Abstract
The incidence of well-differentiated thyroid cancer has seen a worldwide increase in the last three decades. Whether this is due to a ‘true increase’ in incidence or simply increased detection of otherwise subclinical disease remains unclear. The treatment of thyroid cancer revolves around appropriate surgical intervention, minimising complications and the use of adjuvant therapy in select circumstances. Prognostic features and risk stratification are crucial in determining the appropriate treatment. There continues to be considerable debate in several aspects of management in these patients. Level 1 evidence is lacking, and there are limited prospective data to direct therapy, hence limiting decision-making to retrospective analyses, treatment guidelines based on expert opinion and personal philosophies. This overview focuses on the major issues associated with the investigation of thyroid nodules and the extent of surgery. As overall survival in well-differentiated thyroid cancer exceeds 95%, it is important to reduce over-treating the large majority of patients, and focus limited resources on high-risk patients who require aggressive treatment and closer attention. The onus is on the physician to avoid treatment-related complications from thyroid surgery and to offer the most efficient and cost-effective therapeutic option.
Keywords: Differentiated thyroid cancer, medullary thyroid cancer, thyroid cancer
Statement of Search Strategies Used and Sources of Information
Searches were made on Medline and Scopus using thyroid nodule, differentiated thyroid cancer, papillary thyroid cancer and follicular thyroid cancer.
Introduction
There has been a dramatic increase in the incidence of well-differentiated thyroid cancer worldwide over the last three decades [1,2]. In the USA alone, epidemiological data have shown a 2.4-fold increase between 1973 and 2002, and well over 50% of these tumours are below 2 cm [3]. As mortality secondary to thyroid cancer remains unchanged during this time period, it has been speculated that the vast majority of thyroid cancers diagnosed have limited clinical significance. Most thyroid cancer deaths occur secondary to anaplastic, poorly differentiated or medullary thyroid cancers. However, a subset of patients with well-differentiated thyroid cancer are known to have a poorer outcome, and the goal for the clinician is to distinguish this cohort of patients from the vast majority of patients with excellent outcomes. As such, current protocols focus on early diagnosis and risk stratification, treatment individualised to risk groups and follow-up strategies based on prognostic factors.
Well-differentiated thyroid cancers represent more than 90% of thyroid cancers, and comprise two major histological types: papillary and follicular. Despite a number of important clinicopathological differences, these two subtypes are similar in their overall favourable prognosis and the ability to be stratified into low-, intermediate- and high-risk groups based on a number of well-established scoring systems. More than 80% of these cancers have an excellent prognosis, with a 20 year cause-specific mortality rate of <1% [4]. The remaining 20% of patients run a more variable course, and often present with a spectrum of disease, including multiple recurrences, subclinical or clinical metastases, and even death; the mortality rates for patients in this high-risk group range from 50 to 80% at 10 years.
The management of well-differentiated thyroid cancer has always been controversial, mainly because of the prolonged natural history of the disease in most patients. The consequent lack of good level 1 or 2 evidence from prospective trials means that most recommendations and guidelines are based on large, retrospective analyses and expert consensus opinion. Hence, there is a need to accumulate and analyse carefully documented and detailed retrospective analyses from different centres, before circumstantial associations can be made [5,6]. A systematic approach is required to resolve these issues and physicians managing these cancers need to keep abreast of the current developments through well-presented reviews and expert guidelines that are being updated regularly by the National Comprehensive Cancer Network, American Thyroid Association (ATA), British Thyroid Association and European Thyroid Association.
Evaluation of the Thyroid Nodule
The initial presentation for a large majority of well-differentiated thyroid cancer is an asymptomatic thyroid nodule, either noticed fortuitously by the patient or diagnosed incidentally by palpation or imaging. When planning how to investigate these lesions, it is important to note that thyroid nodules are extremely common in the general population: autopsy series suggest that up to 50% of the population have thyroid nodules, of which 5–10% harbour a well-differentiated malignancy. Therefore, the aim of the clinician is to identify patients at risk for well-differentiated thyroid cancer and manage these patients accordingly, while not over-investigating the vast majority of benign lesions. Even when a well-differentiated thyroid cancer is diagnosed, current techniques are unable to differentiate clinically relevant cancers from microcarcinomas that have no bearing on patient outcome. The ATA recently published a comprehensive set of guidelines in the management of patients with thyroid nodules, and differentiated thyroid cancer [7]. The suggested algorithm in investigating these patients is shown in Fig. 1.
Fig. 1.
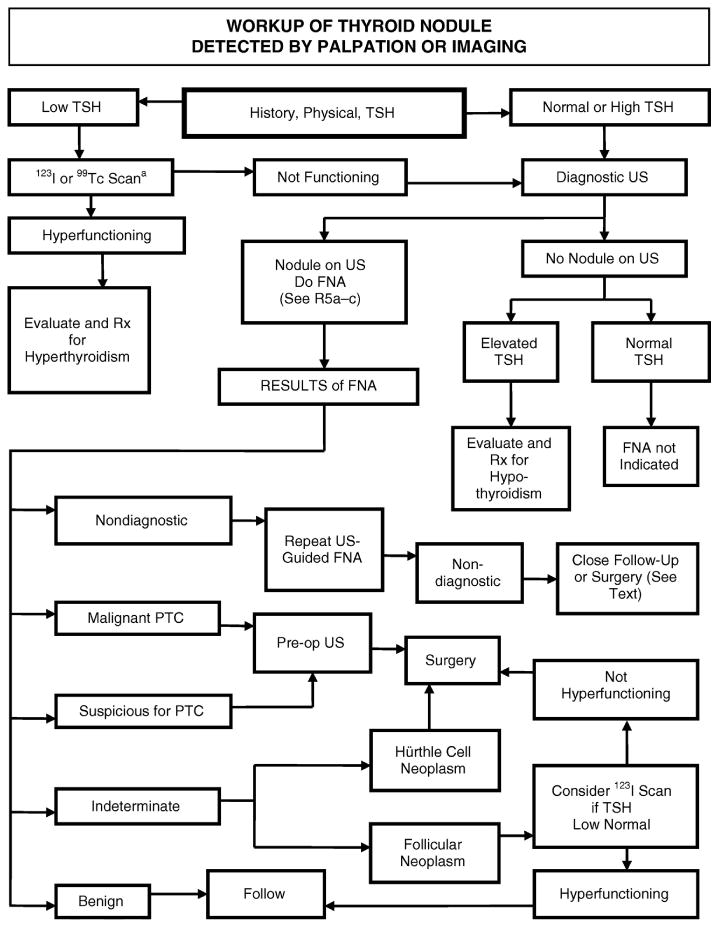
American Thyroid Association suggested algorithm for the management of thyroid nodules (reproduced from [7] with permission from the American Thyroid Association).
Investigating Thyroid Nodules
Although patients occasionally present with symptoms attributable to the thyroid nodule (Table 1), most patients present with asymptomatic nodules, often discovered incidentally through clinical examination or other imaging modalities (ultrasound, carotid duplex scan, computed tomography, magnetic resonance imaging or positron emission tomography [PET]). The major debate with these lesions is when to investigate, the extent of work-up required and which patients require surgical intervention [8–10].
Table 1. Symptoms and signs associated with a thyroid nodule.
| Pain |
| Secondary to bleeding within the thyroid nodule |
|
|
| Compression |
| Dysphagia — oesophageal compression |
| Stridor — tracheal/laryngeal compression |
| Facial oedema — venous congestion |
|
|
| Invasion |
| Hoarseness — invasion of recurrent laryngeal nerve |
|
|
| Toxicity |
| Palpitations |
| Heat intolerance |
| Weight loss |
| Anxiety |
| Tremors |
All patients should have thyroid function tests (at least a [AQ1] TSH level) and undergo a dedicated thyroid ultrasound to evaluate the nodule and remaining thyroid gland. If the serum [AQ1]TSH is below normal, a radionuclide scan should be carried out. The decision to proceed with fine needle aspiration (FNA) biopsy is contingent on a number of clinical factors and imaging findings, as described in Table 2. For incidentalomas, nodule size alone is no longer recommended as a threshold for biopsy, as studies have shown that malignancy rates are equivalent in lesions below and above 1 cm. Instead, currently available guidelines proposed by the Society of Radiologists in Ultrasound suggest that all incidentally discovered nodules with sonographic worrisome features (calcification, irregularlity, solid lesion, hypervascularity) should undergo ultrasound-guided needle biopsy [11,12]. The main suspicious features described are punctate calcification, irregular halo and hypervascularity. The recent ATA guidelines have made detailed recommendations correlating the various sonographic features, and with the need for a FNA biopsy; these are probably the most succinct guidelines to date (Table 3)[7].
Table 2. Criteria for fine needle aspiration biopsy.
| Clinical features | ||
|---|---|---|
| History | Previous irradiation | |
| Age < 20 or > 60 years | ||
| Male | ||
| History of cancer | ||
| Family history of thyroid cancer | ||
| Rapid growth | ||
|
| ||
| Examination | Hard nodule | |
| Single nodule | ||
| Large size >4 cm | ||
| Cervical lymphadenopathy | ||
| Vocal cord palsy | ||
|
| ||
| Imaging | ||
|
| ||
| Ultrasound | Solid nodule | |
| Calcification — micro, macro | ||
| Hypoechogenicity | ||
| Irregular margins, irregular halo | ||
| Central vascular flow | ||
Table 3. American Thyroid Association guidelines for fine needle aspiration recommendations with regards to clinical and sonographic features (adapted from [7]).
| Nodule sonographic or clinical features | Recommended nodule threshold size for fine needle aspiration |
|---|---|
| High-risk history: | |
| Thyroid cancer in first-degree relatives | |
| External beam radiation as a child | |
| Exposure to ionising radiation in childhood or adolescence | |
| Previous thyroid cancer | |
| FDG avidity on positron emission tomography scan | |
|
| |
| Nodule WITH suspicious sonographic features: | >5 mm |
| Microcalcifications, hypoechoic, increased nodular vascularity, infiltrative margins, taller than wide on transverse view | |
|
| |
| Nodule WITHOUT suspicious sonographic features | >5 mm |
|
| |
| Abnormal cervical lymph nodes | All |
|
| |
| Microcalcifications in nodule | ≥1 cm |
|
| |
| Solid nodule | |
|
| |
| AND hypoechoic | >1 cm |
|
| |
| AND iso- or hyperechoic | ≥1–1.5 cm |
|
| |
| Mixed cystic-solid nodule | |
|
| |
| WITH any suspicious ultrasound features | ≥1.5–2 cm |
|
| |
| WITHOUT suspicious ultrasound features | ≥2 cm |
|
| |
| Spongiform nodule | ≥2 cm |
|
| |
| Purely cystic nodule | Fine needle aspiration not indicated |
If the biopsy is reported as suspicious or malignant, the patient will require appropriate thyroidectomy. Otherwise, patients may be followed on clinical grounds with a repeat ultrasound in 6–18 months and if no further progression this can be repeated every 3–5 years.
The presence of a multinodular goiter does not preclude the co-existence of thyroid malignancy. Several studies have indicated that the rate of malignancy is similar in solitary nodules in a normal thyroid gland compared with multinodular goiters [13]. However, in deciding which nodule to biopsy, the clinician should be guided by the ultrasound findings: nodules with suspicious ultrasound features should take precedence over larger but sonographically bland nodules [14,15].
Imaging Thyroid Nodules
Ultrasound has become the gold standard for evaluating thyroid nodules and nodal disease in the lateral neck. As stated before, ultrasound features suggestive of malignancy include an irregular halo, punctate calcification, intra-tumoral hypervascularity and a solid nodule [11,16]. Other worrisome features include hypoechogenicity and nodules that are taller than wide on transverse view. Despite a sensitivity of more than 60–70%, the positive predictive value for each of these features remains less than 15%, hence the need for further evaluation in the appropriate clinical context. The presence of contralateral thyroid nodules is an important sonographic finding. If the patient requires surgery, a total thyroidectomy is usually recommended for bilateral thyroid nodules (even if the contralateral nodules seems to be benign) [17]. Preoperative ultrasound has limited value in evaluating the central compartment nodes. However, ultrasound scans are very useful in evaluating the lateral neck for nodal metastases. Abnormal nodes based on size or sonographic criteria should be evaluated with cross-sectional imaging and needle biopsy. FNA of the lateral compartment nodes may be evaluated by cytology, or the needle washings sent for a thyroglobulin assay. There is a limited role for radionuclide thyroid scans in evaluating nodules in the absence of hyperthyroidism. Decisions regarding surgery are rarely made based on whether a nodule is ‘hot’ or ‘cold’. Cross-sectional imaging, such as computed tomography and magnetic resonance imaging, should be used in patients with compressive or invasive symptoms, especially when a diagnosis of thyroid cancer has already been made. Computed tomography is more accurate in defining the extent of disease, especially when locally invasive [18]. The practice in most centres is to carry out non-contrast computed tomography, although in some circumstances the benefits of using iodinated contrast to delineate the relationship between the tumour and adjacent major structures may outweigh the negative effect of contrast on subsequent radio-iodine therapy.
Fine Needle Aspiration in Investigating Thyroid Nodules
FNA biopsy is the most sensitive and specific preoperative indicator for thyroid malignancies. More than 60% of FNA biopsies show benign disease, averting the need for surgical intervention [14,16,18]. In this case, the accuracy of a benign FNA report is over 90% and increases to 98% if the same result is seen on repeat biopsy. These patients can be followed up with serial ultrasound examinations 6–18 months after the initial FNA. Repeat FNA is only reserved for increasing nodule size (>50% increase by nodule volume or >20% increase in at least two nodule dimensions). Otherwise, subsequent examinations can be delayed to 3–5 yearly intervals [7].
A further 5–10% of biopsies show overt malignancy, necessitating surgery. Interpretation of the remaining 30% of biopsies is more complicated, as most of these are read as suspicious for follicular or Hurthle cell lesions. The diagnosis of a follicular cell lesion or neoplasm continues to be the subject of controversy and leads to difficulty in making appropriate treatment recommendations. Biological markers and immunohistochemistry have no definitive role in routine diagnostic purposes for the moment, although several molecular markers have been suggested to be useful in diagnosing thyroid cancer, including telomerase, beta-galectin and B-Raf [19]. There is an ongoing study to test the accuracy of identifying B-Raf mutations in FNA specimens in predicting for thyroid malignancy. Ultimately, surgical decisions should be made based on clinical and ultrasound features, patient anxiety and the comfort level of the treating physician. Both the patient and the physician should be aware that only 15–20% of these lesions are eventually confirmed to be malignant — either follicular thyroid cancer or follicular variant of papillary thyroid cancer. It is also important that the interpretation of the FNA biopsy be correlated with the clinical context in question, given that FNA has a false-negative rate of 5–10%. If there is a strong clinical suspicion of malignancy, the patient would either require repeat biopsies or surgical intervention.
Positron Emission Tomography Incidentalomas
PET incidentalomas comprise a unique category of lesions that is seen more frequently in the oncology clinics. These are usually diagnosed in patients undergoing PET scans for a previously diagnosed malignancy or screening evaluation for abnormal tumour markers [20]. Patients with focal PET uptake should be aggressively evaluated with an ultrasound and ultrasound-guided biopsy in all cases, as there is a higher incidence of malignancy associated with these lesions (30–50%). More importantly, these FDG-avid lesions tend to have a more aggressive histological subtype (tall cell, insular cell etc) [21,22]. The appropriate management of these lesions requires a balance between the thyroid malignancy and the diagnosis and natural history of the primary pathology for which the PET was carried out.
Surgery for Well-differentiated Thyroid Cancer
Surgery is the mainstay of treatment for well-differentiated thyroid cancer. Indications for surgery include patients where FNA shows overt malignancy as well as atypical follicular lesions, with the latter patients having a 15–20% chance of harbouring a follicular or follicular-variant papillary thyroid cancer. In patients where there is a strong clinical suspicion for malignancy, and who are FNA negative, surgery should be considered to confirm the diagnosis. The aims of surgery are: to eradicate primary and nodal disease, to minimise treatment-related morbidity, to permit accurate staging of the disease, to facilitate radio-iodine therapy, to permit accurate long-term surveillance with [AQ2]radioactive iodine whole body scan and thyroglobulin assay and to minimise the risk of disease recurrence and metastatic spread [7]. The major controversies in the surgical management of thyroid cancer relate to the extent of thyroidectomy and nodal clearance, and these have to be considered in the context of the above goals of thyroid cancer surgery as stated.
Risk Stratification and Extent of Thyroidectomy for Well-differentiated Thyroid Cancer
There has been considerable debate as to the extent of thyroidectomy for well-differentiated thyroid cancer, with regards to the role of total versus less than total thyroidectomy (usually lobectomy and isthmuscectomy) for well-differentiated thyroid cancers [6,17,23]. Proponents of total thyroidectomies argue that this is a safe operation in expert hands, with excellent outcome, limited morbidity, and it removes undiagnosed microscopic multifocal disease in the contralateral lobe. Furthermore, these patients can then undergo radioactive iodine therapy and use thyroglobulin levels as a marker for recurrent disease. Retrospective data extracted from large studies also suggest that there is a statistically significant difference in outcome between total thyroidectomy and lobectomy for tumours >1 cm, although the magnitude of difference is more obvious in tumours >3 cm [6,24]. On the basis of these arguments, many have advocated the routine use of total thyroidectomy in patients with a preoperative diagnosis of well-differentiated thyroid cancer. On the other hand, there are ample data to suggest that up to 80% of patients with well-differentiated thyroid cancer can be cured of disease by thyroid lobectomy alone [23–25]. The latter obviates the need for life-long thyroid replacement, eliminates any possibility of developing permanent hypoparathyroidism and does not risk the contralateral recurrent laryngeal nerve. Furthermore, these patients do not require radio-iodine ablation anyway, or the use of a non-specific tumour marker, such as thyroglobulin.
There have been a range of different risk stratification models proposed, that in principle use similar factors to predict low- and high-risk patients (Table 4) [17]. At Memorial Sloan-Kettering Cancer Center, we use a simplified stratification, dividing patients into low-, intermediate- and high-risk categories (Table 5) to guide our surgical decisions and adjuvant treatment [26]. Patients in the high-risk group or those with an aggressive histological subtype undergo total thyroidectomy followed by adjuvant radioactive iodine ablation. Patients not in the high-risk group but likely to require radioactive iodine would also undergo total thyroidectomy (e.g. patients <45 years old with nodal or distant metastasis). Other indications for total thyroidectomy depend on critical intra-operative evaluation showing the following findings: presence of contralateral thyroid nodules, extrathyroidal extension or central compartment nodal disease. As stated previously, patients in the low-risk group have similar long-term outcome whether they undergo lobectomy or total thyroidectomy, and can hence be adequately treated with a lobectomy and isthmuscetomy, which is the minimal operation required (and in most instances is the maximum operation necessary). Subtotal thyroidectomy should be abandoned as an option for patients with thyroid cancer. Ultimately, the extent of thyroidectomy in these patients depends on the philosophy of the treating surgeon, endrocrinologist and patient preference. Patient preference often has an overriding role to play in these decisions, given the vast amount of information available extolling the virtues of radioactive iodine. Nonetheless, patients in the low-risk group derive no benefit from [AQ2]radioactive iodine and generally do not require [AQ2]radioactive iodine ablation.
Table 4. Prognostic scoring systems used in papillary thyroid cancer. Metastasis refers to distant metastasis and extension/invasion refers to extrathyroidal extension.
| MSKCC | Mayo Clinic | Lahey Clinic | Karolinska | |
|---|---|---|---|---|
| GAMES | AGES | MACIS | AMES | DAMES |
| Grade | Age | Metastasis | Age | DNA |
| Age | Grade | Age | Metastasis | Age |
| Metastasis | Completeness of resection | Metastasis | ||
| Extension | Extension | Invasion | Extension | Extension |
| Size | Size | Size | Size | Size |
Table 5. Risk-group definition in well-differentiated thyroid cancer as per Memorial Sloan-Kettering Cancer Center [26].
| Low risk | Intermediate risk | High risk | ||
|---|---|---|---|---|
| Age (years) | <45 | <45 | >45 | >45 |
| Distant metastasis | M0 | M1 | M0 | M1 |
| Tumour size | <4 cm | >4 cm | <4 cm | >4 cm |
| Histology and grade | Papillary | Follicular and high-grade papillary | Papillary | Follicular and high-grade papillary |
|
| ||||
| 5 year survival (%) | 100 | 96 | 96 | 72 |
| 20 year survival (%) | 99 | 85 | 85 | 57 |
Locally Invasive Thyroid Cancer
Extrathyroidal extension is an important prognostic factor in well-differentiated thyroid cancer. In patients with locally aggressive thyroid cancer, surgical resection of all gross tumour results in the best outcome. Hence, it is incumbent on the thyroid surgeon to determine the extent of local invasion before and during surgery, and to make the appropriate intra-operative decision on the extent of surgical resection [27]. It is important to remember that the best opportunity for cure is during the first surgical procedure. The main principles of thyroid surgery for locally invasive thyroid cancer are listed in Table 6.
Table 6. Principles of surgery for locally invasive thyroid cancer.
| All gross tumour should be removed |
| Preserve functioning structures |
| Preserve vital structures |
| Balance between tumour control and best functioning results |
| Use adjuvant treatment – radioactive iodine, external beam radiotherapy |
Local invasion can be anterior, invading the strap muscles, or posterior, invading the recurrent laryngeal nerves, trachea or oesophagus. Excision of the sternothyroid and sternohyoid muscles is not technically demanding, but requires the surgeon to recognise this possibility. This is especially important in tumours arising from the isthmus, which is only 4 mm in thickness, and hence tend to invade the strap muscles early. Failure to appreciate this possibility may result in inadequate tumour clearance or violating the tumour during surgical exposure. Posterior extension can be more difficult to manage. Recurrent laryngeal nerve invasion with preoperative vocal cord palsy can be managed with resection of the involved nerve, and careful preservation of the contralateral nerve (with or without nerve monitoring). However, in young patients with no preoperative vocal cord paralysis, every effort should be made to dissect the nerve from the tumour. Invasion of the trachea may require tracheal sleeve resection with primary anastomosis. Up to five tracheal rings may be removed, with primary repair after suprahyoid release procedures [28]. Shave procedures should be limited to very superficial invasion, as studies have shown high local recurrence rates secondary to incomplete tumour clearance. Invasion of the cricoid and larynx may require more aggressive approaches, such as cricotracheal resection and reconstruction or vertical hemi-laryngectomies. As there is a higher local recurrence rate, most patients with extensive extrathyroidal extension require adjuvant radioactive iodine and external beam radiotherapy.
Nodal Metastases
Central Compartment Clearance
One of the major debates in intra-operative management of well-differentiated thyroid cancer revolves around the role of prophylactic or elective central compartment clearance [29–31]. Nodal metastases occur in 40–60% of papillary thyroid cancer. However, microscopic disease has a minimal effect on overall survival, although local recurrence rates may be higher. In contrast, bulky nodal disease in high-risk patients affects survival and locoregional recurrence rates. Hence, there is a need to critically evaluate the central compartment intra-operatively in every patient with papillary thyroid cancer. If suspicious or palpable nodal disease is identified, the surgeon should carry out a formal central compartment clearance, taking care not to injure the recurrent laryngeal nerve, and either preserve the inferior parathyroid on its pedicle or auto-transplant in the contralateral sternocleidomastoid muscle.
However, the role of elective central compartment clearance in the absence of overt nodal metastases is unknown. Proponents for elective central compartment clearance argue that it is a safe procedure in expert hands, as meta-analyses show only a slight increase in transient hypoparathyroidism rates, and avoids the need for future manipulation of the tracheoesophageal groove for recurrent disease [32,33]. Opponents argue that the presented statistics are from expert centres and do not represent ‘real world’ complication rates, which may be much higher. Furthermore, there is probably minimal clinical effect from the small volume or microscopic metastatic disease. There are no prospective trials planned to resolve this issue. Hence, surgeon and institutional philosophies will prevail for the moment. The most recent ATA guidelines recommend that prophylactic central compartment clearance should only be carried out in patients with advanced (T3 and T4) primary tumours, but not for smaller (T1 and T2 tumours) in node-negative necks and for follicular thyroid cancer [7].
Lateral Compartment Clearance
Certainly there are no data supporting the routine use of prophylactic lateral neck dissection. If there is clinically apparent disease, patients should undergo a formal modified radical neck dissection, usually sparing the accessory nerve, jugular vein and sternocleidomastoid muscle [33,34]. ‘Berry picking’ of enlarged nodes should be condemned, as this inevitably results in geographical misses and a high nodal recurrence rate. Dissecting the level I region may be spared to avoid injury to the mandibular branch of the facial nerve, as metastasis rates are less than 3% to the submandibular area. Isolated metastases to nodes at levels IIB and VA are also uncommon, and these levels can be spared from extensive dissection, avoiding injury to the accessory nerve [35]. Some surgeons now advocate using ‘compartment-oriented’ dissection and limiting dissections to involved nodal levels only. Although this is conceptually appealing and technically easier, there is a fine line between ‘compartment-oriented’ dissection and ‘berry picking’.
In patients with extensive local disease or multiple positive paratracheal nodes, due diligence should be given to intra-operative assessment of the lower jugular nodal chain, even if presurgical imaging has failed to show nodal disease. If overt nodal disease is identified, selective nodal dissection should be carried out as indicated.
Conclusions
The management of well-differentiated thyroid cancer continues to be a source of debate, predominantly because it is a long-standing disease with good outcome in most patients. A lack of prospective data means that practice guidelines are based on large retrospective series and expert opinion. A concerted effort needs to be made to risk stratify and offer treatment according to risk groups and prognostic factors. Over-treating low-risk patients offers no benefit to the patient and exposes the patient to unnecessary complications. The treatment of well-differentiated thyroid cancer requires a multidisciplinary approach by thyroid surgeons, endocrinologists, oncologists, radiologists, pathologists and nuclear medicine physicians. Treatment philosophies should follow well-established guidelines offered by the National Comprehensive Cancer Network, ATA, British Thyroid Association and other expert organisations, rather than personal philosophies. It is therefore incumbent on the treating physician to keep abreast of existing and future treatment options and revisions in these guidelines. In many cases, the most difficult course of action is watchful waiting, hence doing no harm.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.dos Santos Silva I, Swerdlow AJ. Thyroid cancer epidemiology in England and Wales: time trends and geographical distribution. Br J Cancer. 1993;67:330–340. doi: 10.1038/bjc.1993.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pettersson B, Adami HO, Wilander E, Coleman MP. Trends in thyroid cancer incidence in Sweden, 1958–1981, by histopathologic type. Int J Cancer. 1991;48:28–33. doi: 10.1002/ijc.2910480106. [DOI] [PubMed] [Google Scholar]
- 3.Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973–2002. Jama. 2006;295:2164–2167. doi: 10.1001/jama.295.18.2164. [DOI] [PubMed] [Google Scholar]
- 4.Randolph GW, Thompson GB, Branovan DI, Tuttle RM. Treatment of thyroid cancer: 2007 — a basic review. Int J Radiat Oncol Biol Phys. 2007;69:S92–S97. doi: 10.1016/j.ijrobp.2007.06.027. [DOI] [PubMed] [Google Scholar]
- 5.Hundahl SA, Fleming ID, Fremgen AM, Menck HR. A National Cancer Data Base report on 53,856 cases of thyroid carcinoma treated in the U.S. 1985–1995 [see comments] Cancer. 1998;83:2638–2648. doi: 10.1002/(sici)1097-0142(19981215)83:12<2638::aid-cncr31>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 6.Bilimoria KY, Bentrem DJ, Ko CY, et al. Extent of surgery affects survival for papillary thyroid cancer. Ann Surg. 2007;246:375–381. doi: 10.1097/SLA.0b013e31814697d9. discussion 381–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooper DS, Doherty GM, Haugen BR, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–1214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 8.Atli M, Akgul M, Saryal M, Daglar G, Yasti AC, Kama NA. Thyroid incidentalomas: prediction of malignancy and management. Int Surg. 2006;91:237–244. [PubMed] [Google Scholar]
- 9.Gough J, Scott-Coombes D, Fausto Palazzo F. Thyroid incidentaloma: an evidence-based assessment of management strategy. World J Surg. 2008;32:1264–1268. doi: 10.1007/s00268-008-9503-2. [DOI] [PubMed] [Google Scholar]
- 10.Howlett DC, Speirs A. The thyroid incidentaloma — ignore or investigate? J Ultrasound Med. 2007;26:1367–1371. doi: 10.7863/jum.2007.26.10.1367. [DOI] [PubMed] [Google Scholar]
- 11.Frates MC, Benson CB, Charboneau JW, et al. Management of thyroid nodules detected at US: Society of Radiologists in Ultrasound consensus conference statement. Ultrasound Q. 2006;22:231–238. doi: 10.1097/01.ruq.0000226877.19937.a1. discussion 239–240. [DOI] [PubMed] [Google Scholar]
- 12.Kim EK, Park CS, Chung WY, et al. New sonographic criteria for recommending fine-needle aspiration biopsy of nonpalpable solid nodules of the thyroid. AJR Am J Roentgenol. 2002;178:687–691. doi: 10.2214/ajr.178.3.1780687. [DOI] [PubMed] [Google Scholar]
- 13.Deandrea M, Mormile A, Veglio M, et al. Fine-needle aspiration biopsy of the thyroid: comparison between thyroid palpation and ultrasonography. Endocr Pract. 2002;8:282–286. doi: 10.4158/EP.8.4.282. [DOI] [PubMed] [Google Scholar]
- 14.Leenhardt L, Hejblum G, Franc B, et al. Indications and limits of ultrasound-guided cytology in the management of nonpalpable thyroid nodules. J Clin Endocrinol Metab. 1999;84:24–28. doi: 10.1210/jcem.84.1.5418. [DOI] [PubMed] [Google Scholar]
- 15.Wienke JR, Chong WK, Fielding JR, Zou KH, Mittelstaedt CA. Sonographic features of benign thyroid nodules: interobserver reliability and overlap with malignancy. J Ultrasound Med. 2003;22:1027–1031. doi: 10.7863/jum.2003.22.10.1027. [DOI] [PubMed] [Google Scholar]
- 16.Papini E, Guglielmi R, Bianchini A, et al. Risk of malignancy in nonpalpable thyroid nodules: predictive value of ultrasound and color-Doppler features. J Clin Endocrinol Metab. 2002;87:1941–1946. doi: 10.1210/jcem.87.5.8504. [DOI] [PubMed] [Google Scholar]
- 17.Shaha AR. Controversies in the management of thyroid nodule. Laryngoscope. 2000;110:183–193. doi: 10.1097/00005537-200002010-00001. [DOI] [PubMed] [Google Scholar]
- 18.Shaha AR. Advances in the management of thyroid cancer. Int J Surg. 2005;3:213–220. doi: 10.1016/j.ijsu.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 19.Nikiforova MN, Nikiforov YE. Molecular diagnostics and predictors in thyroid cancer. Thyroid. 2009 doi: 10.1089/thy.2009.0240. [DOI] [PubMed] [Google Scholar]
- 20.Katz SC, Shaha A. PET-associated incidental neoplasms of the thyroid. J Am Coll Surg. 2008;207:259–264. doi: 10.1016/j.jamcollsurg.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 21.Robbins RJ, Wan Q, Grewal RK, et al. Real-time prognosis for metastatic thyroid carcinoma based on 2-[18F]fluoro-2-deoxy-D-glucose-positron emission tomography scanning. J Clin Endocrinol Metab. 2006;91:498–505. doi: 10.1210/jc.2005-1534. [DOI] [PubMed] [Google Scholar]
- 22.Are C, Hsu JF, Ghossein RA, Schoder H, Shah JP, Shaha AR. Histological aggressiveness of fluorodeoxyglucose positron-emission tomogram (FDG-PET)-detected incidental thyroid carcinomas. Ann Surg Oncol. 2007;14:3210–3215. doi: 10.1245/s10434-007-9531-4. [DOI] [PubMed] [Google Scholar]
- 23.Shah JP, Loree TR, Dharker D, Strong EW. Lobectomy versus total thyroidectomy for differentiated carcinoma of the thyroid: a matched-pair analysis. Am J Surg. 1993;166:331–335. doi: 10.1016/s0002-9610(05)80326-5. [DOI] [PubMed] [Google Scholar]
- 24.Shah JP. Re: Extent of surgery affects papillary thyroid cancer. Ann Surg. 2008;247:1082–1083. doi: 10.1097/SLA.0b013e3181758d93. author reply 1083–1084. [DOI] [PubMed] [Google Scholar]
- 25.Shaha A. Treatment of thyroid cancer based on risk groups. J Surg Oncol. 2006;94:683–691. doi: 10.1002/jso.20697. [DOI] [PubMed] [Google Scholar]
- 26.Shah JP, Loree TR, Dharker D, Strong EW, Begg C, Vlamis V. Prognostic factors in differentiated carcinoma of the thyroid gland. Am J Surg. 1992;164:658–661. doi: 10.1016/s0002-9610(05)80729-9. [DOI] [PubMed] [Google Scholar]
- 27.Shaha A. Selective surgical management of well-differentiated thyroid cancer. Ann NY Acad Sci. 2008;1138:58–64. doi: 10.1196/annals.1414.010. [DOI] [PubMed] [Google Scholar]
- 28.Grillo HC, Suen HC, Mathisen DJ, Wain JC. Resectional management of thyroid carcinoma invading the airway. Ann Thorac Surg. 1992;54:3–9. doi: 10.1016/0003-4975(92)91131-r. discussion 9–10. [DOI] [PubMed] [Google Scholar]
- 29.Clayman GL, Shellenberger TD, Ginsberg LE, et al. Approach and safety of comprehensive central compartment dissection in patients with recurrent papillary thyroid carcinoma. Head Neck. 2009;31:1152–1163. doi: 10.1002/hed.21079. [DOI] [PubMed] [Google Scholar]
- 30.Mazzaferri EL, Doherty GM, Steward DL. The pros and cons of prophylactic central compartment lymph node dissection for papillary thyroid carcinoma. Thyroid. 2009;19:683–689. doi: 10.1089/thy.2009.1578. [DOI] [PubMed] [Google Scholar]
- 31.Sugitani I, Fujimoto Y, Yamada K, Yamamoto N. Prospective outcomes of selective lymph node dissection for papillary thyroid carcinoma based on preoperative ultrasonography. World J Surg. 2008;32:2494–2502. doi: 10.1007/s00268-008-9711-9. [DOI] [PubMed] [Google Scholar]
- 32.Chisholm EJ, Kulinskaya E, Tolley NS. Systematic review and meta-analysis of the adverse effects of thyroidectomy combined with central neck dissection as compared with thyroidectomy alone. Laryngoscope. 2009;119:1135–1139. doi: 10.1002/lary.20236. [DOI] [PubMed] [Google Scholar]
- 33.Grubbs EG, Evans DB. Role of lymph node dissection in primary surgery for thyroid cancer. J Natl Compr Canc Netw. 2007;5:623–630. doi: 10.6004/jnccn.2007.0053. [DOI] [PubMed] [Google Scholar]
- 34.Patel KN, Shaha AR. Locally advanced thyroid cancer. Curr Opin Otolaryngol Head Neck Surg. 2005;13:112–116. doi: 10.1097/01.moo.0000156161.82671.43. [DOI] [PubMed] [Google Scholar]
- 35.Shaha AR. Management of the neck in thyroid cancer. Otolaryngol Clin North Am. 1998;31:823–831. doi: 10.1016/s0030-6665(05)70090-6. [DOI] [PubMed] [Google Scholar]


