Abstract
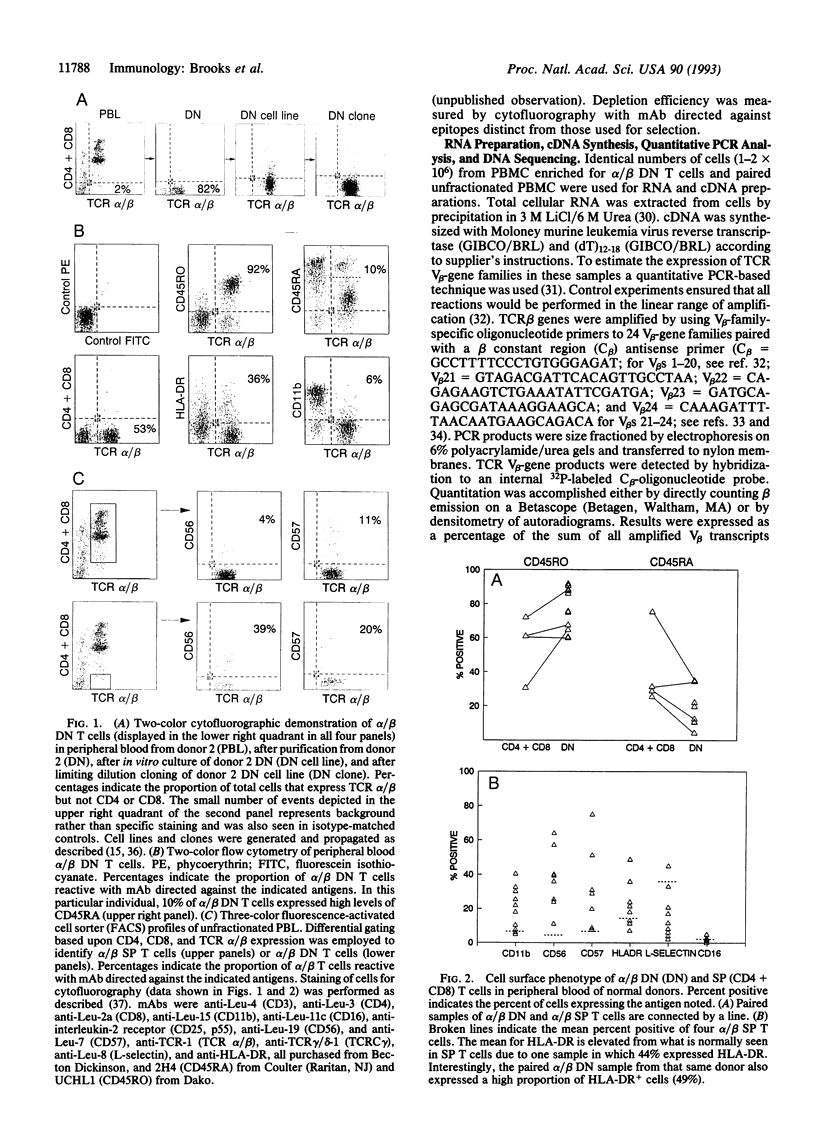
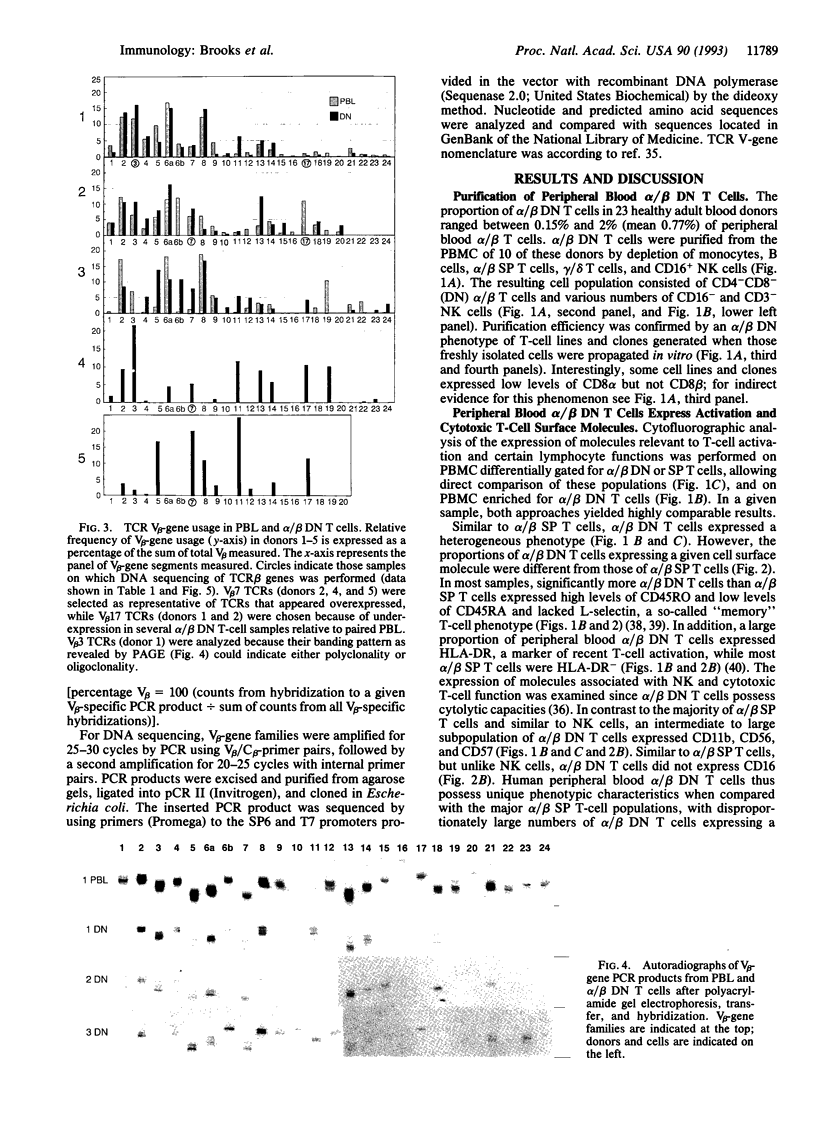
Most human T cells express the TCR alpha/beta and either CD4 or CD8 molecules (single positive, SP); however, small numbers lack CD4 and CD8. In inbred mice, alpha/beta CD4-CD8- (double negative, DN) T cells preferentially express certain beta variable region (V beta) families and may arise via unique developmental pathways. Increased percentages of alpha/beta DN T cells have been identified in some human and murine autoimmune and immunodeficiency diseases. However, their contribution to disease pathology or normal immunity is unknown. To study the cell surface phenotype and TCR diversity of human alpha/beta DN T cells, these cells were isolated from the peripheral blood of healthy adults. The proportion of alpha/beta DN T cells expressing molecules associated with activation (HLA-DR), previous exposure to antigen (CD45RO), and cytotoxic function (CD56, CD57, and CD11b) was increased relative to SP T cells. The TCR V beta repertoire of alpha/beta DN T cells was different from that of alpha/beta SP T cells, although most major gene families were present. For example, higher proportions of V beta 11, a minor gene family in peripheral blood leukocytes, were found in most alpha/beta DN T-cell samples. In contrast to mice, no dominant V beta family was used consistently in different human individuals. Within an individual alpha/beta DN T cells possessed an oligoclonal TCR beta repertoire with conservation of several distinct junctional amino acid motifs with one joined to three different V beta genes in two individuals, suggesting that these cells have undergone a selection process driven by a limited set of ligands. The possibility that they may represent, at least in part, originally SP T cells anergized by down-modulation of CD4 or CD8 must also be entertained. Overall, this study demonstrates that human peripheral blood alpha/beta DN T cells possess unique phenotypic and TCR beta repertoire characteristics when compared with the major alpha/beta SP T cell populations and thus may serve specialized immunologic functions and/or have an unusual origin.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auffray C., Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem. 1980 Jun;107(2):303–314. doi: 10.1111/j.1432-1033.1980.tb06030.x. [DOI] [PubMed] [Google Scholar]
- Band H., Hochstenbach F., McLean J., Hata S., Krangel M. S., Brenner M. B. Immunochemical proof that a novel rearranging gene encodes the T cell receptor delta subunit. Science. 1987 Oct 30;238(4827):682–684. doi: 10.1126/science.3672118. [DOI] [PubMed] [Google Scholar]
- Beverley P. C. Human T cell subsets. Immunol Lett. 1987 Apr;14(4):263–267. doi: 10.1016/0165-2478(87)90001-0. [DOI] [PubMed] [Google Scholar]
- Blumberg R. S., Yockey C. E., Gross G. G., Ebert E. C., Balk S. P. Human intestinal intraepithelial lymphocytes are derived from a limited number of T cell clones that utilize multiple V beta T cell receptor genes. J Immunol. 1993 Jun 1;150(11):5144–5153. [PubMed] [Google Scholar]
- Boyd R. L., Hugo P. Towards an integrated view of thymopoiesis. Immunol Today. 1991 Feb;12(2):71–79. doi: 10.1016/0167-5699(91)90161-L. [DOI] [PubMed] [Google Scholar]
- Brooks E. G., Schmalstieg F. C., Wirt D. P., Rosenblatt H. M., Adkins L. T., Lookingbill D. P., Rudloff H. E., Rakusan T. A., Goldman A. S. A novel X-linked combined immunodeficiency disease. J Clin Invest. 1990 Nov;86(5):1623–1631. doi: 10.1172/JCI114884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks E. G., Wirt D. P., Goldblum R. M., Vaidya S., Asuncion M. T., Patterson J. C., Ware C. F., Klimpel G. R. Double-negative (CD4- CD8-) T cells with an alpha/beta T cell receptor. Non-MHC-restricted cytolytic activity and lymphokine production. J Immunol. 1990 Jun 15;144(12):4507–4512. [PubMed] [Google Scholar]
- Choi Y. W., Kotzin B., Herron L., Callahan J., Marrack P., Kappler J. Interaction of Staphylococcus aureus toxin "superantigens" with human T cells. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8941–8945. doi: 10.1073/pnas.86.22.8941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly J. M., Potter T. A., Wormstall E. M., Hansen T. H. The Lyt-2 molecule recognizes residues in the class I alpha 3 domain in allogeneic cytotoxic T cell responses. J Exp Med. 1988 Jul 1;168(1):325–341. doi: 10.1084/jem.168.1.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crispe I. N., Moore M. W., Husmann L. A., Smith L., Bevan M. J., Shimonkevitz R. P. Differentiation potential of subsets of CD4-8- thymocytes. Nature. 1987 Sep 24;329(6137):336–339. doi: 10.1038/329336a0. [DOI] [PubMed] [Google Scholar]
- Davidson W. F., Dumont F. J., Bedigian H. G., Fowlkes B. J., Morse H. C., 3rd Phenotypic, functional, and molecular genetic comparisons of the abnormal lymphoid cells of C3H-lpr/lpr and C3H-gld/gld mice. J Immunol. 1986 Jun 1;136(11):4075–4084. [PubMed] [Google Scholar]
- Dellabona P., Casorati G., Friedli B., Angman L., Sallusto F., Tunnacliffe A., Roosneek E., Lanzavecchia A. In vivo persistence of expanded clones specific for bacterial antigens within the human T cell receptor alpha/beta CD4-8- subset. J Exp Med. 1993 Jun 1;177(6):1763–1771. doi: 10.1084/jem.177.6.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleit H. B., Wright S. D., Unkeless J. C. Human neutrophil Fc gamma receptor distribution and structure. Proc Natl Acad Sci U S A. 1982 May;79(10):3275–3279. doi: 10.1073/pnas.79.10.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowlkes B. J., Kruisbeek A. M., Ton-That H., Weston M. A., Coligan J. E., Schwartz R. H., Pardoll D. M. A novel population of T-cell receptor alpha beta-bearing thymocytes which predominantly expresses a single V beta gene family. Nature. 1987 Sep 17;329(6136):251–254. doi: 10.1038/329251a0. [DOI] [PubMed] [Google Scholar]
- Gabert J., Langlet C., Zamoyska R., Parnes J. R., Schmitt-Verhulst A. M., Malissen B. Reconstitution of MHC class I specificity by transfer of the T cell receptor and Lyt-2 genes. Cell. 1987 Aug 14;50(4):545–554. doi: 10.1016/0092-8674(87)90027-4. [DOI] [PubMed] [Google Scholar]
- Gay D., Maddon P., Sekaly R., Talle M. A., Godfrey M., Long E., Goldstein G., Chess L., Axel R., Kappler J. Functional interaction between human T-cell protein CD4 and the major histocompatibility complex HLA-DR antigen. Nature. 1987 Aug 13;328(6131):626–629. doi: 10.1038/328626a0. [DOI] [PubMed] [Google Scholar]
- Groh V., Fabbi M., Hochstenbach F., Maziarz R. T., Strominger J. L. Double-negative (CD4-CD8-) lymphocytes bearing T-cell receptor alpha and beta chains in normal human skin. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5059–5063. doi: 10.1073/pnas.86.13.5059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori T., Asou N., Suzushima H., Takatsuki K., Tanaka K., Naito K., Natori H., Oizumi K. Leukaemia of novel gastrointestinal T-lymphocyte population infected with HTLV-I. Lancet. 1991 Jan 12;337(8733):76–77. doi: 10.1016/0140-6736(91)90737-a. [DOI] [PubMed] [Google Scholar]
- Huang L., Crispe I. N. Distinctive selection mechanisms govern the T cell receptor repertoire of peripheral CD4-CD8- alpha/beta T cells. J Exp Med. 1992 Sep 1;176(3):699–706. doi: 10.1084/jem.176.3.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illum N., Ralfkiaer E., Pallesen G., Geisler C. Phenotypical and functional characterization of double-negative (CD4-CD8-) alpha beta T-cell receptor positive cells from an immunodeficient patient. Scand J Immunol. 1991 Nov;34(5):635–645. doi: 10.1111/j.1365-3083.1991.tb01587.x. [DOI] [PubMed] [Google Scholar]
- Kubota H., Okazaki H., Onuma M., Kano S., Hattori M., Minato N. CD3+4-8- alpha beta T cell population with biased T cell receptor V gene usage. Presence in bone marrow and possible involvement of IL-3 for their extrathymic development. J Immunol. 1992 Aug 15;149(4):1143–1150. [PubMed] [Google Scholar]
- Kusunoki Y., Hirai Y., Kyoizumi S., Akiyama M. Evidence for in vivo clonal proliferation of unique population of blood CD4-/CD8- T cells bearing T-cell receptor alpha and beta chains in two normal men. Blood. 1992 Jun 1;79(11):2965–2972. [PubMed] [Google Scholar]
- Lai E., Concannon P., Hood L. Conserved organization of the human and murine T-cell receptor beta-gene families. Nature. 1988 Feb 11;331(6156):543–546. doi: 10.1038/331543a0. [DOI] [PubMed] [Google Scholar]
- Lanier L. L., Le A. M., Cwirla S., Federspiel N., Phillips J. H. Antigenic, functional, and molecular genetic studies of human natural killer cells and cytotoxic T lymphocytes not restricted by the major histocompatibility complex. Fed Proc. 1986 Nov;45(12):2823–2828. [PubMed] [Google Scholar]
- Londei M., Verhoef A., De Berardinis P., Kissonerghis M., Grubeck-Loebenstein B., Feldmann M. Definition of a population of CD4-8- T cells that express the alpha beta T-cell receptor and respond to interleukins 2, 3, and 4. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8502–8506. doi: 10.1073/pnas.86.21.8502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malissen B., Rebai N., Liabeuf A., Mawas C. Human cytotoxic T cell structures associated with expression of cytolysis. I. Analysis at the clonal cell level of the cytolysis-inhibiting effect of 7 monoclonal antibodies. Eur J Immunol. 1982 Sep;12(9):739–747. doi: 10.1002/eji.1830120908. [DOI] [PubMed] [Google Scholar]
- Oksenberg J. R., Panzara M. A., Begovich A. B., Mitchell D., Erlich H. A., Murray R. S., Shimonkevitz R., Sherritt M., Rothbard J., Bernard C. C. Selection for T-cell receptor V beta-D beta-J beta gene rearrangements with specificity for a myelin basic protein peptide in brain lesions of multiple sclerosis. Nature. 1993 Mar 4;362(6415):68–70. doi: 10.1038/362068a0. [DOI] [PubMed] [Google Scholar]
- Picker L. J., Treer J. R., Ferguson-Darnell B., Collins P. A., Buck D., Terstappen L. W. Control of lymphocyte recirculation in man. I. Differential regulation of the peripheral lymph node homing receptor L-selectin on T cells during the virgin to memory cell transition. J Immunol. 1993 Feb 1;150(3):1105–1121. [PubMed] [Google Scholar]
- Plaza A., Kono D. H., Theofilopoulos A. N. New human V beta genes and polymorphic variants. J Immunol. 1991 Dec 15;147(12):4360–4365. [PubMed] [Google Scholar]
- Porcelli S., Morita C. T., Brenner M. B. CD1b restricts the response of human CD4-8- T lymphocytes to a microbial antigen. Nature. 1992 Dec 10;360(6404):593–597. doi: 10.1038/360593a0. [DOI] [PubMed] [Google Scholar]
- Porcelli S., Yockey C. E., Brenner M. B., Balk S. P. Analysis of T cell antigen receptor (TCR) expression by human peripheral blood CD4-8- alpha/beta T cells demonstrates preferential use of several V beta genes and an invariant TCR alpha chain. J Exp Med. 1993 Jul 1;178(1):1–16. doi: 10.1084/jem.178.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimann J. Double-negative (CD4-CD8-), TCR alpha beta-expressing, peripheral T cells. Scand J Immunol. 1991 Dec;34(6):679–688. doi: 10.1111/j.1365-3083.1991.tb01592.x. [DOI] [PubMed] [Google Scholar]
- Robbins P. A., McMichael A. J. Immune recognition of HLA molecules downmodulates CD8 expression on cytotoxic T lymphocytes. J Exp Med. 1991 Jan 1;173(1):221–230. doi: 10.1084/jem.173.1.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson M. A. The human T cell receptor beta-chain gene complex contains at least 57 variable gene segments. Identification of six V beta genes in four new gene families. J Immunol. 1991 Jun 15;146(12):4392–4397. [PubMed] [Google Scholar]
- Sakamoto A., Sumida T., Maeda T., Itoh M., Asai T., Takahashi H., Yoshida S., Koike T., Tomioka H., Yoshida S. T cell receptor V beta repertoire of double-negative alpha/beta T cells in patients with systemic sclerosis. Arthritis Rheum. 1992 Aug;35(8):944–948. doi: 10.1002/art.1780350815. [DOI] [PubMed] [Google Scholar]
- Seman M., Boudaly S., Roger T., Morisset J., Pham G. Autoreactive T cells in normal mice: unrestricted recognition of self peptides on dendritic cell I-A molecules by CD4-CD8- T cell receptor alpha/beta+ T cell clones expressing V beta 8.1 gene segments. Eur J Immunol. 1990 Jun;20(6):1265–1272. doi: 10.1002/eji.1830200611. [DOI] [PubMed] [Google Scholar]
- Shiue L., Gorman S. D., Parnes J. R. A second chain of human CD8 is expressed on peripheral blood lymphocytes. J Exp Med. 1988 Dec 1;168(6):1993–2005. doi: 10.1084/jem.168.6.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivakumar S., Tsokos G. C., Datta S. K. T cell receptor alpha/beta expressing double-negative (CD4-/CD8-) and CD4+ T helper cells in humans augment the production of pathogenic anti-DNA autoantibodies associated with lupus nephritis. J Immunol. 1989 Jul 1;143(1):103–112. [PubMed] [Google Scholar]
- Sneller M. C., Straus S. E., Jaffe E. S., Jaffe J. S., Fleisher T. A., Stetler-Stevenson M., Strober W. A novel lymphoproliferative/autoimmune syndrome resembling murine lpr/gld disease. J Clin Invest. 1992 Aug;90(2):334–341. doi: 10.1172/JCI115867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stashenko P., Nadler L. M., Hardy R., Schlossman S. F. Characterization of a human B lymphocyte-specific antigen. J Immunol. 1980 Oct;125(4):1678–1685. [PubMed] [Google Scholar]
- Takahama Y., Kosugi A., Singer A. Phenotype, ontogeny, and repertoire of CD4-CD8- T cell receptor alpha beta + thymocytes. Variable influence of self-antigens on T cell receptor V beta usage. J Immunol. 1991 Feb 15;146(4):1134–1141. [PubMed] [Google Scholar]
- Takahama Y., Singer A. Post-transcriptional regulation of early T cell development by T cell receptor signals. Science. 1992 Nov 27;258(5087):1456–1462. doi: 10.1126/science.1439838. [DOI] [PubMed] [Google Scholar]
- Toribio M. L., de la Hera A., Regueiro J. R., Márquez C., Marcos M. A., Bragado R., Arnaiz-Villena A., Martínez C. Alpha/beta heterodimeric T-cell receptor expression early in thymocyte differentiation. J Mol Cell Immunol. 1988;3(6):347–362. [PubMed] [Google Scholar]
- Toyonaga B., Mak T. W. Genes of the T-cell antigen receptor in normal and malignant T cells. Annu Rev Immunol. 1987;5:585–620. doi: 10.1146/annurev.iy.05.040187.003101. [DOI] [PubMed] [Google Scholar]
- Wilson R. K., Lai E., Concannon P., Barth R. K., Hood L. E. Structure, organization and polymorphism of murine and human T-cell receptor alpha and beta chain gene families. Immunol Rev. 1988 Jan;101:149–172. doi: 10.1111/j.1600-065x.1988.tb00736.x. [DOI] [PubMed] [Google Scholar]
- Wirt D. P., Brooks E. G., Vaidya S., Klimpel G. R., Waldmann T. A., Goldblum R. M. Novel T-lymphocyte population in combined immunodeficiency with features of graft-versus-host disease. N Engl J Med. 1989 Aug 10;321(6):370–374. doi: 10.1056/NEJM198908103210606. [DOI] [PubMed] [Google Scholar]
- Wu L., Pearse M., Egerton M., Petrie H., Scollay R. CD4-CD8- thymocytes that express the T cell receptor may have previously expressed CD8. Int Immunol. 1990;2(1):51–56. doi: 10.1093/intimm/2.1.51. [DOI] [PubMed] [Google Scholar]
- Yasukawa M., Hato T., Matsumoto M., Takada K., Fujita S., Kobayashi Y. Proliferation of double-negative (CD4-CD8-) T cells bearing T-cell receptor-alpha beta in a haemophiliac with human immunodeficiency virus type 1 infection and factor VIII inhibitor: functional properties of double-negative T-cell receptor-alpha beta+ T cells. Br J Haematol. 1991 Nov;79(3):372–376. doi: 10.1111/j.1365-2141.1991.tb08043.x. [DOI] [PubMed] [Google Scholar]
- Yu D. T., Winchester R. J., Fu S. M., Gibofsky A., Ko H. S., Kunkel H. G. Peripheral blood Ia-positive T cells. Increases in certain diseases and after immunization. J Exp Med. 1980 Jan 1;151(1):91–100. doi: 10.1084/jem.151.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yui K., Wadsworth S., Yellen A., Hashimoto Y., Kokai Y., Greene M. I. Molecular and functional properties of novel T cell subsets in C3H-gld/gld and nude mice. Implications for thymic and extrathymic maturation. Immunol Rev. 1988 Aug;104:121–155. doi: 10.1111/j.1600-065x.1988.tb00761.x. [DOI] [PubMed] [Google Scholar]




