Abstract
We have previously shown that docosahexaenoic acid (DHA) significantly reduced L-Dopa-induced dyskinesia (LID) in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) monkeys (Samadi et al., Ann. Neurol. 59:282–288, 2006). In the present study, we measured for the first time mRNA levels of Nur77, an orphan nuclear receptor that participates to adaptive and/or aberrant dopamine-related behaviors, and retinoid X receptor γ1 (RXRγ1), a putative brain receptor for DHA and transcriptional partner of Nur77, in MPTP monkeys treated with L-Dopa and DHA. The RXRγ1 mRNA is strongly expressed in monkey caudate nucleus and putamen, but no change in levels of RXRγ1 was observed following MPTP and L-Dopa treatments. On the other hand, denervation reduced Nur77 mRNA levels, whereas chronic L-Dopa treatment strongly induced Nur77 transcripts. These modulations are taking placed in substance P positive cells and are associated with both caudate-putamen matrix and striosome compartments. Interestingly, combination of L-Dopa with DHA further increases Nur77 mRNA levels in the anterior caudate-putamen, and mainly in striosomes. This is accompanied by a significant inverse correlation between Nur77 mRNA levels and dyskinetic scores. Taken together, our results show that Nur77 expression is modulated following dopamine denervation and chronic L-Dopa therapy in a non-human primate model of Parkinson’s disease, and suggest that strong modulation of Nur77 expression might be linked to a reduced risk to develop LIDs.
Keywords: NGFI-B, NR4A1, DHA, retinoid X receptor (RXR), MPTP-treated monkey, Parkinson’s disease, L-Dopa-induced dyskinesia (LID), substance P, basal ganglia, caudate nucleus, putamen, striatum
Introduction
Levodopa (L-Dopa) therapy is the most common treatment for Parkinson’s disease (PD). However, in a large proportion of individuals, therapy is hampered by the development of motor complications such as fluctuations, shortening of the motor response (also called wearing off) and dyskinesias. L-Dopa-induced dyskinesia (LID) is an important motor complication of chronic L-Dopa administration, with a prevalence ranging from 45–85% (Brotchie et al., 2005). The development of LIDs over time is a complex process that remains only partially understood, for review see (Brotchie et al., 2005; Calabresi et al., 2008; Cenci, 2007). Importantly, once the brain is primed to elicit dyskinesias, it is difficult to treat parkinsonian symptoms without inducing dyskinesias. LIDs are also extremely difficult to reduce or reverse once they have appeared. In fact, LID can become so severely disabling as to negate any clinical benefit from dopaminergic therapy in advanced PD patients.
Non human primates intoxicated with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) neurotoxin develop a Parkinsonian-like syndrome that is very similar to motor symptoms associated with PD in humans. This model also allows insights about molecular changes taking place subsequently to dopamine neurons loss and further treatment. In addition, this parkinsonian primate model has been proven to mimic particularly well both the initial characteristic of L-Dopa behavioral response and the development of long-term motor complications, such as LID (Calon et al., 2000).
LIDs can be assimilated to a form of pathological learning or plasticity (Bédard et al., 1999; Cenci, 2007). Their delayed appearance and persistence after treatment cessation strongly suggest that long term and possibly permanent basal ganglia circuitry alterations are involved. Therefore, transcription factors, which regulate gene expression, are likely to be involved in these molecular processes. Indeed, chronic Fos proteins of the ΔFosB family of transcription factors in the caudate-putamen, which, when coupled with Jun-D, form AP-1 complexes that can modulate the expression of several genes associated with the generation of LIDs in rodent and primate models of PD (Andersson et al., 1999; Doucet et al., 1996; Pavon et al., 2006). In recent years, we have shown that an orphan transcription factor of the nuclear receptor family, namely Nur77 (also known as Nerve-Growth Factor Inducible gene B (NGFI-B) or NR4A1) might also be associated with movement disorders in rodent models of PD (Sgambato-Faure et al., 2005; St-Hilaire et al., 2006; St-Hilaire et al., 2005; St-Hilaire et al., 2003; van den Munckhof et al., 2006).
Nur77 is a member of the Nur family, which also includes Nurr1 and Nor-1. Basal levels of Nur77 are found in most of dopaminoceptive structures such as the striatum, nucleus accumbens, olfactory tubercle and prefrontal cortex (Xiao et al., 1996; Zetterström et al., 1996b). Its expression is strongly modulated after manipulation of dopamine neurotransmission, for review see (Lévesque and Rouillard, 2007). Unilateral denervation induced by local injection of 6-hydroxydopamine (6-OHDA) in rats produces a complex regulation of Nur77 in the striatum (St-Hilaire et al., 2005; St-Hilaire et al., 2003). The expression of Nur77 transcripts is selectively up-regulated in enkephalin (ENK)-containing cells of the indirect striatal output pathway, whereas Nur77 expression is reduced in dynorphin (DYN)-positive cells of the direct output pathway in the denervated striatum (St-Hilaire et al., 2005; St-Hilaire et al., 2003). Interestingly, subsequent chronic L-DOPA treatment further reduced Nur77 expression in DYN-positive cells in specific striatal areas, whereas it strongly increased Nur77 mRNA levels in this same cell subpopulation in the non-denervated striatum (St-Hilaire et al., 2005; St-Hilaire et al., 2003).
Nur77 can exert its transcriptional activity as a monomer, homodimer or heterodimer with retinoid X receptors (RXR) (Forman et al., 1995; Maira et al., 1999; Zetterström et al., 1996a). Double in situ hybridization labeling indicated that the typical antipsychotic haloperidol strongly increased the co-localization of Nur77 and RXRγ1 isoform in striatal cells (Ethier et al., 2004a). Accordingly, RXR ligands can modulate biochemical and behavioral responses associated with antipsychotic drug administration (Ethier et al., 2004a; Ethier et al., 2004b). For example, haloperidol-induced vacuous chewing movements in mice, which resemble tardive dyskinesias in humans, were exacerbated in animals treated with a synthetic RXR antagonist (HX531), whereas administration of the polyunsaturated fatty acid docosahexaenoic acid (DHA), an endogenous RXR agonist in brain (Mata de Urquiza et al., 2000), significantly reduced haloperidol-induced oro-facial dyskinesias (Ethier et al., 2004b). Interestingly, effects of the RXR agonist and antagonist were abolished in Nur77 knockout mice, indicating that Nur77 is necessary for the activity of these RXR compounds (Ethier et al., 2004b).
Since tardive dyskinesias induced by chronic dopamine receptor blockade with conventional antipsychotic drugs and LIDs may share common biological substrates, we hypothesized that DHA may also reduce LIDs in MPTP monkeys. We previously reported the behavioral data of concomitant administration of DHA with L-Dopa (Samadi et al., 2006). This study showed that DHA significantly reduced LID scores in MPTP-treated monkeys without altering the anti-parkinsonian activity of L-Dopa (Samadi et al., 2006). In the present study, we report, for the first time, the expression of Nur77 and RXRγ1 in non-human primate brains. The data suggest that strong modulation of Nur77 expression might be linked to a reduced risk to develop LIDs.
Material and methods
Animals and treatments
Handling of primates was performed in accordance to the National Institute of Health Guide for the Care and Use of Laboratory Animals. All procedures, including means to minimize discomfort, were reviewed and approved by the Institutional Animal Care Committee of Laval University. Cynomolgus (Macaca Fascicularis) ovariectomized female monkeys weighing 3–5 kg were used in the present study. Animals were housed separately in individual observation cages in a temperature-controlled room and exposed to a 12-hour light/dark cycle. They were fed once daily in the afternoon, and water was provided ad libitum. The number of animals was kept to a minimum, and all efforts were made to avoid animal suffering. Five animals were used as healthy controls, while the other 15 received the neurotoxin MPTP (Sigma-Aldrich Canada, Oakville, Ontario) dissolved in sterile water and injected continuously at 0.5 mg/24 h using Alzet minipumps (Alzet Inc. Cupertino, CA, USA). In general, one month was needed to induce a stable parkinsonian syndrome. The cumulative dose to achieve this goal was in average 18.1 ± 3.8 and 13.9 ± 2.5 mg for L-Dopa and L-Dopa plus DHA groups, respectively (Samadi et al., 2006). The animals were scored several times a week with a disability scale, as previously described (Bélanger et al., 2003). This study was undertaken several months after a stable parkinsonian syndrome had developed (the time after MPTP was 149 ± 63 and 190 ± 42 days for L-Dopa and L-Dopa + DHA groups, respectively) (Samadi et al., 2006). Ten de novo MPTP intoxicated animals were treated with L-Dopa, five received L-Dopa alone, while the others received L-Dopa plus DHA. We used a fixed high daily oral dose of 100/25 mg of L-Dopa/benserazide (Sigma-Aldrich Canada, Oakville, Ontario). For the L-Dopa plus DHA group, MPTP-treated monkeys were first exposed to DHA (100 mg/kg, p.o., in a volume of 20–25 ml according to the weight of the animal) for 3 days before L-Dopa therapy was introduced. Then, combined oral administration of L-Dopa and DHA was performed on a daily basis for 1 month. Locomotor activity, as well as parkinsonian and dyskinetic scores of these animals have been previously reported (Samadi et al., 2006). All animals were sacrificed by an overdose of pentobarbital 4 hours after their last L-Dopa dose.
Tissue preparation
Brains were rapidly removed and stored, as previously described (Morissette et al., 2006). Briefly, they were placed in isopentane cooled in dry ice (−40°C) and kept frozen at −80°C. Hemisected brains were cut into coronal sections of 12μm on a cryostat (−20°C). The slices were thaw-mounted onto SuperFrostPlus (Fisher Scientific Ltd, Nepean, ON, Canada) 75 × 50 mm slides and stored at −80°C until use.
Dopamine concentrations and [125I]RTI-121 autoradiography
Pieces of coronal brain sections were homogenized in 250 μl of 0.1 M HClO4 at 4°C. The homogenate was centrifuged at 10,000×g for 20 min. the supernatants were kept at −80°C. The pellets were dissolved in 100 μl of 0.1 M NaOH for assays of protein content. The concentration of dopamine was measured by high-performance liquid chromatography with electrochemical detection (Morissette et al., 2006). Extent of denervation was also evaluated by measuring the dopamine transporter (DAT) with [125I]RTI-121 (3β-(4-I-iodophenyl)tropane-2β-carboxylic acid isopropyl ester, 2200 Ci/mmol; Mandel, Boston, MA, USA) binding autoradiography. Specific binding was measured using 25 pM of [125I]RTI-121. Non-specific binding was determined by adding 100 nM of Mazindol (Sandoz Pharmaceuticals, Dorval, Qc, Canada) to the incubation buffer (Morissette et al., 2006).
Complementary RNA probe preparation and synthesis
In order to label Nur77 mRNA in monkey brain tissues, we have produced a complementary RNA (cRNA) probe from total RNA of human caudate-putamen tissues. The cRNA probe for Nur77 stems from a 814 bp (nucleotides 19 to 832) fragment of the full-length human cDNA (GeneBank accession no: NM002135) subcloned into pCRII/TOPO and linearized with Bam HI. The RXRγ1 cRNA probe was generated from a 320 bp fragment of the rat full-length RXRγ1 cDNA contained in the pBS-SK+ vector and linearized with EcoRI. Both antisense probes were synthesized with a T7 RNA polymerase. Preliminary experiments indicated that the rat cRNA probe recognized the human RXRγ1 transcript and both sense probes gave no specific signal (data not shown). Synthesis of specific [35S]UTP-labeled cRNA probes for Nur77 and RXRγ1 was performed as previously described (Beaudry et al., 2000; Langlois et al., 2001).
Single in situ hybridization procedure
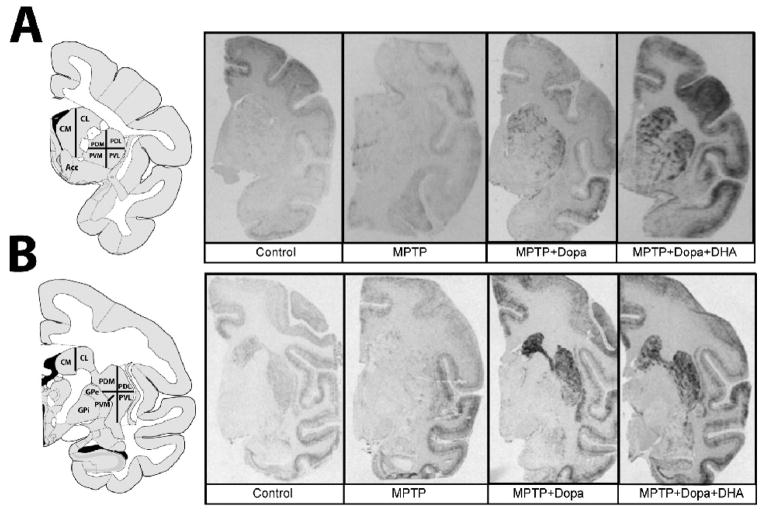
In situ hybridization with tissue sections was done at 58°C overnight in a standard hybridization buffer containing 50% formamide (Beaudry et al., 2000). Tissue sections were then apposed against BiomaxMR (Kodak, New Haven, CT) radioactive sensitive films for 5 to 7 days for Nur77 and 7 to 10 days for RXRγ1 probe. Quantification of the autoradiograms was performed using computerized analysis (ImageJ 1.41o software, Wayne Rasband, NIH). Digital brain images were taken using a Grayscale Digital Camera (Model CFW-1612M, Scion Corporation, Maryland, USA). Optical gray densities were transformed into nCi/g of tissue equivalent using standard curves generated with 14C-microscales (ARC 146A-14C standards, American Radiolabelled Chemicals Inc., St-Louis). Brain areas investigated included anterior and posterior parts of the caudate nucleus and putamen corresponding to Bregma 2.70 to 0.45 (anterior caudate-putamen) and Bregma −6.30 to −8.10 (posterior caudate-putamen) in the atlas of Paxinos, respectively (Paxinos, 2009). Anterior and posterior caudate-putamens have been subdivided into medial and lateral caudate (CM, CL), and dorsolateral (PDL), dorsomedial (PDM), ventrolateral (PVL) and ventromedial putamen (PVM) (Fig. 1). Average levels of labeling for each area were calculated from three adjacent brain sections of the same animals. Background intensities were subtracted from every measurement.
Figure 1.
Representative autoradiograms showing the in situ hybridization signals obtained with the monkey radiolabeled Nur77 mRNA probe. A) Representative examples of monkey brain coronal sections taken at anterior and B) posterior levels of the caudate-putamen in control, MPTP, MPTP + L-Dopa and MPTP + L-Dopa + DHA-treated groups. The left panels illustrate schematic representations of caudate nucleus and putamen subdivision areas used for quantification. Abbreviations are: Acc, nucleus accumbens, lateral (CL) and medial (CM) portions of the caudate nucleus, dorsolateral (PDL), dorsomedial (PDM), ventrolateral (PVL) and ventromedial (PVM) portions of the putamen, GPe, external globus pallidus and GPi, internal globus pallidus.
Double in situ hybridization procedure
The double in situ hybridization procedure was performed as previously described (Beaudry et al., 2000; St-Hilaire et al., 2003). Briefly, double in situ hybridization with a [35S]UTP-labeled Nur77 and a non-radioactive dioxygenin (Dig)-labeled preprotachykinin, which produces substance P (SP), was performed (Krause et al., 1987). The SP neuropeptide is expressed in the same cell population as DYN and was chosen as a marker of the direct striatal pathway because it possesses a higher basal expression than DYN (Gerfen and Young, 1988). The SP probe was obtained from a 200 bp fragment (from nucleotides 78 to 277) of the rat preprotachykinin mRNA into pGEM-4Z vector. The antisense cRNA probe was obtained by plasmid linearization with EcoR I and polymerization with the T7 RNA polymerase. Then, the neuropeptide cRNA probe was labeled with Dig using the Promega Riboprobe System with the Dig-RNA labeling mix (Roche Diagnostics, Laval, Qc, Canada) as previously described (Beaudry et al., 2000; St-Hilaire et al., 2003). Radioactive and Dig-labeled riboprobes were separated from non-hybridized nucleotides on mini Quick spin RNA columns (Roche Diagnostics, Laval, Qc, Canada). Double in situ hybridization was performed under the same conditions as those described for the single hybridization with Nur77. The Dig cRNA probe (SP, 0.75ng/μl) was added in the hybridization mix with the radioactive-labeled probe (4 to 5×106 cpm/μl). After overnight incubation an additional wash step using 50% formamide solution in 2 SSC buffer was performed to reduce non-specific Dig labeling. For Dig revelation, brain sections were blocked with 2% BSA and 0.3% Triton X-100 in buffer A (100 mM Tris-HCl, pH 7.5, 150 mM NaCl), for 30 min at room temperature. Slides were then incubated overnight at 4°C with an anti-Dig antibody conjugated with alkaline phosphatase (Boehringer-Ingelheim Canada, Laval, Qc, Canada). The next day, slides were washed three times in buffer A for 10 min and then in buffer B (100 mM Tris-HCl, pH 9.5, 100 mM NaCl and 50mM MgCl2) for 10 min, and finally incubated in a chromogen solution (buffer B containing 330 μg/ml nitroblue tetrazolium chloride plus 180 μg/ml 5-bromo-4chloro-3indolyl phosphate and 1mM levamisole). Intensity of staining was checked along the incubation. When an adequate staining was obtained, slides were incubated in buffer C (10 mM Tris-HCl, pH 8, 1mM EDTA) for 30 min to stop the reaction. Slides were dried up and then dipped in LM-1 photographic emulsion previously melted at 42°C (for 3h). Two months later, emulsion was developed in D-19 developer (3.5 min) and fixed (5 min) in Rapid Fixer solution (Kodak). Slides were mounted using a water-soluble mounting medium. Single- and double-labeled cells were visualized and manually counted with a Axio Imager A.1 photo microscope (Carl Zeiss Canada Ltd, Montreal, Qc, Canada), at 400x magnification. Neuron counting was performed in 5–10 different fields within the anterior dorsal putamen. Double-labeled cells were identified by the apposition of more than 10 silver grains with the colored product of the Dig reaction. Neuron counting was performed on 4 different sections obtained from a total of 4 animals per group investigated.
Acetylcholinesterase histochemistry
For the demonstration of acetylcholinesterase (AChE) staining, we used the Karnovsky and Roots (1964) method with the modifications described below (Karnovsky and Roots, 1964). Brain sections were incubated in an incubation medium prepared by adding in order 5 mg of acetylthiocholine iodide, 0.1 mM acetate buffer at pH 6, 0.1 M sodium citrate, 30 mM copper sulfate, 1ml distilled water and 5 mM potassium ferricyanide. Slides were incubated for one hour in the incubation medium at 37°C. When the optimal contrast was obtained, slides were rinsed intensively in distilled water to stop the reaction. Slides were then dried up and visualized using a Axiovision Rel 4.6 software (Carl Zeiss Canada Ltd, Montreal, Qc, Canada).
Statistical analysis
Statistical analysis was performed with Prism version 5.0 program (Graph Pad Software Inc. San Diego, CA, USA). All Data were expressed as group mean ± S.E.M. For mRNA levels, data were compared using a one-way analysis of variance (ANOVA). We first performed a test of homogeneity using Bartlett’s test, followed by a logarithmic transformation (when Bartlett’s test was significant) and finally applied a one-way ANOVA on transformed data. When the ANOVA revealed significant differences, a Tukey’s test was performed as post hoc analysis. To investigate the interaction between Nur77 mRNA levels and dyskinetic scores, correlation coefficients were determined by least-squares linear regression and significance was tested using the null hypothesis.
Results
Behavioral data associated with these animals have been presented elsewhere (Samadi et al., 2006). Briefly, all animals included in this study had similar baseline Parkinsonian scores before L-Dopa treatment and their syndrome was stable when dopamine replacement therapy was introduced (Samadi et al., 2006). Animals treated with L-Dopa alone developed intense LIDs (cumulative LID score of 65 ± 13), while MPTP monkeys receiving concomitant administration of DHA with L-Dopa displayed significantly reduced LIDs (cumulative LID scores of 26 ± 12) without significant effect on anti-parkinsonian activity of L-Dopa (Samadi et al., 2006). In addition, L-Dopa- and L-Dopa + DHA-treated MPTP monkeys displayed similar reversal of their parkinsonian symptoms (mean basal parkinsonian scores for L-Dopa- and L-Dopa + DHA-treated groups were 10.5 ± 0.8 and 11.6 ± 0.8, respectively, after 4 weeks of treatment, parkinsonian scores were reduced to 4.2 ± 0.2 and 4.5 ± 0.5 for L-Dopa and L-Dopa + DHA group, respectively) (Samadi et al., 2006). In the present study, de novo MPTP monkeys, i.e. that they did not received other treatment prior this experiment, have been used. Levels of denervation, as measured with [125I]RTI-121 DAT binding, were similar (over 95% reduction) in all MPTP-treated animal groups (Table 1). The data also indicates that chronic L-Dopa and L-Dopa + DHA did not significantly modulate DAT binding levels. Dopamine contents were also similar across all MPTP-treated groups and represent less than 10% of the total caudate-putamen dopamine contents measured in controls (caudate nucleus: Control, 138.1 ± 18.4; MPTP, 1.7 ± 0.9**; MPTP + L-Dopa, 5.3 ± 2.4**; MPTP + L-Dopa + DHA, 5.3 ± 2.4** and putamen: Control, 160.9 ± 10.9; MPTP, 8.9 ± 4.3**; MPTP + L-Dopa, 17.9 ± 4.2**; MPTP + L-Dopa + DHA, 14.6 ± 4.9**; ** p<0.01 vs respective control group). There is no significant residual caudate-putamen dopamine content from conversion of L-Dopa to dopamine at 4h (time of sacrifice) after the last L-Dopa injection since there is no significant difference in dopamine contents between MPTP, MPTP + L-Dopa and MPTP + L-Dopa + DHA groups.
Table 1.
Effect of MPTP lesion and treatments on the dopamine transporter specific binding measured by autoradiography in the caudate nucleus and putamen of monkeys.
| [125I]RTI-121 specific binding (fmol/mg tissue)
|
||||
|---|---|---|---|---|
| Dorsomedial | Ventromedial | Ventrolateral | Dorsolateral | |
| Caudate nucleus | ||||
| Control | 1.081 ± 0.134 | 1.099 ± 0.208 | 0.957 ± 0.088 | 0.984 ± 0.063 |
| MPTP | 0.017 ± 0.007** | 0.056 ± 0.024** | 0.041 ± 0.017** | 0.013 ± 0.004** |
| MPTP+L-Dopa | 0.019 ± 0.005** | 0.032 ± 0.010** | 0.031 ± 0.010** | 0.018 ± 0.005** |
| MPTP+L-Dopa+DHA | 0.032 ± 0.010** | 0.040 ± 0.010** | 0.032 ± 0.010** | 0.024 ± 0.010** |
| Putamen | ||||
| Control | 0.827 ± 0.057 | 0.783 ± 0.043 | 1.025 ± 0.086 | 1.030 ± 0.076 |
| MPTP | 0.093 ± 0.033** | 0.102 ± 0.033** | 0.060 ± 0.020** | 0.060 ± 0.026** |
| MPTP+L-Dopa | 0.067 ± 0.018** | 0.106 ± 0.019** | 0.070 ± 0.020** | 0.040 ± 0.010** |
| MPTP+L-Dopa+DHA | 0.097 ± 0.009** | 0.119 ± 0.018** | 0.066 ± 0.010** | 0.050 ± 0.010** |
Data represent means ± SEM.
p<0.01 vs respective control group.
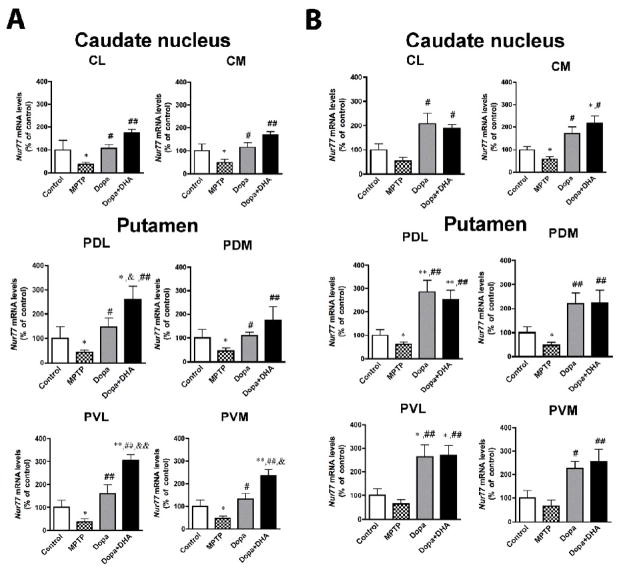
Nur77 mRNA levels are distinctly modulated in L-Dopa- and L-Dopa + DHA-treated MPTP monkeys
Basal Nur77 mRNA levels in the monkey brain were relatively low in anterior and posterior portions of the caudate nucleus and putamen (Fig. 1 and Table 2). Denervation significantly reduced Nur77 mRNA levels in all subterritories of the anterior caudate-putamen (reaching 50 to 65% reduction according to caudate nucleus and putamen subterritories) (Fig. 2A). MPTP significantly reduced Nur77 expression in posterior regions of the medial caudate nucleus (CM), and dorsomedial (PDM) and dorsolateral (PDL) putamen (Fig. 2B). In the anterior caudate-putamen, L-Dopa treatment essentially brought back Nur77 mRNA levels to control values (Fig. 2A). On the other hand, combination of L-Dopa + DHA significantly further increased Nur77 mRNA levels in the putamen, compared to L-Dopa alone (Fig. 2A). These increases correspond to 112, 144 and 102% of Nur77 mRNA levels observed L-Dopa alone groups in PDL, PVL and PVM subterritories, respectively. In the posterior caudate-putamen, L-Dopa alone and L-Dopa + DHA similarly increased Nur77 mRNA levels (Fig. 2B). A 2-fold increase of Nur77 expression is observed in the caudate nucleus of L-Dopa and L-Dopa + DHA treated animals, whereas Nur77 expression reached 3-fold the control values in the putamen (Fig. 2B).
Table 2.
Basal Nur77 mRNA levels in monkey caudate nucleus and putamen in control animals.
|
Nur77 mRNA levels (nCi/g tissue)
|
||
|---|---|---|
| Anterior level | Posterior level | |
| Caudate nucleus | ||
| CM | 101 ± 25 | 78 ± 8 |
| CL | 167 ± 54 | 113 ± 22 |
| Putamen | ||
| PDM | 114 ± 31 | 80 ± 20 |
| PDL | 140 ± 64 | 96 ± 22 |
| PVM | 56 ± 16 | 54 ± 22 |
| PVL | 90 ± 22 | 62 ± 23 |
Data represent means ± SEM. Abreviations: lateral (CL) and medial (CM) portions of the caudate nucleus as well as in dorsolateral (PDL), dorsomedial (PDM), ventrolateral (PVL) and ventromedial (PVM) portions of the putamen. These values were used to establish 100 % control values of Nur77 mRNA levels presented in Figure 2.
Figure 2.
Modulation of Nur77 mRNA levels in L-Dopa-treated MPTP monkeys in the caudate-putamen. Nur77 mRNA levels were measured in control, MPTP, MPTP + L-Dopa and MPTP + L-Dopa + DHA treated monkeys in the anterior (A) and posterior (B) caudate nucleus and putamen. Nur77 mRNA levels were evaluated in lateral (CL) and medial (CM) portions of the caudate nucleus as well as in dorsolateral (PDL), dorsomedial (PDM), ventrolateral (PVL) and ventromedial (PVM) portions of the putamen. Values are expressed in percent (%) of control values. Absolute values in nCi/g tissue of controls are presented in Table 2. Histogram bars represent means ± SEM (N=4–5 per group) (* p < 0.05 and ** p < 0.01 vs Control, # p < 0.05 and ## p < 0.01 vs MPTP and & p < 0.05 and && p < 0.01 vs MPTP + L-Dopa).
Nur77 mRNA levels were undetectable in the ventral globus pallidus (GPv), external globus pallidus (GPe) and internal globus pallidus (GPi), as well as in the substantia nigra and ventral tegmental area in all the groups investigated. As opposed to rodent brain, basal Nur77 mRNA levels were barely detectable in the nucleus accumbens. However, similar effects as in the anterior caudate-putamen were observed after the lesion, L-Dopa alone and L-Dopa + DHA treatments, but with lower magnitudes (data not shown).
Nur77 mRNA levels are selectively modulated in substance P-containing cells
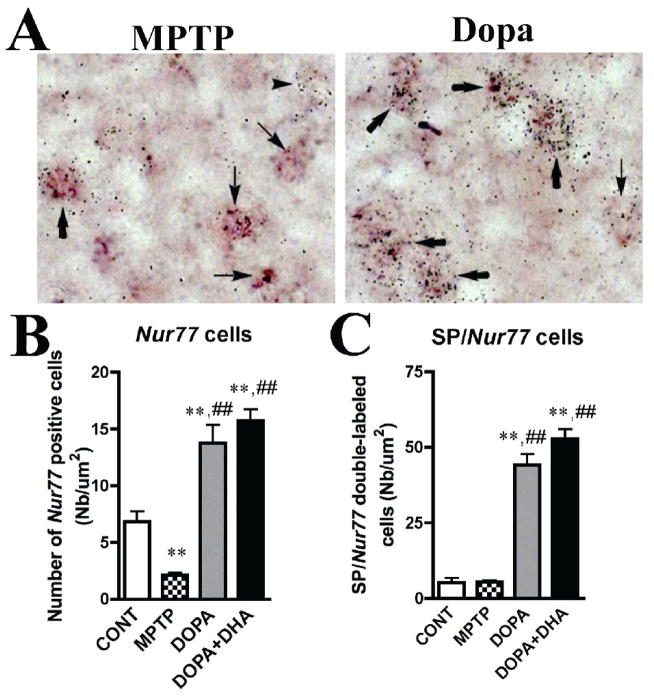
We have previously shown that Nur77 expression is distinctly modulated in the two striatal output pathways (ENK- and DYN-containing cells, respectively) of the striatum in rodent models of PD (St-Hilaire et al., 2003). In order to have a better understanding of the changes taking place in Nur77 mRNA following dopamine depletion in the MPTP-treated monkey, we performed double in situ hybridization labeling with the SP neuropeptide that is expressed in the direct striatal output pathway (Gerfen et al., 1991; Hurd and Herkenham, 1995). We chose this neuropeptide instead of DYN because basal caudate-putamen SP levels are higher than DYN and therefore more easily quantified after double-labeling procedure. Cellular counts were performed in the anterior dorsal putamen. MPTP reduced the number of Nur77-positive cells in this part of the putamen (70% reduction), while L-Dopa alone and L-Dopa + DHA strongly increased the number of Nur77-positive cells per surface unit (2-fold increase compared to control) (Fig. 3A,B). These Nur77 mRNA level changes are consistent with the results observed in the autoradiography analysis (Fig. 2). The number of SP+-cells remained unchanged after MPTP, while it was reduced in L-Dopa alone and L-Dopa + DHA groups (data not shown). L-Dopa and L-Dopa + DHA treatment strongly increased the number of SP/Nur77-positive cells in this portion of the putamen (about 8 to 9-fold increases) (Fig. 3C). Under basal conditions, the percentage of co-localization of Nur77 and SP transcripts mRNA is 41 ± 1 % (the remaining 59% being expressed in SP-negative cells). This observation is similar to what we previously reported in DYN-positive cells in rat striatum (St-Hilaire et al., 2003). The percentage of cells co-expressing SP and Nur77 reached up to 76 ± 3 % and 77 ± 1 % in L-Dopa and L-Dopa + DHA groups, respectively.
Figure 3.
Modulation of Nur77 mRNA levels in L-Dopa-treated MPTP monkeys in substance P (SP) containing cells of the putamen. A) Representative images of the radiolabeled Nur77 mRNA probe (silver grains) and SP transcript labeled with digoxigenin (dark brown staining) in a MPTP and MPTP + L-Dopa-treated animals. Arrowheads indicate single Nur77-positive cells, thin arrows indicate single SP-positive cells and bold arrows indicate double SP/Nur77-positive cells. B) Nur77-positive cell counts in controls (CONT), MPTP, MPTP + L-Dopa and MPTP + L-Dopa + DHA-treated animals. C) SP/Nur77-double labeled cell counts in controls, MPTP, MPTP + L-Dopa and MPTP + L-Dopa + DHA-treated animals. Histogram bars represent mean cell numbers per surface unit (um2) ± SEM (N=4–5 per group) (** p < 0.01 vs CONT and ## p < 0.01 vs MPTP group). All cell counts were performed in the anterior putamen.
Nur77 expression is strongly modulated in the caudate-putamen striosome compartment
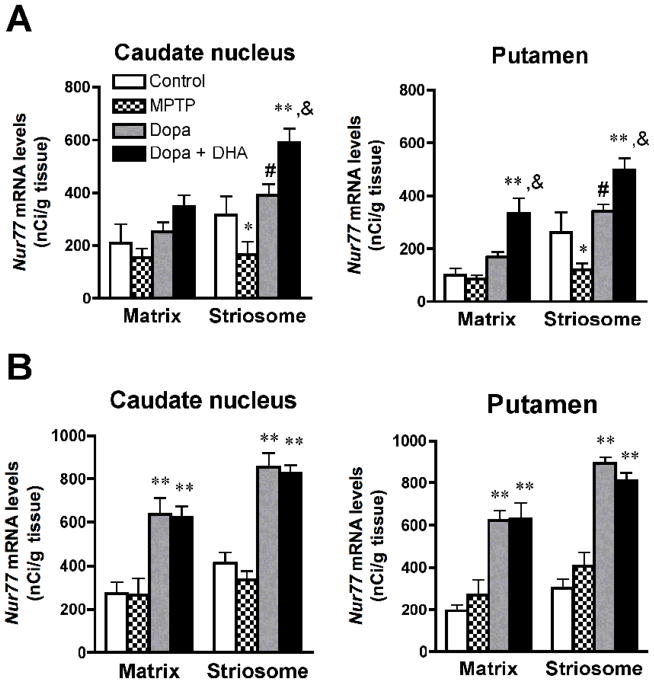
As observed in Figure 1, the expression of Nur77 in the caudate-putamen displays a mosaic-like distribution that resembling to striosomes. To determine striosome-matrix compartimentalization within the caudate-putamen, acetylcholinesterase (AChE) staining was performed on sections adjacent to those used for in situ hybridization. Superpositions of AChE staining and Nur77 mRNA labeling indicate that Nur77 is expressed in both striosome and matrix compartments. However, the expression of Nur77 is about 2-fold higher in striosomes compared to the matrix compartment in the anterior putamen and about 20 to 30% higher in other caudate-putamen subterritories (Fig. 4). MPTP selectively reduced Nur77 mRNA levels in the striosomes in the anterior caudate-putamen (Fig. 4A), while no effect was observed in posterior areas (Fig. 4B). In the anterior caudate-putamen, L-Dopa alone brought back Nur77 mRNA levels to control values in striosomes, while it remained without effect in the matrix (Fig. 4A). Concomitant administration of DHA with L-Dopa further increased Nur77 mRNA levels only in striosomes in the anterior caudate (Fig. 4A), while this treatment further increased Nur77 expression in both striosomes and matrix in the anterior putamen (Fig. 4A). In the posterior caudate-putamen, L-Dopa alone and L-Dopa + DHA similarly increased Nur77 mRNA levels in both striosomes and matrix (Fig. 4B).
Figure 4.
Modulation of Nur77 mRNA levels in striosome and matrix compartments of the anterior (A) and posterior (B) caudate nucleus and putamen in L-Dopa-treated MPTP monkeys. Histogram bars represent means ± SEM in controls, MPTP, MPTP + L-Dopa and MPTP + Dopa + DHA-treated animals (N=4–5 per group, * p < 0.05 and ** p < 0.01 vs control, # p < 0.05 vs MPTP, & p < 0.05 vs MPTP + L-Dopa). For each animal, 3 to 5 striosome and matrix areas were used to average Nur77 mRNA levels in these respective compartments.
Nur77 mRNA levels correlate with LID scores
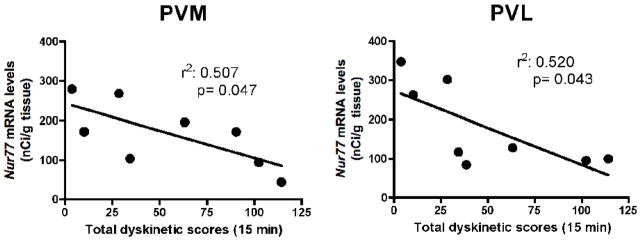
To investigate the relationship between Nur77 mRNA levels and dyskinetic scores, correlation analysis between Nur77 mRNA levels in various subterritories of the caudate nucleus and putamen against the total dyskinetic score for each animal in the L-Dopa- and L-Dopa + DHA-treated groups were computed. Interestingly, we found a significant inverse correlation between Nur77 mRNA levels and individual dyskinetic scores in the anterior PVM and PVL areas (Fig. 5). A similar trend was also observed in the posterior PDM area (r2 = 0.532, p = 0.063). Other caudate-putamen subterritories did not display significant correlations (data not shown).
Figure 5.
Correlation between Nur77 mRNA levels and L-Dopa-induced dyskinesia (LID) scores. We have plotted individual LID scores from MPTP monkeys treated with L-Dopa and L-Dopa + DHA animals with individual Nur77 mRNA levels found in the anterior ventromedial (PVM) and ventrolateral (PVL) portions of the putamen. Linear regression analysis was performed with 95% confidence intervals. Goodness of fit is illustrated with the calculated r2, and the p value statistical analysis indicates whether the slope is significantly different from zero. P values < 0.05 were considered as significant.
RXRγ1 mRNA levels are not modulated by L-dopa and DHA treatments
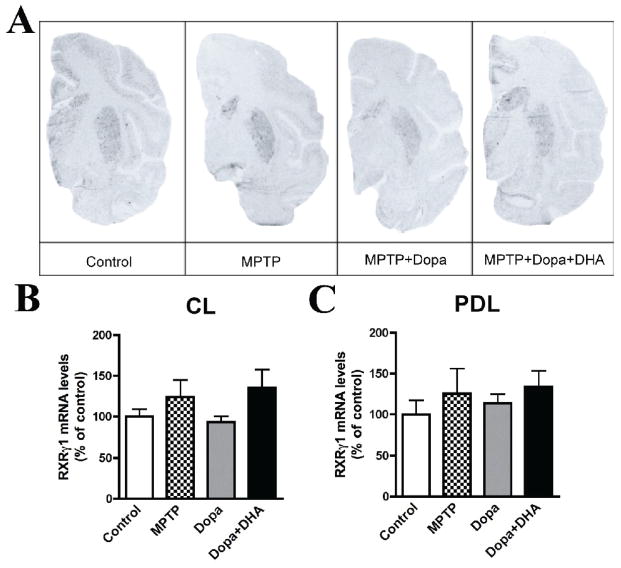
We measured, for the first time, levels of RXRγ1 mRNA in the non-human primate brain. High levels of the RXRγ1 mRNA isoform were detected in non-human primate caudate-putamen (Fig. 6A). As for Nur77, it displayed a striosome/matrix distribution, which was more clearly apparent in posterior caudate-putamen (Fig. 6A). However, RXRγ1 mRNA levels in lateral caudate and putamen were not modulated by any of the treatments in the present experimental paradigm (Fig. 6B,C) and no effect was detected in other caudate-putamen subterritories as well (data not shown).
Figure 6.
Representative autoradiograms of RXRγ1 mRNA levels in L-Dopa-treated MPTP monkeys in the posterior caudate-putamen (A). RXRγ1 mRNA levels were measured in control, MPTP, MPTP + L-Dopa and MPTP + L-Dopa + DHA treated monkeys. B) Quantification of RXRγ1 mRNA levels in lateral (CL) portion of the caudate nucleus and C) dorsolateral (PDL) portion of the putamen. Histogram bars represent means ± SEM (N=4–5 per group).
Discussion
The main findings of the present study is that Nur77 mRNA levels are modulated by denervation and L-Dopa treatment and that Nur77 mRNA levels display an inverse correlation with LID scores. This suggests that Nur77 participates to adaptive events following dopamine denervation and L-Dopa therapy in a non-human primate model of PD. The study also highlights some difference in the modulation of Nur77 expression in the primate compared to rodent models of PD.
In rats bearing a unilateral 6-OHDA lesion, complex modulation of Nur77 mRNA levels were observed in both the intact and lesioned striata (St-Hilaire et al., 2005; St-Hilaire et al., 2003). For example, the expression of Nur77 is selectively up-regulated in ENK-containing cells of the lesioned striatum, while it is down-regulated in DYN-positive cells (St-Hilaire et al., 2003). In DYN cells of the lesioned side of the striatum, L-Dopa treatment further reduced Nur77 expression, while L-Dopa increased Nur77 expression in DYN cells on the intact side of the striatum (St-Hilaire et al., 2003). In the MPTP-treated monkey, we observed only a down-regulation of Nur77 in most of the caudate-putamen subterritories. This suggests that the modulation of Nur77 in ENK cells observed after unilateral 6-OHDA lesion in rodents is absent in MPTP intoxicated primates. The reason for this apparent discrepancy is not known and needs further investigations. Possible explanations may come from differences in the denervation process that exist between the two models (local and unilateral for 6-OHDA vs systemic and bilateral for MPTP), as well as from distinctions between basal ganglia circuitry in rodents and primates. On the other hand, L-Dopa-induced Nur77 expression is observed in SP cells of the caudate-putamen of MPTP monkeys. This observation is consistent with the modulation of Nur77 in DYN-positive cells in the intact side of unilateral 6-OHDA-lesioned rats (St-Hilaire et al., 2003), as well as with some restricted up-regulation of Nur77 following L-Dopa treatment in the ipsilateral striatum observed in another study (Sgambato-Faure et al., 2005). The modulation of Nur77 observed in the intact striatum following L-Dopa treatment in unilaterally 6-OHDA-lesioned rats, which is similar to the present study following systemic MPTP and L-Dopa treatment, possibly results from an effect on intratelencephalic corticostriatal neurons that send massive inputs to both ipsilateral and contralateral striatum (Ballion et al., 2008).
DHA, a polyunsaturated fatty acid of the omega-3 class and an endogenous activator of RXR (Mata de Urquiza et al., 2000), can reduce the severity or delay the appearance of LIDs in MPTP monkeys (Samadi et al., 2006). In mice chronically treated with haloperidol and displaying oro-facial dyskinesias, the anti-dyskinetic property of DHA depends on the presence of Nur77, since DHA remained inactive in Nur77 knockout mice (Ethier et al., 2004b). In the present study, we observe high RXRγ1 levels in the caudate-putamen and inductions of Nur77 in L-Dopa-treated MPTP monkeys are inversely correlated with LID scores. Thus, it is tempting to speculate that a similar mechanism might be involved in the anti-dyskinetic effect of DHA in the present non-human primate model of PD. However, we do not know if the anti-dyskinetic property of DHA is directly link to Nur77-dependent transcriptional activity (through activation of the Nur77/RXR complex). Additional experiments will be required to fully understand the molecular mechanism involved in the anti-dyskinetic property of DHA.
MPTP monkeys rendered parkinsonian displayed elevated amounts of ΔFosB-like proteins and chronic administration of L-Dopa or a selective D1 agonist, which produced dyskinesias, induced a further elevation of ΔFosB-like immunoreactivity (Doucet et al., 1996; Munoz et al., 2008). In addition, treatment with a dopamine D2 agonist or combination of L-Dopa with 5-HT1A and 5-HT1B agonists, which did not produce dyskinesias or reversed LIDs, also reversed the elevation of ΔFosB (Doucet et al., 1996; Munoz et al., 2008). These findings suggest that increased ΔFosB expression, in the striatonigral pathway (expressing SP/DYN neuropeptides), may be associated with the development of dyskinesia. This highlights a fundamental distinction between the modulation of ΔFosB and Nur77 in the MPTP model, since the present data indicate that induction of Nur77 expression is, on the contrary, associated with a reduction of LIDs, suggesting that Nur77 expression is opposed to LID development.
It is interesting to observe that Nur77 expression and LIDs show correlations only in the anterior caudate-putamen. The anterior caudate and putamen receive inputs from the premotor cortex, dorsolateral prefrontal cortex, orbital cortex and dorsal anterior cingulate cortex (Haber et al., 2006). Therefore, the correlation of Nur77 mRNA levels and LIDs in the anterior dorsal striatum suggests that Nur77 might modulate cognitive and learning aspects of motor processes. This is in accordance with the hypothesis that dyskinesias should not be regarded simply as a movement disorder, but also as a limbic and cognitive disorder (Guigoni et al., 2005). In rodents, we did observe a spatial correlation between Nur77 expression and L-DOPA-induced dyskinesia accompanied (whole striatum) or not (lateral part of the striatum) with locomotor sensitization in unilaterally lesioned 6-OHDA rats in a previous report (Sgambato-Faure et al., 2005). However, no attempt to correlate individual Nur77 mRNA levels an intensity of LIDs or locomotor sensitization was made. In addition, the correlation was not performed in more anterior parts of the striatum.
In addition to the segregation into direct and indirect pathways, caudate-putamen projection neurons are segregated functionally and biochemically into two major compartments, termed striosome or patch and matrix (Gerfen, 1984; Graybiel and Ragsdale, 1978). Since we observed a strong co-localization between Nur77 and SP after L-Dopa and L-Dopa + DHA treatments and that immunochemistry of DYN and SP are highly prevalent in striosomes, it is not surprising that these treatments predominantly modulated Nur77 mRNA levels in striosomes. Interestingly, strong DYN-induced expression in anterior caudate-putamen striosomes has been associated with LIDs (Henry et al., 2003), and it has been proposed that a shift towards striosome-predominant activation of IEGs and DYN after chronic drug treatments could promote repetitive behaviors, such as LIDs (Graybiel et al., 2000). The present data suggests that Nur77 might also be included in this LID-associated gene expression pattern. However, as mentioned previously, Nur77 seems to be involved in a pathway that tend to reduce abnormal dopamine-related behaviors as opposed to Fos-related IEGs.
Transcriptome analysis revealed alterations in the expression of more than 100 genes following dopamine denervation and LID installation (Bassilana et al., 2005; Konradi et al., 2004). So, why Nur77 represents an interesting candidate in dyskinesias? There are three main reasons. First, modulations of Nur77 expression were strongly and consistently observed in many different animal models of PD. Second, modulations of Nur77 expression can be observed after chronic denervation and prolonged dopamine replacement therapy, indicating that these modulations do not desensitized over time. Third, by promoting the formation of the Nur77/RXR transcriptional complex (Lévesque and Rouillard, 2007), Nur77 can be viewed as a molecular switch that renders the striatum responsive to RXR agonists, making Nur77-dependent transcriptional activity and RXR an attractive new pharmacological entity aims to control LIDs. We have shown that Nur77 gene ablation alters the modulation of striatal neuropeptides, like neurotensin, normally observed following denervation and L-DOPA therapy (St-Hilaire et al., 2006). Since, neuropeptides have been associated with homeostatic control of dopamine neurotransmission, it is tempting to speculate that the Nur77-dependent (through the Nur77/RXR complex) anti-dyskinetic property might be related, at least in part, with its transcriptional activity on those genes. Interestingly, we recently identified a single nucleotide polymorphism (SNP) in the Nur77 gene that is strongly associated with tardive dyskinesias in a cohort of schizophrenic patients (B. Le Foll, University of Toronto, unpublished data), further reinforcing the relationship between Nur77 and the pathophysiology of dyskinesias.
Acknowledgments
This work was supported by grants from the Canadian Institutes for Health Research (CIHR), the Parkinson Society of Canada (PSC) and the Parkinson’s Disease Foundation (PDF) of United States. P.S. held a post-doctoral fellowship from CIHR-Rx&D.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersson M, Hilbertson A, Cenci MA. Striatal FosB expression is causally linked with L-DOPA-induced abnormal involuntary movements and the associated upregulation of striatal prodynorphin mRNA in a rat model of Parkinson’s disease. Neurobiol Dis. 1999;6:461–474. doi: 10.1006/nbdi.1999.0259. [DOI] [PubMed] [Google Scholar]
- Ballion B, Mallet N, Bezard E, Lanciego JL, Gonon F. Intratelencephalic corticostriatal neurons equally excite striatonigral and striatopallidal neurons and their discharge activity is selectively reduced in experimental parkinsonism. Eur J Neurosci. 2008;27:2313–2321. doi: 10.1111/j.1460-9568.2008.06192.x. [DOI] [PubMed] [Google Scholar]
- Bassilana F, Mace N, Li Q, Stutzmann JM, Gross CE, Pradier L, Benavides J, Ménager J, Bezard E. Unraveling substantia nigra sequential gene expression in a progressive MPTP-lesioned macaque model of Parkinson’s disease. Neurobiol Dis. 2005;20:93–103. doi: 10.1016/j.nbd.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Beaudry G, Langlois MC, Weppe I, Rouillard C, Lévesque D. Contrasting patterns and cellular specificity of transcriptional regulation of the nuclear receptor Nerve Growth Factor-Inducible B by haloperidol and clozapine in the rat forebrain. J Neurochem. 2000;75:1694–1702. doi: 10.1046/j.1471-4159.2000.0751694.x. [DOI] [PubMed] [Google Scholar]
- Bédard PJ, Blanchet PJ, Lévesque D, Soghomonian JJ, Grondin R, Morissette M, Goulet M, Calon F, Falardeau P, Gomez-Mancilla B, Doucet JP, Robertson GS, Di Paolo T. Pathophysiology of L-dopa-induced dyskinesias. Mov Disord. 1999;14:4–8. [PubMed] [Google Scholar]
- Bélanger N, Grégoire L, Tahar AH, Bédard PJ. Chronic treatment with small doses of cabergoline prevents dopa-induced dyskinesias in Parkinsonian monkeys. Mov Disord. 2003;18:1436–1441. doi: 10.1002/mds.10589. [DOI] [PubMed] [Google Scholar]
- Brotchie JM, Lee J, Venderova K. Levodopa-induced dyskinesia in Parkinson’s disease. J Neural Transm. 2005;112:359–391. doi: 10.1007/s00702-004-0251-7. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Di Filippo M, Ghiglieri V, Picconi B. Molecular mechanisms underlying levodopa-induced dyskinesia. Mov Disord. 2008;23(Suppl 3):S570–S579. doi: 10.1002/mds.22019. [DOI] [PubMed] [Google Scholar]
- Calon F, Tahar AH, Blanchet PJ, Morissette M, Grondin R, Goulet M, Doucet JP, Robertson GS, Nestler E, Di Paolo T, Bédard PJ. Dopamine-receptor stimulation: biobehavioral and biochemical consequences. Trends Neurosci. 2000;23 (suppl):S92–S100. doi: 10.1016/s1471-1931(00)00026-4. [DOI] [PubMed] [Google Scholar]
- Cenci MA. Dopamine dysregulation of movement control in L-DOPA-induced dyskinesia. Trends Neurosci. 2007;30:236–243. doi: 10.1016/j.tins.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Doucet JP, Nakabeppu Y, Bédard PJ, Hope BT, Nestler EJ, Jasmin BJ, Chen JS, Iadarola MJ, St-Jean M, Wigle N, Blanchet P, Grondin R, Robertson GS. Chronic alterations in dopaminergic neurotransmission produce a persistent elevation of ΔFosB-like protein(s) in both the rodent and primate striatum. Eur J Neurosci. 1996;8:365–381. doi: 10.1111/j.1460-9568.1996.tb01220.x. [DOI] [PubMed] [Google Scholar]
- Ethier I, Beaudry G, St-Hilaire M, Milbrandt J, Rouillard C, Lévesque D. The transcription factor NGFI-B (Nur77) and retinoids play a critical role in acute neuroleptic-induced extrapyramidal effect and striatal neuropeptide gene expression. Neuropsychopharmacology. 2004a;29:335–346. doi: 10.1038/sj.npp.1300318. [DOI] [PubMed] [Google Scholar]
- Ethier I, Kagechika H, Shudo K, Rouillard C, Lévesque D. Docosahexaenoic acid reduces haloperidol-induced dyskinesias in mice: Involvement of Nur77 and retinoid receptors. Biol Psychiatry. 2004b;56:522–526. doi: 10.1016/j.biopsych.2004.06.036. [DOI] [PubMed] [Google Scholar]
- Forman BM, Umesono K, Chen J, Evans RM. Unique response pathways are established by allosteric interactions among nuclear hormone receptors. Cell. 1995;81:541–550. doi: 10.1016/0092-8674(95)90075-6. [DOI] [PubMed] [Google Scholar]
- Gerfen CR. The neostriatal mosaic: compartmentalization of corticostriatal input and striatonigral output systems. Nature. 1984;311:461–464. doi: 10.1038/311461a0. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, McGinty JF, Young WS., 3rd Dopamine differentially regulates dynorphin, substance P, and enkephalin expression in striatal neurons: in situ hybridization histochemical analysis. J Neurosci. 1991;11:1016–1031. doi: 10.1523/JNEUROSCI.11-04-01016.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen CR, Young WS., 3rd Distribution of striatonigral and striatopallidal peptidergic neurons in both patch and matrix compartments: an in situ hybridization histochemistry and fluorescent retrograde tracing study. Brain Res. 1988;460:161–167. doi: 10.1016/0006-8993(88)91217-6. [DOI] [PubMed] [Google Scholar]
- Graybiel AM, Canales JJ, Capper-Loup C. Levodopa-induced dyskinesias and dopamine-dependent stereotypies: a new hypothesis. Trends Neurosci. 2000;23 (suppl):S71–S77. doi: 10.1016/s1471-1931(00)00027-6. [DOI] [PubMed] [Google Scholar]
- Graybiel AM, Ragsdale CW., Jr Histochemically distinct compartments in the striatum of human, monkeys, and cat demonstrated by acetylthiocholinesterase staining. Proc Natl Acad Sci USA. 1978;75:5723–5726. doi: 10.1073/pnas.75.11.5723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guigoni C, Li Q, Aubert I, Dovero S, Bioulac BH, Bloch B, Crossman AR, Gross CE, Bezard E. Involvement of sensorimotor, limbic, and associative basal ganglia domains in L-3,4-dihydroxyphenylalanine-induced dyskinesia. J Neurosci. 2005;25:2102–2107. doi: 10.1523/JNEUROSCI.5059-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Kim KS, Mailly P, Calzavara R. Reward-related cortical inputs define a large striatal region in primates that interface with associative cortical connections, providing a substrate for incentive-based learning. J Neurosci. 2006;26:8368–8376. doi: 10.1523/JNEUROSCI.0271-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry B, Duty S, Fox SH, Crossman AR, Brotchie JM. Increased striatal pre-proenkephalin B expression is associated with dyskinesia in Parkinson’s disease. Exp Neurol. 2003;183:458–468. doi: 10.1016/s0014-4886(03)00064-5. [DOI] [PubMed] [Google Scholar]
- Hurd YL, Herkenham M. The human neostriatum shows compartmentalization of neuropeptide gene expression in dorsal and ventral regions: an in situ hybridization histochemical analysis. Neuroscience. 1995;64:571–586. doi: 10.1016/0306-4522(94)00417-4. [DOI] [PubMed] [Google Scholar]
- Karnovsky MJ, Roots L. A “direct-Coloring” thiocholine method for cholinesterases. J Histochem Cytochem. 1964;12:219–221. doi: 10.1177/12.3.219. [DOI] [PubMed] [Google Scholar]
- Konradi C, Westin JE, Carta M, Eaton ME, Kuter K, Dekundy A, Lundblad M, Cenci MA. Transcriptome analysis in a rat model of L-DOPA-induced dyskinesia. Neurobiol Dis. 2004;17:219–236. doi: 10.1016/j.nbd.2004.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause JE, Chirgwin JM, Carter MS, Xu ZS, Hershey AD. Three rat preprotachykinin mRNAs encode the neuropeptides substance P and neurokinin A. Proc Natl Acad Sci USA. 1987;84:881–885. doi: 10.1073/pnas.84.3.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlois MC, Beaudry G, Zekki H, Rouillard C, Lévesque D. Impact of antipsychotic drug administration on the expression of nuclear receptors in the neocortex and striatum of the rat brain. Neuroscience. 2001;106:117–128. doi: 10.1016/s0306-4522(01)00248-2. [DOI] [PubMed] [Google Scholar]
- Lévesque D, Rouillard C. Nur77 and retinoid X receptors: crucial factors in dopamine-related neuroadaptation. Trends Neurosci. 2007;30:22–30. doi: 10.1016/j.tins.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maira M, Martens C, Philips A, Drouin J. Heterodimerization between members of the Nur subfamily of orphan nuclear receptors as a novel mechanism for gene activation. Mol Cell Biol. 1999;19:7549–7557. doi: 10.1128/mcb.19.11.7549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mata de Urquiza A, Liu S, Sjöberg M, Zetterström RH, Griffiths W, Sjövall J, Perlmann T. Docosahexaenoic acid, a ligand for the retinoid X receptor in mouse brain. Science. 2000;290:2140–2144. doi: 10.1126/science.290.5499.2140. [DOI] [PubMed] [Google Scholar]
- Morissette M, Dridi M, Calon F, Tahar AH, Meltzer LT, Bédard PJ, Di Paolo T. Prevention of levodopa-induced dyskinesias by a selective NR1A/2B N-methyl-D-aspartate receptor antagonist in parkinsonian monkeys: Implication of preproenkephalin. Mov Disord. 2006;21:9–17. doi: 10.1002/mds.20654. [DOI] [PubMed] [Google Scholar]
- Munoz A, Li Q, Gardoni F, Marcello E, Qin C, Carlsson T, Kirik D, Di Luca M, Bjorklund A, Bezard E, Carta M. Combined 5-HT1A and 5-HT1B receptor agonists for the treatment of L-DOPA-induced dyskinesia. Brain. 2008;131:3380–3394. doi: 10.1093/brain/awn235. [DOI] [PubMed] [Google Scholar]
- Pavon N, Martin AB, Mendialdua A, Moratalla R. ERK phosphorylation and FosB expression are associated with L-DOPA-induced dyskinesia in hemiparkinsonian mice. Biol Psychiatry. 2006;59:64–74. doi: 10.1016/j.biopsych.2005.05.044. [DOI] [PubMed] [Google Scholar]
- Paxinos G. The rherus monkey brain in stereotaxic coordinates. Academic Press; Boston: 2009. [Google Scholar]
- Samadi P, Grégoire L, Rouillard C, Di Paolo T, Lévesque D. Docosahexaenoic acid reduces Levodopa-induced dyskinesias in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine monkeys. Annals Neurol. 2006;59:282–288. doi: 10.1002/ana.20738. [DOI] [PubMed] [Google Scholar]
- Sgambato-Faure V, Buggia V, Gilbert F, Lévesque D, Benabid AL, Berger F. Coordinated and spatial upregulation of Arc in striatonigral neurons correlates with L-dopa-induced behavioral sensitization in dyskinetic rats. J Neuropathol Exp Neurol. 2005;64:936–947. doi: 10.1097/01.jnen.0000186922.42592.b7. [DOI] [PubMed] [Google Scholar]
- St-Hilaire M, Bourhis E, Lévesque D, Rouillard C. Impaired behavioural and molecular adaptations to dopamine denervation and repeated L-DOPA treatment in Nur77 knockout mice. Eur J Neurosci. 2006;24:795–805. doi: 10.1111/j.1460-9568.2006.04954.x. [DOI] [PubMed] [Google Scholar]
- St-Hilaire M, Landry E, Lévesque D, Rouillard C. Denervation and repeated L-DOPA induce complex regulatory changes in neurochemical phenotypes of striatal neurons: Implication of a dopamine D1-dependent mechanism. Neurobiol Dis. 2005;20:450–460. doi: 10.1016/j.nbd.2005.04.001. [DOI] [PubMed] [Google Scholar]
- St-Hilaire M, Landry É, Lévesque D, Rouillard C. Denervation and repeated L-DOPA induce a coordinate expression of the transcription factor NGFI-B in striatal projection pathways in hemi-parkinsonian rats. Neurobiol Dis. 2003;14:98–109. doi: 10.1016/s0969-9961(03)00081-0. [DOI] [PubMed] [Google Scholar]
- van den Munckhof P, Gilbert F, Chamberland M, Lévesque D, Drouin J. Striatal neuroadaptation and rescue of locomotor deficit by L-dopa in Aphakia mice, a model of Parkinson’s disease. J Neurochem. 2006;96:160–170. doi: 10.1111/j.1471-4159.2005.03522.x. [DOI] [PubMed] [Google Scholar]
- Xiao Q, Castillo SO, Nikodem VM. Distribution of messenger RNAs for the orphan nuclear receptors Nurr1 and Nur77 (NGFI-B) in adult rat brain using in situ hybridization. Neuroscience. 1996;75:221–230. doi: 10.1016/0306-4522(96)00159-5. [DOI] [PubMed] [Google Scholar]
- Zetterström RH, Solomin L, Mitsiadis T, Olson L, Perlmann T. Retinoid X receptor heterodimerization and developmental expression distinguish the orphan nuclear receptors NGFI-B, Nurr1, and Nor1. Mol Endocrinol. 1996a;10:1656–1666. doi: 10.1210/mend.10.12.8961274. [DOI] [PubMed] [Google Scholar]
- Zetterström RH, Williams R, Perlmann T, Olson L. Cellular expression of the immediate-early transcription factors Nurr1 and NGFI-B suggests a gene regulatory role in several brain regions including the nigrostriatal dopamine system. Mol Brain Res. 1996b;41:111–120. doi: 10.1016/0169-328x(96)00074-5. [DOI] [PubMed] [Google Scholar]