Abstract
Purpose of this review
Systemic sclerosis (SSc) is an autoimmune disease with fibrosis seen in multiple organs. While not traditionally regarded as a disease of aging, SSc-associated fibrosis shares many of the hallmarks of aging seen in other age-related fibrotic disorders. Here, we review current literature of the potential role of aging and age-related cellular processes in the development of SSc and fibrosis.
Recent findings
Accumulating evidence supports a role for immune dysregulation, epigenetic modifications, cellular senescence, mitochondrial dysregulation and impaired autophagy in fibrosis that occurs in aging and SSc.
Summary
Cellular alterations linked to aging may promote the development and/or progression of SSc-associated fibrosis.
Keywords: Systemic Sclerosis, Fibrosis, Aging
Introduction
Systemic sclerosis (SSc) is a systemic autoimmune disease that often leads to fibrosis in multiple organs, including the skin, heart, vasculature and lungs. Progressive organ fibrosis, particularly in the vasculature and lungs, are major contributors to scleroderma mortality (1). There are several mechanisms which contribute to fibrosis in SSc patients including endothelial dysfunction, innate and adaptive immune responses, endoplasmic reticulum (ER) stress and fibroblast activation (2). SSc is not typically considered as a disease of aging, but in a recent large United States cohort, the peak incidence for SSc was between 45 and 64 years of age (3). Older patients with SSc have increased mortality (4, 5), and patients who present at an older age (>65 years) are at increased risk of pulmonary hypertension, muscle weakness, renal impairment, pulmonary disease and cardiac disease (6, 7).
Much of the literature on fibrosis and aging comes from studies examining other fibrotic diseases, specifically idiopathic pulmonary fibrosis (IPF). IPF is a disease which causes progressive lung fibrosis and is seen almost exclusively in the elderly population (8). The patterns of lung disease seen in SSc and IPF are often different. In IPF, the defining histopathologic pattern is usual interstitial pneumonitis (UIP). In SSc patients, a pattern of non-specific interstitial pneumonitis (NSIP) is more common, although UIP is seen in more advanced cases (9). Despite these differences, both SSc and IPF share many pathobiologic features; these include epithelial and endothelial injury, immune dysregulation, and activated fibroblasts with increased deposition of extracellular matrix (10).
Several hallmarks of cellular aging have been proposed including, genomic instability, telomere attrition, epigenetic alterations, loss of proteostasis, deregulated nutrient sensing, mitochondrial dysfunction, cellular senescence, stem cell exhaustion and altered intercellular communication (11–13). In this manuscript, we explore associations between aging and the pathogenesis of SSc. In particular, we focus on recent studies that highlight the potential role of alterations in immune dysregulation, epigenetic modifications, cellular senescence, mitochondrial dysfunction and impaired autophagy, which are likely to contribute to fibrotic progression in SSc patients.
Immune dysregulation
There is growing evidence of age-related changes in the immune system that lead to immunosenescence (14–16). These changes occur both in the innate and adaptive immune system, and increase susceptibility to infection while decreasing tolerance to self-antigens. Immunosenescence may promote fibrosis in several ways. Immunosenescence can lead to a chronic pro-inflammatory environment (“inflamm-aging”) with increased production of cytokines such as IL-6 and TNFα, and increased levels of neutrophils with enhanced production of reactive oxygen species (ROS). There are also age-related changes in lymphocyte function that might promote a pro-fibrotic environment (16).
There is limited evidence demonstrating a role for immunosenescence in the pathogenesis of SSc. It has been shown that patients with diverse autoimmune disorders, including SSc, are characterized by accelerated aging of the immune system with A decline in T-cell excision circle (TREC) numbers, suggesting increased thymic involution; loss of CD28 expression on both CD4+ and CD8+ T-lymphocytes is a marker of premature aging of lymphocytes in patients with autoimmune disease (17). These CD28null cells are terminally differentiated memory T-cells that are cytotoxic and pro-inflammatory, and are resistant to apoptosis (18, 19). A recent study by Rodriguez-Carrio et al has linked a TNFα polymorphism, TNFArs1800629n (−308 G>A), which is associated with increased production of TNFα and immunosenescence in patients with rheumatoid arthritis. This polymorphism is also associated with increased numbers of CD4+CD28null cells which correlates with worsening clinical outcomes, and it appears that the “immunosenescence phenotype” is reversible with TNFα antagonists **(18). Interestingly, systemic lupus erythematosus patients have also been shown to exhibit immunosenescence with an imbalance in CD4+ T-lymphocytes, and this correlates with an increase incidence in the metabolic syndrome in these patients *(20).
While there is evidence of age-related changes in the adaptive immune system in patients with autoimmune disease and pulmonary fibrosis (16–18, 20), the precise mechanisms by which these changes promote fibrosis are unclear. In a recent study by Pinto et al (21), age-related changes in the innate immune system that drive fibrosis are beginning to be elucidated. This group of investigators demonstrated that changes in the composition, gene expression and functionality of cardiac tissue macrophages with aging, increases susceptibility in mice to age-related cardiac fibrosis **(21). This accumulation of macrophages with low CX3CR1 expression in aged mice is associated with an increase in pro-fibrotic gene expression, including MMP9 and CCL24 (21).
Our understanding of immunosenescence and how age-related changes in both the innate and adaptive immune systems promote fibrosis is still evolving. Understanding how aging triggers self-tolerance, impairs resolution of inflammation and the development/progression of fibrosis will be likely be pivotal in developing novel therapeutics that prevent/impede fibrosis.
Epigenetic Modifications
Epigenetic modifications regulate gene expression without changes to the DNA sequence; these include changes in chromatin structure by DNA methylation and histone modifications, as well as changes to coding and non-coding RNAs. There are examples across multiple species that epigenetic modifications influence longevity, aging, age-related diseases (22).
There is increasing evidence for a role of epigenetic modifications in the pathogenesis of SSc. DNA methylation abnormalities have been demonstrated in fibroblasts, endothelial cells and lymphocytes isolated from SSc patients, and histone modifications have been reported in fibroblasts and B-lymphocytes (23). There is also increasing evidence of role of miRNAs in the pathogenesis of SSc (23).
Sirtuin-1 (Sirt1) is a class III histone deacetylase that regulates the transcription of a large number of genes, primarily through transcriptional repression (24, 25). Two recent studies have highlighted the role of Sirt1 in TGFβ signaling and the development of SSc-associated fibrosis. Zerr et al **(24) demonstrated that dermal fibroblasts from patients with SSc have decreased levels of Sirt1 when compared to controls, a finding that was replicated in a mouse model of skin fibrosis. In this study, activation of Sirt1 enhanced TGFβ signaling and promoted differentiation of fibroblasts into myofibroblasts. Deletion of Sirt1, specifically in fibroblast-like cells, abrogated the effects of TGFβ signaling and led to decreased skin fibrosis (24). Similar results were reported by Wei et al **(25) who demonstrated decreased expression of Sirt1 in skin biopsies and dermal fibroblasts from SSc patients, and inhibition of Sirt1 mediated anti-fibrotic effects in a mouse model of skin fibrosis. Together, these studies support an anti-fibrotic role for Sirt1 and suggest that therapeutic approaches inducing/activating Sirt1 may be beneficial in SSc-associated fibrosis.
NADPH oxidase 4 (Nox4) is a member of the NADPH oxidase family of enzymes that generate ROS for cellular signaling; Nox4 has been implicated in oxidative stress responses and fibrosis in multiple models of fibrosis, including SSc (26–30). In recent work examining epigenetic modification of lung fibroblasts, it was demonstrated that histone modifications involving H4K16Ac mediates age-related increases in Nox4 *(31); additionally, the histone deacetylase inhibitor, suberoylanilide hydroxamic acid (SAHA) leads to apoptosis of myofibroblasts and decreases lung fibrosis in the murine bleomycin-induced model of lung fibrosis *(32).
Both canonical and non-canonical Wnt/β-catenin pathways have been implicated in a variety of fibrotic diseases, including SSc (33). Dees et al (34) recently demonstrated that Wnt signaling is increased in leukocytes and fibroblasts of SSc patients by epigenetic down-regulation of the Wnt antagonists, DKK1 and SFRP1; pharmacologic inhibition of methylation-induced silencing of these antagonists led to a decrease in Wnt signaling.
In summary, age-associated epigenetic modifications can lead to activation of SSc fibroblasts to promote fibrosis. Understanding the role of epigenetic modifications in SSc has the potential to lead to novel anti-fibrotic therapies.
Cellular Senescence
Senescence is a complex process that is defined by an acquisition of irreversible growth arrest; importantly, the accumulation of senescent cells in aging and age-related diseases may have profound implications for tissue homeostasis (35). Critical to the pleiotropic actions of senescent cells is the maintenance of high metabolic activity and elaboration of senescence-associated secretory phenotype (SASP). Interestingly, the SASP associated with senescent fibroblasts has been suggested to promote termination of the wound healing response and mediate anti-fibrotic effects in animal models of liver fibrosis **(36). In contrast, Hecker et al *(30) show that in aged mice with bleomycin-induced lung injury, accumulation of senescent and apoptosis-resistant myofibroblasts is associated with non-resolving fibrosis up to 4 months following injury. In this study, senescence of myofibroblasts was shown to be mediated by an up-regulation of the ROS-generating enzyme, Nox4, and an impaired activation of the Nrf2 antioxidant response pathway; this redox imbalance leads to prolonged senescence and apoptosis resistance leading to persistent fibrosis. Thus, it is important to recognize the differences between acute and chronic senescence in the context of tissue injury-repair (37). Currently, there are limited studies of the role of cellular senescence in the different cell types, including endothelial cells, fibroblasts and immune cells that participate in the pathogenesis of SSc; future studies in this area may provide additional clues to the specific role(s) of senescence in SSc-associated skin and lung fibrosis (38).
Mitochondrial Dysfunction
Decreases in mitochondrial number and mass, changes in mitochondrial DNA and protein levels, and decline in respiratory capacity have all been described in normal aging (39, 40). It is unclear whether this renders cells vulnerable to stress and contributes to disease processes in aging individuals or whether this is a survival mechanism in reparative/regenerative cells (40). There is increasing evidence that mitochondria-generated ROS (mtROS) is important in the pathogenesis of fibrosis (11).
PTEN-induced putative kinase 1 (PINK1) is required for the induction of mitochondrial autophagy (mitophagy) and clearance of damaged/dysfunctional mitochondria. When mitochondria become depolarized, PINK1 accumulates on the surface and begins a signaling cascade that leads to mitophagy (41). PINK1 is also critical for normal mitochondrial function and a deficiency in PINK1 has been linked to dysfunction of the electron transport chain, increased oxidative stress and cellular apoptosis (42). Recent evidence indicates that PINK1 and mitochondrial dysfunction contributes to the pathogenesis of pulmonary fibrosis. Bueno et al **(42) demonstrated that type II alveolar epithelial cells (AECII) from patients with IPF have accumulation of dysfunctional mitochondria and impaired mitophagy; this correlated with decreased levels of PINK1, and PINK1 silencing in normal lung epithelial cells resulted in a profibrotic phenotype. This study also demonstrated that mitochondrial dysfunction led to increased ER stress, vulnerability to AECII apoptosis and fibrosis. In a related study, Patel et al **(43) reported that PINK1 knockout mice are more susceptible to bleomycin-induced lung fibrosis. While these authors also found evidence of mitochondrial dysfunction in IPF lungs, they report increased levels of PINK1 in whole lung tissues from IPF lungs. Furthermore, this study demonstrated that these effects could be induced by TGFβ-mediated increases in mtROS; scavenging of mtROS ameliorated these mitochondrial abnormalities.
Increased production of mtROS may contribute to mitochondrial dysfunction through other mechanisms. It was recently demonstrated that in bleomycin-induced pulmonary fibrosis in Wistar rats, there is acquisition of mtDNA deletions, respiratory chain dysfunction and mtROS production during the development of pulmonary fibrosis *(44). Similarly, Song et al *(45) demonstrated that inhibition of H2O2 or bleomycin-induced mtROS production by astaxanthin (AST) prevents AECII apoptosis, which may mitigate fibrotic responses.
There is a growing body of evidence linking mitochondrial dysfunction to the development of fibrosis. There is limited data on the role of mitochondrial dysfunction in the pathogenesis of SSc-associated fibrosis and other autoimmune diseases, and further research in this area is needed.
Impaired Autophagy
Autophagy is a cellular process whereby intrinsic damaged organelles/proteins or invading pathogens are targeted for lysosomal degradation (46). Autophagy is important in aging since an accumulation of damaged and dysfunctional organelles/proteins occurs during a cell’s lifetime. Therefore, active and effective autophagy is needed for clearance of these abnormal proteins to maintain healthy cellular functions, and enhancing autophagy can promote delay cell aging (47). There is increasing evidence that defective autophagy might play a role in fibrosis associated with both IPF and autoimmune diseases (48–50).
Recent studies support a role for impaired autophagy in lung fibrosis. Cabrera et al *(51) demonstrated that mice deficient in autophagin-1 protease (Atg4b-deficient mice) are more susceptible to bleomycin-induced lung fibrosis; in this study, deficient autophagy led to increased apoptosis of the alveolar and bronchiolar epithelial cells. In studies by Nho and Hergert *(52), abnormalities in the autophagy pathway promoted a fibrotic phenotype of lung fibroblasts when grown on type I collagen matrix. In comparison to normal lung fibroblasts, IPF fibroblasts had less autophagosomes, increased mTOR activity and decreased PTEN expression, thus preventing fibroblast apoptosis.
There is increasing evidence that autophagy is critical in the pathogenesis of SSc. When SSc skin biopsies were compared to healthy controls, SSc samples demonstrated increased autophagy early in the disease course, but decreased autophagy as the disease progressed *(53). Dumit et al **(54) demonstrated that the phenotype of SSc skin fibroblasts and fibroblasts from older patients share similarities; for example, both groups expressed minichromosome maintenance (MCM) helicase proteins to a lesser degree than young, normal skin fibroblasts. This study showed that decreased MCM7 is associated with reduced autophagy, decreased proliferation, and increased accumulation of intracellular proteins in both aged and SSc skin fibroblasts. These findings lend further support for the hypothesis that SSc exhibits an accelerated aging phenotype.
Conclusion
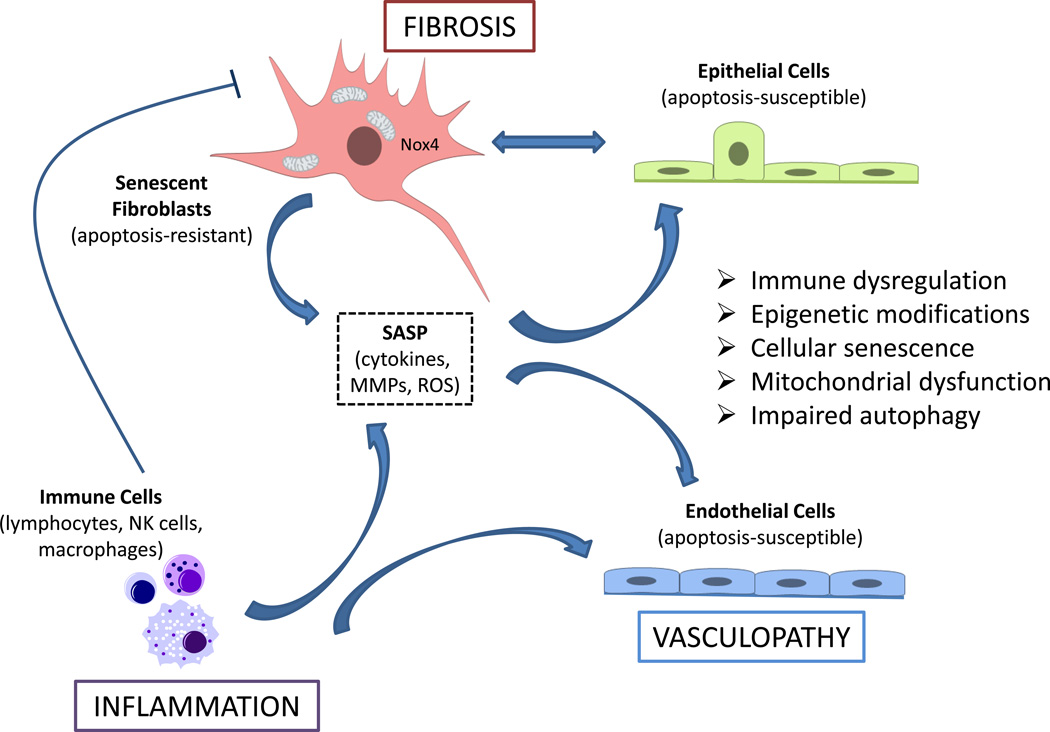
Recent studies provide evidence for aging phenotypes in SSc-associated fibrosis. Aging appears to influence the disease course and mortality in SSc patients, suggesting that aging contributes to disease progression; thus, SSc may be considered a disease process with an accelerated aging phenotype. We have reviewed current evidence for immune dysregulation, epigenetic modifications, cellular senescence, mitochondrial dysfunction and impaired autophagy in the pathogenesis of fibrosis and SSc. Understanding specific roles of these aging-related processes in the altered phenotypes of endothelial cells, immune cells and fibroblasts will provide new opportunities to treat the vasculopathy, inflammation and fibrosis that characterize SSc (Figure 1).
Figure 1.
SSc-associated fibrosis, inflammation and vasculopathy may be perpetuated or accelerated by aging-associated phenotypes that include immune dysregulation, epigenetic modifications, cellular senescence, mitochondrial dysfunction and impaired autophagy. Immune dysfunction, specifically immune-senescence, may fail to clear the accumulation of senescent (myo)fibroblasts that acquire an apoptosis-resistant phenotype. The elaboration of pro-inflammatory cytokines, matrix metalloproteinases (MMPs) and reactive oxygen species (ROS) by the so-called senescence-associated secretory phenotype (SASP) of fibroblasts and immune cells promotes the apoptosis-susceptible phenotype of adjacent epithelial cells and endothelial cells, thus providing a feed-forward mechanism for aberrant tissue remodeling.
Key Points.
Aging is associated with worse outcomes and increased mortality in SSc
Cellular processes such as immune dysregulation, epigenetic modifications, senescence, mitochondrial dysfunction and impaired autophagy may contribute to the genesis and/or progression of inflammation and fibrosis associated with SSc
Further studies are required to define specific roles of aging phenotypes/hallmarks in SSc-associated vasculopathy, inflammation and fibrosis.
Acknowledgements
Financial support and sponsorship
This work was supported by NIH grants, P01 HL114470 and R01 AG046210.
Footnotes
Conflicts of interest
None
References
- 1.Mayes MD, Lacey JV, Jr, Beebe-Dimmer J, Gillespie BW, Cooper B, Laing TJ, et al. Prevalence, incidence, survival, and disease characteristics of systemic sclerosis in a large US population. Arthritis and rheumatism. 2003;48(8):2246–2255. doi: 10.1002/art.11073. [DOI] [PubMed] [Google Scholar]
- 2.Ho YY, Lagares D, Tager AM, Kapoor M. Fibrosis--a lethal component of systemic sclerosis. Nature reviews Rheumatology. 2014;10(7):390–402. doi: 10.1038/nrrheum.2014.53. [DOI] [PubMed] [Google Scholar]
- 3.Furst DE, Fernandes AW, Iorga SR, Greth W, Bancroft T. Epidemiology of systemic sclerosis in a large US managed care population. The Journal of rheumatology. 2012;39(4):784–786. doi: 10.3899/jrheum.111106. [DOI] [PubMed] [Google Scholar]
- 4.Ferri C, Sebastiani M, Lo Monaco A, Iudici M, Giuggioli D, Furini F, et al. Systemic sclerosis evolution of disease pathomorphosis and survival. Our experience on Italian patients' population and review of the literature. Autoimmunity reviews. 2014;13(10):1026–1034. doi: 10.1016/j.autrev.2014.08.029. [DOI] [PubMed] [Google Scholar]
- 5.Strickland G, Pauling J, Cavill C, Shaddick G, McHugh N. Mortality in systemic sclerosis-a single centre study from the UK. Clinical rheumatology. 2013;32(10):1533–1539. doi: 10.1007/s10067-013-2289-0. [DOI] [PubMed] [Google Scholar]
- 6.Manno RL, Wigley FM, Gelber AC, Hummers LK. Late-age onset systemic sclerosis. The Journal of rheumatology. 2011;38(7):1317–1325. doi: 10.3899/jrheum.100956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perez-Bocanegra C, Solans-Laque R, Simeon-Aznar CP, Campillo M, Fonollosa-Pla V, Vilardell-Tarres M. Age-related survival and clinical features in systemic sclerosis patients older or younger than 65 at diagnosis. Rheumatology. 2010;49(6):1112–1117. doi: 10.1093/rheumatology/keq046. [DOI] [PubMed] [Google Scholar]
- 8.Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. American journal of respiratory and critical care medicine. 2011;183(6):788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vivero M, Padera RF. Histopathology of lung disease in the connective tissue diseases. Rheumatic diseases clinics of North America. 2015;41(2):197–211. doi: 10.1016/j.rdc.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 10.Herzog EL, Mathur A, Tager AM, Feghali-Bostwick C, Schneider F, Varga J. Review: interstitial lung disease associated with systemic sclerosis and idiopathic pulmonary fibrosis: how similar and distinct? Arthritis & rheumatology. 2014;66(8):1967–1978. doi: 10.1002/art.38702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thannickal VJ. Mechanistic links between aging and lung fibrosis. Biogerontology. 2013;14(6):609–615. doi: 10.1007/s10522-013-9451-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kennedy BK, Berger SL, Brunet A, Campisi J, Cuervo AM, Epel ES, et al. Geroscience: linking aging to chronic disease. Cell. 2014;159(4):709–713. doi: 10.1016/j.cell.2014.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cannizzo ES, Clement CC, Sahu R, Follo C, Santambrogio L. Oxidative stress, inflamm-aging and immunosenescence. Journal of proteomics. 2011;74(11):2313–2323. doi: 10.1016/j.jprot.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 15.Fulop T, Le Page A, Fortin C, Witkowski JM, Dupuis G, Larbi A. Cellular signaling in the aging immune system. Current opinion in immunology. 2014;29:105–111. doi: 10.1016/j.coi.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 16.Faner R, Rojas M, Macnee W, Agusti A. Abnormal lung aging in chronic obstructive pulmonary disease and idiopathic pulmonary fibrosis. American journal of respiratory and critical care medicine. 2012;186(4):306–313. doi: 10.1164/rccm.201202-0282PP. [DOI] [PubMed] [Google Scholar]
- 17.Thewissen M, Somers V, Venken K, Linsen L, van Paassen P, Geusens P, et al. Analyses of immunosenescent markers in patients with autoimmune disease. Clinical immunology. 2007;123(2):209–218. doi: 10.1016/j.clim.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 18. Rodriguez-Carrio J, Alperi-Lopez M, Lopez P, Alonso-Castro S, Ballina-Garcia FJ, Suarez A. TNFalpha polymorphism as marker of immunosenescence for rheumatoid arthritis patients. Experimental gerontology. 2015;61:123–129. doi: 10.1016/j.exger.2014.12.009.. ** This study provides an example of the genetic basis of the premature immunosenescence of rheumatoid arthrits patients and highlights its potential role in clinical outcomes after TNFα blockade.
- 19.Gilani SR, Vuga LJ, Lindell KO, Gibson KF, Xue J, Kaminski N, et al. CD28 down-regulation on circulating CD4 T-cells is associated with poor prognoses of patients with idiopathic pulmonary fibrosis. PloS one. 2010;5(1):e8959. doi: 10.1371/journal.pone.0008959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ugarte-Gil MF, Sanchez-Zuniga C, Gamboa-Cardenas RV, Aliaga-Zamudio M, Zevallos F, Tineo-Pozo G, et al. Circulating naive and memory CD4+ T cells and metabolic syndrome in patients with systemic lupus erythematosus: data from a primarily Mestizo population. Rheumatology. 2014 doi: 10.1093/rheumatology/keu434.. * This study demonstarted a skewing of CD4(+) T cell subpopulations in SLE patients with metabolic syndrome, potentially linking immunosenescence with cardiovascular morbidity in this subpopulation.
- 21.Pinto AR, Godwin JW, Chandran A, Hersey L, Ilinykh A, Debuque R, et al. Age-related changes in tissue macrophages precede cardiac functional impairment. Aging. 2014;6(5):399–413. doi: 10.18632/aging.100669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brunet A, Berger SL. Epigenetics of aging and aging-related disease. The journals of gerontology Series A, Biological sciences and medical sciences. 2014;69(Suppl 1):S17–S20. doi: 10.1093/gerona/glu042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Altorok N, Almeshal N, Wang Y, Kahaleh B. Epigenetics, the holy grail in the pathogenesis of systemic sclerosis. Rheumatology. 2014 doi: 10.1093/rheumatology/keu155. [DOI] [PubMed] [Google Scholar]
- 24. Zerr P, Palumbo-Zerr K, Huang J, Tomcik M, Sumova B, Distler O, et al. Sirt1 regulates canonical TGF-beta signalling to control fibroblast activation and tissue fibrosis. Annals of the rheumatic diseases. 2014 doi: 10.1136/annrheumdis-2014-205740.. * This study suggests that activation of Sirt1 in fibroblasts is necessary for experimental lung fibrosis, and that suppression of Sirt1, which is already decreased in patints with systemic sclerosis, may offer protection by inhibiting TGF-β signaling.
- 25. Wei J, Ghosh AK, Chu H, Fang F, Hinchcliff ME, Wang J, et al. The Histone Deacetylase Sirtuin 1 Is Reduced in Systemic Sclerosis and Abrogates Fibrotic Responses by Targeting Transforming Growth Factor beta Signaling. Arthritis & rheumatology. 2015;67(5):1323–1334. doi: 10.1002/art.39061.. ** This study indicates that SIRT1 mediates antifibrotic effects, and its reduced tissue expression in patients with systemic sclerosis may contribute to progression of fibrosis
- 26.Sampson N, Berger P, Zenzmaier C. Therapeutic targeting of redox signaling in myofibroblast differentiation and age-related fibrotic disease. Oxidative medicine and cellular longevity. 2012;2012:458276. doi: 10.1155/2012/458276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang F, Liu GS, Dusting GJ, Chan EC. NADPH oxidase-dependent redox signaling in TGF-beta-mediated fibrotic responses. Redox biology. 2014;2:267–272. doi: 10.1016/j.redox.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spadoni T, Svegliati Baroni S, Amico D, Albani L, Moroncini G, Avvedimento EV, et al. A reactive oxygen species-mediated loop maintains increased expression of NADPH oxidases 2 and 4 in skin fibroblasts from patients with systemic sclerosis. Arthritis & rheumatology. 2015;67(6):1611–1622. doi: 10.1002/art.39084. [DOI] [PubMed] [Google Scholar]
- 29.Hecker L, Vittal R, Jones T, Jagirdar R, Luckhardt TR, Horowitz JC, et al. NADPH oxidase-4 mediates myofibroblast activation and fibrogenic responses to lung injury. Nature medicine. 2009;15(9):1077–1081. doi: 10.1038/nm.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hecker L, Logsdon NJ, Kurundkar D, Kurundkar A, Bernard K, Hock T, et al. Reversal of persistent fibrosis in aging by targeting Nox4-Nrf2 redox imbalance. Science translational medicine. 2014;6(231):231ra47. doi: 10.1126/scitranslmed.3008182.. * This study desmonstrated loss of cellular redox homeostasis in senescent myofibroblasts that conferred apoptosis resistance and contributed to progresive fibrosis in an aging model of lung injury, supporting therapeutic strategies targetting redox imbalance in age-associated fibrotic disorders
- 31. Sanders YY, Liu H, Liu G, Thannickal VJ. Epigenetic mechanisms regulate NADPH oxidase-4 expression in cellular senescence. Free radical biology & medicine. 2015;79:197–205. doi: 10.1016/j.freeradbiomed.2014.12.008.. * This study links epigenetic mechanisms with age-associated upregulation of the reactive oxygen species generating enzyme, NOX4, which contributes to senescence/aging and fibrosis
- 32. Sanders YY, Hagood JS, Liu H, Zhang W, Ambalavanan N, Thannickal VJ. Histone deacetylase inhibition promotes fibroblast apoptosis and ameliorates pulmonary fibrosis in mice. The European respiratory journal. 2014;43(5):1448–1458. doi: 10.1183/09031936.00095113.. * This study supports histone deacetylase inhibitors as therapeutic agents for fibrotic diorders by modulating myofibroblast susceptibility to apoptosis.
- 33.Dees C, Distler JH. Canonical Wnt signalling as a key regulator of fibrogenesis - implications for targeted therapies? Experimental dermatology. 2013;22(11):710–713. doi: 10.1111/exd.12255. [DOI] [PubMed] [Google Scholar]
- 34.Dees C, Schlottmann I, Funke R, Distler A, Palumbo-Zerr K, Zerr P, et al. The Wnt antagonists DKK1 and SFRP1 are downregulated by promoter hypermethylation in systemic sclerosis. Annals of the rheumatic diseases. 2014;73(6):1232–1239. doi: 10.1136/annrheumdis-2012-203194. [DOI] [PubMed] [Google Scholar]
- 35.Campisi J. Aging, cellular senescence, and cancer. Annual review of physiology. 2013;75:685–705. doi: 10.1146/annurev-physiol-030212-183653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krizhanovsky V, Yon M, Dickins RA, Hearn S, Simon J, Miething C, et al. Senescence of activated stellate cells limits liver fibrosis. Cell. 2008;134(4):657–667. doi: 10.1016/j.cell.2008.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Munoz-Espin D, Serrano M. Cellular senescence: from physiology to pathology. Nature reviews Molecular cell biology. 2014;15(7):482–496. doi: 10.1038/nrm3823. [DOI] [PubMed] [Google Scholar]
- 38.Piera-Velazquez S, Jimenez SA. Role of cellular senescence and NOX4-mediated oxidative stress in systemic sclerosis pathogenesis. Current rheumatology reports. 2015;17(1):473. doi: 10.1007/s11926-014-0473-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bratic A, Larsson NG. The role of mitochondria in aging. The Journal of clinical investigation. 2013;123(3):951–957. doi: 10.1172/JCI64125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thannickal VJ, Murthy M, Balch WE, Chandel NS, Meiners S, Eickelberg O, et al. Blue journal conference. Aging and susceptibility to lung disease. American journal of respiratory and critical care medicine. 2015;191(3):261–269. doi: 10.1164/rccm.201410-1876PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lionaki E, Markaki M, Palikaras K, Tavernarakis N. Mitochondria, autophagy and age-associated neurodegenerative diseases: New insights into a complex interplay. Biochimica et biophysica acta. 2015 doi: 10.1016/j.bbabio.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 42. Bueno M, Lai YC, Romero Y, Brands J, St Croix CM, Kamga C, et al. PINK1 deficiency impairs mitochondrial homeostasis and promotes lung fibrosis. The Journal of clinical investigation. 2015;125(2):521–538. doi: 10.1172/JCI74942.. ** This study supports mechanistic links between age-related mitochondrial dysfunction, defective mitophagy and lung fibrosis.
- 43. Patel AS, Song JW, Chu SG, Mizumura K, Osorio JC, Shi Y, et al. Epithelial cell mitochondrial dysfunction and PINK1 are induced by transforming growth factor-beta1 in pulmonary fibrosis. PloS one. 2015;10(3):e0121246. doi: 10.1371/journal.pone.0121246.. ** This study demonstrates that the key mitophagy initiating protein, PINK1, ameliorates epithelial cell death and may be necessary to limit fibrogenesis.
- 44.Gazdhar A, Lebrecht D, Roth M, Tamm M, Venhoff N, Foocharoen C, et al. Time-dependent and somatically acquired mitochondrial DNA mutagenesis and respiratory chain dysfunction in a scleroderma model of lung fibrosis. Scientific reports. 2014;4:5336. doi: 10.1038/srep05336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Song X, Wang B, Lin S, Jing L, Mao C, Xu P, et al. Astaxanthin inhibits apoptosis in alveolar epithelial cells type II in vivo and in vitro through the ROS-dependent mitochondrial signalling pathway. Journal of cellular and molecular medicine. 2014;18(11):2198–2212. doi: 10.1111/jcmm.12347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Patel AS, Morse D, Choi AM. Regulation and functional significance of autophagy in respiratory cell biology and disease. American journal of respiratory cell and molecular biology. 2013;48(1):1–9. doi: 10.1165/rcmb.2012-0282TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Madeo F, Zimmermann A, Maiuri MC, Kroemer G. Essential role for autophagy in life span extension. The Journal of clinical investigation. 2015;125(1):85–93. doi: 10.1172/JCI73946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Del Principe D, Vona R, Giordani L, Straface E, Giammarioli AM. Defective autophagy in fibroblasts may contribute to fibrogenesis in autoimmune processes. Current pharmaceutical design. 2011;17(35):3878–3887. doi: 10.2174/138161211798357791. [DOI] [PubMed] [Google Scholar]
- 49.Margaritopoulos GA, Tsitoura E, Tzanakis N, Spandidos DA, Siafakas NM, Sourvinos G, et al. Self-eating: friend or foe? The emerging role of autophagy in idiopathic pulmonary fibrosis. BioMed research international. 2013;2013:420497. doi: 10.1155/2013/420497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Araya J, Kojima J, Takasaka N, Ito S, Fujii S, Hara H, et al. Insufficient autophagy in idiopathic pulmonary fibrosis. American journal of physiology Lung cellular and molecular physiology. 2013;304(1):L56–L69. doi: 10.1152/ajplung.00213.2012. [DOI] [PubMed] [Google Scholar]
- 51. Cabrera S, Maciel M, Herrera I, Nava T, Vergara F, Gaxiola M, et al. Essential role for the ATG4B protease and autophagy in bleomycin-induced pulmonary fibrosis. Autophagy. 2015;11(4):670–684. doi: 10.1080/15548627.2015.1034409.. * This study suggests that the ATG4B protease and autophagy play a crucial role in protecting epithelial cells against bleomycin-induced apoptosis, and that deficiency of this pathway augments fibrosis.
- 52. Nho RS, Hergert P. IPF fibroblasts are desensitized to type I collagen matrix-induced cell death by suppressing low autophagy via aberrant Akt/mTOR kinases. PloS one. 2014;9(4):e94616. doi: 10.1371/journal.pone.0094616.. * This study suggest that an aberrant PTEN/Akt/mTOR axis desensitizes idiopathic pulmonary fibrosis (IPF) fibroblasts from polymerized collagen driven stress by suppressing autophagic activity.
- 53.Frech T, De Domenico I, Murtaugh MA, Revelo MP, Li DY, Sawitzke AD, et al. Autophagy is a key feature in the pathogenesis of systemic sclerosis. Rheumatology international. 2014;34(3):435–439. doi: 10.1007/s00296-013-2827-8. [DOI] [PubMed] [Google Scholar]
- 54. Dumit VI, Kuttner V, Kappler J, Piera-Velazquez S, Jimenez SA, Bruckner-Tuderman L, et al. Altered MCM protein levels and autophagic flux in aged and systemic sclerosis dermal fibroblasts. The Journal of investigative dermatology. 2014;134(9):2321–2330. doi: 10.1038/jid.2014.69.. ** This study demonstrates that systemic sclerosis (SSc) fibroblasts exhibit higher levels of senescence and deficient autophagy, suggesting similarities between SSc-associated fibrosis and the aging process.