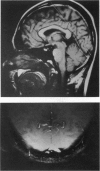
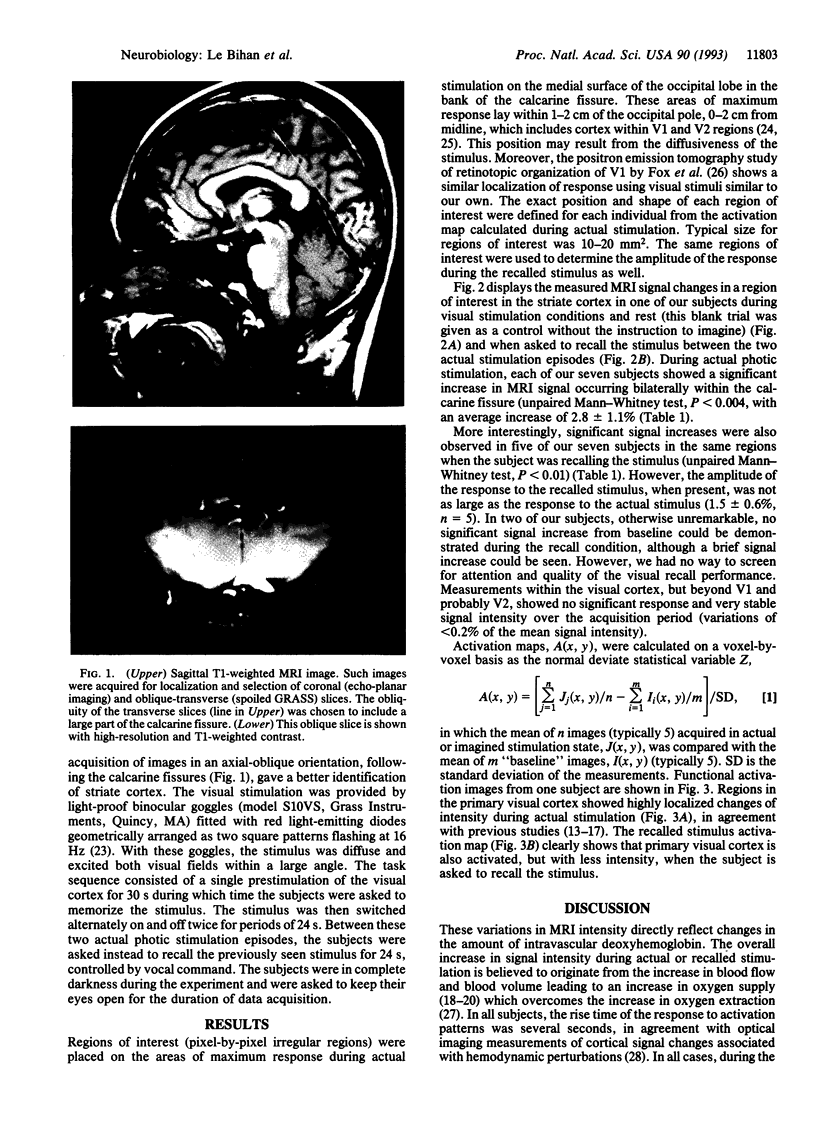
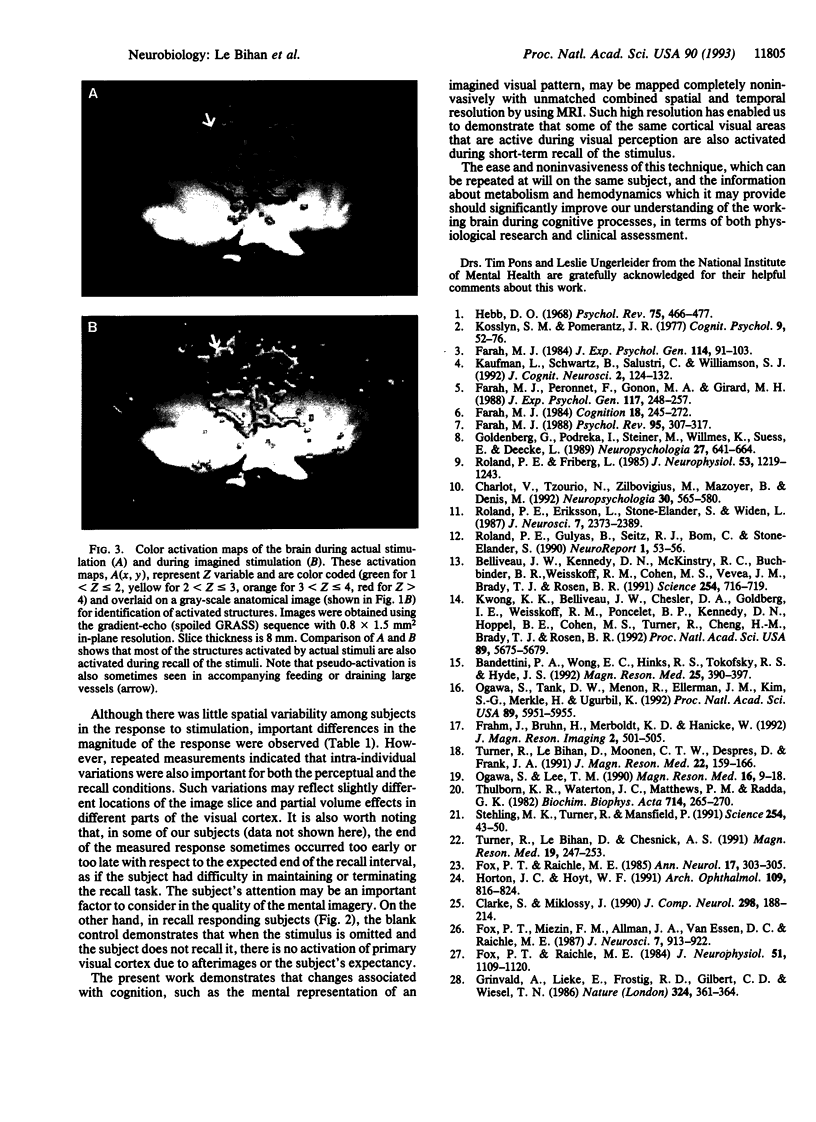
Abstract
The degree to which the process involved in visual perception and visual imagery share a common neuroanatomical substrate is unclear. Physiological evidence for localization of visual imagery early in the visual pathways would have important bearing on current theories of visual processing. A magnetic resonance imaging technique sensitive to regional changes in blood oxygenation was used to obtain functional activation maps in the human visual cortex. During recall of a visual stimulus, focal increases in signal related to changes in blood flow were detected in V1 and V2 cortex in five of seven subjects. These experiments show that the same areas of the early visual cortex that are excited by visual stimulation are also activated during mental representation of the same stimulus. Some of the processes used in topographically mapped cortical areas during visual perception may also be utilized during visual recall.
Full text
PDF
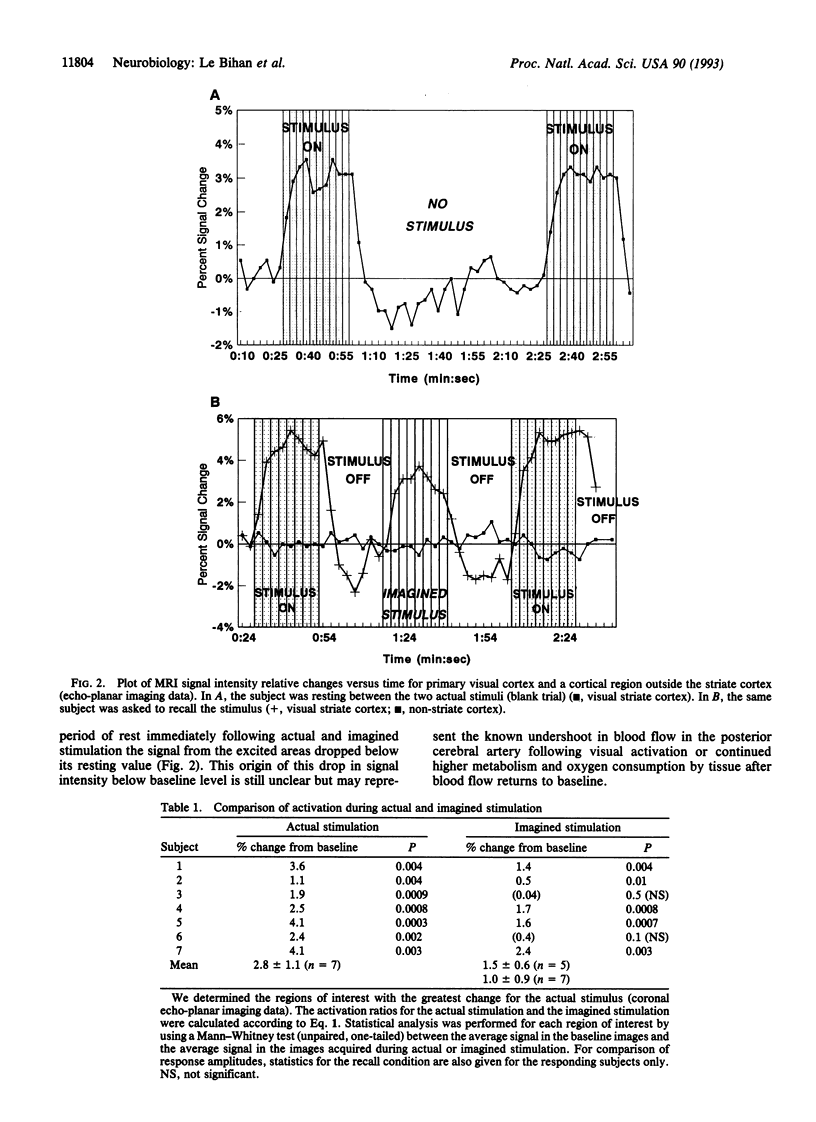
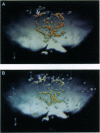
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bandettini P. A., Wong E. C., Hinks R. S., Tikofsky R. S., Hyde J. S. Time course EPI of human brain function during task activation. Magn Reson Med. 1992 Jun;25(2):390–397. doi: 10.1002/mrm.1910250220. [DOI] [PubMed] [Google Scholar]
- Belliveau J. W., Kennedy D. N., Jr, McKinstry R. C., Buchbinder B. R., Weisskoff R. M., Cohen M. S., Vevea J. M., Brady T. J., Rosen B. R. Functional mapping of the human visual cortex by magnetic resonance imaging. Science. 1991 Nov 1;254(5032):716–719. doi: 10.1126/science.1948051. [DOI] [PubMed] [Google Scholar]
- Charlot V., Tzourio N., Zilbovicius M., Mazoyer B., Denis M. Different mental imagery abilities result in different regional cerebral blood flow activation patterns during cognitive tasks. Neuropsychologia. 1992 Jun;30(6):565–580. doi: 10.1016/0028-3932(92)90059-u. [DOI] [PubMed] [Google Scholar]
- Clarke S., Miklossy J. Occipital cortex in man: organization of callosal connections, related myelo- and cytoarchitecture, and putative boundaries of functional visual areas. J Comp Neurol. 1990 Aug 8;298(2):188–214. doi: 10.1002/cne.902980205. [DOI] [PubMed] [Google Scholar]
- Farah M. J. Is visual imagery really visual? Overlooked evidence from neuropsychology. Psychol Rev. 1988 Jul;95(3):307–317. doi: 10.1037/0033-295x.95.3.307. [DOI] [PubMed] [Google Scholar]
- Farah M. J. Psychophysical evidence for a shared representational medium for mental images and percepts. J Exp Psychol Gen. 1985 Mar;114(1):91–103. doi: 10.1037//0096-3445.114.1.91. [DOI] [PubMed] [Google Scholar]
- Farah M. J., Péronnet F., Gonon M. A., Giard M. H. Electrophysiological evidence for a shared representational medium for visual images and visual percepts. J Exp Psychol Gen. 1988 Sep;117(3):248–257. doi: 10.1037//0096-3445.117.3.248. [DOI] [PubMed] [Google Scholar]
- Farah M. J. The neurological basis of mental imagery: a componential analysis. Cognition. 1984 Dec;18(1-3):245–272. doi: 10.1016/0010-0277(84)90026-x. [DOI] [PubMed] [Google Scholar]
- Fox P. T., Miezin F. M., Allman J. M., Van Essen D. C., Raichle M. E. Retinotopic organization of human visual cortex mapped with positron-emission tomography. J Neurosci. 1987 Mar;7(3):913–922. doi: 10.1523/JNEUROSCI.07-03-00913.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox P. T., Raichle M. E. Stimulus rate dependence of regional cerebral blood flow in human striate cortex, demonstrated by positron emission tomography. J Neurophysiol. 1984 May;51(5):1109–1120. doi: 10.1152/jn.1984.51.5.1109. [DOI] [PubMed] [Google Scholar]
- Fox P. T., Raichle M. E. Stimulus rate determines regional brain blood flow in striate cortex. Ann Neurol. 1985 Mar;17(3):303–305. doi: 10.1002/ana.410170315. [DOI] [PubMed] [Google Scholar]
- Frahm J., Bruhn H., Merboldt K. D., Hänicke W. Dynamic MR imaging of human brain oxygenation during rest and photic stimulation. J Magn Reson Imaging. 1992 Sep-Oct;2(5):501–505. doi: 10.1002/jmri.1880020505. [DOI] [PubMed] [Google Scholar]
- Goldenberg G., Podreka I., Steiner M., Willmes K., Suess E., Deecke L. Regional cerebral blood flow patterns in visual imagery. Neuropsychologia. 1989;27(5):641–664. doi: 10.1016/0028-3932(89)90110-3. [DOI] [PubMed] [Google Scholar]
- Grinvald A., Lieke E., Frostig R. D., Gilbert C. D., Wiesel T. N. Functional architecture of cortex revealed by optical imaging of intrinsic signals. 1986 Nov 27-Dec 3Nature. 324(6095):361–364. doi: 10.1038/324361a0. [DOI] [PubMed] [Google Scholar]
- Hebb D. O. Concerning imagery. Psychol Rev. 1968 Nov;75(6):466–477. doi: 10.1037/h0026771. [DOI] [PubMed] [Google Scholar]
- Horton J. C., Hoyt W. F. The representation of the visual field in human striate cortex. A revision of the classic Holmes map. Arch Ophthalmol. 1991 Jun;109(6):816–824. doi: 10.1001/archopht.1991.01080060080030. [DOI] [PubMed] [Google Scholar]
- Kwong K. K., Belliveau J. W., Chesler D. A., Goldberg I. E., Weisskoff R. M., Poncelet B. P., Kennedy D. N., Hoppel B. E., Cohen M. S., Turner R. Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5675–5679. doi: 10.1073/pnas.89.12.5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S., Lee T. M. Magnetic resonance imaging of blood vessels at high fields: in vivo and in vitro measurements and image simulation. Magn Reson Med. 1990 Oct;16(1):9–18. doi: 10.1002/mrm.1910160103. [DOI] [PubMed] [Google Scholar]
- Ogawa S., Tank D. W., Menon R., Ellermann J. M., Kim S. G., Merkle H., Ugurbil K. Intrinsic signal changes accompanying sensory stimulation: functional brain mapping with magnetic resonance imaging. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):5951–5955. doi: 10.1073/pnas.89.13.5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roland P. E., Eriksson L., Stone-Elander S., Widen L. Does mental activity change the oxidative metabolism of the brain? J Neurosci. 1987 Aug;7(8):2373–2389. [PMC free article] [PubMed] [Google Scholar]
- Roland P. E., Friberg L. Localization of cortical areas activated by thinking. J Neurophysiol. 1985 May;53(5):1219–1243. doi: 10.1152/jn.1985.53.5.1219. [DOI] [PubMed] [Google Scholar]
- Roland P. E., Gulyás B., Seitz R. J., Bohm C., Stone-Elander S. Functional anatomy of storage, recall, and recognition of a visual pattern in man. Neuroreport. 1990 Sep;1(1):53–56. doi: 10.1097/00001756-199009000-00015. [DOI] [PubMed] [Google Scholar]
- Stehling M. K., Turner R., Mansfield P. Echo-planar imaging: magnetic resonance imaging in a fraction of a second. Science. 1991 Oct 4;254(5028):43–50. doi: 10.1126/science.1925560. [DOI] [PubMed] [Google Scholar]
- Thulborn K. R., Waterton J. C., Matthews P. M., Radda G. K. Oxygenation dependence of the transverse relaxation time of water protons in whole blood at high field. Biochim Biophys Acta. 1982 Feb 2;714(2):265–270. doi: 10.1016/0304-4165(82)90333-6. [DOI] [PubMed] [Google Scholar]
- Turner R., Le Bihan D., Chesnick A. S. Echo-planar imaging of diffusion and perfusion. Magn Reson Med. 1991 Jun;19(2):247–253. doi: 10.1002/mrm.1910190210. [DOI] [PubMed] [Google Scholar]
- Turner R., Le Bihan D., Moonen C. T., Despres D., Frank J. Echo-planar time course MRI of cat brain oxygenation changes. Magn Reson Med. 1991 Nov;22(1):159–166. doi: 10.1002/mrm.1910220117. [DOI] [PubMed] [Google Scholar]