Abstract
Novel analogs of 1-(N,O-bis[5-isoquinolinesulfonyl]-N-methyl-L-tyrosyl)-4-phenylpiperazine (KN-62,1) were synthesized and found to be potent antagonists in a functional assay, inhibition of ATP-induced K+ efflux in HEK293 cells expressing recombinant human P2X7 receptors. Antagonism of murine P2X7 receptors was also observed. The analogs consisted of L-tyrosine derivatives, of the general structure R1-Tyr(OR2)-piperazinyl-R3, in which three positions were systematically varied in structure through facile acylation reactions. Each of the three positions was optimized in sequence through parallel synthesis alternating with biological evaluation, leading to the identification and optimization of potent P2X7 antagonists. The optimal groups at R1 were found to be large hydrophobic groups, linked to the α-amino position through carbamate, amide, or sulfonamide groups. The benzyloxycarbonyl (Cbz) group was preferred over most sulfonamides and other acyl groups examined, except for quinoline sulfonyl. At R2, an arylsulfonate ester was preferred, and the order of potency was p-tolyl, p-methoxyphenyl, phenyl > α-naphthyl, β-naphthyl. A benzoyl ester was of intermediate potency. Aliphatic esters and carbonate derivatives at the tyrosyl phenol were inactive, while a tyrosyl O-benzyl ether was relatively potent. The most potent P2X7 receptor antagonists identified in this study contained Cbz at the R1 position, an aryl sulfonate at the R2 position, and various acyl groups at the R3 position. At R3, t-butyloxycarbonyl- and benzoyl groups were preferred. The opening of the piperazinyl ring to an ethylene diamine moiety abolished antagonism. In concentration-response studies, a di-isoquinolinyl, Boc derivative, 4 (MRS2306), displayed an IC50 value of 40 nM as an antagonist of P2X7 receptor-mediated ion flux and was more potent than the reference compound 1. Nα-Cbz, Boc-piperazinyl derivatives, 11 (MRS2317), 22 (MRS2326), and 41 (MRS2409) were less potent than 1, with IC50 values of 200–300 nM.
Keywords: ion channels, nucleotides, structure activity relationships, purines, isoquinolines, KN-62
INTRODUCTION
P2 receptors, which are activated by ATP (adenosine 5′-triphosphate) and other purine/pyrimidine nucleotides, consist of two families: G-protein-coupled receptors termed P2Y, of which seven mammalian subtypes have been cloned, and ligand-gated cation channels termed P2X, of which seven mammalian subtypes have been cloned [Fredholm et al., 1997; North and Barnard, 1997; Jacobson et al., in press]. The nomenclature of P2 receptors and their various ligand specificities have been reviewed previously [Jacobson and Knutsen, 2001; Jacobson et al., 1997; Bhagwat and Williams, 1997; Fischer, 1999].
The P2X7 receptor (formerly P2Z receptor) is expressed primarily in blood cells (monocytes, macrophages, and lymphocytes), in the brain (on microglial cells) [Ferrari et al., 1999], and in the salivary gland. Characteristic of the P2X7 receptor is that at high µM concentrations of agonists it forms or activates a large pore in addition to a cation channel. This pore increases permeability indiscriminately to molecules having MW = 900, such as ethidium bromide, which is used as a marker for pore activity. 2′- and 3′-O-(4-benzoylbenzoyl)-ATP (BzATP) is among the most potent agonists at P2X7 receptors, but also has nanomolar potency at P2X1 receptors [Bianchi et al., 1999]. Affinity labeling of the P2X7 receptor in mast cells has been carried out using [3H]-BzATP [Erb et al., 1990].
In macrophages, activation of the P2X7 receptor triggers the processing and release of IL-1β. In the immune system, activation of the P2X7 receptor leads to apoptosis or programmed cell death [Ferrari et al., 1997; Coutinho-Silva et al., 1999; Humphreys et al., 2000]. BzATP (5 mM) caused apoptosis in dendritic cells, which play a significant role in T-cell activation [Coutinho-Silva et al., 1999]. BzATP (1 mM) was very effective in activating the transcription factor NFAT in N9 microglial cells, suggesting purinergic modulation of early inflammatory gene expression in the nervous and immune systems [Ferrari et al., 1999]. Activation of the P2X7 receptor in rat microglia triggers the release of TNF-α [Hide et al., 2000].
Recent reports have emphasized the importance of P2X7 receptors in the immune system and inflammatory processes. It would be very useful to design selective antagonists of high affinity for this receptor. A P2X7 receptor antagonist may be useful in treating septic shock [Hu et. al., 1998] or neurodegenerative diseases, since the receptor activates astrocytes [Sun et al., 1999] and microglial cells [Ferrari et al., 1999; Visentin et al., 1999]. Modulation of the P2X7 receptor may also be beneficial in ophthalmic diseases [Bringmann et al., 2001]. The P2X7 receptor is expressed in high levels in dendritic cells and ATP acting at this site might serve as a signal to downmodulate the immune response [Coutinho-Silva et al., 1999; Ferrari et al., 2000]. A mouse line engineered to have null expression of the P2X7 receptor has been reported [Solle et al., 2001] and may aid in the identification of therapeutic targets for P2X7 receptor antagonists.
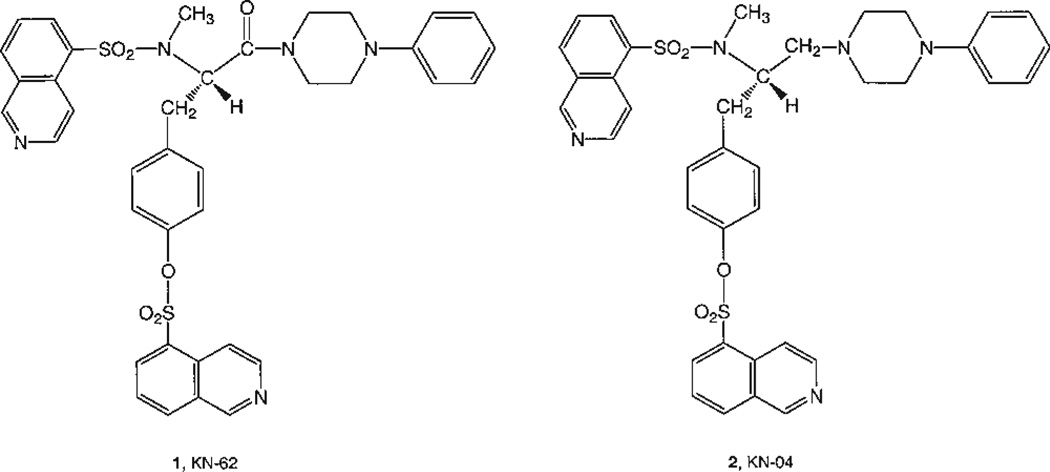
The isoquinoline derivatives (Fig. 1) 1-(N,O-bis[5-isoquinolinesulfonyl]-N-methyl-L-tyrosyl)-4-phenylpiperazine (1, KN-62), and 5-isoquinolinesulfonic acid, 4-[2-[(5-isoquinolinesulfonyl)amino]-3-(4-phenylpiperazine)propyl]phenyl ester (2, KN-04) are potent noncompetitive antagonists at P2X7 receptors [Gargett and Wiley, 1997; Wiley et al., 1998; Humphreys et al., 1998]. Compound 1, but not 2, is also an antagonist of Ca2+/calmodulin-dependent protein kinase II (CaMKII) in the micromolar range [Tokumitsu et al., 1990]. Compound 1 displayed noncompetitive antagonism at P2X7 receptors in HEK293 cells, with an IC50 value of approximately 15 nM [Chessell et al., 1998]. In human leukemic B lymphocytes, compound 1 reduced the rate of permeability increase to larger permeant cations, like ethidium, induced by Bz-ATP with an IC50 of 13.1 nM. Complete inhibition of the flux was observed at 500 nM [Wiley et al., 1998]. Compound 1 had no effect on responses mediated by the P2Y2 receptor of human neutrophils [Gargett and Wiley, 1997] or on calcium mobilization induced by the P2Y1 and P2Y2 receptors naturally expressed in HEK293 human fibroblasts (G. Dubyak, unpubl. obs.). Thus, compound 1 has considerable selectivity for P2X7 receptors within the P2 family.
Fig. 1.
Structures of isoquinoline derivatives reported to be P2X7 receptor antagonists.
Species differences in P2X7 receptor antagonism have been reported [Humphreys et al., 1998; Bianchi et al., 1999]. For example, Brilliant blue G was reported to be a selective antagonist of IC50 values of 10 and 200 nM at the rat and human P2X7 receptors, respectively [Jiang et al., 2000]. Compound 1 at a concentration of 3 µM had no effect on ATP-induced ethidium influx through the rat P2X7 receptor, while the IC50 at the human P2X7 receptor was 0.1 µM [Humphreys et al., 1998].
The binding site for compound 1 resides within the first 335 residues of the human P2X7 receptor [Humphreys et al., 1998]. The precise modeling of this binding site is not feasible at present, due to a lack of a high-resolution template for this ion channel and uncertainty about the oligomeric nature of the channel [Torres et al., 1999]. P2X7 receptors do not form heteromeric receptors with any of the other six P2X subunits and it is not known what types of homomeric assemblies of P2X7 subunits occur [Kim et al., 2001]. P2X7 receptors in brain glia and/or astrocytes appear to be expressed only as monomeric subunits [Kim et al., 2001].
In a study of conformationally constrained analogs of 1 by Baraldi et al. [2000], only one compound showed appreciable activity as a P2X7 antagonist (30-fold weaker than 1) in human macrophage cells. In the present study, we systematically varied all of the substituent groups in a series of tyrosyl analogs of 1, resulting in a qualitative elucidation of structure–activity relationships and an enhancement of potency.
MATERIALS AND METHODS
Chemical Synthesis
Synthetic reagents were purchased from Sigma Chemical Co. (St. Louis, MO) and Aldrich (Milwaukee, WI). 1H-NMR spectra were obtained with a Varian Gemini-300 spectrometer using CD3OD or CDCl3 as a solvent. Low-resolution CI-NH3 (chemical ionization) mass spectra were carried out with a Finnigan 4600 mass spectrometer and high-resolution EI (electron impact) mass spectrometry with a VG7070F mass spectrometry at 6 kV. High-resolution FAB (fast atom bombardment) mass spectrometry was performed with a JEOL SX102 spectrometer using 6-kV Xe atoms following desorption from a glycerol matrix. Compounds 51 and 55a–c were obtained from Calbiochem-Novabiochem (La Jolla, CA).
[N-Fmoc-L-tyrosyl]-Boc-piperazine (52)
A mixture of Fmoc-Tyr-OH (51) (0.4 g, 1 mmol) Bocpiperazine (0.186 g, 1 mmol) and BOP-Cl (0.255 g, 1 mmol) in CH2Cl2 (5 mL) was treated with Et3N (0.28 mL, 2 mmol) and stirred at rt for 5 h. The solvent was removed and the residue obtained was purified using flash chromatography eluting with 10% MeOH in CHCl3 to furnish 52 (0.38 g, 67%) as a solid foam.
1H NMR (CDCl3): δ 7.77 (d, J = 7.3 Hz, 2H), 7.59 (d, J = 7.3 Hz, 2H), 7.40 (t, J = 7.3 Hz, 2H), 7.31 (t, J = 7.3 Hz, 2H), 7.04 (d, J = 7.7 Hz, 2H), 6.74 (d, J = 7.7 Hz, 2H), 5.88 (s, 1H), 5.72 (d, J = 8.4Hz, 1H), 4.90-4.78 (m, 1H), 4.44-4.28 (m, 2H), 4.24-4.16 (m, 2H), 3.6-2.8 (m, 9H), 1.45 (s, 9H).
[L-Tyrosyl]-Boc-piperazine (53)
Compound 52 (0.37 g, 0.65 mmol) was treated with 20% piperidine in DMF (N,N-dimethylformamide) at rt for 10 min for complete reaction. DMF was removed under high vacuum and the residue obtained was purified using flash chromatography eluting with 15% MeOH in CHCl3 to furnish 53 (0.19 g, 54%) as a gum.
1H NMR (CDCl3): δ 6.99 (d, J = 8.2 Hz, 2H), 6.72 (d, J = 8.2 Hz, 2H), 3.9 (t, J = 7.1 Hz, 1H), 3.62-3.20 (m, 6H), 3.10-2.74 (m, 4H), 1.45 (s, 9H).
[N,O-Bis-(quinolinesulfonyl)-L-tyrosyl]-Boc-piperazine and [N,O-Bis-(5-isoquinolinesulfonyl)-L-tyrosyl]-Boc-piperazine (4 and 6)
To a suspension of 8-quinoline sulfonyl chloride or 5-isoquinoline sulfonyl chloride (0.274 g, 1.2 mmol) in anhydrous CH2Cl2 (5 mL) was added a solution of 53 (0.175 g, 0.5 mmol) and Et3N in CH2Cl2 (3 mL) at 0°C, and the mixture stirred at rt for 4 h. The solvent was removed from the reaction mixture under vacuum and the residue obtained was purified using flash chromatography using 5% MeOH in CHCl3 to furnish 0.26 g of 4 and 0.25 g of 6 as a solid foam.
4: 1H NMR (CDCl3): δ 9.43 (s, 1H), 9.34 (s, 1H), 8.84 (d, J = 5.9 Hz, 1H), 8.69 (d, J = 5.4 Hz, 1H), 8.52 (d, J = 5.9 Hz, 1H), 8.54-8.10 (m, 5H), 7.70-7.52 (m, 2H), 6.85 (d, J = 8.2 Hz, 2H), 6.61 (d, J = 8.2 Hz, 2H), 6.00 (bs, 1H), 4.20-4.38 (m, 1H), 3.12-2.50 (m, 10H), 1.45 (s, 9H).
6: 1H NMR (CDCl3): δ 9.25 (d, J = 3.9 Hz, 1H), 9.08 (d, J = 3.9 Hz, 1H), 8.34-8.20 (m, 4H), 8.13 (d, J = 8.1 Hz, 1H), 8.02 (d, J = 8.1 Hz, 1H), 7.68-7.52 (m, 4H), 7.17 (d, J = 9.9 Hz, 1H), 6.90 (d, J = 8.2 Hz, 2H), 6.79 (d, J = 8.2 Hz, 2H), 4.42–4.58 (m, 1H), 3.02-2.26 (m, 10H), 1.44 (s, 9H).
[N,O-Bis-(quinolinesulfonyl)-L-tyrosyl]piperazine and [N,O-Bis-(5-isoquinolinesulfonyl)-L-tyrosyl]piperazine (3 and 5)
Compound 4 or 6 (0.17 g, 0.23 mmol) was treated with 10% TFA in CH2Cl2 at rt for 6 h for complete reaction. Solvent was removed under vacuum and the residue obtained was purified using flash chromatography using 10% MeOH in CHCl3 to furnish 0.12 g of 3 and 5 as a solid foam.
3: 1H NMR (CD3OD): δ 9.41 (s, 1H), 9.30 (s, 1H), 8.73 (d, J = 5.9 Hz, 1H), 8.55-8.05 (m, 7H), 7.66 (ABq, J = 8.1 Hz, 2H), 6.74 (d, J = 8.4 Hz, 2H), 6.27 (d, J = 8.4 Hz, 2H), 4.38-4.22 (m, 1H), 3.80-3.38 (m, 5H), 3.18-2.58 (m, 6H).
5: 1H NMR (CD3OD): δ 9.14 (d, J = 4.3 Hz, 1H), 8.97 (d, J = 4.1 Hz, 1H), 8.52 (d, J = 8.4 Hz, 1H), 8.44-8.22 (m, 4H), 8.17 (d, J = 8.2 Hz, 1H), 7.82-7.58 (m, 4H), 6.94 (d, J = 8.4 Hz, 2H), 6.74 (d, J = 8.4 Hz, 2H), 3.64-2.45 (m, 12H).
[N-Fmoc-O-quinolinesulfonyl-L-tyrosyl]-Boc-piperazine (8)
A suspension of 8-quinoline sulfonyl chloride (0.16 g, 0.71 mmol) in CH2Cl2 was treated with a solution of 52 (0.34 g, 0.60 mmol) and Et3N in CH2Cl2 at 0°C and the mixture was stirred at rt for 6 h. The solvent was removed under vacuum and the crude product was purified using flash chromatography using 5% MeOH in CHCl3 to furnish 0.35 g of 8.
8: 1H NMR (CDCl3): δ 9.26 (s, 1H), 8.3 (t, J = 6.3 Hz, 2H), 8.12 (d, J = 7.9 Hz, 1H), 7.75 (d, J = 7.1 Hz, 2H), 7.64-7.48 (m, 4H), 7.44-7.20 (m, 4H), 7.03 (d, J = 8.3 Hz, 2H), 6.91 (d, J = 8.3 Hz, 2H), 5.61 (d, J = 8.2 Hz, 1H), 4.85-4.65 (m, 1H), 4.45-4.05 (m, 4), 3.55-3.12 (m, 9H), 1.47 (s, 9H).
[O-Quinolinesulfonyl-L-tyrosyl]-Boc-piperazine (9)
Compound 8 (0.3 g, 0.39 mmol) was treated with 20% piperidine in DMF (10 mL) at rt for 10 min. DMF was removed under vacuum and the residue obtained was purified using flash chromatography using 10% MeOH in CHCl3 to furnish 0.19 g of 9 as a solid foam.
9: 1H NMR (CDCl3): δ 9.26 (d, J = 2.8 Hz, 1H), 8.42-8.25 (m, 2H), 8.15 (d, J = 7.9 Hz, 1H), 7.70-7.56 (m, 2H), 7.03 (d, J = 8.3 Hz, 2H), 6.92 (d, J = 8.3 Hz, 2H), 3.92-2.62 (m, 13H), 1.47 (s, 9H).
General Procedure for the Synthesis of 7, 10–18
To a solution of respective R1Cl (0.11 mmol) in anhydrous CH2Cl2 (1 mL) at 0°C was added a solution of 9 (0.03 g, 0.055 mmol), Et3N (0.015 mL, 0.11 mmol) and DMAP (0.007 g, 0.055 mmol) in CH2Cl2 (1 mL) and the mixture stirred for 30 min for complete reaction. The solvent was removed and the crude product was purified by preparative TLC using 5% MeOH in CHCl3 to furnish 7, 10–18.
[N-(5-Isoquinolinesulfonyl)-O-quinolinesulfonyl-L-tyrosyl]-Boc-piperazine (7)
1H NMR (CDCl3): δ 9.45-9.20 (m, 2H), 8.40-8.18 (m, 6H), 7.70-7.52 (m, 4H), 6.83 (d, J = 8.5 Hz, 2H), 6.74 (d, J = 8.5 Hz, 2H), 5.99 (d, J = 9.3 Hz, 1H), 4.40-4.20 (m, 1H), 3.15-2.49 (m, 10H), 1.45 (s, 9H).
[N-Ethyloxycarbonyl-O-quinolinesulfonyl-L-tyrosyl]-Boc-piperazine (10)
1H NMR (CDCl3): δ 9.27 (d, J = 2.4 Hz, 1H), 8.32 (d, J = 7.4 Hz, 2H), 8.15 (d, J = 7.9 Hz, 1H), 7.72-7.52 (m, 2H), 7.04 (d, J = 8.2 Hz, 2H), 6.91 (d, J = 8.2 Hz, 2H), 5.47 (d, J = 7.9 Hz, 1H), 4.82-4.64 (m, 1H), 4.06 (q, J = 7.1 Hz, 2H), 3.52-2.64 (m, 10H), 1.47 (s, 9H), 1.21 9(t, J = 7.1 Hz, 3H).
[N-Cbz-O-quinolinesulfonyl-L-tyrosyl]-Boc-piperazine (11)
1H NMR (CDCl3): δ 9.27 (d, J = 3.9 Hz, 1H), 8.31 (d, J = 8.2 Hz, 2H), 8.14 (d, J = 7.9 Hz, 1H), 7.68-7.52 (m, 2H), 7.44-7.20 (m, 5H), 7.02 (d, J = 7.9 Hz, 2H), 6.90 (d, J = 7.9 Hz, 2H), 5.63 (d, J = 8.5 Hz, 1H), 5.05 (s, 2H), 4.82-4.68 (m, 1H), 3.71 (dd, J = 13.7, 6.87 Hz, 1H), 3.50-2.60 (m, 9H), 1.48 (s, 9H).
[N-Benzoyl-O-quinolinesulfonyl-Ltyrosyl]-Boc-piperazine (12)
1H NMR (CDCl3): δ 9.27 (d, J = 3.8 Hz, 1H), 8.31 (d, J = 7.9 Hz, 2H), 8.14 (d, J = 8.2 Hz, 1H), 7.74 (d, J = 7.6 Hz, 2H), 7.68-7.54 (m, 2H), 7.54-7.34 (m, 3H), 7.08 (d, J = 8.5 Hz, 2H), 6.92 (d, J = 8.2 Hz, 2H), 5.25 (m, 1H), 3.56-2.68 (m, 10H), 1.47 (s, 9H).
[N-Methanesulfonyl-O-quinolinesulfonyl-L-tyrosyl]-Boc-piperazine (13)
1H NMR (CDCl3): δ 9.27 (d, J = 3.9 Hz, 1H), 8.33 (d, J = 7.9 Hz, 2H), 8.15 (d, J = 9.0 Hz, 1H), 7.70-7.55 (m, 2H), 7.06 (d, J = 8.2 Hz, 2H), 6.97 (d, J = 8.2 Hz, 2H), 5.50 (bs, 1H), 4.47 (bs, 1H), 3.66-3.44 (m, 1H), 3.42-2.80 (m, 9H), 2.62 (s, 3H), 1.47 (s, 9H).
[N-Benzenesulfonyl-O-quinolinesulfonyl-L-tyrosyl]-Boc-piperazine (14)
1H NMR (CDCl3): δ 9.25 (s, 1H), 8.36-8.24 (m, 2H), 8.14 (d, J = 7.9 Hz, 1H), 7.74 (d, J = 7.4 Hz, 2H), 7.68-7.38 (m, 5H), 6.97 (d, J = 7.9 Hz, 2H), 6.87 (d, J = 7.9 Hz, 2H), 5.80 (d, J = 9.3 Hz, 1H), 4.34-4.18 (m, 1H), 3.10-2.48 (m, 10H), 1.45 (s, 9H).
[N-Toluenesulfonyl-O-quinolinesulfonyl-L-tyrosyl]-Boc-piperazine (15)
1H NMR (CDCl3): δ 9.26 (d, J = 2.5 Hz, 1H), 8.38-8.24 (m, 2H), 8.15 (d, J = 8.2 Hz, 1H), 7.70-7.54 (m, 4H), 7.32-7.18 (m, 2H), 6.96 (d, J = 8.2 Hz, 2H), 6.88 (d, J = 8.2 Hz, 2H), 5.72 (bs, 1H), 4.24 (s, 1H), 3.18-2.44 (m, 10H), 2.38 (s, 3H), 1.46 (s, 9H).
[N-(4-Methoxybenzenesulfonyl)-O-quinolinesulfonyl-L-tyrosyl]-Boc-piperazine (16)
1H NMR (CDCl3): δ 9.25 (d, J = 3.9 Hz, 1H), 8.31 (d, J = 8.2 Hz, 2H), 8.15 (d, J = 8.2 Hz, 1H), 7.72-7.56 (m, 4H), 7.06-6.80 (m, 6H), 5.70 (d, J = 12.6 Hz, 1H), 4.30-4.14 (m, 1H), 3.83 (s, 3H), 3.22-2.42 (m, 10H), 1.46 (s, 9H).
[N-(1-Naphthylsulfonyl)-O-quinolinesulfonyl-L-tyrosyl]-Boc-piperazine (17)
1H NMR (CDCl3): δ 9.25 (d, J = 2.5 Hz, 1H), 8.52 (d, J = 8.5 Hz, 1H), 8.38-8.22(m, 2H), 8.20-8.08 (m, 2H), 8.04 (d, J = 8.2 Hz, 1H), 7.90 (d, J = 7.7 Hz, 1H), 7.76-7.52 (m, 4H), 7.47 (t, J = 7.7 Hz, 1H), 6.83 (d, J = 8.5 Hz, 2H), 6.75 (d, J = 8.5 Hz, 2H), 5.94 (bs, 1H), 4.30-4.15 (m, 1H), 3.0-2.32 (m, 10H), 1.45 (s, 9H).
[N-(2-Naphthylsulfonyl)-O-quinolinesulfonyl-L-tyrosyl]-Boc-piperazine (18)
1H NMR (CDCl3): δ 9.25 (d, J = 2.2 Hz, 1H), 8.38-8.22 (m, 3H), 8.13 (d, J = 8.2 Hz, 1H), 7.96-7.80 (m, 3H), 7.76-7.52 (m, 5H), 6.95 (d, J = 8.2 Hz, 2H), 6.86 (d, J = 8.2 Hz, 2H), 5.83 (bs, 1H), 4.28 (m, 1H), 3.0-2.70 (m, 5H), 2.70-2.25 (m, 5H), 1.41 (s, 9H).
[N-Cbz-L-tyrosyl]-Boc-piperazine (19)
A mixture of Cbz-Tyr-OH (54) (0.4 g, 1.3 mmol, Aldrich), Boc-piperazine (0.24 g, 1.3 mmol) and BOP-Cl (0.33 g, 1.3 mmol) in anhydrous CH2Cl2 (5 mL) was treated with Et3N (0.36 mL, 2.6 mmol) at rt and stirred for 5 h. The reaction mixture was concentrated under vacuum and the crude product obtained was purified using flash chromatography using 10% MeOH in CHCl3 to furnish 0.4 g of 19.
19: 1H NMR (CDCl3): δ 7.34 (s, 5H), 7.01 (d, J = 8.2 Hz, 2H), 6.72 (d, J = 8.2 Hz, 2H), 6.16 (bs, 1H), 5.70 (d, J = 8.5 Hz, 1H), 5.09 (ABq, J = 12.4 Hz, 2H), 4.90–7.77 (m, 1H), 3.60-3.40 (m, 2H), 3.40-3.14 (m, 4H), 3.08-2.80 (m, 4H), 1.45 (s, 9H).
General Procedure for the Synthesis of 20–31
To a solution of respective R1Cl (0.124 mmol) in anhydrous CH2Cl2 (1 mL) at 0°C was added a solution of 19 (0.03 g, 0.062 mmol), Et3N (0.017 mL, 0.124 mmol) and DMAP (0.007 g, 0.055 mmol) in CH2Cl2 (1 mL) and the mixture stirred for 30 min for complete reaction. The solvent was removed and the crude product was purified by preparative TLC using 5% MeOH in CHCl3 to provide compounds 20–31.
[N-Cbz-O-Methanesulfonyl-L-tyrosyl]-Boc-piperazine (20)
1H NMR (CDCl3): δ 7.4-7.3 (m, 5H), 7.23 (d, J = 8.8 Hz, 2H), 7.19 (d, J = 8.8 Hz, 2H), 5.70 (d, J = 8.2 Hz, 1H), 5.07 (ABq, J = 12.1 Hz, 2H), 4.92-4.80 (m, 1H), 3.60-3.12 (m, 8H), 3.11 (s, 3H), 3.06-2.72 (m, 2H), 1.45 (s, 9H).
[N-Cbz-O-Benzenesulfonyl-L-tyrosyl]-Boc-piperazine (21)
1H NMR (CDCl3): δ 7.82 (d, J = 7.7 Hz, 2H), 7.62- 7.48 (m, 3H), 7.34 (s, 5H), 7.10 (d, J = 7.9 Hz, 2H), 6.88 (d, J = 7.9 Hz, 2H), 5.65 (d, J = 8.5 Hz, 1H), 5.07 (ABq, J = 12.4 Hz, 2H), 4.88-4.76 (m, 1H), 3.60-2.65 (m, 10H), 1.44 (s, 9H).
[N-Cbz-O-Toluenesulfonyl-L-tyrosyl]-Boc-piperazine (22)
1H NMR (CDCl3): δ 7.69 (d, J = 7.9 Hz, 2H), 7.42-7.28 (m, 7H), 7.09 (d, J = 7.9 Hz, 2H), 6.88 (d, J = 7.9 Hz, 2H), 5.65 (d, J = 8.5 Hz, 1H), 5.07 (ABq, J = 12.6 Hz, 2H), 4.90-4.76 (m, 1H), 3.60–3.70 (m, 10H), 2.45 (s, 3H), 1.44 (s, 9H).
[N-Cbz-O-(4-Methoxylbenzenesulfonyl)-L-tyrosyl]-Boc-piperazine (23)
1H NMR (CDCl3): δ 7.83 (d, J = 8.8 Hz, 2H), 7.50-7.40 (m, 5H), 7.20 (d, J = 8.3 Hz, 2H), 7.09 (d, J = 8.8 Hz, 2H), 6.99 (d, J = 8.8 Hz, 2H), 5.75 (d, J = 8.51 Hz. 1H), 5.18 (ABq, J = 12.4 Hz, 2H), 5.0-4.84 (m, 1H), 3.99 (s, 3H), 3.66-2.80 (m, 10H), 1.55 (s,9H).
[N-Cbz-O-(1-Naphthylsulfonyl)-L-tyrosyl]-Boc-piperazine (24)
1H NMR (CDCl3): δ 8.81 (d, J = 8.5 Hz, 1H), 8.13 (d, J = 8.2 Hz, 1H), 8.11-7.94 (m, 2H), 7.92-7.64 (m, 2H), 7.49 (t, J = 7.7 Hz, 1H), 7.32 (s, 5H), 6.99 (d, J = 8.2 Hz, 2H), 6.74 (d, J = 8.2 Hz, 2H), 5.62 (d, J = 8.2 Hz, 1H), 5.05 (ABq, J = 12.1 Hz, 2H), 4.86-4.70 (m, 1H), 3.58-2.64 (m, 10H), 1.47 (s, 9H).
[N-Cbz-O-(2-Naphthylsulfonyl)-L-tyrosyl]-Boc-piperazine (25)
1H NMR (CDCl3): δ 8.38 (s, 1H), 8.05-7.86 (m, 3H), 7.86-7.76 (m, 1H), 7.75-7.45 (m, 2H), 7.32 (s, 5H), 5.61 (d, J = 8.5 Hz, 1H), 5.05 (ABq, J = 12.6 Hz, 2H), 4.88-4.68 (m, 1H), 3.54-2.68 (m, 10H), 1.45 (s, 9H).
[N-Cbz-O-(5-Isoquinolinesulfonyl)-L-tyrosyl]-Boc-piperazine (26)
1H NMR (CDCl3): δ 9.43 (s, 1H), 8.83 (d, J = 6.0 Hz, 1H), 8.55 (d, J = 6.0 Hz, 1H), 8.30-8.21 (m, 2H), 7.67 (t, J = 7.7 Hz, 1H), 7.37-7.30 (m, 5H), 7.02 (d, J = 8.2 Hz, 2H), 6.74 (d, J = 8.2 Hz, 2H), 5.58 (d, J = 8.8 Hz, 1H), 5.05 (ABq, J = 3.3 Hz, 2H), 4.81-4.73 (m, 1H), 3.76-2.75 (m, 10H), 1.46 (s, 9H).
[N,O-Bis-Cbz-L-tyrosyl]-Boc-piperazine (27)
1H NMR (CDCl3): δ 7.48-7.28 (m, 10 H), 7.19 (d, J = 8.2 Hz, 2H), 7.19 (d, J = 8.2 Hz, 2H), 5.68 (d, J = 8.5 Hz, 1H), 5.25 (s, 2H), 5.08 (ABq, J = 12.36 Hz, 2H), 4.94-3.78 (m, 1H), 3.60-2.68 (m, 10H), 1.44 (s, 9H).
[N-Cbz-O-Benzoyl-L-tyrosyl]-Boc-piperazine (28)
1H NMR (CDCl3): δ 8.18 (d, J = 7.1 Hz, 2H), 7.68-7.42 (m, 3H), 7.25 (d, J = 8.2 Hz, 2H), 7.13 (d, J = 8.2 Hz, 2H), 5.78 (d, J = 8.2 Hz, 1H), 5.10 (ABq, J = 12.4 Hz, 2H), 4.88-4.80 (m, 1H), 3.62-2.80 (m, 10H), 1.45 (s, 9H).
[N-Cbz-O-Ethoxycarbonyl-L-tyrosyl]-Boc-piperazine (29)
1H NMR (CDCl3): δ 7.34 (s, 5H), 7.19 (d, J = 8.5 Hz, 2H), 7.09 (d, J = 8.2 Hz, 2H), 5.79 (d, J = 8.5 Hz, 1H), 5.09 (ABq, J = 12.3 Hz, 2H), 4.94-4.74 (m, 1H), 4.29 (q, J = 7.1 Hz, 2H), 3.58-2.70 (m, 10H), 1.44 (s, 9H), 1.38 (t, J = 7.1 Hz, 3H).
[N-Cbz-O-Acetyl-L-tyrosyl]-Boc-piperazine (30)
1H NMR (CDCl3): δ 7.34 (s, 5H), 7.19 (d, J = 8.2 Hz, 2H), 6.99 (d, J = 8.2 Hz, 2H), 5.70 (d, J = 8.5 Hz, 1H), 5.09 (ABq, J = 12.3 Hz, 2H), 4.88-4.84 (m, 1H), 3.51-2.76 (m, 10H), 2.27 (s, 3H), 1.44 (s, 9H).
[N-Cbz-O-Propionyl-L-tyrosyl]-Boc-piperazine (31)
1H NMR (CDCl3): δ 7.34 (s, 5H), 7.18 (d, J = 8.2 Hz, 2H), 6.99 (d, J = 8.2 Hz, 2H), 5.71 (d, J = 8.2 Hz, 1H), 5.09 (ABq, J = 12.6 Hz, 2H), 4.88-4.84 (m, 1H), 3.48-2.78 (m, 10H), 2.57 (q, J = 7.4 Hz, 2H), 1.44 (s, 9H), 1.24 (t, J = 7.4 Hz, 3H).
General Procedure for the Synthesis of 32–40, 42, 43
A solution of 21 (0.03 g, 0.057 mmol), Et3N (0.017 mL, 0.124 mmol) and DMAP (0.007 g, 0.055 mmol) in CH2Cl2 (1 mL) was added to a solution of the respective R1Cl (0.124 mmol) in anhydrous CH2Cl2 (1 mL) at 0° C. The mixture was stirred for 30 min to achieve complete reaction. The solvent was removed and the crude product was purified by preparative TLC using 5% MeOH in CHCl3 to provide compounds 32–40, 42, 43.
[N-Cbz-O-Benzenesulfonyl-L-tyrosyl]toluenesulfonylpiperazine (32)
1H NMR (CDCl3): δ 7.90-7.76 (m, 2H), 7.76-7.64 (m, 1H), 7.64-7.48 (m, 4H), 7.42-7.28 (m, 7H), 7.02 (d, J = 8.5 Hz, 2H), 6.81 (d, J = 8.5 Hz. 2H), 5.57 (d, J = 8.5 Hz, 1H), 5.03 (s, 2H), 4.80-4.64 (m, 1H), 3.90-3.68 (m, 1H), 3.50-2.52 (m, 9H), 2.45 (s, 3H).
[N-Cbz-O-Benzenesulfonyl-L-tyrosyl]methanesulfonylpiperazine (33)
1H NMR (CDCl3): δ 7.85 (d, J = 7.4 Hz, 2H), 7.69 (t, J = 7.4 Hz, 1H), 7.62-7.52 (m, 2H), 7.40-7.28 (m, 5H), 7.14 (d, J = 8.3 Hz, 2H), 6.95 (d, J = 8.5 Hz, 2H), 5.61 (d, J = 8.8 Hz, 1H), 5.08 (t, J = 12.9 Hz, 2H), 4.88-4.72 (m, 1H), 3.70-2.80 (m, 9H), 2.73 (s, 3H), 2.60-2.44 (m, 1H).
[N-Cbz-O-Benzenesulfonyl-L-tyrosyl]benzenesulfonylpiperazine (34)
1H NMR (CDCl3): δ 7.83 (d, J = 7.9 Hz, 2H), 7.76-7.50 (m, 8H), 7.40 (m, 5H), 7.01 (d, J = 8.2 Hz, 2H), 6.79 (d, J = 8.2 Hz, 2H), 5.03 (t, J = 12.6 Hz, 2H), 4.78-4.62 (m, 1H), 3.84-3.64 (m, 1H), 3.44-2.45 (m, 9H).
[N-Cbz-O-benzenesulfonyl-L-tyrosyl]-(4-methoxybenzenesulfonyl)piperazine (35)
1H NMR (CDCl3): δ 7.90-7.80 (m, 2H), 7.74-7.52 (m, 5H), 7.38-7.28 (m, 5H), 7.03 (d, J = 9.1 Hz, 4H), 6.82 (d, J =8.5 Hz, 2H), 5.58 (d, J = 8.5 Hz, 1H), 5.03 (t, J = 12.4 Hz, 2H), 4.78-4.64 (m, 1H), 3.89 (s, 3H), 3.84-3.70 (m, 1H), 3.50-2.52 (m, 9H).
[N-Cbz-O-Benzenesulfonyl-L-tyrosyl]-(1-naphthylsulfonyl)piperazine (36)
1H NMR (CDCl3): δ 8.78 (d, J = 8.24 Hz, 1H), 8.32-8.16 (m, 2H), 8.06 (d, J = 8.2 Hz, 1H), 7.92 (d, J = 8.2 Hz, 2H), 7.84-7.60 (m, 6H), 7.46-7.32 (m, 5H), 7.10 (d, J = 8.2 Hz, 2H), 6.90 (d, J = 8.2 Hz, 2H), 5.64 (d, J = 8.2 Hz, 1H), 5.11 (q, J = 12.4 Hz, 2H), 4.88-4.72 (m, 1H), 3.94-2.82 (m, 10H).
[N-Cbz-O-Benzenesulfonyl-L-tyrosyl]-(2-naphthylsulfonyl)piperazine (37)
1H NMR (CDCl3): δ 8.39 (s, 1H), 8.18-8.0 (m, 3H), 7.91 (d, J = 8.5 Hz, 2H), 7.84-7.70 (m, 4H), 7.70-7.62 (m, 2H), 7.46-7.30 (m, 5H), 7.11 (d, J = 8.5 Hz, 2H), 6.90 (d, J = 8.5 Hz, 2H), 5.63 (d, J = 8.5 Hz, 1H), 5.08 (ABq, J = 12.4 Hz, 2H), 4.86-4.74 (m, 1H), 4.02-3.86 (m, 1H), 3.60-3.15 (m, 4H), 3.10-2.78 (m, 5H).
[N-Cbz-O-Benzenesulfonyl-L-tyrosyl]-Cbz-piperazine (38)
1H NMR (CDCl3): δ 7.80 (d, J = 7.69 Hz, 2H), 7.69-7.58 (m, 1H), 7.58-7.44 (m, 2H), 7.44-7.26 (s, 10H), 7.09 (d, J = 8.2 Hz, 2H), 6.89 (d, J = 8.2 Hz, 2H), 5.62 (d, J = 8.2 Hz, 1H), 5.22-5.0 (m, 4H), 4.88-4.74 (m, 1H), 3.62-2.74 (m, 10H).
[N-Cbz-O-Benzenesulfonyl-L-tyrosyl]ethoxycarbonylpiperazine (39)
1H NMR (CDCl3): δ 7.83 (d, J = 7.4 Hz, 2H), 7.72-7.62 (m, 1H), 7.60-7.48 (m, 2H), 7.42-7.28 (m, 5H), 7.10 (d, J 8.5 Hz, 2H), 6.89 (d, J = 8.5 Hz, 2H), 5.63 (d, J = 8.5 Hz, 1H), 5.07 (ABq, J = 12.4 Hz, 2H), 4.88-4.76 (m, 1H), 4.13 (q, J = 7.1 Hz, 2H), 3.62-2.78 (m, 10H), 1.25 (t, J = 7.1 Hz, 3H).
[N-Cbz-O-Benzenesulfonyl-L-tyrosyl]benzoylpiperazine (40)
1H NMR (CDCl3): δ 7.85 (d, J = 7.7 Hz, 2H), 7.72-7.50 (m, 3H), 7.46-7.28 (m, 10H), 7.12 (d, J = 8.2 Hz, 2H), 6.91 (d, J = 8.2 Hz, 2H), 5.65 (d, J = 8.8 Hz, 1H), 5.07 (ABq, J = 12.4 Hz, 2H), 4.82 (bs, 1H), 3.65-2.85 (m, 10H).
[N-Cbz-O-Benzenesulfonyl-L-tyrosyl]acetylpiperazine (42)
1H NMR (CDCl3): δ 7.83 (d, J = 7.7 Hz, 2H), 7.72-7.64 (m, 1H), 7.55 (t, J = 7.7 Hz, 2H), 7.34 (s, 5H), 7.13 (t, J = 8.7 Hz, 2H), 6.91 (t, J = 8.7 Hz, 2H), 5.64 (bs, 1H), 5.08 (s, 2H), 4.86-4.80 (m, 1H), 3.53-2.94 (m, 10H), 2.08 (s, 3H).
[N-Cbz-O-Benzenesulfonyl-L-tyrosyl]propionylpiperazine (43)
1H NMR (CDCl3): δ 7.82 (d, J = 7.1 Hz, 2H), 7.66 (t, J = 7.1 Hz, 1H), 7.54 (t, J = 7.1 Hz, 2H), 7.33 (s, 5H), 7.12 (t, J = 8.8 Hz, 2H), 6.90 (t, J = 8.8 Hz, 2H), 5.62 (bs, 1H), 5.07 (s, 2H), 4.86-4.78 (m, 1H), 3.58-2.89 (m, 10H), 2.31 (q, J = 7.4 Hz, 2H), 1.12 (t, J = 7.4 Hz, 3H).
[N-Cbz-O-Benzyl-L-tyrosyl]Boc-piperazine (44)
To a mixture of Cbz-Tyr(Bzl)OH (55a) (0.5 g, 1.23 mmol), Boc-piperazine (0.23g, 1.23 mmol), Bop-Cl (0.31 g, 1.23 mmol) in CH2Cl2 (10 mL) was added Et3N (0.343 mL, 2.46 mmol) and stirred at rt for 5 h. The solvent was removed under vacuum and the crude material obtained was purified by flash chromatography using 5% MeOH in CHCl3 to furnish 0.5 g of 44 as a solid foam. 1H NMR (CDCl3): δ 7.46 (m, 5H), 7.08 (d, J = 8.3 Hz, 2H), 6.87 (d, J = 8.5 Hz, 2H), 5.66 (d, J = 8.5 Hz, 1H), 5.08 (ABq, J = 12.1 Hz, 2H), 5.04 (s, 2H), 4.90-4.74 (m, 1H), 3.62-2.70 (m, 10H), 1.44 (s, 9H).
[N-Boc-O-Benzyl-L-tyrosyl]-Boc-piperazine (45)
A mixture of Boc-Tyr(Bzl)OH (55b) (0.03 g, 0.08 mmol), Boc-piperazine (0.015 g, 0.08 mmol), BOP-Cl (0.02 g, 0.08 mmol) in CH2Cl2 (2 mL) was treated with Et3N (0.022 mL, 0.16 mmol) and stirred at rt for 5 h. The solvent was removed under vacuum and the crude material obtained was purified using flash chromatography to furnish 0.30 g of 45 as a solid foam. 1H NMR (CDCl3): δ 7.46-7.28 (m, 5H), 7.09 (d, J = 8.5 Hz, 2H), 6.88 (d, J = 8.5 Hz, 2H), 5.37 (d, J = 8.5 Hz, 1H), 5.04 (s, 2H), 4.84-4.66 (m, 1H), 3.60-2.70 (m, 10H), 1.44 (s, 9H), 1.42 (s, 9H).
[N-Boc-O-Benzenesulfonyl-L-tyrosyl]-Boc-piperazine (46)
Compound 45 (0.02 g, 0.037 mmol) was hydrogenated using Pd/C (0.005 g), H2 at 40 psi for 6 h. The reaction mixture was filtered and concentrated to dryness. A solution of this material (0.012 g, 0.026 mmol), Et3N (0.008 mL, 0.54 mmol) and DMAP (0.003 g, 0.027 mmol) in CH2Cl2 was treated with benzenesulfonyl chloride (0.007 mL, 0.054 mmol) at 0° C and stirred at rt for 30 min. The reaction mixture was concentrated and purified by preparative TLC using 5% MeOH in CHCl3 to furnish 0.01 g of 46 as a solid foam. 1H NMR (CDCl3): δ 7.93 (d, J = 8.2 Hz, 2H), 7.84-7.60 (m, 3H), 7.22 (d, J = 8.2 Hz, 2H), 6.99 (d, J = 8.2 Hz, 2H), 5.44 (s, 1H), 4.94-4.80 (m, 1H), 3.68-3.24 (m, 10H), 1.54 (s, 9H), 1.51 (s, 9H).
[N-Boc-O-Benzyl-N-methyl-L-tyrosyl]-Boc-piperazine (47)
A mixture of Boc-MeTyr(Bzl)OH (55c) (0.5 g, 1.29 mmol), Boc-piperazine (0.24 g, 1.29 mmol), BOP-Cl (0.33 g, 1.29 mmol) in CH2Cl2 (10 mL) was treated with Et3N (0.36 mL, 2.58 mmol) and stirred at rt for 5 h. The solvent was removed under vacuum and the crude material obtained was purified by flash chromatography using 5% MeOH in CHCl3 to furnish 0.5 g of 47 as a solid foam. 1H NMR (CDCl3): δ 7.48-7.22 (m, 5H), 7.14 (d, J = 8.5 Hz, 2H), 6.87 (d, J = 8.5 Hz, 2H), 5.26-5.14 (m, 1H), 5.02 (s, 2H), 3.88-2.84 (m, 10H), 2.79 (s, 3H), 1.45 (s, 9H), 1.35 (s, 9H).
[N-Cbz-O-Benzyl-N-methyl-L-tyrosyl]-Cbz-piperazine (48)
Compound 47 (0.05 g, 0.09 mmol) was treated with 10% TFA in CH2Cl2 (2 mL) at rt for 5 h. The solvent was removed under vacuum and the crude material was purified by preparative TLC to furnish 0.027 g compound. This product (0.027 g, 0.076 mmol) and Et3N (0.042 ml, 0.18 mmol) and DMAP (0.009 g, 0.073 mmol)) in CH2Cl2 (2 mL) was cooled to 0°C and Cbz-Cl was added (0.026 mL, 0.18 mmol) and stirred at rt for 30 min. Solvent was removed under vacuum and the crude material was purified by preparative TLC using 5% MeOH in CHCl3 to furnish 0.025 g of 48 as a solid foam. 1H NMR (CDCl3): δ 7.48-7.24 (m, 15H), 7.13 (d, J = 8.5 Hz, 2H), 6.86 (d, J = 8.5 Hz, 2H), 5.32-5.19 (m, 1H), 5.12 (s, 2H), 5.05 (s, 2H), 5.02 (s, 2H), 3.76-2.98 (m, 10H), 2.92 (s, 3H).
[N-Boc-O-Benzyl-D-tyrosyl]-Boc-piperazine (49)
This compound was prepared starting from Boc-D-Tyr(Bzl)OH (Bachem Bioscience, King of Prussia, PA), by the same method as for the corresponding L-enantiomer, 45.
[N-Cbz-O-Benzyl-L-tyrosyl]-Boc-ethylenediamine (50)
To a mixture of Cbz-Tyr(Bzl)OH (55a) (0.03 g, 0.074 mmol), Boc-ethylenediamine (0.012 mL, 0.074 mmol), Bop-Cl (0.019 g, 0.074 mmol) in CH2Cl2 (2 mL) was added Et3N (0.01 mL, 0.072 mmol) and stirred at rt for 5 h. The solvent was removed under vacuum and the crude material obtained was purified by flash chromatography using 5% MeOH in CHCl3 to furnish 50 as a solid foam. 1H NMR (CDCl3): δ 7.46-7.28 (m, 10H), 709 (d, J = 8.2 Hz, 2H), 6.90 (d, J = 8.2 Hz, 2H), 6.23 (bs, 1H), 5.40-5.22 (m, 1H), 5.18-4.98 (m, 4H), 4.82-4.64 (m, 1H), 4.40-4.22 (m, 1H), 3.38-2.85 (m, 6H), 1.41 (s, 9H).
Pharmacological Analyses
P2X7Receptor Channel Activation
All experiments were performed using adherent HEK293 cells stably transfected with cDNA encoding the human P2X7 receptor. Adherent cells on 12-well polylysine-coated plates were incubated at 37°C in 1 mL physiological salt solution (125 mM NaCl, 5 mM KCl, 1 mM MgCl2, 1.5 mM CaCl2, 25 mM NaHEPES (pH 7.5), 10 mM D-glucose, 1 mg/mL BSA). Antagonists were added from 1,000× stock solutions dissolved in DMSO. Cells were preincubated with antagonists for 15 min prior to stimulation for 10 min with 3 mM ATP (final concentration). Reactions were terminated by rapid aspiration of the extracellular medium in each well. The adherent cells in each well were then extracted overnight with 1 ml 10% HNO3. K+ content in these nitric acid extracts was assayed by atomic absorbance spectrophotometry. Duplicate or triplicate wells were run for all test conditions in each separate experiment.
Several compounds were tested in a similar manner using adherent Bac1.2F5 mouse macrophages that natively express the murine P2X7 receptor.
RESULTS AND DISCUSSIONS
Chemical Synthesis
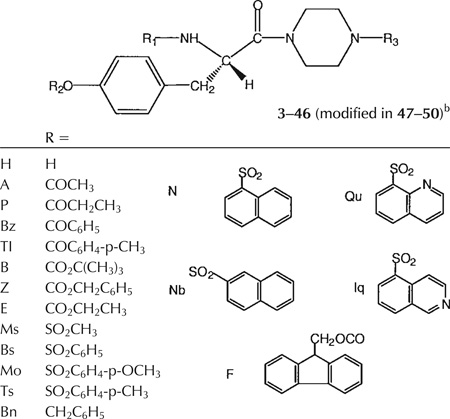
The analogs consisted of L-tyrosine derivatives, of the general structure R1-Tyr(OR2)-piperazinyl-R3 (Tables 1, 2), in which three positions were systematically varied in structure through facile acylation reactions. Each of the three positions was optimized in sequence through parallel synthesis alternating with biological evaluation, consisting of screening at a single concentration (initially 3 µM), leading to the identification and optimization of potent P2X7 antagonists.
TABLE 1.
Synthetic Yields and Characterizations of Tyrosyl Derivatives
| Compound | Formula | % Yield | Analysis* |
|---|---|---|---|
| 3 | C31H29N5O6S2 | 82 | HRMS |
| 4 | c36H37N5O8S2.2H2O | 72 | CHN |
| 5 | C31H29N5O6S2 | 82 | HRMS |
| 6 | C36H37N5O8S2.2H2O | 69 | CHN |
| 7 | C36H37N5O8S2 | 60 | HRMS |
| 8 | C42H42N4O8S | 71 | HRMS |
| 9 | C27H32N4O6S | 90 | HRMS |
| 10 | C30H36N4O8S.1.5H2O | 84 | CHN |
| 11 | C35H38N4O8S.0.5H2O | 73 | CHN |
| 12 | C34H36N4O7S | 71 | CHN |
| 13 | C28H34N4O8S2.1.9H2O | 60 | CHN |
| 14 | C33H36N4O8S2.1.5H2O | 73 | CHN |
| 15 | C34H38N4O8S2.2.1H2O | 65 | CHN |
| 16 | C34H38N4O9S2.2.5H2O | 69 | CHN |
| 17 | C37H38N4O8S2.3.5H2O | 75 | CHN |
| 18 | C37H38N4O8S2.1.5H2O | 85 | CHN |
| 19 | C26H33N3O6 | 63 | HRMS |
| 20 | C27H35N3O8S.0.5H2O | 88 | CHN |
| 21 | C32H37N3O8S.0.75H2O | 68 | CHN |
| 22 | C33H39N3O8S.0.5H2O | 66 | CHN |
| 23 | C33H39N3O9S | 55 | HRMS |
| 24 | C36H39N3O8S.0.5H2O | 63 | CHN |
| 25 | C36H39N3O8S | 58 | CHN |
| 26 | C35H38N4O8S | 62 | HRMS |
| 27 | C34H39N3O8 | 80 | CHN |
| 28 | C33H37N3O7 | 80 | CHN |
| 29 | C29H37N3O8 | 68 | CHN |
| 30 | C28H35N3O7 | 66 | HRMS |
| 31 | C29H37N3O7 | 66 | CHN |
| 32 | C34H35N3O8S2.1H2O | 73 | CHN |
| 33 | C28H31N3O8S2 | 76 | HRMS |
| 34 | C33H33N3O8S2.H2O | 75 | CHN |
| 35 | C34H35N3O9S2.H2O | 67 | CHN |
| 36 | C37H35N3O8S2.1.5H2O | 70 | CHN |
| 37 | C37H35N3O8S2.1.8H2O | 70 | CHN |
| 38 | C35H35N3O8S | 70 | HRMS |
| 39 | C30H33N3O8S.0.5H2O | 72 | CHN |
| 40 | C34H33N3O7S.H2O | 77 | CHN |
| 41 | C37H34N4O7S | 60 | HRMS |
| 42 | C29H31N3O7S.H2O | 68 | CHN |
| 43 | C30H33N3O7S | 69 | CHN |
| 44 | C33H39N3O6.0.2H2O | 71 | CHN |
| 45 | C30H41N3O6.0.2H2O | 69 | CHN |
| 46 | C29H39N3O8S.1H2O | 63 | CHN |
| 47 | C31H43N3O6 | 70 | CHN |
| 48 | C37H39N3O6 | 53 | HRMS |
| 49 | C30H41N3O6.0.2H2O | 70 | CHN |
| 50 | C31H37N3O6 | 60 | CHN |
Elemental analyses of ± 0.4% were considered acceptable. Acceptable tolerance of ± 50 ppm was applied to the high-resolution mass spectral determination.
TABLE 2.
Antagonistic Effects of Tyrosine Derivatives on Function of Human P2X7 Receptors Expressed in HEK293 Cellsa
 | |||
|---|---|---|---|
| Compound | Structure R1R2R3 |
% Inhibition of ATP-induced K releasea |
|
| 1 (KN-62) | 85 ± 9 | (13) | |
| 3 | IqIqH | 14 ± 12 | (3) |
| 4 | IqIqB | 77 ± 24 | (3) |
| 5 | QuQuH | 5± 9 | (3) |
| 6 | QuQuB | 61 ± 30 | (3) |
| 7 | IqQuB | 37 ± 21 | (5) |
| 8 | FQuB | 34 ± 28 | (3) |
| 9 | HQuB | 1± 1 | (3) |
| 10 | EQuB | 5± 7 | (3) |
| 11 | ZQuB | 53 ± 23 | (4) |
| 12 | BzQuB | 13 ± 8 | (3) |
| 13 | MsQuB | 4± 4 | (3) |
| 14 | BsQuB | 8± 3 | (3) |
| 15 | TsQuB | 32 ± 16 | (3) |
| 16 | MoQuB | 26 ± 20 | (4) |
| 17 | NQuB | 16 ± 2 | (3) |
| 18 | NbQuB | 0 | (1) |
| 19 | ZHB | 0 | (1) |
| 20 | ZMsB | 0 | (1) |
| 21 | ZBsB | 59 ± 14 | (3) |
| 22 | ZTsB | 71 ± 30 | (3) |
| 23 | ZMoB | 62 ± 15 | (3) |
| 24 | ZNB | 25 ± 15 | (3) |
| 25 | ZNbB | 43 ± 16 | (4) |
| 26 | ZIqB | 43 | (1) |
| 27 | ZZB | 1 | (1) |
| 28 | ZBzB | 47 ± 21 | (3) |
| 29 | ZEB | 0 | (1) |
| 30 | ZAB | 0 | (1) |
| 31 | ZPB | 4 | (1) |
| 32 | ZBsTs | 0 | (1) |
| 33 | ZBsMs | 0 | (1) |
| 34 | ZBsBs | 0 | (1) |
| 35 | ZBsMo | 0 | (1) |
| 36 | ZBsN | 0 | (1) |
| 37 | ZBsNb | 0 | (1) |
| 38 | ZBsZ | 48 ± 29 | (3) |
| 39 | ZBsE | 24 ± 11 | (3) |
| 40 | ZBsBz | 78 ± 22 | (3) |
| 41 | ZIqBz | 85 ± 10 | (3) |
| 42 | ZBsA | 0 | (1) |
| 43 | ZBsP | 0 | (1) |
| 44 | ZBnB | 45 | (1) |
| 45 | BBnBc | 14 ± 18 | (3) |
| 46 | BBsB | 6 | (1) |
| 47 | BBnBbc | 0± 0 | (3) |
| 48 | ZBnZbc | 8± 7 | (3) |
| 49 | BBnBbc | 4± 3 | (3) |
| 50 | ZBnBb | 0 | (1) |
All experiments were performed using adherent HEK293 cells stably transfected with cDNA encoding the human P2X7 receptor. Adherent cells on 12-well polylysine-coated plates were incubated at 37°C in 1 ml physiological salt solution (125 mM NaCl, 5 mM KCl, 1 mM MgCl2, 1.5 mM CaCl2, 25 mM NaHEPES (pH 7.5), 10 mM D-glucose, 1 mg/ml BSA). Antagonists (3 µM final concentration) were added from 1,000× stock solutions dissolved in DMSO. Cells were preincubated with antagonists for 15 min prior stimulation for 10 min with 3 mM ATP (final concentration). Reactions were terminated by rapid aspiration of the extracellular medium in each well. The adherent cells in each well were then extracted overnight with 1 ml 10% HNO3 and the K+ content in the extracts was assayed by atomic absorbance spectrophotometry. Duplicate or triplicate wells were run for all test conditions in each separate experiment and the measured K+ contents were averaged. Antagonist function was measured by the percent inhibition of the K+ release triggered by 3 mM ATP in paired cells in the absence of antagonist. Data points represent the mean ± SD values obtained; the number of separate experiments is indicated in parentheses. 4, MRS 2306; 6, MRS 2300; 11, MRS 2317; 21, MRS 2328; 22, MRS 2326; 23, MRS 2329; 28, MRS 2333; 38, MRS 2359; 40, MRS 2361; 41, MRS 2409.
47 = Nα-methyl derivative of 45. 48 = Nα-methyl derivative. 49 = D-isomer of 45. 50 = Boc-ethylene diamine (instead of Boc-piperazine) derivative of 44.
No inhibition detected at 30 µM.
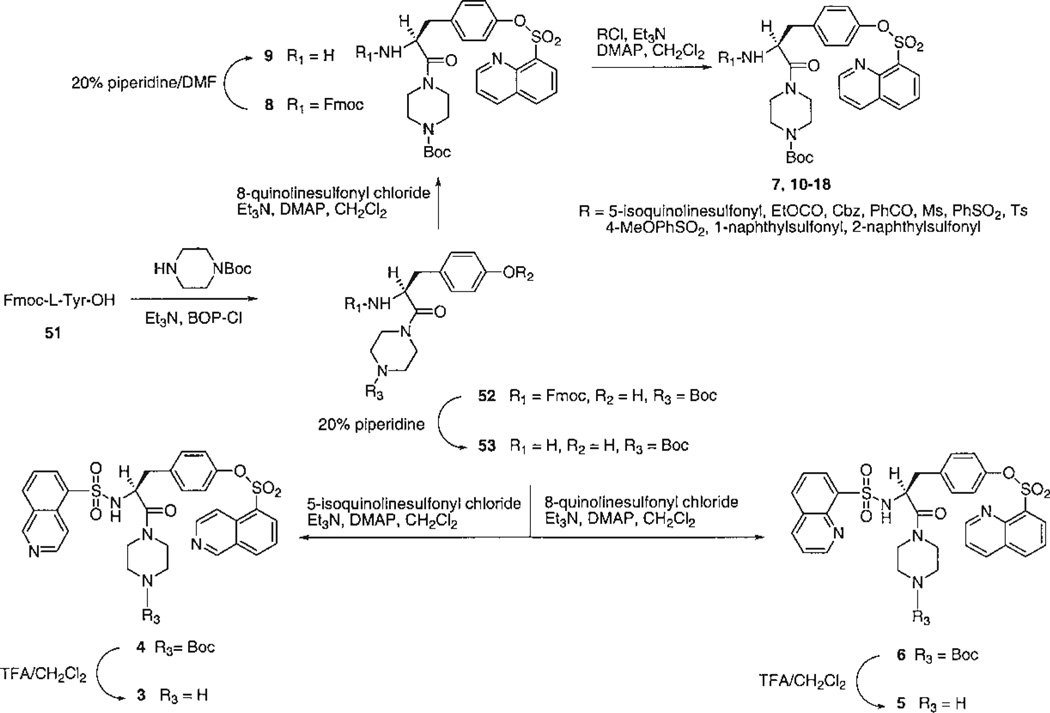
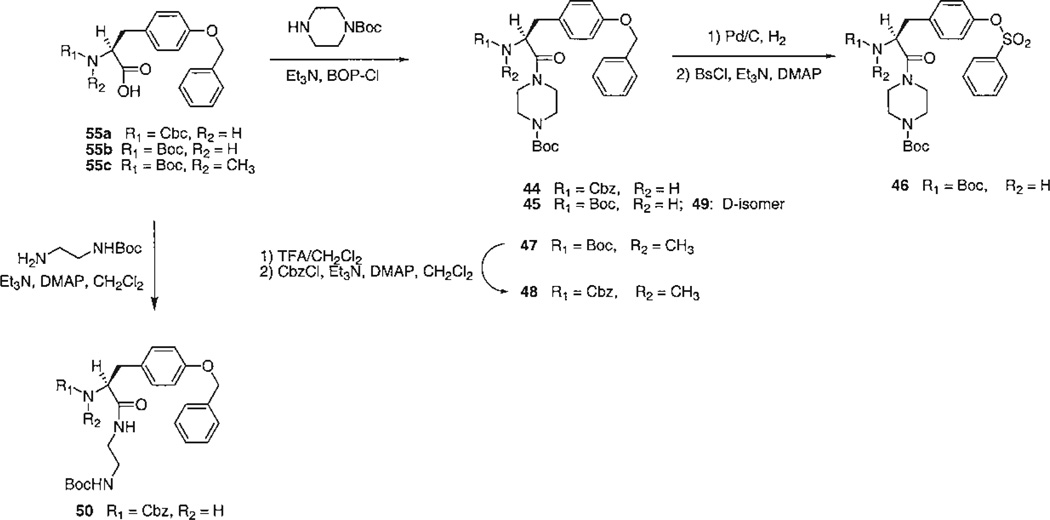
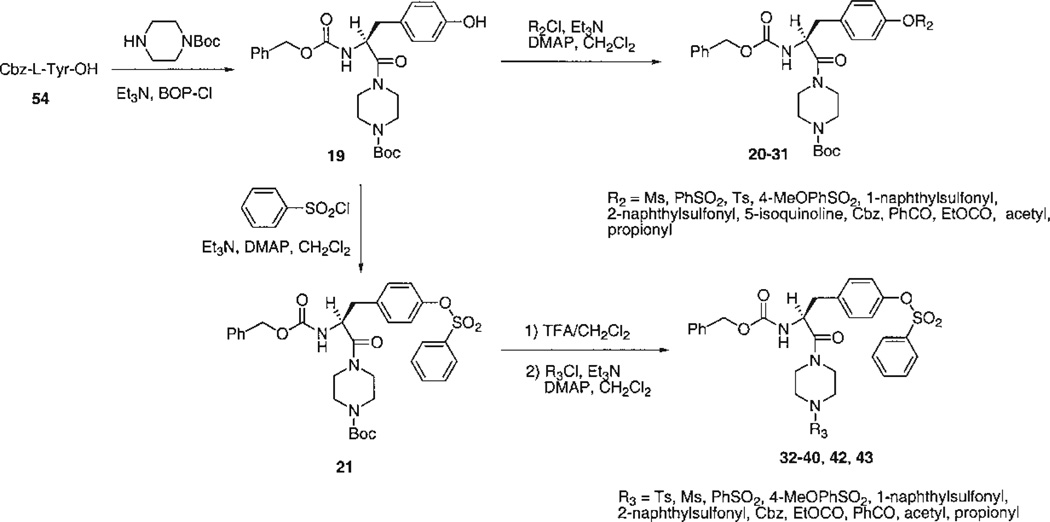
The choice of target compounds and the versatile synthetic routes for systematic substitution around the molecule (Figs. 2–4) were made possible through the ability to replace groups at two positions on the structure of the lead compound, 1. These two positions were the tyrosyl Nα -methyl group, which could be omitted without loss of antagonism, and the N-phenyl-piperazinyl group, which could be replaced conveniently with an N-t-butyloxycarbonyl- (Boc-) -piperazinyl group (see below, potency of 4). Key intermediates that lacked the Nα-Me group, therefore, were the Boc-piperazinyl derivatives, bearing either a N-(9-fluorenylmethyloxycarbonyl-) (Fmoc-), 52 (Fig. 2), or a N-benzyloxycarbonyl (Cbz-), 19 (Fig. 3), protecting group at the Nα-Tyr position. Thus, it was possible to use the Boc both as a group favorable for biological screening, common to many analogs, and also as a protecting group for synthetic intermediates. Amides were formed readily using BOP-Cl (bis(2-oxo-3-oxazolidinyl)phosphinic chloride) as condensing agent. Other amides and sulfonamides were prepared from the corresponding acyl chlorides or sulfonyl chlorides in the presence of DMAP (4-dimethylaminopyridine). Synthetic yields are listed in Table 1.
Fig. 2.
Synthesis of tyrosyl derivatives: introduction of quinoline- and isoquinolinesulfonyl groups and variation of the R1 group.
Fig. 4.
Synthesis of tyrosyl derivatives: miscellaneous variations including N-methyl, D-configuration, and opening of the piperazinyl ring.
Fig. 3.
Synthesis of tyrosyl derivatives: variation of R2 and R3 groups.
The analogs were synthesized in sets, in which one of the “R” positions was varied and the other two were kept constant. Since the reference compound 1 was an isoquinoline derivative, initially a set of quinoline- and isoquinolinesulfonyl derivatives (3–7) was prepared and tested (Fig. 2). An extension of this set (8–18), in which R1 was further varied, contained quinolinesulfonyl at R2, Boc at R3, and various acyl groups at R1. A second set (19–31), in which R2 was varied, contained Cbz at R1 and Boc at R3 (Fig. 3). A third set (32–40, 42, 43), in which R3 was varied, contained Cbz at R1 and benzenesulfonyl at R2. Finally, related derivatives containing O-benzene-sulfonyl tyrosine (44–50) were included (Fig. 4). These derivatives were designed to test the effects of D-Tyr, reintroduction of the Nα -methyl group, and opening the piperazinyl ring, seven analogs containing O-benzyl tyrosine and combinations of Boc and Cbz at R1 and R3. A D-tyrosyl derivative, 49, was prepared by the same method as for the corresponding L-enantiomer, 45. Chemical yields and elemental analyses of the analogs synthesized are shown in Table 1
Biological Activity
The effects of substitution at .R1, R2, and R3 on inhibition of P2X7 receptor-mediated ion flux (Table 2) were compared. Experiments were performed using adherent HEK293 cells stably transfected with cDNA encoding the human P2X7 receptor. Cells were preincubated with antagonists (3 µM) prior to stimulation for 10 min with 3 mM ATP. The percent inhibition of the K+ release was the parameter used to indicate antagonist function.
At the R1 position there was a preference for large hydrophobic groups, linked to the α -amino position through carbamate, amide, or sulfonamide groups. Within the group of 6–18, containing quinolinesulfonyl at R2 and Boc at R3, the derivatives containing quinolinesulfonyl, 6, and Cbz, 11, at the Nα -position were preferred over all other acyl groups and sulfonamides examined and inhibited by >50%. A free NH at the Nα-position, in 9, abolished activity. There appeared to be a sensitivity of the percent inhibition to the precise structure at the R1 position. For example, a toluenesulfonamide, 15, produced greater percent inhibition than the corresponding benzensulfonamide.
At the R2 position, both arylsulfonyl and benzoyl groups led to antagonism, while in the unsubstituted case a sulfonyl group was preferred over an acyl group (cf. 21 and 28). A benzyl ether, 44, having the same substituents at R1 and R3 inhibited to a comparable degree. A free hydroxyl, 19, and a methanesulfonate, 20, were inactive, while a variety of substitution of aryl sulfonates (21–26), including bicyclics, were generally tolerated for antagonism. The approximate rank order of percent inhibition for aryl sulfonates was p-tolyl, 22; p-methoxyphenyl, 23; phenyl, 21 > α -naphthyl, 24; β -naphthyl, 25. A benzoyl ester at the R2 position, 28, inhibited to an intermediate degree. Acyl substitutions at the R2 position that resulted in inactivity included a small carbonate, 29, and small alkyl ester groups, 30 and 31.
A free NH at the R3 position, 3 and 5, resulted in inactivity, thus mainly acylated species were evaluated. At the R3 position, a Boc group, present in many of the derivatives, including 6–31, was well tolerated at the receptor site. Subsequently, for the compounds in which the R3 position was systematically varied, the order of potency was: benzoyl, 40 = Boc, 21, Cbz, 38 > ethyloxycarbonyl, 39. Other analogs in which R1 was Cbz and R2 was benzenesulfonyl were inactive, i.e., R3 consisted of alkyl and aryl sulfonamides, 32–37, and small alkyl amides, 42 and 43. Thus, the structural requirements for the R3 position in order to produce potent antagonists were relatively narrowly defined.
Compounds 45–50 were essentially inactive as human P2X7 receptor antagonists. Thus, the opening of the piperazinyl group to an ethylene diamine moiety, 50, greatly reduced the percent inhibition in comparison to 44. However, it was not possible to adequately evaluate the effects of inversion of configuration of Tyr or Nα -methylation, since the reference compound, 45, was inactive.
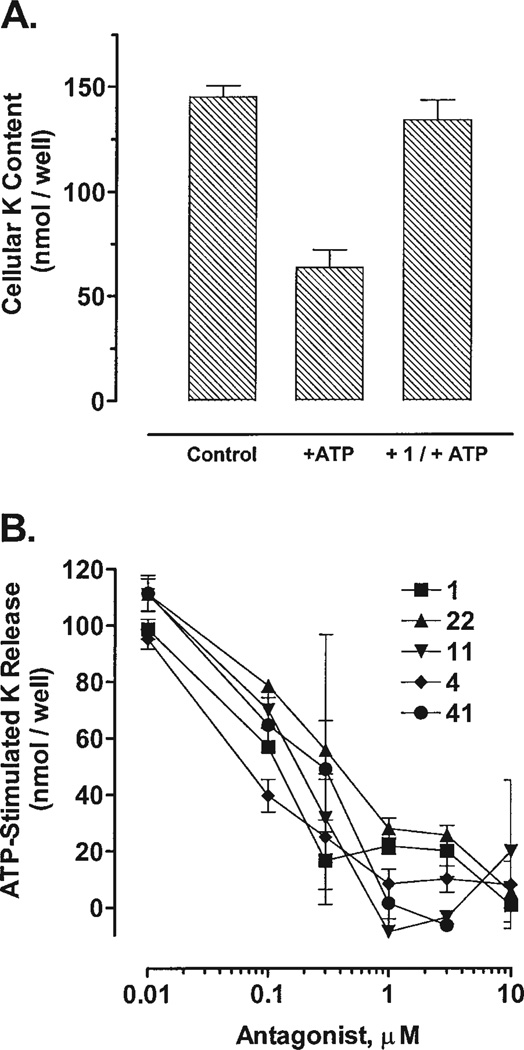
Full concentration-response curves provided a more precise means of comparison among some selected, potent compounds, including the reference compound 1 (Fig. 5A). A di-isoquinolinyl, piperazinyl-Boc derivative, 4, displayed an IC50 of ~40 nM as an antagonist of P2X7 receptor-mediated ion flux (Fig. 5B), and appeared to be more potent than the reference compound, 1. Nα-Cbz-Boc derivatives, 11 and 22, were nearly as potent as 1 as a P2X7 receptor antagonist, with IC50 values of ~200 and ~300 nM, respectively. Compound 41 was also nearly as potent as 1. The IC50 values of 1 and 41 were roughly 100 and 200 nM, respectively.
Fig. 5.
Effects of tyrosyl derivatives on P2X7 receptor-activation in hP2X7-HEK cells. The adherent cells were preincubated with antagonists for 15 min prior to stimulation for 10 min with 3 mM ATP (final concentration). K+ content in these nitric acid extracts was assayed by atomic absorbance spectrophotometry. Duplicate or triplicate wells were run for all test conditions in each separate experiment. A: hP2X7-HEK cells were preincubated with or without 3 µM 1 prior to stimulation with 3 mM ATP. Data points represent the mean (± SD) K+ content from nine separate experiments. B: hP2X7-HEK cells were preincubated with or without the indicated concentrations of selected antagonists prior to stimulation with 3 mM ATP. Data points represent the mean (± SD) K+ contents from triplicate wells in a single experiment. The dashed horizontal lines illustrate the mean K+ content in control cells incubated in the absence of antagonist or ATP. The dotted horizontal lines illustrate the mean K+ content in ATP-stimulated cells that were not preincubated with antagonist. IC50 values are rough estimates from visual inspection of the concentration-response relationships. Hill coefficients were not determined, since previous studies have shown that the lead compound (KN-62,1) represses P2X7 receptor function via complex mechanisms that are not readily amenable to standard ligand-binding analyses.
Several compounds were tested in preliminary experiments as antagonists at the murine P2X7 receptor, expressed natively in Bac1.2F5 mouse macrophages. The degree of inhibition by the tyrosyl derivatives examined was generally greater at murine than at human P2X7 receptors. Percent inhibition measured at 3 µM in the mouse macrophages was: 35 (32), 34 (33), 74 (38), 80 (39), 94 (40), 27 (42), and 28 (43). Thus, four compounds that were inactive at human P2X7 receptors (32, 33, 42, and 43) displayed antagonism at murine P2X7 receptors.
The compounds were not measured as antagonists at other subtypes of P2 receptors. The lead compound, 1, however, is inactive at various other P2 receptors, including P2X4 [Jacobson et al., 2000], P2Y1, and P2Y2 (G. Dubyak, unpubl.). To assess P2 vs. P1 receptor selectivity, binding to adenosine receptors was measured in competitive binding assays [Kim et al., 1998]. Compounds 39 and 41 at 100 µM did not displace radioligand from rat brain adenosine A1 or A2A receptors.
The ability to use Cbz and Boc both as protecting groups and as substituents favoring biological activity suggested a convenient approach to optimizing permutations of acyl groups appended at the three “R” positions. This approach may be described as “sequential parallel synthesis,” of which we have carried out one complete cycle. In subsequent studies the optimization process may be continued in the order: R1-, R2-, R3-, and then back to R1-, and so forth.
Eight novel ligands displaying high percent inhibition (>40% at 3 µM) have been identified, in order of decreasing potency: 41, 40, 4, 22, 23, 6, 21, 11. Modifications at R1 and R2 may not be independent in their effect on potency, since the IqIqB and QuQuB analogs, 4 and 6, were equipotent and both were more potent than the mixed analog, 7.
Since the parent compound, 1, was also reported to be an inhibitor of CaMKII [Tokumitsu et al., 1990] and at other sites [Puhl et al., 1997], activity of selected analogs at these enzymes will have to be examined to establish selectivity for the P2X7 receptor. It will also be interesting to prepare reduced peptide analogs, similar to 2, to examine the role of this carbonyl group in antagonism of both the P2X7 receptor and CaMKII.
Several Tyr derivatives in this study inhibited ion flux to a greater degree at murine vs. human P2X7 receptors. This is the opposite of results reported for the reference compound 1, which was more potent at human than at mouse (BAC1.2f5) P2X7 receptors [Humphreys et al., 1998].
In summary, the present analysis of structure-activity relationships of tyrosyl derivatives as noncompetitive antagonists of the P2X7 receptor has demonstrated that the Tyr Nα -methyl group is unnecessary for antagonism. The three positions selected for extensive modification, i.e., R1 (at Nα-amine), R2 (on Tyr side chain), and R3 (an extension of the Cα -position), were amenable to substitution with various acyl-type groups. The most potent P2X7 receptor antagonists identified in this study contained Cbz at the R1 position, an aryl sulfonate at the R2 position, and various acyl groups at the R3 position. At R1 and R2 groups, aryl substituents were preferred over alkyl. At R3 the structural requirements were the most restrictive of the three positions. Carbonyl, but not sulfonyl, attachment to the piperazinyl ring was allowed and t-butyloxycarbonyl- and benzoyl groups were preferred. Further structure-activity studies are now warranted using a wider range of chemical functionality at these three positions.
Acknowledgments
Contract grant sponsors: Gilead Sciences, Foster City, CA, (R.G.R.), NIH (G.D.); Contract grant number: GM36387.
REFERENCES
- Baraldi PG, Romagnoli R, Tabrizi MA, Falzoni S, di Virgilio F. Synthesis of conformationally constrained analogs of KN62, a potent antagonist of the P2X7-receptor. Bioorg Med Chem Lett. 2000;10:681–684. doi: 10.1016/s0960-894x(00)00083-4. [DOI] [PubMed] [Google Scholar]
- Bhagwat SS, Williams M. P2 purine and pyrimidine receptors: emerging superfamilies of G protein and ligand-gated ion channel receptors. Eur J Med Chem. 1997;32:183–193. [Google Scholar]
- Bianchi BR, Lynch KJ, Touma E, Niforatos W, Burgard EC, Alexander KM, Park HS, Yu H, Metzger R, Kowaluk E, Jarvis MF, van Biesen T. Pharmacological characterization of recombinant human and rat P2X receptor subtypes. Eur J Pharmacol. 1999;376:127–138. doi: 10.1016/s0014-2999(99)00350-7. [DOI] [PubMed] [Google Scholar]
- Bringmann A, Pannicke T, Moll V, Milenkovic I, Faude F, Enzmann V, Wolf S, Reichenbach A. Upregulation of P2X7 receptor currents in Muller glial cells during proliferative vitreoretinopathy. Invest Ophthalmol Vis Sci. 2001;42:860–807. [PubMed] [Google Scholar]
- Chessell IP, Simon J, Hibell AD, Michel AD, Barnard EA, Humphrey PPA. Cloning and functional characterization of the mouse P2X7 receptor. FEBS Lett. 1998;439:26–30. doi: 10.1016/s0014-5793(98)01332-5. [DOI] [PubMed] [Google Scholar]
- Coutinho-Silva R, Persechini PM, Bisaggio RD, Perfettini JL, Neto AC, Kanellopoulos JM, Motta-Ly I, Dautry-Varsat A, Ojcius DM. P2Z/P2X7 receptor-dependent apoptosis of dendritic cells. Am J Physiol. 1999;276:C1139–C1147. doi: 10.1152/ajpcell.1999.276.5.C1139. [DOI] [PubMed] [Google Scholar]
- Erb L, Lustig KD, Ahmed AH, Gonzalez FA, Weisman GA. Covalent incorporation of 3′-O-(4-benzoyl)benzoyl-ATP into a P2 purinoceptor in transformed mouse fibroblasts. J Biol Chem. 1990;265:7424–7431. [PubMed] [Google Scholar]
- Ferrari D, Chiozzi P, Falzoni S, Dal Susino M, Collo G, Buell G, Di Virgilio F. ATP-mediated cytotoxicity in microglial cells. Neuropharmacology. 1997;36:1295–1301. doi: 10.1016/s0028-3908(97)00137-8. [DOI] [PubMed] [Google Scholar]
- Ferrari D, Stroh C, Schulze-Osthoff K. P2X7/P2Z purinoreceptor-mediated activation of transcription factor NFAT in microglial cells. J Biol Chem. 1999;274:13205–13210. doi: 10.1074/jbc.274.19.13205. [DOI] [PubMed] [Google Scholar]
- Ferrari D, La Sala A, Chiozzi P, Morelli A, Falzoni S, Girolomoni G, Idzko M, Dichmann S, Norgauer J, Di Virgilio F. The P2 purinergic receptors of human dendritic cells: identification and coupling to cytokine release. FASEB J. 2000;14:2466–2476. doi: 10.1096/fj.00-0031com. [DOI] [PubMed] [Google Scholar]
- Fischer B. Therapeutic applications of ATP-(P2) receptors agonists and antagonists. Exp Opin Ther Patents. 1999;9:385–399. [Google Scholar]
- Fredholm BB, Abbracchio MP, Burnstock G, Dubyak GR, Harden TK, Jacobson KA, Schwabe U, Williams M. Toward a revised nomenclature for P1 and P2 receptors. Trends Pharm Sci. 1997;18:79–82. doi: 10.1016/s0165-6147(96)01038-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gargett CE, Wiley JS. The isoquinoline derivative KN-62 a potent antagonist of the P2Z-receptor of human lymphocytes. Br J Pharmacol. 1997;120:1483–1490. doi: 10.1038/sj.bjp.0701081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hide I, Tanaka M, Inoue A, Nakajima K, Kohsaka S, Inoue K, Nakata Y. Extracellular ATP triggers tumor necrosis factor-alpha release from rat microglia. J Neurochem. 2000;75:965–972. doi: 10.1046/j.1471-4159.2000.0750965.x. [DOI] [PubMed] [Google Scholar]
- Hu Y, Fisette PL, Denlinger LC, Guadarrama AG, Sommer JA, Proctor RA, Bertics PJ. Purinergic receptor modulation of lipopolysaccharide signaling and inducible nitric-oxide synthase expression in RAW 264.7 macrophages. J Biol Chem. 1998;273:27170–27175. doi: 10.1074/jbc.273.42.27170. [DOI] [PubMed] [Google Scholar]
- Humphreys BD, Virginio C, Surprenant A, Rice J, Dubyak GR. Isoquinolines as antagonists of the P2X7 nucleotide receptor: high selectivity for the human versus rat receptor homologues. Mol Pharmacol. 1998;54:22–32. doi: 10.1124/mol.54.1.22. [DOI] [PubMed] [Google Scholar]
- Humphreys BD, Rice J, Kertesy SB, Dubyak GR. Stress-activated protein kinase/JNK activation and apoptotic induction by the macrophage P2×7 nucleotide receptor. J Biol Chem. 2000;275:26792–26798. doi: 10.1074/jbc.M002770200. [DOI] [PubMed] [Google Scholar]
- Jacobson KA, Knutsen LJS. P1 and P2 purine and pyrimidine receptors. In: Abbracchio MP, Williams M, editors. Handbook of experimental pharmacology. 151/I. Germany: Springer-Verlag: Berlin: Purinergic and pyrimidinergic signalling I; 2001. pp. 129–175. [Google Scholar]
- Jacobson KA, Kim Y-C, Camaioni E, van Rhee AM. Structure activity relationships of P2 receptor agonists and antagonists. In: Turner JT, Weisman G, Fedan J, editors. The P2 nucleotide receptors. Series “The receptors.”. Clifton, NJ: Humana Press; 1997. pp. 81–107. [Google Scholar]
- Jacobson KA, King BF, Burnstock G. Pharmacological characterization of P2 (nucleotide) receptors. Celltransmissions. 2000;16:3–16. [Google Scholar]
- Jacobson KA, Jarvis MF, Williams M. Perspective: purine and pyrimidine (P2) receptors as drug. Targets. J Med Chem. 2002 doi: 10.1021/jm020046y. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang LH, Mackenzie AB, North RA, Surprenant A. Brilliant blue G selectively blocks ATP-gated rat P2X7 receptors. Mol Pharmacol. 2000;58:82–88. [PubMed] [Google Scholar]
- Kim Y-C, de Zwart M, Chang L, Moro S, von Frijtag Drabbe Künzel JK, Melman N, IJzerman AP, Jacobson KA. Derivatives of the triazoloquinazoline adenosine antagonist (CGS15943) having high potency at the human A2B and A3 receptor subtypes. J Med Chem. 1998;41:2835–2841. doi: 10.1021/jm980094b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Spelta V, Sim J, North RA, Surprenant A. Differential assembly of rat purinergic P2X7 receptor in immune cells of the brain and periphery. J Biol Chem. 2001 doi: 10.1074/jbc.M102253200. [DOI] [PubMed] [Google Scholar]
- North RA, Barnard EA. Nucleotide receptors. Curr Opin Neurobiol. 1997;7:346–357. doi: 10.1016/s0959-4388(97)80062-1. [DOI] [PubMed] [Google Scholar]
- Puhl HL, Raman PS, Williams CL, Aronstam RS. Inhibition of M3 muscarinic acetylcholine receptor-mediated Ca2+ influx and intracellular Ca2+ mobilization in neuroblastoma cells by the Ca2+/ calmodulin-dependent protein kinase inhibitor 1-[N,O-bis(5-isoquinolinesulfonyl)-N-methyl-L-trosyl]-4-phenylpiperazine (KN-62) Biochem Pharmacol. 1997;53:1107–1114. doi: 10.1016/s0006-2952(97)00089-0. [DOI] [PubMed] [Google Scholar]
- Solle M, Labasi J, Perregaux DG, Stam E, Petrushova N, Koller BH, Griffiths RJ, Gabel CA. Altered cytokine production in mice lacking P2X7 receptors. J Biol Chem. 2001;276:125–132. doi: 10.1074/jbc.M006781200. [DOI] [PubMed] [Google Scholar]
- Sun SH, Lin LB, Hung AC, Kuo JS. ATP-stimulated Ca2+ influx and phospholipase δ activities of a rat brain-derived type-2 astrocyte cell line, RBA-2, are mediated through P2X7 receptors. J Neurochem. 1999;73:334–343. doi: 10.1046/j.1471-4159.1999.0730334.x. [DOI] [PubMed] [Google Scholar]
- Tokumitsu H, Chijiwa T, Hagiwara M, Mizutani A, Terasawa M, Hidaka H. KN-62, 1-[N,O-bis(5-isoquinolinesulfonyl)-N-methyl-L-tyrosyl]-4-phenylpiperazine, a specific inhibitor of Ca2+/calmodulin-dependent protein kinase II. J Biol Chem. 1990;265:4315–4320. [PubMed] [Google Scholar]
- Torres GE, Egan TM, Voigt MM. Hetero-oligomeric assembly of P2X receptor subunits. Specificities exist with regard to possible partners. J Biol Chem. 1999;274:6653–6659. doi: 10.1074/jbc.274.10.6653. [DOI] [PubMed] [Google Scholar]
- Visentin S, Renzi M, Frank C, Greco A, Levi G. Two different ionotropic receptors are activated by ATP in rat microglia. J Physiol. 1999;519:723–736. doi: 10.1111/j.1469-7793.1999.0723n.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley S, Gargett CE, Zhang W, Snook MB, Jamieson GP. Partial agonists and antagonists reveal a second permeability state of human lymphocyte P2Z/P2X7 channel. Am J Physiol. 1998;275:C1224–C1231. doi: 10.1152/ajpcell.1998.275.5.C1224. [DOI] [PubMed] [Google Scholar]