Abstract
We used active and passive surveillance to estimate nontyphoidal Salmonella (NTS) infection during 2012 in Guangdong Province, China. Under passive surveillance, for every reported NTS infection, an estimated 414.8 cases occurred annually. Under active surveillance, an estimated 35.8 cases occurred. Active surveillance provides remarkable advantages in incidence estimate.
Keywords: Nontyphoidal Salmonella infection, Salmonella, epidemiology, burden of illness pyramid model, bacteria, Guangdong Province, China, foodborne illness
Gastrointestinal illness or diarrhea caused by foodborne pathogens, such as nontyphoidal Salmonella (NTS), is a global public health concern (1). Many countries (e.g., the United States, England and Wales, Australia, Canada, Jordan, and Japan) have estimated the incidence of gastrointestinal illness caused by specific pathogens (2–8). However, in China, information is limited about the incidence of specific foodborne pathogens.
In 2003, China initiated a national, internet-based disease reporting system called the National Notifiable Disease Reporting System (NNDRS). This system legally requires routine reporting from all medical institutions and public health units of a list of infectious diseases. In this system, diarrheal pathogens other than Vibiro cholerae and Shigella dysenteriae, such as Salmonella spp., Escherichia coli, and Listeria spp., are reported as “other infectious diarrhea”; information about etiology is provided as an additional comment (9). Because NNDRS is passive, few reports include laboratory confirmation. According to previous data from passive surveillance, <1,000 NTS cases were reported in Guangdong Province annually since 2009, representing only a small proportion of actual infections.
In 2009, Guangdong Provincial Center for Disease Control and Prevention (Guangdong CDC) established laboratory-based active surveillance for NTS infection. In 2012, this system covered more than half of the Guangdong Province prefectures, capturing 61.5% of the population served by 27 sentinel hospitals (21 general hospitals and 6 specialized hospitals, including pediatric and gynecologic). In the surveillance system, patients with >3 loose stools in a 24-hour period plus fever, vomiting, or abdominal pain who visited the sentinel hospitals were enrolled as cases, and fecal samples were collected. The sentinel hospitals were required to forward Salmonella isolates to Guangdong CDC, along with epidemiologic data, for analysis. Culture-confirmed cases were then reported to NNDRS with pathogen information. Based on the pyramid model of burden of illness, we used data from active and passive surveillance to estimate NTS infection and to clarify the advantages and disadvantages of each system (2,7,10).
The Study
The estimation requires multiple steps. First, a person must have symptoms severe enough for medical care (multiplier 1). Second, the physician must collect patients’ specimens (multiplier 2) and forward them for testing by bacterial culture (multiplier 3). Third, the sample test result must be positive (multiplier 4), and the confirmed case must be reported (multiplier 5) (2,7,8).
To obtain multiplier 1, we conducted a 12-month population-based household survey during March 1, 2012–February 28, 2013 (approved by the Ethics Committee of Guangdong CDC). Respondents were randomly selected from 4 districts in western, eastern, and central Guangdong Province. The case definition was the same as that for active surveillance. We used a standard questionnaire to collect information about diarrhea in the previous 4 weeks. The incidence rate of diarrhea was 0.1081 (95% CI 0.1004–0.1158) episodes/person-year; 38.6% of the household survey respondents with diarrhea sought medical care. Multipliers 2 and 3 were based on data from sentinel hospitals and comprised the overall number of diarrhea cases, samples collected, and samples submitted for culture during the year. A total of 75,583 (45.3%) samples of 166,729 registered diarrhea cases in the sentinel hospitals were collected, of which 22,577 (29.9%) were tested. Laboratories of sentinel hospitals cultured samples for Salmonella in accordance with standard protocol provided by the national reference laboratory by using MacConkey agar as plating medium. According to a proficiency testing program, the Salmonella isolation sensitivity rate of these laboratories was 87.5% (multiplier 4). The numbers of Salmonella isolates identified and reported to NNDRS as NTS infectious diarrhea by all sentinel hospitals yielded the proportion of cases reported (648/1,061, 61.1%) (multiplier 5). We estimated the number of infections using the above 5 multipliers. Thus, active surveillance for each reported NTS infection identified 35.8 cases. We also analyzed multipliers of specialized hospitals (Table 1).
Table 1. Active and passive surveillance multipliers used to determine the incidence of nontyphoidal Salmonella infections, Guangdong Province, China, 2012*.
| Surveillance steps | Active surveillance |
Passive surveillance, by age group, y |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Overall | General hospitals | Specialized hospitals | Overall | 0–4 | 5–24 | 25–44 | 45–64 | >65 | ||
| Multiplier 1: Patient seeks medical care | 2.59 | 2.59 | 2.59 | 2.59 | 1.25 | 5.0 | 8.0 | 2.0 | 3.0 | |
| Multiplier 2: Physician obtains samples | 2.21 | 2.01 | 2.31 | 2.21 | 2.21 | 2.21 | 2.21 | 2.21 | 2.21 | |
| Multiplier 3: Samples tested for Salmonella | 3.35 | 2.45 | 6.73 | 3.35 | 3.35 | 3.35 | 3.35 | 3.35 | 3.35 | |
| Multiplier 4: Positive laboratory test result | 1.14 | 1.14 | 1.14 | 2.07 | 2.07 | 2.07 | 2.07 | 2.07 | 2.07 | |
| Multiplier 5: Confirmed cases reported | 1.64 | 1.60 | 1.82 | 10.45 | 10.45 | 10.45 | 10.45 | 10.45 | 10.45 | |
| Overall | 35.8 | 23.3 | 83.7 | 414.8 | 200.1 | 800.6 | 1,280.9 | 320.2 | 480.4 | |
*Incidence is cases per 100,000 persons.
In the population survey–based passive surveillance system, multiplier 1 was the same as for active surveillance. According to a comparison with samples from the submission proportion in a survey of physician-diagnosed diarrhea in Guangdong Province during 2009 (Mann-Whitney test, p = 0.246) (11), and a comparison between medical institutions that charged and did not charge for testing (Kolmogorov-Smirnov test, p = 0.837), the proportion of samples submitted and tested from active surveillance were also used as estimates of passive surveillance. The average test sensitivity of sentinel laboratories before active surveillance began was used as an estimate of all medical institutions (i.e., the sensitivity of passive surveillance [48.2%]). Using numbers of Salmonella isolates in Guangdong Province from laboratory data, and number of reported NTS cases by all medical institutions, we determined the proportion of reported NTS was 9.6% (991/10,360). Thus, for each reported NTS case under passive surveillance, 414.8 cases actually occurred. Multipliers of 5 age groups also were presented (Table 1).
To generate a more robust estimate, we conducted uncertainty and sensitivity analyses (online Technical Appendix, http://wwwnc.cdc.gov/EID/article/22/4/15-1372-Techapp1.pdf) on passive surveillance data using Monte Carlo simulation (@Risk 6.0; Palisade, Ithaca, NY, USA) (12). We used β distribution to describe the uncertainty of proportions and negative binominal distribution to estimate the number of cases. The sensitivity analysis helped determine factors that provide higher uncertainty in the estimate.
The uncertainty analysis model predicted a 411.9 (95% CI 308.4–592.7) overall multiplier and estimated that 408,499 (95% CI 302,899–591,901) Salmonella cases occurred per year when the overall multiplier was applied to the 991 reported NTS cases, resulting in 391.6 (95% CI 290.3–567.4) cases/100,000 persons in 2012. Incidence for 5 age groups was also estimated (Table 2). The rank correlation of various factors in the model showed that patients seeking medical care provided the highest uncertainty in the overall estimate (influence rate 96%) (Figure).
Table 2. Results of Monte Carlo simulation characterizing the uncertainty about the number of cases and the passive surveillance multipliers and the annual reported and estimated cases and incidence of nontyphoidal Salmonella infections, Guangdong Province, China, 2012.
| Age group, y | Annual no. cases, median (95% CI) | Multipliers, median (95% CI) | No. cases |
Annual incidence, cases/100,000 |
|||
|---|---|---|---|---|---|---|---|
| Reported | Estimated (95% CI) | Reported | Estimated (95% CI) | ||||
| 0–4 | 87,195 (68,367–138,725) | 209.6 (168.5–332.1) | 415 | 87,195 (68,367–138,725) | 6.13 | 1,287.9 (1,009.8–2,049.0) | |
| 5–24 | 222,558 (90,222–1,376,278) | 605.3 (248.9–3720.3) | 367 | 222,558 (90,222–1,376,278) | 0.90 | 547.7 (222.0–3387.1) | |
| 25–44 | 119,409 (49,292–488,534) | 1038.3 (436.4–4250.9) | 115 | 119,409 (49,292–488,534) | 0.32 | 333.4 (137.6–1,364.1) | |
| 45–64 | 18,577 (11,400–36,234) | 320.2 (216.8–603.2) | 58 | 18,577 (11,400–36,234) | 0.40 | 126.6 (77.7–247.0) | |
|
>65 |
16,421
(8,351–42,273) |
457.1
(259.7–1156.5) |
36 |
16,421
(8,351–42,273) |
|
0.56 |
255.1
(129.7–656.8) |
| Overall | 408,499 (302,899–591,901) | 411.9 (308.4–592.7) | 991 | 408,499 (302,899–591,901) | 0.95 | 391.6 (290.3–567.4) | |
Figure.
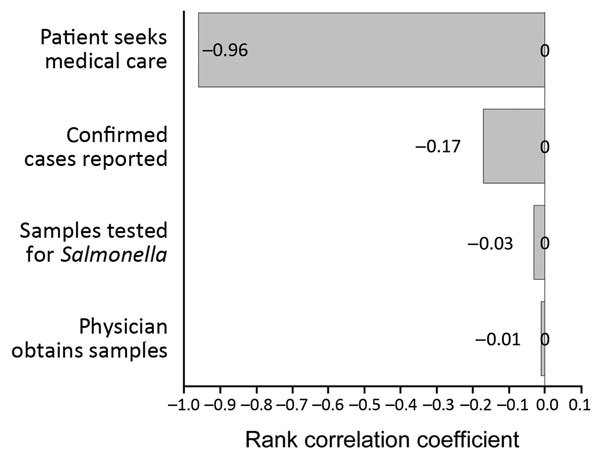
Rank correlations for the total number of nontyphoidal Salmonella cases in the population (tornado diagram), Guangdong Province, China, 2012.
Conclusions
Our estimated NTS incidence was lower than the incidence in China as determined from a literature review (626.5 cases/100,000 persons) (13) but close to that in the United States (352.1 cases/100,000 persons) (3). However, incidences for persons <5 years of age and 5–24 years of age in our study were higher than those for persons in China and the United States, highlighting that Salmonella represents a major health problem in Guangdong Province, especially among younger persons. Our estimated active surveillance rate (35.8) of NTS infections per reported case is similar to estimates in the United States (38.6 and 39) (2,10) but different from those for England (3.2), Jordan (278), and Japan (63) (7,8,14). Such differences might be due to differences in methods used and to actual differences in Salmonella infections.
With fewer missing cases and less underestimation, active surveillance has lower overall multipliers than passive surveillance, indicating smaller surveillance artifacts and more accurate incidence estimate and presents remarkable advantages over passive surveillance. The estimate for active surveillance also showed that if we seek to reduce uncertainty in the overall estimate, we should first focus on encouraging patients to seek medical care.
Our study provides policymakers in China with a reference for the importance of Salmonella incidence and calls for balanced surveillance on both foodborne infections and foods and enlarging active surveillance scales. More surveillance guidelines need to be developed to help physicians identify timing of sampling, tests, and performance. Laws requiring reporting of foodborne diseases and pathogens need to be enacted to increase quantity and quality of reporting. The result suggests that to increase care seeking and sample submission, government health insurance schemes should be further developed to cover diagnostic tests and treatments of diseases of public health significance.
Uncertainty and sensitivity analysis and study limitations for a study of nontyphoidal Salmonella infection, Guangdong Province, China, 2012.
Acknowledgments
We thank professionals from prefectural and county level CDCs for the household survey and physicians at sentinel hospitals for data collection. We also thank Olga Henao, Dana Cole, and Shua Chai for suggestions on the study and Jianghui Zhu and Xiaoyu Song for statistical review.
The study was supported by the China–US Collaborative Program on Emerging and Re-emerging Infectious Diseases (subproject 6) (1U2GHH00018-01) and Guangdong Medical Research Foundation (sponsored projectA2013069).
Biography
Ms. Xi Huang is a master of medicine and works as a public health physician at the Guangdong CDC, Guangdong, China. Her research interests include foodborne disease surveillance, outbreak detection, and epidemiologic investigation.
Footnotes
Suggested citation for this article: Huang X, Huang Q, Dun Z, Huang W, Wu S, Liang J, et al. Nontyphoidal Salmonella infection, Guangdong Province, China, 2012. Emerg Infect Dis. 2016 Apr [date cited]. http://dx.doi.org/10.3201/eid2204.151372
Preliminary results from this study were presented at the 2015 International Conference on Emerging Infectious Diseases (board 189), August 24–26, 2015, Atlanta, Georgia, USA.
These authors contributed equally to this article.
References
- 1.Majowicz SE, Musto J, Scallan E, Angulo FJ, Kirk M, O’Brien SJ, et al. The global burden of nontyphoidal Salmonella gastroenteritis. Clin Infect Dis. 2010;50:882–9 and. 10.1086/650733 [DOI] [PubMed] [Google Scholar]
- 2.Voetsch AC, Van Glider TJ, Angulo FJ, Farley MM, Shallow S, Marcus R, et al. FoodNet estimate of the burden of illness caused by nontyphoidal Salmonella infections in the United States. Clin Infect Dis. 2004;38(Suppl 3):S127–34 and. 10.1086/381578 [DOI] [PubMed] [Google Scholar]
- 3.Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson MA, Roy SL, et al. Foodborne illness acquired in the United States—major pathogens. Emerg Infect Dis. 2011;17:7–15 and. 10.3201/eid1701.P11101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adak GK, Meakins SM, Yip H, Lopman BA, O’Brien SJ. Disease risks from foods, England and Wales, 1996–2000. Emerg Infect Dis. 2005;11:365–72 . 10.3201/eid1103.040191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hall G, Kirk MD, Becker N, Gregory JE, Unicomb L, Millard G, et al. Estimating foodborne gastroenteritis, Australia. Emerg Infect Dis. 2005;11:1257–64 and. 10.3201/eid1108.041367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomas MK, Majowicz SE, Sockett PN, Fazil A, Pollari F, Doré K, et al. Estimated numbers of community cases of illness due to Salmonella, Campylobacter and verotoxigenic Escherichia coli: pathogen-specific community rates. Can J Infect Dis Med Microbiol. 2006;17:229–34 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gargouri N, Walke H, Belbeisi A, Hadadin A, Salah S, Ellis A, et al. Estimated burden of human Salmonella, Shigella, and Brucella infections in Jordan, 2003–2004. Foodborne Pathog Dis. 2009;6:481–6 . 10.1089/fpd.2008.0192 [DOI] [PubMed] [Google Scholar]
- 8.Kubota K, Iwasaki E, Inagaki S, Nokubo T, Sakurai Y, Komatsu M, et al. The human health burden of foodborne infections caused by Campylobacter, Salmonella, and Vibrio parahaemolyticus in Miyagi Prefecture, Japan. Foodborne Pathog Dis. 2008;5:641–8 and. 10.1089/fpd.2008.0092 [DOI] [PubMed] [Google Scholar]
- 9.Varma JK, Wu S, Feng Z. Detecting and controlling foodborne infections in humans: lessons for China from the United States experience. Glob Public Health. 2012;7:766–78 and. 10.1080/17441692.2011.641988 [DOI] [PubMed] [Google Scholar]
- 10.Chalker RB, Blaser MJ. A review of human salmonellosis: III. Magnitude of Salmonella infection in the United States. Rev Infect Dis. 1988;10:111–24 and. 10.1093/clinids/10.1.111 [DOI] [PubMed] [Google Scholar]
- 11.Ke B, Ran L, Wu S, Deng X, Ke C, Feng Z, et al. Survey of physician diagnostic and treatment practices for patients with acute diarrhea in Guangdong Province, China. Foodborne Pathog Dis. 2012;9:47–53 and. 10.1089/fpd.2011.0964 [DOI] [PubMed] [Google Scholar]
- 12.Powell M, Ebel E, Schlosser W. Considering uncertainty in comparing the burden of illness due to foodborne microbial pathogens. Int J Food Microbiol. 2001;69:209–15 and. 10.1016/S0168-1605(01)00495-0 [DOI] [PubMed] [Google Scholar]
- 13.Mao X, Hu J, Liu X. Estimation on disease burden of foodborne nontyphoid salmonellosis in China using literature review method. Chinese Journal of Disease Control and Prevention. 2011;15:622–5. [Google Scholar]
- 14.Wheeler JG, Sethi D, Cowden JM, Wall PG, Rodrigues LC, Tompkins DS, et al. Study of infectious intestinal disease in England: rates in the community, presenting to general practice, and reported to national surveillance. The Infectious Intestinal Disease Study Executive. BMJ. 1999;318:1046–50 and. 10.1136/bmj.318.7190.1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Uncertainty and sensitivity analysis and study limitations for a study of nontyphoidal Salmonella infection, Guangdong Province, China, 2012.


