Abstract
Background
There are numerous health-related quality of life (HRQol) measurements used in coronary heart disease (CHD) in the literature. However, only values assessed with preference-based instruments can be directly applied in a cost-utility analysis (CUA).
Objective
To summarize and synthesize instrument-specific preference-based values in CHD and the underlying disease-subgroups, stable angina and post-acute coronary syndrome (post-ACS), for developed countries, while accounting for study-level characteristics, and within- and between-study correlation.
Methods
A systematic review was conducted to identify studies reporting preference-based values in CHD. A multivariate meta-analysis was applied to synthesize the HRQoL values. Meta-regression analyses examined the effect of study level covariates age, publication year, prevalence of diabetes and gender.
Results
A total of 40 studies providing preference-based values were detected. Synthesized estimates of HRQoL in post-ACS ranged from 0.64 (Quality of Well-Being) to 0.92 (EuroQol European”tariff”), while in stable angina they ranged from 0.64 (Short form 6D) to 0.89 (Standard Gamble). Similar findings were observed in estimates applying to general CHD. No significant improvement in model fit was found after adjusting for study-level covariates. Large between-study heterogeneity was observed in all the models investigated.
Conclusions
The main finding of our study is the presence of large heterogeneity both within and between instrument-specific HRQoL values. Current economic models in CHD ignore this between-study heterogeneity. Multivariate meta-analysis can quantify this heterogeneity and offers the means for uncertainty around HRQoL values to be translated to uncertainty in CUAs.
Introduction
A large number of health-related quality of life (HRQoL) measures for patients with coronary heart disease (CHD) is available in the literature[1–4]. Those measures either describe the HRQoL of patients suffering from CHD overall or distinguish across patients suffering from one of the underlying forms of CHD, specifically stable angina or post-acute coronary syndrome (post-ACS). This interest in estimating the level of HRQoL in CHD is mainly due to its increasing economic and clinical burden [5], the number of CHD prevention and treatment strategies available, and the necessity to assess the impact of a treatment on HRQoL for use as input parameter in cost-utility analyses (CUAs) [6,7].
From the abundance of HRQoL measurements in CHD, only the preference-based HRQoL values can be directly applied in CUA [6,8]. These values express the individual’s preference for living with CHD compared to other health states on an interval scale where the value of zero is assigned to death, and the value of one to full health [7]. Preference-based values can be generated with direct elicitation techniques, such as the time trade-off (TTO), the standard gamble (SG) and the rating scale (RS). Another approach is based on multi-attribute questionnaires, such as the EuroQol 5D (EQ-5D), Short form 6D (SF-6D), health utility index (HUI) and the quality of well-being (QWB) scale [7].
There is significant variation in the published literature with respect to the preference-based HRQoL value of patients with CHD [1,9–11]. Methodological differences between direct preference-based techniques such as the exact specifications of the questions on individuals’ preferences, have been shown to have considerable impact on HRQoL values [7]. Some of the differences in HRQoL values measured with preference-based multi-attribute instruments reflect the variation across instruments on the sensitivity of different health attributes across different severity levels as well as the use of different direct valuation techniques [7]. Finally, HRQoL values can largely vary due to differences in study-level covariates such as patients’ characteristics (e.g. underlying CHD forms, age, and comorbidities), types of treatment applied or time points of measuring HRQoL relative to the disease onset or treatment initiation.
All the aforementioned differences in the underlying methodology indicate that the choice of the HRQoL value to be applied in CUA needs to consider the impact of both instrument-specific properties and study-level covariates. Notably, when selecting a HRQoL value from the published literature, an evidence synthesis can provide better estimates around the mean and variance of HRQoL to inform CUA than a single study value. The application of meta-analysis on HRQoL values is straightforward when all values are measured with the same instrument. However, a number of studies provide multiple and correlated HRQoL values measured in the same population but using different instruments. Conducting separate univariate meta-analyses for each HRQoL is inappropriate as ignoring the within-study correlation might lead to biased mean and standard error (SE) estimates [12,13]. Instead, multivariate meta-regression analysis is recommended [12,13]. Therefore, in this study we aim to systematically summarize and synthesize the published preference-based HRQoL values in CHD and its underlying disease-subgroups (i.e. stable angina and post-ACS) for developed countries. To account for the underlying differences in the instruments used to measure the HRQoL, and the correlation between instrument-specific values both within and between studies, the synthesis of values is conducted on an instrument-specific level. Additionally, the impact of study-level covariates on HRQoL is explored in regression analysis.
Methods
Data collection
This study was conducted following the PRISMA guidelines and a checklist for their application in provided in S1 Appendix. Relevant studies reporting HRQoL in CHD were independently screened and systematically reviewed by two team members [JS and PP]. Studies were searched using MEDLINE and EMBASE. The search terms used were: (“coronary disease” or “coronary heart disease” or “myocardial infarction” or “angina” or “acute coronary syndrome”) and (“utility” or “quality of life” or “outcome assessment”) and ("Health Utilities Index" or "quality of well-being" or "rating scale" or "standard gamble" or "time trade-off" or "15D" or "SF-6D" or "EQ-5D" or "HALex"). See S2 Appendix for details of search strategy for MEDLINE and EMBASE. The search was limited to studies applying to developed countries published between 1990 and November 2014. The last search was conducted on the 15th of December 2014. By including only studies from developed countries, we aimed to limit the variation on HRQoL associated with the relation between socio-economic status and HRQoL [14]. Additionally, the references of the identified articles and other systematic reviews were searched for relevant studies not included in the above-mentioned databases (snowballing). Studies were considered eligible for this analysis if they: 1) applied a preference-based instrument of measuring HRQoL (TTO, SG, RS, EQ-5D, SF-6D, SF-15D, HUI, QWB and HALex) 2) reported mean preference-based HRQoL values measured three months or more after the initiation of CHD-treatment or after the onset of CHD, 3) reported standard deviations (SDs) and sample sizes or confidence intervals (CIs)/SEs of those measurements. Duplicate studies were excluded as well as editorials, letters, clinical conference abstracts, reviews and studies that reported median but not mean HRQoL values. Disagreements were resolved through discussion.
Data from the eligible studies were extracted in duplicate by two team members [JS and PP]. Any uncertainties were resolved by discussion. Data were extracted regarding study origin (authors, publication year, country), study design, participants (age, study sample, percentage of men, percentage of diabetics, underlying form of CHD) as well as the HRQoL (mean value, SD or CI/SE of the mean), type of instrument for measuring HRQoL and correlation coefficients between instrument-specific values. Moreover, for the HRQoL measured with the EQ-5D, we recorded a ‘tariff’ applied to the EQ-5D questionnaire data to generate preferences. Tariffs present valuation sets for EQ-5D health states derived using the TTO technique in nationality-specific population samples [7].
Assumptions and data adjustments
We distinguished between two underlying CHD subgroups: stable angina and post-ACS. The stable angina and the post-ACS groups comprised of stable angina patients and patients with unstable angina or myocardial infarction, respectively, in whom HRQoL was measured at least three months after diagnosis or treatment initiation. Our analysis was limited to HRQoL values measured three months after onset of CHD or treatment initiation hypothesizing that the impact of the acute disease onset or a treatment effect on HRQoL will be stabilized by then. Additionally, all the values identified, including the ones subgrouped to stable angina, post-ACS and the ones for which information on patients’ characteristics were insufficient to provide their allocation to either of the two underlying CHD subgroups, were analysed together as a general CHD group.
Furthermore, no distinction was made between the HRQoL values measured in patient subgroups other than the ones reflecting underlying CHD subgroups (e.g. different treatment or socio-economic subgroups). In cases where studies provided HRQoL values in a subgroup classification that was not of interest, the HRQoL values across subgroups were synthesized to provide a weighted mean and variance estimate for a specific CHD form per study.
Statistical model
A multivariate meta-analysis was used to estimate synthesized, instrument-specific HRQoL estimates in post-ACS, stable angina and general CHD [12]. This approach accounts for the correlation (both within and between studies) between HRQoL values assessed with different instruments and was extended to a multivariate meta-regression analysis to account for the impact of study-level covariates where appropriate. The general structure of the model applied is presented below in matrix form.
| (1) |
Here yi stands for a vector of size p whose elements comprise the instrument-specific HRQoL values for study i, Xi is a matrix of p instruments and k covariates, βi is the vector of regression coefficients of size k, δi is a vector of random-effects terms of size p and εi is a vector of random sampling errors of size p. We assumed that δi~MVN(0,Δ), where
| (2) |
Here Δ represents the between-study variance–covariance matrix and its elements are , the between-study instrument-specific variance, and ρτ(jj′), the between-study correlation coefficient assessed when measuring the HRQoL values with j and j′ instruments. Additionally, it was assumed that εi~MVN(0,Si), where
| (3) |
The matrix Si is the within-study variance–covariance matrix with elements , the within-study instrument-specific variance and ρjj′, the within-study correlation coefficient.
A common problem in multivariate meta-regression is the presence of missing data for variables of interest in eligible studies. In our analysis, missing data were anticipated for some of the yi elements and their corresponding variances, as well as for some within-study correlation coefficients. Missing values in yi may occur when HRQoL in a particular study was not estimated with all the instruments included in the meta-regression. We resolved this by setting the missing values in yi as equal to zero and their corresponding variances equal to an arbitrary large number (1,000) (11). In this way the contribution of these values to the summarized HRQoL estimate was insignificant. The problem related to missing values of ρjj′ was resolved by retrieving correlation estimates from a more general population without severe comorbidities. Elements of ρjj′ that still remained missing were assumed to be equal to zero [13]. In order to observe the impact of ignoring the presence of correlation and to account for possibly different values of correlation coefficients, we undertook a sensitivity analysis by assuming correlation coefficients to take values of 0 and 0.5 [15].
In the regression analysis, the covariates incorporated in Xi were examined for their statistical significance and their impact on reducing some of the between-study heterogeneity on those HRQoL values. Age, publication year, prevalence of diabetes and gender were the covariates examined. Following reasons were employed when choosing covariates: age and gender were previously shown to impact HRQoL [16]; diabetes is a commonly present comorbidity in patients with CHD, and is indicated to be associated with a reduced level of HRQoL (Coffey et al, 2002)[17]; publication year was explored to assess a possible improvement in HRQoL through time. There are no guidelines to suggest the minimal number of studies per outcome of interest (i.e. an instrument-specific HRQoL value) required for the regression analysis to be plausible in the multivariate setting. For this reason, we adopted the guidelines for the univariate setting that suggest a data set sample size of approximately 10 measurements may be sufficient for conducting a regression analysis with one covariate at a time [15]. The regression analysis was limited to those outcomes where at least 10 measurements were available.
In order to indicate the extent of heterogeneity in the true population level of HRQoL that is unexplained by study-level covariates and random sampling error in the multivariate setting, we calculated the and statistics, recently suggested by Jackson et al [18]. We used to measure the impact heterogeneity for all HRQoL estimates jointly and the statistic to measure the level of heterogeneity both jointly and separately for instrument-specific HRQoL estimates [18]. For the estimation of the , the R2 statistic was used as a basis. In the multivariate setting, the R2 statistic can be interpreted as the ratio of the volumes of CIs for summarized estimates under the random effect model and the volumes of CIs for summarized estimates under the fixed effects models [18]. Under such a notation of the R2 statistic, Jackson et al. suggested that the can be estimated as [18]. Furthermore, the statistic, was estimated as , where the H2 statistic represents the ratio of a generalized version of Cohran’s Q statistic and its associated degrees of freedom [18].
The multivariate meta-regression analysis was performed using the package mvmeta [19] in the statistical software R (version 2.15.3) [20].
Results
Study characteristics
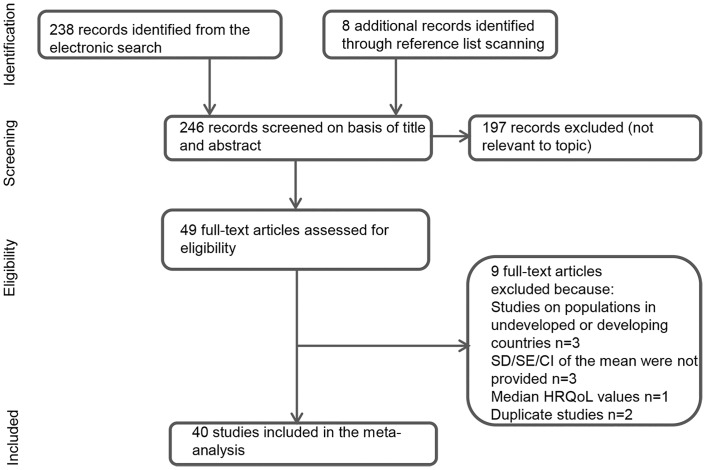
The process of study selection and extraction is presented in a PRISMA flow chart (Fig 1). A total of 40 eligible studies representing over 30,575 patients were identified after the inclusion and exclusion criteria were applied. A list of all potentially-eligible studies with reasons for exclusion is presented in S3 Appendix. The main characteristics of these studies are detailed in Table 1 [1–4,9–11,16,21–52]. The studies were published between 1991 and 2014, with most of them between 2005 and 2010. Of the 40 studies included, 10 referred to the UK, 8 to the US, 6 to the multiple country settings, 4 to Germany, 3 to Canada and Finland, 2 to Norway and Sweden, and 1 to each of Australia and Korea. Various study designs were implemented, ranging from randomised clinical trial to different observational study designs. Of the HRQoL values present in those 40 studies, 31% were observed in patients with stable angina and 29% in patients with post-ACS. The remaining 40% of the HRQoL values were associated with patients suffering from any form of CHD (including stable angina and post-ACS). The majority of patients were men (71%) with an average age of 65.35 years. There was large variation in patients’ characteristics, such as the presence and prevalence of various comorbidities (e.g. prevalence of diabetes was in range 7–38%), and treatment under evaluation (e.g. surgical procedures, pharmacological treatment or cardiovascular rehabilitation) across the studies. When reported, the time of HRQoL assessment from disease onset or treatment initiation was between 4 months and 10 years.
Fig 1. PRISMA flow diagram summarizing the study selection process.
HRQoL, health-related quality of life; SD, standard deviation; SE, standard error; CI, confidence interval.
Table 1. Summary of studies reporting preference-based values in developed countries in coronary heart disease.
| Author (Year), Country | Study design | CHD subgroup | Study sample (Sample size) | Instrument | Age | HRQoL value (SE) | Time (months)* | Men (%) | Diabetics (%) |
|---|---|---|---|---|---|---|---|---|---|
| Al Ruzzeh[21](2008), UK | RCT | Stable angina | Randomised to OPCAB (n = 66) | EQ-5D UK | 64 | 0.6580 (0.2500) | 6 | 84 | 21 |
| Randomised to CABG-CPB (n = 55) | 64 | 0.6540 (0.2100) | 83 | 24 | |||||
| Ascione (2004), UK[22] | RCT | ACS | Patients with previous MI or requirement for bypass surgery randomised to CABG-CPB (n = 151) | EQ-5D UK | 64 | 0.8200 (0.0230) | 36 | 81 | 33 |
| Patients with previous MI or requirement for bypass surgery randomised to OPCAB (n = 164) | EQ-5D UK | 66 | 0.8100 (0.0187) | 36 | 83 | 43 | |||
| Bakhai[23](2012), France, Spain, UK | Prospective observational cohort study | ACS | Patients undergoing PCI (N = 1140) | EQ-5D* | 62 | 0.8100 (0.0071) | 12 | 78 | NA |
| Bohmer[24](2011), Norway | Open RCT | ACS | Randomised to early invasive: angiography and PCI (n = 134) | 15D | 61 | 0.8890 (0.0138) | 7 | 80 | 6 |
| Randomised to late invasive: angiography and PCI (n = 132) | 62 | 0.8720 (0.0158) | 71 | 8 | |||||
| Burstrom[25](2001), Sweden | Retrospective cross-sectional survey | Stable angina | Respondents with self-reported angina (N = 191) | EQ-5D UK | NA | 0.7000 (0.0180) | NA | NA | NA |
| Respondents with self-reported angina (N = 180) | RS | NA | 0.6900 (0.0150) | NA | NA | NA | |||
| Chong[26](2009), Australia | Cross-sectional survey (prisoner population) | Stable angina | Respondents with self-reported angina (n = 81) | SF-6D | NA | 0.6440 (0.0146) | NA | NA | NA |
| Respondents with self-reported angina & MT (n = 17) | 0.6200 (0.0410) | ||||||||
| Cohen[27](2011), Europe and North America | Prospective substudy as part of RCT | chd | Randomised to CABG (n = 810) | EQ-5D US | 66 | 0.8470 (0.0054) | 6 | 79 | 39 |
| Randomised to PCI (n = 815) | 66 | 0.8610 (0.0052) | 76 | 38 | |||||
| Denvir[28](2006), UK | Prospective observational study | chd | Patients from high SES group undergoing PCI (n = 876) | EQ-5D* | 62 | 0.7500 (0.0088) | 12 | 69 | 10 |
| Patients from low SES group undergoing PCI (n = 462) | 62 | 0.6300 (0.0140) | 63 | 13 | |||||
| Dunning (2008), UK[29] | Prospective cross-sectional | chd | Patients undergoing CABG (N = 621) | EQ-5D* | 71 | 0.7000 (0.0123) | 120 | NA | NA |
| Ellis[30](2005), US | Cross-sectional survey | ACS | Patients with history of ACS (N = 490) | EQ-5D US | 66 | 0.8100 (0.0081) | 6 | 71 | NA |
| Fryback[31](1993), US | Longitudinal cohort study | Stable angina | Respondents with self-reported angina (n = 68) | QWB | 64 | 0.6600 (0.0015) | 12 | NA | NA |
| ACS | Respondents with self-reported ACS (n = 20) | 64 | 0.6400 (0,0175) | ||||||
| Garster[1](2009), US | Cross-sectional random-digit-dialled survey | chd | Respondents with self-reported CHD not taking chest pain MT (n = 265) | EQ-5D US | 70 | 0.8200 (0.0092) | NA | 57 | 30 |
| Respondents with self-reported CHD currently taking chest pain MT (n = 218) | 69 | 0.7400 (0.0142) | 49 | 47 | |||||
| Respondents with self-reported CHD not taking chest pain MT (n = 265) | HUI3 | 70 | 0.7500 (0.0154) | 57 | 30 | ||||
| Respondents with self-reported CHD currently taking chest pain MT (n = 218) | 69 | 0.5600 (0.0237) | 49 | 47 | |||||
| Respondents with self-reported CHD not taking chest pain MT (n = 265) | SF-6D | 70 | 0,7500 (0,0080) | 57 | 30 | ||||
| Respondents with self-reported CHD currently taking chest pain MT (n = 218) | 69 | 0.6700 (0.0102) | 49 | 47 | |||||
| Respondents with self-reported CHD not taking chest pain MT (n = 265) | QWB | 70 | 0.5800 (0.0086) | 57 | 30 | ||||
| Respondents with self-reported CHD currently taking chest pain MT (n = 218) | 69 | 0.5200 (0.0095) | 49 | 47 | |||||
| Respondents with self-reported CHD not taking chest pain MT (n = 265) | HUI2 | 70 | 0.8000 (0.0203) | 57 | 30 | ||||
| Respondents with self-reported CHD currently taking chest pain MT (n = 218) | 69 | 0.6900 (0.0277) | 49 | 47 | |||||
| Respondents with self-reported CHD not taking chest pain MT(n = 265) | HALex | 70 | 0.6800 (0.0275) | 57 | 30 | ||||
| Respondents with self-reported CHD currently taking chest pain MT(n = 218) | 69 | 0.5000 (0.0289) | 49 | 47 | |||||
| Griffin[32](2007), UK | Prospective observational study | chd | Patients undergoing CABG (n = 100) | EQ-5D UK | 65 | 0.6600 (0.0310) | 72 | 78 | 13 |
| Patients undergoing PCI (n = 108) | 65 | 0.6500 (0,0289) | |||||||
| Patients receiving MT (n = 131) | 65 | 0.6100 (0.0262) | |||||||
| Kattainen[33](2005), Finland | Longitudinal observational study | chd | Patients undergoing CABG (n = 393) | 15D | 63 | 0.8580 (0.0004) | 6 | 73 | 20 |
| Patients undergoing PCI (n = 153) | 61 | 0.8240 (0,0007) | 67 | ||||||
| Kiessling[9](2005), Sweden | Prospective RCT | chd | Patients with CAD randomised to CML GP attending seminars—supported lipid-lowering strategy (n = 45) | EQ-5D UK | 65 | 0.8000 (0.0042) | 24 | 82 | 11 |
| Patients with CAD randomised to CML GP following local guidelines—supported lipid-lowering strategy (n = 43) | 64 | 0.7600 (0.0070) | 88 | 14 | |||||
| Patients with CAD randomised to CML specialist group—supported lipid-lowering strategy (n = 167) | 61 | 0.7600 (0.0014) | 74 | 16 | |||||
| Kim[2](2005), UK | RCT | ACS | Patients randomised to maximal MT plus early coronary arteriography with possible myocardial revascularization (n = 806) | EQ-5D UK | 63 | 0.7520 (0.0090) | 12 | 61 | 15 |
| Patients randomised to maximal MT plus ischemia- or symptom-provoked angiography and revascularization (n = 820) | 62 | 0.7360 (0.0100) | 64 | 12 | |||||
| Kramer[10](2012), Germany | Quasi-experimental design | chd | CHD patients undergoing developers treatment pathway (n = 128) | EQ-5D Europe | 69 | 0.7812 (0.0153) | 6 | 79 | NA |
| CHD patients undergoing users treatment pathway (n = 70) | 69 | 0.6936 (0.0251) | 61 | NA | |||||
| CHD patients undergoing controls treatment pathway (n = 92) | 71 | 0.6645 (0.0265) | 59 | NA | |||||
| Lacey[34](2003), UK | Retrospective longitudinal survey | ACS | Post-MI patients (N = 222) | EQ-5D UK | 63 | 0.7180 (0.0163) | 12 | 75 | NA |
| Lee (2014), Korea[35] | Cross-sectional survey | chd | Respondents with self-reported CHD (N = 708) | EQ-5D Korea | 64 | 0.831 (0.0090) | 82 | 53 | 27 |
| Loponen[36](2009), Finland | Prospective observational study | Stable angina | Patients undergoing CABG (n = 213) | 15D | 67 | 0.8579 (0.0075) | 6 | 79 | 26 |
| Patients undergoing PCI (n = 208 | 65 | 0.8456 (0.0073) | 69 | 18 | |||||
| Nichol (1996), Canada[37] | Observational survey-based study | Stable angina | Respondents undergoing elective cardiac catheterization (n = 41) | SG | 58 | 0.8300 (0.0422) | NA | 87 | NA |
| Norris[3](2008), Canada | Prospective longitudinal cohort study | chd | Women undergoing catheterization (n = 479) | EQ-5D* | 67 | 0.8000 (0.0046) | 12 | 0 | 21 |
| Men undergoing catheterization (n = 1727) | 65 | 0.9000 (0.0024) | 12 | 100 | 22 | ||||
| Nowels[4](2005), US | Cross-sectional study | ACS | Post-MI patients (CCSG class I) (n = 67) | EQ-5D UK | 65 | 0.7800 (0.0244) | 6 | 69 | NA |
| Post-MI patients (CCSG class II) (n = 17) | 65 | 0.7200 (0.0289) | |||||||
| Pettersen[38](2008), Norway | Cohort study survey-based | ACS | Post-MI patients with LVEF>50% (n = 160) | EQ-5D UK | 64 | 0.8300 (0.0142) | 30 | 71 | 4 |
| Post-MI patients with LVEF = 40–50% (n = 53) | 65 | 0.7200 (0.0371) | |||||||
| Post-MI patients with LVEF<40%) (n = 30) | 66 | 0.7600 (0.0256) | |||||||
| Ose[11](2012), Austria, Belgium, UK, France, Germany, Netherlands, Slovenia and Switzerland | Cross-sectional observational study | chd | CHD patients (n = 2656) | EQ-5D Europe | 68 | 0.7300 (0.0043) | NA | 70 | NA |
| Puskas[39](2004), US | RCT | Stable angina | Randomised to OPCAB (n = 77) | EQ-5D UK | 63 | 0.7900 (0.0285) | 12 | 78 | 33 |
| Randomised to CABG (n = 79) | 64 | 0.8040 (0.0259) | 77 | 33 | |||||
| Saarni[40](2006), Finland | Survey-based, stratified cluster, sampling design | chd | Respondents with self-reported CHD (n = 555) | 15D | 70 | 0.8210 (0.0050) | NA | 53 | NA |
| Respondents with self-reported CHD (n = 555) | EQ-5D UK | 70 | 0.6840 (0.0120) | ||||||
| Schweikert[41](2009), Germany | Observational survey-based study | ACS | Patients with history of MI (N = 2950) | EQ-5D UK | 68 | 0.8650 (0.0028) | 109 | 79 | NA |
| Schweikert[42](2009), Germany | Comprehensive cohort design | ACS | Patients undergoing CR (inpatient setting) (n = 100) | EQ-5D Europe | 58 | 0.8910 (0.0183) | 12 | 79 | 17 |
| Patients undergoing CR (outpatient setting) (n = 47) | 55 | 0.9410 (0.0257) | 76 | 14 | |||||
| Serruys[43](2001), the ARTS (multicentre 19 countries) | RCT | Stable angina | Randomised to PCI (n = 593) | EQ-5D UK | 62 | 0.8600 (0.0066) | 6 | 77 | 19 |
| Randomised to CABG (n = 579) | 62 | 0.8600 (0.0062) | 76 | 16 | |||||
| Shah[44](2009), US | Observational survey-based study | ACS | Patients (70%) undergoing PCI (N = 32) | EQ-5D US | 89 | 0.7800 (0.0071) | 14 | 38 | 17 |
| Sharples[45](2007), UK | RCT | Stable angina | Patients with suspected or known CAD undergoing angiography (N = 898) | EQ-5D UK | 62 | 0.8020 (0.0035) | 6 | 69 | 13 |
| Patients with suspected or known CAD undergoing angiography (N = 898) | SF-6D | 62 | 0.6425 (0,0012) | ||||||
| Shrive[46](2007), Canada | Prospective longitudinal cohort study | chd | Patients (70%) undergoing PCI (N = 1954) | EQ-5D US | NA | 0.8700 (0.0034) | 12 | 77 | 15 |
| Patients (70%) undergoing PCI (N = 1954) | EQ-5D UK | NA | 0.8300 (0.0045) | ||||||
| Stafford (2011), UK[47] | Cross-sectional survey | Stable angina | Respondents with self-reported angina (n = 717) | EQ-5D UK | NA | 0.7110 (0.0265) | NA | NA | NA |
| ACS | Respondents with self-reported MI (n = 550) | EQ-5D UK | NA | 0.6360 (0.0171) | NA | NA | NA | ||
| Sullivan[16](2006), US | Survey-based, stratified cluster design | ACS | Respondents with self-reported MI (n = 244) | EQ-5D US | 62 | 0.7040 (0.0168) | NA | NA | NA |
| Stable angina | Respondents with self-reported angina (n = 228) | 69 | 0.6950 (0.0201) | ||||||
| Tsevat (1991), US[48] | Observational survey-based study | ACS | Survivors of MI (N = 80) | TTO | 61 | 0.8700 (0.0026) | 12 | 79 | NA |
| Visser[49](1994), UK | Comparative study | Stable angina | Angina patients (NYHA I) receiving MT against chest pain (n = 10) | QWB | 65 | 0.6800 (0.0316) | NA | 73 | NA |
| Angina patients (NYHA II) receiving MT against chest pain (n = 25) | 66 | 0.6200 (0.0180) | |||||||
| Angina patients (NYHA III) receiving MT against chest pain (n = 21) | 67 | 0.6200 (0.0262) | |||||||
| Weintraub (2008), US and Canada[50] | RCT | Stable angina | Randomised to PCI (n = 701) | SG | 63 | 0.9300 (0.0064) | 6 | 85 | 32 |
| Randomised to MT (n = 665) | SG | 63 | 0.9300 (0.0058) | 6 | 85 | 35 | |||
| Werdan[51](2012), Germany | Non-interventional, multicentre open-label prospective study | Stable angina | Angina patients receiving MT (ivabradine) (n = 2330) | EQ-5D UK | 66 | 0.8270 (0.0041) | 4 | 59 | 33 |
| Winkelmayer[52](2006), UK, Ireland and Netherlands | Prospective and cross-sectional design as a part of RCT | ACS | MI patients receiving MT (pravastatin) (N = 546) | HUI3 | 75 | 0.7350 (0.0111) | NA | 48 | 11 |
* The time point of measuring HRQoL relative to the disease onset or treatment application
HRQoL, quality of life; SE, standard error; ACS, acute coronary syndrome; CHD, coronary heart disease; HALex, Health and Activity Limitation Index; HUI, health utility index; QWB, quality of well-being; RS, rating scale; SG, standard gamble; TTO, time trade-off; UK, United Kingdom; US, United States; RCT, randomized clinical trial; NA, not available; MI, myocardial infarction; MT, medical treatment; CABG, coronary artery bypass graft; CPB, cardiopulmonary bypass; OPCAB, off-pump coronary artery bypass; SES, socio-economic status; CAD, coronary artery disease; CML, case method learning; CR, cardiac rehabilitation; GP, general practitioner; PCI, percutaneous coronary intervention; CCSG; Canadian Cardiovascular Society Classification for Angina Pectoris; LVEF; left ventricular ejection fraction
The most commonly applied instrument for measuring HRQoL values was the EQ-5D (63.5%), while 15D, QWB, SF-6D, HUI, SG, TTO, RS and HALex were less prevalent (7.7, 7.7, 5.8, 5.8, 3.8, 1.9, 1.9 and 1.9%, respectively). The values measured with the EQ-5D instrument varied by the TTO “tariffs” utilized (i.e. UK, US, Europe and Korea). The scoring of most of the EQ-5D values was based on the UK “tariff” (53%) while US, European and Korean “tariff” were less present (19, 8, 8 and 3%, respectively). Three studies provided no explicit information on the EQ-5D scoring “tariff” used, however, in order to allow for the evidence synthesis these values were grouped together with the UK “tariffs”. The values measured with the HUI instrument were presented in the studies as both mark 2 (i.e. HUI2) and mark 3 (i.e. HUI3).
Finally, the correlation coefficients between the instrument-specific HRQoL values, necessary for conducting a multivariate meta-analysis on the data set formed, were reported in only one of the studies included in the data set [31]. Therefore, some of the correlation coefficients were retrieved from other studies on cardiovascular patients or general populations without severe comorbidities (S1 Table) [31,53,54–57]. Nevertheless, a great number of within the instrument-specific correlation coefficients remained missing.
Multivariate meta-analysis estimates in post-ACS, stable angina, and general CHD
Table 2 summarizes the instrument-specific estimates synthesized through multivariate meta-analysis in the post-ACS, stable angina subgroups, and general CHD assessed on the full data set. The values for estimates synthesized in post-ACS ranged from 0.64 (QWB) to 0.92 (EQ-5D European”tariff”), while in stable angina estimates ranged from 0.64 (SF-6D) to 0.89 (SG). In general CHD, the values ranged from 0.60 (HALex) to 0.89 (SG). Between-study SDs and variance-covariance matrices for HRQoL in the post-ACS and stable angina subgroups, and general CHD, when these parameters could be estimated, are reported in S2–S4Tables.
Table 2. Post-acute coronary syndrome, stable angina, and general CHD parameter estimates for HRQoL and multivariate heterogeneity statistics using multivariate meta-analysis.
| Instrument | N | Post-ACS subgroup | N | Stable angina subgroup | N | CHD (full dataset) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 15D | 1 | 0.8816 (0.0074) | 1 | 0.8515 (0.0037) | 4 | 0.8495 (0.0069) | 99.9% | |||||
| EQ-5D Europe | 1 | 0.9170 (0.0105) | 3 | 0.7915 (0.0625) | 99.7% | |||||||
| EQ5D Korea | 1 | 0.8310 (0.0090) | ||||||||||
| EQ-5D UK | 8 | 0.7638 (0.0246) | 99.3% | 7 | 0.7792 (0.0250) | 99.4% | 22 | 0.7591 (0.0122) | 99.7% | |||
| EQ-5D US | 3 | 0.7662 (0.0308) | 97.3% | 1 | 0.6950 (0.0201) | 7 | 0.8012 (0.0128) | 98.0% | ||||
| HALex | 1 | 0.5967 (0.0075) | ||||||||||
| HUI2 | 1 | 0.7626 (0.0061) | ||||||||||
| HUI3 | 1 | 0.7350 (0.0111) | 2 | 0.7259 (0.0118) | 63.5% | |||||||
| QWB | 1 | 0.6400 (0.0175) | 2 | 0.6517 (0.0097) | 97.5% | 4 | 0.6287 (0.0189) | 99.4% | ||||
| RS | 1 | 0.6900 (0.0150) | ||||||||||
| SF-6D | 2 | 0.6413 (0.0017) | 51.9% | 3 | 0.6859 (0.0131) | 99.3% | ||||||
| SG | 2 | 0.8889 (0.0492) | 99.9% | 2 | 0.8889 (0.0492) | 99.9% | ||||||
| TTO | 1 | 0.8700 (0.0026) | 1 | 0.8700 (0.0026) | ||||||||
| 86.8% | 70.7% | 68.1% | 92.7% | 91.2% | 93.9% |
N presents the number of instrument-specific HRQoL values used for estimation.
All model coefficients with the level of significance p < 0.001 are presented in bold.
Standard errors of parameter estimates are showed in parentheses.
ACS, acute coronary syndrome; CHD, coronary heart disease; HRQoL, health-related quality of life; UK, United Kingdom; US, United States; HALex, Health and Activity Limitation Index; HUI, health utility index; QWB, quality of well-being; RS, rating scale; SG, standard gamble; TTO, time trade-off.
In this evidence synthesis, some of the instrument-specific HRQoL values included in the data set were present only as single inputs. Notably, the output of the multivariate meta-analysis in the aforementioned cases reflected the initial instrument-specific HRQoL inputs. Because the EQ-5D UK”tariff” values were the only instrument-specific values available as multiple inputs in both post-ACS and stable angina subgroups, a relatively fair comparison of the level of summarized HRQoL between the two subgroups would only be possible for this instrument-specific subgroup. This comparison indicated slightly lower estimates in post-ACS (i.e. 0.76) than the ones in stable angina (i.e. 0.78).
Large variations are noticeable across instrument-specific estimates of HRQoL in CHD presented in Table 2. The highest level of HRQoL was observed for the SG, TTO and 15D estimates, which were followed by slightly lower EQ-5D and HUI estimates, while the RS, SF-6D, QWB and HALex were the instruments with lowest HRQoL estimates.
Interestingly, substantial unexplained between-study but within-instrument heterogeneity was observed in all the multivariate meta-analysis models with the most excessive levels of heterogeneity observed in general CHD assessed on the full dataset (Table 2). There was a general agreement between both and statistics on the level of heterogeneity.
Regression analysis
The EQ-5D UK “tariff” values were the only instrument-specific values available with more than 10 observations per instrument used and therefore in line with our requirement for regression analysis. This led to reducing the analysis from a multivariate to a univariate meta-regression. Table 3 presents the EQ-5D UK “tariff” estimates synthesized through a univariate meta-regression where the impact of disease subgroup, age, publication year, and prevalence of diabetes and of men was examined on the HRQoL. Here, increasing age was found to generally reduce the level of HRQoL while prevalence of diabetes and higher proportion of men increased its level. The impact of publication year was inconclusive. However, none of the covariates examined was found to provide a significant improvement in the model fit nor did they reduce the between-study heterogeneity (Table 3).
Table 3. Parameter estimates and heterogeneity statistics for the EQ-5D UK “tariff” estimates using univariate meta-regression.
| Model number | Model | Post-ACS | I2 | Model number | Angina | I2 | Model number | General CHD | I2 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | b0 | 0.7587 (0.0215) | 99.6% | 5 | 0.7542 (0.0178) | 99.5% | 9 | 0.7652(0.0165) | 99.6% |
| bDisease type | 0.0165 (0.0339) | 0.0512 (0.0396) | |||||||
| bAge | -0.0051 (0.0059) | -0.0035 (0.0057) | -0.0056 (0.0057) | ||||||
| 2 | b0 | 0.7588 (0.0347) | 99.7% | 6 | 0.7497 (0.0355) | 99.6% | 10 | 0.7604 (0.0326) | 99.7% |
| bDisease type | 0.0059 (0.0333) | 0.0270 (0.0348) | |||||||
| bPublication year | -0.0002 (0.0052) | 0.0005 (0.0051) | -0.0001 (0.0050) | ||||||
| 3 | b0 | 0.7275 (0.0510) | 99.7% | 7 | 0.7405 (0.0443) | 99.7% | 11 | 0.7340 (0.0426) | 99.7% |
| bDisease type | 0.0125 (0.0457) | 0.0306 (0.0450) | |||||||
| bDiabetes | 0.0029 (0.0024) | 0.0017 (0.0026) | 0.0027 (0.0022) | ||||||
| 4 | b0 | 0.5982 (0.1456) | 99.7% | 8 | 0.5572 (0.1325) | 99.7% | 12 | 0.5964 (0.1407) | 99.7% |
| bDisease type | 0.0028 (0.0325) | 0.0540 (0.0331) | |||||||
| bMen | 0.0024 (0.0020) | 0.0028 (0.0018) | 0.0025 (0.0019) |
All model coefficients with the level of significance p < 0.001 are presented in bold.
Standard errors of parameter estimates are showed in parentheses.
For regression models 1–4, an intercept is provided assigned with b0, coefficients bDisease type (referring to post-ACS) and in model 1 bAge, in model 2 bPublication year, in model 3 bDiabetes and in model 4 bMen. The number of EQ-5D values in post-ASC available for models 1–4 was 8.
For regression models 5–8, an intercept is provided assigned with b0, coefficients bDisease type (referring to stable angina) and in model 5 bAge, in model 6 bPublication year, in model 7 bDiabetes and in model 8 bMen. The number of EQ-5D values in stable angina available for models 5–8 was 7.
For regression models 9–12, an intercept is provided assigned with b0, coefficients in model 9 bAge, in model 10 bPublication year, in model 11 bDiabetes and in model 12 bMen. The number of EQ-5D values in CHD in general available for models 19–12 was 22.
Example for interpretation: Model 4 –summary EQ-5D UK “tariff” estimate in men with post-ACS would be 0.6034 (i.e 0.5982+0.0028+0.0024), and 0.601 in women with similar characteristics (0.5982+0.0028).
ACS, acute coronary syndrome; CHD, coronary heart disease; UK, United Kingdom; b0, intercept.
Sensitivity analysis on the correlation coefficients between instrument-specific values was undertaken. The result of this sensitivity analysis was that the model was overall robust to different values of the correlation coefficients (S5 Table). Moreover, the SEs of instrument-specific estimates were generally insensitive to ignoring the correlation between the instrument-specific values or setting the values of correlation coefficients to 0.5.
Discussion
This is the first study that systematically summarized and synthesized instrument-specific preference-based HRQoL values in CHD, and its underlying disease-forms, post-ACS and stable angina in developed countries. Pooled mean HRQoL values were estimated and a large variation was observed both within and between the instrument-specific values. This variation could be explained by the large underlying heterogeneity in the study populations, and the observed and unobserved variation between the HRQoL instruments. Other factors possibly include the impact of treatment applied, initial (acute) disease severity level, national and socio-economic characteristics, various comorbidities present or the time of assessment. Moreover, the fact that TTO “tariffs” derived in nationality-specific population samples for the preference-based scoring of the EQ-5D vary across countries [58], suggests possible larger variations in the HRQoL values in CHD patients from various national or multinational settings. Additional arguments emphasize that unobservable differences in cultural or socio-economic status may also be present in representatives of general population samples selected for the assessment of tariffs [59]. The consequence of this would then be a greater underlying variability in HRQoL values in CHD assessed with instruments utilizing those tariffs. In essence, the aforementioned concerns may have a direct impact on the generalizability of country-specific HRQoL values to various national or multinational settings.
The regression analysis indicated no significant association between available study-level covariates and HRQoL estimates. Caution is needed in the interpretation of those findings, especially when low power for testing the effect of study-level covariates on the pooled HRQoL estimate is present—namely, only 22 EQ-5D UK “tariff” values were available for the regression-analysis. However, the reduction and increase in HRQoL observed with advancing age and higher proportion of men, respectively is in line with other published information [60]. Surprisingly, studies with a higher proportion of patients with diabetes had a higher average HRQoL estimate what contrasts the finding by Xie et al. [61]. This may be due to the missing information of the prevalence of diabetes across the studies as well as an example of ecological fallacy.
The variation between the instruments in the HRQoL estimates observed in our study is in agreement with the findings from other studies that demonstrated the differences across instrument-specific HRQoL values [62–64](Fryback et al 2009)[65]. Similarly to the study by Johnson et al, we observed higher levels of HRQoL when summarizing the EQ-5D US “tariffs” compared to the UK “tariffs”[58]. Additionally, SG values commonly exceed TTO values and RS values, a finding that was also confirmed in our study [66,67]. Furthermore, our synthesized HUI3 estimates in CHD were lower compared to the EQ-5D estimates, but similar to the findings of O’Brien et al. in patients at increased risk of sudden cardiac death and receiving implantable defibrillator therapy[63]. In our study, both the summarized and study-level SF-6D values were lower than EQ-5D values in CHD what contrasts the observations of Brazier et al.[1,45]. We observed the lowest level of HRQoL in CHD for estimates measured with the QWB and the HALex, what is in line with the patterns of values in general US population measured with six preference-based instruments by Fryback et al.[65] Differences such as the valuation technique, bounds of scale and sensitivity to change after treatment are only some of potential reasons for the variation between the instrument-specific HRQoL estimates [62–64].
For the evidence synthesis we applied multivariate meta-analysis given that when information on various instrument-specific values is sparse, it allows for “borrowing of strength” from the values available by accounting for the correlation between them [12,13]. Though such an approach may provide more precise summarized estimates [12,13] than a meta-analysis where the correlation is ignored, this did not hold in our study due to a high between-study variation.
Potentials for direct comparisons of our analysis to other synthesized HRQoL values are limited due to the lack of studies meta-analysing preference-based values in CHD. A meta-analysis of 84 studies identified to address HRQoL in cardiac patients by Kinney et al. may be one potential comparator to our study [68]. Kinney et al. investigated the effect of pharmacological, surgical, nursing or other treatment on HRQoL and found a small positive effect of treatment (i.e. standardised mean difference (d) = 0.31). Despite certain similarity in the patient populations investigated between the two studies can be acknowledged, numerous differences such as the study inclusion criteria (i.e. any measurement of HRQoL including the ones of single health attributes), choice of study effect size (i.e. standardised mean difference), the period of data collection (i.e. 1987–1991) and the methodology used for conducting meta-analysis (i.e. fixed-effect model) hamper adequate comparisons.
Another comparator to our study may be a review by Dyer et al. on the EQ-5D values in cardiovascular disease (CVD)[69]. Dyer et al. summarized and stratified the EQ-5D values across different CVD subgroups (e.g. ischemic heart disease (IHD) such as angina/myocardial infarction/CHD, heart failure etc.) and, when feasible, across three severity level categories defined by the percentage of patients in a given group in class III/IV of NYHA or CCS class[69]. Their stratification of the EQ-5D values with IHD collected at baseline resulted in the range of values from 0.45 for moderate/severe angina to 0.80 for mild angina. The authors did not synthesise these values across different severity levels due to the high heterogeneity observed (i.e. I2 of 82–96%), but suggested more rigorous study inclusion criteria and the possibility to expand their data set with more recent publications (i.e. studies published after 2008) as a method to reduce some of the heterogeneity observed[69]. However, the more rigorous inclusion criteria that our study proposed such as the inclusion of only mean HRQoL values measured in patients in stable or post-acute disease state, and incorporating HRQoL values from a wider publication range (i.e. 1990–2014) in the data set, did not reduce the high heterogeneity observed across studies. Notably, the heterogeneity indices observed in the study by Dyer et al. and the ones observed between the EQ-5D values in our study cannot be directly compared[69]. Differences between the two data sets and the method for disease stratification (i.e. post-ACS and stable angina subgroups vs. CCS class categories reported in ICH) are limiting such a comparison.
Our analysis is confronted with certain limitations. The main limitation of our study is that we analysed study-level and not patient-level data. Analysing patient-level data might provide significant improvements in our analysis. If detailed information on patients currently nonclassified specific CHD from was available, this would allow for a more accurate disease-specific allocation of HRQoL values. Conducting the multivariate meta-analysis on such a data set could possibly lead to estimates with lower level of between-study heterogeneity. Another limitation of our study was that we conducted the meta-regression analysis only on the subset of the EQ-5D UK “tariff” values due to a relatively small number of studies providing other instrument-specific values. This regression analysis was also limited with the respect to the variety of covariates investigated. Covariates such as patients’ socioeconomic status, presence of comorbidities other than diabetes or the impact of treatment applied were not investigated in the regression analysis due to their scarce information across the studies [70]. Expectedly, this study was confronted with the missing information on within-study correlation coefficients. This was solved by retrieving the correlation coefficients reported in other studies on cardiovascular patients or general populations. The sensitivity of the study results on the correlation coefficients utilized was tested in the sensitivity analysis. Furthermore, the publication bias was not formally assessed using a funnel plot due to the small number of instrument-specific HRQoL values included in this study as well as considerable heterogeneity. Also, publication bias is not expected in our meta-analysis given that the HRQoL values are commonly measured as secondary study outcomes and, therefore, are not expected to impact the decision to publish. Finally, information on HRQoL in CHD measured with non-preference-based and disease-specific instruments was not included in our analysis.
Importantly, our study did not aim to investigate what the most appropriate and reliable instrument to measure HRQoL in CHD is, but rather to summarize and synthesize all the available evidence of preference-based instrument-specific values. The decision on the most robust instrument-specific estimate to be applied in a CUA depends not only on the appropriateness and reliability of an instrument to measure HRQoL in CHD but also on its agreement with instrument-specific values available for other health states modelled in the CUA. Furthermore, some decision-makers might argue that considering the previously discussed reasons for country- and centre-specific variability in HRQoL values, one should simply choose a single country-specific and CHD-form specific HRQoL value. However, in the case where multiple country-specific values exist or even a single such value is unavailable, the decision on the most robust value becomes more complex and needs to rely on an evidence-synthesis exercise Moreover, although distinguishing between underlying CHD-forms seems as more clinically relevant, CUAs in both primary and secondary prevention of CHD often model a general CHD health state [71–74]. An evidence synthesis of all available HRQoL values may again be considered to select a robust HRQoL estimate in CHD. Such an estimate could then reflect more appropriately the complex nature of CHD and its various manifestations. This motivated us to consider an evidence-synthesis of HRQoL values in overall CHD.
Finally, characterizing the between study heterogeneity not only provides a better mean estimate for HRQoL values used in CUA but it also provides a better understanding on the uncertainty around the HRQoL value and how this translates into uncertainty around the CUA outcomes. This uncertainty, as we showed in our study, is considerable and as it is mostly found between studies, it is ignored when a single value from an individual study is selected. Dias et al. proposed that in the presence of between-study heterogeneity, using the predictive distribution is the appropriate way to characterize parameter uncertainty when embedding synthesized evidence in CUA [75]. Researchers using the findings from this study for economic evaluation purposes will therefore have to rely in generating values that incorporate both within- and between-study standard deviation (predictive distribution) provided in the results section of this article.
Conclusions
This study represents the first evidence synthesis of instrument-specific preference-based HRQoL values in post-ACS, stable angina, and CHD in general. Considerable differences in mean HRQoL estimates were observed both within and between the instruments. These differences characterized by large between-study heterogeneity may be explained by both the observed and unobserved methodological differences across instruments and underlying study-level characteristics. Current CUAs in CHD ignore this between-study study heterogeneity. Therefore, multivariate meta-analysis can facilitate quantifying this heterogeneity for HRQoL estimates and offer the means for uncertainty around HRQoL values to be translated to uncertainty in economic models.
Supporting Information
(DOC)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Garster NC, Palta M, Sweitzer NK, Kaplan RM, Fryback DG. Measuring health-related quality of life in population-based studies of coronary heart disease: comparing six generic indexes and a disease-specific proxy score. Quality of Life Research. 2009;(9):1239–1247. 10.1007/s11136-009-9533-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim J, Henderson RA, Pocock SJ, Clayton T, Sculpher MJ, Fox KAA. Health-related quality of life after interventional or conservative strategy in patients with unstable angina or non–ST-segment elevation myocardial infarction: one-year results of the third Randomized Intervention Trial of unstable Angina (RITA-3). J Am Coll Cardiol. 2005;(2):221–228. [DOI] [PubMed] [Google Scholar]
- 3.Norris CM, Spertus JA, Jensen L, Johnson J, Hegadoren KM, Ghali WA. Sex and gender discrepancies in health-related quality of life outcomes among patients with established coronary artery disease. Circulation: Cardiovascular Quality and Outcomes. 2008;(2):123–130. [DOI] [PubMed] [Google Scholar]
- 4.Nowels D, McGloin J, Westfall JM, Holcomb S. Validation of the EQ-5D quality of life instrument in patients after myocardial infarction. Quality of life research. 2005;(1):95–105. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization. World Health Organization. Fact sheet No 310 'Top ten causes of death'. 2008; Available: http://www.who.int/mediacentre/factsheets/fs310/en/index.html. Accessed 16 November 2012.
- 6.Drummond M. Introducing economic and quality of life measurements into clinical studies. Ann Med. 2001;(5):344–349. [DOI] [PubMed] [Google Scholar]
- 7.Drummond MF, Sculpher MJ, Torrance GW. Methods for the economic evaluation of health care programmes.: Oxford University Press, USA; 2005. [Google Scholar]
- 8.National Institute for Health and Care Excellence. Guide to the methods of technology appraisal 2013. 2013; Available: http://publications.nice.org.uk/guide-to-the-methods-of-technology-appraisal-2013-pmg9. Accessed 08 October 2013. [PubMed]
- 9.Kiessling A, Zethraeus N, Henriksson P. Cost of lipid lowering in patients with coronary artery disease by case method learning. Int J Technol Assess Health Care. 2005;(02):180–186. [PubMed] [Google Scholar]
- 10.Kramer L, Hirsch O, Schlößler K, Träger S, Baum E, Donner-Banzhoff N. Associations between demographic, disease related, and treatment pathway related variables and health related quality of life in primary care patients with coronary heart disease. Health and quality of life outcomes. 2012;(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ose D, Rochon J, Campbell SM, Wensing M, van Lieshout J, Uhlmann L, et al. Secondary prevention in patients with coronary heart diseases: what factors are associated with health status in usual primary care? PloS one. 2012;(12):e51726 10.1371/journal.pone.0051726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jackson D, Riley R, White IR. Multivariate meta‐analysis: Potential and promise. Stat Med. 2011;(20):2481–2498. 10.1002/sim.4172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mavridis D, Salanti G. A practical introduction to multivariate meta-analysis. Stat Methods Med Res. 2012;. [DOI] [PubMed] [Google Scholar]
- 14.Niedzwiedz CL, Katikireddi SV, Pell JP, Mitchell R. Socioeconomic inequalities in the quality of life of older Europeans in different welfare regimes. Eur J Public Health. 2014;(3):364–370. 10.1093/eurpub/cku017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to meta-analysis.: Wiley; 2011. [Google Scholar]
- 16.Sullivan PW, Ghushchyan V. Preference-based EQ-5D index scores for chronic conditions in the United States. Medical Decision Making. 2006;(4):410–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coffey JT, Brandle M, Zhou H, Marriott D, Burke R, Tabaei BP, et al. Valuing health-related quality of life in diabetes. Diabetes Care. 2002;(12):2238–2243. [DOI] [PubMed] [Google Scholar]
- 18.Jackson D, White IR, Riley RD. Quantifying the impact of between‐study heterogeneity in multivariate meta‐analyses. Stat Med. 2012;(29):3805–3820. 10.1002/sim.5453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.CRAN project. Multivariate meta-analysis and meta-regression 0.3.1. Package ‘mvmeta’. 2012; Available: http://cran.r-project.org/web/packages/mvmeta/mvmeta.pdf. Accessed 16 November 2012.
- 20.R. The R foundation for statistical computing, Rversion 2.14.0. 2011.
- 21.Al‐Ruzzeh S, Epstein D, George S, Bustami M, Wray J, Ilsley C, et al. Economic Evaluation of Coronary Artery Bypass Grafting Surgery With and Without Cardiopulmonary Bypass: Cost‐Effectiveness and Quality‐Adjusted Life Years in a Randomized Controlled Trial. Artif Organs. 2008;(11):891–897. 10.1111/j.1525-1594.2008.00647.x [DOI] [PubMed] [Google Scholar]
- 22.Ascione R, Reeves BC, Taylor FC, Seehra HK, Angelini GD. Beating heart against cardioplegic arrest studies (BHACAS 1 and 2): quality of life at mid-term follow-up in two randomised controlled trials. Eur Heart J. 2004;(9):765–770. [DOI] [PubMed] [Google Scholar]
- 23.BAKHAI A, FERRIERES J, IÑIGUEZ A, SARTRAL M, BELGER M, SCHMITT C, et al. Clinical Outcomes, Resource Use, and Costs at 1 Year in Patients with Acute Coronary Syndrome Undergoing PCI. J Interv Cardiol. 2012;. [DOI] [PubMed] [Google Scholar]
- 24.Bøhmer E, Kristiansen IS, Arnesen H, Halvorsen S. Health and cost consequences of early versus late invasive strategy after thrombolysis for acute myocardial infarction. European Journal of Cardiovascular Prevention & Rehabilitation. 2011;(5):717–723. [DOI] [PubMed] [Google Scholar]
- 25.Burström K, Johannesson M, Diderichsen F. Swedish population health-related quality of life results using the EQ-5D. Quality of Life Research. 2001;(7):621–635. [DOI] [PubMed] [Google Scholar]
- 26.Chong CAKY, Li S, Nguyen GC, Sutton A, Levy MH, Butler T, et al. Health-state utilities in a prisoner population: a cross-sectional survey. Health and quality of life outcomes. 2009;(Articl). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cohen DJ, Van Hout B, Serruys PW, Mohr FW, Macaya C, Den Heijer P, et al. Quality of life after PCI with drug-eluting stents or coronary-artery bypass surgery. N Engl J Med. 2011;(11):1016–1026. 10.1056/NEJMoa1001508 [DOI] [PubMed] [Google Scholar]
- 28.Denvir M, Lee A, Rysdale J, Walker A, Eteiba H, Starkey I, et al. Influence of socioeconomic status on clinical outcomes and quality of life after percutaneous coronary intervention. J Epidemiol Community Health. 2006;(12):1085–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dunning J, Waller JR, Smith B, Pitts S, Kendall SW, Khan K. Coronary artery bypass grafting is associated with excellent long-term survival and quality of life: a prospective cohort study. Ann Thorac Surg. 2008;(6):1988–1993. 10.1016/j.athoracsur.2008.02.024 [DOI] [PubMed] [Google Scholar]
- 30.Ellis JJ, Eagle KA, Kline-Rogers EM, Erickson SR. Validation of the EQ‐5D in patients with a history of acute coronary syndrome*. Current Medical Research and Opinion. 2005;(8):1209–1216 [DOI] [PubMed] [Google Scholar]
- 31.Fryback DG, Dasbach EJ, Klein R, Klein BEK, Dorn N, Peterson K, et al. The Beaver Dam Health Outcomes study Initial Catalog of Health-state Quality Factors. Medical Decision Making. 1993;(2):89–102. [DOI] [PubMed] [Google Scholar]
- 32.Griffin S, Barber J, Manca A, Sculpher M, Thompson S, Buxton M, et al. Cost effectiveness of clinically appropriate decisions on alternative treatments for angina pectoris: prospective observational study. BMJ. 2007;(7594):624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kattainen E, Sintonen H, Kettunen R, Merilainen P. Health-related quality of life of coronary artery bypass grafting and percutaneous transluminal coronary artery angioplasty patients: 1-year follow-up. Int J Technol Assess Health Care. 2005;(2):172–179. [PubMed] [Google Scholar]
- 34.Lacey E, Walters S. Continuing inequality: gender and social class influences on self perceived health after a heart attack. J Epidemiol Community Health. 2003;(8):622–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee HT, Shin J, Lim YH, Kim KS, Kim SG, Kim JH, et al. Health-Related Quality of Life in Coronary Heart Disease in Korea: The Korea National Health and Nutrition Examination Survey 2007 to 2011. Angiology. 2014. [DOI] [PubMed] [Google Scholar]
- 36.Loponen P, Luther M, Korpilahti K, Wistbacka JO, Huhtala H, Laurikka J, et al. HRQoL after coronary artery bypass grafting and percutaneous coronary intervention for stable angina. Scandinavian Cardiovascular Journal. 2009;(2):94–99. 10.1080/14017430802395450 [DOI] [PubMed] [Google Scholar]
- 37.Nichol G, Llewellyn-Thomas HA, Thiel EC, Naylor CD. The relationship between cardiac functional capacity and patients' symptom-specific utilities for angina: some findings and methodologic lessons. Med Decis Making. 1996;(1):78–85. [DOI] [PubMed] [Google Scholar]
- 38.Pettersen KI, Kvan E, Rollag A, Stavem K, Reikvam A. Health-related quality of life after myocardial infarction is associated with level of left ventricular ejection fraction. BMC cardiovascular disorders. 2008;(1):28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Puskas JD, Williams WH, Mahoney EM, Huber PR, Block PC, Duke PG, et al. Off-pump vs conventional coronary artery bypass grafting: early and 1-year graft patency, cost, and quality-of-life outcomes. JAMA. 2004;(15):1841–1849. [DOI] [PubMed] [Google Scholar]
- 40.Saarni SI, Härkänen T, Sintonen H, Suvisaari J, Koskinen S, Aromaa A, et al. The impact of 29 chronic conditions on health-related quality of life: a general population survey in Finland using 15D and EQ-5D. Quality of Life Research. 2006;(8):1403–1414. [DOI] [PubMed] [Google Scholar]
- 41.Schweikert B, Hunger M, Meisinger C, Konig HH, Gapp O, Holle R. Quality of life several years after myocardial infarction: comparing the MONICA/KORA registry to the general population. Eur Heart J. 2009;(4):436–443. 10.1093/eurheartj/ehn509 [DOI] [PubMed] [Google Scholar]
- 42.Schweikert B, Hahmann H, Steinacker JM, Imhof A, Muche R, Koenig W, et al. Intervention study shows outpatient cardiac rehabilitation to be economically at least as attractive as inpatient rehabilitation. Clinical research in cardiology. 2009;(12):787–795. 10.1007/s00392-009-0081-6 [DOI] [PubMed] [Google Scholar]
- 43.Serruys PW, Unger F, Sousa JE, Jatene A, Bonnier HJRM, Schönberger JPAM, et al. Comparison of coronary-artery bypass surgery and stenting for the treatment of multivessel disease. N Engl J Med. 2001;(15):1117–1124. [DOI] [PubMed] [Google Scholar]
- 44.Shah P, Najafi AH, Panza JA, Cooper HA. Outcomes and quality of life in patients≥ 85 years of age with ST-elevation myocardial infarction. Am J Cardiol. 2009;(2):170–174. 10.1016/j.amjcard.2008.08.051 [DOI] [PubMed] [Google Scholar]
- 45.Sharples L, Hughes V, Crean A, Dyer M, Buxton M, Goldsmith K, et al. Cost-effectiveness of functional cardiac testing in the diagnosis and management of coronary artery disease: a randomised controlled trial. The CECaT trial.: Gray Pub. 2007. [DOI] [PubMed] [Google Scholar]
- 46.Shrive FM, Ghali WA, Johnson JA, Donaldson C, Manns BJ. Use of the US and UK Scoring Algorithm for the EuroQol-5D in an Economic Evaluation of Cardiac Care. Med Care. 2007;(3):269–273. [DOI] [PubMed] [Google Scholar]
- 47.Stafford M, Soljak M, Pledge V, Mindell J. Socio-economic differences in the health-related quality of life impact of cardiovascular conditions. Eur J Public Health. 2012;(3):301–305. 10.1093/eurpub/ckr007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tsevat J, Goldman L, Lamas GA, Pfeffer MA, Chapin CC, Connors KF, et al. Functional status versus utilities in survivors of myocardial infarction. Med Care. 1991;(11):1153–1159. [DOI] [PubMed] [Google Scholar]
- 49.Visser M, Fletcher A, Parr G, Simpson A, Bulpitt C. A comparison of three quality of life instruments in subjects with angina pectoris: the Sickness Impact Profile, the Nottingham Health Profile, and the Quality of Well Being Scale. J Clin Epidemiol. 1994;(2):157–163. [DOI] [PubMed] [Google Scholar]
- 50.Weintraub WS, Boden WE, Zhang Z, Kolm P, Zhang Z, Spertus JA, et al. Cost-Effectiveness of Percutaneous Coronary Intervention in Optimally Treated Stable Coronary PatientsCLINICAL PERSPECTIVE. Circulation: Cardiovascular Quality and Outcomes. 2008;(1):12–20. [DOI] [PubMed] [Google Scholar]
- 51.Werdan K, Ebelt H, Nuding S, Höpfner F, Hack G, Müller-Werdan U. Ivabradine in combination with beta-blocker improves symptoms and quality of life in patients with stable angina pectoris: results from the ADDITIONS study. Clinical Research in Cardiology. 2012;1–9. [DOI] [PubMed] [Google Scholar]
- 52.Winkelmayer WC, Benner JS, Glynn RJ, Schneeweiss S, Wang PS, Brookhart MA, et al. Assessing health state utilities in elderly patients at cardiovascular risk. Medical decision making. 2006;(3):247–254. [DOI] [PubMed] [Google Scholar]
- 53.Lalonde L, Clarke AE, Joseph L, Mackenzie T, Grover SA. Comparing the psychometric properties of preference-based and nonpreference-based health-related quality of life in coronary heart disease. Quality of Life Research. 1999;(5):399–409. [DOI] [PubMed] [Google Scholar]
- 54.Franks P, Hanmer J, Fryback DG. Relative disutilities of 47 risk factors and conditions assessed with seven preference-based health status measures in a national US sample: toward consistency in cost-effectiveness analyses. Med Care. 2006;(5):478–485. [DOI] [PubMed] [Google Scholar]
- 55.Honkalampi T, Sintonen H. Do the 15D scores and time trade-off (TTO) values of hospital patients' own health agree? Int J Technol Assess Health Care. 2010;(01):117–123. [DOI] [PubMed] [Google Scholar]
- 56.Moock J, Kohlmann T. Comparing preference-based quality-of-life measures: results from rehabilitation patients with musculoskeletal, cardiovascular, or psychosomatic disorders. Quality of Life Research. 2008;(3):485–495. 10.1007/s11136-008-9317-6 [DOI] [PubMed] [Google Scholar]
- 57.Bosch JL, Hunink MM. Comparison of the Health Utilities Index Mark 3 (HUI3) and the EuroQol EQ-5D in patients treated for intermittent claudication. Quality of Life Research. 2000;(6):591–601. [DOI] [PubMed] [Google Scholar]
- 58.Johnson JA, Luo N, Shaw JW, Kind P, Coons SJ. Valuations of EQ-5D health states: are the United States and United Kingdom different? Med Care. 2005;(3):221–228. [DOI] [PubMed] [Google Scholar]
- 59.Havranek EP, Steiner JF. Valuation of health states in the US versus the UK: two measures divided by a common language? Med Care. 2005;(3):201–202. [DOI] [PubMed] [Google Scholar]
- 60.Emery CF, Frid DJ, Engebretson TO, Alonzo AA, Fish A, Ferketich AK, et al. Gender differences in quality of life among cardiac patients. Psychosom Med. 2004;(2):190–197. [DOI] [PubMed] [Google Scholar]
- 61.Xie J, Wu EQ, Zheng ZJ, Sullivan PW, Zhan L, Labarthe DR. Patient-reported health status in coronary heart disease in the United States: age, sex, racial, and ethnic differences. Circulation. 2008;(5):491–497. 10.1161/CIRCULATIONAHA.107.752006 [DOI] [PubMed] [Google Scholar]
- 62.Van Stel HF, Buskens E. Comparison of the SF-6D and the EQ-5D in patients with coronary heart disease. Health Qual Life Outcomes. 2006;20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.O'Brien BJ, Spath M, Blackhouse G, Severens J, Dorian P, Brazier J. A view from the bridge: agreement between the SF‐6D utility algorithm and the Health Utilities Index. Health Econ. 2003;(11):975–981. [DOI] [PubMed] [Google Scholar]
- 64.Brazier J, Roberts J, Tsuchiya A, Busschbach J. A comparison of the EQ‐5D and SF‐6D across seven patient groups. Health Econ. 2004;(9):873–884. [DOI] [PubMed] [Google Scholar]
- 65.Fryback DG, Dunham NC, Palta M, Hanmer J, Buechner J, Cherepanov D, et al. US norms for six generic health-related quality-of-life indexes from the National Health Measurement study. Med Care. 2007;(12):1162–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bleichrodt H. A new explanation for the difference between time trade‐off utilities and standard gamble utilities. Health Econ. 2002;(5):447–456. [DOI] [PubMed] [Google Scholar]
- 67.Bleichrodt H, Johannesson M. Standard gamble, time trade-off and rating scale: experimental results on the ranking properties of QALYs. J Health Econ. 1997;(2):155–175. [DOI] [PubMed] [Google Scholar]
- 68.Kinney MR, Burfitt SN, Stullenbarger E, Rees B, DeBolt MR. Quality of life in cardiac patient research: a meta-analysis. Nurs Res. 1996;(3):173–180. [DOI] [PubMed] [Google Scholar]
- 69.Dyer MTD, Goldsmith KA, Sharples LS, Buxton MJ. A review of health utilities using the EQ-5D in studies of cardiovascular disease. Health and Quality of Life Outcomes 2010. 8:13 10.1186/1477-7525-8-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pinquart M, Sörensen S. Influences of socioeconomic status, social network, and competence on subjective well-being in later life: a meta-analysis. Psychol Aging. 2000;(2):187 [DOI] [PubMed] [Google Scholar]
- 71.Gillespie P, O'Shea E, Murphy AW, Byrne MC, Byrne M, Smith SM, et al. The cost-effectiveness of the SPHERE intervention for the secondary prevention of coronary heart disease. Int J Technol Assess Health Care. 2010;(03):263–271. [DOI] [PubMed] [Google Scholar]
- 72.Pignone M, Earnshaw S, Tice JA, Pletcher MJ. Aspirin, statins, or both drugs for the primary prevention of coronary heart disease events in men: a cost–utility analysis. Ann Intern Med. 2006;(5):326–336. [DOI] [PubMed] [Google Scholar]
- 73.Pletcher MJ, Lazar L, Bibbins-Domingo K, Moran A, Rodondi N, Coxson P, et al. Comparing impact and cost-effectiveness of primary prevention strategies for lipid-lowering. Ann Intern Med. 2009;(4):243–254. [DOI] [PubMed] [Google Scholar]
- 74.Greving J, Visseren F, de Wit G, Algra A. Statin treatment for primary prevention of vascular disease: whom to treat? Cost-effectiveness analysis. BMJ. 2011. [DOI] [PubMed] [Google Scholar]
- 75.Dias S, Welton NJ, Sutton AJ, Ades AE. Evidence synthesis for decision making 5: the baseline natural history model. Med Decis Making. 2013;(5):657–670. 10.1177/0272989X13485155 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.