Abstract
Different patterns of expression of the transcription factors of Nur77 and Nor-1 are induced following acute administration of typical and atypical antipsychotic drugs. The pharmacological profile of atypical antipsychotics suggests that serotonergic and/or adrenergic receptors might contribute to these reported differences. In order to test this possibility, we examined the abilities of serotonin 5-HT1A and 5-HT2A/2C, and α1- and α2-adrenergic receptor drugs to modify the pattern of Nur77 (NR4A1) and Nor-1 (NR4A3) mRNA expression induced by haloperidol. Various groups of mice were treated with either saline, DOI, a 5-HT2A/2C agonist, MDL11939, a 5-HT2A antagonist, 8-OH-DPAT, a 5-HT1A agonist, prazosin, an α1-adrenergic antagonist and idazoxan, an α2-adrenergic antagonist, alone or in combination with haloperidol. The 5-HT2A/2C agonist DOI alone significantly increased Nur77 expression in the medial striatum and nucleus accumbens. DOI reduced Nor-1 expression, while MDL11939 increased the expression of this transcript in the cortex. Prazosin reduced Nur77 expression in the dorsal striatum and nucleus accumbens. Interestingly, 8-OH-DPAT and MDL11939 partially prevented haloperidol-induced Nur77 up-regulation, while MDL11939 completely abolished Nor-1 expression in the striatum. In addition, MDL11939 decreased haloperidol-induced Nur77 and Nor-1 mRNA levels in the ventral tegmental area. On the contrary, idazoxan (α2 antagonist) consistently potentiated haloperidol-induced Nur77, but not Nor-1 mRNA levels in the striatum, whereas prazosin (α1 antagonist) remained without effect. Taken together, these results show the ability of a 5-HT1A agonist or a 5-HT2A antagonist to reduce haloperidol-induced Nur77 and Nor-1 striatal expression, suggesting that these serotonin receptor subtypes participate in the differential pattern of gene expression induced by typical and atypical antipsychotic drugs.
Keywords: Antipsychotic drug, in-situ hybridization, noradrenalin, serotonin, striatum
Introduction
Nuclear receptors are a vast family of proteins that regulate gene transcription. These receptors provide multicellular organisms with a means to directly control gene expression in response to a wide range of developmental and physiological cues, as well as to internal and environmental stimuli. The NR4A subgroup is constituted of three closely related receptors (collectively called Nurs) ; i.e. Nur77 [NR4A1; also known as nerve-growth-factor inducible gene B (NGFI-B) and TR3], Nurr1 (NR4A2) and Nor-1 (NR4A3) (for review, see Maxwell & Muscat, 2005). Nurs are classified as early response genes and are induced by a wide range of stimuli, including growth factors, cytokines, peptide hormones, stress and neurotransmitters. Their ability to sense and rapidly respond to changes in the cellular environment appears to be a hallmark of this subgroup. The NR4A subgroup members are expressed in various part of the brain, but a close neuroanatomical association can be observed with the dopamine system. Basal expression of Nurr1 is found in the hippocampus, hypothalamus, cortex and most notably in midbrain areas containing dopamine producing neurons, i.e. the substantia nigra (SN) and ventral tegmental area (VTA) (Gofflot et al. 2007; Zetterström et al. 1996). Contrary to Nurr1, which is enriched in the mesencephalon, Nur77 and Nor-1 are mainly expressed in the forebrain, i.e. the olfactory bulb and tubercle, cortex, striatum, nucleus accumbens, hippocampal formation, hypothalamus and amygdala (Beaudry et al. 2000; Gofflot et al. 2007; Ponnio & Conneely, 2004; Werme et al. 2000; Zetterström et al. 1996). Their expression patterns suggest an involvement in various brain functions including cognition, emotion, reward, motivated behaviour, learning, memory and locomotion. Unfortunately, only a few of these functions have been thoroughly investigated so far.
Although gene targets for Nur77 and Nor-1 were not systematically characterized in the central nervous system, some evidence strongly suggest that neuropeptides known to be related to the dopamine system such as enkephalin and neurotensin might represent transcriptional targets for Nur77 (Ethier et al. 2004a; St-Hilaire et al. 2006). In addition, we have shown that catechol-O-methyltransferase (COMT) mRNA levels and activity are reduced in Nur77-deficient mice, suggesting that Nur77 could modulate COMT expression and participates in the control of enzymatic degradation of dopamine (Gilbert et al. 2006).
Antipsychotic drugs currently used in the treatment of schizophrenia can be classified as either typical or atypical antipsychotic drugs or neuroleptics. Typical neuroleptics such as haloperidol have a high propensity to cause a variety of extrapyramidal motor symptoms (Casey, 1991). New-generation atypical antipsychotics such as clozapine and olanzapine are defined as drugs active in the treatment of schizophrenia but with a lesser propensity to induce motor symptoms (Serretti et al. 2004). Haloperidol, a typical neuroleptic, increased Nur77 and Nor-1 mRNA levels in the striatum, a brain region involves in the control of locomotion (Beaudry et al. 2000; Maheux et al. 2005; Werme et al. 2000). Using Nur77 and Nor-1 induction patterns by various typical and atypical antipsychotic drugs, we have shown that modulation of Nur77 and Nor-1 mRNA levels can be used to calculate an index predictive of the typical vs. atypical profile of antipsychotic drugs (Maheux et al. 2005). Inductions of Nurs (Nur77 and Nor-1) can be correlated with dopamine D2 and D3 receptor affinities and serotonin (5-HT) 5-HT2A/D2 affinity ratios could also be used to predict Nur77 and Nor-1 patterns of expression. Interestingly, Nur77 mRNA up-regulation is maintained upon chronic typical antipsychotic drug treatments without any apparent desensitization, suggesting that Nur77 not only participates in the initiation of a neuroadaptive signalling cascade, but also in more prolonged effects (Beaudry et al. 2000; Langlois et al. 2001). As opposed to Nurr1, mRNA levels of Nur77 and Nor-1 are extremely low in the SN and VTA in basal conditions in the adult brain (Maheux et al. 2005). However, their expression can be significantly increased in these brain areas by administration of both typical and atypical antipsychotic drugs (Maheux et al. 2005). These data strongly suggest that Nur77 and Nor-1 expression is tightly regulated in central dopamine systems (for a review, see Lévesque & Rouillard, 2007).
It is generally recognized that the atypical profile of antipsychotic drugs is associated with their additional interaction with 5-HT receptor subtypes, i.e. blockade of 5-HT2A and agonism at the 5-HT1A receptors (Ichikawa & Meltzer, 2000; Ichikawa et al. 2001; Meltzer & Huang, 2008; Meltzer et al. 2003). These pharmacological properties are thought to be responsible for their lower propensity to generate extrapyramidal motor side-effects. Indeed, blockade of 5-HT2A/2C or activation of 5-HT1A in combination with administration of a typical neuroleptic was shown to prevent haloperidol-induced catalepsy (Ohno et al. 2008, 2009). Interaction of antipsychotic drugs with 5-HT2A and/or 5-HT1A receptors has also been shown to increase cortical dopamine release (Ichikawa & Meltzer, 2000; Ichikawa et al. 2001; Meltzer & Huang, 2008; Meltzer et al. 2003), suggesting that the effects on 5-HT receptor subtypes might also contribute to their antipsychotic clinical profile in addition to reducing motor side-effects.
Most antipsychotic drugs also interact with α1- and α2-adrenergic receptor subtypes. Although recent reports indicates that Nur77 and Nor-1 can be modulated by β-adrenergic receptor activity in the periphery (Maxwell & Muscat, 2005; Myers et al. 2009; Pearen et al. 2008) and by adrenergic drugs in the pineal gland (Humphries et al. 2004), no data is available on the modulation of Nur77 and Nor-1 by selective α-adrenergic receptors in the central nervous system. While most antipsychotic drugs display a strong α1-adrenergic receptor interaction, some atypical antipsychotic drugs such as clozapine and risperidone also display high affinity for the α2-adrenergic receptor subtype (Bymaster et al. 1996; Schotte et al. 1996). Interestingly, α2-adrenergic receptor blockade enhanced cortical dopamine release, reduced dopamine D2 receptor antagonist-induced conditioned avoidance response, in addition to reversing haloperidol-induced catalepsy (Hertel et al. 1999; Invernizzi et al. 2003; Wadenberg et al. 2007). Thus, α2-adrenergic receptors might also contribute to the clinical profile of these atypical antipsychotic drugs.
Distinct patterns of immediate-early gene (IEG) modulation have also been associated with the respective clinical profiles of typical and atypical antipsychotic drugs (Beaudry et al. 2000; Maheux et al. 2005; Robertson et al. 1994; Werme et al. 2000). An important number of studies on the effects of typical and atypical antipsychotics on the region-specific expression of IEG, such as Fos and Nur families, have been performed. Specifically, cortical IEG expression by atypical antipsychotics, such as clozapine, has been speculated to be indicative of beneficial effects against negative symptoms of schizophrenia, whereas typical antipsychotics, such as haloperidol, induce striatal IEG expression, an effect that is thought to be related to the extrapyramidal side-effects liability of dopamine D2 receptor antagonists (Bruins Slot et al. 2009; Maheux et al. 2005; Merchant & Dorsa, 1993; Robertson et al. 1994).
Since Nur77 and Nor-1 are distinctly modulated by typical and atypical antipsychotic drugs (Maheux et al. 2005) and Nur77 is closely related to antipsychotic drug motor effects such as catalepsy (acute Parkinsonism) and tardive dyskinesia (Ethier et al. 2004a, b), we conducted a series of pharmacological investigations aimed at exploring the contribution of serotonergic and adrenergic receptor subtypes in the pattern of expression Nur77 and Nor-1 induced by the conventional antipsychotic drug haloperidol. We report that 5-HT1A and 5-HT2A/2C receptors contribute significantly to the gene expression patterns induced by this antipsychotic drug.
Materials and methods
Animals
The experiments were performed on male wild-type C57BL/6 mice (Charles River, Canada) weighing 20–25 g. Animals were housed in groups of five per cage. They were maintained on a 12-h light/dark cycle (lights on 07:00 hours) under controlled temperature (24 °C) and humidity (40–50%). Food and drinking water were available ad libitum. Handling of mice was performed in accordance with the Canadian Guide for the Care and Use of Laboratory Animals and all procedures, including means to minimize discomfort, were approved by the institutional Animal Care Committee of University of Montreal. In order to minimize the possibility of stress-induced Nur expression, mice were handled for about 3 d prior to the day of experiment and we initiated strict environmental controls on all experimental procedures, including the use of one animal handler and injecting animals within the same room. After drug administration, the animals were kept in their home cage until anaesthetized and sacrificed.
Drugs
A 5 mg/ml solution of haloperidol was obtained commercially from Sabex Inc. (Canada) and diluted in saline to its final concentration. (±)DOI-HCl [(±) [1-(2,5-dimethoxy-4-iodophenyl)-aminopropane]-hydrochloride], (±)-8-OH-DPAT-HBr [8-hydroxy-2-(di-n-propylamino)-tetralin]-hydrobromide], prazosin and idazoxan were obtained from Sigma-Aldrich Canada Inc. (Canada). MDL11939 (α-phenyl-1-(2-phenylethyl)-4-piperidine-methanol), was purchased from Tocris Bioscience (USA).
Experimental protocols
Mice were acutely treated intraperitoneally (i.p.) with the different drugs in a final volume of 0.5 ml. Two series of experiments were performed in this study. Each group of animals consisted of five mice. For serotonin agents, treatments were: saline, DOI, a 5-HT2A/C agonist (2.5 mg/kg), 8-OH-DPAT, a selective 5-HT1A agonist (2.5 mg/kg), MDL11939, a 5-HT2A antagonist (2 mg/kg), haloperidol, a typical antipsychotic drug (0.5 mg/kg), DOI+haloperidol, 8-OH-DPAT+ haloperidol and MDL11939+haloperidol. For adrenergic agents, treatments were: saline, prazosin, a selective α1-adrenergic antagonist (1 mg/kg), idazoxan, a selective α2-adrenergic antagonist (1 mg/ kg), haloperidol (0.5 mg/kg), prazosin+haloperidol and idazoxan+haloperidol. All serotonergic or adrenergic drugs and saline were administered 30 min before saline (control groups) or haloperidol, so that all animals had received two injections. The animals were sacrificed by decapitation under CO2 anaesthesia 1 h after saline (controls) or haloperidol drug administration. All drug dosages and the time of sacrifice were based on data in the literature showing modulations of Nur77, Nor-1 or other IEGs, such as c-fos, and preliminary work from our laboratory (Beaudry et al. 2000; Gervais et al. 1999; Maheux et al. 2005; Marcus et al. 2005; Tremblay et al. 1998; Wadenberg et al. 2000). After decapitation, brains were rapidly removed and immediately immersed into cold isopentane (−40 °C) for a few seconds and kept at −80 °C until used.
In-situ hybridization procedure
Cryostat coronal brain sections (12 μm) were mounted onto Snowcoat X-tra™ slides (Canada) and stored at −80 °C until used. Brain sections were fixed in 4% paraformaldehyde at 4 °C for 20 min. Specific [35S]UTP-radiolabelled complementary RNA (cRNA) probes were used. The Nur77 probe preparation and radiolabelling have been described in detail elsewhere (Beaudry et al. 2000; Ethier et al. 2004a). The mouse Nor-1 probe was generated from a PCR fragment of 393 bp (from nucleotides 572 to 964) subcloned into pBluscript SK+ linearized with HindIII to generate to antisense cRNA (Maheux et al. 2005). Single-stranded riboprobes were synthesized and labelled using Promega riboprobe kit (Promega, USA), [35S]UTP (PerkinElmer Inc., Canada) and the RNA polymerase T7. In-situ hybridization of riboprobes with tissue sections was performed at 56–58 °C, overnight, in a standard hybridization buffer containing 50% formamide (Beaudry et al. 2000; Ethier et al. 2004a; Langlois et al. 2001; Maheux et al. 2005). Tissue sections were then apposed against BiomaxMR (Kodak, USA) radioactive sensitive films for 2–5 d.
Quantification analysis
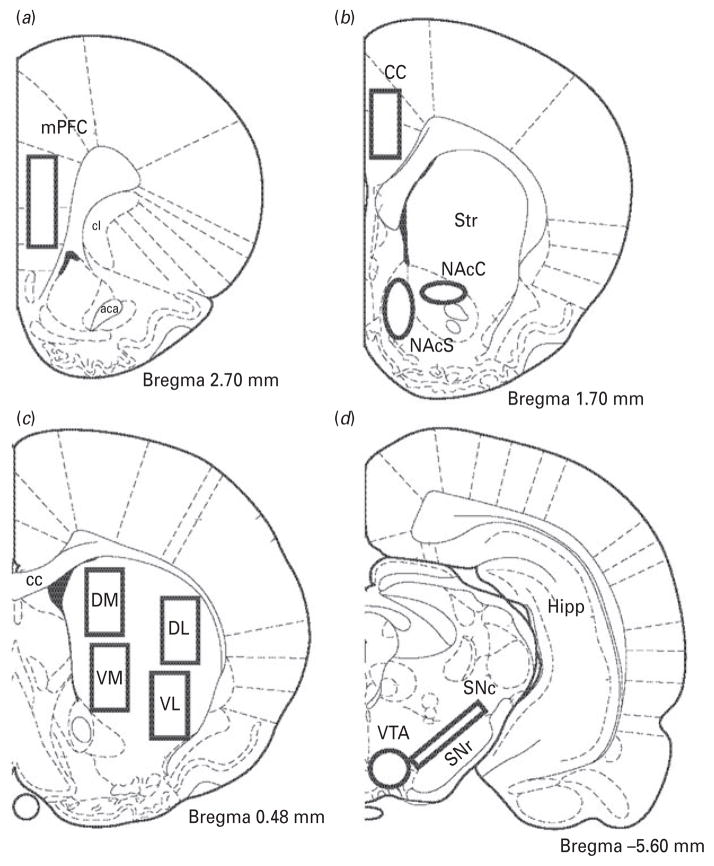
Levels of radioautographic labelling on films were quantified by computerized densitometry. Digitized brain images were obtained by a CCD camera model XC-77 (Sony) equipped with a 60-mm f/2.8D (Nikon) magnification lens. Images were analysed using the Image J 1.43u software (Wayne Rasband, NIH). Optical densities of autoradiograms were transformed to nCi/g of tissue using [14C] radioactivity standards (ARC 146A-14C standards, American Radiolabelled Chemicals Inc., USA). Brain areas investigated included the dorsolateral (StDL), dorsomedial (StDM), ventrolateral (StVL) and ventromedial (StVM) portions of the striatum, the shell (NAcS) and core (NAcC) of the nucleus accumbens, medial prefrontal cortex (mPFC), cingulate cortex (CC), substantia nigra pars compacta (SNc) and VTA. Figure 1 illustrates the exact coordinates and brain areas used for quantification of mRNA levels.
Fig. 1.
Schematic illustration of the mouse brain regions selected for quantitative analysis of Nur mRNA levels. Blank boxes indicate the sampled areas in (a) the medial prefrontal cortex (mPFC), (b) nucleus accumbens core (NAcC), nucleus accumbens shell (NAcS), and cingulate cortex (CC), (c) dorsomedial (DM), dorsolateral (DL), ventromedial (VM) and ventrolateral (VL) portions of the striatum, and (d) substantia nigra pars compacta (SNc) and ventral tegmental area (VTA). Corresponding Bregma levels are indicated in respective diagrams. Other abbreviations shown are : aca, anterior commissure ; cl, clustrum; Str, striatum; cc, corpus callosum; Hipp, hippocampus; SNr, substantia nigra pars reticulata.
Statistical analysis
For each animal and for all brain regions investigated, we measured Nur mRNA levels on four different sections. Average signals from both brain hemispheres were made. All data were then expressed as group mean±S.E.M. from five animals per group. Homogeneity of variances was first determined with Bartlett’s χ2 test, and square root or log data transformation was performed to increase homogeneity when necessary. Statistical analyses of mRNA level variances were performed using a one-way ANOVA. When a significant variance analysis was observed, Tukey’s test was performed as post-hoc analysis. Statistical analyses and graphs were performed with GraphPad Prism version 4.0 software (GraphPad Software Inc., USA).
Results
As previously reported, there is significant basal expression of Nur77 and Nor-1 mRNA in the mouse forebrain including the StDM, StDL, mPFC, CC and NAc. Interestingly, Nur77 and Nor-1 mRNA are barely detectable in the SN/VTA complex in untreated animals (Maheux et al. 2005; Zetterström et al. 1996). As previously observed, haloperidol induced strong upregulations of Nur77 and Nor-1 mRNA levels in the StVL and StVM and in the SN/VTA complex (Maheux et al. 2005). In this study, haloperidol only upregulated Nor-1 in the PFC and CC. The absolute Nur77 and Nor-1 mRNA values in the vehicle-treated animals expressed in nCi/g of tissue are presented in Table 1. These values have been used to determine % of control levels presented in the following figures and tables.
Table 1.
Nur77 and Nor-1 mRNA levels in vehicle-treated animals in the brain areas analysed
| Brain areas | mRNA levels (nCi/g tissue)
|
|
|---|---|---|
| Nur77 | Nor-1 | |
| mPFC | 389±46 | 123±12 |
| CC | 445±33 | 106±16 |
| NAcS | 103±13 | 42±4 |
| NAcC | 151±21 | 29±2 |
| StDM | 207±11 | 70±6 |
| StDL | 177±14 | 19±2 |
| StVM | 105±8 | 24±4 |
| StVL | 38±7 | 31±4 |
| SNc | 4±2 | 8±1 |
| VTA | 6±2 | 5±2 |
CC, Cingulate cortex ; mPFC, medial prefrontal cortex ; NAcC, nucleus accumbens core ; NAcS, nucleus accumbens shell ; SNc, substantia nigra pars compacta ; StDM, dorsomedial striatum; StDL, dorsolateral striatum ; StVM, ventromedial striatum ; StVL, ventrolateral striatum; VTA, ventral tegmental area.
Values represent mean±S.E.M. from vehicle-treated animals (n=5).
Effects of serotonergic drugs on haloperidol-induced Nur expression
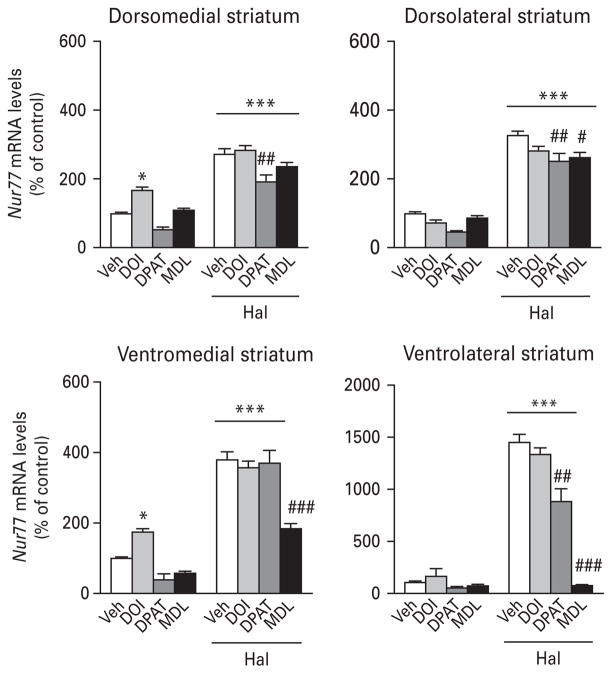
Treatment of animals with the 5-HT2A/2C agonist DOI alone increased Nur77 mRNA levels in the NAc (Table 2) and medial striatum (Fig. 2, left panels). However, DOI remained without effect on haloperidol-induced Nur77 mRNA levels in all brain areas investigated (Fig. 2, Table 2). Administration of the 5-HT1A agonist 8-OH-DPAT alone did not alter Nur77 expression, but significantly reduced haloperidol-induced Nur77 mRNA levels in the striatum (except in the StVM portion) and NAcS (Fig. 2, Table 2). The 5-HT2A antagonist MDL11939 also remained inactive when administered alone, except for a small increase in the CC (Table 2). But, it significantly reduced haloperidol-induced Nur77 expression in the striatum, except for the StDM subterritory (Fig. 2). MDL11939 also decreased haloperidol-induced Nur77 expression in the VTA, but remained without effect in the SNc (Table 2). We did not observe any significant modulation of Nur77 in the PFC or CC (Table 2).
Table 2.
Effects of serotonin drugs on Nur77 expression in extra-striatal areas
| Veh | DOI | 8-OH-DPAT | MDL11939 | Hal | DOI+Hal | 8-OH-DPAT+Hal | MDL11939+Hal | |
|---|---|---|---|---|---|---|---|---|
| Nur77 mRNA levels (% of vehicle) | ||||||||
| mPFC | 100±12 | 133±14 | 125±11 | 149±11 | 148±12 | 122±19 | 115±7 | 143±15 |
| CC | 100±7 | 133±8 | 100±12 | 143±11* | 116±7 | 100±11 | 140±13 | 135±6 |
| NAcS | 100±12 | 275±36* | 233±32 | 153±18 | 338±21** | 434±39*** | 562±75***## | 442±38*** |
| NAcC | 100±14 | 218±26* | 149±39 | 209±31 | 267±42** | 347±27*** | 305±31*** | 323±23*** |
| SNc | 100±58 | 264±103 | 201±129 | 63±36 | 2146±342*** | 1777±235*** | 1996±303*** | 1726±337*** |
| VTA | 100±40 | 144±43 | 93±17 | 111±31 | 1418±155*** | 1186±180*** | 1024±37*** | 848±115***## |
CC, Cingulate cortex ; Hal, haloperidol; mPFC, medial prefrontal cortex ; NAcC, nucleus accumbens core ; NAcS, nucleus accumbens shell ; SNc, substantia nigra pars compacta ; VTA, ventral tegmental area ; Veh, vehicle.
Values represent mean±S.E.M. from five animals per group
p<0.05,
p<0.01,
p<0.001 vs. Veh group;
p<0.01 vs. haloperidol group.
Fig. 2.
Histograms illustrating the effect of 5-HT drugs on haloperidol-induced Nur77 mRNA levels in the mouse striatum. Values are expressed as percentage of change compared to vehicle-treated animals (control) and represent mean±S.E.M. Each group included five animals. See Materials and methods section for dose regimens. Veh, Vehicle ; DPAT, 8-OH-DPAT; MDL, MDL11939 ; Hal, haloperidol (* p<0.05, *** p<0.001 vs. Veh; # p<0.05, ## p<0.01, ### p<0.001 vs. Hal).
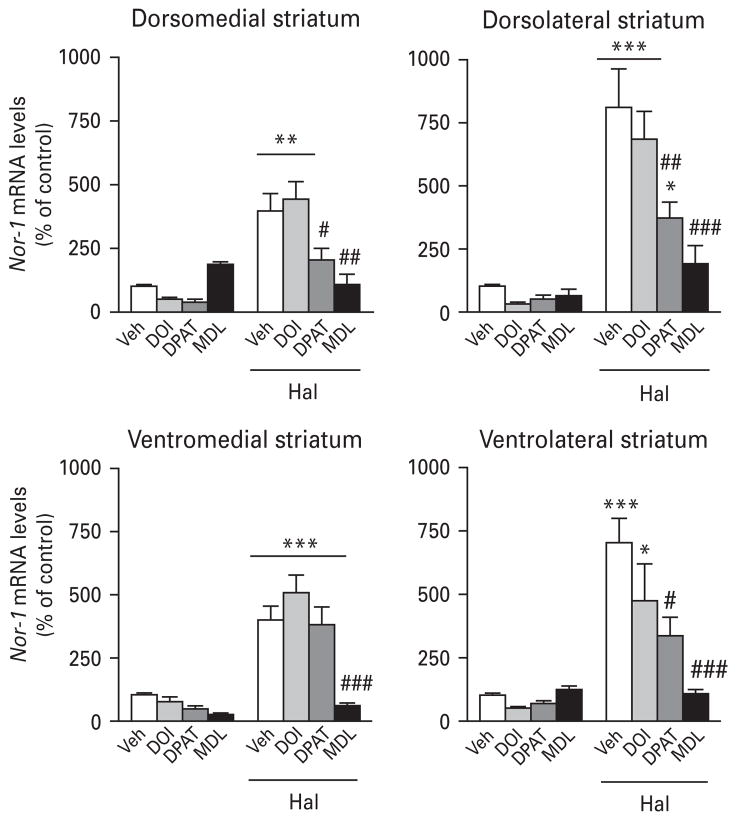
DOI alone significantly reduced Nor-1 mRNA levels in the PFC and CC, whereas MDL11939 significantly up-regulated Nor-1 expression in the cortex (Table 3). However, DOI did not alter haloperidol-induced Nor-1 expression in any of the brain areas investigated (Fig. 3, Table 3). The 5-HT1A agonist 8-OH-DPAT alone did not alter the expression of Nor-1 in the brain area investigated (Fig. 3, Table 3). However, pre-treatment with the 5-HT1A agonist was able to reduce haloperidol-induced Nor-1 mRNA levels in many brain areas, including the cortex, VTA and striatum (Fig. 3, Table 3). The most striking effects were observed with pre-treatment with the 5-HT2A antagonist MDL11939, which strongly reduced or totally prevented haloperidol-induced Nor-1 in all the brain areas investigated, except for the SNc (Fig. 3, Table 3).
Table 3.
Effects of serotonin drugs on Nor-1 expression in extra-striatal areas
| Veh | DOI | 8-OH-DPAT | MDL11939 | Hal | DOI+Hal | 8-OH-DPAT+Hal | MDL11939+Hal | |
|---|---|---|---|---|---|---|---|---|
| Nor-1 mRNA levels (% of vehicle) | ||||||||
| mPFC | 100±10 | 35±3* | 71±18 | 194±27* | 241±38* | 236±50* | 115±16# | 68±20## |
| CC | 100±15 | 34±9* | 58±15 | 220±45* | 228±34* | 211±56* | 119±20# | 41±9## |
| NAcS | 100±9 | 46±16 | 180±58 | 257±58 | 589±60** | 526±131** | 773±80*** | 101±19### |
| NAcC | 100±8 | 80±25 | 124±40 | 314±76* | 654±94*** | 642±148** | 407±66** | 108±22### |
| SNc | 100±18 | 118±46 | 47±7 | 42±17 | 975±162*** | 682±88** | 889±135*** | 743±125*** |
| VTA | 100±36 | 159±47 | 103±19 | 123±34 | 1608±188*** | 1307±199*** | 1128±41***# | 911±114***## |
CC, Cingulate cortex ; Hal, haloperidol; mPFC, medial prefrontal cortex ; NAcC, nucleus accumbens core ; NAcS, nucleus accumbens shell ; SNc, substantia nigra pars compacta ; VTA, ventral tegmental area ; Veh, vehicle.
Values represent mean±S.E.M. from five animals per group
p<0.05,
p<0.01,
p<0.001 vs. Veh group;
p<0.05,
p<0.01,
p<0.001 vs. haloperidol group.
Fig. 3.
Histograms illustrating the effect of 5-HT drugs on haloperidol-induced Nor-1 mRNA levels in the mouse striatum. Values are expressed as percentage of change compared to vehicle-treated animals (control) and represent mean±S.E.M. Each group included five animals. See Materials and methods for dose regimens. Veh, Vehicle ; DPAT, 8-OH-DPAT; MDL, MDL11939 ; Hal, haloperidol (* p<0.05, ** p<0.01, *** p<0.001 vs. Veh; # p<0.05, ## p<0.01, ### p<0.001 vs. Hal).
Effects of adrenergic drugs on haloperidol-induced Nur expression
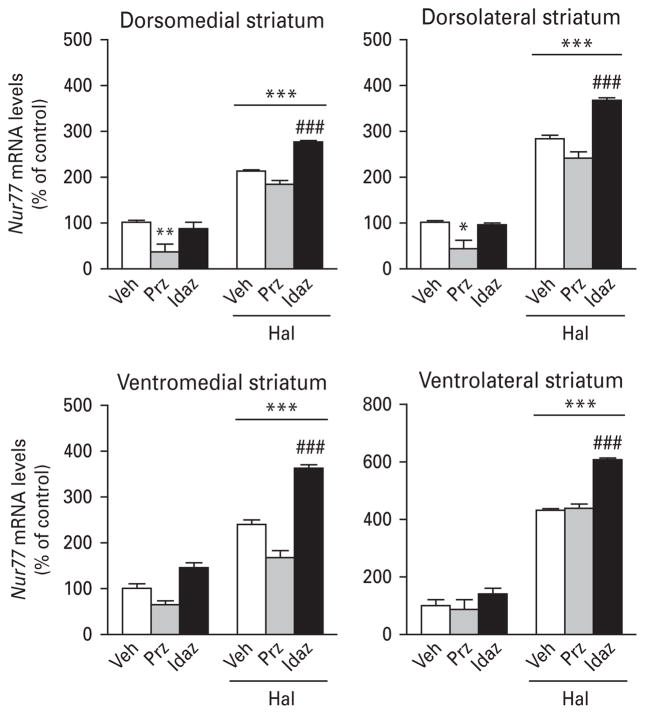
Treatment with prazosin (an α1-adrenergic antagonist) alone significantly reduced basal Nur77 mRNA levels in the NAc (Table 4) and dorsal striatum (Fig. 4, top panels), while idazoxan (an α2-adrenergic antagonist) had no effect in all brain regions investigated (Fig. 4, Table 4). On the other hand, pre-treatment with idazoxan potentiated haloperidol-induced Nur77 expression in the SNc, NAcS and all striatal subterritories (Fig. 4, Table 4). Pre-treatment with prazosin also induced a potentiation of haloperidol-induced Nur77 mRNA levels in the VTA (Table 4) and a small but significant increase in the NAcC (Table 4). But, prazosin had no effect on haloperidol-induced Nur77 expression in all striatal subterritories (Fig. 4).
Table 4.
Effects of adrenergic drugs on Nur77 expression in extra-striatal areas
| Veh | Prazosin | Idazoxan | Haloperidol | Prazosin+Hal | Idazoxan+Hal | |
|---|---|---|---|---|---|---|
| Nur77 mRNA levels (% of vehicle) | ||||||
| mPFC | 100±14 | 73±4 | 151±10 | 116±9 | 131±9 | 150±18 |
| CC | 100±16 | 62±16 | 125±16 | 109±11 | 124±8 | 148±9 |
| NAcS | 100±12 | 41±20* | 77±14 | 221±11*** | 216±4*** | 304±10***## |
| NAcC | 100±1 | 38±4** | 96±5 | 250±9*** | 197±6***## | 263±6*** |
| SNc | 100±23 | 132±35 | 141±20 | 772±80*** | 827±93*** | 1094±119***# |
| VTA | 100±18 | 113±18 | 104±11 | 475±60*** | 707±69***# | 618±55*** |
CC, Cingulate cortex ; Hal, haloperidol; mPFC, medial prefrontal cortex ; NAcC, nucleus accumbens core ; NAcS, nucleus accumbens shell ; SNc, substantia nigra pars compacta; VTA, ventral tegmental area ; Veh, vehicle.
Values represent mean±S.E.M. from five animals per group
p<0.05,
p<0.01,
p<0.001 vs. Veh group;
p<0.05,
p<0.01 vs. haloperidol group.
Fig. 4.
Histograms illustrating the effect of adrenergic drugs on haloperidol-induced Nur77 mRNA levels in the mouse striatum. Values are expressed as percentage of change compared to vehicle-treated animals (control) and represent mean±S.E.M. Each group included five animals. See Materials and methods section for dose regimens. Veh, Vehicle ; Prz, prazosin; Idaz, idazoxan; Hal, haloperidol (* p<0.05, ** p<0.01, *** p<0.001 vs. Veh; ### p<0.001 vs. Hal).
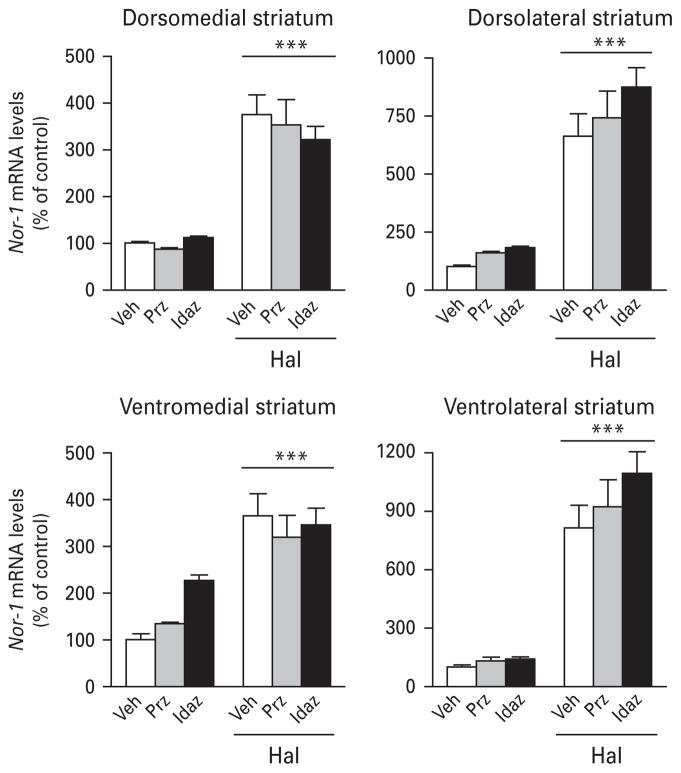
Contrary to Nur77 expression, adrenergic drugs had no effect on basal or haloperidol-induced Nor-1 expression (Fig. 5), except for the α2-adrenergic antagonist idazoxan, which potentiated haloperidol-induced Nor-1 expression in the NAcS (Table 5). Idazoxan also selectively reduced haloperidol-induced Nor-1 in the VTA, whereas prazosin remained without effect (Table 5).
Fig. 5.
Histograms illustrating the effect of adrenergic drugs on haloperidol-induced Nor-1 mRNA levels in the mouse striatum. Values are expressed as percentage of change compared to vehicle-treated animals (control) and represent mean±S.E.M. Each group included five animals. See Materials and methods section for dose regimens. Veh, Vehicle ; Prz, prazosin ; Idaz, idazoxan; Hal, haloperidol (*** p<0.001 vs. Veh).
Table 5.
Effects of adrenergic agents on Nor-1 expression in extra-striatal areas
| Veh | Prazosin | Idazoxan | Hal | Prazosin+Hal | Idazoxan+Hal | |
|---|---|---|---|---|---|---|
| Nor-1 mRNA levels (% of vehicle) | ||||||
| mPFC | 100±11 | 114±7 | 110±5 | 72±3 | 110±14 | 110±17 |
| CC | 100±8 | 110±11 | 109±9 | 81±7 | 129±10# | 88±9 |
| AcSh | 100±4 | 113±11 | 109±16 | 312±29*** | 384±21*** | 452±36***## |
| AcC | 100±10 | 127±14 | 117±7 | 333±29*** | 366±53*** | 304±21*** |
| SNc | 100±17 | 89±29 | 85±6 | 208±29* | 216±24* | 195±19* |
| VTA | 100±14 | 77±22 | 72±19 | 177±15* | 175±8* | 95±8# |
CC, Cingulate cortex ; Hal, haloperidol; mPFC, medial prefrontal cortex ; NAcC, nucleus accumbens core ; NAcS, nucleus accumbens shell ; SNc, substantia nigra pars compacta; VTA, ventral tegmental area ; Veh, vehicle.
Values represent mean±S.E.M. from five animals per group
p<0.05,
p<0.001 vs. Veh group;
p<0.05,
p<0.01 vs. haloperidol group.
Discussion
We and others have previously shown that typical and atypical antipsychotic drugs induced distinct patterns of expression of Nur77 and Nor-1mRNA levels in brain areas related to their clinical efficacy, i.e. the PFC, CC, NAc, striatum and SN/VTA complex (Beaudry et al. 2000; Bruins Slot et al. 2009; Maheux et al. 2005; Werme et al. 2000). These transcription factors are strongly up-regulated in striatal areas associated with locomotor functions by typical antipsychotic drugs, whereas atypical drugs induced only mild effects in these areas. Given the preferentially high affinity of atypical antipsychotics for some 5-HT receptors and most notably for 5-HT1A and 5-HT2A receptors, it has been postulated for decades that 5-HT and its receptors are key mediators for the actions of atypical antipsychotics (Meltzer, 1999). In support of this hypothesis, we show here that a 5-HT1A receptor agonist or a 5-HT2A antagonist can reduce haloperidol-induced Nur77 and Nor-1 mRNA up-regulation in the striatum. As previously demonstrated with c-fos expression (Bruins Slot et al. 2009; Ohno et al. 2008; Tremblay et al. 1998), the present results demonstrate that it is possible to transform the pattern of expression of Nur77 and Nor-1 induced by haloperidol into a pattern resembling that induced by atypical antipsychotics simply with the addition of drugs targeting these 5-HT receptor subtypes. We also demonstrate that adrenergic receptor blockade is not involved. On the contrary, α2-adrenergic receptor blockade potentiates the effect of haloperidol on Nur77 mRNA levels in the striatum.
Interestingly, our results suggest that both 5-HT2A and α1-adrenergic receptors, but not 5-HT1A receptors, are involved in Nur expression in basal conditions. Briefly, basal expression of Nur77 in the striatum is modulated by 5-HT2A and α1-adrenergic drugs, whereas basal expression of Nor-1 in the cortex is modulated only by 5-HT2A receptors. Concomitant activation of serotonin 5-HT2A receptors has no effect, but blockade of 5-HT2 receptors strongly reduced haloperidol-induced Nur77 expression in the ventral striatum, VTA, and almost totally abolished haloperidol-induced expression of Nor-1 in all the brain areas investigated, except within the SN. The regionally selective effect of MDL11939 (5-HT2A antagonist) might be explained in part by the medioventral to dorsolateral gradients of expression of this 5-HT2 receptor subtype (Appel et al. 1990; Laprade et al. 1996). The α1-adrenergic antagonist prazosin had no effect on haloperidol-induced Nur77 expression, whereas the α2-adrenergic receptor antagonist idazoxan potentiated haloperidol-induced Nur77 expression in the NAcS, the entire striatum and SN. No effect was observed on Nor-1 expression. Activation of 5-HT1A receptors had no effect on basal expression of Nur77 and Nor-1. However, when these nuclear receptors are up-regulated by haloperidol, both 5-HT1A agonism or 5-HT2A antagonism can be involved, whereas adrenergic receptors are not. On the other hand, haloperidol-induced Nur77 and Nor-1 expression are further up-regulated in the NAcS by a 5-HT1A agonist. Taken together these results on gene transcription are consistent with the beneficial effect of 5-HT1A and 5-HT2A receptor subtypes in reducing dopamine nigrostriatal pathway activity, while promoting dopamine mesolimbic pathway activity.
Therefore, our results clearly indicate that 5-HT receptors contribute to the distinct transcriptional patterns induced by typical and atypical antipsychotic drugs. But how? There are at least two possibilities. First, it could be an indirect effect through the modulation of dopamine release. Indeed, there is considerable evidence obtained from microdialysis and electrophysiological studies that 5-HT2A receptor antagonists modulate differentially nigrostriatal, mesolimbic and mesocortical dopamine systems (Meltzer et al. 2003). Administration of selective D2 antagonists to rodents produces large increases in extracellular dopamine concentrations in the striatum and NAc and only a modest one in the PFC (Meltzer et al. 2003). Administration of a selective 5-HT2A receptor antagonist alone had little effect on dopamine release in any of these brain regions. However, when the 5-HT2A and the D2 antagonists are combined, there is an increase in dopamine release in the PFC, but no change in the striatum (Andersson et al. 1995; Liégeois et al. 2002; Westerink et al. 2001). These differential effects on regional dopamine release are thought to be involved in both gene expression patterns and in the differential clinical profile of atypical antipsychotic drugs. A second possibility is a direct effect on gene expression pattern via their effects on various receptor subtypes. We and others have previously demonstrated that Nur77 and Nor-1 can be modulated by 5-HT1A and 5-HT2A/2C receptors (present data and Bruins Slot et al. 2009; Gervais et al. 1999). We show here that DOI, a 5-HT2A/2C agonist, administered alone increased Nur77 in NAc and medial striatum (limbic portions of the striatal complex), while it reduced Nor-1 in the PFC and CC. On the other hand, MDL11939, a preferential antagonist of the 5-HT2A receptor, increased Nor-1 in cortical areas. This strongly suggests that Nur77 and Nor-1 are differentially modulated by 5-HT neurotransmission in the mouse forebrain. However, DOI did not alter haloperidol-induced Nur77 and Nor-1 expression patterns. This indicates that activation of 5-HT2 receptors participates in the tonic basal expression of Nur77, but it is the blockade of 5-HT2 that is responsible for the reduction of haloperidol-induced Nur77 expression. In addition, haloperidol-induced Nor-1 mRNA levels are strongly modulated by 5-HT antagonists in striatal and extra-striatal brain areas, suggesting that this transcription factor is particularly sensitive to manipulation of 5-HT neurotransmission. It is interesting to note that vector delivery of a Nor-1 short-hairpin RNA (shRNA) in the brain ameliorated depressive-like behaviours in Wistar–Kyoto rats (Schaffer et al. 2010).
It is also interesting to observe that in absence of D2 blockade, the 5-HT2 antagonist increased Nor-1 mRNA levels, while it reduced haloperidol-induced Nor-1 expression in the cortex. At the present time, we have no explanation for this paradoxical effect of MDL11393. The 5-HT1A agonist (8-OH-DPAT) is also able to reduce haloperidol-induced Nor-1 expression in the cortex. Modulations of the haloperidol-induced pattern of Nor-1 expression in the cortex are reminiscent of the modulation of dopamine release in the cortex upon combined administration of a 5-HT1A agonist or a 5-HT2A antagonist with a dopamine D2 receptor antagonist, as previously discussed. Interestingly, these effects were not observed with Nur77, suggesting that Nor-1 might be a better marker of atypicality in the cortex compared to Nur77.
It has been shown that striatal 5-HT2 receptors exert a positive control on basal dopamine release and mediate a tonic inhibitory serotonergic tone on dopamine neurons in the VTA. Consequently, administration of 5-HT2 receptor antagonists can directly increase dopamine release in the NAc and the PFC, and 5-HT2 agonists can suppress dialysate levels of dopamine in the frontal cortex (Gobert & Millan, 1999; Millan et al. 1998). Interestingly, MDL11939 (5-HT2 antagonist) displayed a selective effect on Nur77 and Nor-1 mRNA levels in the VTA, compared to the SN indicating that a 5-HT2 antagonist can also exert a selective transcriptional activity within dopamine neurons of the mesolimbic and mesocortical pathways.
8-OH-DPAT, a 5-HT1A agonist, tends to reduce Nur77 in the striatum, which is consistent with a previous report (Gervais et al. 1999). Although we used a higher dose of 8-OH-DPAT, modulations of haloperidol-induced Nur77 and Nor-1 expression in the striatum by this 5-HT1A agonist are also consistent with the report of Bruins Slot and colleagues (2009). The partial activity of the 5-HT1A agonist might result from interactions at both pre-synaptic autoreceptor and post-synaptic sites by the present 8-OH-DPAT dose. These effects are also similar to previous data on striatal Fos immunohistochemistry or c-fos mRNA levels (Bruins Slot et al. 2009; Ohno et al. 2008; Tremblay et al. 1998). One of the proposed mechanism for these effects is a reduction of the activity of 5-HT projections (through stimulation of pre-synaptic 5-HT1A autoreceptors or blockade of post-synaptic 5-HT2A receptors) that inhibit dopaminergic nigrostriatal neurons, thus increasing striatum dopamine levels, which partially or totally overcome the blockade of D2 receptors by the antipsychotic drug.
Post-synaptic α1- and α2-adrenergic receptors are highly expressed in the cerebral cortex, while presynaptic α2-adrenergic receptors are also present in noradrenergic terminals and locus coeruleus neurons. Therefore, they are well placed to exert an important modulation on dopamine neurotransmission (Shen & Gundlach, 2000). The present results indicate that Nur77, but not Nor-1 expression, can be modulated by an α2-adrenergic antagonist in the mouse forebrain in the presence of haloperidol, whereas the α1-adrenergic drug remained without effect in the striatum and cortex. Similar data were obtained using c-fos expression (Fink-Jensen et al. 1995) or catalepsy behaviour (Wadenberg et al. 2000, 2007). Interestingly, it has been shown that prazosin can selectively modulate the firing pattern of dopamine neurons in the VTA (Grenhoff & Svensson, 1993). We also observed a selective transcriptional activity on Nur77 by prazosin in the VTA, while idazoxan had a preferential activity in the SN. Potentiation of the effect of haloperidol by idazoxan was surprising because it has been shown that α2-adrenergic antagonists are able to reduce haloperidol-induced catalepsy (Invernizzi et al. 2003; Wadenberg et al. 2007). However, the mechanism of the effect of α2-adrenergic receptors might be complex, since 5-HT neurotransmission seems to be involved in the effect of idazoxan (Invernizzi et al. 2003). In addition, the contribution of other receptor targets, such as histamine H1, 5-HT6, 5-HT7 or muscarinic M1–M4, cannot be excluded. Further experiments will be necessary to determine their contributions to Nur77 and Nor-1 gene expression patterns. Muscarinic M1–M4 receptors are of particular interest (Conn et al. 2009), but co-administration of scopolamine, a muscarinic M1–M4 antagonist, failed to reduced haloperidol-induced Nur77 expression in the striatum (J. Maheux & D. Lévesque, unpublished observations).
In summary, our results clearly indicate that 5-HT receptors contribute to the distinct transcriptional patterns induced by typical and atypical antipsychotic drugs. While the exact nature of the influence of antipsychotic drug-induced Nur77 and Nor-1 expression on downstream in-vivo responses remains to be clarified, the region-specific modulations of these transcription factors may constitute useful markers of antipsychotic drug activity and to help predict the clinical profile of potential antipsychotic drugs in development.
Acknowledgments
We acknowledge the support of grants from the Canadian Institutes for Health Research (CIHR) and the Stanley Medical Research Institute (SMRI). J.M. holds a Michael Smith honorific fellowship for research on schizophrenia of the CIHR.
Footnotes
Statement of Interest
None.
References
- Andersson JL, Nomikos GG, Marcus M, Hertel P, et al. Ritanserin potentiates the stimulatory effects of raclopride on neuronal activity and dopamine release selectivity in the mesolimbic dopaminergic system. Naunyn-Schmiedebergs Archives of Pharmacology. 1995;352:374–385. doi: 10.1007/BF00172774. [DOI] [PubMed] [Google Scholar]
- Appel NM, Mitchell WM, Garlick RK, Glennon RA, et al. Autoradiographic characterization of (+/−)-1-(2,5-dimethoxy-4-[125I]iodophenyl)-2-aminopropane ([125I]DOI) binding to 5-HT2 and 5-HT1c receptors in rat brain. Journal of Pharmacology & Experimental Therapeutics. 1990;255:843–857. [PubMed] [Google Scholar]
- Beaudry G, Langlois M-C, Weppe I, Rouillard C, et al. Contrasting patterns and cellular specificity of transcriptional regulation of the nuclear receptor nerve growth factor-inducible B by haloperidol and clozapine in the rat forebrain. Journal of Neurochemistry. 2000;75:1694–1702. doi: 10.1046/j.1471-4159.2000.0751694.x. [DOI] [PubMed] [Google Scholar]
- Bruins Slot LA, Lestienne F, Grevoz-Barret C, Newman-Tancredi A, et al. F15063, a potential antipsychotic with dopamine D2/D3 receptor antagonist and 5-HT1A receptor agonist properties : influence on immediate-early gene expression in rat prefrontal cortex and striatum. European Journal of Pharmacology. 2009;620:27–35. doi: 10.1016/j.ejphar.2009.08.019. [DOI] [PubMed] [Google Scholar]
- Bymaster FP, Calligaro DO, Falcone JF, Marsh RD, et al. Radioreceptor binding profile of the atypical antipsychotic olanzapine. Neuropsychopharmacology. 1996;14:87–96. doi: 10.1016/0893-133X(94)00129-N. [DOI] [PubMed] [Google Scholar]
- Casey DE. Neuroleptic drug-induced extrapyramidal syndromes and tardive dyskinesia. Schizophrenia Research. 1991;4:109–120. doi: 10.1016/0920-9964(91)90029-q. [DOI] [PubMed] [Google Scholar]
- Conn PJ, Jones CK, Lindsley CW. Subtype-selective allosteric modulators of muscarinic receptors for the treatment of CNS disorders. Trends in Pharmacological Sciences. 2009;30:148–155. doi: 10.1016/j.tips.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ethier I, Beaudry G, St-Hilaire M, Milbrandt J, et al. The transcription factor NGFI-B (Nur77) and retinoids play a critical role in acute neuroleptic-induced extrapyramidal effect and striatal neuropeptide gene expression. Neuropsychopharmacology. 2004a;29:335–346. doi: 10.1038/sj.npp.1300318. [DOI] [PubMed] [Google Scholar]
- Ethier I, Kagechika H, Shudo K, Rouillard C, et al. Docosahexaenoic acid reduces haloperidol-induced dyskinesias in mice: involvement of Nur77 and retinoid receptors. Biological Psychiatry. 2004b;56:522–526. doi: 10.1016/j.biopsych.2004.06.036. [DOI] [PubMed] [Google Scholar]
- Fink-Jensen A, Ludvigsen TS, Korsgaard N. The effect of clozapine on Fos protein immunoreactivity in the rat forebrain is not mimicked by the addition of alpha1-adrenergic or 5HT2 receptor blockade to haloperidol. Neuroscience Letters. 1995;194:77–80. doi: 10.1016/0304-3940(95)11731-b. [DOI] [PubMed] [Google Scholar]
- Gervais J, Soghomonian J-J, Richard D, Rouillard C. Dopamine and serotonin interactions in the modulation of the expression of the immediate-early transcription factor, nerve growth factor-inducible B, in the striatum. Neuroscience. 1999;91:1045–1054. doi: 10.1016/s0306-4522(98)00688-5. [DOI] [PubMed] [Google Scholar]
- Gilbert F, Morissette M, St-Hilaire M, Paquet B, et al. Nur77 gene knockout alters dopamine neuron biochemical activity and dopamine turnover. Biological Psychiatry. 2006;60:538–547. doi: 10.1016/j.biopsych.2006.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobert A, Millan MJ. Serotonin (5-HT)2A receptor activation enhances dialysate levels of dopamine and noradrenaline, but not 5-HT, in the frontal cortex of freely-moving rats. Neuropharmacology. 1999;38:315–317. doi: 10.1016/s0028-3908(98)00188-9. [DOI] [PubMed] [Google Scholar]
- Gofflot F, Chartoire N, Vasseur L, Heikkinen S, et al. Systematic gene expression mapping clusters nuclear receptors according to their function in the brain. Cell. 2007;131:405–418. doi: 10.1016/j.cell.2007.09.012. [DOI] [PubMed] [Google Scholar]
- Grenhoff J, Svensson TH. Prazosin modulates the firing pattern of dopamine neurons in rat ventral tegmental area. European Journal of Pharmacology. 1993;233:79–84. doi: 10.1016/0014-2999(93)90351-h. [DOI] [PubMed] [Google Scholar]
- Hertel P, Fagerquist MV, Svensson TH. Enhanced cortical dopamine output and antipsychotic-like effects of raclopride by α2 adrenoceptor blockade. Science. 1999;286:105–107. doi: 10.1126/science.286.5437.105. [DOI] [PubMed] [Google Scholar]
- Humphries A, Weller J, Klein D, Baler R, et al. NGFI-B (Nur77/Nr4a1) orphan nuclear receptor in rat pinealocytes : circadian expression involves an adrenergic-cyclic AMP mechanism. Journal of Neurochemistry. 2004;91:946–955. doi: 10.1111/j.1471-4159.2004.02777.x. [DOI] [PubMed] [Google Scholar]
- Ichikawa J, Ishii H, Bonaccorso S, Fowler WL, et al. 5-HT2A and D2 receptor blockade increases cortical DA release via 5-HT1A receptor activation : a possible mechanism of atypical antipsychotic-induced cortical dopamine release. Journal of Neurochemistry. 2001;76:1521–1531. doi: 10.1046/j.1471-4159.2001.00154.x. [DOI] [PubMed] [Google Scholar]
- Ichikawa J, Meltzer HY. The effect of serotonin1A receptor agonism on antipsychotic drug-induced dopamine release in rat striatum and nucleus accumbens. Brain Research. 2000;858:252–263. doi: 10.1016/s0006-8993(99)02346-x. [DOI] [PubMed] [Google Scholar]
- Invernizzi RW, Garavaglia C, Samanin R. The α2-adrenoceptor antagonist idazoxan reverses catalepsy induced by haloperidol in rats independent of striatal dopamine release : role of serotonergic mechanisms. Neuropsychopharmacology. 2003;28:872–879. doi: 10.1038/sj.npp.1300119. [DOI] [PubMed] [Google Scholar]
- Langlois M-C, Beaudry G, Zekki H, Rouillard C, et al. Impact of antipsychotic drug administration on the expression of nuclear receptors in the neocortex and striatum of the rat brain. Neuroscience. 2001;106:117–128. doi: 10.1016/s0306-4522(01)00248-2. [DOI] [PubMed] [Google Scholar]
- Laprade N, Radja F, Reader TA, Soghomonian JJ. Dopamine receptor agonists regulate levels of the serotonin 5-HT2A receptor and its mRNA in a subpopulation of rat striatal neurons. Journal of Neuroscience. 1996;16:3727–3736. doi: 10.1523/JNEUROSCI.16-11-03727.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lévesque D, Rouillard C. Nur77 and retinoid X receptors : crucial factors in dopamine-related neuroadaptation. Trends in Neurosciences. 2007;30:22–30. doi: 10.1016/j.tins.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liégeois J-F, Ichikawa J, Meltzer HY. 5-HT2A receptor antagonism potentiates haloperidol-induced dopamine release in rat medial prefrontal cortex and inhibits that in the nucleus accumbens in a dose-dependent manner. Brain Research. 2002;947:157–165. doi: 10.1016/s0006-8993(02)02620-3. [DOI] [PubMed] [Google Scholar]
- Maheux J, Ethier I, Rouillard C, Lévesque D. Induction patterns of transcription factors of the Nur family (Nurr1, Nur77 and Nor-1) by typical and atypical antipsychotics in the mouse brain : implication for their mechanism of action. Journal of Pharmacology & Experimental Therapeutics. 2005;313:460–473. doi: 10.1124/jpet.104.080184. [DOI] [PubMed] [Google Scholar]
- Marcus MM, Jardemark KE, Wadenberg ML, Langlois X, et al. Combined α2 and D2/3 receptor blockade enhances cortical glutamatergic transmission and reverses cognitive impairment in the rat. International Journal of Neuropsychopharmacology. 2005;8:315–327. doi: 10.1017/S1461145705005328. [DOI] [PubMed] [Google Scholar]
- Maxwell MA, Muscat GEO. The NR4A subgroup: immediate early response genes with pleiotropic physiological roles. Nuclear Receptor Signaling. 2005;4:e002. doi: 10.1621/nrs.04002. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer HY. The role of serotonin in antipsychotic drug action. Neuropsychopharmacology. 1999;21 (Suppl):S106–S115. doi: 10.1016/S0893-133X(99)00046-9. [DOI] [PubMed] [Google Scholar]
- Meltzer HY, Huang M. In vivo actions of atypical antipsychotic drug on serotonergic and dopaminergic systems. Progress in Brain Research. 2008;172:177–197. doi: 10.1016/S0079-6123(08)00909-6. [DOI] [PubMed] [Google Scholar]
- Meltzer HY, Li Z, Kaneda Y, Ichikawa J. Serotonin receptors : their key role in drugs to treat schizophrenia. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2003;27:1159–1172. doi: 10.1016/j.pnpbp.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Merchant KM, Dorsa DM. Differential induction of neurotensin and c-fos gene expression by typical vs. atypical antipsychotics. Proceedings of the National Academy of Sciences USA. 1993;90:3447–3451. doi: 10.1073/pnas.90.8.3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan MJ, Dekeyne A, Gobert A. Serotonin (5-HT)2C receptors tonically inhibit dopamine (DA) and noradrenaline (NA), but not 5-HT, release in the frontal cortex in vivo. Neuropharmacology. 1998;37:953–955. doi: 10.1016/s0028-3908(98)00078-1. [DOI] [PubMed] [Google Scholar]
- Myers SA, Eriksson N, Burow R, Wang SC, et al. β-adrenergic signaling regulates NR4A nuclear receptor and metabolic gene expression in multiple tissues. Molecular and Cellular Endocrinology. 2009;309:101–108. doi: 10.1016/j.mce.2009.05.006. [DOI] [PubMed] [Google Scholar]
- Ohno Y, Shimizu S, Imaki J. Effects of tandospirone, a 5-HT1A agonistic anxiolytic agent, on haloperidol-induced catalepsy and forebrain Fos expression in mice. Journal of Pharmacological Sciences. 2009;109:593–599. doi: 10.1254/jphs.08313fp. [DOI] [PubMed] [Google Scholar]
- Ohno Y, Shimizu S, Imaki J, Ishihara S, et al. Anticataleptic 8-OH-DPAT preferentially counteracts with haloperidol-induced Fos expression in the dorsolateral striatum and the core region of the nucleus accumbens. Neuropharmacology. 2008;55:717–723. doi: 10.1016/j.neuropharm.2008.06.005. [DOI] [PubMed] [Google Scholar]
- Pearen MA, Myers SA, Raichur S, Ryall JG, et al. The orphan nuclear receptor, NOR-1, a target of β-adrenergic signaling, regulates gene expression that controls oxidative metabolism in skeletal muscle. Endocrinology. 2008;149:2853–2865. doi: 10.1210/en.2007-1202. [DOI] [PubMed] [Google Scholar]
- Ponnio T, Conneely OM. Nor-1 regulates hippocampal axon guidance, pyramidal cell survival, and seizure susceptibility. Molecular and Cellular Biology. 2004;24:9070–9078. doi: 10.1128/MCB.24.20.9070-9078.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson GS, Matsumura H, Fibiger HC. Induction patterns of Fos-like immunoreactivity in the forebrain as predictors of atypical antipsychotic activity. Journal of Pharmacology & Experimental Therapeutics. 1994;271:1058–1066. [PubMed] [Google Scholar]
- Schaffer DJ, Tunc-Ozcan E, Shukla PK, Volenec A, et al. Nuclear orphan receptor Nor-1 contributes to depressive behavior in the Wistar-Kyoto rat model of depression. Brain Research. 2010;1362:32–39. doi: 10.1016/j.brainres.2010.09.041. [DOI] [PubMed] [Google Scholar]
- Schotte A, Janssen PFM, Gommeren W, Luyten WHML, et al. Risperidone compared with new and reference antipsychotic drugs: in vitro and in vivo receptor binding. Psychopharmacology. 1996;124:57–73. doi: 10.1007/BF02245606. [DOI] [PubMed] [Google Scholar]
- Serretti A, De Ronchi D, Lorenzi C, Berardi D. New antipsychotics and schizophrenia : a review on efficacy and side effects. Current Medicinal Chemistry. 2004;11:343–358. doi: 10.2174/0929867043456043. [DOI] [PubMed] [Google Scholar]
- Shen PJ, Gundlach AL. Differential modulatory effects of α- and β-adrenoceptor agonists and antagonists on cortical immediate-early gene expression following focal cerebrocortical lesion-induced spreading depression. Brain Research Molecular Brain Research. 2000;83:133–144. doi: 10.1016/s0169-328x(00)00216-3. [DOI] [PubMed] [Google Scholar]
- St-Hilaire M, Bourhis E, Lévesque D, Rouillard C. Impaired behavioural and molecular adaptations to dopamine denervation and repeated L-DOPA treatment in Nur77 knockout mice. European Journal of Neuroscience. 2006;24:795–805. doi: 10.1111/j.1460-9568.2006.04954.x. [DOI] [PubMed] [Google Scholar]
- Tremblay P-O, Gervais J, Rouillard C. Modification of haloperidol-induced pattern of c-fos expression by serotonin agonists. European Journal of Neuroscience. 1998;10:3546–3555. doi: 10.1046/j.1460-9568.1998.00372.x. [DOI] [PubMed] [Google Scholar]
- Wadenberg ML, Hertel P, Fernholm R, Hygge Blakeman K, et al. Enhancement of antipsychotic-like effects by combined treatment with the α1-adrenoceptor antagonist prazosin and the dopamine D2 receptor antagonist raclopride in rats. Journal of Neural Transmission. 2000;107:1229–1238. doi: 10.1007/s007020070036. [DOI] [PubMed] [Google Scholar]
- Wadenberg ML, Wiker C, Svensson TH. Enhanced efficacy of both typical and atypical antipsychotic drugs by adjunctive α2 adrenoceptor blockade: experimental evidence. International Journal of Neuropsychopharmacology. 2007;10:191–202. doi: 10.1017/S1461145706006638. [DOI] [PubMed] [Google Scholar]
- Werme M, Ringholm A, Olson L, Brené S. Differential patterns of induction of NGFI-B, Nor1 and c-fos mRNAs in striatal subregions by haloperidol and clozapine. Brain Research. 2000;863:112–119. doi: 10.1016/s0006-8993(00)02109-0. [DOI] [PubMed] [Google Scholar]
- Westerink BHC, Kawahara Y, De Boer P, Geels C, et al. Antipsychotic drugs classified by their effects on the release of dopamine and noradrenaline in the prefrontal cortex and striatum. European Journal of Pharmacology. 2001;412:127–138. doi: 10.1016/s0014-2999(00)00935-3. [DOI] [PubMed] [Google Scholar]
- Zetterström RH, Williams R, Perlmann T, Olson L. Cellular expression of the immediate-early transcription factors Nurr1 and NGFI-B suggests a gene regulatory role in several brain regions including the nigrostriatal dopamine system. Molecular Brain Research. 1996;41:111–120. doi: 10.1016/0169-328x(96)00074-5. [DOI] [PubMed] [Google Scholar]