Abstract
Eight examples of biosynthetic pathways wherein a natural enzyme has been identified and claimed to function as a catalyst for the [4+2] cycloaddition reaction, namely, Diels-Alderases, are briefly reviewed. These are discussed in the context of the mechanistic challenges associated with the technical difficulty of proving that the net formal [4+2] cycloaddition under study, indeed proceeds through a synchronous, mechanism and that the putative biosynthetic enzyme deploys the pericyclic transition state required for a Diels-Alder cycloaddition reaction.
1. Introduction
Among the family of concerted pericyclic reactions governed and understood by the theoretical underpinnings of the conservation of orbital symmetry rules advanced by Woodward and Hoffmann, the [4 + 2] cycloaddition reaction, namely the Diels-Alder reaction, is among the most useful and widely studied reactions in synthetic organic chemistry. A vast array of publications concerning the synthetic utility, mechanism, catalysis and theoretical bases of the Diels-Alder reaction are evident in the literature. An area of particular controversy concerns the fundamental question regarding the existence of biosynthetic enzymes that have evolved to catalyze this tremendously important synthetic construction. Our laboratory previously published a review of biosynthetic Diels-Alder constructions in 2003 covering primarily secondary metabolites that had been suggested by researchers to arise by potential biosynthetic Diels-Alder reactions, both enzyme-catalyzed and non-enzyme-catalyzed.[1] Oikawa has since contributed in highlighting developments in this area by publishing a review in 2005.[2] Kelly provided a more detailed overview of potential Diels-Alderases in 2008, and Liu et al. has provided insight as to the challenges workers face in securing mechanistically, a Diels-Alderase in Nature.[3] Herein, we survey and provide a contextual perspective of recent publications claiming to have identified biosynthetic genes coding for the expression of biosynthetic enzymes that catalyze the Diels-Alder reaction in the formation of primary or secondary metabolites.
Frontier molecular orbital (FMO) theory and molecular dynamics studies have been used to describe and understand the Diels-Alder reaction.[4-6] The highest occupied molecular orbital (HOMO) of the diene has perfect molecular orbital (MO) overlap with the lowest occupied molecular orbital (LUMO) of the dienophile, allowing for electron flow to form two new bonds. The neutral [4 + 2] cycloaddition of 1, 3-butadiene with ethylene experiences restricted HOMOdiene-LUMOdienophile interaction.[4] This limitation requires catalytic and/or electronic support in order for a favorable reaction to occur.[5] Most commonly, an increase in the nucleophilicity of the diene or the electrophilicity of the dienophile is found sufficient. Increasing the electron density of the diene raises its’ HOMO and LUMO to a higher energy while decreasing the electron density of the dienophile has the adverse effect.[4] This strengthens the HOMOdiene-LUMOdienophile interaction creating a favorable forward reaction.[6] To further increase reactivity, the use of lewis acids is common.[4, 6] The interaction between the lewis acid and the electron-withdrawing group of the dienophile further stabilizes its HOMO and LUMO by polarization of the alpha, beta double bond in turn making the dienophile more electrophilic and therefore, the diene more nucleophilic.[7]This is well represented in early work by Kojima and Inukai in the reaction of methyl acrylate with isoprene in the presence of Aluminum trichloride (AlCl3).[7] The HOMO and LUMO of the dienophile are lowered and the electron densities of the orbitals are redistributed by the coordination of AlCl33.[6] Experimental evidence represents an increase in reaction rate of the catalytic reaction, by 105, compared to that of the uncatalyzed reaction expressing higher yields, lower reaction temperatures, and shorter reaction times.[7]
Manipulating the electronics of the addends also plays a significant role in increasing regioselectivity. This is exemplified in the aforementioned work by Kojima and Inukai.[7] The AlCl3-catalyzed reaction of isoprene with acrylate results in a 97:3 regioselective ratio of para:meta products due to the electronic reorganization of Lewis-acidic bound acrylate and the electron-donating effect of the alkyl group on the 2-position of diene.[7] The regioselectivity of this reaction was increased from an 80:20 para:meta ratio for the uncatalyzed reaction of isoprene with methylacrylate.[6-8]
The electron-withdrawing group (EWG) on the dienophile, relative to the diene substitutents, aids in the stereochemistry established by the Diels-Alder reaction. While the stereochemical relationships on the addends are preserved, secondary interactions between the EWG and the diene determine the relative stereochemistry in a stereoselective transformation. Early work by Houk and Strozier demonstrates this along with the continued effect of a lewis-acid.[6, 9]. In the [4 + 2] cycloaddition of cyclopentadiene with methyl acrylate, the stereoselective reaction occurs in a 82:18 endo:exo ratio.[6] Studies from the 1970's suggested the explanation for the stereoselective reaction was due to secondary orbital overlap interactions. A later consensus on this subject is discussed later in the manuscript. Enhanced stereoselectivity can be achieved for the lewis-acid catalyzed reaction due to the distribution of electron density within the molecular orbitals of the dienophile. This is exemplified in the work provided by Houk in the reaction of cyclopentadiene and methyl acrylate. The addition of AlCl3 increased the stereoselectivity to a 99:1 ratio of endo:exo.[6]
2. Diels Alder Reaction Mechanism
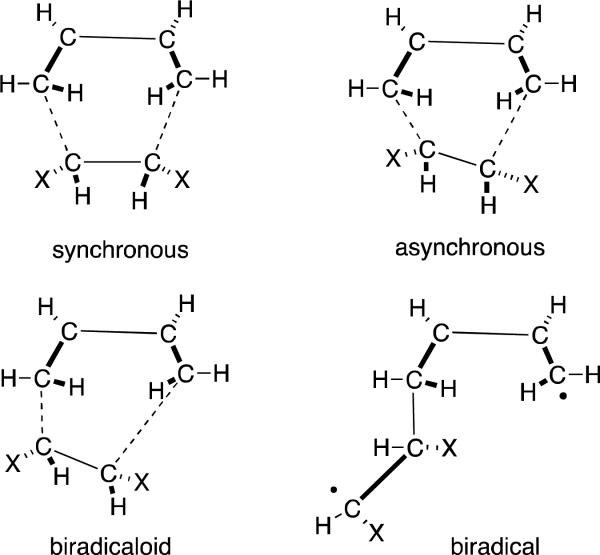
The mechanistic argument on whether the Diels-Alder reaction proceeds via a concerted or stepwise mechanism dates back to studies carried out by Woodward et al. suggesting a synchronous concerted transition state.[10] Early theoretical calculations of kinetic isotope effects and reaction rates were found not to be conclusive,[11] and the mechanism of the [4+2] cycloaddition reaction has been extensively studied experimentally and theoretically by numerous researchers. Figure 1 represents the four transition state (TS) structures through which the net redox-neutral [4+2] cycloaddition may be envisioned to proceed.
Figure 1.
Possible Transition Structures in the Diels-Alder reaction
Based on earlier calculations conducted with lower levels of theory by Dewar et. al.,[11-12] the Diels-Alder reaction has been rationalized to occur through a biradical intermediate suggesting that multi-bond reactions cannot proceed via a synchronous transition state.[13] This theory was subsequently contradicted as Houk et al. showed that geometries are more sensitive to electron correlation than to the basis set as it exceeds 3-21G.[14] Further investigations by Houk et al. based on Becke3LYP/6-31G* calculations suggested the concerted transition state is lower in energy than the biradical transition state in the retro-Diels-Alder reaction of norbornene,[15] while highly substituted dienes were found to favor a stepwise mechanism.[16]
2.1 The [4+2] Cycloaddition Reaction with Symmetrical Addends
The simplest or “parent” Diels-Alder reaction involves 1,3-butadiene with ethylene to form cyclohexene (Figure 2). Early high-level ab initio and RH calculations by Burke et al. and Townshend et al. predicted a symmetrical transition state, with perfect synchronicity of formation of the two new C-C bonds.[17] Using the highest level of theory (STO-3G and 4-31G basis sets at CAS1 and CAS2 level) at the time, ab initio calculations by Bernardi et al. suggested a synchronous transition state for the parent Diels-Alder reaction.[18] While the basis sets for the MC-SCF study was limited, Becke3LP and CASSCF calculations at the higher 6-31G* level of theory and CASSCF calculations at the STO-3G level of theory predict a synchronous transition structure for the reaction of ethylene with butadiene (Figure 2).[14, 18] The calculated transition structure for the reaction of ethylene with butadiene was favored over a stepwise mechanism by 5 kcal/mol in calculations by Houk et al.[14] Secondary kinetic isotope effects interrogated by Gajewski et al., suggested the reaction of nearly symmetrical dienes and dienophiles is nearly synchronous.[19] These ‘nearly synchronous’ addends refer to alkyl substituents, none of which have significant electronic activating or deactivating effects.
Figure 2.
Parent Diels-Alder Reaction. Bond lengths in Angstroms. a) CASSCF/STO-3G calculated transition structures from Bernardi et al. 1985. CAS2 and CAS1 are in parentheses and lower-level 4-31G basis set calculations are in brackets. b) CASSCF/STO-3G calculated transition structures by Houk et al. 1993.
2.2 DA Reaction with Unsymmetrical Addends
The first ab initio calculations and semi-emperical studies on a reaction of 1,3-butadiene with an unsymmetrical dienophile were performed by Houk et al. on the reaction of acrolein and 1,3-butadiene.[20] Ab initio quantum mechanical calculations at the 3-21G basis set suggested an asynchronous TS (Figure 3).[20]
Figure 3.
Orbital calculations for the asymmetric endo transition structure in the reaction of acrolein with butadiene.
These calculations suggested that substituents, particularly ones that form an asymmetric diene, create asynchronicity within the transition state. RHF calculations predicted one substituent to give a 0.3 Å difference in the bond lengths of the forming new sigma bonds within the transition state.[20] Orbital calculations are given for the endo transition structure as they were found most favorable. While the theoretical underpinnings for endo preferences are not clear, secondary orbital overlap interactions (now largely assumed moot based on theoretical work) as well as dipolar, electrostatic and van der Waals forces have been attributed to govern endo-selectivity.[20]
Further investigations by Bernardi et al. with MC-SCF studies at the minimal STO-3G level predict that reaction of vinylcyclobutane and 1,3-butadiene undergoes a two-step asynchronous (diradicaloid nature) mechanism. This suggested that steric effects can significantly increase the amount of asynchronicity.[21] Based on symmetry, McIver has also concluded that it is highly unlikely that a symmetric transition structure would be relevant.[22]
2.3 D-A Reactions with Activating Symmetrical Addends
While recent studies reveal that Diels-Alder reactions with unsymmetrical addends undergo cycloaddition through asynchronous transition states, it was accepted that reactions with symmetrical addends would undergo cycloaddition through synchronous or nearly synchronous transition states.[11, 23] Singleton et al., was interested if this would apply to doubly activated dienophiles,[23] due to the observation of attenuated activation by a second activating group.[11, 24] Reaction of 1,3-butadiene with bis(boryl)-acetylene is predicted by density functional theory to undergo cycloaddition through a highly unsymmetrical transition state.[23] Becke3LYP calculations with a 6-31G* basis set predicted the symmetrical structure, forced Cs symmetry, to be 6.5 kcal/mol higher in energy than the unsymmetrical transition structure.[23] Due to the size of the boron groups and the favorable HOMO-LUMO overlap of the first boryl group with the π-system of 1,3-butadiene, further investigation with other activating groups was found necessary and the results were somewhat surprising.[23] Becke3LYP/6-31G* theoretical calculations predicted unsymmetrical and nearly symmetrical transition states for the reaction of acetylenedicarboxylate with isoprene (Figure 4),[23] and an unsymmetrical transition state with cyclopentadiene.[25]
Figure 4.
Becke3LYP/6-31G* calculations for the asynchronous endo and exo transition structures for the reaction of acetylene dicarboxylic acid with isoprene by Singleton et al. 2001.
Experimental kinetic isotope effects support asynchronous character as well as theoretical kinetic isotope effects for the reaction of acetylene dicarboxylate with isoprene.[23, 26] The asynchronous character of the symmetrical reaction can be explained by the difference in activation by the two activating groups. Stronger activation by the first group over that of the second group accounts for the asynchronous nature of the transition state.
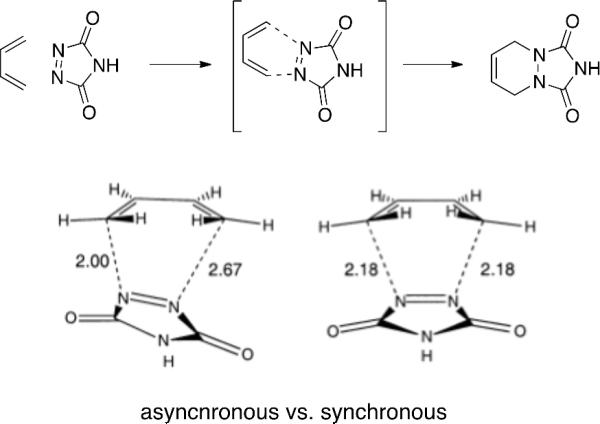
Becke3LYP/6-31G* calculations for the reaction with the highly reactive parent triazolinedione (TAD) represents a highly unsymmetrical transition state being favored by 1.1 kcal/mol by Singleton et al. (Figure 5)[23] and 1.0 kcal/mol by Houk et. al.[16] over the second-order saddle point.
Figure 5.
Becke3LYP/6-31G* calculated asynchronous and forced Cs symmetry saddle point synchronous transition structures for the reaction of TAD with 1,3-butadiene by Singleton et al. 2001. Asynchronous TS is 1.1 kcal/mol lower in energy than synchronous TS.
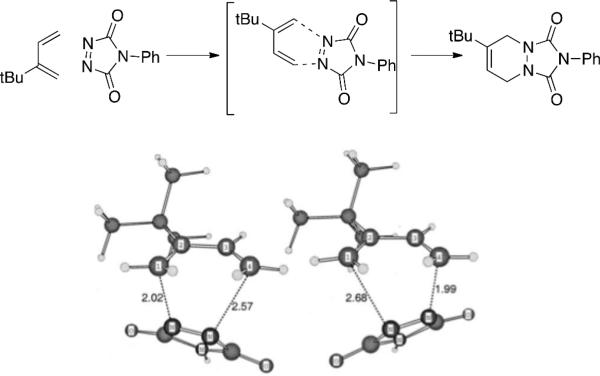
While Houk et al. found an alternative stepwise diradicaloid mechanism for the reaction of TAD with tert-butyl butadiene 3 kcal/mol lower in energy[16] than the concerted transition state, Singleton et al. suggests the reaction can undergo a stepwise or concerted asynchronous transition structure.[23] Calculated kinetic isotope effects of phenyltriazolinedione (PTAD) suggest very little asynchronicity due to the closeness in experimental proton KIE. Theoretical calculations suggest a different interpretation. Beck3LYP/6-31G* calculations suggested a highly asynchronous transition state (TS) (Figure 6) while predicted KIEs follow suit. The concern remains if a symmetrical transition structure can account for the observed kinetic isotope effects. Fortunately, calculated isotope effects of the synchronous transition structure are symmetrical and do not agree with the experimental values. Therefore, due to agreement of theoretical isotope effects with the highly unsymmetrical Becke3LYP/6-31G* calculation transition state, reaction of PTAD with butadiene supports highly asynchronous mechanisms and asymmetrical transition states.[23]
Figure 6.
Becke3LYP/6-31G* calculated asynchronous endo- and exo- transition structures for the reaction of PTAD with tert-butyl-butadiene
It can be concluded that while there has been a lot of attention towards establishing a specific reaction mechanism for the Diels-Alder reaction, many questions still remain. Theoretical evidence suggests that symmetrical substrates undergo cycloaddition via a concerted transition state. Determination of the synchronicity versus asynchronicity of the transition state appears to be highly substituent dependent. The presence of activating groups, which is most common in the putative primary or secondary metabolite substrates to be discussed below, creates intrinsic asymmetry and attendant asynchronicity within the transition structure. It should also be appreciated that the “concertedness” of a putative Diels-Alder reaction, whether it be a generated by synthetic chemistry or by a putative enzyme-catalyzed or enzyme-mediated system, lie on a continuum with symmetrical and concerted on one end to highly asynchronous and non-concerted on the other.
3. Diels-Alderases
The possible involvement of Diels-Alder reactions in biosynthesis has been an area of intense interest, and attempts to identify enzymes that might function as catalysts for the Diels-Alder reaction has been an active area of research since the late 1970's.[27] While numerous examples of non-enzyme-catalyzed biosynthetic Diels-Alder constructions are evident in the literature,[1b] the question of evolutionary selection pressure leading to the creation of enzymes that have specifically evolved to catalyze the [4+2] cycloaddition reaction via a true pericyclic transition state have attracted the attention of numerous research groups, particularly in the past twenty years. These will be briefly surveyed below.
3.1. Solanapyrone Synthase
Ichihara and co-workers performed feeding studies in Alternaria solani, that provided evidence of oxidation of prosolanapyrone 1 to prosolanapyrone 2 (Figure 7), followed by a subsequent [4+2] cycloaddition in the biosynthesis of Solanapyrones A (4) and B (3),[28] Ichihara et al. isolated a partially purified protein that was named Solanapyrone synthase and were the first to claim the identification of a natural Diels-Alderase.[27]
Figure 7.
Biosynthesis of Solanapyrone A (4) and B (3) with Solanapyrone synthase as putative cyclase.
The enzymatic and non-enzymatic reaction of prosolanapyrone 2 was monitored by HPLC analysis utilizing ultraviolet (UV) and circular dicrhoism (CD) detectors. The reaction did occur without presence of crude enzyme, but produced a 3:97 ratio of exo:endo adducts; whereas in the presence of the partially purified enzyme, the reaction proceeded, from 1 and 2, in about a 6:1 exo-selective ratio. A control experiment with denatured enzyme was carried out to establish the reactivity of the enzyme. While the reaction does occur non-enzymatically, the enzyme-catalyzed reaction proceeded 4.1 times faster with an increase in enzyme concentration.[27] The question remained whether the two-step reaction was carried out by one or two enzymes. Further investigations by Oikawa et al. suggest that Solanapyrone synthase is an oxidase that catalyzes the oxidation of 1 to aldehyde 2, followed by Diels-Alder cyclization in a two-step manner.[29] In this instance, conformational restriction of the in situ-generated aldehyde 2, seems a plausible mechanistic explanation for the differences in endo:exo-selectivity between the protein-free and protein-mediated reactions.
More recently, Oikawa and co-workers have succeeded in cloning and functionally expressing Solanapyrone synthase in the heterologous host Aspergillus oryzae. Using purified enzyme, they were able to confirm that this single enzyme effects the net two-electron oxidation of Prosolanapyrone 1 to the achiral substrate aldehyde 2, and mediates the formal [4+2] cycloaddition forming optically pure Solanapyrone.[30] These workers concluded that: “Expression and purification of SPS confirmed that this single protein catalyzes both the oxidation and the [4+2] cycloaddition, probably acting as a Diels–Alderase.”
The Solanapyrone system therefore also introduces the concept that the “Diels-Alderase” function of Solanapyrone synthase may be an accidental or spontaneous manifestation of producing the reactive aldehyde substrate (2) in the conformationally restricted and chiral environment of the active site of this oxidase. In other words, Solanapyrone synthase is functionally constituted as an oxidase and the “Diels-Alderase” activity is a subsequent fortuitous characteristic of the primary biochemical purpose of the enzyme's major function to oxidize a primary alcohol to an aldehyde. It is significant that the in situ-generated aldehyde substrate 2 is achiral, and the enzymatic product is optically pure. This outcome clearly demonstrates that the enzyme pre-organizes the transition state conformation to proceed in an enantioselective and diastereoselective manner. The high stereoselectivity of the enzyme-mediated process does not, de novo, provide experimental support for a Diels-Alder transition state and mechanism, but seems highly likely based on the corresponding protein-free reaction. The implications of this conceptual framework will be discussed in more detail below.
3.2. Lovastatin Nonaketide Synthase
Vederas, Hutchinson and co-workers have studied the fascinating formation of the decalin system (7) in the biosynthesis of the HMG CoA-reductase inhibitor Lovastatin (10).[31] The Lovastatin nonaketide synthase (LNKS) was shown to participate in the synthesis of polyketides,[32] particularly pyrones 5 and 6,[33] and the formation of Dihydromonacolin L (9; see Figure 8), with the lovC protein, in the biosynthesis of Lovastatin.[31]
Figure 8.
Biosynthesis of Lovatastatin (10) with LNKS as cyclase.
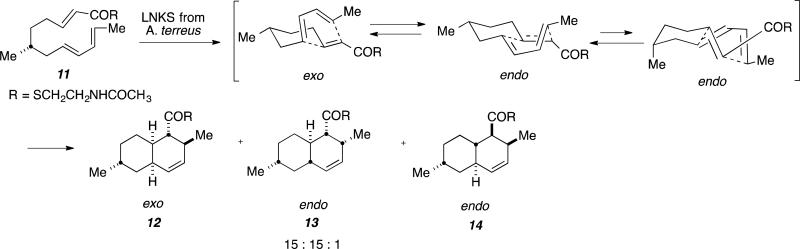
To interrogate the involvement of this enzyme in the formation of decalin 7, incubation ofpurified, recombinant enzyme was submitted to reaction with 11.[31] Investigation with NMR studies revealed formation of 12 and 13 from 11, non-enzymatically and formation of 12, 13, and 14 enzymatically in the presence of LNKS in a 15:15:1 ratio, respectively (Figure 9).[31] The stereochemistry of endo-product 13, corresponds to the natural stereochemistry in the biosynthesis of Lovastatin. The authors concluded that the endo transition state is a manifestation of van der Waals interactions occurring in the hydrophobic active site of the enzyme.[31]
Figure 9.
Model reaction with LNKS as cyclase in the enzymatic reaction of 11.
This seminal work was the first to describe the cloning, functional expression and isolation of a pure enzyme involved in a putative secondary metabolite Diels-Alder reaction. However, as in virtually all of the systems studied prior to and subsequent to the LNKS work, rigorous biophysical evidence corroborating a concerted, pericyclic transition state mechanism for the construction of the decalin ring system in Lovastatin biosynthesis has not been secured. Indeed, alternative step-wise and/or highly asynchronous mechanisms might indeed be operative and await further, deeper experimental interrogation.
3.3. Macrophomate Synthase
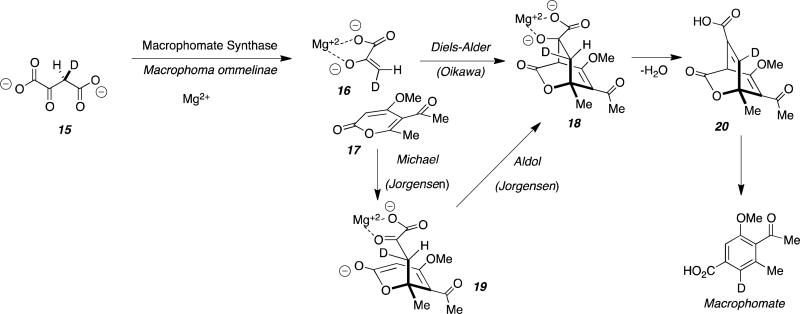
The biosynthesis of Macrophomate has been the subject of particular controversy and was first proposed by Oikawa, et al., to proceed via an enzyme-catalyzed intermolecular Diels-Alder reaction. The putative Diels-Alderase, Macrophomate synthase (MPS) was demonstrated by Oikawa, et al., to catalyze a multi-step reaction from 2-pyrone (17) and oxaloacetate 15 to ultimately generate Macrophomate. These workers postulated that the key transformation proceeded via the intermolecular Diels-Alder reaction of enol pyruvate 16 and pyrone 17 to yield the unstable bicyclo[2.2.2] Diels-Alder adduct 18.[34] Dehydration of 18 to 20 and decarboxylation with concomitant aromatization through the proposed enzyme-catalyzed Diels-Alder reaction mechanism is illustrated in Figure 10.[34]
Figure 10.
Proposed biosynthesis of macrophomate where macrophomate synthase functions as cyclase.
The authors speculated that, upon coordination with Mg2+, decarboxylation of 15 occurs to form enolpyruvate 16. Intermolecular Diels-Alder cycloaddition was proposed to furnish the bicyclo[2.2.2] species 18 which, followed by dehydration and decarboxylation provides Macrophomate. In order to determine the stereochemical outcome of the Mg2+-dependent enzymatic cycloadditon proposed, deuterium-labeled oxaloacetate and NMR spectroscopy revealed retention of the deuterium in the final Macrophomate product.[34] This revealed the high stereospecificity of the transformation that was ascribed as evidence for a concerted process. While the experimental evidence based on NMR studies is only circumstantial, the authors concluded that the enzymatic reaction proceeded via the Diels-Alder route due to the high stereospecificity observed.[34] It should be noted that these studies only revealed that the net process was highly stereospecific and does not conclusively corroborate a concerted or pericyclic mechanism catalyzed by MPS.
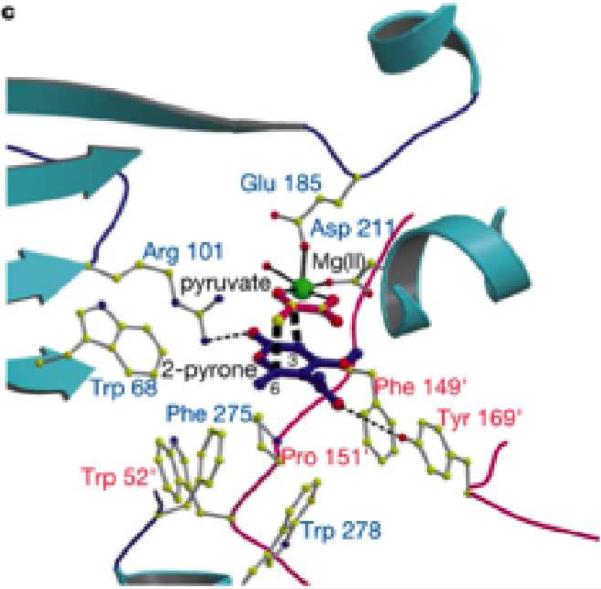
Protein X-ray crystallography studies by Oikawa and co-workers provided further insight into the active site of MPS.[35] A binding model was built by Oikawa et al. based on the transition structure of the presumed Diels-Alder cyclization and structural insights from the crystal structure (Figure 11). The carbonyl oxygen of 2-pyrone can hydrogen bond to Arg101 and the acyl oxygen can hydrogen bond to Tyr169 which undergoes π-stacking with the Phe149 residue, placing Tyr169 in the correct orientation.[35] Experimental evidence with two mutants MPS proteins represents the importance of hydrogen-bonding in the active site, providing further support for the binding site represented in Figure 11.[35]
Figure 11.
Model active site of MPS with binding of 15 and 17 utilizing crystal structure information and proposed transition structure.
Jorgensen and co-workers investigated this reaction from a theoretical perspective and conducted mixed quantum and molecular mechanics (QM/MM) combined with Monte Carlo simulations and free-energy perturbation (FEP) calculations and found a lower energy step-wise reaction pathway involving intermolecular Michael addition through intermediate 19, followed by intramolecular aldol condensation to provide the same bicyclo[2.2.2]intermediate 18 proposed by the Oikawa group.[36] The Jorgensen investigations revealed that the Diels-Alder TS model is 17.7 and 12.1 kcal/mol less stable than the Michael and aldol TSs in the stepwise route, respectively. Jorgensen states in their paper: “Therefore, this work indicates that the Michael-aldol mechanism is the route used by MPS to catalyze the second step of the overall transformation, and that the enzyme is not a natural Diels-Alderase, as claimed by Ose and co-workers (Nature 2003, 422, 185-189; Acta Crystallogr. 2004, D60, 1187-1197).”
Significantly, Hilvert and co-workers subsequently demonstrated that MPS was a very competent aldolase, corroborating the second arm of the Jorgensen Michael-aldol mechanism.[37] Hilvert et al., demonstrated that MPS is highly homologous to a known aldolase DDG (2-dehydro-3deoxygalactarate) and concluded that this lends plausibility to the two-step Michael-aldol alternative originally proposed by Jorgensen.[37] This work, taken in context with the penetrating theoretical insights of Jorgensen, reveals that extreme care must be taken when presuming that a putative Diels-Alder transformation, (wherein the substrates appear to be structurally viable Diels-Alder substrates), form a product that ostensibly appears to be the result of a concerted [4+2] cycloaddition. It is clear that products can arise by distinct, step-wise, non-concerted steps that are mechanistically orthogonal to a bona fide Diels-Alder constructions.
3.4. Riboflavin Synthase
Riboflavin synthase of Escherichia coli is an enzyme that has been extensively studied and appears to be responsible for multiple functions in the biosynthesis of the essential enzymatic co-factor Riboflavin.[38] This enzyme catalyzes the fascinating transfer of a four-carbon unit derived from one of the two equivalents of the common substrate, 6,7-dimethyl-8-ribityllumazine (22), culminating in the formation of Riboflavin and 5-amino-6-ribitylamino-2,4(1H,3H)-pyrimidinedione (26). The mechanism proposed by Bacher, et al., is illustrated in Figure 12 and features the key intermolecular Diels-Alder condensation between 22 and the oxidized diene species 23 derived from 22 to form 24 – a substance that these workers have isolated and structurally characterized.
Figure 12.
Proposed biosynthesis of Riboflavin via a key [4+2] cycloaddition to provide the isolated and structurally characterized intermediate 24.
Bacher proposes that the enzyme specifically functions as a Diels-Alderase, but there has been no experimental data published supporting this provocative mechanistic hypothesis. The authors discuss that the electronics of the proposed Diels-Alder substrates favor an inverse-electron-demand [4+2] reaction wherein overlap of the HOMO of the dienophile (22) and the LUMO of the diene (23). No corresponding protein-free reaction was reported that would have provided additional mechanistic insights.
Computational work reported later by Houk et al., provided theoretical evidence for other possible mechanistic routes.[39] Density functional theory calculations were used to evaluate the following mechanistic pathways: nucleophilic catalysis, hydride transfer followed by subsequent Diels-Alder, hydrogen atom transfer, and a nucleophilic addition mechanism.[39] While the Diels-Alder transformation cannot yet be ruled out, the alternative pathways are more likely to proceed with nucleophilic addition being of the lowest energy. Although, it should be noted that due to high-energy intermediates such as 22, and the likelihood of a stepwise mechanism, the synthesis of Riboflavin probably does not proceed via a Diels-Alderase although it formally constitutes a “[4+2]” construction.[39]
Access to the purified enzyme provides ample opportunity to study the detailed mechanism of the very interesting heterodimerization of 22 where kinetic isotope effects and double isotope fractionation would be particularly revealing. It is also appreciated that tedious, expensive and time-consuming experiments are required to conduct such a rigorous mechanistic inquiry; a challenge encountered by all workers in this field.
3.5. Spinosyn A Biosynthesis
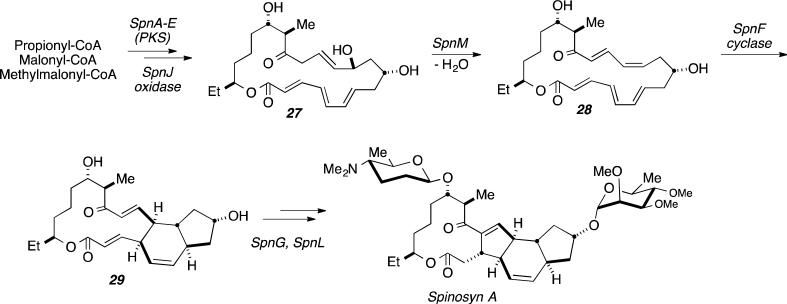
The fascinating biosynthesis of Spinosyn A, was recently investigated by Liu et al., and these workers identified the polyketide synthase expressed from the SpnF gene of Saccharopolyspora spinosa suggesting SpnF serves as a natural Diels-Alderase that catalyzes a [4+2] cycloaddition in the formation of the cyclohexene ring core of Spinosyn A.[40] The authors suggested that this represents the first example of an enzyme whose sole purpose is to catalyze a Diels-Alder reaction, and the basis for such a claim in the context of the Lovastatin biosynthesis discussed above resides on the fact that LovB is a multi-functional PKS. The biosynthetic gene cluster of S. spinosyn for Spinosyn A also includes SpnJ, SpnL, SpnM, which were also initially proposed to code for an enzyme that converts product 27 to 29 (Figure 13).[40]
Figure 13.
Biosynthesis of Spinosyn A (24) where SpnF has been proposed as the putative cyclase.
To elucidate if the SpnF cyclase was a true Diels-Alderase, conversion of 27 to cyclized product 29 was observed after a two hour incubation period with the SpnM gene product and monitored by HPLC.[40] Analysis by NMR, mass spectrometry and further investigation by HPLC of the reaction time-course led to the discovery of intermediate 28, identified by spectral analysis.[40] The presence of the monocyclic macrolactone intermediate represents a possible 2-step process that includes a dehydration step followed by the cycloaddition as shown in Figure 13.[40] To determine if the enzyme expressed by the SpnM gene catalyzes both the dehydration and cycloaddition steps, dependence of the rate of each step on the concentration of the SpnM-coded enzyme was determined by HPLC (consumption vs. time).[40] Rate enhancement of the dehydration step was observed with an increase in SpnM gene product concentration and the cyclization step was unaffected.[40] While data from Michaelis-Menten kinetics and first order kinetics suggest the cyclization can take place non-enzymatically, the remaining genes from the gene cluster were in turn interrogated. Incubation of SpnF with 28 produced the desired bicyclic species 29 in twenty minutes, which was compared to the two hour non-enzymatic reaction time.[40] In order to measure the effect of the SpnF enzyme on the rate of cyclization, HPLC was used with a fixed concentration of SpnM with time as a function of SpnF. Michaelis-Menten kinetics established that SpnF encoded a potential Diels-Alderase in the catalytic conversion of 28 to 29 with an apparent kcat of 14 +/− 1.6 min−1 for an approximated rate enhancement of 500-fold.[40]
Recent X-ray crystal structure elucidation of the enzyme by Keatinge-Clay et al. was obtained to probe further the putative mechanism of the SpnF-coded PKS-catalyzed cyclization.[41] While the enzyme of interest resembles S-adenosylmethionine (SAM)-dependent methyltransferases (SAM MTs), the activity of SAM MTs could give insight to the activity of the stand-alone enzyme. The activity of SAM MTs in these particular cyclization reactions is currently unknown.[41a] The authors conclude however, that: “The mechanisms by which SpnF and SpnL catalyse their respective cyclization reactions are a point of interest. The SpnF-catalysed endo mode syn-addition of an alkenyl to a dienyl functionality seems consistent with a Diels–Alder reaction; however, confirmation of this hypothesis will require demonstrating that the reaction progresses through a single pericyclic transition state such as [6]. Therefore, a stepwise [4+2] cycloaddition mechanism, for example, one involving a dipolar intermediate such as [7], cannot at present be ruled out.” This comment again gets to the heart of the purely academic argument pervasive in this field regarding the high-resolution details of the synchronicity of the C-C bond-forming step and the exact nature of the transition state.
3.6. Biosynthesis of Pyrroindomycins
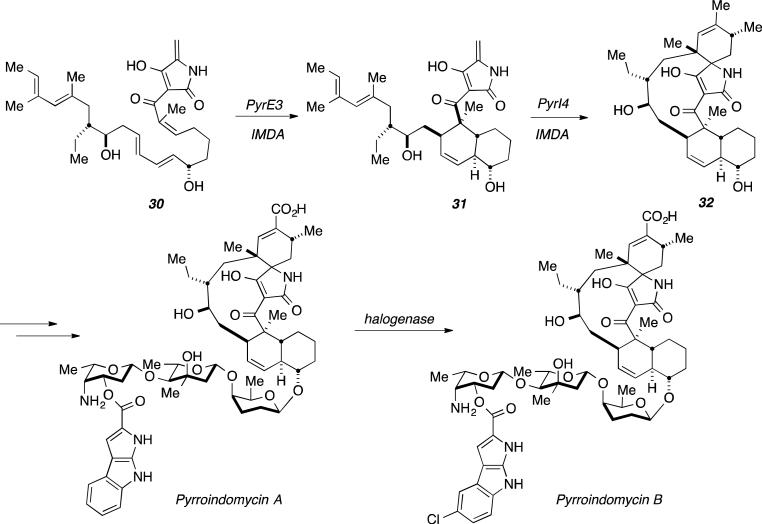
Biosynthetic studies of the naturally occurring pyrroindomycins, the first naturally-occurring spirotetramates, isolated by Ding et al., suggests that the pentacyclic core is constructed via two distinct, sequential [4+2] cycloadditions.[42] Bioinformatic analysis of the previously established biosynthetic gene cluster provided two candidates, pyrE3 and pyrI4.[42c] Known functions of enzymes homologous to PyrE3 and PyrI4 suggested that PyrI4 to be responsible for the formation of spiro-indole 32, via dialkydecalin (31) construction by PyrE3 (Figure 14).[42c]
Figure 14.
Proposed biosynthesis of Pyrroindomycins with PyrE3 and PyrI4 as cyclases.
Deactivation of PyrE3 resulted in the isolation of intermediate 30 while inactive enzyme, PyrE3 and PyrI4, failed to generate Pyrroindomycin A and Pyrroindomycin B.[42c] Structure elucidation of 30 was confirmed by NMR and mass spectrometry analysis and is consistent with the previously proposed biosynthesis.[42c] Further investigation required addition of both purified enzymes, PyrE3 and PyrI4 to intermediate 30 with no additional cofactors.[42c] The enzymes collectively converted 30 into 32, validated by structural characterization.
Based on initial gene disruption and metabolite accumulation studies, the individual function of PyrE3 and PyrI4 was investigated. Incubation of 30 with PyrE3 resulted in essentially complete conversion into 31, but no reaction occurred in the presence of PyrI4 alone. ESI-MS analysis and site-directed mutagenesis studies were successful in identifying and subsequently determining the dependence of flavin adenine dinucleotide (FAD) for PyrE3 activity. It was assumed that FAD influences the geometry of the active site during the PyrE3-mediated cycloaddition, which was further supported by electronic circular dichroism (ECD) spectra analysis of related proteins in the presence and absence of the FAD cofactor. Intermediate 31 did not convert into 32 upon incubation in aqueous media without the presence of PyrI4.[42c] While this suggests a non-spontaneous, and specifically enzyme-mediated [4+2] reaction, initial studies revealed a dependence of PyrI4 activity on the dialkyldecalin system represented as intermediate 31. Steady-state kinetics indicated a moderate efficiency for the enzymatic conversion of 30 into 31 and 31 into 32.
3.7 Thiazolyl Peptide Biosynthesis
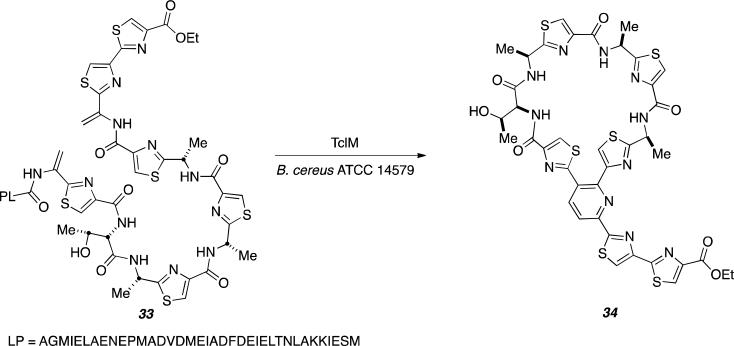
Recent investigations of the remarkably complex thiazolyl peptide biosynthesis by Bowers and co-workers, suggests that a single enzyme, TclM catalyzes the final macrocyclization step that was previously suggested to proceed via a [4+2] cycloaddition between dehydroalanine residues (Dha).[43] These investigators synthesized Thiocillin 33 to interrogate the enzymatic reaction with TclM in hopes of forming cycloaddition product 34 (Figure 15).[43]
Figure 15.
Biosynthesis of Thiocillin 34 via TclM as cyclase.
Substrate 33 was incubated with purified TclM gene for 20 hours and monitored by high-resolution quadrupole time-of-flight (QTOF) LC/MS and UV-vis. Overtime, a new absorption peak was observed as well as full consumption of the intermediate indicative of substrate 33. The new peak was potentially 34 as the wavelength observed is consistent with that of thiazolyl peptides with the same trisubstituted pyridine core. Further investigation by NMR spectroscopy shows evidence of aromatic protons from the pyridine ring and observation by LC/MS shows an m/z that matches the desired product as well as appropriate fragmentation patterns.[43]
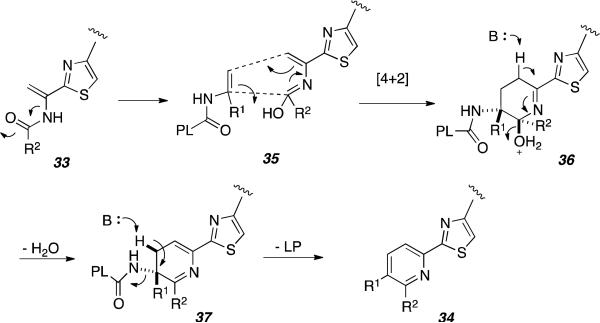
While it is evident that TclM catalyzes the formation of the tri-substituted core present in product 34, the nature of the mechanism in its formation remains unclear. Figure 16 represents the proposed [4+2] cycloaddition mechanism for the net transformation of 33 to 34.[43] The authors were careful to designate this assembly as a “formal [4+2] cycloaddition” and did not over-state the case for TclM necessarily representing a true Diels-Alderase. TclM also apparently conducts the aromatization to the final pyridine ring as putative intermediates corresponding to the intermediate species 36 and 37 were not detectable. Here again, access to the recombinant enzyme provides an ideal opportunity to study the mechanism of this fascinating macrocyclic assembly at high resolution that might involve the intermediacy of a biosynthetic Diels-Alder construction.
Figure 16.
Proposed mechanism for the transformation of 25 to 26 in presence of TclM.
3.8. Stephacidin-Notoamide and Paraherquamide Biosynthesis
Our laboratories have been studying the biosynthesis of a large family of fungal alkaloids including the dioxopiperazine-containing Stephacidins, Notoamides, Brevianamides, and the related monooxopiperazine alkaloids including the Paraherquamides, Marcfortines, Malbrancheamides and Asperparalines for some time.[44] All of these alkaloids contain a bicyclo[2.2.2]diazaoctane core that is the result of the net oxidative cyclization of a reverse-isoprene moiety to the two α-carbons of the amino acid units, typically derived from tryptophan and proline or a proline analog. Biomimetic laboratory intramolecular Diels-Alder reactions, which occur at ambient temperature and below in aqueous media, provide indirect support for the putative biosynthetic assembly processes.
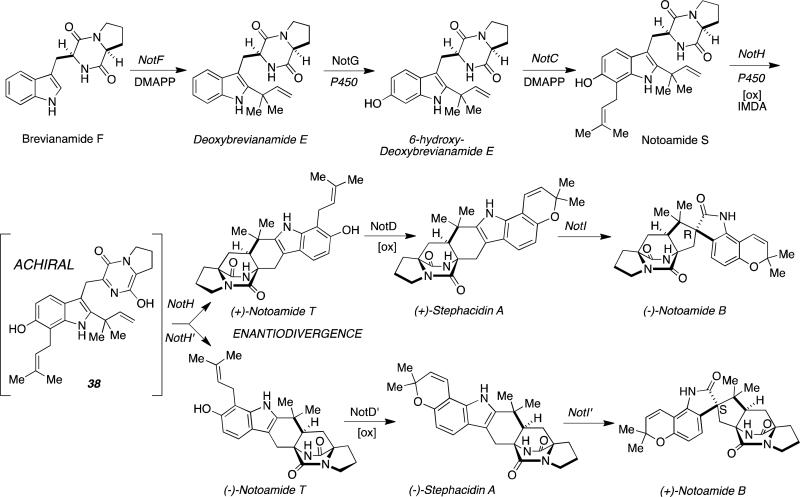
What is further remarkable about Stephacidin-Notoamide biosynthesis, is the observation that two distinct pairs of terrestrial and marine-derived orthologous Aspergillus sp. produce opposite enantiomers of Stephacidin A and Notoamide B (100% er). We have postulated that the key enantiodivergent step is also the formal [4+2] cycloaddition reaction that all experimental evidence indicates arises through a common achiral azadiene (38) as shown in Figure 17.
Figure 17.
Proposed enantiodivergent biogenesis of the Stephacidins and Notoamides through a formal [4+2] cycloaddition.
Whole genome sequencing and bioinformatics analyses of the identified gene clusters has revealed that the conversion of Notoamide S into Notoamide T, appears to be conducted by a cytochrome P450 NotH.[44d] 4 That the putative intermediate IMDA substrate 38 is achiral, and that the final natural products are optically pure, strongly implicates pre-organization of the isoprene moiety relative to the azadiene in the active site of the enzyme. It is also noteworthy that the identical Diels-Alder reaction has been successfully conducted through a biomimetic total synthesis producing racemic Notoamide T in a 2.4:1 syn:anti-diasteromeric ratio.
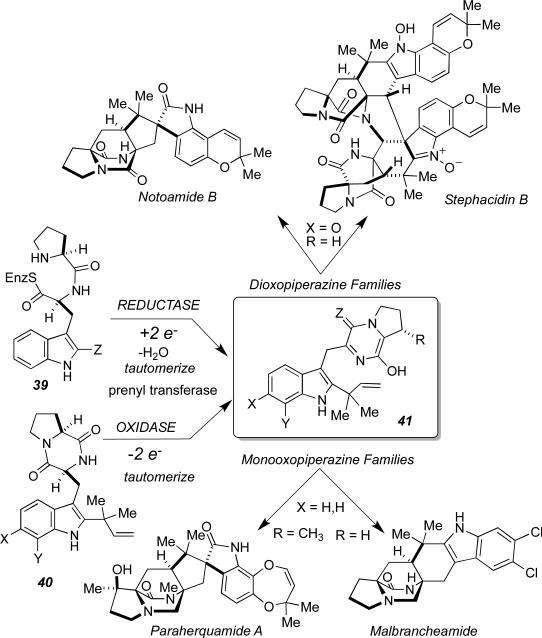
We have recently determined that the biosynthesis of the monooxopiperazine alkaloids (such as Paraherquamide and Malbrancheamide) and the dioxopiperazine alkaloids (such as Stephacidin and Notoamide B) arise by distinct pathways, but appear to converge on the formation of an azadiene intermediate (38) that suffers a net formal [4+2] cycloaddition to form the bicyclo[2.2.2]diazaoctane ring system, albeit in two distinct oxidation states (Figure 18, Z = O or H2). Based on our current understanding of the pathway and biosynthetic enzyme function, we hypothesize that the primary function of the enzymes responsible for fashioning the bicyclo[2.2.2]diazaoctane ring systems, are oxidation (for the diooxopiperazines) and possibly prenyl transfer or reduction for the monooxopiperazines, and are not specifically cyclases. Attempts to functionally express NotH in a heterologous host have not been successful to date and constitute a major focus of our current efforts. In both families of alkaloids, a reactive intermediate is formed in the chiral and conformationally restricted environment of the enzyme active site, which presumably pre-organizes the conformation of the azadiene relative to the isoprene moiety resulting in highly enantioselective and diastereoselective cycloadditions. Here as in other systems discussed in this article, the cycloaddition reaction appears to be a fortuitous “auxiliary reaction” leading to new molecular complexity. There is ample evidence that chemical diversification in secondary metabolism is driven by these fascinating “experiments” in enzyme evolution.[45]
Figure 18.
Unified biosynthesis of the monooxopiperazine and dioxopiperazine families of alkaloids containing a bicyclo[2.2.2]diazaoctane ring system.
3.9 Versipelostatin
The synthesis of polyketides is attractive due to the wide variety of pharmaceutical drugs developed from this class of compounds. Their structural variety includes a spirotetronate core, which is proposed to arise from an IMDA.[46] Figure 19 shows the biosynthetic transformation of Versipelostatin (43). To test this proposition, inactivation of the VstJ gene resulted in loss of Versipelostatin (43) formation, as shown by liquid chromatography-mass spectrometry (LC/MS) analysis.[46] Further analysis with incubation of purified enzyme with synthesized substrate 44 and Michaelis-Menten kinetics reveal the non-spontaneous catalytic character of VstJ in the biosynthesis of the spirotetramate.[46] Activation of the dienophile and stabilization of the cis-diene must be provided by organization and hydrogen bonding within the active site, respectively.
Figure 19.
Biosynthesis of Versipelostatin (43) with VstJ as cyclase.
4. Conclusion
There are at least nine biosynthetic systems to date that have been identified to contain natural enzymes claimed to function as the key cyclase in biosynthetic transformations that appear to constitute biosynthetic formal [4+2] cycloadditions or Diels-Alder-type constructions. Despite the increasing sophistication of mechanistic tools – both theoretical and experimental - to study the concerted nature of putative enzyme-mediated and/or specifically enzyme-catalyzed biosynthetic Diels-Alder constructions, much remains to be investigated and rigorous, and corroborating biophysical evidence remains elusive.
The fundamental mechanistic conundrum for Diels-Alderases is the issue of turnover. Virtually all known enzymatic reactions lower the activation energy to effect rate acceleration by stabilizing the structure, charge and geometry of the developing transition state, which does not typically resemble the product structure. For the Diels-Alder reaction however, the transition state and the product are highly homologous structurally, which would manifest as product inhibition begging the question of how turnover is achieved. This very problem of turnover was evident in the work of Schultz and Hilvert who designed and generated catalytic antibodies for the Diels-Alder reaction using transition state mimics as haptens.[48]
In the case of systems where the putative Diels-Alderase is an enzyme that evolved to perform a distinct biochemical transformation, such as oxidation, generating the reactive substrate in the chiral environment of the active site, turnover is presumably not affected by product inhibition. In such systems, such as the Solanapyrone and Stephacidin biosyntheses discussed above, conformational restriction of the reactive substrate as it is generated in the active site, is pre-disposed to undergo the subsequent “side-reaction” of cycloaddition. The entropic benefit of conformational restriction certainly must contribute to increase the rate of the cycloaddition step relative to the laboratory reaction and would also plausibly impact stereoselectivity relative to the laboratory reaction. In fact, the relative changes in stereoselectivity between the cycloadition reaction in the presence and absence of enzyme has been used, perhaps incorrectly, as “experimental evidence” to support the pericyclic mechanism of these enzyme-mediated transformations. Extreme care should be exercised in this regard when comparing the laboratory reaction (where feasible) to the protein-mediated transformation. Differences in stereoselectivity only reveal that the protein-mediated reaction has a distinct stereoselectivity and does not penetrate the subtle mechanistic details of the protein-mediated reaction.
These authors remain circumspect as to whether a true Diels-Alderase exists that evolved to specifically catalyze the Diels-Alder reaction through the concerted, synchronous pericyclic transition state required. Yet this becomes essentially a philosophical argument as the experimental rigor required to prove that a pericyclic transition state is operative to the exclusion of alternative step-wise, nonsynchronous or highly asynchronous mechanisms will inevitably loom. In particular, non-enzymatic, synthetic chemical Diels-Alder reactions where the spectrum of substrates, which can undergo cycloaddition through a strictly concerted, synchronous mechanism controlled by orbital symmetry considerations, typically require symmetrical substrates, rarely found in natural products studied to date in this context. The polyketide synthases identified in the Pyrroindomycin and Spinosyn biosyntheses, appear to constitute enzymes identified to catalyze a formal [4+2] cycloaddition reaction as their sole function.47 Chemists have been irresistibly drawn to the biosynthetic Diels-Alder construction for the allure of penetrating how Nature has been able to exploit so many chemical reactions familiar in synthetic chemistry to assemble molecular complexity and molecular diversity in primary metabolites and especially secondary metabolites. That Nature might have “accidentally” stumbled upon the Diels-Alder reaction or, more precisely based on the current body of experimental evidence, formal [4+2] cycloaddition reactions, in the course of secondary metabolic enzyme tailoring is certainly evident and numerous examples of non-enzyme-catalyzed biosynthetic Diels-Alder constructions are known.2 The biological activity of such natural substances, if providing an adaptive benefit to the producing organism, furnishes the evolutionary driving force to preserve, propagate and modify such enzymes. This field remains an area worthy of additional experimentation, theoretical analysis and pedagogical vetting. As one of the most significant reactions in synthetic organic chemistry, it is very exciting to contemplate the extent to which Nature has interrogated formal [4+2] cycloaddition constructions. What remains as an open, and largely unresolved issue, is the extent to which formal [4+2] constructions mediated and/or catalyzed enzymatically, proceed through concerted, or mostly synchronous bond constructions as required for a true pericyclic transformation. The same problems here in the formal [4+2] cycloadditions evident in Nature, permeated an extensive series of papers on chorismate mutase, formally an enzyme-catalyzed Claisen rearrangement.
Acknowledgements
This paper is dedicated to the memory of Professor Robert Burns Woodward and his myriad accomplishments and contributions to science. Our research work in this field has been supported by the National Institutes of Health Grant RO1 CA070375. The authors also wish to acknowledge Professor Craig Townsend, Professor John C. Vederas, and Professor Christopher Walsh for stimulating discussions and insight.
Biography
 Robert M. Williams was born in New York in 1953 and attended Syracuse University where he received the B.A. degree in Chemistry in 1975. He obtained the Ph.D. degree in 1979 at MIT (W.H. Rastetter) and was a post-doctoral fellow at Harvard (1979-1980; R.B. Woodward/Yoshito Kishi). He joined Colorado State University in 1980 and was named a University Distinguished Professor in 2002. His interdisciplinary research program (over 300 publications) at the chemistry-biology interface is focused on the total synthesis of biomedically significant natural products, biosynthesis of secondary metabolites, studies on antitumor drug-DNA interactions, HDAC inhibitors, amino acids and peptides.
Robert M. Williams was born in New York in 1953 and attended Syracuse University where he received the B.A. degree in Chemistry in 1975. He obtained the Ph.D. degree in 1979 at MIT (W.H. Rastetter) and was a post-doctoral fellow at Harvard (1979-1980; R.B. Woodward/Yoshito Kishi). He joined Colorado State University in 1980 and was named a University Distinguished Professor in 2002. His interdisciplinary research program (over 300 publications) at the chemistry-biology interface is focused on the total synthesis of biomedically significant natural products, biosynthesis of secondary metabolites, studies on antitumor drug-DNA interactions, HDAC inhibitors, amino acids and peptides.
References
- 1.a Laschat S. Angew. Chem. Int. Ed. 1996;35:289–291. [Google Scholar]; b Stocking EM, Williams RM. Angew. Chem. Int. Ed. 2003;42:3078–3115. doi: 10.1002/anie.200200534. [DOI] [PubMed] [Google Scholar]
- 2.Kim HJ, Ruszczycky MW, Liu HW. Curr. Opin. Chem. Biol. 2012;16:124–131. doi: 10.1016/j.cbpa.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.a Kelly WL. Org. Biomol. Chem. 2008;6:483–4493. doi: 10.1039/b814552k. [DOI] [PubMed] [Google Scholar]; b Oikawa H. Bull. Chem. Soc. Jpn. 2005;78:537–554. [Google Scholar]
- 4.Sauer J, Sustmann R. Angew. Chem. Int. Ed. 1980;19:779–807. [Google Scholar]
- 5.a Houk KN. J. Am. Chem. Soc. 1973;95:4092–4094. [Google Scholar]; b Houk KN, Strozier R. J. Am. Chem. Soc. 1973;94:4094–4096. [Google Scholar]
- 6.Houk KN. Proc. Natl. Acad. Sci. USA. 2012;109:12860–12865. doi: 10.1073/pnas.1209316109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inukai T, Kojima TJ. J. Org. Chem. 1966;30:1121–1123. [Google Scholar]
- 8.Houk KN. Acc. Chem. Res. 1975;8:361–369. [Google Scholar]
- 9.Hoffmann R, Woodward RB. J. Am. Chem. Soc. 1965;87:4388–4389. [Google Scholar]
- 10.Woodward RB, Katz T. Tetrahedron. 1959;5:70. [Google Scholar]
- 11.Dewar MJS, Olivella S, Rzepa HS. J. Am. Chem. Soc. 1978;100:5650–5660. [Google Scholar]
- 12.a Dewar MJS, Griffin AC, Kirschner S. J. Am. Chem. Soc. 1974;96:6225–6226. [Google Scholar]; b Dewar MJS, Olivella S, Stewart JJP. J. Am. Chem. Soc. 1986;108:5771–5779. doi: 10.1021/ja00279a018. [DOI] [PubMed] [Google Scholar]
- 13.Dewar MJS. J. Am. Chem. Soc. 1984;106:209–219. [Google Scholar]
- 14.Li Y, Houk KN. J. Am. Chem. Soc. 1993;115:7478–7485. [Google Scholar]
- 15.Beno BR, Wilsey S, Houk KN. J. Am. Chem. Soc. 1999;121:4816–4826. [Google Scholar]
- 16.Chen JS, Houk KN, Foote CS. J. Am. Chem. Soc. 1998;120:12303–12309. [Google Scholar]
- 17.a Townshend RE, Ramunni G, Segal G, Hehre WJ, Salen L. J. Am. Chem. Soc. 1976;98:2190–2098. [Google Scholar]; b Burke LA, Leroy G, Sana M. Theor. Chim. Acta. 1975;40:313. [Google Scholar]
- 18.Bernardi F, Bottini A, Robb MA, Field MJ, Hillier IH, Guest MF. J. Chem. Soc., Chem. Commun. 1985:1051–1052. [Google Scholar]
- 19.Gajewski JJ, Peterson KB, Kagel JR, Huang YCJ. J. Am. Chem. Soc. 1989;111:9078–9081. [Google Scholar]
- 20.Loncharich RJ, Brown FK, Houk KN. J. Org. Chem. 1989;54:1129–1135. [Google Scholar]
- 21.Bernardi F, Bottoni A, Field MJ, Guest MF, Hillier IH, Robb MA, Venturini A. J. Am. Chem. Soc. 1988;110:3050–3055. [Google Scholar]
- 22.a McIver JW. J. Am. Chem. Soc. 1975;97:3632. [Google Scholar]; b J W. McIver. Acc. Chem. Res. 1974;7:72. [Google Scholar]
- 23.Singleton DA. Tetrahedron. 2001;57:5149–5160. [Google Scholar]
- 24.Hancock RA, Wood BF. J. Chem. Soc., Chem. Commun. 1988:351–353. B. F. [Google Scholar]
- 25.Froese RDJ, Coxon JM, West SC, Morokuma K. J. Org. Chem. 1997;62:6991–6996. [Google Scholar]
- 26.Singleton DA, Thomas AA. J. Am. Chem. Soc. 1995;117:9357–9358. [Google Scholar]
- 27.Oikawa H, Katayama K, Suzuki Y, Ichihara A. J. Chem. Soc., Chem. Commun. 1995:1321–1322. [Google Scholar]
- 28.Oikawa H, Suzuke Y, Naya A, Katayama K, Ichihara A. J. Am. Chem. Soc. 1994;116:3605–3606. [Google Scholar]
- 29.a Katayama K, Kobayashi T, Oikawa H, Honma M;, Ichihara A. Biochim. Biophys. Acta. 1998;1384:387–395. doi: 10.1016/s0167-4838(98)00040-5. [DOI] [PubMed] [Google Scholar]; b Oikawa H, Kobayashi T, Katayama K, Suzuki Y, Ichihara A. J. Org. Chem. 1998;63:8748–8756. [Google Scholar]
- 30.Kashara K, Miyamoto, T. T, Fujimoto T, Oguri H, Okiwano T, Oikawa H, Ebizuka Y, Fujii I. ChemBioChem. 2010;11:1245–1252. doi: 10.1002/cbic.201000173. [DOI] [PubMed] [Google Scholar]
- 31.Auclair K, Sutherland A, Kennedy J, Witter D,J, de Heever JPV, Hutchinson CR, Vederas JC. J. Am. Chem. Soc. 2000;122:11519–11520. [Google Scholar]
- 32.Ma SM, Li JW, Choi JW, Zhou H, Lee KK, Moorthie VA, Xie X, Kealey JT, Da Silva NA, Vederas JC, Tang Y. Science. 2009;326:589–593. doi: 10.1126/science.1175602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kennedy J, Auclair K, Kendrew SG, Park C, Vederas JC, Hutchinson CR. Science. 1999;284:1368–1372. doi: 10.1126/science.284.5418.1368. [DOI] [PubMed] [Google Scholar]
- 34.Watanabe K, Mie T, Ichihara A, Oikawa H, Honma M. J. Biol. Chem. 2000;275:38393–38401. doi: 10.1074/jbc.M003119200. [DOI] [PubMed] [Google Scholar]
- 35.Ose T, Watanabe H, Yao M, Tanaka I, Watanabe K, Mie T, Honma M, Oikawa H. Nature. 2003;422:185–189. doi: 10.1038/nature01454. [DOI] [PubMed] [Google Scholar]
- 36.Guimaraes CRW, Udier-Blagovic M, Jorgensen WL. J. Am. Chem. Soc. 2005;127:3577–3588. doi: 10.1021/ja043905b. [DOI] [PubMed] [Google Scholar]
- 37.Serafimov JM, Gillingham D, Kuster S, Hilvert D. J. Am. Chem. Soc. 2008;130:7798–7799. doi: 10.1021/ja8017994. [DOI] [PubMed] [Google Scholar]
- 38.a Eberhardt N, Kemter K, Richter G, Cushman M, Bacher A. Eur. J. Biochem. 2001;268:4315–4323. doi: 10.1046/j.1432-1327.2001.02351.x. [DOI] [PubMed] [Google Scholar]; b Kim R, Illarionov B, Joshi M, Cushman M, Lee, Eisenreich W, Fischer M, Bacher A. J. Am. Chem. Soc. 2010;132:2983–2990. doi: 10.1021/ja908395r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Breugst M, Eschenmoser A, Houk KN. J. Am. Chem. Soc. 2013;135:6658–6668. doi: 10.1021/ja402099f. [DOI] [PubMed] [Google Scholar]
- 40.Kim HJ, Ruszczycky MW, Choi SH, Liu YN, Liu HW. Nature. 2011;473:109–112. doi: 10.1038/nature09981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.a Fage CD, Isiorho EA, Liu Y, Wagner DT, Liu H. Nature. 2015;11:256–260. doi: 10.1038/nchembio.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Fage CD, Isiorho EA, Liu Y, Wagner DT, Liu H, Keatinge-Clay AT. Nature Chem. Biol. 2015;11:256–260. doi: 10.1038/nchembio.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.a Ding W, Williams DR, Northcote P, Siegel MM, Tsao R, Ashcroft J, Morton GO, Alluri M, Abbanat D, Maiese WM, Ellestad GA. J. Antibiot. 1994;47:1250–1257. doi: 10.7164/antibiotics.47.1250. [DOI] [PubMed] [Google Scholar]; b Singh MP, Petersen PJ, Jacobus NV, Mroczenski-Wildey MJ, Maiese WM, Greenstein M, Steinberg DA. J. Antibiot. 1994;47:1258–1265. doi: 10.7164/antibiotics.47.1258. [DOI] [PubMed] [Google Scholar]; c Tian Z, Sun P, Yan Y, Wu Z, Zheng Q, Zhou S, Zhang H, Yu F, Jia X, Chen D, Mandi A, Kurtan T, Liu W. Nat. Chem. Biol. 2015;11:259–268. doi: 10.1038/nchembio.1769. [DOI] [PubMed] [Google Scholar]
- 43.Wever WJ, Bogart JW, Baccile JA, Chan AN, Schroeder FC, Bowers AA. J. Am. Chem. Soc. 2015;137:3494–3497. doi: 10.1021/jacs.5b00940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.a Li S, Srinivasan K, Tran H, Yu F, Finefield JM, Sunderhaus JD, McAfoos TJ, Tsukamoto S, Williams RM, Sherman DH. Med. Chem. Commun. 2012;3:987–996. doi: 10.1039/C2MD20029E. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Finefield JM, Frisvad J, Williams RM. J. Nat. Prod. 2012;75:812–833. doi: 10.1021/np200954v. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Sunderhaus JD, Williams RM. Israel J. Chem. 2011;51:442–452. doi: 10.1002/ijch.201100016. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Ding Y, de Wet JR, Cavalcoli J, Li S, Greshock TJ, Miller KA, Finefield JM, Sunderhaus JD, McAfoos T, Tsukamoto S, Williams RM, Sherman DH. J. Am. Chem. Soc. 2010;132:12733–12740. doi: 10.1021/ja1049302. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Townsend CA. ChemBioChem. 2011;12:2267–2269. doi: 10.1002/cbic.201100431. [DOI] [PubMed] [Google Scholar]
- 45.Gu L, Wang B, Kulkarni A, Geders TW, Grindberg RV, Gerwick L, Hakansson K, Wipf P, Smith JL, Gerwick WH, Sherman DH. Nature Chem. Biol. 2009;459:731–735. doi: 10.1038/nature07870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hashimoto T, Hashimoto J, Teruya K, Hirano T, Shin-ya K, Ikeda H, Liu H, Nishiyama M, Kuzuyama T. J. Am. Chem. Soc. 2015;137:572–575. doi: 10.1021/ja510711x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hashimoto T, Hashimoto J, Teruya K, Hirano T, Shin-ya K, Ikeda H, Liu HW, Nishiyama M, Kuzuyama T. J. Am. Chem. Soc. 2014;137:572–575. doi: 10.1021/ja510711x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.a Romesberg FE, Spiller B, Schultz PG, Stevens RC. Science. 1998;279:1929–1934. doi: 10.1126/science.279.5358.1929. [DOI] [PubMed] [Google Scholar]; b Xu J, Deng Q, Chen J, Houk KN, Bartek J, Hilvert D, Wilson IA. Science. 1999;286:2345–2349. doi: 10.1126/science.286.5448.2345. [DOI] [PubMed] [Google Scholar]; c Piatesi A, Hilvert D. ChemBioChem. 2004;5:460–466. doi: 10.1002/cbic.200300806. [DOI] [PubMed] [Google Scholar]; d Braisted AC, Schultz PG. J. Am. Chem. Soc. 1990;112:7430–7431. [Google Scholar]; e Hilvert D, Hill KW, Nared KD, Auditor MTM. J. Am. Chem. Soc. 1989;111:9261–9262. [Google Scholar]; f Piatesti A, Aldag C, Hilvert D. J. Mol. Biol. 2008;377:993–1001. doi: 10.1016/j.jmb.2008.01.069. [DOI] [PubMed] [Google Scholar]; g Verdino P, Aldag C, Hilvert D, Wilson IA. PNAS. 2008;105:11725–11730. doi: 10.1073/pnas.0801783105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.a Ganem B. Angew. Chem. Int. Ed. 1996;35:936–945. [Google Scholar]; b Belasco JG, Albery WJ, Knowles JR. J. Am. Chem. Soc. 1983;105:2475–2477. [Google Scholar]; c Sogo SG, Widlanski TS, Hoare JH, Grimshaw CE, Berchtold GA, Knowles JR. J. Am. Chem. Soc. 1984;106:2701–2703. [Google Scholar]; d Guimarães CRW, Repasky MP, Chandrasekhar, TiradoRives J, Jorgensen WL. J. Am. Chem. Soc. 2003;125:6892–6899. doi: 10.1021/ja021424r. [DOI] [PubMed] [Google Scholar]; e Wright SK, DeClue MS, Mandal A, Lee L, Wiest O, Cleland WW, Hilvert D. J. Am. Chem. Soc. 2005;127:12957–12964. doi: 10.1021/ja052929v. and references cited therein. [DOI] [PMC free article] [PubMed] [Google Scholar]