To the Editor: Leishmania infantum is endemic to northeastern Brazil. It is responsible for visceral leishmaniasis (VL), a major emerging health problem in urban areas. Transmission occurs predominantly by the Lutzomyia longipalpis sand fly, but transfusion-associated VL, caused by L. infantum, has been reported from southern Europe and, by L. donovani, on the Indian subcontinent (1,2). Most L. infantum infections are asymptomatic (3), raising concern that the parasite could be present in donated blood from otherwise healthy residents in areas to which it is endemic (4).
VL caused by L. infantum is endemic to 20 of Brazil’s 27 states; an annual average of 3,553 cases occur nationwide, with 54% of all cases reported from Brazil’s northeastern region. The state of Ceará historically ranks first or second in number of cases; an annual average of 467 cases were reported during the last decade (5). Thirty-eight percent of cases were reported from Fortaleza, the capital, where 28.4% of the state’s population resides. Over a 10-year-period, 277 (7.8%) persons with VL have died, and 109 (39%) of VL-related deaths have occurred in Fortaleza.
Sixty-nine percent of blood donors for Ceará reside in Fortaleza. To determine the prevalence of Leishmania infection among healthy blood donors, we tested blood donated to the State of Ceará Public Blood Bank. Compulsory serologic testing was also done for Trypanosoma cruzi (Chagas disease), hepatitis B and C, Treponema pallidum (syphilis), human T-cell lymphotropic virus types 1 and 2, and HIV-1 and -2. In the blood bank, 60% of units are centrifuged to separate the buffy coat in preparation for platelet separation. During May–November 2011, we randomly selected 431 buffy coats and tested them for Leishmania spp. by ELISA and PCR. To separate plasma from cells, 10 mL of buffy coat was centrifuged in Ficoll-Hypaque (Histopaque −1077, Sigma-Aldrich, São Paulo, Brazil). We tested plasma for leishmanial IgG by ELISA using a modified protocol of Evans et al. (6). IOC/L2906 L. infantum strain (MHOM/BR/2002/LPC-RPV) was used as the source of promastigote antigens. In addition, DNA was extracted from the mononuclear cell preparation, and PCR was performed with primers 150 (5′-GGG[G/T]AGGGGCGTTCT[G/C]CGAA-3′) and 152 (5′-[G/C][G/C][G/C][A/T]CTAT[A/T]TTACACCAACCCC-3′) that target the 120-bp conserved region of the Leishmania kDNA minicircle present in all Leishmania spp (7). As a positive control, kDNA was extracted from L. amazonensis promastigotes, strain BA-125 (MHOM/BR/87), characterized by PCR and isoenzymes (8). All PCR-positive samples were purified and sent to Ludwig Biotec (Alvorada, Brazil) for sequencing by ACTGENE-Molecular Analysis. The Federal University of Ceará Ethics Committee approved this study.
Buffy coats from 57 (13.2%) serum samples from 431 donors were positive for leishmanial IgG, and 20 (4.6%) were positive for Leishmania spp. DNA. Sequencing of all PCR-positive samples confirmed the Leishmania genus. Three donors tested positive by both ELISA and PCR. Overall, the prevalence of leishmanial infection was 17.1% of blood donors. Eighty of the 431 units tested positive for >1 of compulsorily screened infections and were rejected. Of the remaining 351 that were negative for co-infection, 43 (12.2%) were positive for leishmanial IgG and 15 (4.3%) for Leishmania spp. DNA. Two donors were positive for both by ELISA and PCR. The prevalence of Leishmania infection among blood units accepted for transfusion was 16%.
The results demonstrate a surprisingly high prevalence of Leishmania infection in blood donors in Fortaleza, several times higher than that other diseases for which blood is screened (Figure). In a recent study in Salvador, Brazil (9), 5.4% of blood donors had leishmanial antibodies, of which 68% were positive by its PCR targeting kDNA amplification.
Figure.
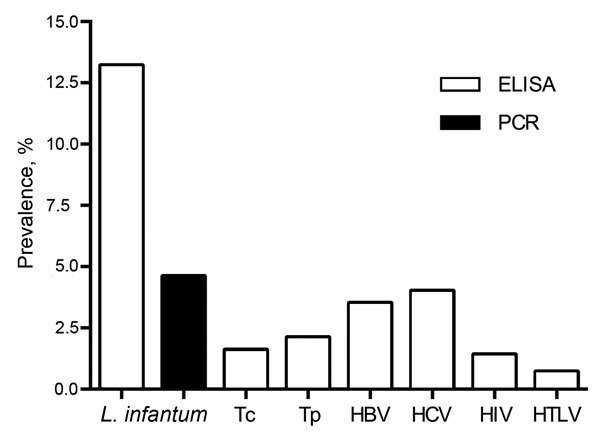
Comparison of the prevalence of Leishmania infantum as tested by PCR and ELISA and of other infections compulsorily tested in 431 blood donors in Fortaleza, state of Ceará, northeastern Brazil. HBV, hepatitis B virus; HCV, hepatitis C virus; HTLV, human T-cell lymphotropic virus; Tc, Trypanosoma cruzi; Tp, Treponema pallidum.
The percentages of antibody- or PCR-positive units capable of transmitting Leishmania and the outcomes are unknown. Viable Leishmania might not be in the blood of all PCR-positive donors, and even when present, the inoculum might be reduced by removal of infected circulating mononuclear phagocytes in the buffy coat, or parasites might be affected by steps involved in preparation or storage. However, if we consider units that test positive by PCR as being potentially infectious, the number of recipients at risk is of substantial concern. For example, in 2011, there were 99,933 blood donations to the State of Ceará Public Blood Bank. After compulsory screening for the other bloodborne pathogens, 93,238 units were accepted for transfusion. Extrapolating from the PCR-positive rate of 4.3%, a total of 4,009 recipients possibly were exposed to infection. Further studies are needed to determine whether recipients of blood from donors who are PCR positive and/or leishmanial antibody positive become infected with L. infantum. Persons with advanced AIDS or other immunosuppressive conditions seemingly would be at greatest risk for VL.
In Brazil, legislation requires that all blood for transfusion be tested for T. cruzi ., hepatitis B and C, T. pallidum, human T-cell lymphotropic virus types 1 and 2, and HIV-1 and -2. As additional information becomes available, screening for L. infantum also might be advisable to reduce the possibility of the recipient becoming infected, developing VL, and possibly being a reservoir of infection in the community (10), particularly in Ceará and other regions where the prevalence of L. infantum infection is high.
Acknowledgments
We thank Luciana Carlos for all her help.
This work was supported by CNPq, Brazil (grant 477705/2010-3).
Footnotes
Suggested citation for this article: Monteiro DCS, Sousa AQ, Lima DM, Fontes RM, Praciano CC, Frutuoso MS, et al. Leishmania infantum infection in blood donors, northeastern Brazil [letter]. Emerg Infect Dis. 2016 Apr [date cited]. http://dx.doi.org/10.32032/eid2204.150065
References
- 1.Cummins D, Amin S, Halil O, Chiodini PL, Hewitt PE, Radley-Smith R. Visceral leishmaniasis after cardiac surgery. Arch Dis Child. 1995;72:235–6. 10.1136/adc.72.3.235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dey A, Singh S. Transfusion transmitted leishmaniasis: a case report and review of the literature. Indian J Med Microbiol. 2006;24:165–70 . [PubMed] [Google Scholar]
- 3.Evans TG, Teixeira MJ, McAuliffe IT, Vasconcelos I, Vasconcelos AW, Sousa AQ, et al. Epidemiology of visceral leishmaniasis in northeast Brazil. J Infect Dis. 1992;166:1124–32. 10.1093/infdis/166.5.1124 [DOI] [PubMed] [Google Scholar]
- 4.Luz KG, da Silva VO, Gomes EM, Machado FC, Araujo MA, Fonseca HE, et al. Prevalence of anti-Leishmania donovani antibody among Brazilian blood donors and multiply transfused hemodialysis patients. Am J Trop Med Hyg. 1997;57:168–71 . [DOI] [PubMed] [Google Scholar]
- 5.Ministry of Health. Brazil. Epidemiological situation. LV—cases [cited 2015 Dec 1]. http://portalsaude.saude.gov.br/index.php/o-ministerio/principal/leia-mais-o-ministerio/726-secretaria-svs/vigilancia-de-a-a-z/leishmaniose-visceral-lv/11334-situacao-epidemiologica-dados
- 6.Evans TG, Krug EC, Wilson ME, Vasconcelos A, Alencar JE, Pearson RD. Evaluation of antibody responses in American visceral leishmaniasis by ELISA and immmunoblot. Mem Inst Oswaldo Cruz. 1989;84:157–66. 10.1590/S0074-02761989000200003 [DOI] [PubMed] [Google Scholar]
- 7.de Oliveira CI, Báfica A, Oliveira F, Favali CB, Correa T, Freitas LA, et al. Clinical utility of polymerase chain reaction–based detection of Leishmania in the diagnosis of American cutaneous leishmaniasis. Clin Infect Dis. 2003;37:e149–53. 10.1086/379610 [DOI] [PubMed] [Google Scholar]
- 8.Barral A, Pedral-Sampaio D, Grimaldi Júnior G, Momen H, McMahon-Pratt D, Ribeiro de Jesus A, et al. Leishmaniasis in Bahia, Brazil: evidence that Leishmania amazonensis produces a wide spectrum of clinical disease. Am J Trop Med Hyg. 1991;44:536–46 . [DOI] [PubMed] [Google Scholar]
- 9.Fukutani KF, Figueiredo V, Celes FS, Cristal JR, Barral A, Barral-Netto M, et al. Serological survey of Leishmania infection in blood donors in Salvador, northeastern Brazil. BMC Infect Dis. 2014;14:422. 10.1186/1471-2334-14-422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Costa CH, Gomes RB, Silva MR, Garcez LM, Ramos PK, Santos RS, et al. Competence of the human host as a reservoir for Leishmania chagasi. J Infect Dis. 2000;182:997–1000. 10.1086/315795 [DOI] [PubMed] [Google Scholar]


