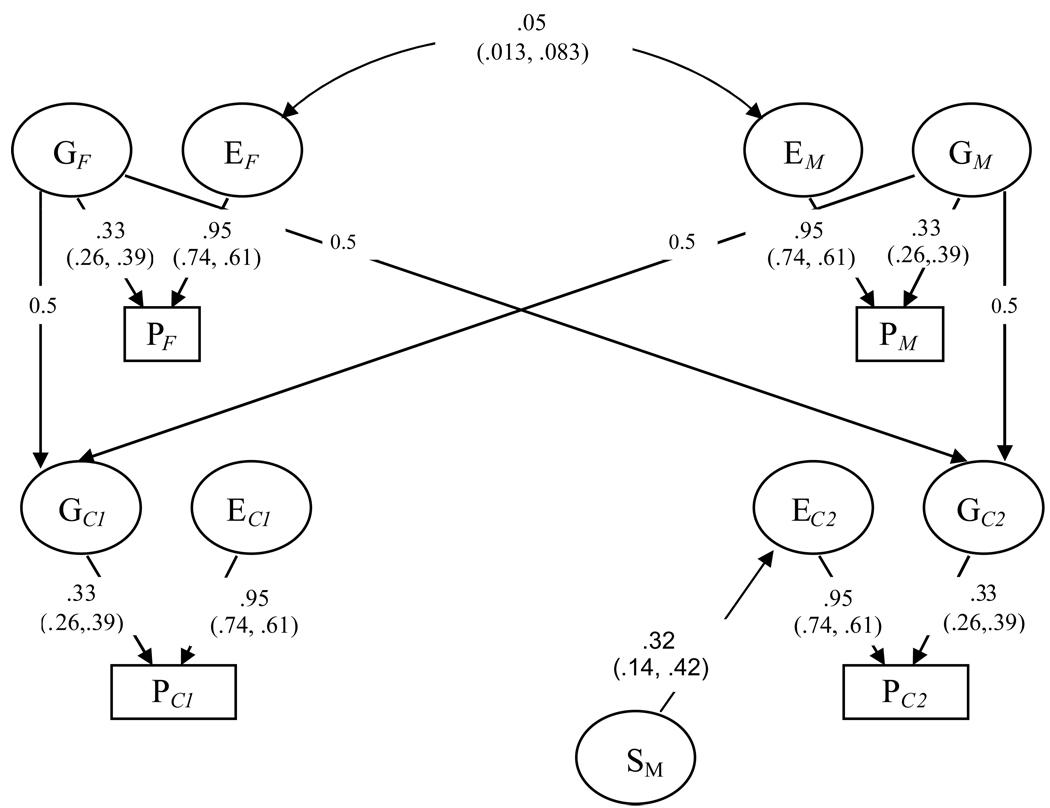
Model 1 is the full model including genetic and environmental factors influencing tinnitus in parents and children and a common environmental sibling effect in children. In model 2, the genetic effects are constrained to be equal across sexes with a minimal loss in fit. The sibling effect could not be constrained to be equal across sexes without significant loss of fit (model 3, 4, 7 and 8), but the sibling effect for females could be dropped from the model without any substantial loss of fit (model 5 and 9). The high AIC value for model 10 illustrates that the genetic effect is small but significant. The best fitting model (model 9) including equal genetic effects for males and females, unique environmental effects and sibling effect for males is illustrated in Figure 2.
Figure 2.
Path diagram illustrating the best fitting model for tinnitus including equal genetic and environmental effects in males and females, sibling effects in males and social homogamy on tinnitus for a nuclear family including parents with two children. Capital letters G, E in circles denote the latent variables for genetic and environmental effects, respectively. S denotes latent environmental factors common to same sex siblings, P denotes phenotype (tinnitus). Subscript F=female, M=male, C1=child number 1 (female in this diagram), C2=child number 2 (male in this diagram). 95 % confidence interval for the effects are given in parenthesis below each estimate.
Heritability (g2) of 0.11 was found in males and females. Environmental effects shared by siblings were only found in males.
Discussion
This study addressed the importance of genetic factors in tinnitus using a large, population based cohort of Norwegian nuclear families. We found a heritability of 0.11, meaning that the relative importance of genetic factors in tinnitus is low.
When interpreting these findings, the following limitations should be taken into consideration. To date the vast majority of traits studied with quantitative population genetic methods have shown moderate to high heritability. High heritability has been taken as evidence for high validity, or at least high reliability. Reversing this reasoning, low heritability could raise suspicion of low measurement precision. However, our questions about tinnitus are straightforward and appear to have face validity; also the test-retest reliability is satisfactory. Our prevalence is comparable with similar studies from other countries 3, 7, 14, 15. Even if our measure should only be moderately valid and, say, capture only half the population variance in tinnitus, the true heritability would only be the double of our estimate (0.22 for males and females), which is still quite low
On the other hand, tinnitus is a symptom described in a heterogeneous group of diseases and thus the heritability could differ substantially depending on the biological nature of the underlying disease. The data available do not allow separation into different clinical subgroups of tinnitus, and our phenotype under study is also undoubtedly heterogeneous. Therefore our results should be understood as average values across different types of tinnitus rather than valid for all types of tinnitus. But if our measure represents both highly heritable and non-heritable forms of tinnitus one would expect our heritability estimate to be at least moderate. The very low heritability found in the present study does not suggest that any prevalent type of tinnitus is highly heritable.
Data from nuclear families do not allow separating effect of environmental transmission from parents to offspring and genetic effect (heritability). The estimates presented are the upper limit of the heritability estimates and may be confounded by environmental parent-offspring transmission. If there is an effect of family environment, our heritability estimate is somewhat inflated.
Many subjects in the data set are included in more than one family relation (e.g., a subject could be a sister in one family and a mother in another) introducing dependency between the observations. Somewhat inflated chi-squared values in the testing of nested models have not affected our results, however, since all parameters except genetic effect and sibling environmental effects shared by brothers could be fixed at zero. These two effects were significant beyond doubt, with chi-square differences compared to nested models 18.66, d.f=2, p<0.0001 for g and 6.51, d.f=1, p=0.011 for eM.
The small but significant spouse correlation of 0.044 for tinnitus was modelled as social homogamy; mates assort because they belong to the same social groupings or strata where the members are exposed to the same environmental risk factors (e.g. noise exposure). This specification may not be entirely realistic, but the specification of this low partner correlation hardly matters for the parameter estimates.
We found a small but significant environmental sibling effect only present in males. This is consistent with results for noise induced hearing loss in the same sample. Hearing impairment due to occupational noise and noise from recreational sources, including from gunfire or shooting, could be demonstrated in males but not in females 11. This male exposure is likely to aggregate in families to some extent and may well have contributed somewhat to tinnitus as well as to hearing loss.
Our heritability estimate is lower than the heritability of 0.39 (95% CI 0.03–0.75) for females in a previous report from Peterson et al. 8. No significant genetic effect for males was found in this study. The result is based a small sample of 478 twin pairs aged 70 to 100 years with the majority of tinnitus cases aged between 70 and 80 years old. The effect of age was not taken into account in the analysis. The correlations reported for males are very low indicating a “negative” heritability. However, if pooling the correlations across sex, which would seem reasonable in such a small sample, the correlation pattern indicates a much lower heritability estimate which is in good agreement with the results from the present study.
In conclusion, we find a comparatively low the heritability for tinnitus and a sibling environmental effect only present in males. This result needs to be replicated, using other measures of tinnitus and other types of family data. Our results do not necessarily mean that genetic effects are unimportant for all forms of tinnitus as this symptom can arise from a wide variety of underlying diseases. Considering the heterogeneous aetiology of tinnitus, rather than searching for the genes responsible for tinnitus in general, efforts to identify subgroups of individuals affected by tinnitus with specific aetiologies should be made. Our results do not support the spending of large amounts of time and resources to identify the genes coding for tinnitus in general.
Acknowledgements
The study was funded by the National Institute on Deafness and Other Communication Disorders (NIDCD), National Institutes of Health, Bethesda, Maryland, USA, research contract No. N01-DC-6-2104. The Nord Trøndelag Health Study (HUNT), of which the Hearing Loss Study is a part, was conducted in collaboration with the National Institute of Public Health, Oslo, The National Health Screening, Oslo, Nord-Trøndelag County Council, and the Norwegian University of Technology and Science, Trondheim. The Nord-Trøndelag County Health Officer and the Community Health Officer in Levanger and in other municipalities provided organisational and other practical support. We also thank the NTHLS team for their diligence.
Footnotes
Preliminary results were presented at the 30th Congress of Nordic Association of Otolaryngology, June 13th, 2008.
References
- 1.Lockwood AH, Salvi RJ, Burkard RF. Tinnitus. New England Journal of Medicine. 2002 Sep 19;347(12):904–910. doi: 10.1056/NEJMra013395. [DOI] [PubMed] [Google Scholar]
- 2.Konig O, Schaette R, Kempter R, Gross M. Course of hearing loss and occurrence of tinnitus. Hearing Research. 2006;221:59–64. doi: 10.1016/j.heares.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 3.Sindhusake D, Golding M, Newall P, Rubin G, Jakobsen K, Mitchell P. Risk factors for tinnitus in a population of older adults: the blue mountains hearing study. Ear & Hearing. 2003 Dec 1;24:501–507. doi: 10.1097/01.AUD.0000100204.08771.3D. [DOI] [PubMed] [Google Scholar]
- 4.Jastreboff PJ. Phantom auditory perception (tinnitus): mechanisms of generation and perception. Neuroscience Research. 1990 Aug;8(4):221–254. doi: 10.1016/0168-0102(90)90031-9. [DOI] [PubMed] [Google Scholar]
- 5.Jastreboff PJ, Jastreboff MM. Tinnitus retraining therapy: a different view on tinnitus. Orl; Journal of Oto-Rhino-Laryngology and its Related Specialties. 2006;68:23–30. doi: 10.1159/000090487. [DOI] [PubMed] [Google Scholar]
- 6.Hendrickx JJ, Huyghe JR, Demeester K, et al. Familial aggregation of tinnitus: a European multicentre study. B-ENT. 2007;3(Suppl 7):51–60. [PubMed] [Google Scholar]
- 7.Hoffman HJ, Reed GW. Chapter 3. Epidemiology of tinnitus. In: Snow JB Jr, editor. Tinnitus: theory and management. Hamilton [Ont.]: BC Decker Inc; 2004. pp. 16–41. [Google Scholar]
- 8.Petersen HC, Andersen T, Frederiksen H, Hoffman HJ, Christensen K. The heritability of tinnitus - a twin study (Poster) Aarhus (Denmark): Nordic Epidemiology Congress. 2002 [Google Scholar]
- 9.Neale MC, Cardon LR. Methodology for genetic studies of twins and families. 1ed ed. Boston: 1992. [Google Scholar]
- 10.Sand PG, Langguth B, Kleinjung T, Eichhammer P. Genetics of chronic tinnitus. Progress in Brain Research. 2007;166:159–168. doi: 10.1016/S0079-6123(07)66014-2. [DOI] [PubMed] [Google Scholar]
- 11.Tambs K, Hoffman HJ, Borchgrevink HM, Holmen J, Samuelsen SO. Hearing loss induced by noise, ear infections, and head injuries: results from the Nord-Trondelag Hearing Loss Study. International Journal of Audiology. 2003 Mar;42(2):89–105. doi: 10.3109/14992020309078340. [DOI] [PubMed] [Google Scholar]
- 12.R Development Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; 2005. [Google Scholar]
- 13.Akaike H. Factor-Analysis and Aic. Psychometrika. 1987;52(3):317–332. [Google Scholar]
- 14.Johansson MS, Arlinger SD. Prevalence of hearing impairment in a population in Sweden. International Journal of Audiology. 2003 Jan;42(1):18–28. doi: 10.3109/14992020309056081. [DOI] [PubMed] [Google Scholar]
- 15.Demeester K, van Wieringen A, Hendrickx JJ, et al. Prevalence of tinnitus and audiometric shape. B-ENT. 2007;3(Suppl 7):37–49. [PubMed] [Google Scholar]