Abstract
We aimed to estimate the association between sleep duration trajectories and body composition in adolescents. We used data from participants of the 1993 Pelotas (Brazil) Birth Cohort Study who were later followed up at age 18 years (response rate of 81.3%). At the time, 3974 adolescents had complete data on body composition, which was assessed by air displacement plethysmography. Sleep duration was self-reported by participants at ages 11 and 18 years. Analyses were sex-stratified. The mean sleep duration at 11 years was 9.7 (SD 1.4) and 8.4 (SD 1.9) at 18 years. Sleep duration was dichotomized as inadequate (<8 hours/day) or adequate (≥8 hours/day). Mean body mass, fat mass, and fat-free mass indices at 18 years were 23.4 kg/m2 (SD 4.5), 6.1 kg/m2 (SD 3.9) and 17.3 kg/m2 (SD 2.5), respectively. Girls who reported inadequate sleep duration at 11 years of age, but adequate sleep duration at 18, on average experienced an increase in body mass index (β = 0.39 z-scores; 95% CI 0.13, 0.65), fat mass index (β = 0.30 z-scores; 95% CI 0.07, 0.53), and fat-free mass index (β = 0.24 z-scores; 95% CI 0.08, 0.39) compared to those who had adequate sleep duration at both time points. The results suggest that changes in sleep duration across adolescence may impact body composition in later adolescence and that this may differ by sex.
Introduction
Adolescent obesity is an important public health issue worldwide [1]. In Brazil, overweight prevalence among adolescents has steadily increased over the past 30 years. In 2008–2009, 20.5% of adolescents were classified as overweight, six times the prevalence among boys and three times among girls in 1974–1975 [2]. The prevalence of obesity in the same period has increased from 0.4% to 5.9% among boys and 0.7% to 4.0% among girls [2]. The physical and psychosocial consequences of adolescent obesity have been well documented [3, 4].
The causes for the rise of the obesity epidemic are not obvious because it has biological, economic, social and cultural determinants. While the most proximal cause of excessive accumulation of body fat is a positive energy balance, this imbalance is triggered by a number of other factors. One hypothesized factor is sleep duration, which has been linked to obesity risk and body composition in several recent epidemiological studies [5–8]. Further, the increasing prevalence of overweight and/or obesity has coincided with a reduction in sleep duration in modern societies [9]. For example, data from the USA show that adults have on average reduced their sleep duration by one to two hours, and that more than a third of young adults are sleeping less than seven hours [10], a phenomenon also reported among children and adolescents [11, 12]. A study conducted with young people aged 10–19 years in the state of São Paulo, Brazil, showed that 39% of them slept eight hours or less [13].
The precise biological mechanisms that mediate the relationship between sleep and body composition are unknown. However, both laboratory and population-based studies have suggested pathways such as decreased leptin levels, elevated ghrelin levels, tiredness and increased opportunity for food intake [14, 15]. These mechanisms could lead to appetite stimulation, increased energetic intake and decreased physical activity, promoting weight gain [14, 15].
We conducted sex-stratified analyses based on evidence for sex differences in the association between sleep duration and obesity in adolescents [16, 17]. We postulated that short sleep duration during adolescence would predict BMI and fat mass in late adolescence, and that the effect would be stronger in girls compared with boys. We also hypothesized that adequate sleep duration during adolescence would be associated with fat-free mass gain, and that the effect would be stronger in boys compared with girls.
Since adolescence has been identified as a risk period for sleep-related problems [18, 19], and it is a phase characterized by changes in body composition [20], we aimed estimate the relationship between sleep duration trajectories and body mass, fat mass, and fat-free mass indices during adolescence, using data from the 1993 Birth Cohort of Pelotas, Brazil.
Methods
Subjects
All 5365 children born in hospitals of the urban area of Pelotas, Southern Brazil, in 1993 were recruited for a birth cohort study (n = 5265). The resultant birth cohort consisted of 5249 live births (16 declined to participate) [21]. These participants have been followed on several occasions [22]. In the present analyses we use data from two follow-up visits.
All cohort members were followed up in 2004–2005, at the age of 11 years. Those who completed the interviews, added to those known to have died, made up 87.5% (n = 4452) of the original cohort. Adolescents and their mothers were interviewed during home visits.
At the 18-year follow-up, we interviewed 4106 adolescents, for a response rate of 81.3%. More detailed information about the study can be found elsewhere [22]. In the present study we included 3974 adolescents for whom body composition measurements were available.
This study was approved by the Ethics Committee of the Medicine School of the Federal University of Pelotas in an official letter numbered 05/11. Written informed consent was obtained prior to each follow-up.
Measurements
Weight, height, and body composition (fat mass and fat-free mass) were evaluated when participants were 18 years of age. Fat mass and fat-free mass were obtained by air displacement plethysmography (BOD POD®). We calculated their respective indices by dividing fat mass and fat-free mass (in kg) by height (in m2). Since fat mass and fat-free mass indices take into account the height they improve the reading in individuals with different heights [23]. Body mass index (BMI) was similarly calculated by dividing body weight (kg) by height (m) squared.
Regarding the exam, subjects wore top and shorts (spandex), and a silicone swimming cap. Weight was measured by a high precision scale (0.01 kg) part of the BOD POD® machine. Height was measured twice by trained researcher using a Harpenden metal stadiometer, to the nearest mm. According to the World Health Organization (WHO) BMI-for-age and sex reference in z score [24] normal, overweight, and obesity were defined as BMI-for-age ≤ +1 SD, > + 1 SD and > + 2 SD, respectively.
Sleep duration at 11 and 18 years old was collected by asking the adolescents two questions: "What time do you usually fall asleep on weekdays?" and "What time do you usually wake up on weekdays?" Sleep duration was calculated as the time difference between the two answers, and was categorized as <8 or ≥8 h per day, according to National Sleep Foundation’s recommendations [25].
Statistical analyses
BMI, fat mass index and fat-free mass index were standardized as sample-specific z-scores and analyzed as continuous variables. Sleep duration trajectories were created based on the combination of sleep duration levels at 11 and 18 years of age and was classified as always adequate (≥8 h); adequate-inadequate (≥8 h, <8 h); inadequate-adequate (<8 h, ≥8 h); and always inadequate (<8 h).
Crude and adjusted analyses were performed using linear regression. Possible confounders added to the model were: maternal skin color, gestational weight gain [26], pregnancy smoking, pregnancy alcohol consumption, birth order, type of delivery, family income at birth, maternal education at birth, maternal age at birth, birth weight, physical activity at 11 years, and screen time at 11 years. Physical activity was evaluated using a questionnaire to measure commuting to and from school, and leisure time activities [27]. The questionnaire also included information on screen time. The mean time spent in front of TV, videogame, and computer (in a typical week) was noted separately for weekdays and weekends. Screen time variable was constructed by adding the weighted mean screen time (TV + videogame + computer), assigning weight 5 to weekdays and weight 2 to weekends and dividing the result by 7 to obtain the mean time in minutes per day.
All analyses were stratified by sex and performed using Stata version 12.1 (Stata Corp., College Station, Texas, USA).
Results
Table 1 gives the participant characteristics. Two-thirds of the adolescents´ mothers had inadequate gestational weight gain (66.0%), and almost one-third reported smoking during pregnancy (32.8%) and had caesarean sections (31.1%). The median physical activity at 11 years was 285 minutes per week (IQR: 140 to 540). Also, the mean of maternal education at birth was almost seven years (SD 3.5) and adolescent screen time at 11 years was 4.3 hours per day (SD 2.7). The average sleep duration at 11 and 18 years was 9.7 (SD 1.4) and 8.4 (SD 1.9) hours, respectively. In relation to the outcomes studied, the means of BMI, fat mass index and fat-free mass index were 23.4 kg/m2 (SD 4.5), 6.1 kg/m2 (SD 3.9) and 17.3 kg/m2 (SD 2.5), respectively.
Table 1. Description of variables studied by sex.
1993 Pelotas (Brazil) Birth Cohort Study (n = 3974).
| Categorical variables | Total | Male | Female | ||||||||
| n (%) | n (%) | n (%) | |||||||||
| Maternal skin color | |||||||||||
| White | 3057 (77.0) | 1527 (77.4) | 1530 (76.5) | ||||||||
| Black | 735 (18.5) | 352 (17.8) | 383 (19.2) | ||||||||
| Other | 180 (4.5) | 94 (4.8) | 86 (4.3) | ||||||||
| Gestational weight gain | |||||||||||
| Adequate | 1291 (34.0) | 642 (34.2) | 649 (33.8) | ||||||||
| Inadequate | 2509 (66.0) | 1237 (65.8) | 1272 (66.2) | ||||||||
| Pregnancy smoking | |||||||||||
| No | 2671 (67.2) | 1341 (68.0) | 1330 (66.5) | ||||||||
| Yes | 1303 (32.8) | 632 (32.0) | 671 (33.5) | ||||||||
| Pregnancy alcohol consumption | |||||||||||
| No | 3773 (94.9) | 1886 (95.6) | 1887 (94.3) | ||||||||
| Yes | 201 (5.1) | 87 (4.4) | 114 (5.7) | ||||||||
| Birth order | |||||||||||
| Firstborn | 1580 (39.8) | 773 (39.2) | 807 (40.3) | ||||||||
| Second | 1190 (30.0) | 591 (30.0) | 599 (30.0) | ||||||||
| Third | 628 (15.8) | 300 (15.2) | 328 (16.4) | ||||||||
| Forth or later | 573 (14.4) | 307 (15.6) | 266 (13.3) | ||||||||
| Type of delivery | |||||||||||
| Normal | 2738 (68.9) | 1360 (68.9) | 1378 (68.9) | ||||||||
| Caesarean | 1236 (31.1) | 613 (31.1) | 623 (31.1) | ||||||||
| BMI at 11 years | |||||||||||
| Normal | 2952 (76.6) | 1412 (74.5) | 1540 (78.7) | ||||||||
| Overweight | 448 (11.6) | 189 (10.0) | 259 (13.2) | ||||||||
| Obesity | 453 (11.8) | 294 (15.5) | 159 (8.1) | ||||||||
| BMI at 18 years | |||||||||||
| Normal | 2881 (72.7) | 1467 (74.6) | 1414 (70.9) | ||||||||
| Overweight | 680 (17.2) | 318 (16.2) | 362 (18.1) | ||||||||
| Obesity | 400 (10.1) | 180 (9.2) | 220 (11.0) | ||||||||
| Continuous variables | Total | Male | Female | ||||||||
| n | Median | IQR | n | Median | IQR | n | Median | IQR | |||
| Family income (MMW) | 3907 | 2.6 | 1.5–4.6 | 1945 | 2.5 | 1.5–4.5 | 1962 | 2.9 | 1.5–4.8 | ||
| Physical activity at 11 years (minutes/week) | 3733 | 285 | 140–540 | 1846 | 370 | 180–660 | 1887 | 220 | 110–420 | ||
| n | Mean | SD | n | Mean | SD | n | Mean | SD | |||
| Maternal education (completed years) | 3967 | 6.8 | 3.5 | 1969 | 6.8 | 3.5 | 1998 | 6.8 | 3.5 | ||
| Maternal age at birth (years) | 3973 | 26.1 | 6.4 | 1972 | 26.1 | 6.5 | 2001 | 26.1 | 6.3 | ||
| Birth weight (grams) | 3969 | 3181.8 | 527.7 | 1970 | 3249.2 | 534.7 | 1999 | 3115.4 | 512.2 | ||
| Screen time at 11 years (hours/day) | 3844 | 4.3 | 2.7 | 1885 | 4.5 | 2.9 | 1959 | 4.2 | 2.5 | ||
| Sleep duration at 11 years (hours) | 3849 | 9.7 | 1.4 | 1889 | 9.6 | 1.4 | 1960 | 9.7 | 1.4 | ||
| Sleep duration at 18 years (hours) | 3955 | 8.4 | 1.9 | 1962 | 8.1 | 1.8 | 1993 | 8.8 | 1.9 | ||
| BMI (kg/m2) | 3974 | 23.4 | 4.5 | 1973 | 23.4 | 4.2 | 2001 | 23.5 | 4.8 | ||
| Fat mass index (kg/m2) | 3974 | 6.1 | 3.9 | 1973 | 4.2 | 3.1 | 2001 | 8.0 | 3.6 | ||
| Fat-free mass index (kg/m2) | 3974 | 17.3 | 2.5 | 1973 | 19.1 | 1.9 | 2001 | 15.5 | 1.6 | ||
Maximum percentage of unknown observations: (n = 241; 6.1%) for the physical activity at 11 years variable.
MMW: Monthly minimum wages.
SD: Standard deviation.
IQR: Interquartile range.
BMI: Body mass index.
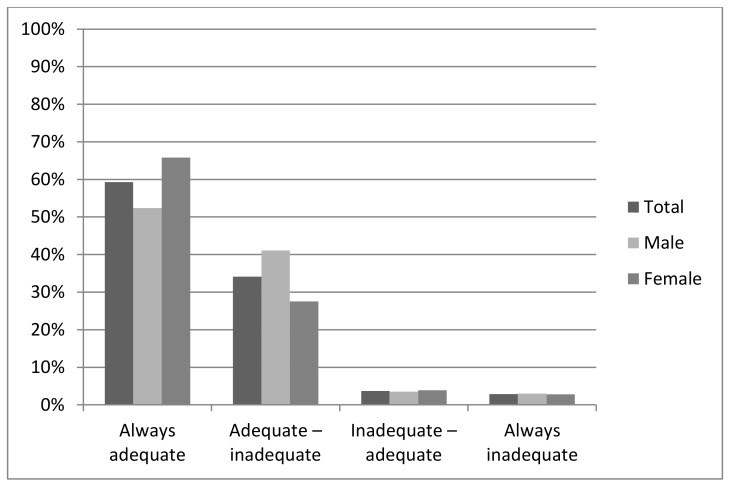
We observed that more than one-third of the adolescents reported adequate sleep at 11 years of age but inadequate sleep at 18 (41.1% and 27.5% among males and females, respectively). On the other hand, only 3.7% of the adolescents improved their sleep duration (Fig 1).
Fig 1. Prevalence of sleep duration trajectories.
1993 Pelotas (Brazil) Birth Cohort Study.
Crude and adjusted analyses of the association between sleep duration trajectories during adolescence and the outcomes stratified by sex are shown in the Table 2. In the adjusted models, change in sleep duration remained associated with BMI and fat mass index in females. Girls who increased their sleep duration from 11 to 18 years of age showed an increase of 0.39 z-scores (95% CI 0.13, 0.65) and 0.30 z-scores (95% CI 0.07, 0.53) in BMI and fat mass index, respectively, when compared to those who always had adequate sleep duration. Fat-free mass index was associated with change in sleep duration in both sexes. Boys who went from adequate to inadequate sleep duration during adolescence had an increase of 0.09 z-scores (95% CI 0.01, 0.16) in fat-free mass index, whereas girls who improved their sleep duration from 11 to 18 years of age showed an increase of 0.24 z-scores (95% CI 0.08, 0.39) in fat-free mass index compared to adolescents who had adequate sleep duration in both follow-ups.
Table 2. Crude and adjusted analyses of association between sleep duration trajectories (between 11 and 18 years) and outcomes (in z-scores) stratified by sex.
1993 Pelotas (Brazil) Birth Cohort Study.
| Crude analyses | Adjusted analyses* | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | |||||||||
| n | β (95% CI) | p† | n | β (95% CI) | p† | β (95% CI) | p† | R2Ɨ | β (95% CI) | p† | R2Ɨ | |
| BMI | ||||||||||||
| Sleep duration trajectories | 0.041 | 0.015 | 0.203 | 0.03 | 0.029 | 0.03 | ||||||
| Always adequate | 982 | Reference | 1279 | Reference | Reference | Reference | ||||||
| Adequate–inadequate | 775 | 0.09 (0.001;0.18) | 543 | -0.03 (-0.14;0.08) | 0.08 (-0.01;0.17) | -0.01 (-0.12;0.10) | ||||||
| Inadequate–adequate | 65 | 0.18 (-0.06;0.42) | 76 | 0.39 (0.14;0.63) | 0.13 (-0.13;0.39) | 0.39 (0.13;0.65) | ||||||
| Always inadequate | 56 | 0.27 (0.01;0.52) | 54 | -0.02 (-0.31;0.26) | 0.19 (-0.07;0.45) | -0.03 (-0.33;0.26) | ||||||
| Fat mass index | ||||||||||||
| Sleep duration trajectories | 0.037 | 0.034 | 0.174 | 0.03 | 0.049 | 0.02 | ||||||
| Always adequate | 982 | Reference | 1279 | Reference | Reference | Reference | ||||||
| Adequate–inadequate | 775 | 0.04 (-0.03;0.12) | 543 | -0.03 (-0.12;0.06) | 0.03 (-0.05;0.11) | -0.03 (-0.13;0.07) | ||||||
| Inadequate–adequate | 65 | 0.25 (0.04;0.45) | 76 | 0.30 (0.09;0.52) | 0.23 (0.0003;0.45) | 0.30 (0.07;0.53) | ||||||
| Always inadequate | 56 | 0.20 (-0.58;-0.47) | 54 | -0.02 (0.44;0.54) | 0.13 (-0.09;0.36) | -0.05 (-0.31; 0.21) | ||||||
| Fat-free mass index | ||||||||||||
| Sleep duration trajectories | 0.020 | 0.026 | 0.023 | 0.03 | 0.030 | 0.06 | ||||||
| Always–adequate | 982 | Reference | 1279 | Reference | Reference | Reference | ||||||
| Adequate–inadequate | 775 | 0.10 (0.03;0.17) | 543 | -0.01 (-0.07;0.06) | 0.09 (0.01;0.16) | 0.03 (-0.04;0.10) | ||||||
| Inadequate–adequate | 65 | -0.05 (-0.24;0.13) | 76 | 0.23 (0.08;0.38) | -0.11 (-0.32;0.09) | 0.24 (0.08;0.39) | ||||||
| Always inadequate | 56 | 0.17 (-0.03;0.36) | 54 | -0.005 (-0.18;0.17) | 0.14 (-0.07;0.34) | 0.03 (-0.15;0.20) | ||||||
BMI: Body mass index; CI: Confidence interval.
*For family income, maternal education, maternal skin colour, maternal age at birth, gestational weight gain, pregnancy smoking, pregnancy alcohol consumption, birth order, type of delivery, birth weight, physical activity at 11 years, and screen time at 11 years.
†Wald test for heterogeneity.
ƗAdjusted R-squared.
Discussion
Among girls, we found an association between sleep duration and BMI, fat mass index and fat-free mass index. In contrast, in boys, we found a very weak association between sleep duration and fat-free mass index. Previous studies have been inconsistent. Another cohort study including adolescents found no longitudinal association between sleep duration and body fat percentage in both sexes; however, in the cross-sectional analyses at 17 years a positive association was found among girls only [28]. Similarly, two cross-sectional studies showed a positive association between sleep duration and body fat percentage in female adolescents only [29, 30]. On the other hand, a longitudinal study found no relationship between total sleep and BMI or body fat percentage in either boys and girls [31].
To the best of our knowledge, this is the first study that examined the relationship between sleep duration and fat-free mass in adolescents. Sleep is an active process in the brain that is necessary for restorative functions and hormone secretion, particularly during development period [32], and thus it may play an important role in the accrual of fat-free mass. More studies are necessary to better understand this finding.
Storfer-Isser et al. performed an exploratory analysis to understand sex differences in the association between sleep duration and BMI finding that morning leptin levels were significantly higher among girls than boys [17]. Another important point to be considered is the sex differences in the physical and sedentary behaviors. During the adolescence, for example, physical activity levels decrease most notably among girls [33] and regular exercise may help promote suffıcient sleep [34].
The sex distinctions observed in our study may be due to the differences in the physiology of puberty in males and females as they relate to changes in body composition [35]. We have hypothesized that the effect of sleep duration might be masked in girls because they suffer greater changes in fat mass during adolescence compared to boys [36]. During puberty, boys experience rapid increases in fat-free mass and reduced fat mass, whereas girls gain considerable amounts of fat but relatively little fat-free mass. These differences may be largely due to hormones secretion as testosterone and oestrogen. Sex steroid hormones play important roles in the accumulation, metabolism and distribution of adipose tissue [37]. For example, testosterone and oestrogen facilitate fat deposition in the abdominal and gluteo-femoral regions, respectively [38]. Testosterone is also important for the increase in lean mass that occurs during puberty, especially in boys [39].
The strengths of our study include the prospective design, since there are few studies with adolescents based on longitudinal analyses; the large sample size; the high response rates; and the utilization of air displacement plethysmography as method for evaluating fat mass and fat-free mass.
Limitations include potential selection biases due to loss to follow-up. As described in detail previously [22], at 18-year-old-follow-up, participants with lower socioeconomic status, a worse nutritional profile, and those whose mothers had no schooling were less likely to be followed up. It is important to highlight, however, that the magnitude of these differences is modest, thus minimizing the probability of bias [22]. Another possible limitation is the methodology used to estimate sleep duration. However, a study of high school adolescents in the USA found high correlations between actigraphy and self-reported bedtimes (r = 0.70) and wake-up times (r = 0.77) during weekdays [40]. Thus, even with limitations on the accurate quantification of sleep time, it seems that our measure is good enough to discriminate the participants according to their sleep duration. Additionally, it is possible that a measurement error might have given rise to non-differential misclassification and the associations between sleep and body composition may be underestimated. Finally, although the differences in sleep duration at 11 years might reflect differences in pubertal development, and differences in developmental tempo will be related to later body composition outcomes, we do not have information of pubertal status at 11-year follow-up.
In conclusion, our findings suggest sex differences in the association between sleep duration and body composition in adolescents. Girls who improved their sleep duration during adolescence showed higher BMI, fat mass index and fat-free mass index compared to those who always had adequate sleep duration. Longitudinal studies are also useful for better understanding this relationship in adolescence. We recommend further research using valid and accurate measurements of sleep duration and body composition.
Acknowledgments
This article is based on data from the study "Pelotas Birth Cohort, 1993" conducted by Postgraduate Program in Epidemiology at Universidade Federal de Pelotas with the collaboration of the Brazilian Public Health Association (ABRASCO) and the Brazilian National Research Council (CNPq). From 2004 to 2013, the Wellcome Trust supported the 1993 birth cohort study. The European Union, National Support Program for Centers of Excellence (PRONEX), and the Brazilian Ministry of Health supported previous phases of the study.
Data Availability
All relevant data are within the paper.
Funding Statement
The authors have no support or funding to report.
References
- 1.Bibiloni Mdel M, Pons A, Tur JA. Prevalence of overweight and obesity in adolescents: a systematic review. ISRN Obes. 2013;2013:392747 10.1155/2013/392747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The Brazilian Institute of Geography and Statistics (2010) Consumer Expenditure Survey—POF 2008–2009: anthropometry and nutritional status of children, teenagers and adult in Brazil. IBGE; Available: http://www.ibge.gov.br/english/estatistica/populacao/condicaodevida/pof/2008_2009_encaa/default.shtm [Google Scholar]
- 3.Bjorge T, Engeland A, Tverdal A, Smith GD. Body mass index in adolescence in relation to cause-specific mortality: a follow-up of 230,000 Norwegian adolescents. Am J Epidemiol. 2008;168(1):30–7. 10.1093/aje/kwn096 [DOI] [PubMed] [Google Scholar]
- 4.Daniels SR. Complications of obesity in children and adolescents. Int J Obes (Lond). 2009;33 Suppl 1:S60–5. 10.1038/ijo.2009.20 [DOI] [PubMed] [Google Scholar]
- 5.Gupta NK, Mueller WH, Chan W, Meininger JC. Is obesity associated with poor sleep quality in adolescents? American journal of human biology: the official journal of the Human Biology Council. 2002;14(6):762–8. 10.1002/ajhb.10093 [DOI] [PubMed] [Google Scholar]
- 6.Wells JC, Hallal PC, Reichert FF, Menezes AM, Araujo CL, Victora CG. Sleep patterns and television viewing in relation to obesity and blood pressure: evidence from an adolescent Brazilian birth cohort. Int J Obes (Lond). 2008;32(7):1042–9. Epub 2008/03/19. 10.1038/ijo.2008.37 [DOI] [PubMed] [Google Scholar]
- 7.Seegers V, Petit D, Falissard B, Vitaro F, Tremblay RE, Montplaisir J, et al. Short sleep duration and body mass index: a prospective longitudinal study in preadolescence. Am J Epidemiol. 2011;173(6):621–9. Epub 2011/02/10. 10.1093/aje/kwq389 [DOI] [PubMed] [Google Scholar]
- 8.Silva GE, Goodwin JL, Parthasarathy S, Sherrill DL, Vana KD, Drescher AA, et al. Longitudinal association between short sleep, body weight, and emotional and learning problems in Hispanic and Caucasian children. Sleep. 2011;34(9):1197–205. Epub 2011/09/03. 10.5665/SLEEP.1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chamorro RA, Duran SA, Reyes SC, Ponce R, Algarin CR, Peirano PD. [Sleep deprivation as a risk factor for obesity]. Rev Med Chil. 2011;139(7):932–40. Epub 2011/11/05. doi: /S0034-98872011000700017. [PubMed] [Google Scholar]
- 10.National Sleep Foundation. International Bedroom Poll First to Explore Sleep Differences among Six Countries 2013. Available: http://www.sleepfoundation.org/article/press-release/national-sleep-foundation-2013-international-bedroom-poll.
- 11.Dollman J, Ridley K, Olds T, Lowe E. Trends in the duration of school-day sleep among 10- to 15-year-old South Australians between 1985 and 2004. Acta Paediatr. 2007;96(7):1011–4. 10.1111/j.1651-2227.2007.00278.x [DOI] [PubMed] [Google Scholar]
- 12.Van Cauter E, Knutson KL. Sleep and the epidemic of obesity in children and adults. Eur J Endocrinol. 2008;159 Suppl 1:S59–66. Epub 2008/08/23. 10.1530/EJE-08-0298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bernardo MPSL, Pereira EF, Louzada FM, D’Almeida V. Sleep duration in adolescents of different socioeconomic status. Jornal Brasileiro de Psiquiatria. 2009;58(4):231–7. [Google Scholar]
- 14.Knutson KL, Spiegel K, Penev P, Van Cauter E. The metabolic consequences of sleep deprivation. Sleep Med Rev. 2007;11(3):163–78. Epub 2007/04/20. doi: S1087-0792(07)00020-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taheri S. The link between short sleep duration and obesity: we should recommend more sleep to prevent obesity. Arch Dis Child. 2006;91(11):881–4. Epub 2006/10/24. 10.1136/adc.2005.093013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knutson KL. Sex differences in the association between sleep and body mass index in adolescents. The Journal of pediatrics. 2005;147(6):830–4. 10.1016/j.jpeds.2005.07.019 [DOI] [PubMed] [Google Scholar]
- 17.Storfer-Isser A, Patel SR, Babineau DC, Redline S. Relation between sleep duration and BMI varies by age and sex in youth age 8–19. Pediatric obesity. 2012;7(1):53–64. 10.1111/j.2047-6310.2011.00008.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carskadon MA, Harvey K, Duke P, Anders TF, Litt IF, Dement WC. Pubertal changes in daytime sleepiness. Sleep. 1980;2(4):453–60. [DOI] [PubMed] [Google Scholar]
- 19.Taylor DJ, Jenni OG, Acebo C, Carskadon MA. Sleep tendency during extended wakefulness: insights into adolescent sleep regulation and behavior. Journal of sleep research. 2005;14(3):239–44. 10.1111/j.1365-2869.2005.00467.x [DOI] [PubMed] [Google Scholar]
- 20.Cameron N. Human growth and development USA: Elsevier Science; 2002. [Google Scholar]
- 21.Victora CG, Araujo CL, Menezes AM, Hallal PC, Vieira Mde F, Neutzling MB, et al. Methodological aspects of the 1993 Pelotas (Brazil) Birth Cohort Study. Revista de saude publica. 2006;40(1):39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goncalves H, Assuncao MC, Wehrmeister FC, Oliveira IO, Barros FC, Victora CG, et al. Cohort profile update: The 1993 Pelotas (Brazil) birth cohort follow-up visits in adolescence. Int J Epidemiol. 2014;43(4):1082–8. 10.1093/ije/dyu077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.VanItallie TB, Yang MU, Heymsfield SB, Funk RC, Boileau RA. Height-normalized indices of the body's fat-free mass and fat mass: potentially useful indicators of nutritional status. Am J Clin Nutr. 1990;52(6):953–9. [DOI] [PubMed] [Google Scholar]
- 24.World Health Organization. Growth reference data for 5–19 years: Geneva: World Health Organization; 2007. [Google Scholar]
- 25.Hirshkowitz M, Whiton K, Albert SM, Alessi C, Bruni O, DonCarlos L, et al. National Sleep Foundation’s sleep time duration recommendations: methodology and results summary. Sleep Health: Journal of the National Sleep Foundation. 2015;1(1):40–3. 10.1016/j.sleh.2014.12.010 [DOI] [PubMed] [Google Scholar]
- 26.Ministério da Saúde. Pré-natal e puerpério Atenção qualificada e humanizada: 5a ed. Brasília: Ministério da Saúde; 2005. [Google Scholar]
- 27.Craig CL, Marshall AL, Sjostrom M, Bauman AE, Booth ML, Ainsworth BE, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381–95. 10.1249/01.MSS.0000078924.61453.FB [DOI] [PubMed] [Google Scholar]
- 28.Araujo J, Severo M, Ramos E. Sleep duration and adiposity during adolescence. Pediatrics. 2012;130(5):e1146–54. Epub 2012/10/03. 10.1542/peds.2011-1116 [DOI] [PubMed] [Google Scholar]
- 29.Garaulet M, Ortega FB, Ruiz JR, Rey-Lopez JP, Beghin L, Manios Y, et al. Short sleep duration is associated with increased obesity markers in European adolescents: effect of physical activity and dietary habits. The HELENA study. Int J Obes (Lond). 2011;35(10):1308–17. Epub 2011/07/28. 10.1038/ijo.2011.149 [DOI] [PubMed] [Google Scholar]
- 30.Jiang YR, Spruyt K, Chen WJ, Mei H, Sun WQ, Wang Y, et al. Associations between parent-reported sleep duration and adiposity in Chinese early adolescents. J Public Health (Oxf). 2015;37(2):277–85. 10.1093/pubmed/fdu049 [DOI] [PubMed] [Google Scholar]
- 31.Lytle LA, Murray DM, Laska MN, Pasch KE, Anderson SE, Farbakhsh K. Examining the longitudinal relationship between change in sleep and obesity risk in adolescents. Health Educ Behav. 2013;40(3):362–70. Epub 2012/09/18. 10.1177/1090198112451446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dahl RE, Lewin DS. Pathways to adolescent health sleep regulation and behavior. J Adolesc Health. 2002;31(6 Suppl):175–84. [DOI] [PubMed] [Google Scholar]
- 33.Kimm SY, Glynn NW, Kriska AM, Barton BA, Kronsberg SS, Daniels SR, et al. Decline in physical activity in black girls and white girls during adolescence. N Engl J Med. 2002;347(10):709–15. 10.1056/NEJMoa003277 [DOI] [PubMed] [Google Scholar]
- 34.Foti KE, Eaton DK, Lowry R, McKnight-Ely LR. Sufficient sleep, physical activity, and sedentary behaviors. Am J Prev Med. 2011;41(6):596–602. 10.1016/j.amepre.2011.08.009 [DOI] [PubMed] [Google Scholar]
- 35.Zafon C. Oscillations in total body fat content through life: an evolutionary perspective. Obes Rev. 2007;8(6):525–30. 10.1111/j.1467-789X.2007.00377.x [DOI] [PubMed] [Google Scholar]
- 36.Veldhuis JD, Roemmich JN, Richmond EJ, Rogol AD, Lovejoy JC, Sheffield-Moore M, et al. Endocrine control of body composition in infancy, childhood, and puberty. Endocr Rev. 2005;26(1):114–46. 10.1210/er.2003-0038 [DOI] [PubMed] [Google Scholar]
- 37.Wells JC. Sexual dimorphism of body composition. Best Pract Res Clin Endocrinol Metab. 2007;21(3):415–30. 10.1016/j.beem.2007.04.007 [DOI] [PubMed] [Google Scholar]
- 38.Norgan NG. The beneficial effects of body fat and adipose tissue in humans. Int J Obes Relat Metab Disord. 1997;21(9):738–46. [DOI] [PubMed] [Google Scholar]
- 39.Bogin B. Patterns of human growth Cambridge: Cambridge University Press; 1999. [Google Scholar]
- 40.Wolfson AR, Carskadon MA, Acebo C, Seifer R, Fallone G, Labyak SE, et al. Evidence for the validity of a sleep habits survey for adolescents. Sleep. 2003;26(2):213–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.