Abstract
Background
The efficacy and safety of warfarin therapy for stroke prevention in atrial fibrillation (AF) depends on the time in therapeutic range (TTR). We aimed to assess the predictive ability of SAMe-TT2R2 score in Chinese AF patients on warfarin, whose TTR is notoriously poor.
Methods and Results
This is a single-centre retrospective study. Patients with non-valvular AF on warfarin diagnosed between 1997 and 2011 were stratified according to SAMe-TT2R2 score, and TTR was calculated using Rosendaal method. The predictive power of SAMe-TT2R2 scores for good TTR i.e. >70% was assessed. We included 1,428 Chinese patients (mean age 76.2±8.7 years, 47.5% male) with non-valvular AF on warfarin. The mean and median TTR were 38.2±24.4% and 38.8% (interquartile range: 17.9% and 56.2%) respectively. TTR decreased progressively with increasing SAMe-TT2R2 score (p = 0.016). When the cut-off value of SAMe-TT2R2 score was set to 2, the sensitivity and specificity to predict TTR<70% were 85.7% and 17.8%, respectively. The corresponding positive and negative predictive values were 10.1% and 92.0%. After a mean follow-up of 4.7±3.6 years, 338 patients developed an ischemic stroke (4.96%/year). Patients with TTR≥70% had a lower annual risk of ischemic stroke of 3.67%/year compared with than those with TTR<70% (5.13%/year)(p = 0.08). Patients with SAMe-TT2R2 score ≤2 had the lowest risk of annual risk of ischemic stroke (3.49%/year) compared with those with SAMe-TT2R2 score = 3 (4.56%/year), and those with SAMe-TT2R2 score ≥4 (6.41%/year)(p<0.001). There was also a non-significant trend towards more intracranial hemorrhage with increasing SAMe-TT2R2 score.
Conclusions
The SAMe-TT2R2 score correlates well with TTR in Chinese AF patients, with a score >2 having high sensitivity and negative predictive values for poor TTR. Ischemic stroke risk increased progressively with increasing SAMe-TT2R2 score, consistent with poorer TTRs at high SAMe-TT2R2 scores.
Introduction
Warfarin therapy effectively reduces ischemic stroke and mortality amongst patients with non-valvular atrial fibrillation (AF).[1–3] The efficacy and safety of warfarin, however, very much depends on the quality of anticoagulation control, as assessed by the time in therapeutic range (TTR), with the proportion of time spent within therapeutic range of 2.0–3.0.[4–7] It is generally accepted that patients on warfarin should spend more than 65%, or even 70%, of time with INR between 2–3 to obtain the benefit as well as safety of the therapy.[8, 9] In Asian countries, anticoagulation control is notoriously poor, both in real world practice and in randomized clinical trials.[10]
Recently, a new clinical score, the SAMe-TT2R2 score was derived and validated using a primarily white Caucasian population to predict the likelihood of AF patients on warfarin of having a good TTR (with SAMe-TT2R2 score 0–2).[11] Given that non-Caucasian race already confers 2 points in this score, the SAMe-TT2R2 score requires validation and/or re-calibration in a non-Caucasian population.
In this study, we aimed, for the first time, to evaluate the ability of SAMe-TT2R2 score in predicting the quality of anticoagulation control (as reflected by TTR) in an Asian population.
Methods
Study Design and Patients
This was a retrospective study based on a hospital-based AF registry as previously described, [2–4, 12, 13] and was approved by the Institutional Review Board of the University of Hong Kong / Hospital Authority Hong Kong West Cluster. Consent was waived as all the data were analyzed anonymously. Briefly, patients ≥18 years of age diagnosed to have AF in Queen Mary Hospital, Hong Kong, from July 1997 to December 2011, were identified via a computerized database of clinical management system. Patients were excluded from the current study if they had significant valvular heart disease (i.e. prosthetic heart valve, rheumatic heart disease), less than 10 retrievable INR measurements, or interruption of warfarin for >2 weeks. Those with incomplete data or missing follow up were excluded from this study.
Definitions
The SAMe-TT2R2 score (S: Sex [female] [1 point]; A: age <60 years [1 point]; Me: Medical History [>2 of the following comorbidities: hypertension, diabetes, coronary artery disease/myocardial infarction, peripheral arterial disease, congestive heart failure, previous stroke, pulmonary disease, hepatic or renal disease] [1 [point]; T: Treatment [interacting drugs e.g. Amiodarone for rhythm control] [1 point]; T: Tobacco use (within 2 years) [2 points]; and R: Race [non-white] [2 points] was calculated for each individual.[11] Since all patients in our study were Chinese i.e. non-White population, the minimum score will be 2 points. Subsequent occurrence of risk factor contributory to the SAMe-TT2R2 score had not been taken into account.
In addition, ischemic stroke risk was estimated at baseline using the CHA2DS2-VASc score (C: congestive heart failure [1 point]; H: hypertension [1 point]; A2: age 65–74 years [1 point] and age ≥75 years [2 points]; D: diabetes mellitus [1 point]; S: prior stroke or transient ischemic attack [2 points]; VA: vascular disease [1 point]; and Sc: sex category [female] [1 point]) as described in recent guidelines.[14] Likewise, the HAS-BLED score was calculated at baseline as a measure of bleeding risk.[15] Uncontrolled hypertension was defined as systolic blood pressure >160 mmHg at baseline and subsequent visit-to-visit changes in systolic blood pressure had not been taken in account. Similarly, liver disease as determined by the derangement in liver biochemistry and renal disease as determined by serum creatinine level were only assessed at baseline, subsequent changes had not been taken into account.
According to the center’s protocol, INR was measured every 8 weeks and more frequently when INR was not within the therapeutic range. The TTR was calculated for each patient using Rosendaal method, [16] in which INR was assumed to change in a linear manner between measurements, and INR values on the days with no measurement were interpolated. However, INR measurements within the first 6 weeks of warfarin therapy were excluded from this analysis due to the more frequent INR testing and large fluctuation in measurements during initial warfarin adjustment. The percentage of time during which a patient had an INR within 2.0–3.0 was taken as the TTR. According to the European guidelines, [9] a TTR≥70% was considered the criterion for ‘good anticoagulation control’.[8]
Statistical Analysis
Continuous and discrete variables are expressed as mean ± standard derivation and percentages, respectively. Statistical comparisons of the baseline clinical characteristics were performed using Student’s t test, Mann-Whitney U test, or one-way ANOVA as appropriate. Binary and linear hazards regression models were used to calculate hazard ratios (HRs) of some predictive factors and their 95% confidence interval (CIs) for poor anticoagulation control, TTR<70%. The predictive performance of the SAMe-TT2R2 score for ischemic stroke was assessed using the c-statistics (area under the curve). The c-statistic for receiver operating characteristic curve was calculated using Analyze-It for Excel with the Delong-Delong comparison for c-statistic. The c-statistic integrates measures of sensitivity and specificity of the range of a variable. Ideal prediction yields a c-statistic of 1.00 whereas a value of <0.5 indicates that the prediction is no better than chance. Calculations were performed using SPSS software (version 21.0) and MedCal (version 13.1.2). All tests were two-sided, and p-values were considered significant if <0.05.
Results
We included 1,428 Chinese patients (mean age 76.2±8.7 years, 47.5% male) with non-valvular AF on warfarin [Table 1]. The mean and median TTR overall were 38.2±24.4% and 38.8% (interquartile range: 17.9% and 56.2%) respectively. During the 14-year study period, the mean INR improved from 37.3±23.7% between 1997 and 2001 and 36.8±24.1% between 2002 and 2007, to 43.1±26.0% between 2008 and 2011. As in the original derivation cohort and subsequent external validation cohort, TTR decreased progressively with increasing SAMe-TT2R2 score (p = 0.016) (Fig 1). Even amongst patients with the lowest SAMe-TT2R2 score, i.e., 2, TTR was only 41.0±23.3%, which decreased to 39.0±25.0% and 36.0±24.0% amongst patients with SAMe-TT2R2 score of 3, and ≥4 respectively.
Table 1. Baseline characteristics.
| All (n = 1,428) | TTR | p-value1 | ||
|---|---|---|---|---|
| ≥70% (n = 154) | <70% (n = 1,274) | |||
| Mean age, (yrs) | 76.2±8.7 | 73.8±9.2 | 76.4±8.6 | <0.01* |
| Age<60 years, n (%) | 48 (3.4) | 11 (7.1) | 37 (2.9) | <0.01* |
| Female, n (%) | 671 (52.5) | 79 (51.3) | 671 (52.7) | 0.75 |
| HT, n (%) | 922 (64.6) | 102 (66.2) | 820 (64.4) | 0.65 |
| DM, n (%) | 387 (27.1) | 32 (20.8) | 355 (27.9) | 0.06 |
| Tobacco use (within 2 yrs), n (%) | 71 (5.0) | 11 (7.1) | 60 (4.7) | 0.19 |
| Dialysis, n (%) | 29 (2.0) | 0 (0) | 29 (2.3) | 0.07 |
| Heart failure, n (%) | 367 (25.7) | 335 (26.3) | 32 (20.8) | 0.14 |
| CAD, n (%) | 407 (28.5) | 365 (28.6) | 42 (27.3) | 0.72 |
| PAD, n (%) | 102 (7.1) | 13 (8.4) | 89 (7.0) | 0.51 |
| Stroke/TIA, n (%) | 496 (34.7) | 68 (44.2) | 428 (33.6) | <0.01* |
| CHA2DS2-VASc | ||||
| Mean CHA2DS2-VASc | 4.2±1.6 | 4.1±1.5 | 4.2±1.6 | 0.34 |
| Median CHA2DS2-VASc (IQR) | 4 (3, 5) | 4 (3, 5) | 4 (3, 5) | 0.42 |
| Mean HAS-BLED | 2.3±0.9 | 2.3±0.9 | 2.3±0.9 | 0.83 |
| Treatment with interacting drugs | 94 (6.6) | 10 (6.5) | 84 (6.6) | 0.96 |
| SAMe-TT2R2 score | 0.02* | |||
| 2, n (%) | 254 (17.8) | 22 (14.3) | 232 (18.2) | |
| 3, n (%) | 646 (45.2) | 80 (51.9) | 566 (44.4) | |
| 4, n (%) | 442 (31.0) | 41 (26.6) | 401 (31.5) | |
| 5, n (%) | 75 (5.3) | 7 (4.5) | 68 (5.3) | |
| 6, n (%) | 11 (0.8) | 4 (2.6) | 7 (0.5) | |
Abbreviations: CAD: Coronary artery disease; DM: diabetes mellitus; IQR: interquartile range; PAD: peripheral arterial disease; TIA: transient ischemic attack.
1p-value for comparison between patients with TTR ≥70% and TTR<70%.
* p-value <0.05
Fig 1. Relation between different SAMe-TT2R2 scores and the time of therapeutic range (TTR) in Chinese AF patients.
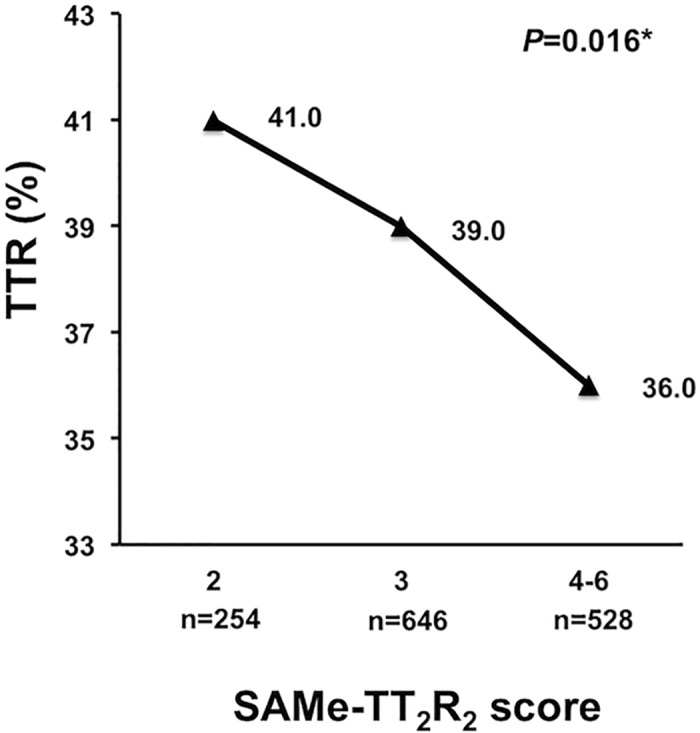
In the whole cohort, only 154 patients (10.7%) had good anticoagulation control (i.e., TTR≥70%) and were younger (73.8±9.2 years vs. 76.4±8.6 years, p<0.01), had a higher proportion with age <60 years (7.1% vs. 2.9%, p<0.01), and previous stroke/transient ischemic stroke (44.2% vs. 33.6%, p<0.01), compared with those with TTR<70%. Table 2 summarizes the HRs and the corresponding 95% CIs of baseline characteristics to poor TTR (TTR<70%) in both univariate and multivariate analysis. Of note, diabetes mellitus and heart failure were independently associated with TTR <70%, whereas age less than 60 years, previous stroke or transient ischemic attack, and lower SAMe-TT2R2 score appeared to be associated with good TTR (Table 2).
Table 2. Association between baseline factors and poor time in therapeutic range (TTR) <70%).
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Age<60 years | 0.39 (0.19–0.80) | 0.008* | 0.39 (0.18–0.84) | 0.016* |
| Female | 1.06 (0.76–1.48) | 0.75 | ||
| HT | 0.92 (0.64–1.31) | 0.65 | ||
| DM | 1.47 (0.98–2.21) | 0.06 | 1.57 (1.02–2.41) | 0.04* |
| Tobacco use within 2 yrs | 0.64 (0.33–1.25) | 0.19 | ||
| Heart failure | 1.36 (0.90–2.05) | 0.14 | 1.33 (0.87–2.04) | 0.19 |
| CAD | 1.07 (0.74–1.56) | 0.72 | ||
| PAD | 0.82 (0.44–1.50) | 0.51 | ||
| Stroke/TIA | 0.64 (0.46–0.90) | 0.01* | 0.64 (0.45–0.90) | 0.01* |
| CHA2DS2-VASc | 1.05 (0.95–1.17) | 0.34 | ||
| HAS-BLED | 1.02 (0.84–1.24) | 0.83 | ||
| Treatment with interacting drugs | 1.02 (0.52–2.00) | 0.96 | ||
| SAMe-TT2R2 score | 0.037* | 0.15 | ||
| 2, n (%) | Reference | Reference | ||
| 3, n (%) | 0.67 (0.41–1.10) | 0.12 | 0.58 (0.35–0.96) | 0.04* |
| 4, n (%) | 0.93 (0.54–1.60) | 0.79 | 0.77 (0.43–1.38) | 0.39 |
| ≥ 5, n (%) | 0.65 (0.30–1.40) | 0.27 | 0.67 (0.28–1.60) | 0.47 |
* p-value <0.05
When the cutoff value of SAMe-TT2R2 score was set to 2, the sensitivity and specificity to predict TTR≥70% were 85.7% and 18.2%, respectively. The corresponding positive and negative predictive values were 11.2% and 91.3%. The Youden index was 0.039. If the cutoff value to predict TTR≥70% was set to 3, the sensitivity and specificity to detect TTR≥70% were lower, being 66.2% and 37.4%, respectively; however, the positive and negative predictive values were similar, being 11.8% and 89.7%, respectively. The Youden index for the cutoff of 3 was 0.036.
After a mean follow-up of 4.7±3.6 years, 338 patients developed an ischemic stroke with an annual incidence of 4.96%/year. Patients with TTR≥70% had a lower annual risk of ischemic stroke of 3.67%/year compared with than those with TTR<70% 5.13%/year (p = 0.08). The SAMe-TT2R2 score showed a significant association with annual risk of ischemic stroke. Fig 2 shows a Kaplan Meier analysis of ischemic stroke amongst patients with different strata of SAMe-TT2R2 score (Log-rank: 16.0, P<0.001). Patients with SAMe-TT2R2 score ≤2 had the lowest risk of annual risk of ischemic stroke (3.49%/year) compared with those with SAMe-TT2R2 score = 3 (4.56%/year), and those with SAMe-TT2R2 score ≥4 (6.41%/year)(p<0.001). Fig 3 summarizes HR and the corresponding 95% CIs of different strata of SAMe-TT2R2 score for ischemic stroke. At follow up, there were altogether 63 intracranial haemorrhages with annual incidence of 0.90%/year. There was a non-significant trend towards more events with increasing SAMe-TT2R2 score, with events rates at scores 2, 3 and ≥4 being 0.77%/year, 0.96%/year (HR: 1.23, 95% CI: 0.62–2.45), and 0.90%/year (HR: 1.09, 95% CI: 0.52–2.26), respectively. However, the area under the curve of the SAMe-TT2R2 score for stroke prediction was only 0.543 (95% CI: 0.52–0.57) with the Youden index of 0.08.
Fig 2. Kaplan-Meier estimates of ischemic stroke-free survival in Chinese AF patients with different SAMe-TT2R2 scores.
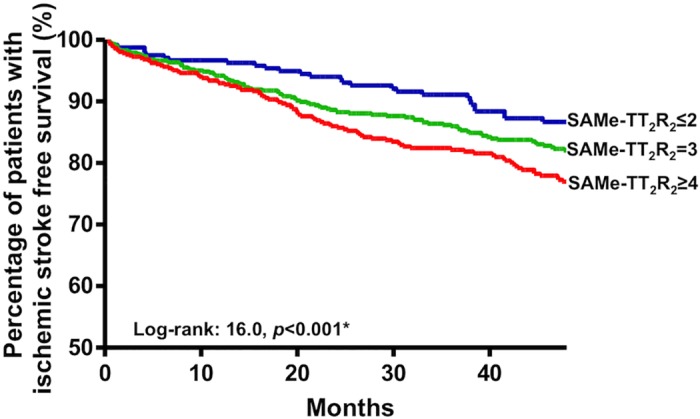
Fig 3. Hazard Ratios of different strata of SAMe-TT2R2 scores on ischemic stroke.
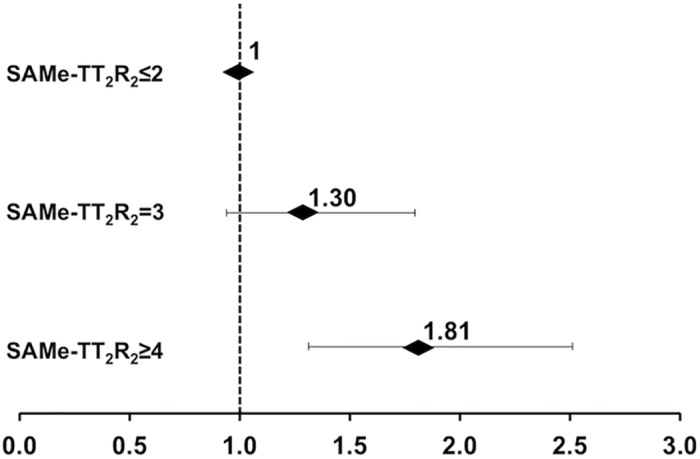
Horizontal lines represent 95% confident intervals (CIs) around point estimates.
Discussion
In this study, we have shown for the first time that amongst Chinese AF patients on warfarin, the SAMe-TT2R2 correlated with TTR in Chinese AF patients, with a SAMe-TT2R2 score >2 having a high sensitivity and negative predictive value for good TTR. Second, recalibration of the score in this non-Caucasian population did not improve its sensitivity. Third, the incidence of ischemic stroke increased progressively with increasing SAMe-TT2R2 score, consistent with poor TTRs at high SAMe-TT2R2 scores.
The importance of good quality anticoagulation control amongst patients on warfarin therapy typically with a TTR above 65% to 70% cannot be emphasized. In Asian population including Chinese, quality of anticoagulation has generally been poor [10], with a median TTR amongst Chinese AF patients being as low as 38.8% in the present cohort. Poor TTR undermines not only the efficacy of the therapy (more ischemic stroke), but also the safety (more intracranial bleeding).[4] Indeed, this might partly explain the prevailing perception of warfarin as an ineffective-and-yet-dangerous drug amongst Chinese clinicians, leading to gross underutilization of the therapy in Chinese and resulting is major missed opportunities for stroke prevention.
The SAMe-TT2R2 score, which was initially derived and validated in the white Caucasian population, facilitates decision making by clinicians to help predict the likelihood of achieving good quality anticoagulation control following the initiation of warfarin therapy.[11] In general, patients are expected to have good TTR when the SAMe-TT2R2 score is 0–2, and are at risk of suboptimal anticoagulation control when the SAMe-TT2R2 score >2. Rather than imposing a ‘trial of warfarin’ to see if high TTRs can be achieved, and putting such inception cohort patients at risk of suboptimal INRs and increased stroke risk (by 70%)[17], those patients with SAMe-TT2R2 score >2 could be targeted upfront for better follow up and educational efforts [18], or alternative anticoagulant strategies (e.g. non-vitamin K antagonist oral anticoagulants (NOACs)). Given the overall poor TTR in the Chinese AF population, NOACs should perhaps be considered as the first line antithrombotic agents for stroke prevention in non-valvular AF; warfarin therapy might be considered only when NOAC is contraindicated as in patients with end-stage renal disease.
Similar to the original derivation cohort, [11] an increase in the SAMe-TT2R2 score likewise resulted in a decrease in TTR in our cohort of Chinese AF patients and as many other non-Asian cohorts have shown, higher stroke rates [6, 19]. For the same SAMe-TT2R2 score, Chinese AF patients still appear to have a poorer TTR compared with white Caucasians [20], implying that other strategies (e.g. NOACs) may be better options. As rightly predicted by the score, around 90% of patients in the present cohort with the SAMe-TT2R2 score ≥2 did not have good anticoagulation control. This is consistent with epidemiological and trial data showing that Asians seem to do poorly on warfarin with higher risks of thromboembolism and bleeding (particularly intracranial haemorrahge)[10]. Albeit qualitatively consistent, deviating the default cutoff value of 2 from the original derivation cohort would still make the score less useful in Chinese AF population, with a lowered sensitivity and negative predictive value by such recalibration.
Although the inclusion of an ethnicity component into the SAMe-TT2R2 score appears to a practical way to improve the predictive power of the score, this may oversimplify the observed difference in TTR down to ethnicity per se. The reasons underlying poor TTR may be highly population-specific, ranging from genetic, dietary, behavioural to even health care provision system. Individual factors constituting the SAMe-TT2R2 score may affect the TTR in different ways in different ethnic groups with different life-style, value and belief. Most obviously, in the primarily Caucasian population, younger age represents a risk factor for poor TTR whereas in Hong Kong Chinese, younger age may in fact be associated with better understanding to diet restriction and importance of TTR, which may then in turn translate into a better TTR. Likewise in primarily Caucasian population, previous stroke was somehow associated with poor TTR, but amongst Chinese in Hong Kong, patients with previously stroke had a better TTR, which may reflect a more compliant lifestyle after stroke. Unfortunately, we lack a parallel Caucasian cohort in Hong Kong for comparison. For the SAMe-TT2R2 score, being Chinese is undoubtedly associated with poor TTR but higher SAMe-TT2R2 score may be the result of a combination of certain protective factors and risk factors for poor TTR, thus may contribute to either better or poorer TTR. Additional cultural-specific factors such as the frequency of traditional Chinese medicine use may be necessary to further improve the performance of the SAMe-TT2R2 score, but this could be remedied by extending the T criterion of the SAMe-TT2R2 score for ‘Treatment [interacting drugs]’ to include ‘Treatment [interacting drugs] or high intake of diet/foods that interfere with warfarin’. Some of the clinical factors within the SAMe-TT2R2 score are also risk factors for stroke, but the SAMe-TT2R2 score remains a simple validated score that has been shown to predict labile INRs, thromboembolism, death and serious bleeding events [19].
Limitations
This study is limited by its registry-based and single-centre observational design in primarily hospital-based patients. The variance in the management including the overall quality and facilities offered to patients for anticoagulation control might differ over the study period of 14 years. Information about the frequency and magnitude of warfarin dose changes during follow up was not recorded in this cohort. We were also suboptimally powered for serious bleeding events, including intracranial haemorrhage. The choice of target TTR of 70% instead of 65% as suggested by the NICE guideline in the UK is somewhat arbitrary. However, even the target TTR is to be lowered to 65%, the percentage of patients achieving the target TTR remains very small (14.8%).
Conclusion
The SAMe-TT2R2 score correlates well with TTR in Chinese AF patients, with a score >2 having high sensitivity and negative predictive values for poor TTR. The risk of ischemic stroke increased progressively with increasing SAMe-TT2R2 score, consistent with poorer TTRs at high SAMe-TT2R2 scores.
Data Availability
All relevant data are within the manuscript.
Funding Statement
Dr. Lip received financial assistance for his work as a consultant for Bayer, Astellas, Merck, AstraZeneca, Sanofi, BMS/Pfizer, Daiichi-Sankyo, Medtronic, Biotronik, Portola and Boehringer Ingelheim. He also received financial assistance for his work on the speakers bureau for Bayer, BMS/Pfizer, Boehringer Ingelheim, Medtronic, Daiichi-Sankyo and Sanofi Aventis. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Hart RG, Pearce LA, Aguilar MI. Adjusted-dose warfarin versus aspirin for preventing stroke in patients with atrial fibrillation. Annals of internal medicine. 2007;147(8):590–2. Epub 2007/10/17. . [DOI] [PubMed] [Google Scholar]
- 2.Lau KK, Chan PH, Yiu KH, Chan YH, Liu S, Chan KH, et al. Roles of the CHADS2 and CHA2DS2-VASc scores in post-myocardial infarction patients: Risk of new occurrence of atrial fibrillation and ischemic stroke. Cardiology journal. 2014. 10.5603/CJ.a2014.0034 . [DOI] [PubMed] [Google Scholar]
- 3.Siu CW, Tse HF. Net clinical benefit of warfarin therapy in elderly Chinese patients with atrial fibrillation. Circulation Arrhythmia and electrophysiology. 2014;7(2):300–6. 10.1161/CIRCEP.113.000858 . [DOI] [PubMed] [Google Scholar]
- 4.Ho CW, Ho MH, Chan PH, Hai JJ, Cheung E, Yeung CY, et al. Ischemic stroke and intracranial hemorrhage with aspirin, dabigatran, and warfarin: impact of quality of anticoagulation control. Stroke. 2015;46(1):23–30. Epub 2014/11/20. 10.1161/strokeaha.114.006476 . [DOI] [PubMed] [Google Scholar]
- 5.Schmitt L, Speckman J, Ansell J. Quality assessment of anticoagulation dose management: comparative evaluation of measures of time-in-therapeutic range. Journal of thrombosis and thrombolysis. 2003;15(3):213–6. 10.1023/B:THRO.0000011377.78585.63 . [DOI] [PubMed] [Google Scholar]
- 6.Wan Y, Heneghan C, Perera R, Roberts N, Hollowell J, Glasziou P, et al. Anticoagulation control and prediction of adverse events in patients with atrial fibrillation: a systematic review. Circulation Cardiovascular quality and outcomes. 2008;1(2):84–91. Epub 2009/12/25. 10.1161/circoutcomes.108.796185 . [DOI] [PubMed] [Google Scholar]
- 7.Gallego P, Roldan V, Marin F, Romera M, Valdes M, Vicente V, et al. Cessation of oral anticoagulation in relation to mortality and the risk of thrombotic events in patients with atrial fibrillation. Thrombosis and haemostasis. 2013;110(6):1189–98. Epub 2013/10/08. 10.1160/th13-07-0556 . [DOI] [PubMed] [Google Scholar]
- 8.De Caterina R, Husted S, Wallentin L, Andreotti F, Arnesen H, Bachmann F, et al. Vitamin K antagonists in heart disease: current status and perspectives (Section III). Position paper of the ESC Working Group on Thrombosis—Task Force on Anticoagulants in Heart Disease. Thrombosis and haemostasis. 2013;110(6):1087–107. Epub 2013/11/15. 10.1160/th13-06-0443 . [DOI] [PubMed] [Google Scholar]
- 9.European Heart Rhythm A, European Association for Cardio-Thoracic S, Camm AJ, Kirchhof P, Lip GY, Schotten U, et al. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). European heart journal. 2010;31(19):2369–429. Epub 2010/08/31. 10.1093/eurheartj/ehq278 . [DOI] [PubMed] [Google Scholar]
- 10.Chiang CE, Wang KL, Lip GY. Stroke prevention in atrial fibrillation: an Asian perspective. Thrombosis and haemostasis. 2014;111(5):789–97. Epub 2014/02/07. 10.1160/th13-11-0948 . [DOI] [PubMed] [Google Scholar]
- 11.Apostolakis S, Sullivan RM, Olshansky B, Lip GY. Factors affecting quality of anticoagulation control among patients with atrial fibrillation on warfarin: the SAMe-TT(2)R(2) score. Chest. 2013;144(5):1555–63. Epub 2013/05/15. 10.1378/chest.13-0054 . [DOI] [PubMed] [Google Scholar]
- 12.Nuhrich J, Steven D, Berner I, Rostock T, Hoffmann B, Servatius H, et al. Impact of Biatrial Defragmentation in Patients with Paroxysmal Atrial Fibrillation: Results from A Randomized Prospective Study. Heart rhythm: the official journal of the Heart Rhythm Society. 2014. Epub 2014/06/08. 10.1016/j.hrthm.2014.06.002 . [DOI] [PubMed] [Google Scholar]
- 13.Huang D, Anguo L, Yue WS, Yin L, Tse HF, Siu CW. Refinement of ischemic stroke risk in patients with atrial fibrillation and CHA2 DS2 -VASc score of 1. Pacing and clinical electrophysiology: PACE. 2014;37(11):1442–7. 10.1111/pace.12445 . [DOI] [PubMed] [Google Scholar]
- 14.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137(2):263–72. Epub 2009/09/19. 10.1378/chest.09-1584 . [DOI] [PubMed] [Google Scholar]
- 15.Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138(5):1093–100. Epub 2010/03/20. 10.1378/chest.10-0134 . [DOI] [PubMed] [Google Scholar]
- 16.Rosendaal FR, Cannegieter SC, van der Meer FJ, Briet E. A method to determine the optimal intensity of oral anticoagulant therapy. Thromb Haemost. 1993;69(3):236–9. Epub 1993/03/01. . [PubMed] [Google Scholar]
- 17.Azoulay L, Dell'Aniello S, Simon TA, Renoux C, Suissa S. Initiation of warfarin in patients with atrial fibrillation: early effects on ischaemic strokes. European heart journal. 2014;35(28):1881–7. 10.1093/eurheartj/eht499 . [DOI] [PubMed] [Google Scholar]
- 18.Clarkesmith DE, Pattison HM, Lip GY, Lane DA. Educational intervention improves anticoagulation control in atrial fibrillation patients: the TREAT randomised trial. PloS one. 2013;8(9):e74037 10.1371/journal.pone.0074037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lip GY, Haguenoer K, Saint-Etienne C, Fauchier L. Relationship of the SAMe-TT2R2score to poor quality anticoagulation, stroke, clinically relevant bleeding and mortality in patients with atrial fibrillation. Chest. 2014. Epub 2014/04/12. 10.1378/chest.13-2976 . [DOI] [PubMed] [Google Scholar]
- 20.Poli D, Antonucci E, Testa S, Lip GY. A prospective validation of the SAMe-TT2R2 score: how to identify atrial fibrillation patients who will have good anticoagulation control on warfarin. Internal and emergency medicine. 2014;9(4):443–7. Epub 2014/03/22. 10.1007/s11739-014-1065-8 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the manuscript.


