Abstract
This review summarizes a set of experimental approaches with which we explore fighting behavior in crayfish and the importance of aminergic systems in its control. Our results illustrate that agonistic behavior in crustaceans can be characterized within a quantitative framework, that different types of behavioral plasticity in aggressive behavior are in need of physiological explanation, and that pharmacological intervention involving serotonergic systems produces characteristic changes in fighting. Moreover, we attempt to identify changes in neurochemistry during the acquisition of social status. Many of the studies presented here summarize ongoing work. Nonetheless, results to date complement and extend previous detailed physiological, morphological and biochemical studies exploring the roles of amines in aggression.
Keywords: Neurochemistry, Aggression, Crustacea, Serotonin (5-HT), Selective serotonin reuptake inhibitor (SSRI)
Introduction
The depths of northern, aquatic, cold-temperate environments are ruled by well-armed and well-armored crustaceans. Clawed decapods, such as crayfish, lobsters and their close phylogenetic kin, are arguably the most successful predators and scavengers of benthic habitats, with weights from a few hundred grams (crayfish) up to 20+ kg (lobsters), and occurring in very high numbers under suitable conditions. For instance, Orconectes rusticus, the species of crayfish studied here, reach densities well in excess of 20 individuals/m2 in Lake Ontario [Stewart and Haynes, 1994]. Even in lobsters, where numbers have declined in recent decades due to intensive fishing, historical records tell a story of plenty. Referred to as a ‘poor man’s dinner’ they were gathered in large numbers at low tide [Wood, 1635] and individuals weighing around 5 kg were common [Gould, 1841].
Despite their numbers in a given area, these animals lead a solitary existence, occupy shelters singly, and most meetings are agonistic in nature. So, aside from mating behavior in some species [Atema, 1986] and pair formation in others [Seibt, 1974], clawed decapods remain as originally characterized ‘a solitary, aggressive animal in its natural habitat’ [Herrick, 1909]. It is likely that the existence of many close neighbors plays an integral role in crayfish biology as individuals frequently encounter conspecifics during their daily forays – we regularly observe agonistic encounters as well as matings between individual O. rusticus who roam the stream bottom during the day at our collecting sites on the Portage River near Bowling Green, Ohio, USA.
With conspicuous, potentially lethal claws [Marden, 1973], the resolution of intraspecific conflict in this group demands a measured strategy. In close accordance with predictions of game theory models of fighting, behaviors during agonistic encounters feature a number of different stereotyped patterns, proceed with an escalating sequence of intensities, and end when one of the combatants withdraws. Shelters play a central role in crayfish [Capelli and Hamilton, 1984; Ranta and Lindstrom, 1993] and lobster biology [Karnofsky et al., 1989; Figler et al., 1998], providing protection from predation as well as aiding in access to food and mates [Hyatt, 1983; Atema, 1986]. Protective shelter is of particular importance for early juveniles, where attacks by predators are common [Lavalli and Barshaw, 1986; Barshaw and Lavalli, 1988]. Fighting behavior in our seminatural observation aquaria (fig. 1] occurs during acquisition or defense of a burrow in many instances, but also ensues when individuals encounter each other during regular forays away from cover. Given an arena size and features designed using appropriate ethological considerations [Goessmann et al., 2000; Huber et al., 2001], encounters also readily occur with similar characteristics in a simplified scenario and lacking any obvious resource, suggesting an inherent disposition for agonism towards conspecific opponents.
Fig. 1.
Two male crayfish (Orconectes rusticus) face each other during an agonistic interaction in our 600 l seminatural aquarium. Individuals appear to test each other’s ability for physical superiority in a series of stereotyped, behavioral maneuvers. Unrestrained use of the claws only occurs in rare occasions and is largely limited to the later stages of an encounter.
In a typical scenario, intensity of fighting increases in step-wise fashion beginning with threat displays upon first contact, followed by phases of ritualized aggression, restrained use of claws, and in rare instances ending in brief periods of unbridled combat [Bruski and Dunham, 1987; Huber and Kravitz, 1995; Huber et al., 2001]. The presence of such a structured behavioral system, combined with an opportunity to bring the analysis to the level of individual neurons, thus offers us unique opportunities for exploring fundamental issues of interactions between aggression, dominance, and amine neurochemistry in this group. Work in our laboratories has employed interdisciplinary approaches to explore a variety of issues in aggression, ranging from self-structuring properties in hierarchy formation to a search for its underlying neurochemical mechanisms. In close collaborations with the labs of Edward A. Kravitz (Harvard Medical School, Boston, Mass., USA) and Barbara Beltz (Wellesley College, Wellesley, Mass., USA) four objectives have guided our research: (1) we have developed a quantitative behavioral framework for fighting behavior in crayfish; (2) characterized behavioral sources of variation in need of physiological explanation; (3) examined the behavioral consequences of pharmacological amine manipulations, and (4) attempted to identify changes in neurochemistry that accompany behavioral differences of social status.
A Behavioral Framework for the Study of Crayfish Fighting
Dyadic conflict among crayfish features behavior that is distinctly structured [Bovbjerg, 1953], utilizes a number of stereotyped behavior patterns, and progresses according to a strict set of rules [Huber and Kravitz, 1995; Huber and Delago, 1998]. The early stages of crayfish encounters commonly include threat displays and ritualized aggressive acts. In a typical scenario encounters then continue with behaviors where claws are used in restrained fashion. If at that point fights have remained inconclusive (i.e. both individuals are still contesting the outcome), then the conflict may escalate to brief episodes of unrestrained use of the claws. The fight ends with the withdrawal of one opponent. The winner continues to initiate further bouts until the subordinate consistently retreats from the advances of the dominant. Behavioral ecological considerations provide a useful theoretical framework for an understanding of such behavior [Bovbjerg, 1956]. Game theory models of fighting [Parker and Rubenstein, 1981; Enquist and Leimar, 1983; Leimar and Enquist, 1984] predict that species equipped with damaging weapons will match characteristics predictive of eventual success in stepwise fashion and with escalating intensities. During this process individuals of similar strength acquire increasingly detailed information concerning the opponent’s strength and fighting ability at a reduced risk [Dingle, 1983; Smith and Dunham, 1990, 1996; Rutherford et al., 1996]. By restricting injurious use of claws to cases of close matches in essential variables, this effectively reduces an individual’s potential for sustaining physical damage. Assessment strategies thus predict that duration and progress of a fight is contingent upon a series of individual ‘decisions’, namely, whether an individual initiates an encounter, escalates to a higher intensity, retaliates if an opponent escalates, or retreats from further fighting. Consistent with its use in behavioral ecology, the term ‘decision’ is not meant to imply the operation of cognitive mechanisms. With success reinforced through evolutionary processes, it refers rather to the fitness advantage that a particular strategy confers upon its bearer. Using such concepts in a descriptive sense, decisions of individuals can thus be characterized as the probability with which a particular strategy will occur in a given context. In essence our work focuses to a lesser degree on what the animal does at any particular moment, but rather it attempts to describe in what way an animal approaches a given situation [Huber et al., 2001]. Ultimately this approach is used to provide an estimate of the individual’s internal motivational state [Huber and Delago, 1998].
Paired dominance relationships form when the outcome of agonistic bouts creates a lasting polarity and subsequent social interactions become increasingly predictable [Francis, 1988; Drews, 1993; Guiasu and Dunham, 1997]. In crayfish, subordinates rarely engage dominants either due to a decrease in aggressive state, individual recognition of a proven superior opponent [Vannini and Gherardi, 1981], or, most likely, the detection of an opponent’s relative dominance status [Winston and Jacobson, 1978; Francis, 1988; Zulandt-Schneider et al., 2000]. Even in group situations, fighting consists mainly of paired encounters and interactions involving three or more individuals are rare. Repeated dyadic conflicts thus create a web of social relationships among members of a group [Bovbjerg, 1956; Lowe, 1956]. A hierarchy forms with self-organizing properties in (near) linear fashion [Vannini and Sardini, 1971; Atema and Cobb, 1980; Copp, 1986; Issa et al., 1999] with future success contingent upon previous outcomes [Goessmann et al., 2000] through winner/loser effects (e.g. Burk, 1979; Chase et al., 1994; Hollis et al., 1995; Daws et al., 2001].
Characterization of Behavioral Plasticity in Need of Physiological Explanation
Quantitative behavioral techniques summarized above allow us to characterize behavioral changes that result from a variety of contexts, such as winning or losing a single encounter, fighting at different levels of intensity, or occupying high social status for various lengths of time. When pairs or groups of individuals are placed into an aquarium, the number of agonistic challenges, their mean duration and maximum intensity reached, are initially high but then decreased steadily as a hierarchy develops among them [Copp, 1986; Issa et al., 1999]. Sex and relative differences in body- and claw size (i.e. asymmetries in excess of 10%) are important predictors of dominance [Evans and Shehadi-Moacdieh, 1988; Barki et al., 1992; Figler et al., 1995; Pavey and Fielder, 1996]. However, in meetings of similarly-sized individuals, early wins in one or several of these component agonistic bouts created a lasting polarity for future fighting [Issa et al., 1999]. Consistent with effects reported from a wide range of species [Beaugrand et al., 1991; Jackson, 1991; Dugatkin, 1997, Hsu and Wolf, 1999], crayfish who recently prevailed in an interaction subsequently demonstrated an increased likelihood to win again, even in encounters with unknown conspecifics. Similarly, crayfish who experienced a recent loss exhibited a decreased likelihood of acquiring further wins. Thus with a given social experience, crayfish can be conditioned towards dominant or subordinate status. For example, initial dominance relations can be reversed by repeatedly providing subordinates with wins against third parties and the previously dominant one with repeated losses [Daws et al., 2001]. In such ‘winner’ and ‘loser’ effects, the aggressive state of subordinates is lowered while that of dominant individuals increases [Francis, 1983; Beacham and Newman, 1987; Chase et al., 1994].
In groups, linear hierarchies emerged which became increasingly stable over time. Winning influenced subsequent fighting behavior at two distinct time scales. In the short term, recent winners became less likely to retreat, even though other characteristics of fighting, such as chances for initiating, escalating or retaliating, remained essentially unchanged. Secondly, individuals who had occupied dominant positions for days became increasingly likely to escalate to higher intensities early in the encounter. Both effects biased the outcome of future interactions such that winning enhanced further success and losing decreased an individual’s subsequent chances for dominance. Moreover, social conditioning as a result of previous wins or losses influenced both the dynamics of fighting behavior and the outcome of subsequent interactions on a timeframe of up to several days [Goessmann et al., 2000].
Characterization of the Effects of Amine Manipulations
Numerous physiological mechanisms have been implicated in the control of variation in fighting behavior. Despite differences in the particulars, serotonin is strongly implicated in a neuromodulatory role for aggression across a wide variety of invertebrate and vertebrate taxa, including humans [Brunner et al., 1993a, b; Chen et al., 1994; Saudou et al., 1994; Nielsen et al., 1994, 1995; Kravitz, 2000]. Support has emerged from a broad range of experimental approaches including correlative evidence [Mehlman et al., 1994, 1995; Blumensohn et al., 1995; Reisner et al., 1996], studies of family histories [Brunner et al., 1993a, b; Coccaro et al., 1994; Virkkunen et al., 1995], dietary or neurotoxic amine depletion [Vergnes et al., 1988; Giammanco et al., 1990; Cleare and Bond, 1995; Doernberg et al., 2001], receptor pharmacology [Bell and Hobson, 1994; Mühlenkamp et al., 1995; Olivier et al., 1995], application of re-uptake inhibitors [Olivier et al., 1989; Hilakivi-Clarke and Goldberg, 1993; Fuller, 1995, 1996], acute serotonin treatment [Puciowski et al., 1985; Maler and Ellis, 1987; Raleigh et al., 1991], and genetic knock-out studies [Saudou et al., 1994; Cases et al., 1995]. However, the nature of these relationships is not simple, and unraveling the precise role of the amine in aggressive behavior has proven particularly difficult. For example, disruption of monamine oxidase A (MAOA), an enzyme involved in biogenic amine inactivation, leads to a marked increase in aggression and violence in rats [Cases et al., 1995] and humans [Brunner et al., 1993a, b], although our current understanding of serotonergic systems would have predicted the opposite. However, aside from serotonin, MAOA also metabolizes dopamine and other amines involved in affect, raising the potential for complex interactions between different neuromodulatory systems. A major caveat in studying amine systems is the inherent flexibility, dynamic nature, and feedback properties within different behavioral and physiological contexts [Yeh et al., 1997; Beltz et al., 1998; Bradbury, 2000].
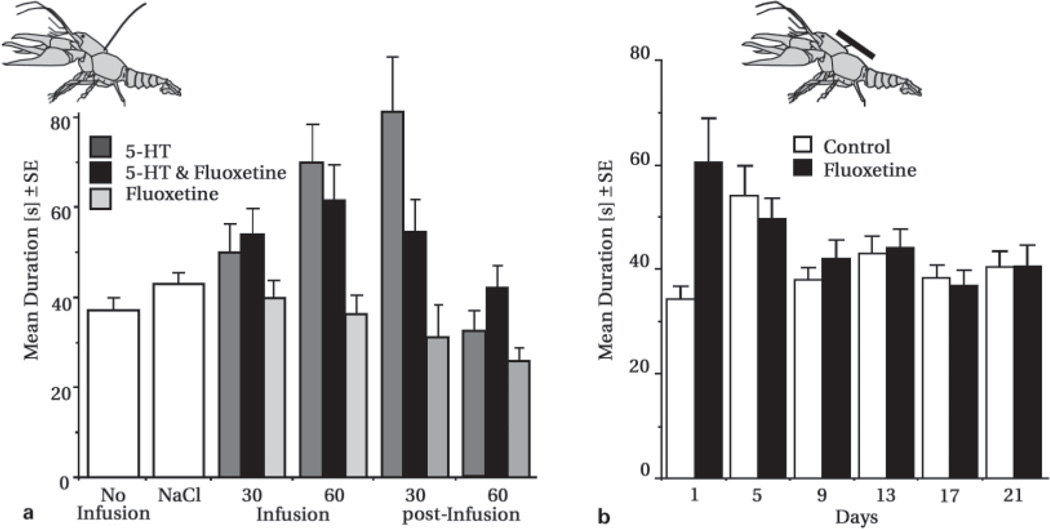
In lobsters, crayfish, and other decapod crustaceans, activation of serotonin systems is closely associated with aggressive or dominant behavior [Antonsen and Paul, 1997; Edwards and Kravitz, 1997; Kravitz, 2000; Sneddon et al., 2000; Tierney et al., 2000]. Such treatment altered decisions to retreat from opponents [Huber et al., 1997b; Huber and Delago, 1998], without changing the way fights were initiated, how they escalated, or their final outcome (fig. 2a]. Treated subordinates continued to re-engage their opponents in extended bouts of fighting, even in situations that carry a substantial risk of injury. The most robust behavioral effects of serotonin emerged with continued infusion into freely-moving crayfish via a fine-bore canula and syringe pump. Aggressive state increased slowly with a peak after over one hour [Huber et al., 1997a]. Behavioral effects resulting from single or repeated injections of amine pulses proved somewhat less salient – possibly because such experimental treatments require that the individual is at the same time captured, restrained, pierced with a needle, and injected with a large bolus of serotonin. This may affect a variety of neurochemical (e.g. stress) systems and thereby confound any effects associated with the intended treatment itself. Moreover, serotonin-mediated effects appear to require a loading of pre-synaptic terminals (see below) with persisting augmentation of basal levels. Single pulses are metabolized rapidly [Huber et al., 1997a] and may thus be less efficient in producing behavioral effects. In addition to any natural, underlying complexities, methodological differences are thus likely to contribute to the diverse range of behavioral effects that have been reported for amine manipulations in decapods (e.g. Peeke et al., 2000]. Continuously infusing lower peak levels of serotonin [Huber et al., 1997a, b; Huber and Delago, 1998] may be preferable as it offers a less intrusive method of treatment, and may thus be less prone to artifacts. Moreover, while single injections may suffice to measure how in the short term a substance affects instances of behavior such as wins/loses, continuous amine-infusion may provide a better means for studying behavioral phenomena at longer time scales. Serotonin-associated changes in behavior accompanying such treatment closely resemble key characteristics observed in individuals who have recently won an encounter [Goessmann et al., 2000], suggesting the existence of a potential link.
Fig. 2.
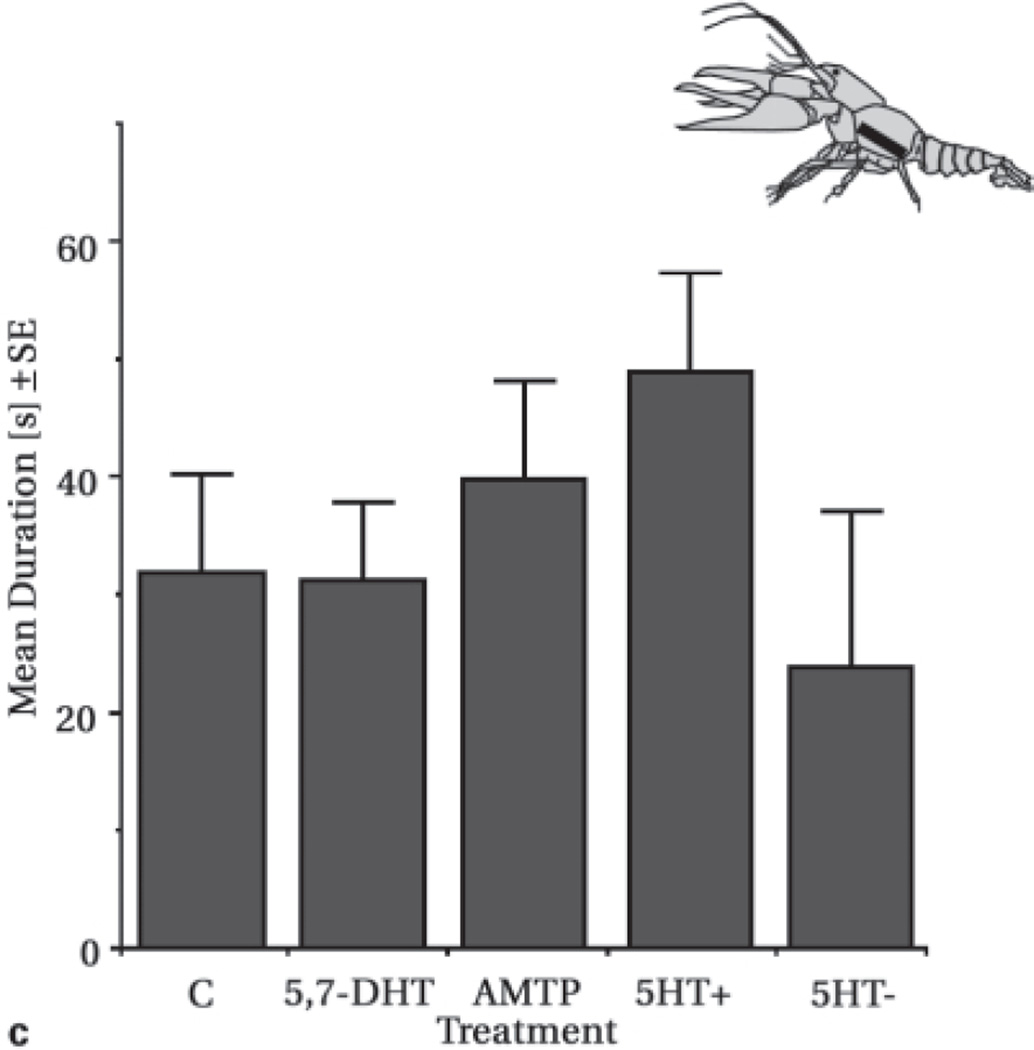
Composite figure illustrates differences in fight duration resulting from pharmacological manipulations of crayfish serotonin systems. a Fine-bore fused silica capillaries were used to infuse serotonin, fluoxetine or both substances together into freely-moving, subordinate animals (all infused at 3 µg/min). Serotonin infusion resulted in longer fighting that persisted well after the infusion pump was turned off (F(5,2888) = 14.777, p < 0.001). Multivariate statistical techniques (i.e. discriminant function analysis) revealed that longer bouts of fighting resulted from a decreased likelihood of retreat. Infusion of fluoxetine alone did not enhance aggression (F(5,2513) = 1.130, p = 0.342), however, co-infused with serotonin resulted in a pronounced reduction of serotonin fight-enhancing effects (F(5,2941) = 7.653, p < 0.001). b Chronic infusion of fluoxetine via osmotic minipumps increased duration of fighting during the early stages of treatment compared to animals receiving vehicle only ([treatment] F(1,2642) = 22.160, p < 0.001; [day] F(5,2642) = 2.9463, p = 0.018; [treatment*day] F(5,2642) = 3.724, p = 0.002). As with acute serotonin infusion, these differences in fighting were due to a decrease in the probability for retreat. c Duration of fighting in individuals which received chronic silastic implants containing either 5-HT synthesis inhibitors (5,7-dihydroxytryptamine or alpha-methyltryptamine) or serotonin at one of two different rates. No significant differences in fight duration existed among these groups (F(4,314) = 1.06, p = 0.374).
Additional, distinct changes in behavior have been identified within the context of social interactions (see section II above) and are in need of physiological explanations. Injection of octopamine (i.e. the phenol analogue of norepinephrine) into squat lobsters was accompanied by an increase in escape behavior [Antonsen and Paul, 1997]. A linkage between steroid hormones and changes in the rate of escalation seems likely. Studies reporting such effects have focused on the (steroid controlled) molt cycle [Tamm and Cobb, 1978, 1980; Steger and Caldwell, 1983]; correlated measures of aggressiveness with the profile of ecdysteroids such as 20-hydroxyecdysone [Baldaia et al., 1984; Graf and Delbecque, 1987; Snyder and Chang, 1991]; and increased aggression in stomatopods and lobsters by direct injection of ecdysone (Bollingbroke and Kass-Simon, 2001; Caldwell, pers. comm.).
In crayfish and lobsters serotonin is removed from the hemolymph within a few minutes, yet behavioral changes associated with amine infusions persist well beyond that. Observed changes in aggressive state may thus be due to one or several mechanisms [Huber et al., 1997a, b] including: (1) long lasting changes at post-synaptic sites located within decision-making centers of the nervous system; (2) increased release after ‘extra’ serotonin has accumulated in nerve terminals during the period when hemolymph serotonin levels were high; (3) the appearance of ‘behaviorally active’ serotonin metabolites in the hemolymph; or (4) other neuronal mechanisms. We have begun to explore the possible role of these mechanisms in agonistic decision making using pharmacological manipulations with (1) application of fluoxetine [Huber et al., 1997a; Huber and Delago, 1998, Delago et al., in review]; (2) neurochemical disruption using the serotonin synthesis inhibitor 5,7-dihydroxytryptamine [Benton et al., 1997; Panksepp and Huber, in review]; and (3) chronic infusion of serotonin [Panksepp and Huber, in review].
Acute fluoxetine alone did not affect fighting of subordinate animals, but fight-enhancing effects of serotonin were greatly reduced (fig. 2a] in its presence [Huber et al., 1997a, b; Huber and Delago, 1998]. Fluoxetine, the active ingredient of Prozac, is a highly effective antidepressant in humans, however, its pharmacological profile and precise mode of action are in dispute. We used osmotic mini-pumps to deliver continuous infusions of fluoxetine to both crayfish and lobsters for 21 days (fig. 2b]. During chronic fluoxetine exposure animals were randomly paired with opponents at intervals of 3–4 days. Such treatment produced subtle, aggression-enhancing effects, particularly during the initial days of treatment [Delago et al., in review]. With behavioral effects generally similar to those associated with acute infusions of serotonin, individuals were less likely to retreat from larger opponents, resulting in longer, more intense fighting.
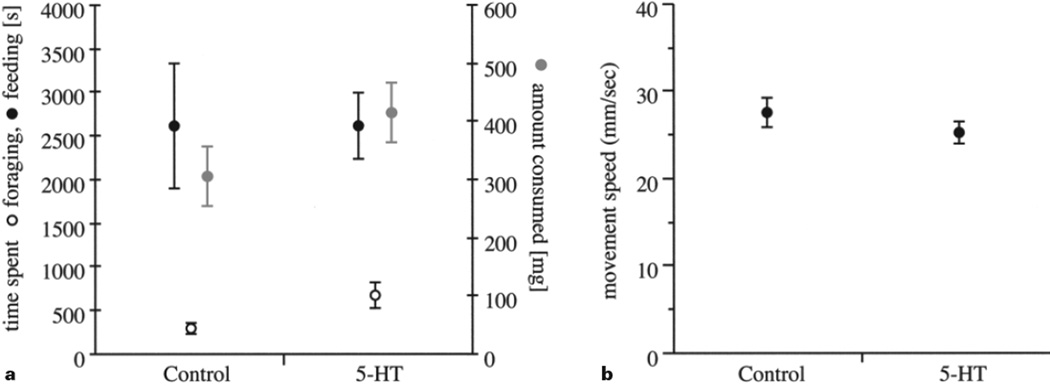
As with any effect of pharmacological manipulation, specificity is always a concern. Namely, aside from those behaviors of primary focus, how many other aspects of behavior are altered as a function of the treatment [Peeke et al., 2000]? We have further explored the behavioral specificity of serotonin treatment in crayfish by examining its effects on foraging and feeding behavior, as well as on movement patterns and space usage in an open field paradigm. Much like agonistic behavior, the acts of locating and consuming food are organized into separate behavioral stages that can be readily distinguished and quantified. The behavioral sequence begins with the distal sensation of waterborne chemicals through the antennules. If motivated, orientation towards the food source follows. When in close proximity, the crayfish then switches to local search strategies for precise localization of the source [Moore and Grills, 1999]. Rapid approach at close range and retrieval of the food item are followed by consumption until satiety. Injections of serotonin did not alter the latency to find a food source, the time spent feeding, or the amount of food eaten (fig. 3a]. Moreover, we explored the effects of serotonin treatment on general locomotor arousal by measuring a variety of spatial parameters describing the movements of individual crayfish in an arena (e.g. total distance traveled, speed of movement, space utilization). Single injections of serotonin did not alter movement patterns or the usage of space in the arena (fig. 3b].
Fig. 3.
Behavioral effects of acute treatment with serotonin. Measures of feeding and locomotion were obtained for crayfish (Orconectes rusticus) that had been injected with 3 µg serotonin per gram body weight (1 mg serotonin:1 ml 125 mM saline) or with an equal volume of vehicle only. a Following treatment, individuals (weights: 6.2–22.2 g), which had not been fed for 14 days prior to the experiment were placed under a mesh cage (85 mm2) on one end of an observation tank (584 × 305 mm). Consistent flow was provided by two water inlets located at the opposite end of the aquarium and a familiar food source (5 mm section of earthworm) was placed at the upstream end of the tank. Following a 30-min acclimation period the cage was removed allowing the animals to move freely around the aquarium. The amount of time to locate the food source (i.e. search time), time spent feeding once the food item had been found (i.e. feeding time) and the total amount consumed were quantified. One-way ANOVAs with α adjusted for three comparisons using Dunn-Sedàk (α’ = 0.017) revealed no significant differences between groups in search time (F(1,19) = 5.16, p’ = 0.036), time spent feeding (F(1,19) = 0.01, p’ = 0.996) or the total amount consumed (F(1,19) = 2.14, p’ = 0.161). b Treated individuals (weights: 8.1–17.0 g) were acclimated for 30 min in a gravel lined, observation aquarium (400 mm2) by having a circular flowerpot (diameter = 100 mm) placed over them. The flowerpot was raised and the individual’s movements were videotaped from above for 60 min. Locations (x and y coordinates) were obtained every 1.5 s from a series of digitized frames using video-tracking software (freely available at http://caspar.bgsu.edu/~software/java/ based on Quicktime for Java libraries). The speed of locomotion was calculated as the average straight-line distance between consecutive captures for frames in which animals had moved. ANOVA revealed no significant main effect for treatment (F(1,64) = 0.621, p = 0.434).
The results from experiments with chronic fluoxetine treatment indicated the need for a more thorough examination of the time course of plasticity in amine function. We have since explored the effects of chronic amine manipulations by exposing crayfish to serotonin and 5,7-dihydroxy-tryptamine via silastic implants [Panksepp and Huber, in review]. Biochemical as well as behavioral changes accompanying such manipulations were counter-intuitive and suggested the action of compensatory mechanisms (fig. 2c]. Treatment with 5,7-dihydroxytryptamine for 5–16 days greatly reduced serotonin levels in all sections of the nervous system except the brain. However, different rates of chronic serotonin treatment did not similarly increase its levels in the central nervous system. Long-term enhancement of serotonin levels may have boosted inactivating processes, and it is possible that compensatory processes effectively counteracted our experimental interventions. Despite amine depletion, behavioral differences did not match our initial predictions of a decrease in aggressive state. Crayfish treated with 5,7-dihydroxytryptamine fought with behavioral characteristics that were indistinguishable from those of controls. Moreover, marked differences in the rate of escalation were observed in individuals that had received serotonin despite the treatment’s inefficacy to raise absolute levels. Specifically, infusion at a slow rate increased aggressive state, while a faster infusion rate effectively reduced fighting.
Characterization of Changes in Amine Levels during Initiation, Establishment and Reinforcement of Dominance Relationships in Crayfish
Studies from this lab and others suggest the existence of complex links between aggressive behavior and neurochemical processes. Although considerable attention has thus far been focused on the behavioral effects resulting from experimental manipulations of amine function, amine correlates of aggressive state and social status have received much less attention. It is likely that behavioral and physiological levels of organization exert feed-back effects onto each other. Differences in the concentration of neuromodulators as a function of social status have been reported in blood [Rose et al., 1971, 1975; Raleigh et al., 1984, Schuhr, 1987; Knoll and Egberink-Alink, 1989; Shively et al., 1991] and brain regions throughout a range of vertebrate taxa [Kemble et al., 1990; Summers et al., 1997; Øverli et al., 1998; Winberg and Lepage, 1998].
In crustaceans, social status influenced both concentrations of 5-HT in hemolymph [Sneddon et al., 2000] and the efficacy of modulators at identified synapses [Yeh et al., 1996, 1997; Krasne et al., 1997]. Neurons within local circuits controlling tail flip, a common behavior of retreat [Glanzman and Krasne, 1983, 1986; Bustamante and Krasne, 1991], exhibited reduced responsiveness in the presence of serotonin, as well as changes in their excitability [Krasne et al., 1997]. Pharmacological evidence suggests that differences in social status produce altered expression of serotonin receptor subtype populations [Yeh et al., 1996, 1997]. Our recent work has examined whether central nervous system amine levels reflect the acquisition of social status in the short-term, as well as long-term increases in body size during adult growth. Using HPLC with electrochemical detection we have explored such relationships for individual segments of the crayfish nervous system. Experimental conditions were selected to maximize differences in behavior between dominants and subordinates [Yue, 2001, Yue and Huber, in prep.]. Towards this goal, two crayfish were housed together and able to interact for 24 h. A single shelter was introduced after the initial determination of dominance. In this study no changes in amines accompanied an almost doubling of adult body weights from 5.8–10.3 grams. Amine levels thus do not increase in proportion with the rest of the body, and it seems that adult crayfish continue to grow around central aminergic systems. Allometric growth patterns in CNS structures are not unusual, having been reported in other systems [Hochner and Spira, 1987; Bloomfield and Hitchcock, 1991; Hill et al., 1994], and may be accompanied by surprising physiological properties [Edwards et al., 1994a, b]. Furthermore, results indicate that after 24 h no significant differences in the CNS levels of DA and 5HT were detected among dominant, subordinate, and control individuals [Yue, 2001; Yue and Huber, in prep.). Absolute levels of CNS are determined by the collective action of activating, transporting, releasing and inactivating processes which are also under circadian control [Castanon-Cervantes et al., 1999]. This work thus allows us to view only one small part of the picture, and interpreting a lack of change in absolute levels is difficult; 5-HT and DA systems may well respond to social experience at other time frames [Summers and Greenberg, 1995], involve increased production that is matched by increased turnover [Blanchard et al., 1991; Winberg et al., 1991; Winberg and Nilsson, 1993; Summers et al., 1997], or act via post-synaptic processes [Yeh et al., 1997]. Moreover, a view of amine neuromodulatory systems as dynamic entities [Hebert and Gerhardt, 1998] rather than long-term, static characteristics is also consistent with results from the chronic amine manipulations reported here.
Conclusions
Discussions of aggression in animal behavior have, at least in recent decades, focused predominantly on the evolutionary significance of conflict [Caldwell and Dingle, 1975; Fry, 1980; Schulz, 1986; Ratnieks, 1991], the ultimate consequences associated with different behavioral strategies [Parker, 1974; Maynard-Smith, 1979], or individual properties which may confer an advantage to the owner [Lamprecht, 1986; Matsuzawa and Shiraishi, 1992; Fairbanks, 1994]. Although there has been significant progress in research concerned with the ultimate consequences of behavior, other areas have yielded results more slowly. This is particularly true with regard to the physiological roles played by neuromodulatory substances for motivational elements [Tinbergen, 1951], behavioral states [Bryson, 1971; Blanchard and Blanchard, 1989], and dispositions for fighting [Lorenz, 1966]. The rediscovery of neurochemical factors has thus recently led to renewed inclusion of proximate mechanisms and physiological variables into mainstream ethology [van Staaden, 1998]. Due to their special characteristics, the rediscovery of interdisciplinary approaches for understanding behavioral causation has proven particularly fruitful in the case of neuromodulators. An increasingly dynamic view of such systems closely matches ideas that have previously emerged from work on the behavioral effects of steroid hormones [Arnold and Schlinger, 1993; Henrich and Brown, 1995; Ottinger and Abdelnabi, 1997], the network properties of amines/peptides [Johnson and Harris-Warrick, 1990; Katz and Frost, 1997; Stevenson and Meuser, 1997; Hoffman et al., 1998], and the functional characteristics of amine/peptide systems in specific social contexts [Fox et al., 1997; Yeh et al., 1997]. Such neuromodulatory substances do not ‘produce’ behavior per se but rather fine tune, or modulate, ongoing activity. In addition, serotonergic systems are likely influenced by the behavioral and experiential background of an individual through feedback mechanisms [Raleigh et al., 1984; Winberg et al., 1997]. Thus, in order to examine the particular roles of neuromodulators, it is essential that we combine studies of physiology, biochemistry, and molecular genetics, with quantitative behavioral analyses.
This review has summarized the experimental approaches with which we are attempting to link amines and their metabolites to aggression in crustaceans. Our work has (1) characterized the behavioral rules governing fighting in clawed decapod crustaceans, (2) estimated the contribution of learned plasticity for such behavior, (3) evaluated decisions during fighting in terms of game theory models, (4) identified the number and characteristics of individual sources of behavioral heterogeneity, (5) supported the significance of serotonin function for decisions involving retreat, (6) illustrated how the outcome of encounters influences fighting behavior in subsequent encounters, and (7) how the repeated application of this type of social conditioning leads to the formation of linear hierarchies through self-structuring.
Changes in serotonergic function appear to be an important physiological determinant of aggression in decapods. Raising levels of serotonin, either directly by infusion or by blocking its re-uptake, resulted in enhanced fighting. Important questions remain concerning the particular neurons responsible for such observed changes in decision-making. We have recently shown that serotonergic input from a pair of deutocerebral giant serotonergic neurons in the supra-esophageal ganglion is essential for the development and growth of central processing centers [Benton et al., 1997]. Further explorations of these neurons and their target synaptic regions will be required to assess whether they may be the source of higher order decisions, such as when to give up in an agonistic encounter.
Results presented above, coupled with those of other investigators, argue for a highly dynamic association between serotonin and aggressive behavior in decapods [Doernberg et al., 2001], although our understanding is far from complete. Consistent with recent reports of compensatory mechanisms in murine genetic knock-out models of behavior [Hen, 2000], we suspect the actions of similar, yet-unidentified mechanisms for the behavioral results reported here. The previously mentioned caveat in working with the crustacean species of our research focus thus reflects a caution common to all aspiring to understand amine systems in general. Changes in behavior that are regulated by biogenic amines, whether experimentally or naturally induced, do not seem to be controlled solely by the molecule itself, but rather through a dynamic orchestration of the system in which they are imbedded [Kravitz, 1988]. Such a ‘systems view’ of behavior requires that causal explanations be framed within the molecule’s own unique physiological and behavioral context. Moreover, exploration of a neuromodulators functional role in healthy and diseased nervous systems requires that we arrive at an integrated understanding of their effects for complex behaviors [Lederhendler and Shulkin, 2000], such as aggression. Future studies linking molecular and biochemical changes to pharmacological manipulations of serotonin and behavioral plasticity will help bridge the gap between the conclusion of flexibility and specific aspects of the serotonin system in decapod crustaceans.
Acknowledgments
We would like to thank Dr. Alisdair Daws for help in the analysis of spatial data. We are grateful to Drs. Edward A. Kravitz, Moira van Staaden and two anonymous reviewers who have provided valuable criticism on this paper. This research was supported by grants to RH and PM (NSF IBN-9874608) and to RH (NSF DBI-0070334 and NIH MH62557-01).
References
- Antonsen BL, Paul DH. Serotonin and octopamine elicit stereotypical agonistic behaviors in the squat lobster Munida quadrispina (Anomura, Galatheidae) J. Comp. Physiol. A. 1997;181:501–510. [Google Scholar]
- Arnold AP, Schlinger BA. Sexual-differentiation of brain and behavior: the zebra finch is not just a flying rat. Brain Behav. Evol. 1993;42:231–241. doi: 10.1159/000114157. [DOI] [PubMed] [Google Scholar]
- Atema J. Review of sexual selection and chemical communication in the lobster, Homarus americanus. Can. J. Fish. Aquat. Sci. 1986;43:2283–2290. [Google Scholar]
- Atema J, Cobb JS. Social behavior. In: Cobb JS, Phillips BF, editors. The Biology and Management of Lobsters. New York: Academic Press; 1980. pp. 409–450. [Google Scholar]
- Baldaia L, Porcheron P, Coimbra J, Cassier P. Ecdysteroids in the shrimp Palaemon serratus: relations with molt cycle. Gen. Comp. Endocrinol. 1984;55:437–443. doi: 10.1016/0016-6480(84)90015-7. [DOI] [PubMed] [Google Scholar]
- Barki A, Karplus I, Goren M. Effects of size and morphotype on dominance hierarchies and resource competition in the freshwater prawn Macrobrachium rosenbergii. Anim. Behav. 1992;44:547–555. [Google Scholar]
- Barshaw DE, Lavalli KL. Predation upon postlarval lobsters (Homarus americanus) by cunners (Tautogolabrus adspersus) and mud crabs (Neopanope sayi) on three different substrates: eelgras, mud and rocks. Mar. Ecol. Prog. Ser. 1988;48:119–123. [Google Scholar]
- Beacham JL, Newman JA. Social experience and the formation of dominance relationships in the pumpkinseed sunfish, Lepomis gibbosus. Anim. Behav. 1987;35:1560–1563. [Google Scholar]
- Beaugrand J, Goulet C, Payette D. Outcome of dyadic conflict in male green swordtail fish, Xiphophorus helleri: effects of body size and prior dominance. Anim. Behav. 1991;41:417–424. [Google Scholar]
- Bell R, Hobson H. 5-HT1A receptor influences on rodent social and agonistic behavior: a review and empirical study. Neurosci. Biobehav. Rev. 1994;18:325–338. doi: 10.1016/0149-7634(94)90046-9. [DOI] [PubMed] [Google Scholar]
- Beltz B, Richards K, Marder E. The serotonin transporter and receptor mature prior to serotonin appearance in embryonic STG: a borrowed transmitter hypothesis. Soc. Neurosci. Abstr. 1998;24:107. [Google Scholar]
- Benton J, Huber R, Ruchhoeft M, Helluy S, Beltz B. Serotonin depletion by 5,7-dihydroxytryptamine alters deutocerebral development in the lobster, Homarus americanus. J. Neurobiol. 1997;33:357–373. doi: 10.1002/(sici)1097-4695(199710)33:4<357::aid-neu2>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard DC. Attack and defense in rodents as ethoexperimental models for the study of emotion. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 1989;13(Suppl):S3–S14. doi: 10.1016/0278-5846(89)90105-x. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Cholvanich P, Blanchard RJ, Clow DW, Hammer RP, Rowlett JK. Serotonin, but not dopamine, metabolites are increased in selected brain-regions of subordinate male-rats in a colony environment. Brain Res. 1991;568:61–66. doi: 10.1016/0006-8993(91)91379-f. [DOI] [PubMed] [Google Scholar]
- Bloomfield SA, Hitchcock PF. Dendritic arbors of large-field ganglion cells show scaled growth during expansion of the goldfish retina: a study of morphometric and electrotonic properties. J. Neurosci. 1991;11:910–917. doi: 10.1523/JNEUROSCI.11-04-00910.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumensohn R, Ratzoni G, Weizman A, Israeli M, Greuner N, Apter A, Tyano S, Biegon A. Reduction in serotonin 5HT2 receptor binding on platelets of delinquent adolescents. Psychopharmacology. 1995;118:354–356. doi: 10.1007/BF02245966. [DOI] [PubMed] [Google Scholar]
- Bolingbroke M, Kass-Simon G. 20-Hydroxyecdysone causes increased aggressiveness in female American lobsters, Homarus americanus. Hormones and behavior. 2001;39:144–156. doi: 10.1006/hbeh.2001.1642. [DOI] [PubMed] [Google Scholar]
- Bovbjerg RV. Dominance order in the crayfish Orconectes virilis (Hagen) Physiol. Zool. 1953;26:173–178. [Google Scholar]
- Bovbjerg RV. Some factors affecting aggressive behavior in crayfish. Physiol. Zool. 1956;29:127–136. [Google Scholar]
- Bradbury CW. Acute and chronic dopamine dynamics in a nonhuman primate model of recreational drug use. J. Neurosci. 2000;20:7109–7115. doi: 10.1523/JNEUROSCI.20-18-07109.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner HG, Nelen M, Breakfield XO, Ropers HH, van-Oost BA. Abnormal behavior associated with a point mutation in the structural gene for monoamine oxidase A. Science. 1993a;262:578–580. doi: 10.1126/science.8211186. [DOI] [PubMed] [Google Scholar]
- Brunner HG, Nelen MR, van-Zandvoort P, Abeling NG, van-Gennip AH, Wolters EC, Kuiper MA, Ropers HH, van-Oost BA. X-linked borderline mental retardation with prominent behavioral disturbance: phenotype, genetic localization, and evidence for disturbed monoamine metabolism [see comments] Am. J. Human Genet. 1993b;52:1032–1039. [PMC free article] [PubMed] [Google Scholar]
- Bruski CA, Dunham DW. The importance of vision in agonistic communication of the crayfish Orconectes rusticus. I. An analysis of bout dynamics. Behaviour. 1987;63:83–107. [Google Scholar]
- Bryson G. Biogenic amines in normal and abnormal behavioral states. Clin. Chem. 1971;17:5–26. [PubMed] [Google Scholar]
- Burk TE. Analysis of Social Behavior in Crickets. Oxford, UK: Univ. Oxford Press; 1979. [Google Scholar]
- Bustamante J, Krasne FB. Effects of octopamine on transmission at the first synapse of the crayfish lateral giant escape reaction pathway. J. Comp. Physiol. A. 1991;169:369–377. [Google Scholar]
- Caldwell R, Dingle H. Ecology and evolution of agonistic behavior in stomatopods. Naturwissenschaften. 1975;62:214–222. [Google Scholar]
- Capelli GM, Hamilton PA. Effects of food and shelter on aggressive activity in the crayfish Orconectes rusticus. J. Crustacean Biol. 1984;4:252–260. [Google Scholar]
- Cases O, Seif I, Grimsby J, Gaspar P, Chen K, Pournin S, Muller U, Aguet M, Babinet C, Shih JC, Demaeyer E. Aggressive behavior and altered amounts of brain serotonin and norepinephrine in mice lacking MAOA. Science. 1995;268:1763–1766. doi: 10.1126/science.7792602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castanon-Cervantes O, Batelle B-A, Fanjul-Moles ML. Rhythmic changes in the serotonin content of the brain and eyestalk of crayfish during development. J. Exp. Biol. 1999;202:2823–2830. doi: 10.1242/jeb.202.20.2823. [DOI] [PubMed] [Google Scholar]
- Chase ID, Bartolomeo C, Dugatkin LA. Aggressive interactions and inter-contest interval -how long do winners keep winning. Anim. Behav. 1994;48:393–400. [Google Scholar]
- Chen C, Rainnie DG, Greene RW, Tonegawa S. Abnormal fear response and aggressive behavior in mutant mice deficient for alpha-calcium-calmodulin kinase II. Science. 1994;266:291–294. doi: 10.1126/science.7939668. [DOI] [PubMed] [Google Scholar]
- Cleare AJ, Bond AJ. The Effect of tryptophan depletion and enhancement on subjective and behavioral aggression in normal-male subjects. Psychopharmacology. 1995;118:72–81. doi: 10.1007/BF02245252. [DOI] [PubMed] [Google Scholar]
- Coccaro EF, Silverman JM, Klar HM, Horvath TB, Siever LJ. Familial correlates of reduced central serotonergic system function in patients with personality disorders. Arch. Gen. Psychiatry. 1994;51:318–324. doi: 10.1001/archpsyc.1994.03950040062008. [DOI] [PubMed] [Google Scholar]
- Copp NH. Dominance hierarchies in the crayfish Procambarus clarkii [Girard, 1852] and the question of learned individual recognition (Decapoda, Astacidea) Crustaceana. 1986;51:9–24. [Google Scholar]
- Daws AG, Grills J, Konzen K, Moore PA. Previous experiences alter the outcome of aggressive interactions between males in the crayfish, Procambarus clarkii. J. Mar. Freshwater Behav. Physiol. 2001 in press. [Google Scholar]
- Delago A, Kravitz E, Huber R. Effects of chronic fluoxetine in agonistic behavior of crayfish and lobsters. in review. [Google Scholar]
- Dingle H. Strategies of agonistic behaviour in crustacea. In: Rebach S, Dunham DW, editors. Studies of Adaptation: The Behavior of Higher Crustacean. New York: Wiley; 1983. pp. 85–111. [Google Scholar]
- Doernberg SB, Cromarty SI, Heinrich R, Beltz BS, Kravitz EA. Agonistic behavior in naive juvenile lobsters depleted of serotonin by 5,7-dihydroxytryptamine. J. Comp. Physiol. A. 2001;187:91–103. doi: 10.1007/s003590000178. [DOI] [PubMed] [Google Scholar]
- Drews C. The concept and definition of dominance in animal behavior. Behaviour. 1993;125:283–313. [Google Scholar]
- Dugatkin LA. Winner and loser effects and the structure of dominance hierarchies. Behav. Ecol. 1997;8:583–587. [Google Scholar]
- Edwards DH, Kravitz EA. Serotonin, social status and aggression. Curr. Opin. Neurobiol. 1997;7:812–819. doi: 10.1016/s0959-4388(97)80140-7. [DOI] [PubMed] [Google Scholar]
- Edwards DH, Fricke RA, Barnett LD, Yeh SR, Leise EM. The onset of response habituation during the growth of the lateral giant neuron of crayfish. J. Neurophysiol. 1994a;72:890–898. doi: 10.1152/jn.1994.72.2.890. [DOI] [PubMed] [Google Scholar]
- Edwards DH, Yeh SR, Barnett LD, Nagappan PR. Changes in synaptic integration during the growth of the lateral giant neuron of crayfish. J. Neurophysiol. 1994b;72:899–908. doi: 10.1152/jn.1994.72.2.899. [DOI] [PubMed] [Google Scholar]
- Enquist M, Leimar O. Evolution of fighting behaviour: decision rules and assessment of relative strength. J. Theor. Biol. 1983;102:387–410. [Google Scholar]
- Evans DL, Shehadi-Moacdieh M. Body size and prior residency in staged encounters between female prawns, Palaemon elegans Rathke (Decapoda: Palaemonidae) Anim. Behav. 1988;36:452–455. [Google Scholar]
- Fairbanks WS. Dominance, age and aggression among female pronghorn, Antilocapra americana (Family, Antilocapridae) Ethology. 1994;97:278–293. [Google Scholar]
- Figler MH, Finkelstein JE, Twum M, Peeke HVS. Intruding male red swamp crayfish, Procambarus clarkii, immediately dominant members of established communities of smaller mixed-sex conspecifics. Aggr. Behav. 1995;21:225–236. [Google Scholar]
- Figler MH, Peeke HVS, Chang ES. Shelter-related aggression between adult male conspecific intruders and resident maternal American lobsters (Homarus americanus) with eggs at different stages of embryogenesis. Mar. Freshwater Behav. Physiol. 1998;31:151–166. [Google Scholar]
- Fox HE, White SA, Kao MHF, Fernald RD. Stress and dominance in a social fish. J. Neurosci. 1997;17:6463–6469. doi: 10.1523/JNEUROSCI.17-16-06463.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis RC. Experiential effects on agonistic behavior in the paradise fish, Macropodus opercularis. Behaviour. 1983;85:292–313. [Google Scholar]
- Francis RC. On the relationship between aggression and social dominance. Ethology. 1988;78:223–237. [Google Scholar]
- Fry DP. The evolution of aggression and the level of selection controversy. Aggr. Behav. 1980;6:69–90. [Google Scholar]
- Fuller RW. Serotonin uptake inhibitors: uses in clinical therapy and in laboratory research. Prog. Drug. Res. 1995;45:167–204. doi: 10.1007/978-3-0348-7164-8_5. [DOI] [PubMed] [Google Scholar]
- Fuller RW. Fluoxetine effects on serotonin function and aggressive behavior. Ann. NY Acad. Sci. 1996;794:90–97. doi: 10.1111/j.1749-6632.1996.tb32512.x. [DOI] [PubMed] [Google Scholar]
- Giammanco S, Ernandes M, Lopez-de-Onate R, Paderni MA. Short term diet of precooked corn meal almost lacking in tryptophan and interspecific rat-mouse aggressive behaviour. Arch. Int. Physiol. Biochim. 1990;98:23–26. doi: 10.3109/13813459009115733. [DOI] [PubMed] [Google Scholar]
- Glanzman DL, Krasne FB. Serotonin and octopamine have opposite modulatory effects on the crayfish’s lateral giant escape reaction. J. Neurosci. 1983;3:2263–2269. doi: 10.1523/JNEUROSCI.03-11-02263.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glanzman DL, Krasne FB. 5,7-Dihy-droxytryptamine lesions of crayfish serotonin-containing neurons: effect on the lateral giant escape reaction. J. Neurosci. 1986;6:1560–1569. doi: 10.1523/JNEUROSCI.06-06-01560.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goessmann C, Hemelrijk C, Huber R. The formation and maintenance of crayfish hierarchies: behavioral and self-structuring properties. Behav. Ecol. Sociobiol. 2000;48:418–428. [Google Scholar]
- Gould X. Report on the Invertebra of Massachusetts: Comprising the Mollusca, Crustacea, Annelida & Radiata. Cambridge, Mass: The Cotes; 1841. [Google Scholar]
- Graf F, Delbecque JP. Ecdysteroid titers during the molt cycle of Orchestia cavimana (Crustacea, Amphipoda) Gen. Comp. Endocrinol. 1987;65:23–33. doi: 10.1016/0016-6480(87)90218-8. [DOI] [PubMed] [Google Scholar]
- Guiasu RC, Dunham DW. Initiation and outcome of agonistic contests in male form I Cambarus robustus Girard, 1852 crayfish (Decapoda, Cambaridae) Crustaceana. 1997;70:480–496. [Google Scholar]
- Hebert MA, Gerhardt GA. Normal and drug-induced locomotor behavior in aging: comparison to evoked DA release and tissue content in Fischer 344 rats. Brain Res. 1998;797:42–54. doi: 10.1016/s0006-8993(98)00370-9. [DOI] [PubMed] [Google Scholar]
- Hen R. Differential contributions of pre-and postsynaptic 5-HT1A receptors to the effects of anxiolytic drugs: a tissue specific knock-out study. Soc. Neurosci. Abstracts. 2000;26:583. [Google Scholar]
- Henrich VC, Brown NE. Insect nuclear receptors: a developmental and comparative perspective. Insect Biochem. Molec. Biol. 1995;25:881–897. doi: 10.1016/0965-1748(95)00030-y. [DOI] [PubMed] [Google Scholar]
- Herrick FH. The natural history of the American lobster. Bull. U.S. Bur. Fish. 1909;29:149–408. [Google Scholar]
- Hilakivi-Clarke LA, Goldberg R. Effects of tryptophan and serotonin uptake inhibitors on behavior in male transgenic transforming growth factor alpha mice. Eur. J. Pharmacol. 1993;237:101–108. doi: 10.1016/0014-2999(93)90098-3. [DOI] [PubMed] [Google Scholar]
- Hill AA, Edwards DH, Murphey RK. The effect of neuronal growth on synaptic integration. J. Comput. Neurosci. 1994;1:239–254. doi: 10.1007/BF00961736. [DOI] [PubMed] [Google Scholar]
- Hochner B, Spira ME. Preservation of motoneuron electrotonic characteristics during postembryonic growth. J. Neurosci. 1987;7:261–270. doi: 10.1523/JNEUROSCI.07-01-00261.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman BJ, Hansson SR, Mezey E, Palkovits M. Localization and dynamic regulation of biogenic amine transporters in the mammalian central nervous system. Frontiers Neuroendocrinol. 1998;19:187–231. doi: 10.1006/frne.1998.0168. [DOI] [PubMed] [Google Scholar]
- Hollis KL, Dumas MJ, Singh P, Fackelman P. Pavlovian conditioning of aggressive behavior in Blue Gourami Fish (Trichogaster trichopterus) -Winners become winners and losers stay losers. J. Comp. Psychol. 1995;109:123–133. [Google Scholar]
- Hsu YY, Wolf LL. The winner and loser effect: integrating multiple experiences. Anim. Behav. 1999;57:903–910. doi: 10.1006/anbe.1998.1049. [DOI] [PubMed] [Google Scholar]
- Huber R, Delago A. Serotonin alters decisions to withdraw in fighting crayfish, Astacus astacus: the motivational concept revisited. J. Comp. Physiol. A. 1998;182:573–583. [Google Scholar]
- Huber R, Kravitz EA. A quantitative analysis of agonistic behavior in juvenile American lobsters (Homarus americanus L) Brain Behav. Evol. 1995;46:72–83. doi: 10.1159/000113260. [DOI] [PubMed] [Google Scholar]
- Huber R, Daws AG, Tuttle SB, Panksepp JB. Quantitative Techniques for the Study of Crustacean Aggression. Berlin: Springer-Verlag; 2001. [Google Scholar]
- Huber R, Orzeszyna M, Pokorny N, Kravitz EA. Biogenic amines and aggression: experimental approaches in crustaceans. Brain Behav. Evol. 1997a;50:60–68. doi: 10.1159/000113355. [DOI] [PubMed] [Google Scholar]
- Huber R, Smith K, Delago A, Isaksson K, Kravitz EA. Serotonin and aggressive motivation in crustaceans: altering the decision to retreat. Proc. Nat. Acad. Sci. USA. 1997b;94:5939–5942. doi: 10.1073/pnas.94.11.5939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyatt GW. Qualitative and quantitative dimensions of crustacean aggression. In: Rebach S, Dunham DW, editors. Studies in Adaptation: The Behaviour of Higher Crustacea. New York: Wiley & Sons; 1983. pp. 113–139. [Google Scholar]
- Issa FA, Adamson DJ, Edwards DH. Dominance hierarchy formation in juvenile crayfish Procambarus clarkii. J. Exper. Biol. 1999;202:3497–3506. doi: 10.1242/jeb.202.24.3497. [DOI] [PubMed] [Google Scholar]
- Jackson WM. Why do winners keep winning. Behav. Ecol. Sociobiol. 1991;28:271–276. [Google Scholar]
- Johnson BR, Harris-Warrick RM. Aminergic modulation of graded synaptic transmission in the lobster stomatogastric ganglion. J. Neurosci. 1990;10:2066–2076. doi: 10.1523/JNEUROSCI.10-07-02066.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnofsky EB, Atema J, Elgin RH. Field observations of social behavior, shelter use, and foraging in the lobster, Homarus americanus. Biol. Bull. 1989;176:239–246. doi: 10.2307/1541982. [DOI] [PubMed] [Google Scholar]
- Katz PS, Frost WN. Removal of spike frequency adaptation via neuromodulation intrinsic to the Tritonia escape swim central pattern generator. J. Neurosci. 1997;17:7703–7713. doi: 10.1523/JNEUROSCI.17-20-07703.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemble ED, Blanchard DC, Blanchard RJ. Effects of regional amygdaloid lesions on flight and defensive behaviors of wild black rats (Rattus rattus) Physiol. Behav. 1990;48:1–5. doi: 10.1016/0031-9384(90)90251-x. [DOI] [PubMed] [Google Scholar]
- Knoll BW, Egberink-Alink ST. Androgens, progestagens and agonistic behaviour: a review. Vet. Q. 1989;11:1775–1781. doi: 10.1080/01652176.1989.9694205. [DOI] [PubMed] [Google Scholar]
- Krasne FB, Shamsian A, Kulkami R. Altered excitability of the crayfish lateral giant escape reflex during agonistic encounters. J. Neurosci. 1997;17:709–716. doi: 10.1523/JNEUROSCI.17-02-00709.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz EA. Hormonal control of behavior: amines and the biasing of behavioral output in lobsters. Science. 1988;241:1775–1781. doi: 10.1126/science.2902685. [DOI] [PubMed] [Google Scholar]
- Kravitz EA. King Solomon Lecture, Serotonin and aggression: insights gained from a lobster model system and speculations on the role of amine neurons in a complex behavior. J. Comp. Physiol. A. 2000;359:1–18. doi: 10.1007/s003590050423. [DOI] [PubMed] [Google Scholar]
- Lamprecht J. Structure and causation of the dominance hierarchy in a flock of barheaded geese (Anser indicus) Behaviour. 1986;96:26–48. [Google Scholar]
- Lavalli KL, Barshaw DE. Burrows protect postlarval lobsters Homarus americanus from predation by the non-burrowing cunner Tautogolabrus adspersus, but not from the burrowing mud crab Neopanope texani. Mar. Ecol. Prog. Ser. 1986;32:13–16. [Google Scholar]
- Lederhendler I, Shulkin J. Behavioral neuroscience: challenges for the era of molecular biology. Trends Neurosci. 2000;23:451–454. doi: 10.1016/s0166-2236(00)01636-2. [DOI] [PubMed] [Google Scholar]
- Leimar O, Enquist M. Effects of asymmetries in owner-intruder conflicts. J. Theor. Biol. 1984;111:475–491. [Google Scholar]
- Lorenz KZ. On Aggression. Brace, New York: Harcourt; 1966. [Google Scholar]
- Lowe M. Dominance-subordinance relationships in the crayfish Cambarellus shufeldti. Tulane Stud. Zool. 1956;4:139–170. [Google Scholar]
- Maler L, Ellis WG. Inter-male aggressive signals in weakly electric fish are modulated by monoamines. Behav. Brain Res. 1987;25:75–81. doi: 10.1016/0166-4328(87)90046-5. [DOI] [PubMed] [Google Scholar]
- Marden L. The American Lobster, delectable cannibal. Nat. Geo. Mag. 1973;1973:462–487. [Google Scholar]
- Matzuzawa Y, Shiraishi T. Relationship between aggressive behavior and social dominance in a small herd of goats. Anim. Sci. Technol. 1992;63:503–513. [Google Scholar]
- Maynard-Smith J. Game theory and the evolution of behaviour. Proc. R. Soc. Lond. B Biol. Sci. 1979;205:475–488. doi: 10.1098/rspb.1979.0080. [DOI] [PubMed] [Google Scholar]
- Mehlman PT, Higley JD, Faucher I, Lilly AA, Taub DM, Vickers J, Suomi SJ, Linnoila M. Low CSF 5-HIAA concentrations and severe aggression and impaired impulse control in nonhuman primates. Am. J. Psychiatry. 1994;151:1485–1491. doi: 10.1176/ajp.151.10.1485. [DOI] [PubMed] [Google Scholar]
- Mehlman PT, Higley JD, Faucher I, Lilly AA, Taub DM, Vickers J, Suomi SJ, Linnoila M. Correlation of CSF 5-HIAA concentration with sociality and the timing of emigration in free-ranging primates. Am. J. Psychiatry. 1995;152:907–913. doi: 10.1176/ajp.152.6.907. [DOI] [PubMed] [Google Scholar]
- Moore PA, Grills J. Chemical orientation to food by the crayfish, Orconectes rusticus: influence by hydrodynamics. Anim. Behav. 1999;58:953–963. doi: 10.1006/anbe.1999.1230. [DOI] [PubMed] [Google Scholar]
- Mühlenkamp F, Lucion A, Vogel WH. Effects of selective serotonergic agonists on aggressive behavior in rats. Pharmacol. Biochem. Behav. 1995;50:671–674. doi: 10.1016/0091-3057(95)00351-7. [DOI] [PubMed] [Google Scholar]
- Nielsen DA, Goldman D, Virkkunen M, Tokola R, Rawlings R, Linnoila M. Suicidality and 5-hydroxyindoleacetic acid concentration associated with a tryptophan hydroxylase polymorphism. Arch. Gen. Psychiatry. 1994;51:34–38. doi: 10.1001/archpsyc.1994.03950010034005. [DOI] [PubMed] [Google Scholar]
- Olivier B, Mos J, van-der-Heyden J, Hartog J. Serotonergic modulation of social interactions in isolated male mice. Psychopharmacology. 1989;97:154–156. doi: 10.1007/BF00442239. [DOI] [PubMed] [Google Scholar]
- Olivier B, Mos J, Vanoorschot R, Hen R. Serotonin receptors and animal models of aggressive behavior. PharmacoPsychiatry. 1995;28:80–90. doi: 10.1055/s-2007-979624. [DOI] [PubMed] [Google Scholar]
- Ottinger MA, Abdelnabi MA. Neuroendocrine systems and avian sexual differentiation. Amer. Zool. 1997;37:514–523. [Google Scholar]
- Øverli O, Winberg S, Damsgard B, Jobling M. Food intake and spontaneous swimming activity in arctic char (Salvelinus alpinus): role of brain serotonergic activity and social interactions. Can. J. Zool. 1998;76:1366–1370. [Google Scholar]
- Panksepp JB, Huber R. Chronic alterations in serotonin function: dynamic neurochemical properties in agonistic behavior of the crayfish, Orconectes rusticus. doi: 10.1002/neu.10035. in review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker GA. Assessment strategy and the evolution of fighting behaviour. J. Theor. Biol. 1974;47:223–243. doi: 10.1016/0022-5193(74)90111-8. [DOI] [PubMed] [Google Scholar]
- Parker GA, Rubenstein DI. Role assessment, reserve strategy, and the acquisition of information in asymmetric animal conflicts. Anim. Behav. 1981;29:221–240. [Google Scholar]
- Pavey CR, Fielder DR. The influence of size differential on agonistic behavior in the fresh-water crayfish, Cherax cuspidatus (Decapoda, Parastacidae) J. Zool. 1996;238:445–457. [Google Scholar]
- Peeke HVS, Blank GS, Figler MH, Chang ES. Effects of exogenous serotonin on a motor behavior and shelter competition in juvenile lobsters (Homarus americanus) J. Comp. Physiol. A. 2000;186:575–582. doi: 10.1007/s003590000113. [DOI] [PubMed] [Google Scholar]
- Puciowski O, Plaznik A, Kostowski W. Aggressive behavior inhibition by serotonin and quipazine injected into the amygdala in the rat. Behav. Neural Biol. 1985;43:58–68. doi: 10.1016/s0163-1047(85)91496-7. [DOI] [PubMed] [Google Scholar]
- Raleigh MJ, Brammer GL, McGuire MT, Yuwiler A. Social and environmental influences on blood serotonin concentrations in monkeys. Arch. Gen. Psychiatry. 1984;41:405–410. doi: 10.1001/archpsyc.1984.01790150095013. [DOI] [PubMed] [Google Scholar]
- Raleigh MJ, Mcguire MT, Brammer GL, Pollack DB, Yuwiler A. Serotonergic mechanisms promote dominance acquisition in adult male vervet monkeys. Brain Res. 1991;559:181–190. doi: 10.1016/0006-8993(91)90001-c. [DOI] [PubMed] [Google Scholar]
- Ranta E, Lindstrom K. Body-size and shelter possession in mature signal crayfish, Pacifastacus leniusculus. Ann. Zool. Fennici. 1993;30:125–132. [Google Scholar]
- Ratnieks FLW. Evolution of discriminatory aggression in marine invertebrates. J. Theor. Biol. 1991;152:557–565. doi: 10.1016/s0022-5193(05)80397-2. [DOI] [PubMed] [Google Scholar]
- Reisner IR, Mann JJ, Stanley M, Huang YY, Houpt KA. Comparison of cerebrospinal fluid monoamine metabolite levels in dominant aggressive and nonaggressive dogs. Brain Res. 1996;714:57–64. doi: 10.1016/0006-8993(95)01464-0. [DOI] [PubMed] [Google Scholar]
- Rose RM, Berstein IS, Gordon TP. Consequences of social conflict on plasma testosterone levels in rhesus monkeys. Psycho-som. Med. 1975;37:50–61. doi: 10.1097/00006842-197501000-00006. [DOI] [PubMed] [Google Scholar]
- Rose RM, Holaday JW, Bernstein IS. Plasma testosterone, dominance rank and aggressive behaviour in male rhesus monkeys. Nature. 1971;231:366–368. doi: 10.1038/231366a0. [DOI] [PubMed] [Google Scholar]
- Rutherford PL, Dunham DW, Allison V. Antennule use and agonistic success in the crayfish Orconectes rusticus [Girard, 1852] (Decapoda, Cambaridae) Crustaceana. 1996;69:117–122. [Google Scholar]
- Saudou F, Amara DA, Dierich A, LeMeur M, Ramboz S, Segu L, Buhot M-C, Hen R. Enhanced aggressive behavior in mice lacking 5HT1B receptor. Science. 1994;265:1875–1878. doi: 10.1126/science.8091214. [DOI] [PubMed] [Google Scholar]
- Schuhr B. Social structure and plasma corticosterone level in female albino mice. Physiol. Behav. 1987;40:689–693. doi: 10.1016/0031-9384(87)90269-1. [DOI] [PubMed] [Google Scholar]
- Schulz H. Agonistic behavior, territorial behavior, and courtship display of the little bustard Tetrax tetrax. J. Ornithol. 1986;127:125–204. [Google Scholar]
- Seibt U. Mechanisms and sensory capacities underlying the pair bond in the shrimp Hymenocera picta Dana [Ger] Z. Tierpsych. 1974;35:337–351. [PubMed] [Google Scholar]
- Shively CA, Brammer GL, Kaplan JR, Raleigh MJ, Manuck SB. The complex relationship between behavioral attributes, social status, and whole-blood serotonin in male Macaca fascicularis. Am. J. Primatol. 1991;23:99–112. doi: 10.1002/ajp.1350230204. [DOI] [PubMed] [Google Scholar]
- Smith MR, Dunham DW. Chela posture and vision -Compensation for sensory deficit in the crayfish Orconectes-Propinquus [Girard] (Decapoda, Cambaridae) Crustaceana. 1990;59:309–313. [Google Scholar]
- Smith MR, Dunham DW. Antennae mediate agonistic physical contact in the crayfish Orconectes rusticus [Girard, 1852] (Decapoda, Cambaridae) Crustaceana. 1996;69:668–674. [Google Scholar]
- Sneddon LU, Taylor AC, Huntingford FA, Watson DG. Agonistic behaviour and biogenic amines in shore crabs Carcinus meanas. J. Exp. Biol. 2000;203:537–545. doi: 10.1242/jeb.203.3.537. [DOI] [PubMed] [Google Scholar]
- Snyder MJ, Chang ES. Ecdysteroids in relation to the molt cycle of the American lobster, Homarus americanus. I. Hemolymph titers and metabolites. Gen. Comp. Endocrinol. 1991;81:133–145. doi: 10.1016/0016-6480(91)90133-q. [DOI] [PubMed] [Google Scholar]
- Steger R, Caldwell RL. Intraspecific deception by bluffing: a defense strategy of newly molted stomatopods (Arthropoda: crustacea) Science. 1983;221:558–560. doi: 10.1126/science.221.4610.558. [DOI] [PubMed] [Google Scholar]
- Stevenson PA, Meuser S. Octopaminergic innervation and modulation of a locust flight steering muscle. J. Exp. Biol. 1997;200:633–642. doi: 10.1242/jeb.200.3.633. [DOI] [PubMed] [Google Scholar]
- Stewart TW, Haynes JM. Benthic macroinvertebrate communities of southwestern Lake Ontario following invasion of Dreissena. J. Great Lakes Res. 1994;20:479–493. [Google Scholar]
- Summers CH, Greenberg N. Activation of central biogenic amines following aggressive interaction in male lizards, Anolis carolinensis. Brain Behav. Evol. 1995;45:339–349. doi: 10.1159/000113561. [DOI] [PubMed] [Google Scholar]
- Summers TR, Hunter AL, Summers CH. Female social reproductive roles affect central monoamines. Brain Res. 1997;767:272–278. doi: 10.1016/s0006-8993(97)00604-5. [DOI] [PubMed] [Google Scholar]
- Tamm GR, Cobb SJ. Behavior and the crustacean molt cycle: changes in aggression of Homarus americanus. Science. 1978;200:79–81. doi: 10.1126/science.200.4337.79. [DOI] [PubMed] [Google Scholar]
- Tamm GR, Cobb JS. Aggression, dominance and the crustacean molt cycle. Am. Zool. 1980;20:727A. doi: 10.1126/science.200.4337.79. [DOI] [PubMed] [Google Scholar]
- Tierney AJ, Mangiamele LA, Blanck JK, Moll SF, Thysen JA. Effects of serotonin receptor agonists on posture and aggressive behavior in crayfish. Soc. Neurosci. Abstr. 2000;26:1763. [Google Scholar]
- Tinbergen N. The Study of Instinct. Oxford, UK: Oxford Univ. Press; 1951. [Google Scholar]
- van Staaden MJ. Ethology: at 50 and beyond. Trends Ecol. Evol. 1998;13:6–8. doi: 10.1016/s0169-5347(97)01254-8. [DOI] [PubMed] [Google Scholar]
- Vannini M, Gherardi F. Dominance and individual recognition in Potamon fluviatile (Decapoda, Brachyura): possible role of visual cues. Mar. Behav. Physiol. 1981;8:13–20. [Google Scholar]
- Vannini M, Sardini A. Aggressivity and dominance in river crab Potamon fluviatile (Herbst) Monit. Zool. Ital. 1971;5:173–213. [Google Scholar]
- Vergnes M, Depaulis A, Boehrer A, Kempf E. Selective increase of offensive behavior in the rat following intrahypothalamic 5,7-DHT-induced serotonin depletion. Behav. Brain Res. 1988;29:85–91. doi: 10.1016/0166-4328(88)90055-1. [DOI] [PubMed] [Google Scholar]
- Virkkunen M, Goldman D, Nielsen DA, Linnoila M. Low brain serotonin turnover rate (low CSF 5-HIAA) and impulsive violence. J. Psychiatry Neurosci. 1995;20:271–275. [PMC free article] [PubMed] [Google Scholar]
- Winberg S, Lepage O. Elevation of brain 5-HT activity, POMC expression, and plasma cortisol in socially subordinate rainbow trout. Am. J. Physiol. Regul. Integr C. 1998;43:R645–R654. doi: 10.1152/ajpregu.1998.274.3.R645. [DOI] [PubMed] [Google Scholar]
- Winberg S, Nilsson GE. Time course of changes in brain serotonergic activity and brain tryptophan levels in dominant and subordinate juvenile Arctic charr. J. Exp. Biol. 1993;179:181–195. [Google Scholar]
- Winberg S, Nilsson GE, Olsen KH. Social rank and brain levels of monoamines and monoamine metabolites in Arctic charr, Salvelinus alpinus (L) J. Comp. Physiol. A. 1991;168:241–246. [Google Scholar]
- Winberg S, Winberg Y, Fernald RD. Effect of social rank on brain monoaminergic activity in a cichlid fish. Brain Behav. Evol. 1997;49:230–236. doi: 10.1159/000112994. [DOI] [PubMed] [Google Scholar]
- Winston ML, Jacobson S. Dominance and effects of strange conspecifics on aggressive interactions in the hermit crab Pagurus longicarpus (Say) Anim. Behav. 1978;26:184–191. doi: 10.1016/0003-3472(78)90018-0. [DOI] [PubMed] [Google Scholar]
- Wood W. New England’s Prospect. The Cotes, reprinted by Univ. Amherst, Mass: Massachusetts Press; 1635. (1977) [Google Scholar]
- Yeh S-R, Fricke RA, Edwards DH. The effect of social experience on serotonergic modulation of the escape circuit of crayfish. Science. 1996;271:366–369. doi: 10.1126/science.271.5247.366. [DOI] [PubMed] [Google Scholar]
- Yeh S-R, Musolf BE, Edwards DH. Neuronal adaptations to changes in the social dominance status of crayfish. J. Neurosci. 1997;17:697–708. doi: 10.1523/JNEUROSCI.17-02-00697.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue Z. Ph.D. Diss. Bowling Green, Ohio: Bowling Green State University; 2001. Neurochemical systems and behavior: social status and the search for amine correlates in crayfish. [Google Scholar]
- Yue Z, Huber R. Social status and amine levels in crayfish, Orconectes rusticus. in prep. [Google Scholar]
- Zulandt-Schneider RA, Schneider RWS, Moore PA. Recognition of dominance status by chemoreception in the red swamp crayfish, Procambarus clarkii. J. Chem. Ecol. 1999;25:781–794. [Google Scholar]