Abstract
Background
Notch signaling plays role in stem cell biology, tumor formation, angiogenesis, and cell death. Targeting Notch pathway could serve as a therapeutic strategy in cancer. Little is known about the differential role of various components of the Notch pathway in salivary AdCC.
Methods
To investigate the association of the Notch pathway in AdCC carcinogenesis, we analyzed the Notch receptors (Notch-1, Notch-2, Notch-4) and Notch ligands (Jagged-1, Delta) expressions.
Results
The results showed elevated expression levels of all five proteins in AdCC tissue relative to normal salivary gland tissues. Jagged-1/Notch-2 co-expression was significantly associated with increased patient survival rate. The elevated expression level of these Notch receptors and ligands in AdCC points to Notch signaling as a key player in AdCC pathogenesis.
Conclusions
Our data provide evidence for relationship between Jagged-1/Notch-2 co-expression and better overall patient survival with AdCC. Targeting Notch signaling pathway may provide therapeutic benefits for these patients.
Keywords: adenoid cystic, carcinoma, Notch, cancer stem cells, targeted therapy, Notch-2/ Jagged-1
Introduction
Adenoid cystic carcinoma (AdCC) is one of the most common salivary gland malignancies1–3. The majority of these tumors occur in the minor salivary glands; and the oral cavity is the most frequent site. AdCC arises from the intercalated ducts, which are composed of inner ductal epithelial and outer myoepithelial cells. In its tubular and cribriform patterns, this tumor recapitulates the dual involvement of epithelial and myoepithelial neoplastic cells.
The histogenesis of AdCCs is uncertain, although an origin from stem cells with multidirectional differentiation is possible. AdCC is classified into three histologic subtypes: solid, cribriform, and tubular. Grading is difficult, as a tumor may show evidence of more than one subtype. The tubular and cribriform patterns are known for their indolent behavior, whereas progression to the solid phenotype has been associated with the loss of myoepithelial cells and aggressive biology. Myoepithelial cells may play a role in restraining the aggressive biological behavior of (solid) AdCCs. The current dogma is that myoepithelial cells rarely transform but, when they do, the tumors derived from them are always low grade. In breast cancer, myoepithelial cells have been labeled “natural tumor suppressors” owing to their negative effects on tumor cell growth, invasion, and angiogenesis, which are achieved via the secretion of protease inhibitors and downregulation of matrix metalloproteinase levels4. The tumor-suppressive function of myoepithelial cells is progressively lost during the in situ-to-invasive breast carcinoma transition.
The morphologic heterogeneity of human salivary gland tumors belies the traditional concept of tumor progression through stepwise alterations in multiple molecular and cellular pathways5, 6. The traditional hypothesis is that a subpopulation of intratumoral cells - cancer stem cells (CSCs) - is uniquely capable of propagating the tumor, and relies on the hierarchical model to explain tumor heterogeneity and behavior5. The stochastic or clonal evolution model of tumor progression, however, suggests that while tumor cells are equally capable of propagating the tumor, distinct intratumoral populations may undergo clonal evolution in response to certain microenvironmental factors, thereby creating tumor heterogeneity.
CSCs appear to drive both tumorigenesis and metastasis, so successful treatment of patients will require elimination of these cells. However, in breast cancer, CSCs appear to be resistant to standard radiation and chemotherapy 7. Therefore, new therapeutic approaches need to be developed to target this population. One strategy is to target critical signaling pathways for self-renewal and differentiation, and several candidate pathways have been identified, including Notch, Wnt, and Hedgehog. Notch proteins are a family of transmembrane receptors that play a fundamental role in cell fate decisions. In mammals, there are four Notch receptors (Notch 1–4) and five ligands (Jagged-1 and -2, Delta-like-1, -3, and -4). Notch signaling is initiated by receptor- ligand interactions between neighboring cells that result in cleavage of the intracellular domain by the gamma-secretase complex. The intracellular domain then translocates to the nucleus. where it activates transcription via CSL (also termed CBF-1 or RBP-Jx). CSL/Notch interactions induce target gene expression, including expression of members of the Hes and Hey families of transcription factors that ultimately prevent cell differentiation8–10.
To determine whether the Notch pathway plays a role in adenoid cystic carcinogenesis, we undertook a detailed immunohistochemical analysis of human salivary AdCC and normal salivary tissue samples to compare the protein expression levels of Notch family members.
Materials and Methods
Archival formalin-fixed paraffin blocks of AdCC from 199 patients accessioned at The University of Texas MD Anderson Cancer Center between 1988 and 2006 formed this study (using appropriate written informed consent obtained after approval by the institutional review board). The material for tissue microarray was created using two 1.0-mm-diameter cores of spatially different areas of representative tumor from each paraffin block. Pathologic patterns and the phenotypic expression of Notch-1, Notch-2, Notch-4, Jagged-1, and Delta-like (DLL1) were recorded independently and were compared with clinical factors, including sex, age, and tumor stage along with demographic and clinical outcomes.
Immunohistochemical and immunoreactivity analysis
Immunohistochemical analysis for Notch-1, Notch-2, Notch-4, Jagged-1, and Delta (DLL1) was performed using the Bond Max staining protocol (Vision Biosystems, Norwell,) on 4-µm paraffin sections of the tissue microarray material. In brief, following dewaxing, washing, and rehydration of the slides through xylene and graded alcohols, Tris-EDTA buffer was used for antigen retrieval. Slides were subsequently treated with 3% hydrogen peroxide to block endogenous peroxidase. Following incubation with the primary antibodies against Notch-1, Notch-2, Notch-4, Jagged-1, and Delta (DLL1) (Cell Signaling, 1:100 dilution), the secondary conjugate antibodies were applied (Cell Signaling). Finally, each specimen-containing slide was developed using the chromogen DAB and counterstained with hematoxylin.
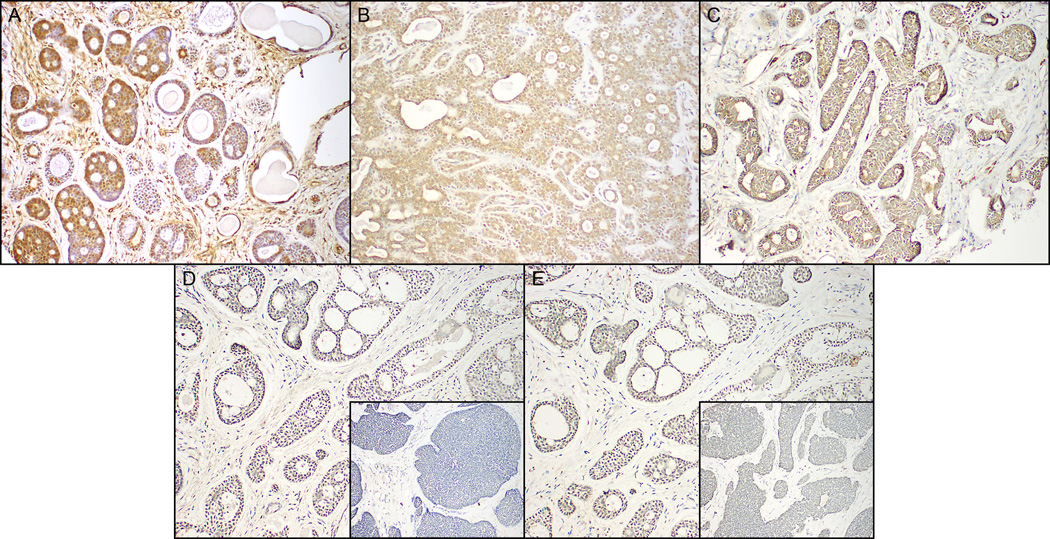
Notch-1, Notch-2, Notch-4, Jagged-1, Delta (DLL-1) immunohistochemical features from each sample were evaluated by Head and Neck pathologists. Subcellular localization was predominantly nuclear and cytoplasmic for receptors Notch-1, Notch-2 and Notch-4 and cytoplasmic or membranous for ligands Jagged-1 and Delta-1 (Figure 1). Lesional tissues with strong staining in >50% of the neoplastic cells were scored as strongly positive. Weak or strong staining in <50% of the cells was scored as weakly positive. Less than 5% staining was scored as negative
Figure 1.
In AdCCs, similar patterns of immunostaining were present with Notch-1 (A), Notch-4 (B), Delta (DLL1) (C), and with Notch-2 (D), Jagged-1 (E) (Fig 1 D, E- inset showing weak/ lack of immunoreactivity within solid type AdCC).
Statistical analysis
Descriptive statistics for scaled values and frequencies of study patients within the categories for each of parameter of interest were enumerated with the assistance of commercial statistical software. Correlations between biomarkers and between biomarkers and end points were assessed by Pearson’s Chi-square test or, where there were fewer than 10 items in any cell of a 2 × 2 grid, by the two-tailed Fisher exact test. Curves describing overall survival were generated by the Kaplan-Meier product limit method. The statistical significance of differences between the actuarial curves was tested by the log rank test. Follow-up time was the time from the first appointment at The University of Texas MD Anderson Cancer Center for the primary tumor of concern until the date of last contact or death. Calculated p values of <0.05 were considered significant. These statistical tests were performed with the assistance of Statistica (StatSoft, Inc.) and SPSS for Windows (SPSS Inc.) statistical software applications.
Results
Demographic and pathologic findings
The study cohort comprised 100 women and 99 men, who ranged in age from 15 to 86 years (median age, 52 years). Tumor sites included the parotid gland (29 patients), hard palate (28), maxillary sinus (26), submandibular and sublingual glands (20), and various minor salivary gland sites (96). According to AJCC staging criteria, 5 patients had stage I, 22 had stage II, 7 had stage III, and 31 had stage IV disease. Information on staging was not available for 122 patients, and 13 patients had no record of staging.
Histopathologic and clinical findings
The tissue cores showed at least 2 distinctive patterns within and between cores of the same case. We considered a pattern to be predominant if it was present in more than 50% of a given tumor. Among the 194 tumors for which a predominant type could be ascertained, 57 were tubular (29.4%), 109 (56.2%) had predominantly cribriform patterns (composed of epithelial and myoepithelial neoplastic cells), and 28 (14.4%) had a solid pattern (mostly devoid of myoepithelial cells). Of the 156 patients with information on metastasis, 67 (43%) developed distant metastasis, and 89 (57%) had no evidence of metastasis. In addition, of all 199 patients, 30 (15.0%) died in less than 3 years, and 117 (58.5%) survived more than 3 years; for the remainder no survival data were available.
Notch-1, Notch-2, Notch-4, Jagged-1, and Delta expression levels
In normal salivary ductal cells, Notch-1, Notch-2, Notch-4, Jagged-1, and Delta (DLL1) were weakly expressed or were absent in the cytoplasm of, whereas faint cytoplasmic expression was noted in cells of the intercalated and striated ducts.
In AdCCs, similar patterns of staining were present with Notch-1, Notch-4, Delta (DLL1) (Figure 1 A,B,C), and with Notch-2, Jagged-1 (Fig 1 D, E- inset showing weak/ lack of immunoreactivity within solid type AdCC). AdCCs were immunoreactive for Notch-1 (149 of 175 biomarkers; 85%), Notch-4 (149/164; 90%), Delta (DLL1) (129 of 161; 80%), 57% for Jagged 1 (93/162; 57%), and 34% for Notch-2 (55/161; 34%) (Table 1). These results revealed an overall increase in the expression of several Notch receptors and ligands in salivary AdCC compared with normal salivary tissue, and that multiple Notch receptors and ligands are upregulated simultaneously in any given cancer tissue.
Table 1.
Differential expression of selected markers in adenoid cystic carcinoma patients.
| Marker | AdCC (−)ve | AdCC (+)ve |
|---|---|---|
| Notch-1 | 26/ 175 (15%) |
149/175 (85%) |
| Notch-4 | 24/164 (15%) |
149/164 (85%) |
| DLL1 | 32/161 (20%) |
129/ 161 (80%) |
| Notch-2 | 106/161 (66%) |
55/161 (34%) |
| Jagged-1 | 69/162 (43%) |
93/162 (57%) |
Clinicopathologic correlations
No significant statistical correlations were found between individual markers and clinicopathologic parameters.
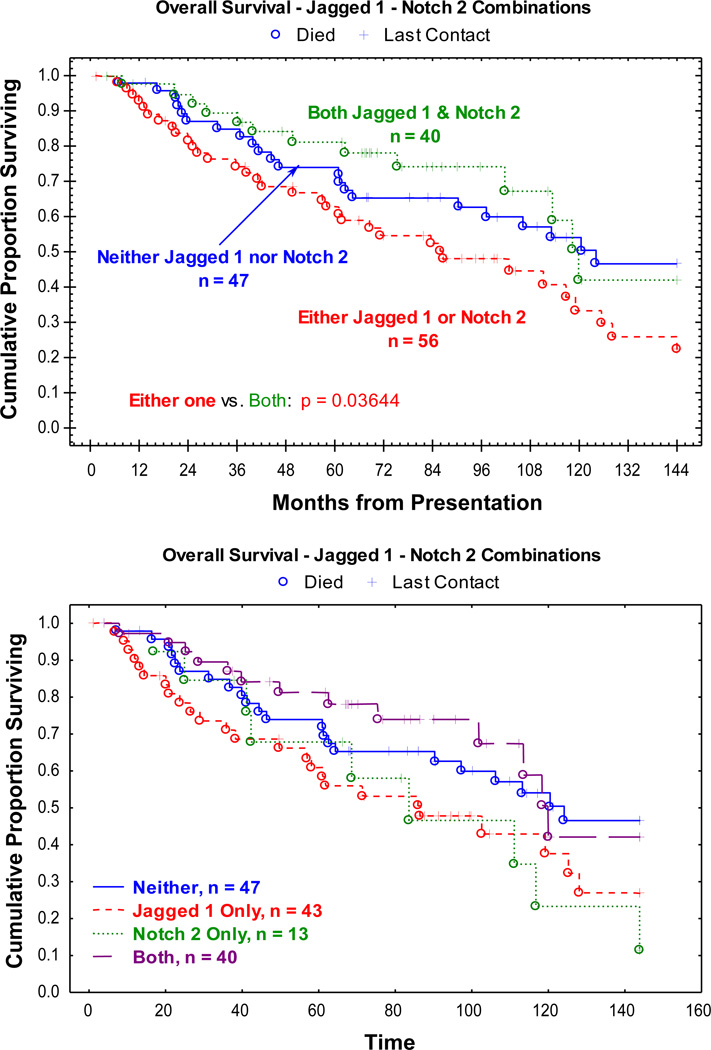
The combination of Notch-2 and Jagged-1 was significantly associated with vital status at last contact within 12 years of tumor presentation (p = 0.036) (Figure 2). The worst survival rate was associated with having one of the markers, and the best survival rate with both of them (Figure. 2). This finding may be explained in that in a setting with both a receptor (e.g., Notch-2) and a ligand (e.g., Jagged-1) there is a “tumor suppressive” effect. On the other hand, when a Jagged ligand activates other Notch paralogs (e.g., Notch-1 or -4), the biological behavior tends to be more aggressive. Such a pattern would account for Jagged-1 alone having the poor prognostic significance were observed.
Figure 2.
The Kaplan-Meier plots of the overall survival population. The combination of Notch-2 and Jagged-1 was significantly associated with vital status at last contact within 12 years of tumor presentation (p = 0.036). The worst survival rate was associated with having one of the markers, and the best survival rate with both of them.
Discussion
To understand the role of Notch pathway in human AdCC, we undertook a detailed immunohistochemical analysis to determine the protein expression level of Notch family members in these tumors. Our results indicate that there is a general increase in the expression of several Notch pathway members in salivary AdCC compared with normal salivary tissue. Although no significant statistical correlation was found between individual markers and clinicopathologic parameters, the combination of Jagged 1 and Notch 2 showed a significant p value for vital status- the worst survival was associated with having one of the markers and the best survival was in the group that had both of them. Hypothetically, in a setting that has a ligand (Jagged-1) and Notch-2 together, there is a “tumor suppressive” effect, while when Jagged activates other Notch paralogs (such as Notch-1 and Notch-4), one more commonly gets a more aggressive biological behavior. This would account for Jagged-1 without Notch-2 having a poor prognostic significance.
Our data provides the first direct evidence for a relationship between Jagged-1/Notch-2 co-expression and better overall patient survival in human salivary AdCC.
CSCs are defined as a subset of self-sustaining cancer cells with the exclusive ability to maintain the tumor. CSCs are capable of i) differentiation, giving rise to heterogeneous progeny, ii) self-renewal, which maintains a stem cell pool for expansion, and iii) homeostatic control, ensuring regulation of CSC functions and accounting for the tissue specificity of CSCs. Inherent stem cell properties – such as self-renewal, multipotency, and increased proliferative capacity – allow CSC populations to maintain a pluripotent phenotype, while also producing a heterogeneous tumor5, 6. Stem cells in hematopoietic malignancies have been well characterized, but specific CSCs in solid tumors have not yet been fully defined. CSCs have been reported in solid tumors of various organs, including the breast, brain, prostate, lung, colon, pancreas, liver, and skin11. The breast CSC model, with well-defined biomarkers, has been used to further explore molecular signatures and signaling pathways11, 12. CSCs appear to drive both tumorigenesis and metastasis, and successful treatment of patients will require elimination of these cells. This is complicated, however, by evidence that in breast cancer, CSCs are resistant to standard radiation and chemotherapy. Therefore, new therapeutic approaches need to be developed to target this population. One strategy is to target critical signaling pathways for self renewal and differentiation, and several candidate pathways have been identified including Notch, Wnt, and Hedgehog.
Accumulating evidence supports the CSC hypothesis in AdCC 13–15. When applicable to AdCC, the CSC concept will allow for a better understanding of the cell biologic traits, such as chemoresistance and metastasis, of this aggressive tumor.
Dysregulation of the Notch signaling pathway has been demonstrated in a variety of tumors, including breast cancer, and studies have shown that Notch inhibition with gamma-secretase inhibitors can kill breast cancer cell lines in vitro and in vivo. As Notch signaling has been implicated in both mammary stem cell and breast CSC self-renewal, it is possible that gamma-secretase inhibitors may provide therapeutic benefit by affecting not only bulk tumor cells but also CSCs. These inhibitors are already in early clinical development in breast cancers 9–12. It is our hypothesis that salivary AdCC contains CSC-like cells and that the number and/or self-renewal of CSC-like cells can be suppressed by inhibition of the Notch signaling.
The standard treatment of primary AdCC is complete surgical excision. 6–20 Selected patients with close or microscopically positive margins and those with perineural invasion may undergo post-operative radiotherapy. Approximately 25% of patients with AdCC develop recurrence or metastasis in 5 to 10 years. Treatment for patients with locally advanced, recurrent, or metastatic AdCC is limited. Multiple small clinical trials using single, combined, or targeted biological agents (or combinations thereof) have been conducted, but the results have been variable and the treatments generally unsuccessful. These trials have used small cohorts, heterogeneous tumor histologies, and no surrogate biological end points for therapy effectiveness. Therefore, the role of systemic therapy for advanced AdCC is empirical and undefined 21–23.
The interaction of various signaling pathways in the development of salivary AdCC has been a subject of intense research for many years. In this study, we demonstrated that several Notch receptors and ligands are overexpressed in salivary AdCC. We provide the first direct evidence for a relationship between Jagged-1/Notch-2 co-expression and better overall patient survival in human salivary AdCC. Targeting the Notch signaling pathway may provide therapeutic benefits.
Acknowledgments
AdCC tissue microarrays were constructed and provided by Dr. Adel K. El-Naggar’s laboratory (the National Institute of Health, National Institute of Dental and Craniofacial Research and Rare Disease Research grant number UO1DE019765 (AKEN). This study was supported in part by MDACC start-up funds (DB).
Abbreviations
- AdCC
adenoid cystic carcinoma
- CSC
cancer stem cells
Footnotes
Disclosure of potential conflict of interest- None
References
- 1.Leivo I. Insights into a complex group of neoplastic disease: advances in histopathologic classification and molecular pathology of salivary gland cancer. Acta Oncol. 2006;45(6):662–668. doi: 10.1080/02841860600801316. [DOI] [PubMed] [Google Scholar]
- 2.Speight PM, Barrett AW. Salivary gland tumours. Oral Dis. 2002;8(5):229–240. doi: 10.1034/j.1601-0825.2002.02870.x. [DOI] [PubMed] [Google Scholar]
- 3.van Heerden WF, Raubenheimer EJ. Intraoral salivary gland neoplasms: a retrospective study of seventy cases in an African population. Oral Surg Oral Med Oral Pathol. 1991;71(5):579–582. doi: 10.1016/0030-4220(91)90366-k. [DOI] [PubMed] [Google Scholar]
- 4.Barsky SH, Karlin NJ. Myoepithelial cells: autocrine and paracrine suppressors of breast cancer progression. J Mammary Gland Biol Neoplasia. 2005;10(3):249–260. doi: 10.1007/s10911-005-9585-5. [DOI] [PubMed] [Google Scholar]
- 5.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414(6859):105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 6.Wicha MS, Liu S, Dontu G. Cancer stem cells: an old idea--a paradigm shift. Cancer Res. 2006;66(4):1883–1890. doi: 10.1158/0008-5472.CAN-05-3153. discussion 95–6. [DOI] [PubMed] [Google Scholar]
- 7.Fillmore CM, Kuperwasser C. Human breast cancer cell lines contain stem-like cells that self-renew, give rise to phenotypically diverse progeny and survive chemotherapy. Breast Cancer Res. 2008;10(2):R25. doi: 10.1186/bcr1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Devaney J, Stirzaker C, Qu W, et al. Epigenetic deregulation across chromosome 2q14.2 differentiates normal from prostate cancer and provides a regional panel of novel DNA methylation cancer biomarkers. Cancer Epidemiol Biomarkers Prev. 2011;20(1):148–159. doi: 10.1158/1055-9965.EPI-10-0719. [DOI] [PubMed] [Google Scholar]
- 9.Dontu G, Al-Hajj M, Abdallah WM, Clarke MF, Wicha MS. Stem cells in normal breast development and breast cancer. Cell Prolif. 2003;36(Suppl 1):59–72. doi: 10.1046/j.1365-2184.36.s.1.6.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dontu G, El-Ashry D, Wicha MS. Breast cancer, stem/progenitor cells and the estrogen receptor. Trends Endocrinol Metab. 2004;15(5):193–197. doi: 10.1016/j.tem.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 11.Charafe-Jauffret E, Ginestier C, Monville F, et al. [Stem cells and epithelial cancers: the example of breast cancer] Ann Pathol. 2008;28(1):S30–S32. doi: 10.1016/j.annpat.2008.09.005. Spec No 1. [DOI] [PubMed] [Google Scholar]
- 12.Charafe-Jauffret E, Monville F, Ginestier C, Dontu G, Birnbaum D, Wicha MS. Cancer stem cells in breast: current opinion and future challenges. Pathobiology. 2008;75(2):75–84. doi: 10.1159/000123845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ding LC, She L, Zheng DL, et al. Notch-4 contributes to the metastasis of salivary adenoid cystic carcinoma. Oncol Rep. 2010;24(2):363–368. doi: 10.3892/or_00000868. [DOI] [PubMed] [Google Scholar]
- 14.Sun S, Wang Z. ALDH high adenoid cystic carcinoma cells display cancer stem cell properties and are responsible for mediating metastasis. Biochem Biophys Res Commun. 2010;396(4):843–848. doi: 10.1016/j.bbrc.2010.04.170. [DOI] [PubMed] [Google Scholar]
- 15.Zhou JH, Hanna EY, Roberts D, Weber RS, Bell D. ALDH1 immunohistochemical expression and its significance in salivary adenoid cystic carcinoma. Head Neck. 2012 doi: 10.1002/hed.23003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dodd RL, Slevin NJ. Salivary gland adenoid cystic carcinoma: a review of chemotherapy and molecular therapies. Oral Oncol. 2006;42:759–769. doi: 10.1016/j.oraloncology.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 17.Gilbert J, Li Y, Pinto HA, et al. Phase II trial of taxol in salivary gland malignancies (E1394): a trial of the Eastern Cooperative Oncology Group. Head Neck. 2006;28:197–204. doi: 10.1002/hed.20327. [DOI] [PubMed] [Google Scholar]
- 18.Laurie SA, Licitra L. Systemic therapy in the palliative management of advanced salivary gland cancers. J Clin Oncol. 2006;24:2673–2678. doi: 10.1200/JCO.2005.05.3025. [DOI] [PubMed] [Google Scholar]
- 19.Kokemueller H, Swennen G, Brueggemann N, Brachvogel P, Eckardt A, Hausamen JE. Epithelial malignancies of the salivary glands: clinical experience of a single institution-a review. Int J Oral Maxillofac Surg. 2004;33:423–432. doi: 10.1016/j.ijom.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 20.Kokemuller H, Bruggemann N, Brachvogel P, Eckardt A. [Malignant epithelial salivary gland tumors. Clinical review of 2 decades] Mund Kiefer Gesichtschir. 2004;8:191–201. doi: 10.1007/s10006-004-0531-2. [DOI] [PubMed] [Google Scholar]
- 21.Khan AJ, DiGiovanna MP, Ross DA, et al. Adenoid cystic carcinoma: a retrospective clinical review. Int J Cancer. 2001;96(3):149–158. doi: 10.1002/ijc.1013. [DOI] [PubMed] [Google Scholar]
- 22.Krams M, Parwaresch R, Sipos B, Heidorn K, Harms D, Rudolph P. Expression of the c-kit receptor characterizes a subset of neuroblastomas with favorable prognosis. Oncogene. 2004;23(2):588–595. doi: 10.1038/sj.onc.1207145. [DOI] [PubMed] [Google Scholar]
- 23.Perez-Soler R. Phase II clinical trial data with the epidermal growth factor receptor tyrosine kinase inhibitor erlotinib (OSI-774) in non-small-cell lung cancer. Clin Lung Cancer. 2004;6(Suppl 1):S20–S23. doi: 10.3816/clc.2004.s.010. [DOI] [PubMed] [Google Scholar]