Abstract
Background
Enteroendocrine L-cells synthesise and release the gut hormone glucagon-like peptide-1 (GLP-1) in response to food transit. Deletion of the tumour suppressor kinase LKB1 from proglucagon-expressing cells leads to the generation of intestinal polyps but no change in circulating GLP-1 levels. Here, we explore the role of the downstream kinase AMP-activated protein kinase (AMPK) in these cells.
Method
Loss of AMPK from proglucagon-expressing cells was achieved using a preproglucagon promoter-driven Cre (iGluCre) to catalyse recombination of floxed alleles of AMPKα1 and α2. Oral and intraperitoneal glucose tolerance were measured using standard protocols. L-cell mass was measured by immunocytochemistry. Hormone and peptide levels were measured by electrochemical-based luminescence detection or radioimmunoassay.
Results
Recombination with iGluCre led to efficient deletion of AMPK from intestinal L- and pancreatic alpha-cells. In contrast to mice rendered null for LKB1 using the same strategy, mice deleted for AMPK displayed an increase (WT: 0.05 ± 0.01, KO: 0.09±0.02%, p<0.01) in L-cell mass and elevated plasma fasting (WT: 5.62 ± 0.800 pg/ml, KO: 14.5 ± 1.870, p<0.01) and fed (WT: 15.7 ± 1.48pg/ml, KO: 22.0 ± 6.62, p<0.01) GLP-1 levels. Oral, but not intraperitoneal, glucose tolerance was significantly improved by AMPK deletion, whilst insulin and glucagon levels were unchanged despite an increase in alpha to beta cell ratio (WT: 0.23 ± 0.02, KO: 0.33 ± 0.03, p<0.01).
Conclusion
AMPK restricts L-cell growth and GLP-1 secretion to suppress glucose tolerance. Targeted inhibition of AMPK in L-cells may thus provide a new therapeutic strategy in some forms of type 2 diabetes.
Introduction
Release of hormones from enteroendocrine cells in response to food transit through the gut, and the consequent activation of insulin release beyond that prompted by the rise in blood glucose alone, is responsible for the incretin effect during feeding [1,2]. L-cells make up less than 1% of the epithelial cells lining the intestinal wall, but are vital for normal physiology and energy metabolism [3,4]. L-cells are thus responsible for the synthesis and secretion of glucagon-like peptide-1 (GLP-1), GLP-2, peptide YY (PYY) and oxyntomodulin via the action of prohormone convertases (PC) 1/3 on proglucagon [5]. Although the mechanisms which trigger secretion from L-cells in response to nutrients are debated [6], roles for sodium-glucose co-transporters (SGLTs), ATP-sensitive K+ (KATP) channels and an array of G-protein-coupled receptors have all been implicated.
GLP-1 receptors (GLP1R) are present on the pancreatic beta-cell and agonism at these receptors by L-cell-derived peptides, or by stabilised analogues such as liraglutide [7], is of considerable therapeutic interest in the treatment of type 2 diabetes (T2D). Binding of GLP-1 to GLP1R on pancreatic beta-cells triggers cAMP synthesis and downstream signalling by Protein kinase A (PKA) and Exchange Protein Activated by cAMP-2 (EPAC2), to activate insulin secretion [8,9]. Although a matter of debate [10], enhanced ATP synthesis [11], closure of KATP channels and Ca2+ influx may also play a role [12]. Whether the effects of GLP-1 are chiefly achieved through an action of the circulating hormone [13], or reflect an paracrine reflex loop triggered by GLP1 released in the gut [14,15], is also contested.
Released from pancreatic alpha-cells, glucagon is generated by the action of prohormone convertases (PC) 2 on proglucagon, and serves as the main anti-hypoglycaemic hormone in mammals [16]. Whilst elevated secretion of the hormone contributes to hyperglycemia in earlier stages of Type 2 diabetes T2D [17], impaired release is observed in patients living with Type 1 diabetes (T1D) and in long-standing T2D [18].
AMP-activated protein kinase (AMPK) is an evolutionarily-conserved fuel-sensitive serine/threonine protein kinase and cellular nutrient sensor implicated in the regulation of energy homeostasis [19] [20]. AMPK exists as a heterotrimeric complex comprising a catalytic α (α1and α2; encoded by Prkaa1 and Prkaa2) subunit, a scaffold β (Prkab; β1 and β2) subunit and a regulatory g (Prkag; γ1, γ2 or γ3) subunit [21,22]. The AMPK complex is activated in response to elevated intracellular AMP:ATP [23] and ADP:ATP ratios [24]. Responses to these changes in nucleotide ratios are chiefly, though not exclusively, controlled by alterations in the susceptibility to phosphorylation by upstream kinases, notably liver kinase B1 (LKB1; STK11) and Ca2+ / calmodulin-dependent kinase kinase II (CaMKKII) [25], of threonine-172 in the key catalytic loop of the α-subunit [26]. We have previously shown that changes in AMPK activity are important for the regulation of both insulin [27,28] and glucagon [29,30] secretion by glucose and other nutrients. Whether this enzyme is also important in the control of hormone secretion from L-cells is unknown.
LKB1 is a tumour suppressor deleted in Peutz-Jeghers syndrome (PJS), a condition characterised by the appearance of hamartomatous polyps in the gut and increased risk cancer [31]. In addition to phosphorylating AMPK, LKB1 is also responsible for the activation for a further 12 downstream kinases of the AMPK-related kinase (AMPK-RK) family [32]. We have previously observed [33] that deletion of LKB1 from proglucagon-expressing cells, achieved by crossing animals bearing a glucagon promoter-driven Cre recombinase (iGLuCre) to mice with floxed LKB1alleles, leads to the development of large gastro-duodenal polyps, reminiscent of those seen in PJS [31]. This leads to premature death from ~ 14 weeks as a result of impaired gastric emptying. Lineage tracing revealed that LKB1 ablation caused an epithelial-to-mesenchymal transition (EMT) in LKB1 null proglucagon-expressing cells, and consequently the formation of a population of smooth muscle-like cells which expanded to form polyps.
The present study was therefore designed with two goals in mind. Firstly, to determine whether the suppressive effect of LKB1 on EMT in L-cells or their progenitors is mediated by AMPK or via other AMPK-RKs [32], and secondly to explore the role of AMPK in controlling circulating GLP-1 levels.
Materials and Methods
Materials
Unless indicated otherwise, reagents were purchased from Sigma (Poole, Dorset, U.K.).
Methods
Generation of mice selectively lacking AMPK α1 and α2 in proglucagon-producing cells
Mice homozygous for AMPKα1fl/fl were first crossed to mice homozygous for AMPKα2fl/fl. The resulting double heterozygous AMPKα1fl/+:α2fl/+ mice were crossed with iGluCre expressing animals [34], where ~100 kB of the 5’ flanking region of the mouse proglucagon promoter drives Cre expression. The latter provides efficient recombination both in L-cells and in pancreatic alpha-cells, with a minor degree of recombination also in pancreatic beta-cells [35]. The above strategy generated triple heterozygous iGluCre:AMPKα1fl/+:α2fl/+-positive mice. The latter were bred with AMPKα1fl/fl:α2fl/fl mice to produce iGluAMPKdKO animals and further crossed to AMPKα1fl/fl:α2fl/fl animals to generate littermate controls. As previously reported using STOP-flox-tdRFP reporter mice [34,35], recombination catalysed with this Cre deleter strain occurs in > 75% of pancreatic α cells, ~ 70% of intestinal L-cells. Low levels of recombination were also found in the olfactory bulb and hind brain [35]. All mice were kept on a C57/BL6 background.
Mouse maintenance and diet
Mice were housed in cages with 2–6 mice per cage in a pathogen free facility with a 12 hour light and dark cycle. Animals had ad libitum access to standard mouse chow diet (Research Diet, New Brunswick, NJ). All in vivo procedures were conducted in accordance with U.K. Home Office regulations (Animal Scientific Procedures Act of 1986, Home Office Project License number PPL 70/06608, holder Dr Isabelle Leclerc), with approval from the local ethical committee (Animal Welfare and Ethics Review Board, AWERB), at the Central Biological Services (CBS) unit at the Hammersmith Campus of Imperial College London. Animals were euthanized at the end of the study (i.e. immediately after the completion of the indicated protocols) by cervical dislocation followed by termination of the circulation by decapitation. The study used a total of 42 experimental animals.
Oral (OGTT) and intraperitoneal (IPGTT) glucose tolerance tests
Mice were fasted overnight (15 h) and given free access to water. Glucose (1 g/kg body weight) was administered via either oral gavage or intraperitoneal injection. Blood was sampled from the tail vein at 0, 15, 30, 45, 60, 90 and 120 min. after glucose administration. Blood glucose was measured with an automatic glucometer (Accuchek; Roche, Burgess Hill, UK).
Plasma glucagon and insulin measurement
For measurement of glucagon and insulin levels in vivo, 100μl of blood was collected from the tail vein into heparin-coated tubes (Sarstedt, Beaumont Leys, UK). Plasma was separated by sedimentation at 10,000 g for 20 min (4°C). Plasma glucagon or insulin levels were measured in 50 μL aliquots by radioimmunoassay with competitive 125I-labelled glucagon or insulin (Millipore, Watford, UK).
Measurement of plasma GLP-1
Mice were fasted overnight (15 h), or fed ab libitum, and 50μl blood collected from the tail vein into EDTA-coated tubes. Plasma was separated by centrifugation at 4°C, 13,200 r.p.m. for 20 min. and total GLP-1 levels assayed using a two-site microtitre plate-based immunoassay with electrochemical luminescence detection (Meso Scale Discovery Kit, Gaithersburg, MD).
Insulin tolerance tests
Mice were fasted for 5 h with free access to water before intraperitoneal injection with 0.75 U/kg bovine insulin (Sigma, Dorset,UK). Blood glucose levels were measured at 0, 15, 30, 45 and 60 min.
Immunohistochemistry of pancreatic and intestinal sections
Isolated pancreata and sections of the ileum were fixed in 10% (vol/vol) buffered formalin and embedded in paraffin wax within 24 h of removal. Details of the subsequent immunohistochemical analysis are provided under S2 File (Supplementary Methods).
Pancreatic islet isolation
Islets were isolated as described previously [36]. Further details are provided under S2 File (Supplementary Methods).
Glucagon secretion
Glucagon secretion was measured from groups of 12 size-matched islets per well essentially as described [30]. Further details are provided under Supplementary Methods.
Statistics
Data were analysed using GraphPad PRISM 6.0 software. Significance was tested using unpaired Student’s two-tailed t-tests with Bonferroni post-tests for multiple comparisons, or two-way ANOVA as indicated. P<0.05 was considered significant and errors signify ± SEM.
Results
Deletion of the AMPK α-subunits improves oral but not intraperitoneal glucose tolerance
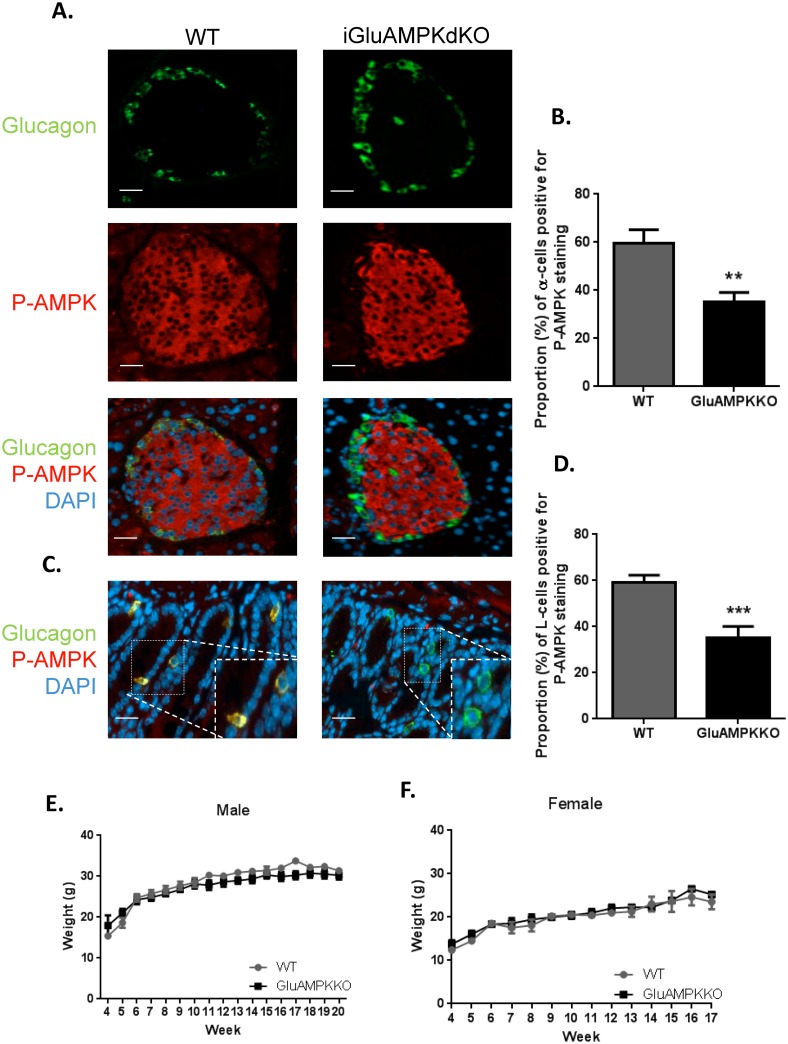
Fig 1A demonstrates the deletion of AMPK from pancreatic alpha-cells in iGluCre:AMPKα1:α2KO (subsequently referred to as iGluAMPKdKO) mice by immunocytochemical analysis. The percentage of alpha-cells co-stained with anti-P-AMPK α1/α2 and anti-glucagon antibodies was calculated (Fig 1B) and revealed a ~2-fold decrease in P-AMPK levels in the null mice as assessed in terms of the proportion of pAMPK immuno-positive cells (WT: 59.6% ± 5.57, KO 35.1% ± 4.04, P<0.01). Pancreatic slices were also co-stained for P-AMPK and insulin demonstrating that AMPK was not deleted in beta-cells (S1 Fig, S1 File, WT; 83.5% ± 5.19, KO; 76.8 ± 2.07, P>0.05).
Fig 1. AMPK is deleted selectively in pancreatic α-cells and enteroendocrine L-cells in iGlu AMPKdKO mice.
(A) Immunofluorescent staining of pancreatic islets using P-AMPKα1/α2 (red) and proglucagon (green) antibody in iGluAMPKdKO mice versus WT littermates, (B) percentage of α-cells co-staining for AMPK and glucagon. (C) Immunofluorescent staining of ileum slices staining with the above antibodies to quantify AMPK knock-down in enteroendocrine cells (D), n = 3 mice/genotype (all male). Body weight was measured weekly in (E) male and (F) female iGluAMPKdKO and WT mice, weeks 4–20. N = 9–11 mice/genotype, **P<0.01, ***P<0.001, by unpaired Student’s t-test. Data are expressed as means ± SEM.
Both AMPKα isoforms were detected at the mRNA level in purified wild-type L-cells across the intestine, with α1 predominating (S2 Fig). By immunocytochemical analysis of sections of the ileum, P-AMPK was detected in a smaller proportion of L-cells from iGluAMPKdKO mice (P<0.001) versus WT littermates (35.3% ± 4.75 and 59.3% ± 2.95, respectively, Fig 1C and 1D), consistent with recombination in the majority of L-cells.
Body weight was measured weekly in WT and KO mice from weaning (4 weeks) until 20 weeks of age in males, and 17 weeks of age in females (Fig 1E and 1F) and revealed no differences between genotypes. iGluAMPKdKO mice appeared healthy and were not bloated in appearance, in contrast to LKB1 KO mice produced using the same proglucagon promoter-driven Cre to achieve recombination of floxed Lkb1 alleles [33].
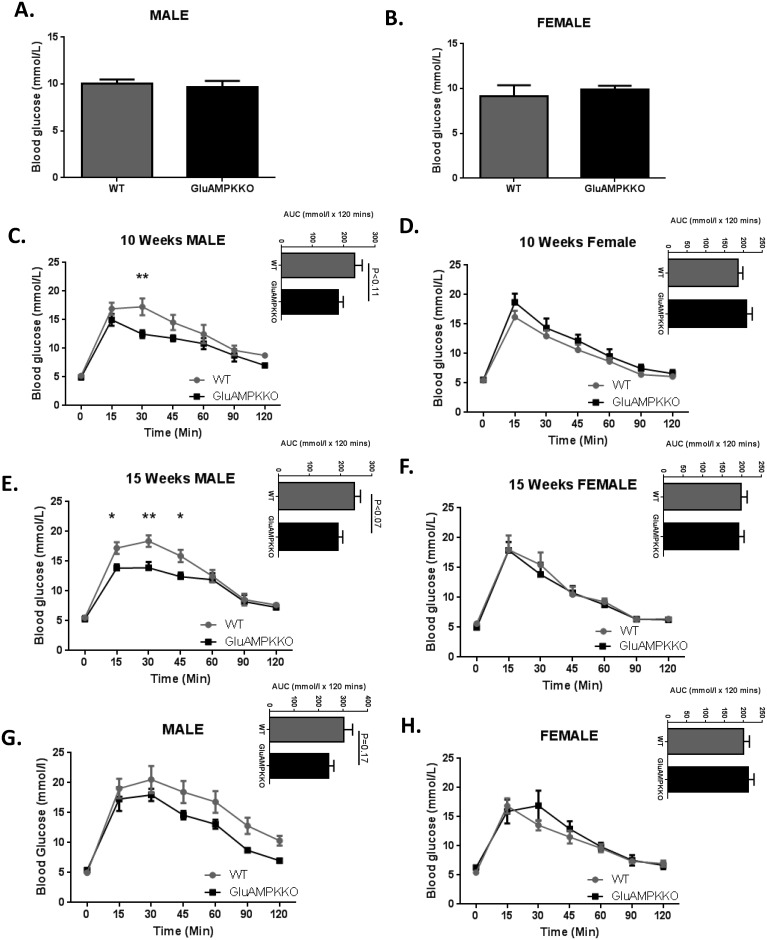
When animals were given free access to food, glycaemia was unchanged in iGluAMPKdKO mice versus WT littermates (Fig 2A and 2B). During oral glucose tolerance tests (OGTT), glycaemia was found to be significantly improved in 10 week old male mice at the 30 min. time point (P<0.01) in iGluAMPKdKO mice compared to WT littermates (12.5 ± 0.70 mmol/l and 17.3 ± 1.48 mmol/l, respectively; Fig 2C). Glucose tolerance was enhanced further in 15 week-old male mice 15, 30 and 45 min. after gavage (P<0.05, 0.01, 0.05, respectively) in iGluAMPKdKO mice compared to WT littermates (15 min, 13.8 ± 0.64 vs 17.2 ± 0.97 mmol/l; 30 min: 13.9 ± 0.97 vs 18.4 ± 0.94 mmol/l and 45 min, 12.4 ± 0.69 vs 15.9 ± 1.02 mmol/l, respectively; Fig 2E). Glucose tolerance was unchanged in female KO mice at both 10 and 15 weeks of age during OGTT (Fig 2D and 2F). Intraperitoneal glucose tolerance tests (IPGTTs) on the same cohorts of mice at 20 weeks revealed no significant differences (Fig 2G and 2H).
Fig 2. AMPKα1 and -α2 deletion in proglucagon expressing cells improves glucose tolerance after oral administration of glucose in male mice but is unchanged after glucose injection.
(A,B) Glycaemia was measured in male and female iGluAMPKdKO and WT mice when fed ad libitum. n = 6–9 mice/genotype/sex. (C,D) Glucose was administered using oral gavage (1 g/kg) after mice were fasted overnight and blood glucose levels measured at 0, 15, 30, 45, 60, 90 and 120 min. after glucose administration in 10 week old male and female mice. (E, F) Oral glucose tolerance tests repeated in 15 week old male and female iGluAMPKdKO and WT mice. Area under the curve (AUC) is displayed at the top right of panels. (G,H) IPGTTs were performed on 20 week old male and female mice. n = 5–9 mice/sex/genotype, *P<0.05, **P<0.01, by 2-way ANOVA. Data are expressed as means ± SEM.
AMPKα1 and α2 deletion in proglucagon-expressing cells has no effect on insulin tolerance or circulating insulin levels
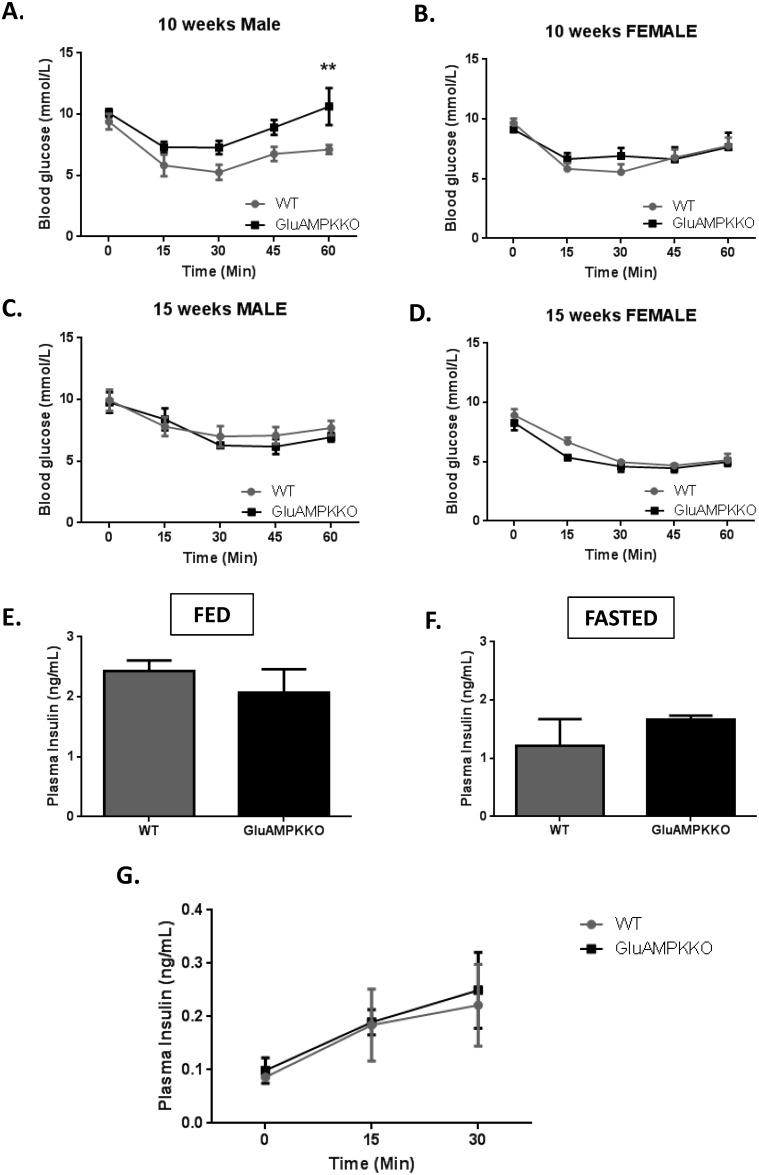
Insulin tolerance tests performed in 10 and 15 week-old male and female mice (Fig 3A–3D) revealed no differences in insulin sensitivity between iGluAMPKdKO mice and their WT littermates except at the 60 min. time point in 10 week-old male mice. Here, blood glucose was significantly higher (P<0.01) in iGluAMPKdKO mice compared to WT animals (10.6 ± 1.52 and 7.12 ± 0.36 mmol/l, respectively), possibly indicating an enhanced counter-regulatory response in the null mice.
Fig 3. AMPKα1 and -α2 deletion has no or minor effects on glycaemia after insulin injection, or on circulating insulin levels.
(A,B) Intraperitoneal insulin tolerance tests (ITTs) were performed on 10 week old male and female mice after mice after fasting for 5 h. Blood glucose levels were measured 0, 15, 30, 45 and 60 min. after insulin injection. (C,D) ITTs were repeated on 15 week old male and female mice, n = 5–9 mice/sex/genotype. **P<0.01, by 2-way ANOVA. (E,F) Plasma insulin levels were measured in iGluAMPKdKO and WT mice when fed as normal or after being fasted overnight and (G) at 0, 15 and 30 min. after glucose injection. n = 3 mice/genotype, mixed male and female. Data expressed as means ± SEM.
Levels of insulin in the plasma of these mice fed ad libitum or after overnight fast (Fig 3E and 3F) revealed no changes in insulin levels between iGluAMPKdKO mice and WT littermate controls (Fed: 2.07 ± 0.39 ng/ml, 2.43 ± 0.18 ng/ml, respectively; Fasted: 1.67 ± 0.06 ng/ml, 1.21ng/ml ± 0.46, respectively). Plasma insulin levels were also not different between null and control mice when measured 0, 15 and 30 min. after glucose injection (Fig 3G).
Deletion of AMPKα1 and α2 in proglucagon-expressing cells results in increased L-cell mass and elevated circulating GLP-1 levels
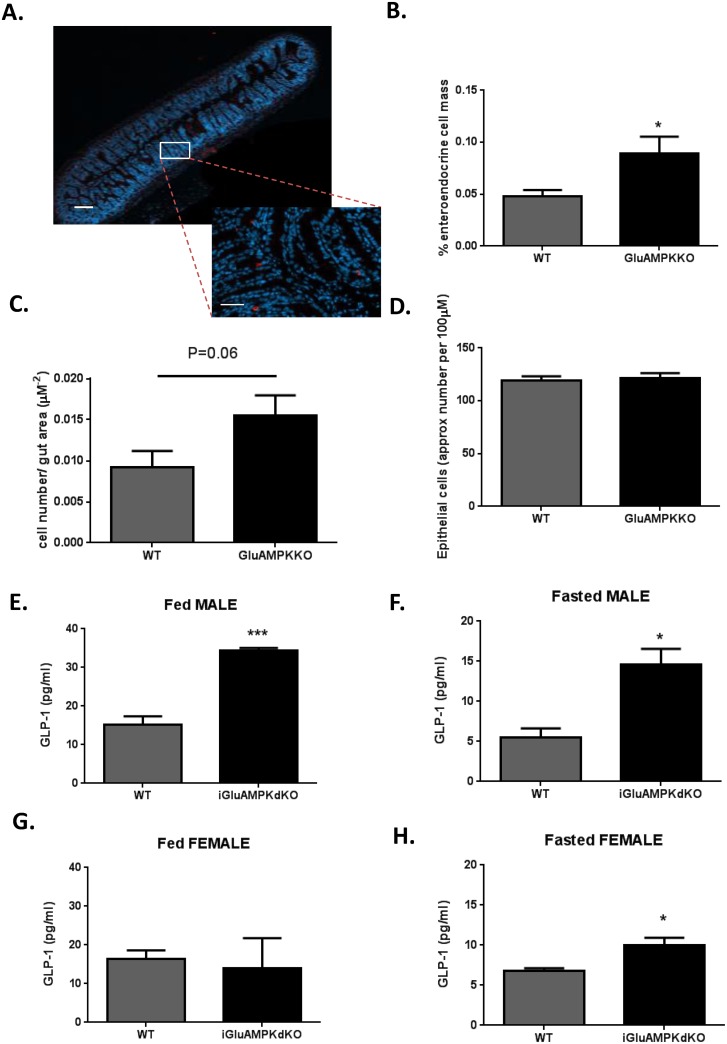
The improvement in oral (Fig 2B–2E) but not intraperitoneal (Fig 2G and 2H) glucose tolerance is suggestive of an improved incretin response in iGluAMPKdKO mice versus controls. To determine whether this may, at least in part, reflect elevated GLP-1 secretion from L-cells in the former, L-cell mass and circulating GLP-1 levels were measured. Sections of the ileum of iGlu AMPKdKO mice and WT littermates were stained with anti-proglucagon antibody, allowing the proportion of proglucagon-expressing cells to be determined (Fig 4A and 4B). This approach revealed an 80% increase in overall L-cell mass in male iGluAMPKdKO (0.09% ± 0.02) versus WT littermates (0.05% ± 0.01, n = 3 mice/genotype, 5 slides/mouse and 15–30 islets counted/slide; P<0.05). A tendency towards an increase in individual cell number per unit area (0.015 ± 0.002 for KO and 0.009 ± 0.002 μm-2 for WT, p = 0.06, n = 3 animals/genotype) was also apparent (Fig 4C). No differences in overall epithelial cell number/unit area, as estimated through nuclear staining, were detected (Fig 4D).
Fig 4. L-cell mass and GLP-1 secretion are enhanced in iGluAMPKdKO mice.
(A) Immunohistochemical analysis of an ileum section from an iGluAMPKdKO mouse; red staining represents proglucagon (GLP-1) staining in an enteroendocrine cell, dotted lines represent magnified section. (B) Percentage of proglucagon staining cells in the ileum. (C) L-cell count/area of gut, P = 0.06 by unpaired Student’s t-test. (D) Approximate endothelial cell count for the whole gut section; n = 3 mice/genotype (all male). (E,G) GLP-1 levels in the plasma of fed or fasted male and (F,H) female mice, n = 3 mice/sex/genotype. *P<0.05, **P<0.01, ***P<0.001 by unpaired Student’s t-test. Data are expressed as means ± SEM.
Plasma GLP-1 levels were also measured in iGluAMPKdKO mice and littermate controls and were significantly elevated in male KO mice versus controls both after ad libitum feeding (KO, 34.33 ± 0.72 pg/ml versus WT, 15.1 ± 2.25 pg/ml, n = 3 mice/ genotype, p<0.001, Fig 4E) or after overnight fast (KO 14.6 ± 1.95 pg/ml vs WT 5.47 ± 1.17 pg/ml, Fig 4F). By contrast, for female mice, no differences between iGluAMPKdKO and control animals were observed in the fed state (KO, 13.9 ± 7.83 pg/ml versus WT, 16.4 ± 2.19 pg/ml, n = 3 mice/genotype, p>0.05, Fig 4G) whereas a modest elevation in plasma GLP-1 was measured in the fasted state (KO, 10.0 ± 0.88 pg/ml vs WT, 6.77 ± 0.37 pg/ml, respectively, p<0.05, Fig 4H).
AMPKα1, -α2 deletion in pancreatic alpha-cells has no effect on alpha-cell mass or glucagon secretion
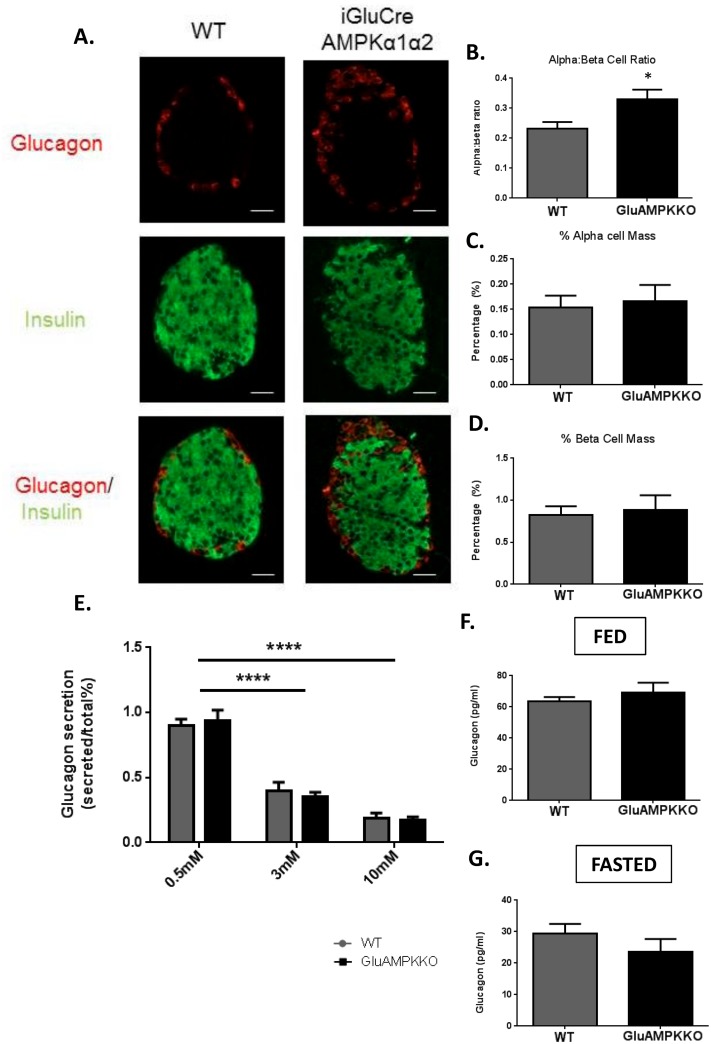
To investigate whether AMPKα1 and α2 play a role in pancreatic islet cell growth or survival, pancreatic sections from male mice were co-stained with insulin and glucagon in order to measure pancreatic beta- and alpha-cell mass, respectively, as well as the alpha:beta cell ratio (Fig 5A–5D). Though the alpha:beta cell ratio was increased in AMPKα1α2KO mice versus WT littermates (P<0.05), no changes were found in overall alpha- or beta-cell mass.
Fig 5. AMPKα1,α2KO has no effect on α-cell mass or glucagon secretion in male mice.
(A) Immunofluorescent staining of pancreatic sections using guinea pig anti-insulin (1:200; green) or rabbit anti-glucagon (1:100; red) antibodies from iGluAMPKdKO or WT mice. (B) Alpha:beta cell ratio, (C) % alpha-cell mass, (D) % beta-cell mass; n = 3 mice/genotype (all male), (E) glucagon secretion, measured from 12 size-matched islets incubated in 0.5, 3 or 10 mmol/l glucose and measured using radioimmunoassay, (F,G) levels of glucagon in the plasma of iGluAMPKdKO and WT mice when fed or fasted overnight. n = 6 mice/genotype, *P<0.05, by Student’s t-test; ****P<0.0001 by 2-way ANOVA. Data are expressed as means ± SEM.
We next determined whether deleting the AMPKα subunits in pancreatic alpha-cells might affect glucagon secretion in vitro. Here, isolated islets were incubated in 0.5, 3 or 10 mmol/l glucose (Fig 5E). Similar to alpha-cell mass, AMPKα1/α2 deletion exerted no effect on glucagon secretion. Plasma glucagon levels were measured in fed and fasted mice (Fig 5F and 5G) and, consistent with earlier results [30] using an alternative proglucagon promoter, were unaffected in iGluAMPKdKO mice in comparison to WT littermates.
Discussion
The first aim of this study was to determine whether deleting AMPKα1 and α2 in proglucagon-expressing cells would phenocopy the effect of deleting LKB1 in these cells using the same iGluCre transgene [33] and produce large gastro-duodenal PJS like-polyps. In marked contrast to our earlier findings [33], iGluAMPKdKO mice showed no signs of tumour development or premature death, suggesting that members of the AMPK-related kinase (AMPK-RK) family [32], rather than canonical AMPK α1 or α2-containing complexes, are responsible for the suppression of polyposis from proglucagon-expressing cells [37]. Possible candidates include Par1b/MARK2 [38], which controls cell polarity and is implicated in beta cell hyperplasia after LKB1 deletion [39,40], as well as NUAK1/SNARK [41] and NUAK2 [42].
Deletion of AMPKα1 and α2 in L-cells improves GLP-1 secretion and glucose tolerance
In further contrast to the effects of deleting LKB1 in proglucagon-expressing cells using the iGluCre deleter strain, male mice lacking both AMPK catalytic subunits in these cells, including enteroendocrine L-cells, displayed improved oral glucose tolerance (Fig 2C and 2E). Interestingly, glucose tolerance during IPGTT was not significantly affected in iGluAMKdKO mice (Fig 2G and 2H), although it should be noted that the latter tests were performed in the same animals at a slightly later stage (at 20 vs 15 weeks of age). These findings suggest that the incretin response is enhanced in the iGluAMPKdKO model. Providing direct evidence for this view, GLP-1 release into the plasma was significantly increased in iGluAMPKdKO mice, as assessed in fed and fasted mice (Fig 4E–4H). Although we were not able to detect differences in fasting or fed plasma insulin levels between WT and KO mice (Fig 3E and 3F), nor altered plasma insulin during IPGTTs (Fig 3G), lower glucose levels were observed in male mice during OGTTs (Fig 2C and 2E) and are indicative of enhanced beta cell function, as expected given elevated circulating GLP-1 levels.
We have also considered the possibility that the above observations might suggest a local action of GLP-1 on enteric neurons close to the site of release which then initiates an paracrine loop to regulate hepatic glucose production [43]. However, in the latter study the acute action of metformin, an AMPK activator, in the duodenum was to reduce hepatic glucose production via an AMPK-dependent mechanism. Metformin has also previously been found to increase plasma active GLP-1 levels in wild-type rats [44], as well as in rats lacking dipeptidyl peptidase IV (DPPIV) [45], and to enhance GLP-1 secretion from a human-derived intestinal L-cell line (NCI-H716) [46]. These observations argue for an acute stimulation of GLP-1 release in response to the biguanide. The apparent contrast between the present study and earlier findings [43] [45] may thus suggest that metformin acts (1) on GLP-1 secretion independently of AMPK, as recently reported in studies of hepatic gluconeogenesis [47], or (2) that there may be significant differences between the impact of acute and chronic activation of L-cell AMPK on a “gut-brain-liver axis”, with the latter acting chiefly to alter cell mass.
Interestingly, the above changes in oral glucose tolerance were apparent in male but not female mice and reflected more minor changes in circulating GLP-1 levels. Whilst the reasons for these differences must remain speculative, they may include greater variance in the measurements for females (e.g. due to reproductive cycles), differences in feeding patterns or sexual dimorphism in the expression of GLP-1, insulin or other receptors.
AMPKα1 and α2 deletion from pancreatic alpha-cells does not alter alpha-cell mass or glucagon secretion but increases L-cell mass
In the present study no changes in alpha- or beta-cell mass were observed in iGluAMPKdKO mice consistent with previous findings using the shorter glucagon promoter-Cre to drive deletion in these cells [30]. Nonetheless, and reflecting the greater statistical power to detect small differences, we did observe a small increase in alpha to beta cell ratio in the null mice, suggesting some degree of hyperplasia (or hypertrophy) of alpha cells (or a decrease in in beta cell number/mass). Future studies, on larger cohorts, will be needed to explore these findings in more detail and might be supported by measurements of hepatic glucose output or pyruvate tolerance as further estimates of glucagon action.
AMPK is a regulator of the mTOR complex, that goes on to phosphorylate the mTORC1 components Raptor and TSC2 [48] which are involved in the regulation of cell size [49] and proliferation. Interestingly, a clear increase in total L-cell mass, expressed as a fraction of intestinal surface, was apparent (Fig 4A and 4B) alongside a strong tendency towards an increase, of similar magnitude, in the number of L-cells per unit area. Future investigations will be required to explore the mechanisms involved, which might conceivably include the activation of mTORC1 as a result of AMPK deletion. Additionally, Carbohydrate Response Element Binding Protein (ChREBP), a transcriptional regulator of proglucagon gene expression and carbohydrate metabolism in L-cells [50], is also inhibited by AMPK [51], and may further increase GLP-1 synthesis and secretion.
Consistent with previous findings [30] in which both AMPK α1 and α2 were deleted in the alpha-cell using a shorter (1.6 kB) preproglucagon promoter [52] to drive Cre expression, we obtained no evidence for changes in circulating glucagon levels after ablation of AMPK activity. Of note, the longer proglucagon promoter used in the present study deletes additionally in enteroendocrine L- and probably other proglucagon-expressing cells throughout the body (e.g. in the brain stem and olfactory bulb) [35]. Thus, we show that deleting both AMPK α-subunits in pancreatic alpha-cells has little effect on glucagon release in response to low glucose levels in vitro or in vivo. Likewise, in the iGluAMPKdKO mice, glucagon secretion was not enhanced in iGluAMPKdKO mice versus controls during hypoglycaemia induced by insulin injection (results not shown). It should be noted, however, that when AMPK α1, but not α2, was deleted selectively in the alpha-cell with the shorter proglucagon promoter-driven Cre, impaired glucagon secretion was observed both in vivo during hypoglycemic clamp and in vitro [30]. Furthermore, AMPK activation was previously found to regulate glucagon secretion in vitro in a study using clonal αTC1-9 cells [29]. In the latter study, AMPK expression was stimulated using pharmacological agents such as metformin, and resulted in enhanced glucagon secretion at both high and low glucose concentrations. One possible explanation for the apparent discrepancy between the present and earlier studies [30] is that complete inactivation of AMPK in the α-cell in vivo leads to more robust compensatory changes than seen after AMPKα1 deletion alone.
Conclusions
Whilst not required prevent polyposis [33], AMPK limits expansion of the L-cell population and GLP-1 release, processes which may provide a new therapeutic avenue for the treatment of T2D.
Supporting Information
(TIFF)
(TIFF)
(DOCX)
(DOCX)
Acknowledgments
We thank Professors David Carling and Graham Williams (Imperial College) for useful discussion and Dr Isabelle Leclerc with support in animal maintenance.
Abbreviations
- AMPK
AMP-activated protein kinase
- EMT
epithelial-to-mesenchymal transition
- GLP-1
glucagon-like peptide-1
- GLP1R
GLP-1 receptor
- iGluCre
preproglucagon promoter-driven Cre recombinase
- ITT
insulin tolerance test
- IPGTT, OGTT
intraperitoneal or oral glucose tolerance test
- LKB1
liver kinase B1
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
GAR was supported by a Wellcome Trust (http://www.wellcome.ac.uk/) Senior Investigator Award (WT098424AIA), an MRC (http://www.mrc.ac.uk/) Programme Grant (MR/J0003042/1) and a Royal Society (https://royalsociety.org/) Wolfson Research Merit Award. SZ-V received a Merck Sharp & Dohme (MSD)/European Foundation for the Study of Diabetes (EFSD, http://www.europeandiabetesfoundation.org/) award. This work was also supported by the Innovative Medicines Initiative (http://www.imi.europa.eu/index_en.html) Joint Undertaking under Grant Agreement 155005 (IMIDIA), resources of which are composed of financial contribution from the European Union's Seventh Framework Programme (FP7/2007-2013) and European Federation of Pharmaceutical Industries and Associations (EFPIA) companies. Financial support was provided by the pharmaceutical company, Astra Zeneca, under the terms of the IMIDIA consortium. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Meier JJ, Nuack MA (2015) Incretin-based therapies: where will we be 50 years from now? Diabetologia 58(8): 1745–1750. 10.1007/s00125-015-3608-6 [DOI] [PubMed] [Google Scholar]
- 2.Holst JJ (2013) Enteroendocrine secretion of gut hormones in diabetes, obesity and after bariatric surgery. Curr Opin Pharmacol 13(6): 983–988. 10.1016/j.coph.2013.09.014 [DOI] [PubMed] [Google Scholar]
- 3.Drucker DJ (2015) Deciphering metabolic messages from the gut drives therapeutic innovation: the 2014 Banting Lecture. Diabetes 64(2): 317–326. 10.2337/db14-1514 [DOI] [PubMed] [Google Scholar]
- 4.Parker HE Gribble FM, Reimann F (2014) The role of gut endocrine cells in control of metabolism and appetite. Exp Physiol 99(9): 1116–1120. 10.1113/expphysiol.2014.079764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heller RS A G (1995) Intra-islet regulation of hormone secretion by glucagon-like peptide-1-(7–36) amide. Am J Physiol 269(6 Pt 1): G852–860. [DOI] [PubMed] [Google Scholar]
- 6.Gribble FM, Reimann F (2015) Enteroendocrine Cells: Chemosensors in the Intestinal Epithelium. Annu Rev Physiol. [DOI] [PubMed] [Google Scholar]
- 7.Drucker DJ Nauck MA (2006) The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 368(9548): 1696–1705. [DOI] [PubMed] [Google Scholar]
- 8.Light PE Manning-Fox J, Riedel MJ, Wheeler MB (2002) Glucagon-like peptide-1 inhibits pancreatic ATP-sensitive potassium channels via a protein kinase A- and ADP-dependent mechanism. Mol Endocrinol 16(9): 2135–2144. [DOI] [PubMed] [Google Scholar]
- 9.Béguin P, Nagashima K, Nishimura M, Gonoi T, Seino S. (1999) PKA-mediated phosphorylation of the human K(ATP) channel: separate roles of Kir6.2 and SUR1 subunit phosphorylation. EMBO J 18(17): 4722–4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leech CA, Dzhura I, Chepurny OG, Kang G, Schwede F, Genieser HG, et al. (2011) Molecular physiology of glucagon-like peptide-1 insulin secretagogue action in pancreatic β cells. Prog Biophys Mol Biol 107(2): 236–247. 10.1016/j.pbiomolbio.2011.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hodson DJ, Mitchell RK, Bellomo EA, Sun G, Vinet L, Meda P, et al. (2013) Lipotoxicity disrupts incretin-regulated human beta cell connectivity. J Clin Invest 123: 4182–4194. 10.1172/JCI68459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hodson DJ, Mitchell RK, Marselli L, Pullen TJ, Gimeno Brias S, Semplici F, et al. (2014) ADCY5 couples glucose to insulin secretion in human islets. Diabetes 63: 3009–3021. 10.2337/db13-1607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lamont BJ, Li Y, Kwan E, Brown TJ, Gaisano H, Drucker DJ (2012) Pancreatic GLP-1 receptor activation is sufficient for incretin control of glucose metabolism in mice. J Clin Invest 122(1): 388–402. 10.1172/JCI42497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steinert RE, Beglinger C, Langhans W (2015) Intestinal GLP-1 and satiation-from man to rodents and back. Int J Obes (Lond). [DOI] [PubMed] [Google Scholar]
- 15.Donath MY, Burcelin R (2013) GLP-1 effects on islets: hormonal, neuronal, or paracrine? Diabetes Care 36 Suppl 2: S145–148. 10.2337/dcS13-2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murlin JR Clough H, Gibbs CBF, Stokes AM (1923) Aqueous extracts of the pancreas. I Influence on the carbohydrate metabolism of depancreatized animals. J Biol Chem 56: 253–296. [Google Scholar]
- 17.Müller WA Faloona GR, Aguilar-Parada E, Unger RH. (1970) Abnormal alpha-cell function in diabetes. Response to carbohydrate and protein ingestion. N Engl J Med 283(3): 109–115. [DOI] [PubMed] [Google Scholar]
- 18.Cryer PE (2002) The pathophysiology of hypoglycaemia in diabetes. Diabetes Nutr Metab 15(5): 330–333. [PubMed] [Google Scholar]
- 19.Shaw RJ, Lamia KA, Vasquez D, Koo SH, Bardeesy N, Depinho RA, et al. (2005) The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science 310(5754): 1642–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kahn BB, Alquier T, Carling D, Hardie DG. (2005) AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab 1(1): 15–25. [DOI] [PubMed] [Google Scholar]
- 21.Kemp BE, Stapleton D, Campbell DJ, Chen ZP, Murthy S, Walter M, et al. (2003) AMP-activated protein kinase, super metabolic regulator. Biochem Soc Trans 31(Pt 1): 162–168. [DOI] [PubMed] [Google Scholar]
- 22.Stapleton D, Woollatt E, Mitchelhill KI, Nicholl JK, Fernandez CS, Michell BJ, et al. (1997) AMP-activated protein kinase isoenzyme family: subunit structure and chromosomal location. FEBS Lett 409(3): 452–456. [DOI] [PubMed] [Google Scholar]
- 23.Hardie DG, Scott JW, Pan DA, Hudson ER. (2003) Management of cellular energy by the AMP-activated protein kinase system. FEBS Lett 546(1): 113–120. [DOI] [PubMed] [Google Scholar]
- 24.Xiao B, Sanders MJ, Underwood E, Heath R, Mayer FV, Carmena D, et al. (2011) Structure of mammalian AMPK and its regulation by ADP. Nature 472(7342): 230–233. 10.1038/nature09932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hawley SA, Pan DA, Mustard KJ, Ross L, Bain J, Edelman AM, et al. (2005) Calmodulin-dependent protein kinase kinase-beta is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab 2(1): 9–19. [DOI] [PubMed] [Google Scholar]
- 26.Hawley SA, Boudeau J, Reid JL, Mustard KJ, Udd L, Mäkelä TP, et al. (2003) Complexes between the LKB1 tumor suppressor, STRAD alpha/beta and MO25 alpha/beta are upstream kinases in the AMP-activated protein kinase cascade. J Biol 2(4): 28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.da Silva Xavier G, Leclerc I, Varadi A, Tsuboi T, Moule SK, Rutter GA. (2003) Role for AMP-activated protein kinase in glucose-stimulated insulin secretion and preproinsulin gene expression. Biochem J 371(Pt 3): 761–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun G, Tarasov AI, McGinty J, McDonald A, da Silva Xavier G, Gorman T, et al. (2010) Ablation of AMP-activated protein kinase alpha1 and alpha2 from mouse pancreatic beta cells and RIP2.Cre neurons suppresses insulin release in vivo. Diabetologia 53(5): 924–936. 10.1007/s00125-010-1692-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leclerc I, Sun G, Morris C, Fernandez-Millan E, Nyirenda M, Rutter GA. (2011) AMP-activated protein kinase regulates glucagon secretion from mouse pancreatic alpha cells. Diabetologia 54(1): 125–134. 10.1007/s00125-010-1929-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun G, da Silva Xavier G, Gorman T, Priest C, Solomou A, Hodson DJ, et al. (2015) LKB1 and AMPKα1 are required in pancreatic alpha cells for the normal regulation of glucagon secretion and responses to hypoglycemia. Mol Metab 4(4): 277–286. 10.1016/j.molmet.2015.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giardiello FM, Welsh SB, Hamilton SR, Offerhaus GJ, Gittelsohn AM, Booker SV, et al. (1987) Increased risk of cancer in the Peutz-Jeghers syndrome. N Engl J Med 316(24): 1511–1514. [DOI] [PubMed] [Google Scholar]
- 32.Lizcano JM, Goransson O, Toth R, Deak M, Morrice NA, Boudeau J, et al. (2004) LKB1 is a master kinase that activates 13 kinases of the AMPK subfamily, including MARK/PAR-1. EMBO J 23(4): 833–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zac-Varghese S, Trapp S, Richards P, Sayers S, Sun G, Bloom SR, et al. (2014) The Peutz-Jeghers kinase LKB1 suppresses polyp growth from intestinal cells of a proglucagon-expressing lineage in mice. Dis Model Mech 7(11): 1275–1286. 10.1242/dmm.014720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parker HE, Adriaenssens A, Rogers G, Richards P, Koepsell H, Reimann F, et al. (2012) Predominant role of active versus facilitative glucose transport for glucagon-like peptide-1 secretion. Diabetologia 55(9): 2445–2455. 10.1007/s00125-012-2585-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Soedling H, Hodson DJ, Adrianssens AE, Gribble FM, Reimann F, Trapp S, et al. (2015) Limited impact on glucose homeostasis of leptin receptor deletion from insulin- or proglucagon-expressing cells. Mol Metab 4(9): 619–630. 10.1016/j.molmet.2015.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ravier MA, and Rutter G. A. (2010) Isolation and culture of mouse pancreatic islets for ex vivo imaging studies with trappable or recombinant fluorescent probes. Methods Mol Biol 633: 171–184. 10.1007/978-1-59745-019-5_12 [DOI] [PubMed] [Google Scholar]
- 37.Monteverde T, Muthalagu N, Port J, and Murphy DJ. (2015) Evidence of cancer-promoting roles for AMPK and related kinases. FEBS J. [DOI] [PubMed] [Google Scholar]
- 38.Cohen D, Tian Y, Müsch A. (2007) Par1b promotes hepatic-type lumen polarity in Madin Darby canine kidney cells via myosin II- and E-cadherin-dependent signaling. Mol Biol Cell 18(6): 2203–2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Granot Z, Swisa A, Magenheim J, Stolovich-Rain M, Fujimoto W, Manduchi E, et al. (2009) LKB1 regulates pancreatic beta cell size, polarity, and function. Cell Metab 10(4): 296–308. 10.1016/j.cmet.2009.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fu A., Ng AC, Depatie C., Wijesekara N., He Y., Wang G. S., et al. (2009) Loss of Lkb1 in adult beta cells increases beta cell mass and enhances glucose tolerance in mice. Cell Metabolism 10: 285–295. 10.1016/j.cmet.2009.08.008 [DOI] [PubMed] [Google Scholar]
- 41.Kusakai G, Suzuki A, Ogura T, Kaminishi M, Esumi H (2004) Strong association of ARK5 with tumor invasion and metastasis. J Exp Clin Cancer Res 23(2): 263–268. [PubMed] [Google Scholar]
- 42.Namiki T, Tanemura A, Valencia JC, Coelho SG, Passeron T, Kawaguchi M, et al. (2011) AMP kinase-related kinase NUAK2 affects tumor growth, migration, and clinical outcome of human melanoma. Proc Natl Acad Sci U S A 108(16): 6597–6602. 10.1073/pnas.1007694108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Duca FA, Cote CD, Rasmussen BA, Zadeh-Tahmasebi M, Rutter GA, Filippi BM, et al. (2015) Metformin activates a duodenal Ampk-dependent pathway to lower hepatic glucose production in rats. Nat Medicine 21(5): 506–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mulherin AJ, Oh AH, Kim H, Grieco A, Lauffer LM, and Brubaker PL (2011) Mechanisms underlying metformin-induced secretion of glucagon-like peptide-1 from the intestinal L cell. Endocrinology 152: 4610–4619. doi: 4610.1210/en.2011-1485. Epub 2011 Oct 4614. 10.1210/en.2011-1485 [DOI] [PubMed] [Google Scholar]
- 45.Yasuda N, Inoue T, Nagakura T, Yamazaki K, Kira K, Saeki T, et al. (2002) Enhanced secretion of glucagon-like peptide 1 by biguanide compounds. Biochem Biophys Res Commun 298: 779–784. [DOI] [PubMed] [Google Scholar]
- 46.Kim MH, Jee JH, Park S, Lee MS, Kim KW, and Lee MK (2014) Metformin enhances glucagon-like peptide 1 via cooperation between insulin and Wnt signaling. J Endocrinol 220: 117–128. doi: 110.1530/JOE-1513-0381. Print 2014 Feb. 10.1530/JOE-13-0381 [DOI] [PubMed] [Google Scholar]
- 47.Foretz M, Hebrard S, Leclerc J, Zarrinpashneh E, Soty M, Mithieux G, et al. (2010) Metformin inhibits hepatic gluconeogenesis in mice independently of the LKB1/AMPK pathway via a decrease in hepatic energy state. J Clin Invest 120: 2355–2369. doi: 2310.1172/JCI40671. Epub 42010 Jun 40623. 10.1172/JCI40671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kennedy HJ, Pouli AE, Ainscow EK, Jouaville LS, Rizzuto R, Rutter GA. (1999) Glucose generates sub-plasma membrane ATP microdomains in single islet beta-cells. Potential role for strategically located mitochondria. J Biol Chem 274(19): 13281–13291. [DOI] [PubMed] [Google Scholar]
- 49.Olsen HL, Theander S, Bokvist K, Buschard K, Wollheim CB, Gromada J. (2005) Glucose stimulates glucagon release in single rat alpha-cells by mechanisms that mirror the stimulus-secretion coupling in beta-cells. Endocrinology 146(11): 4861–4870. [DOI] [PubMed] [Google Scholar]
- 50.Trabelsi MS, Daoudi M, Prawitt J, Ducastel S, Touche V, Sayin SI, et al. (2015) Farnesoid X receptor inhibits glucagon-like peptide-1 production by enteroendocrine L cells. Nat Commun 6: 7629 10.1038/ncomms8629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kawaguchi T, Osatomi K, Yamashita H, Kabashima T, Uyeda K (2002) Mechanism for fatty acid "sparing" effect on glucose-induced transcription: regulation of carbohydrate-responsive element-binding protein by AMP-activated protein kinase. J Biol Chem 277(6): 3829–3835. [DOI] [PubMed] [Google Scholar]
- 52.Herrera PL (2000) Adult insulin- and glucagon-producing cells differentiate from two independent cell lineages. Development 127(11): 2317–2322. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIFF)
(TIFF)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.