Abstract
A primary goal of our research is to explore proximate mechanisms important in recruiting adaptive social behaviors. For instance, if one of three different behaviors may be expressed in a particular set of circumstances, how do neurochemical mechanisms bias behavior towards the expression of one act in lieu of the other possibilities? In this article, we review recent results suggesting that serotonin may play such a role in the control of aggression in crayfish. First, we summarize techniques that have been optimized for sensitive characterization of neurochemical profiles in crayfish. Then, borrowing concepts from behavioral ecology, we review a framework for quantitative investigation, which regards behavior as a set of individual decisions, each with a particular probability for occurrence, a motivational context, and controlled by its own distinct neurochemical mechanisms.
Keywords: HPLC, electrochemical detection, serotonin (5-HT), biogenic amine, social dominance
Introduction
A fundamental goal for contemporary neuroscience is to understand how neurochemical substrates foster behavioral, motivational, and affective states. During the last half-century, the emergence of a vast collection of neurotransmitters and neuropeptides has challenged investigators to explore the precise interactions between such neurochemicals, brain systems, and behavior. Moreover, identifying the functional role of such molecules has benefited immensely from the existence of phylogenetic homologies. For instance, the respective roles of glutamate and gamma-aminobutyric acid as excitatory and inhibitory neurotransmitters were first established in studies of decapod crustaceans (Iversen et al., 1967; Taraskevich, 1971). Our understanding of how neurochemical mechanisms bias behavior in ethological contexts, however, is fragmentary at best. Although biogenic amines are commonly recognized for their substantial involvement in a spectrum of behaviors, a comprehensive framework linking neuromodulator function with motivational aspects of behavior has remained elusive.
The indolamine serotonin has received a disproportionate amount of attention for its role in aggression (Dyakonova and Schürmann, 1999; Higley et al., 1996; Kravitz, 2000; Overli et al., 1999), social dominance (Bloch et al., 2000; Matter et al., 1998; Raleigh et al., 1991; Winberg et al., 1993), feeding (Heinz et al., 1996; Meguid et al., 2000; Rozenboim et al., 1999), and sexual behavior (Evans et al., 1998; Meston and Frohlich, 2000; Uphouse, 2000), as well as being implicated in the etiology of affective disorders ranging from depression to schizophrenia (Blier and de Montigny, 1999; Kroeze and Roth, 1998; Lucki 1998; Sivam, 1996; Yadid et al., 2000). As might be expected when many behaviors are represented by a single neural system, the anatomical and physiological properties of serotonin systems are inherently difficult to study. In most species, serotonin neurons are situated along the neuroaxis, with extensive projections branching across large areas of nervous tissue (Azmitia and Segal, 1978; Beltz and Kravitz, 1987; Lidov and Molliver, 1982). Serotonin neurons and their targets are endowed with a full complement of receptors, each with a unique coupling mechanism and binding profile (Hen, 1993; Kroeze and Roth, 1998; Tierney, 2001). Electrophysiological studies demonstrate that serotonergic neurons cycle in the range of 0.5–3.0 Hz as slow-rhythm oscillators (Ma et al., 1992; Wang and Aghajanian, 1977) with depolarization commonly followed by autoinhibition (Heinrich et al., 1999; Wang and Aghajanian, 1977). Furthermore, depending on the precise context and species, serotonin can serve as a trophic factor during development, a classical neurotransmitter, a neuromodulator, or a neurohormone.
Serotonin systems are implicated as key physiological mechanisms in the control of agonistic behavior and social dominance in species ranging from ants (Kostowski et al., 1975) to humans (Lesch and Merschdorf, 2000) with many taxa in between (Blanchard et al., 1991; Dornberg et al., 2001; Dyakonova and Schürmann, 1999; Korzan et al., 2000; Maler and Ellis, 1987; Raleigh et al., 1991; Reisner et al., 1996). In different species, investigators have utilized a variety of experimental approaches by studying the physiological correlates of social status or aggressive state (Blanchard et al., 1991; Eloffson et al., 2000; Higley et al., 1996), applying pharmacological interventions (Fuller, 1996; Larson and Summers, 2001; Olivier et al., 1989; Raleigh et al., 1991), or adopting gene-knockout techniques (Cases et al., 1995; Chen et al., 1994; Saudou et al., 1994). However, as with all behaviors, serotonin's precise role in aggression is unknown, and controversy surrounds seemingly incompatible interpretations. Attempts at resolving such issues have focused on phylogenetic differences where increased serotonin function is often considered to lower aggression in vertebrates, while the opposite scenario seems to hold true for invertebrate taxa (Edwards and Kravitz, 1997; Weiger, 1997).
Although it is possible that the role of serotonin systems in aggression underwent such a sign change during early vertebrate evolution, disagreement also arises from the use of different, often unstated, definitions when dealing with the concept of aggression. When addressed at the individual or paired level, aggression has been considered from a variety of disparate perspectives; including aggressive state, risk taking, lack of control for impulsiveness or violence, negative effects of stress, an ability to win single agonistic encounters, or achieving and maintaining social dominance. If the rules that govern behavioral intricacies in social relations are to be described, it becomes essential to unambiguously specify the precise phenomena under study. Moreover, it is beneficial to consider how social structures have been shaped during their evolutionary history. In invertebrates, dominance is primarily a result of physical superiority, while in many vertebrates it is determined additionally by the formation of coalitions and by differential treatment of kin. However, important differences exist even within vertebrate social systems, where overt aggression may be a main route to high social rank in some taxa (Blanchard et al., 1991; Larson and Summers, 2001; Winberg and Nilsson, 1993), but not in others (Raleigh et al., 1991). Since the utility and expression of agonistic behavior varies greatly as a function of the particular social system under study, it is clear that general theories attempting to explain aggression should be invoked with great care.
Recent work in our laboratory centers on using crayfish to study links between serotonin, aggression, and social dominance (Huber et al., 2001a). Unlike many vertebrate social systems; social rank among crayfish is not complicated by social coalitions or pair bonds (Goessmann et al., 2000; Issa et al., 1999). Individuals primarily maintain a solitary existence, where conflict between conspecifics occurs in attempts to obtain or defend resources such as shelter (Ranta and Lindström, 1993), food (Ranta and Lindström, 1992; Stocker and Huber, 2001), or mates (Berrill and Arsenault 1982, 1984). However, even in the absence of a resource, crayfish exhibit an aggressive predisposition (Bruski and Dunham, 1987; Goessmann et al., 2000; Issa et al., 1999) that may be due to selective pressures of living in habitats that can reach densities in excess of fifty individuals/m2 (Berrill, 1978; Stewart and Haynes, 1994; personal observation). Agonistic meetings between crayfish are characterized by a series of highly structured behavioral acts with escalation governed by strict rules (Huber et al., 2001b). Typically, fights progress through several conspicuous stages that include ritualized visual displays, antennae whipping, claw lock, wrestling, and if size asymmetries are small, brief periods of unbridled claw use (Bovbjerg, 1953; Bruski and Dunham, 1987; Huber and Kravitz, 1995; Rubenstein and Hazlett, 1973). Such behavioral characteristics are consistent with predictions derived from game theory (Austad, 1989; Enquist and Leimar, 1983; Maynard-Smith and Price, 1973; Parker and Rubenstein, 1981) and are thus well suited for quantitative assessment of individual agonistic encounters (Huber et al., 2001b).
The presence of a highly structured behavioral framework presents new vistas for exploring the interface between behavior and its underlying physiological mechanisms. Serotonin is likely to play a role in the control of aggression between clawed decapod crustaceans such as crayfish and lobsters (Kravitz, 2000). A small population of serotonin-containing neurons (Beltz and Kravitz, 1983; Real and Czternasty, 1990) is involved in controlling characteristic postures of dominant animals (Harris-Warrick and Kravitz, 1984; Ma et al., 1992) and the generation of tail flips, a common form of retreat (Edwards et al., 1999; Glanzman and Krasne, 1983; Yeh et al., 1996, 1997). Moreover, work from several labs indicates an association between serotonin systems and aggression in clawed-crustacean species (Antonsen and Paul, 1997; Doernberg et al., 2001; Krasne et al., 1997; Sneddon et al., 2000; Tierney 2000; Yeh et al., 1997). In collaborations with others, our lab has employed several approaches for exploring the relationships between serotonin and fighting behavior in crayfish. Specifically, we have (1) developed a framework for identifying sources of behavioral variation in need of physiological explanation, (2) explored the behavioral consequences of acute and chronic pharmacological interventions of the crayfish serotonin system, and (3) attempted to relate variation in amine levels to social status. Quantitative verification of amine manipulations is an important aspect of such studies. Towards this goal, we have optimized chromatographic techniques for sensitive detection of biogenic amines in crustacean nervous tissues and hemolymph. In this article, we review our efforts to relate pharmacologically induced as well as naturally occurring changes in crayfish aggression to serotonin using high performance liquid chromatography with electrochemical detection.
General Overview of Quantitative Neurochemistry
High performance liquid chromatography coupled with an electrochemical detection system (HPLC-ED) offers a highly selective and sensitive tool for analyzing substances in catechol- and indolamine pathways. HPLC-ED allows separation of a complex mixture of neurochemical constituents, as well as determination of the number, identity, and quantity present in crustacean nervous system tissues. Based on surface properties of their functional groups, analytes are differentially adsorbed onto a non-polar stationary phase (i.e., a C18-chromatography column) until eluted with a sufficiently polar mobile phase. Non-polar substances are retained longer in the stationary phase, while polar and ionic molecules are not adsorbed and thus elute near the void volume.
Under most elution conditions, serotonin, dopamine, and other aromatic amines of interest exist as positively charged ions (basic pKa of 4–7). Retaining such molecules sufficiently long for analysis thus entails the additional electrostatic action of a negatively charged ion-pairing counter-ion (e.g., an anionic detergent). Control of retention time for these compounds, as well as their relative order of elution, depends on the amount of organic modifier and ion pairing agent, and the electrical charge of functional groups at the mobile phase pH. Electrochemical detectors measure the current produced by chemical reactions in which molecules lose (oxidation) or gain (reduction) electrons as they pass through a potential applied to an analytical cell. Aromatic amines are quite suitable for such detection systems, as they are readily oxidized at low applied potentials. At any given potential, the size of the resulting current is proportional to the concentration of the molecule that is being reacted. Moreover, characteristic current/voltage relationships of compounds produce distinct profiles of peak heights at different potentials and thereby aid in their identification.
Over the last several years, we have optimized chromatographic techniques for the analysis of crayfish central nervous system homogenates (Benton et al., 1997; Panksepp and Huber, 2002; Yue and Huber, unpublished data) using reversed-phase, ion-pair HPLC coupled with a single-cell amperometric system or, more recently, with a four-channel, flow-through coulometric detection instrument. Crayfish are anesthetized in ice, followed by a rapid dissection of the ventral nerve cord. Tissues are placed into 1.5-ml microfuge tubes containing 200 μl 0.1N perchloric acid (PCA), mechanically disrupted with a motor driven homogenizer (Kontes pelleting tool) equipped with a Teflon pestle, and cellular debris and denatured proteins are pelleted with a tabletop centrifuge (Capsulefug, 6200RPM, 15 minutes). Aliquots (20 μl; supernatant diluted 10–100 fold with mobile phase) are applied directly to an HPLC system consisting of a solvent delivery pump (Jasco PU 1580), injector (Rheodyne 9725I), with 20-μl sample loop, pulse dampener (ESA PEEK), and analytical column (Spherisorb S3ODS2, 10 cm, ø4.6 nm). The mobile phase contains 50 mM sodium phosphate (6.9 g/l monobasic anhydrous, FW 120.0; Sigma S-0751), 5 mM heptanesulfonic acid (1g/l sodium salt; FW: 202.2; Sigma H-2766), and vol/vol 15% MeOH and 7% Acetonitrile as organic modifiers. The final solvent buffer is adjusted to pH 2.8 with concentrated phosphoric acid (ACS reagent; FW: 98.0; Sigma P-6560), filtered through a 0.22-μm filter, and operated under ambient temperatures at a flow rate of 1.0 ml/min. Detection is achieved with a 4-channel electrochemical cell controller (ESA Coulochem II with 2 additional potentiostat modules, ESA Inc., Chelmsford, MA), conditioning cell (ESA 5021, ESA Inc., Chelmsford, MA), and analytical cell array (4-channel coulometric cell array, ESA Inc., Chelmsford, MA).
Potentials for the serially arranged cells are set at progressively higher levels, so each coulometric cell functions as a screening electrode for subsequent cells. Potentials and their typical background currents are, respectively, set for conditioning cell (800 nA @ 35 mv), detector 1 (5 nA @ 300 mv), detector 2 (32 nA @ 420 mv), detector 3 (25 nA @ 540 mv), and detector 4 (177 nA @ 660 mv). Analog signals from the cell controller are recorded with 4-channel analog/digital converter hardware (MacLab/s4, CB Sciences) and multi channel strip chart software (MacLab Chart v3.3.3, CB Sciences) on an Apple Macintosh computer. A 6V circuit monitors the position of the injector and automatically triggers the start of recording when a switch from “load” to “inject” is detected. Recorded chromatograms are analyzed with chromatography software (MacLab Peaks v 1.3.1, CB Sciences).
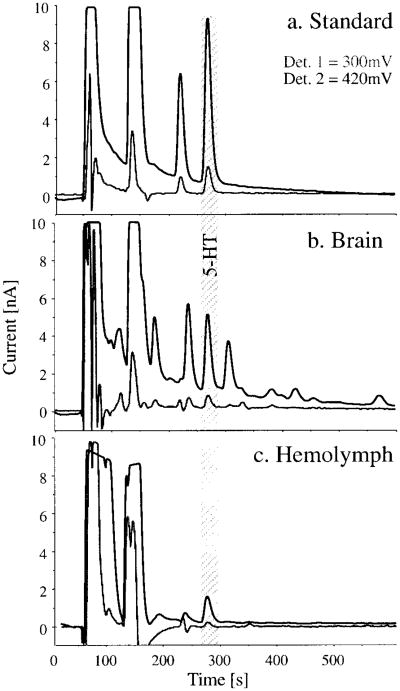
Identification of amines is based on (1) comparison with external standards by retention time under several different mobile phase conditions, (2) the ratio of peak currents between different detectors, and (3) adding known amounts of substance to the sample. Known standards are dissolved in 0.1N PCA at a concentration of 1 mg/ml with final concentrations (50 pg amine/20 μl) in mobile phase made from stock each day. Detection limits are < 1 pg serotonin or dopamine injected on column. Recovery rates are close to 100% and no further correction is applied. Standards are applied every fifth sample, and at the beginning and end of each analytical session. Figure 1 illustrates chromatograms of the first and second channel for crayfish supraesophageal ganglion (i.e., brain) and hemolymph.
Fig. 1.
Representative chromatograms for injections of a 5-HT standard, a perchloric acid extract of brain homogenate, and a hemolymph sample from crayfish, Orconectes rusticus. The mobile phase contained 50 mM sodium phosphate, 5 mM heptanesulfonic acid, 15% MeOH, and 7% Acetonitrile at pH 2.8. A 4-channel coulometric cell array was operated with test electrodes set at 300 mV (channel 1), 420 mV (channel 2), 540 mV (channel 3), and 660 mV (channel 4). a: Applied to the column were 50 pg of 5-HT (269.5 s), N-acetyl 5-HT (234.3 s), and other substances. b: The supraesophageal ganglion (brain) of one female (4.2 g) was dissected, homogenized in 200 μl of 0.1N perchloric acid, centrifuged, and the supernatant diluted 1.20 times with mobile phase. c: A 40-μl hemolymph sample from a female crayfish (9.5 g) was collected, precipitated in 10 μl 0.5N PCA, centrifuged, and the supernatant diluted 1:100 times with mobile phase. Pictured are currents on channels 1 (dark grey) and 2 (black) at a full current range of 10 nA. The ratio of peak areas on different detectors (in nA seconds) is diagnostic for each substance. In addition to eluting at the same time, ratios (det1/det2) for the peak at 269.5 seconds in tissues match that of the standard with 14% (standard), 13% (brain), and 15% (hemolymph), respectively.
Acute Serotonin Treatment in Crayfish
Experimental alteration of central nervous system serotonin alters aggressive motivation in many taxa (Doernberg et al., 2001; Dyakonova and Schürmann, 1999; Fuller, 1996; Larson and Summers, 2001; Maler and Ellis, 1987; Raleigh et al., 1991). In particular, close associations between serotonin systems and aggressive state have been demonstrated in clawed decapod crustaceans (Antonsen and Paul, 1997; Kravitz, 2000; Sneddon et al., 2000; Tierney, 2000). Our first attempts to examine effects of serotonin manipulations on fighting behavior included pairing crayfish (Astacus astacus) with large size asymmetries (>30%) and securing a fine-bore, fused silica cannula into the pericardial sinus of the smaller individual (Huber et al., 1997a,b; Huber and Delago, 1998). Initially, pairs established a dominance relationship that invariably resulted in continued retreat of the smaller opponent. Following a saline-control that fostered no associated behavioral changes, the pump was switched to infusion of serotonin. Such treatment resulted in marked changes in the behavior of the subordinate crayfish, an effect that continued well after the pump had been turned off. Infused animals again re-engaged their larger opponents; fights lasted longer and reached higher levels of intensity compared to controls. Multivariate techniques (e.g., discriminant function analysis) revealed that serotonin treatment had specifically altered the decision to retreat from an opponent, without affecting how likely the animal was to initiate fights, how individual fights progressed to higher intensities, or in the case of large size asymmetries, the eventual establishment of social rank. Furthermore, it was likely that serotonin reuptake mechanisms played a crucial role in this behavioral change as the behavioral effect of serotonin was blocked when fluoxetine was infused concurrently (Huber et al., 1997a,b; Huber and Delago, 1998).
To further explore the physiological time course of the experimental treatment (in A. astacus), a second group of animals was injected with a bolus of serotonin for which the half-life in hemolymph was estimated at10.1 minutes using HPLC-ED. Taken together, the behavioral and neurochemical data illustrated that an altered decision to retreat was evident once serotonin levels had stabilized around 10 μM, and persisted even after a large amount of serotonin had been cleared from the hemolymph. The delay between turning the pump on/off and the respective appearance/decline of behavioral effects, in conjunction with data from the fluoxetine infusions, suggested that such changes in aggressive motivation required prolonged loading of presynaptic terminals and were possibly mediated through the activity of slow acting, metabotropic receptors.
Acute serotonin treatment in crayfish exhibits a unique behavioral specificity: treated animals continue to engage larger opponents in prolonged bouts of fighting even in instances that carry substantial risk of injury. We postulated that such treatment specifically alters properties and/or activity of neurons present at key sites for agonistic “decision making” in the crayfish central nervous system (Huber and Delago, 1998). It is important to note, however, that interpretations from other studies of decapod “aggression” (e.g., Peeke et al., 2000) seemingly disagree with any role for serotonin in decapod fighting behavior and rather suggest serotonin treatment may indirectly affect social interaction through altered muscle coordination. Such a discrepancy provides an excellent opportunity to address the previously mentioned caveat of viewing aggression from multiple perspectives (see Introduction), and thus may help to underscore what can go astray when different behavioral concepts are conflated. Figure 2 illustrates how seemingly subtle differences in independent and dependent variables may, upon closer examination, produce appreciably different experimental conditions.
Fig. 2.
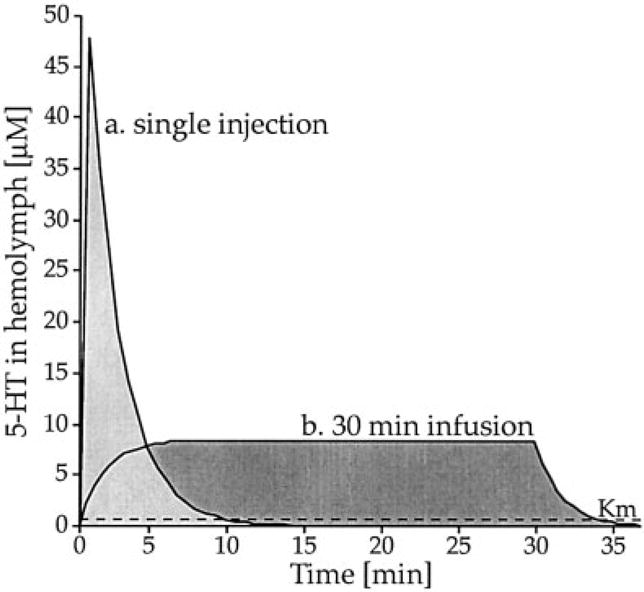
Model of temporal dynamics for hemolymph 5-HT concentrations illustrate the particular differences for seemingly similar pharmacological treatments. Curves represent 5-HT levels in hemolymph following administration of a total of 90 μg 5-HT into a 30-g crayfish (Orconectes rusticus) with approximately 8 ml of hemolymph in the form of (a) a single injection or via (b) continuous infusion into the pericardial sinus for 30 minutes. Calculations were obtained from rate and amount of 5-HT administered, as well as from empirical measures of its half-life in the circulation (t1/2 = 254 s at 21°C; see data presented in text). A single-3 μg/g body weight injection of 5-HT raises hemolymph concentrations to a peak of 48 μM, which then declines rapidly. Less than 1% of injected 5-HT remains after 10 minutes. Continuous infusion, in contrast, stabilizes hemolymph concentrations around 8 μM 5-HT. Injections of radioactively labeled sucrose into the pericardial cavity indicated that substances of interest are evenly distributed throughout the body within 30 seconds (unpublished data). Comparison of the area under each curve indicates differences in the amount of 5-HT available over time for these experimental conditions. Seemingly similar treatments (e.g., administration of 90 μg 5-HT using different techniques) may actually, upon closer inspection, be creating different neurochemical contexts. Although both neurochemical treatments are intended to address a similar question, they are not doing so by an equivalent means of investigation. Such issues become especially relevant considering the importance of loading presynaptic terminals through uptake mechanisms (Huber and Delago, 1998), with a Km value for decapod nervous tissue of 0.66 μM (Livingstone et al., 1981). Moreover, a variable related to crayfish and lobster aggression (e.g., excitability of a neuronal circuit that controls tail flip) can achieve opposite states depending on both the duration and concentration of 5-HT exposure (Teshiba et al., 2001). In attempts to resolve apparent differences in experimental findings, consideration of a series of questions should prove helpful. How much substance was injected and over what time frame? What was the site of pharmacological administration (abdominal muscle, pericardial cavity, or ventral blood sinus)? Were behavioral effects resulting from 5-HT treatment measured during treatment or well after? Is there an underlying motivational construct that might explain both engaging a physically superior opponent and not fleeing from a novel situation?
Chronic Serotonin Pharmacology in Crayfish
Since the discovery of the tricyclic antidepressants (Kuhn, 1958) and monoamine oxidase inhibitors (Bloch et al., 1954), interest in and use of drugs that alter biogenic amine systems have grown considerably. Initial discoveries were followed by the emergence of a large group of therapeutic compounds (Blier and de Montigny, 1999; Nestler, 1998) that share the common biochemical property of direct affinity for monoamine systems. However, the particular benefits conferred by such therapies are not manifest in a single physiological axis, but rather through several, often unexpected, physiological and behavioral alterations (Berry et al., 1996; Kalsner, 2000; Nestler, 1998; Rossby et al., 1995; Silva and Brandao, 2000; Sivam, 1995; Smith et al., 2000; Yadid et al., 2000). With regard to such pharmacotherapies, amine modulatory systems may be more appropriately viewed as initiating mechanisms for a cascade of large scale neurochemical, hormonal, and anatomical changes (Duman et al., 1997; Jacobs et al., 2000; Kudryavtseva and Avgustinovich, 1998; McEwen, 2000; McKittrick et al., 2000; Nestler, 1998). Irrespective of their (direct or indirect) association with affective disorders, biogenic amines clearly play a critical role in the control of motivational elements underlying fundamental behaviors such as feeding or aggression. To understand the actions of amine-modifying drugs in such behavioral contexts, it is essential to examine how motivational plasticity parallels alterations in amine function.
We began this task by examining the agonistic behavior patterns of crayfish that received continuous infusions of fluoxetine (Prozac) over a 3-week period via osmotic mini-pumps (Delago et al., unpublished data). The behavioral effects were in general agreement with findings from experiments with acute serotonin infussions: treated animals exhibited a behavioral profile characterized by a decreased willingness to retreat, resulting in more intense and prolonged fighting, particularly during the initial days of treatment. The behavioral effects accompanying chronic fluoxetine treatment, however, were less pronounced than those associated with acute serotonin infusion.
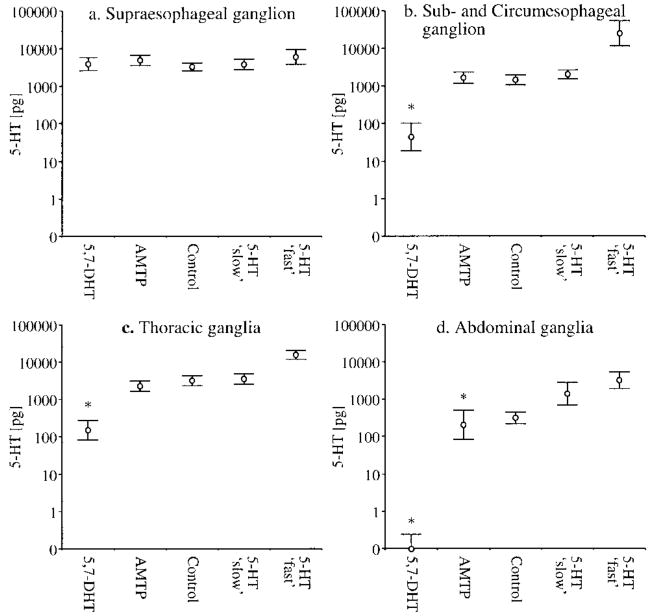
To further explore differences in fighting behavior resulting from chronic augmentation or disruption of the crayfish (Orconectes rusticus) central serotonin system, we used silastic tube implants containing serotonin, or the serotonin-depleting compounds 5,7-dihydroxytryptamine and alpha-methyltryptophan (Panksepp and Huber, 2002). Following behavioral trials carried out across a range of exposure periods, the entire nervous system was assayed for serotonin with HPLC-ED (Fig. 3). 5,7-dihydroxytryptamine was present at high levels in all segments of the central nervous system and resulted in significant depletion in all tissues, except brain. Alpha-methyltryptophan treatment was much less effective in depleting serotonergic stores. Counter to our predictions, chronic infusion of serotonin at two different rates (0.60 and 7.81 μg/hour) did not augment baseline serotonin levels in the central nervous system. Findings were even more counter-intuitive once the agonistic behavior of treated crayfish was included. Serotonin-depleted animals were indistinguishable from controls, whereas differences in fighting behavior were detected between experimental groups where serotonin content had not changed in absolute terms (i.e., animals receiving serotonin at a lower rate escalated more quickly and those treated with serotonin at a faster rate escalated more slowly).
Fig. 3.
5-HT content is plotted for individual segments of the crayfish central nervous system following chronic treatment with pharmacological compounds. Silastic tubes (15.0 mm length, 0.635 mm Ø) were loaded with crystals of 5,7-dihydroxytryptamine (5,7-DHT), α-methyltryptophan (AMTP), 5-HT (“fast” = 7.81 μg/hr and “slow” = 0.60 μg/hr), or left empty (control). Tubes were implanted into the crayfish thoracic body cavity and individuals were socially isolated across a range of time periods (1-5 weeks) before fighting a randomly selected, size-matched conspecific. Following behavioral trials, experimental animals were anaesthetized on ice and the entire ventral nerve cord was dissected out. Individual segments were assayed for 5-HT content with HPLC-ED. a: supraesophageal ganglion; F(4,52) = 1.54, P = 0.205; b: sub- and circumesophogeal ganglion; F(4,52) = 69.94, P < 0.001; c: thoracic ganglia (1–5); F(4,51) = 34.95, P < 0.001; d: abdominal ganglia (1–6); F(4,53) = 10.46, P < 0.001. Measures of 5-HT were log-transformed to reduce heteroscedasticity of treatment variances. Post-hoc differences between individual groups were identified with Tukey's HSD test (α = 0.05) and are indicated with an asterisk.
Results of the experiments reported above strongly suggested the activation of compensatory mechanisms that appear to have counteracted our pharmacological interventions. As has been demonstrated in other species, neuronal compensation can be manifested at many levels; including synthesis (Sivam, 1995; Stachowiak et al., 1986), amine release (Hall et al., 1999; Lent, 1984), metabolic activity (Ase et al., 2000; Fickbohm et al., 2000), and receptor turnover (Patel et al., 1996; Woo et al., 1996). To begin addressing such possibilities in crayfish, we compared the rate of serotonin clearance from hemolymph between individuals chronically treated with serotonin-filled silastic implants and controls that received empty silastic tubes. Seven days after implantation, crayfish (O. rusticus) were challenged with a single injection of serotonin (1.415 μg/g body weight) into the abdominal musculature and the rate of clearance was estimated from HPLC-ED measures obtained 8 minutes later. Serotonin was removed rapidly from the hemolymph of control individuals (mean t1⁄2 = 254 s at 21°C) and no significant differences were detected in this measure (F(118) = 0.09, P = 0.766) for individuals that had carried chronic 5-HT implants. It thus appears the mechanisms that inactivate and remove serotonin from the hemolymph are not a primary site for compensatory changes in this species. Other potential levels of compensation include modulation of release, central metabolism, or receptor turnover (Cooper et al., 2001).
Amine Correlates of Social Status in Crayfish
Serotonin and its metabolites are closely associated with both aggressive state and social rank (Blanchard et al., 1991; Higley et al., 1989; Matter et al., 1998; Winberg and Nilsson, 1993). Moreover, serotonin concentration in crustacean hemolymph (Sneddon et al., 2000) and its efficacy for receptors on a neuronal circuit controlling tail flip (Yeh et al., 1997), varies as a function of social status. Recently, we examined whether social status is reflected by changes in the concentration of biogenic amines in the crayfish central nervous system (Huber et al., 2001a; Yue and Huber, unpublished data).
In a series of experiments, conditions were chosen to maximize the behavioral differences associated with social dominance. Pairs of socially-naive crayfish were closely matched for body weight, carapace length, and claw size, and remained together continuously for 24 hours. Following a series of initial agonistic interactions, fighting subsided and a dominant animal emerged with little challenge from the subordinate. No reversals in dominance were observed during the 24 hours following initial pairing. Central nervous system levels of serotonin and dopamine were subsequently measured with HPLC-ED in dominant and subordinate individuals, as well as in size-matched controls. No significant differences in amine levels were detected between these three groups (Fig. 4). Much like the pharmacological experiments mentioned above, physiological changes related to social status may be expressed over different time frames or involve metabolism (Blanchard et al., 1991; Summers et al., 1997; Winberg et al., 1991; Winberg and Nilsson, 1993), changes in receptor populations (Flugge et al., 1997; McKittrick et al., 1995; Yeh et al., 1997) or release (Sneddon et al., 2000).
Fig. 4.
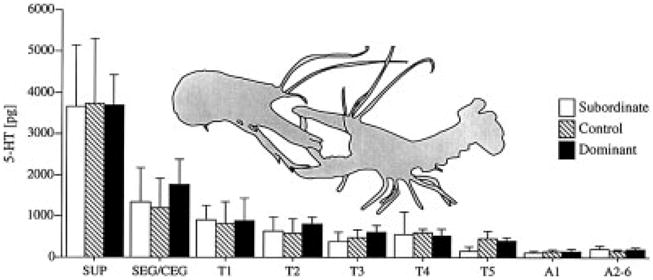
Ten groups of three size-matched crayfish were randomly selected: two crayfish were placed in an observation aquarium and allowed to interact for 24 hours, while the third individual served as a control. Following determination of social status 5-HT and dopamine in individual central nervous system segments were measured using HPLC-ED. Kruskal Wallis analysis of variance (α= 0.05) revealed no significant differences in 5-HT (illustrated above) or dopamine (results not shown) content for dominant, subordinate, or control crayfish across any individual central nervous system tissues.
Conclusions
A common misconception is the notion that a particular monoamine system is the physiological underpinning of a specific behavioral state. For instance, neurotoxic lesions or gene “knockouts” are often expected to obliterate the behavior in question. However, such neuromodulatory systems do not produce behavior per se; they rather appear to fine-tune ongoing activity by modulating neuronal environments towards a changed probability of adaptive responsiveness (Kravitz, 1988). Destruction of a modulatory, neurochemical system should thus not alter the patterns of behavior themselves, but only their modulation within a given set of circumstances.
A main challenge for understanding biogenic amine systems is the inclusion of contextual specificity. This requires that we reevaluate the orthodox assumption of a one-way causal route from brain to behavior. For instance, it is likely that many of the reported behavioral effects associated with biogenic amines hinge on the precise social (Berton et al., 1999; Ison et al., 1996; Yeh et al, 1997), genetic (Cases et al., 1995; Rilke et al., 1998; Yadid et al., 2000) and developmental (Ferris, 2000; Schenk et al., 1987) context in which they are expressed. Likewise, in both healthy and diseased states many amine modulated behaviors may obtain specificity through interactions with other aminergic and peptidergic systems (Bradwejn and Koszycki, 1991; Ferris and Delville, 1994; Jacobs and Fornal, 1999; Meguid et al., 2000; Sivam, 1996). It is, therefore, essential that multi-disciplinary and integrated approaches are applied to such problems, where quantitative neurochemistry, pharmacology, and molecular biology are combined with quantitative behavioral analyses (Lederhendler and Shulkin, 2000).
Towards this goal, work from our lab has illustrated that serotonin reuptake mechanisms are specifically involved in decisions to retreat during agonistic encounters between crayfish. Moreover, in accordance with studies in vertebrates (Ase et al., 2000, Gingrich and Hen, 2000, Pan et al., 2001), powerful compensatory mechanisms are ultimately manifested at the behavioral level following chronic amine manipulations in crayfish. In conjunction with experiments addressing the acquisition of social status in crayfish, our studies identifying biochemical changes induced by pharmacological disruption of crayfish serotonin systems and those of others (e.g., Teshiba et al., 2001), support a view of biogenic amines as individual elements embedded in a dynamically orchestrated system, rather than simply as static neurochemical characteristics.
High performance liquid chromatography with electrochemical detection has contributed greatly to our ability to identify, and partially resolve, the questions and assumptions raised in this article. In a much more general sense inextricably linked to our experimental paradigm is the acceptance that a pharmacological basis for the study, as well as therapy, of motivational states has become a major facet of behavioral neuroscience in the 21st century. To truly understand how behavioral and neural processes are modified with this cornucopia of molecules, it will be essential to not only characterize the induction of transient and persistent alterations in nervous system biochemistry, but to identify how the nervous system responds to the altered state that we, as experimenters, produce.
Acknowledgments
We are thankful to Huberlab members (K. Davis, K. Hock, A. Pytel, A. Stocker, and D.A.W.S.) and S. Tuttle for helpful comments and suggestions on earlier versions of this manuscript. We also appreciate the helpful input of two anonymous reviewers. Work reported in this paper was supported by grants to R.H. (NSF IBN-9874608, NSF DBI-0070334, and NIH MH62557-01).
Grant sponsor: NSF; Grant numbers: IBN-9874608, DBI-0070334; Grant sponsor: NIH; Grant number: MH62557-01.
References
- Antonsen BL, Paul DH. Serotonin and octopamine elicit sterotypical agonistic behaviors in the squat lobster Munidia quadrispina (Anomura, Galatheidae) J Comp Physiol A. 1997;181:501–510. [Google Scholar]
- Ase AR, Reader TA, Hen R, Riad M, Descarries L. Altered serotonin and dopamine metabolism in the CNS of serotonin 5-HT1A or 5-HT1B receptor knockout mice. J Neurochem. 2000;75:2415–2426. doi: 10.1046/j.1471-4159.2000.0752415.x. [DOI] [PubMed] [Google Scholar]
- Austad SN. Game theory and the evolution of animal contests. Trends Ecol Evol. 1989;4(1):2–3. [Google Scholar]
- Azmitia EC, Segal M. An autoradiographic analysis of the differential ascending projections of the dorsal and median raphe nuclei in the rat. J Comp Neurol. 1978;179:641–688. doi: 10.1002/cne.901790311. [DOI] [PubMed] [Google Scholar]
- Beltz BS, Kravitz EA. Mapping of serotonin-like immunoreactivity in the lobster nervous system. J Neurosci. 1983;3:585–602. doi: 10.1523/JNEUROSCI.03-03-00585.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltz BS, Kravitz EA. Physiological identification, morphological analysis and development of identified serotonin-proctolin containing neurons in the lobster ventral nerve chord. J Neurosci. 1987;7:533–547. doi: 10.1523/JNEUROSCI.07-02-00533.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton J, Huber R, Ruchhoeft M, Helluy S, Beltz B. Serotonin depletion by 5,7 dihydroxytryptamine alters deutocerebral development in the lobster, Homarus americanus. J Neurobiol. 1997;33:357–373. doi: 10.1002/(sici)1097-4695(199710)33:4<357::aid-neu2>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Berrill M. Distribution and ecology of crayfish in the Kawartha Lakes region of southern Ontario Can J Zool. 1978;56:166–177. [Google Scholar]
- Berrill M, Arsenault M. Spring breeding of a northern temperate crayfish Orconectes rusticus. Can J Zool. 1982;60:2641–2645. [Google Scholar]
- Berrill M, Arsenault M. The breeding behavior of a northern temperate orconectid crayfish, Orconectes rusticus. Anim Behav. 1984;32:333–339. [Google Scholar]
- Berry SA, Shah MC, Khan N, Roth, BL Rapid agonist-induced internalization of the 5-hydroxytryptamine 2A receptor occurs via the endosome pathway in vitro. Mol Pharmacol. 1996;50:306–313. [PubMed] [Google Scholar]
- Berton O, Durand M, Aguerre S, Mormède, Chaouloff F. Behavioral, neuroendocrine and serotonergic consequences of single social defeat and repeated fluoxetine pretreatment in the lewis rat strain. Neurosci. 1999;92:327–341. doi: 10.1016/s0306-4522(98)00742-8. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Panrapee C, Blanchard RJ, Clow DW, Hammer RP, Jr, Rowlett JK, Bardo MT. Serotonin, but not dopamine, metabolites are increased in selected brain regions of subordinate male rats in a colony environment. Brain Res. 1991;568:61–66. doi: 10.1016/0006-8993(91)91379-f. [DOI] [PubMed] [Google Scholar]
- Blier P, de Montigny C. Serotonin and drug-induced therapeutic responses in major depression, obsessive compulsive and panic disorders. Neuropsychopharmacol. 1999;21(2S):91–98. doi: 10.1016/S0893-133X(99)00036-6. [DOI] [PubMed] [Google Scholar]
- Bloch RG, Dooneief AS, Buchberg AS, Spellman S. The clinical effect of isoniazid and iproniazid in the treatment of pulmonary tuberculosis. Ann Int Med. 1954;40:881–900. doi: 10.7326/0003-4819-40-5-881. [DOI] [PubMed] [Google Scholar]
- Bloch G, Simon T, Robinson GE, Hefetz A. Brain biogenic amines and reproductive dominance in bumble bees (Bombus terrestris) J Comp Physiol A. 2000;186:261–268. doi: 10.1007/s003590050426. [DOI] [PubMed] [Google Scholar]
- Bovbjerg RV. Dominance order in the crayfish Orconectes virilis (Hagen) Physiol Zool. 1953;26:127–136. [Google Scholar]
- Bradwejn J, Koszycki D. Imipriamine antagonism of the panogenic effects of cholecystokinin tetrapeptide in panic disorder patients. Am J Psychiat. 1991;151:261–263. doi: 10.1176/ajp.151.2.261. [DOI] [PubMed] [Google Scholar]
- Bruski CA, Dunham DW. The importance of vision in agonistic communication of crayfish Orconectes rusticus: an analysis of bout dynamics. Behaviour. 1987;63:83–107. [Google Scholar]
- Cases O, Seif I, Grimsby J, Gaspar P, Chen K, Pournin S, Muller U, Aguet M, Babinet C, Shih JC, De Maeyer E. Aggressive behavior and altered amounts of brain serotonin and norepinephrine in mice lacking MAOA. Science. 1995;268:1763–1766. doi: 10.1126/science.7792602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Rainnie D, Greene RW, Tonegawa S. Abnormal fear response and aggressive behavior in mutant mice deficient for alpha-calcium-calmodulin kinase II. Science. 1994;266:291–294. doi: 10.1126/science.7939668. [DOI] [PubMed] [Google Scholar]
- Cooper RL, Chase RJ, Tabor J. Altered responsiveness to 5-HT at the crayfish neuromuscular junction due to chronic-p-CPA and m-CPP treatment. Brain Res. 2001;916:143–151. doi: 10.1016/s0006-8993(01)02885-2. [DOI] [PubMed] [Google Scholar]
- Doernberg SB, Cromarty SI, Heinrich R, Beltz BS, Kravitz EA. Agonistic behavior in naive juvenile lobsters depleted of serotonin by 5,7-dihydroxytryptamine. J Comp Physiol A. 2001;187:91–103. doi: 10.1007/s003590000178. [DOI] [PubMed] [Google Scholar]
- Duman RS, Heninger GR, Nestler EJ. A molecular and cellular theory of depression. Arch Gen Psychiat. 1997;54:597–606. doi: 10.1001/archpsyc.1997.01830190015002. [DOI] [PubMed] [Google Scholar]
- Dyakonova VE, Schürmann FW. Effects of serotonergic and opiodergic drugs on escape behaviors and social status of male crickets. Naturwissenschaften. 1999;86:435–437. doi: 10.1007/s001140050647. [DOI] [PubMed] [Google Scholar]
- Edwards DH, Kravitz EA. Serotonin, social status and aggression. Current Opin Neurobiol. 1997;7:812–817. doi: 10.1016/s0959-4388(97)80140-7. [DOI] [PubMed] [Google Scholar]
- Edwards DH, Heitler WJ, Krasne FB. Fifty years of a command neuron: the neurobiology of escape behavior in the crayfish. Trend Neurosci. 1999;22:153–160. doi: 10.1016/s0166-2236(98)01340-x. [DOI] [PubMed] [Google Scholar]
- Elofsson UO, Mayer I, Damsgård B, Winberg S. Intermale competition in sexually mature arctic charr: effects on brain monoamines, endocrine stress responses, sex hormone levels, and behavior. Gen Comp Endocrinol. 2000;118:450–460. doi: 10.1006/gcen.2000.7487. [DOI] [PubMed] [Google Scholar]
- Enquist M, Leimar O. Evolution of fighting behaviour: decision rules and assessment of relative strength. J Theor Biol. 1983;102:387–410. [Google Scholar]
- Evans BP, Stoffolano JG, Yin CM, Meyer JS. The effects of injection of amphetamine on female insemination in the black blow fly Phormia regina (Diptera Calliphoridae) Physiol Entomol. 1998;23:20–24. [Google Scholar]
- Ferris CF. Adolescent stress and neural plasticity in hamsters: a vasopressin-serotonin model of inappropriate aggressive behaviour. Exp Physiol. 2000;85:85S–90S. doi: 10.1111/j.1469-445x.2000.tb00011.x. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Delville Y. Vasopressin and serotonin interactions in the control of agonistic behavior. Psychoneuroendocrinol. 1994;19:593–601. doi: 10.1016/0306-4530(94)90043-4. [DOI] [PubMed] [Google Scholar]
- Fickbohm DJ, Spitzer N, Katz PS. Serotonin homeostasis in the brain of Tritonia Diomedea. Soc Neurosci Abstr. 2000;26:343. 5. [Google Scholar]
- Flugge G, Ahrens O, Fuchs E. Monoamine receptors in the prefrontal cortex of Tupaia belangeri during chronic psychosocial stress. Cell Tiss Res. 1997;288:1–10. doi: 10.1007/s004410050787. [DOI] [PubMed] [Google Scholar]
- Fuller RW. The influence of fluoxetine on aggressive behavior. Neuropsychopharmacology. 1996;14:77–81. doi: 10.1016/0893-133X(95)00110-Y. [DOI] [PubMed] [Google Scholar]
- Gingrich JA, Hen R. The broken mouse: the role of development, plasticity and environment in the interpretation of phenotypic changes in knockout mice. Curr Opin Neurbiol. 2000;10:46–152. doi: 10.1016/s0959-4388(99)00061-6. [DOI] [PubMed] [Google Scholar]
- Glanzman DL, Krasne FB. Serotonin and octopamine have opposite modulatory effects on the crayfish's lateral giant escape reaction. J Neurosci. 1983;3:2263–2269. doi: 10.1523/JNEUROSCI.03-11-02263.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goessmann C, Hemelrijk C, Huber R. The formation and maintenance of crayfish hierarchies: behavioral and self-structuring properties. Behav Ecol Sociobiol. 2000;48:418–428. [Google Scholar]
- Hall FS, Devries AC, Fong GW, Huang S, Pert A. Effects of 5,7-dihydroxytryptamine depletion of tissue serotonin levels on extracellular serotonin in the striatum assessed with in vivo microdialysis: relationship to behavior. Synapse. 1999;33:16–25. doi: 10.1002/(SICI)1098-2396(199907)33:1<16::AID-SYN2>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Harris-Warrick R, Kravitz EA. Cellular mechanisms for modulation of posture by octopamine and serotonin in the lobster. J Neurosci. 1984;4:1976–1993. doi: 10.1523/JNEUROSCI.04-08-01976.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich R, Cromarty SI, Horner M, Edwards DH, Kravitz EA. Autoinhibition of serotonin cells: an intrinsic regulatory mechanism sensitive to the pattern of usage of the cells. Proc Natl Acad Sci USA. 1999;96:2473–2478. doi: 10.1073/pnas.96.5.2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz CA, Zangerl AR, Berenbaum MR. Effects of natural and synthetic neuroactive substances on the growth and feeding of cabbage looper, Trichoplusia ni. Entomol Exp App. 1996;80:443–451. [Google Scholar]
- Hen R. Structural and functional conservation of serotonin receptors throughout evolution. EXS. 1993;63:266–278. doi: 10.1007/978-3-0348-7265-2_14. [DOI] [PubMed] [Google Scholar]
- Higley JD, Mehlman PT, Taub DM, Higley SB, Suomi SJ, Linnoila M, Vickers JH. Cerebrospinal fluid monoamine and adrenal correlates of aggression in free-ranging rhesus monkeys. Arch Gen Psychiat. 1989;49:436–441. doi: 10.1001/archpsyc.1992.01820060016002. [DOI] [PubMed] [Google Scholar]
- Higley JD, King ST, Jr, Hasert MF, Champoux M, Suomi SJ, Linnoila M. Stability of interindividual differences in serotonin function and its relationship to serve aggression and competent social behavior in rhesus macaque females. Neuropsychopharmacology. 1996;14:67–76. doi: 10.1016/S0893-133X(96)80060-1. [DOI] [PubMed] [Google Scholar]
- Huber R, Delago A. Serotonin alters decisions to withdraw in fighting crayfish, Astacus astacus: the motivational concept revisited. J Comp Physiol A. 1998;182:573–583. [Google Scholar]
- Huber R, Kravitz EA. A quantitative analysis of agonistic behavior in juvenile american lobsters (Homarus americanus) Brain Behav Evol. 1995;46:72–83. doi: 10.1159/000113260. [DOI] [PubMed] [Google Scholar]
- Huber R, Smith K, Delago A, Isaksson K, Kravitz EA. Serotonin and aggressive motivation in crustaceans: altering the decision to retreat. Proc Nat Acad Sci USA. 1997a;94:5939–5942. doi: 10.1073/pnas.94.11.5939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber R, Orzeszyna M, Pokorny N, Kravitz EA. Biogenic amines and aggression: experimental approaches in crustaceans. Brain Behav Evol. 1997b;50(Suppl 1):60–68. doi: 10.1159/000113355. [DOI] [PubMed] [Google Scholar]
- Huber R, Panksepp JB, Yue X, Delago A, Moore P. Dynamic interactions of behavior and amine neurochemistry in acquisition and maintenance of social rank in crayfish. Brain Behav Evol. 2001a;57:271–282. doi: 10.1159/000047245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber R, Daws A, Tuttle S, Panksepp JB. Quantitative behavioral techniques for the study of crustacean aggression. In: Wiese K, Schmidt M, editors. Physiology of the crustacean nervous system. Berlin: Springer; 2001b. pp. 30–45. [Google Scholar]
- Issa FA, Adamson DJ, Edwards DH. Dominance hierarchy formation in juvenile crayfish Procambarus clarkii. J Exp Biol. 1999;202:3497–3506. doi: 10.1242/jeb.202.24.3497. [DOI] [PubMed] [Google Scholar]
- Ison M, Fachinelli C, Rodríguez ELE. Effect of the ICV injection of 5,7-di-hydroxytryptamine on the aggressive behavior of dominant and submissive pigeons (Columba livia) Pharmacol Biochem Behav. 1996;53:951–955. doi: 10.1016/0091-3057(95)02005-5. [DOI] [PubMed] [Google Scholar]
- Iversen LL, Kravitz EA, Otsuka M. Release of gamma-aminobutyric acid (GABA) from lobster inhibitory neurones. J Physiol. 1967;188:21P–22P. [PubMed] [Google Scholar]
- Jacobs BL, Fornal CA. Activity of serotonergic neurons in behaving animals. Neuropsychopharmacology. 1999;21:9S–15S. doi: 10.1016/S0893-133X(99)00012-3. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, van Prague H, Gage FH. Adult brain neurogenesis and psychiatry: a novel theory of depression. Mol Psychiat. 2000;5:262–269. doi: 10.1038/sj.mp.4000712. [DOI] [PubMed] [Google Scholar]
- Kalsner S. The question of feedback at the somadendritic region and antidepressant drug action. Brain Res Bull. 2000;52:467–473. doi: 10.1016/s0361-9230(00)00289-6. [DOI] [PubMed] [Google Scholar]
- Korzan WJ, Summers TR, Summers CH. Monoaminergic activities of limbic regions are elevated during aggression: influence of sympathetic social signalling. Brain Res. 2000;970:170–178. doi: 10.1016/s0006-8993(00)02420-3. [DOI] [PubMed] [Google Scholar]
- Kostowski W, Markowska L, Markiewicz L. On the role of serotonin in aggressive behaviour of ants Genus formica. J Pharmacol Pharmacy (Poland) 1975;27:237–239. [PubMed] [Google Scholar]
- Krasne FB, Shamsian A, Kulkarni R. Altered excitability of the crayfish lateral giant escape reflex during agonistic encounters. J Neurosci. 1997;17(2):709–716. doi: 10.1523/JNEUROSCI.17-02-00709.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz EA. Hormonal control of behavior amines and the biasing of behavioral output in lobsters. Science. 1988;241:1775–1780. doi: 10.1126/science.2902685. [DOI] [PubMed] [Google Scholar]
- Kravitz EA. Serotonin and aggression insights gained from a lobster model system and speculations on the role of amine neurons in a complex behavior. J Comp Physiol A. 2000;186:221–238. doi: 10.1007/s003590050423. [DOI] [PubMed] [Google Scholar]
- Kroeze WK, Roth BL. The molecular biology of serotonin receptors: therapeutic implications for the interface of mood and psychosis. Biol Psychiat. 1998;44:1128–1142. doi: 10.1016/s0006-3223(98)00132-2. [DOI] [PubMed] [Google Scholar]
- Kudryavtseva NN, Avgustinovich DF. Behavioral and physiological markers of experimental depression induced by social conflicts (DISC) Aggress Behav. 1998;24:271–286. [Google Scholar]
- Kuhn R. The treatment of depressive states with G22355 (imipramine hydrochloride) Am J Psychiat. 1958;115:459–464. doi: 10.1176/ajp.115.5.459. [DOI] [PubMed] [Google Scholar]
- Larson ET, Summers CH. Serotonin reverses dominant social status. Behav Brain Res. 2001;121:95–102. doi: 10.1016/s0166-4328(00)00393-4. [DOI] [PubMed] [Google Scholar]
- Lederhendler I, Shulkin J. Behavioral neuroscience: challenges for the era of molecular biology. Trends Neurosci. 2000;23:451–454. doi: 10.1016/s0166-2236(00)01636-2. [DOI] [PubMed] [Google Scholar]
- Lent CM. Quantitative effects of a neurotoxin upon serotonin levels within tissue compartments of the medicinal leech. J Neurobiol. 1984;15:309–323. doi: 10.1002/neu.480150502. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Merschdorf U. Impulsivity aggression and serotonin a molecular psychobiological perspective. Behav Sci Law. 2000;18:581–604. doi: 10.1002/1099-0798(200010)18:5<581::aid-bsl411>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Lidov HGW, Molliver ME. Immunocytochemical study of the development of serotonergic neurons in the rat CNS. Brain Res Bull. 1982;9:559–604. doi: 10.1016/0361-9230(82)90164-2. [DOI] [PubMed] [Google Scholar]
- Livingstone MS, Schaeffer SF, Kravitz EA. Biochemistry and ultrastructure serotonin nerve endings in the lobster: serotonin and octopamine are contained in different nerve endings. J Neurobiol. 1981;12:27–54. doi: 10.1002/neu.480120104. [DOI] [PubMed] [Google Scholar]
- Lucki I. The spectrum of behaviors influenced by serotonin. Biol Psychiat. 1998;44:151–165. doi: 10.1016/s0006-3223(98)00139-5. [DOI] [PubMed] [Google Scholar]
- Ma P, Beltz B, Kravitz EA. Serotonin-containing neurons in lobsters: their role as gain-setters in postural control mechanisms. J Neurophysiol. 1992;68:36–53. doi: 10.1152/jn.1992.68.1.36. [DOI] [PubMed] [Google Scholar]
- Maler L, Ellis WG. Inter-male aggressive signals in weakly electric fish are modulated by monoamines. Behav Brain Res. 1987;25:75–81. doi: 10.1016/0166-4328(87)90046-5. [DOI] [PubMed] [Google Scholar]
- Matter JM, Ronan PJ, Summers CH. Central monoamines in free-ranging lizards: differences associated with social roles and territoriality. Brain Behav Evol. 1998;51:23–32. doi: 10.1159/000006526. [DOI] [PubMed] [Google Scholar]
- Maynard-Smith J, Price GR. The logic of animal conflict. Nature. 1973;246:15–18. [Google Scholar]
- McKittrick CR, Blanchard DC, Blanchard RJ, McEwen BS, Sakai RR. Serotonin receptor binding in a colony model of chronic social stress. Biol Psychiat. 1995;37:383–393. doi: 10.1016/0006-3223(94)00152-s. [DOI] [PubMed] [Google Scholar]
- McKittrick CR, Magarinos M, Blanchard DC, Blanchard RJ, McEwen BS, Sakai RR. Chronic social stress reduces dendritic arbors in CA3 of hippocampus and decreases binding to serotonin transporter sites. Synapse. 2000;36:85–94. doi: 10.1002/(SICI)1098-2396(200005)36:2<85::AID-SYN1>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Effects of adverse experience for brain structure and function. Biol Psychiat. 2000;48:721–731. doi: 10.1016/s0006-3223(00)00964-1. [DOI] [PubMed] [Google Scholar]
- Meguid MM, Fetissov SO, Varma M, Sato T, Zhang L, Laviano A, Rossi-Fanelli F. Hypothalamic dopamine and serotonin in the regulation of food intake. Nutrition. 2000;16:843–857. doi: 10.1016/s0899-9007(00)00449-4. [DOI] [PubMed] [Google Scholar]
- Meston CM, Frohlich PF. The neurobiology of sexual function. Arch Gen Psychiat. 2000;57:1012–1030. doi: 10.1001/archpsyc.57.11.1012. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Antidepressant treatments in the 21st century. Biol Psychiat. 1998;44:526–533. doi: 10.1016/s0006-3223(98)00095-x. [DOI] [PubMed] [Google Scholar]
- Olivier B, Mos J, van der Heyden J, Hartog J. Serotonergic modulation of social interactions in isolated male mice. Psychopharmacology. 1989;97:154–156. doi: 10.1007/BF00442239. [DOI] [PubMed] [Google Scholar]
- Overli O, Harris CA, Winberg S. Short-term effects of fights for social dominance and the establishment of dominant-subordinate relationships on brain monoamines and cortisol in rainbow trout. Brain Behav Evol. 1999;54:263–275. doi: 10.1159/000006627. [DOI] [PubMed] [Google Scholar]
- Pan Y, Gembom E, Peng W, Lesch KP, Mossner R, Simantov R. Plasticity in serotonin uptake in primary neuronal cultures of serotonin transporter knockout mice. Dev Brain Res. 2001;126:125–129. doi: 10.1016/s0165-3806(00)00145-0. [DOI] [PubMed] [Google Scholar]
- Panksepp JB, Huber R. Chronic alterations in serotonin function dynamic neurochemical properties in agonistic behavior of the crayfish Orconectes rusticus. J Neurobiol. 2002;50:276–290. doi: 10.1002/neu.10035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker GA, Rubenstein DI. Role assessment, reserve strategy, and the acquisition of information in asymmetric animal conflicts. Anim Behav. 1981;29:221–240. [Google Scholar]
- Patel TD, Azmitia EC, Zhou FC. Increased 5-HT 1A receptor immunoreactivity in the rat hippocampus following 5, 7 DHT lesions in the cingulum bundle and fimbriafornix. Behav Brain Res. 1996;73:319–323. doi: 10.1016/0166-4328(96)00122-2. [DOI] [PubMed] [Google Scholar]
- Peeke HVS, Blank GS, Figler MH, Chang ES. Effects of exogenous serotonin on a motor behavior and shelter competition in juvenile lobsters (Homarus americanus) J Comp Physiol A. 2000;186:575–582. doi: 10.1007/s003590000113. [DOI] [PubMed] [Google Scholar]
- Ranta E, Lindström K. Power to hold sheltering burrows by juveniles of the signal crayfish, Pasifastacus leniusculus. Ethology. 1992;92:217–226. [Google Scholar]
- Ranta E, Lindström K. Body-size and shelter possession in mature signal crayfish, Pacifastacus leniusculus. Ann Zool Fennici. 1993;30:125–132. [Google Scholar]
- Raleigh MJ, McGuire MT, Brammer GL, Pollack DB, Yuwiler A. Serotonergic mechanisms promote dominance acquisition in adult male vervet monkeys. Brain Res. 1991;559:181–190. doi: 10.1016/0006-8993(91)90001-c. [DOI] [PubMed] [Google Scholar]
- Real D, Czternasty G. Mapping of serotonin like immunoreactivity in the ventral nerve cord of crayfish. Brain Res. 1990;521:203–212. doi: 10.1016/0006-8993(90)91544-q. [DOI] [PubMed] [Google Scholar]
- Reisner IR, Mann JJ, Stanley M, Huang YY, Houpt KA. Comparison of cerebrospinal fluid monoamine metabolite levels in dominant-aggressive and non-aggressive dogs. Brain Res. 1996;714:57–64. doi: 10.1016/0006-8993(95)01464-0. [DOI] [PubMed] [Google Scholar]
- Rilke O, Freier D, Jähkel M, Oehler J. Dynamic alterations of serotonergic metabolism and receptors during social isolation of low- and high-active mice. Pharmacol Biochem Behav. 1998;59:891–896. doi: 10.1016/s0091-3057(97)00509-1. [DOI] [PubMed] [Google Scholar]
- Rossby SP, Nalepa I, Huang M, Perrin C, Burt AM, Schmidt DE, Gillespie, Sulser F. Norepinephrine-independent regulation of GRII mRNA in vivo by a tricyclic antidepressant. Brain Res. 1995;687:79–82. doi: 10.1016/0006-8993(95)00459-4. [DOI] [PubMed] [Google Scholar]
- Rozenboim I, Kapowska E, Robinzon B, Uni Z. Effects of fenfluramine on body weight, feed intake, and reproductive activities of broiler breeder hens. Poultry Sci. 1999;78:1768–1772. doi: 10.1093/ps/78.12.1768. [DOI] [PubMed] [Google Scholar]
- Rubenstein DI, Hazlett B. Examination of the agonistic behaviour of the crayfish Orconectes virilis by character analysis. Behaviour. 1973;20:193–216. [Google Scholar]
- Saudou F, Amara DA, Dierich A, LeMeur M, Ramboz S, Segu L, Buhot MC, Hen R. Enhanced aggressive behavior in mice lacing 5-HT 1B receptor. Science. 1994;265:1875–1878. doi: 10.1126/science.8091214. [DOI] [PubMed] [Google Scholar]
- Schenk S, Larcelle G, Gorman K, Amit Z. Cocaine self-administration in rats influenced by environmental conditions: implications for the etiology of drug abuse. Neurosci Lett. 1987;81:227–231. doi: 10.1016/0304-3940(87)91003-2. [DOI] [PubMed] [Google Scholar]
- Silva RCB, Brandão ML. Acute and chronic effects of gepirone and fluoxetine in rats tested in the elevated plus-maze: an ethological analysis. Pharmacol Biochem Behav. 2000;65:209–216. doi: 10.1016/s0091-3057(99)00193-8. [DOI] [PubMed] [Google Scholar]
- Sivam SP. GBR-12909-induced self-injurious behavior: role of dopamine. Brain Res. 1995;690:259–263. doi: 10.1016/0006-8993(95)00604-o. [DOI] [PubMed] [Google Scholar]
- Sivam SP. Dopamine, serotonin and tachykinin in self-injurious behavior. Life Sci. 1996;58(26):2367–2375. doi: 10.1016/0024-3205(96)00121-x. [DOI] [PubMed] [Google Scholar]
- Smith TD, Kuczenski R, George-Friedman K, Malley JD, Foote SL. In vivo microdialysis assessment of extracellular serotonin and dopamine levels in awake monkeys during sustained fluoxetine administration. Synapse. 2000;38:460–470. doi: 10.1002/1098-2396(20001215)38:4<460::AID-SYN11>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Sneddon LU, Taylor AC, Huntingford FA, Watson DG. Agonistic behaviour and biogenic amines in shore crabs Carcinus maenas. J Exp Biol. 2000;230:537–545. doi: 10.1242/jeb.203.3.537. [DOI] [PubMed] [Google Scholar]
- Stachowiak MK, Stricker LM, Jacoby JH, Zigmond MJ. Increased tryptophan hydroxylase activity in serotonergic nerve terminals spared by 5,7-dihydroxytryptamine. Biochem Pharmacol. 1986;35:1241–1248. doi: 10.1016/0006-2952(86)90266-2. [DOI] [PubMed] [Google Scholar]
- Stewart TW, Haynes JM. Benthic macroinvertebrate communities of southwestern Lake Ontario following invasion of Dreissena. J Great Lakes Res. 1994;20:479–493. [Google Scholar]
- Stocker AM, Huber R. Fighting strategies in crayfish Orconectes rusticus (Decapoda, Cambaridae) differ with hunger state and the presence of food cues. Ethology. 2001;107:727–736. [Google Scholar]
- Summers TR, Hunter AL, Summers CH. Female social reproductive roles affect central monoamines. Brain Res. 1997;767:272–278. doi: 10.1016/s0006-8993(97)00604-5. [DOI] [PubMed] [Google Scholar]
- Taraskevich PS. Reversal potentials of L-glutamate and the excitatory transmitter at the neuromuscular junction of the crayfish. Biophysica Acta. 1971;241:700–703. doi: 10.1016/0005-2736(71)90071-x. [DOI] [PubMed] [Google Scholar]
- Teshiba T, Shamsian A, Yashar B, Yeh SR, Edwards DH, Krasne FB. Dual and opposing modulatory effects of serotonin on crayfish lateral and giant escape command neurons. J Neurosci. 2001;21:4523–4529. doi: 10.1523/JNEUROSCI.21-12-04523.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tierney AJ. Effects of serotonin receptor agonists on posture and aggressive behavior in crayfish. Soc Neurosci Abstr. 2000;26:657. 11. [Google Scholar]
- Tierney AJ. Structure and function of invertebrate 5-HT receptors: a review. Comp Biochem Physiol A. 2001;128:791–804. doi: 10.1016/s1095-6433(00)00320-2. [DOI] [PubMed] [Google Scholar]
- Uphouse L. Female gonadal hormones, serotonin, and sexual receptivity. Brain Res Rev. 2000;33:242–257. doi: 10.1016/s0165-0173(00)00032-1. [DOI] [PubMed] [Google Scholar]
- Wang RY, Aghajanian GK. Antidromically identified serotonergic neurons in the rat midbrain raphe: evidence for collateral inhibition. Brain Res. 1977;132:186–93. doi: 10.1016/0006-8993(77)90719-3. [DOI] [PubMed] [Google Scholar]
- Weiger WA. Serotonergic modulation of behaviour: a phylogenetic overview. Biol Rev Camb Phil Soc. 1997;72:61–95. doi: 10.1017/s0006323196004975. [DOI] [PubMed] [Google Scholar]
- Winberg S, Nilsson GE, Olsen KH. Social rank and brain levels of monoamines and monoamine metabolites in Arctic charr, Salvelinus alpinus (L.) J Comp Physiol A. 1991;168:241–246. [Google Scholar]
- Winberg S, Nilsson GE. Time-course of changes in brain serotonergic activity and brain tryptophan levels in dominant and subordinate juvenile arctic charr. J Exp Biol. 1993;179:181–195. [Google Scholar]
- Winberg S, Carter CG, McCarthy ID, He ZY, Nilsson GE, Houlihan DF. Feeding rank and brain serotonergic activity in rainbow trout Onocorhynchus mykiss. J Exp Biol. 1993;179:197–211. [Google Scholar]
- Woo CC, Wilson DA, Sullivan RM, Leon M. Early locus coruleus lesions increase the density of B adernergic receptors in the rat main olfactory bulb of rats. Int J Dev Neurosci. 1996;14:913–919. doi: 10.1016/s0736-5748(96)00041-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadid G, Nakash R, Deri I, Tamar G, Kinor N, Gispan I, Zangen A. Elucidation of the neurobiology of depression insights from a novel genetic animal model. Prog Neurobiol. 2000;62:353–378. doi: 10.1016/s0301-0082(00)00018-6. [DOI] [PubMed] [Google Scholar]
- Yeh SR, Fricke RA, Edwards DH. The effect of social experience on serotonergic modulation of the escape circuit of crayfish. Science. 1996;271:366–369. doi: 10.1126/science.271.5247.366. [DOI] [PubMed] [Google Scholar]
- Yeh SR, Musolf BE, Edwards DH. Neuronal adaptations to changes in the social dominance status of crayfish. J Neurosci. 1997;17:697–708. doi: 10.1523/JNEUROSCI.17-02-00697.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]