Abstract
The plant pathogenic bacterium Ralstonia solanacearum injects more than 70 effector proteins (virulence factors) into the host plant cells via the needle-like structure of a type III secretion system. The type III secretion system effector proteins manipulate host regulatory networks to suppress defense responses with diverse molecular activities. Uncovering the molecular function of these effectors is essential for a mechanistic understanding of R. solanacearum pathogenicity. However, few of the effectors from R. solanacearum have been functionally characterized, and their plant targets remain largely unknown. Here, we show that the ChaC domain-containing effector RipAY/RSp1022 from R. solanacearum exhibits γ-glutamyl cyclotransferase (GGCT) activity to degrade the major intracellular redox buffer, glutathione. Heterologous expression of RipAY, but not other ChaC family proteins conserved in various organisms, caused growth inhibition of yeast Saccharomyces cerevisiae, and the intracellular glutathione level was decreased to ∼30% of the normal level following expression of RipAY in yeast. Although active site mutants of GGCT activity were non-toxic, the addition of glutathione did not reverse the toxicity, suggesting that the toxicity might be a consequence of activity against other γ-glutamyl compounds. Intriguingly, RipAY protein purified from a bacterial expression system did not exhibit any GGCT activity, whereas it exhibited robust GGCT activity upon its interaction with eukaryotic thioredoxins, which are important for intracellular redox homeostasis during bacterial infection in plants. Our results suggest that RipAY has evolved to sense the host intracellular redox environment, which triggers its enzymatic activity to create a favorable environment for R. solanacearum infection.
Keywords: plant defense, redox regulation, Saccharomyces cerevisiae, thioredoxin, type III secretion system (T3SS)
Introduction
Numerous bacterial pathogens of plants and animals inject virulence proteins, so-called effectors, directly into the host cell cytoplasm through specialized secretion apparatuses, such as the type III secretion system (T3SS).2 The translocated T3SS effector proteins manipulate diverse host cellular processes, including cytoskeletal reorganization, signal transduction, gene expression, vesicular trafficking, autophagy, and DNA replication, to promote infection and ultimately cause disease (1, 2). Although the identification of the biochemical activities of these effector proteins is essential to understand the molecular mechanism of pathogenesis, it has been very difficult to predict the molecular functions based on their primary sequences because many effector proteins have limited similarity to known proteins and some effectors can be considered “convergently evolved” mimics of their eukaryotic cell counterparts (3). Furthermore, thanks to extensive genome sequencing programs, coupled with robust computational predictions of sequence motifs characteristic of effector proteins, the number of putative effectors identified in the genomes from many bacterial pathogens is continuously increasing in the database (4). For the above reasons, a function-based method to efficiently analyze effector proteins is desired. Interestingly, effector proteins have been observed to confer a growth inhibition phenotype when heterologously expressed in the yeast Saccharomyces cerevisiae (5, 6). This growth inhibition is thought to be the consequence of the effector-induced compromise of cellular processes conserved between yeast and higher eukaryotes. For example, Lesser and Miller (7) showed that the Yersinia effector YopE, which functions as a Rho GTPase-activating protein (RhoGAP), blocks actin polarization and cell cycle progression through its RhoGAP activity. Importantly, growth inhibition is a genetically tractable phenotype, and it provides a variety of means to investigate modes of action of these effectors toward host cell targets.
Ralstonia solanacearum, which is a widely distributed soil-borne phytopathogen, causes lethal bacterial wilt of more than 200 plant species, including economically important crops, such as tomato, potato, and tobacco (8). Among the pathogenicity determinants of this bacterium, T3SS was shown to play an essential role in pathogenicity because the corresponding mutants were avirulent on host plants (9). In general, compared with animal pathogens, bacterial plant pathogens, such as Xanthomonas spp. or Pseudomonas syringae, contain larger numbers (∼30–40) of effectors, but the R. solanacearum effector repertoire is exceptionally large, probably due to its wide host range (10). Postgenomic functional analyses using regulation-based approaches and/or T3SS-translocon assays have identified the nearly complete repertoire of 70–75 effectors in the reference strain GMI1000 and the phylogenetically close strain RS1000 (11). The investigation of the R. solanacearum strain complex pangenome also identified additional families of likely effector proteins with no homology to other previously identified effectors, and thus the current estimated number of effector families is ∼110 among 11 strains representative of the biodiversity of the R. solanacearum strain complex (12). Individual R. solanacearum strains typically possess around 60–75 effectors. Effector repertoire comparison revealed a group of 32 core effectors present in 10 of 11 strains (13). To date, only a few R. solanacearum effectors have been assigned molecular functions and targets (14–16), but most of these effectors remain functionally uncharacterized.
In this study, we screened R. solanacearum effectors using a yeast expression system and identified RipAY as an effector whose expression causes growth inhibition in yeast. RipAY, which is one of the R. solanacearum core effectors, has previously been shown experimentally to be an effector injected into host plant cells via T3SS (17), but the molecular function of this effector has yet to be characterized. Bioinformatics analysis revealed that RipAY contains a ChaC domain, which is a conserved domain found in all phyla examined but whose molecular function was totally unknown when we started our study. Recently, it has been reported that yeast and mammalian ChaC domain-containing proteins exhibit γ-glutamyl cyclotransferase (GGCT) activity specifically to degrade glutathione (18). We demonstrated that RipAY exhibits robust GGCT activity and significantly decreases intracellular glutathione in yeast. Surprisingly, we failed to detect GGCT activity of recombinant RipAY expressed in Escherichia coli. However, we could detect robust GGCT activity when RipAY was activated by yeast or plant thioredoxins. Both glutathione and thioredoxins are important for maintaining cellular redox homeostasis and also indispensable for proper activation of plant innate immunity during pathogen infection (19, 20). Together, our data provide new evidence that R. solanacearum perturbs the host redox environment to allow bacterial infection.
Experimental Procedures
Strains, Plasmids, and Media
Descriptions of the strains and plasmids used in this study are presented in Tables 1–3. Escherichia coli DB3.1 (Life Technologies, Inc.) was used for the construction and amplification of the GatewayTM vectors, and E. coli DH5α or JM109 was the bacterial host for all of the other plasmids constructed. Coding sequences were amplified by PCR using KOD-Plus-Neo polymerase (Toyobo) or PrimeSTAR GXL polymerase (Takara Bio). Plasmids were sequenced to ensure that no mutations were introduced due to manipulations. Yeast transformation was performed using the lithium acetate method (21). Mutant constructs were generated by site-directed mutagenesis (22) and confirmed by sequencing. The media used for yeast culture were synthetic dextrose (SD) medium (2% glucose, 0.67% yeast nitrogen base without amino acids) and synthetic galactose (SGal) medium (2% galactose, 0.67% yeast nitrogen base without amino acids). Appropriate amino acids and bases were added to SD or SGal medium as necessary. Yeast cells were cultured at 26 °C unless otherwise stated.
TABLE 1.
Strains used in this study
| Strain | Genotype | Reference or source |
|---|---|---|
| S. cerevisiae | ||
| BY4741 | MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 | Research Genetics |
| BY4742 | MATα his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 | Research Genetics |
| BY4743 | BY4741/BY4742 | Research Genetics |
| MTY654 | BY4741 dug3Δ::KanMX4 ecm38Δ::KanMX4 gcg1Δ::KanMX4 | This study |
| MTY674 | BY4741 trx1Δ::KanMX4 | This study |
| MTY676 | BY4741 trx2Δ::KanMX4 | This study |
| MTY678 | BY4741 trx3Δ::KanMX4 | This study |
| MTY680 | BY4741 trx1Δ::KanMX4 trx2Δ::KanMX4 | This study |
| MTY761 | BY4741 trx1Δ::KanMX4 trx2Δ::KanMX4 trx3Δ::KanMX4 | This study |
| gsh1Δ/gsh1Δ | BY4743 gsh1Δ::KanMX4/gsh1Δ::KanMX4 | Research Genetics |
| AH109 | MATa trp1-901 leu2-3 112 ura3-52 his3-200 gal4Δ gal80Δ LYS2::GAL1UAS-GAL1TATA-HIS3 MEL1 GAL2UAS-GAL2TATA-ADE2 URA3::MEL1UAS-MEL1TATA-LacZ | Clontech |
| R. solanacearum | ||
| OE1-1 | Wild-type, pathogenic to tobacco, eggplant isolate | Ref. 25 |
| RK5081 | OE1-1 ΔhrcU-1 | This study |
| RK7101 | OE1-1 ΔripAY-4 | This study |
TABLE 2.
S. cerevisiae plasmids used in this study
| Plasmids | Construct | Reference or source |
|---|---|---|
| pMT751 | URA3, 2μ, PGAL1-attR1-Cmr-ccdB-attR2-GFP | Ref. 33 |
| pMT830 | URA3, 2μ, Ptet-off-attR1-Cmr-ccdB-attR2-GFP | Ref. 33 |
| pMT1373 | URA3, 2μ, PGAL1-attR1-Cmr-ccdB-attR2-Protein A | This study |
| pGBKT7 | TRP1, 2μ, yeast two-hybrid GAL4 DNA-BD vector | Clontech |
| pGADT7 | LEU2, 2μ, yeast two-hybrid GAL4 AD vector | Clontech |
| pACT2 | LEU2, 2μ, yeast two-hybrid GAL4 AD vector | Clontech |
| pMT731 | pACT2; GAL4 AD-attR1-Cmr-ccdB-attR2 | This study |
| pMT922 | pMT830; URA3, 2μ, Ptet-off-GFP | Ref. 33 |
| pRE85 | pMT751; URA3, 2μ, PGAL1-R. sonalacearum RipAY-GFP | This study |
| pRE97 | pMT751; URA3, 2μ, PGAL1-R. sonalacearum RSc0782-GFP | This study |
| pRE98 | pMT751; URA3, 2μ, PGAL1-H. sapiens Chac2-GFP | This study |
| pRE99 | pMT751; URA3, 2μ, PGAL1-P. syringae PSPTO_5239-GFP | This study |
| pRE100 | pMT751; URA3, 2μ, PGAL1-S. cerevisiae GCG1-GFP | This study |
| pRE161 | pMT751; URA3, 2μ, PGAL1-A. citrulli Aave_4606-GFP | This study |
| pRE173 | pMT751; URA3, 2μ, PGAL1-A. thaliana AT1G44790/GGCT2;3-GFP | This study |
| pRE174 | pMT751; URA3, 2μ, PGAL1-A. thaliana AT4G31290/GGCT2;2-GFP | This study |
| pRE175 | pMT751; URA3, 2μ, PGAL1-A. thaliana AT5G26220/GGCT2;1-GFP | This study |
| pRE86 | pMT751; URA3, 2μ, PGAL1-RipAYE216Q-GFP | This study |
| pRE164 | pMT751; URA3, 2μ, PGAL1-RipAY2Y129A-GFP | This study |
| pRE165 | pMT751; URA3, 2μ, PGAL1-RipAYL130A-GFP | This study |
| pRE166 | pMT751; URA3, 2μ, PGAL1-RipAYS131A-GFP | This study |
| pRE167 | pMT751; URA3, 2μ, PGAL1-RipAYL132A-GFP | This study |
| pRE168 | pMT751; URA3, 2μ, PGAL1-RipAYL130G-GFP | This study |
| pSF38 | pMT751; URA3, 2μ, PGAL1-RipAYC333S-GFP | This study |
| pRE257 | pMT1373; URA3, 2μ, PGAL1-S. cerevisiae GCG1-Protein A | This study |
| pRE258 | pMT1373; URA3, 2μ, PGAL1-RipAY-Protein A | This study |
| pRE259 | pMT1373; URA3, 2μ, PGAL1-RipAYE216Q-Protein A | This study |
| pSF25 | pGBKT7; TRP1, 2μ, PADH1-GAL4 DNA-BD-RipAYE216Q | This study |
| pSF37 | pGBKT7; TRP1, 2μ, PADH1-GAL4 DNA-BD-RipAYE216Q,C333S | This study |
| pRE249 | pGADT7; LEU2, 2μ, PADH1-GAL4 AD-A. thaliana TRX h2 | This study |
| pRE250 | pGADT7; LEU2, 2μ, PADH1-GAL4 AD-A. thaliana TRX h3 | This study |
| pSF50 | pMT731; LEU2, 2μ, PADH1-GAL4 AD-A. thaliana TRX h5 | This study |
| pSF51 | pMT731; LEU2, 2μ, PADH1-GAL4 AD-A. thaliana TRX h5C39S,C42S | This study |
| pSF52 | pMT731; LEU2, 2μ, PADH1-GAL4 AD-S. cerevisiae TRX1 | This study |
| pMT1416 | pMT731; LEU2, 2μ, PADH1-GAL4 AD-R. solanacearum TrxA | This study |
| pTEF1-416-HGT1 | pRS416; URA3, CEN, PTEF1-S. cerevisiae HGT1 | Ref. 31 |
TABLE 3.
E. coli plasmids used in this study
| Plasmids | Construct | Reference or source |
|---|---|---|
| pET23d | Amp, PT7, the C-terminal His6 tag fusion vector | Novagen, Inc. |
| pDEST17 | Amp, PT7, the Gateway N-terminal His6 tag fusion vector | Life Technologies, Inc. |
| pMT1371 | pET23d: PT7-attR1-Cmr-ccdB-attR2-Protein A | This study |
| pSF16 | pET23d: PT7-R. solanacearum RipAY-His6 | This study |
| pSF17 | pET23d: PT7-S. cerevisiae GCG1-His6 | This study |
| pSF16 | pET23d: PT7-R. solanacearum RipAY-His6 | This study |
| pSF40 | pET23d: PT7-S. cerevisiae TRX1-His6 | This study |
| pSF49 | pDEST17: PT7-Met-His6-A. thaliana TRX h5 | This study |
| pMT1293 | pET23d: PT7-S. cerevisiae DUG1-His6 | This study |
| pMT1409 | pDEST17: PT7-Met-His6-A. thaliana TRX h2 | This study |
| pMT1410 | pDEST17: PT7-Met-His6-A. thaliana TRX h3 | This study |
| pMT1411 | pDEST17: PT7-Met-His6-A. thaliana TRX h5C39S,C42S | This study |
| pMT1415 | pDEST17: PT7-Met-His6-R. solanacearum TrxA | This study |
| pMT1368 | pMT1371: PT7-Protein A | This study |
| pRE254 | pMT1371: PT7-S. crevisiae GCG1-Protein A | This study |
| pRE255 | pMT1371: PT7-R. solanacearum RipAY-Protein A | This study |
| pRE256 | pMT1371: PT7-R. solanacearum RipAYE216Q-Protein A | This study |
| pRE496 | pMT1371: PT7-R. solanacearum RipAYC333S-Protein A | This study |
Phylogenetic Analysis
Phylogenetic trees of ChaC domain-containing proteins, including RipAY or thioredoxins from various organisms, were created with MEGA6 (23). The amino acid sequences of ChaC proteins or thioredoxins from the NCBI database were aligned with ClustalW, from which a maximum likelihood phylogenetic tree was created.
Homology Modeling and Structure Analysis
The RipAY protein sequence was used for fold recognition using the Phyre2 server. This search identified human GGCT (C7 orf24, Protein Data Bank entries 2PN7 and 2RBH) as the best template. We used the alignment provided by the Phyre2 server to construct models for RipAY using CueMol molecular graphics software.
Production and Purification of Recombinant Proteins in E. coli
For the purification of the His6-tagged recombinant proteins, Rosetta-gami B (DE3) cells (EMD) carrying pMT1293, pMT1409, pMT1410, pMT1411, pMT1415, pSF16, pSF17, pSF40, or pSF49 plasmid were grown in 600 ml of Luria-Bertani (LB) medium with 34 μg/ml chloramphenicol and 100 μg/ml ampicillin at 37 °C to an A600 of 0.8. The bacteria were then moved to 18 °C for 30 min, and the protein expression was induced by the addition of isopropyl-1-thio-β-d-galactopyranoside to 0.3 mm for 6 h. The cells were collected by centrifugation at 10,000 × g for 5 min. The cell pellet was resuspended in 30 ml of binding buffer (50 mm NaH2PO4, 500 mm NaCl, 5 mm imidazole, pH 8.0) containing 1 mm PMSF and disrupted by sonication. The lysate was cleared by centrifugation at 10,000 × g for 30 min at 4 °C, and the cleared lysate was applied to the HisTrap FF 1-ml column (GE Healthcare) equilibrated with binding buffer. The column was then washed with a 20-column volume of binding buffer and eluted with elution buffer (50 mm NaH2PO4, 500 mm NaCl, 300 mm imidazole, pH 8.0). Fractions containing the recombinant His6-tagged fusion proteins were collected. Purified His6-tagged thioredoxins were treated with 20 mm dithiothreitol (DTT) at room temperature for 15 min to reduce thioredoxins. Purified proteins were subsequently dialyzed into PBS, and protein concentrations were determined using a micro-BCA protein assay kit (Thermo Scientific).
Measurement of Cys-Gly Peptidase (Dug1)-coupled GGCT Activity of His6-tagged RipAY and Gcg1 Purified from E. coli
GGCT activity toward glutathione as substrate was carried out using a Dug1-coupled assay as described previously (18) with some modifications. In brief, 0.4 μm Gcg1 or 0.04 μm RipAY with appropriate concentrations of the cell lysates from various organisms or thioredoxins purified from E. coli was incubated with glutathione for 20 min at 37 °C in 50 μl of reaction mixture containing 50 mm Tris-HCl (pH 8.0). The reaction was terminated by the addition of 20 μl of 0.25 n HCl and then neutralized with the addition of 8.4 μl of 1 m Tris and made up to 100 μl with 50 mm Tris-HCl (pH 8.0). Ten microliters of the reaction mixture was then transferred to a new tube to which was added 2 μl of Dug1 reaction mixture; this converts the Cys-Gly dipeptide into free cysteine and glycine. The Dug1 reaction mixture consisted of 20 μm MnCl2 and 1 μg of recombinant S. cerevisiae Dug1p, a Cys-Gly peptidase purified from E. coli. This reaction mixture was further incubated for 1 h at 37 °C. The free cysteine generated was measured by acidic ninhydrin solution (20 g of ninhydrin dissolved in acetic acid and HCl that are mixed in a 3:2 ratio) and added to the reaction mixture, and the reaction mixture was incubated at 100 °C for 10 min to develop the pink color. Two hundred microliters of colored solutions was transferred to a 96-well plate, and the A570 was measured using a microplate reader (Bio-Rad). The assay was performed in triplicates, and data are mean ± S.E.
Measurement of GGCT Activity of Protein A-tagged RipAY and Gcg1
GCG1, ripAYWT, or ripAYE216Q genes on pDONR221 donor vector were cloned into the Protein A-tagging gateway destination vectors, pMT1371 for E. coli or pMT1373 for S. cerevisiae, by the LR reaction. E. coli Rosetta gami B (DE3) cells carrying pMT1371-GCG1, ripAYWT, ripAYE216Q, or ripAYC333S plasmids were cultured in 100 ml of LB medium containing 34 μg/ml chloramphenicol and 100 μg/ml ampicillin at 37 °C to an A600 of 0.8. The bacteria were then moved to 18 °C for 30 min, and the protein expression was induced by the addition of isopropyl-1-thio-β-d-galactopyranoside to 0.3 mm for 6 h. E. coli cells expressing Protein A-tagged Gcg1, RipAYWT, RipAYE216Q, or RipAYC333S proteins were suspended in IPP150 buffer (10 mm Tris-HCl, pH 8.0, 150 mm NaCl, 50 mm EDTA) and disrupted by sonication to generate the E. coli lysates. S. cerevisiae cells carrying pMT1373-GCG1, ripAYWT, or ripAYE216Q plasmids were cultured in 20 ml of SD (−Ura) liquid medium at 26 °C to an A600 of 1.0, and then cells were washed twice with sterilized water and resuspended in 20 ml of SGal (−Ura) liquid medium and cultured at 26 °C for 17 h to induce the protein expressions. S. cerevisiae cells expressing Protein A-tagged Gcg1, RipAYWT, or RipAYE216Q proteins were suspended in 500 μl of 1 m sorbitol, 50 mm EDTA solution containing 10 mm DTT with 1 mg of Zymolyase-20T (Nakarai Tesque Inc.) to obtain spheroplasts. Spheroplasts were washed once with 1 m sorbitol, 50 mm EDTA and resuspended with 1 ml of IPP150 buffer containing 2 μm pepstatin A and 1 mm PMSF and homogenized with a Dounce homogenizer (10 strokes) on ice to generate the S. cerevisiae lysates. The lysates from E. coli or S. cerevisiae cells were cleared by centrifugation at 10,000 × g for 30 min at 4 °C, and 50 μl of IgG-Sepharose beads (50%, v/v) were added into the cleared lysate and incubated for 2 h at 4 °C on a rotating wheel. The beads were washed three times with the same buffer. The Protein A-tagged fusion protein-immobilized beads were used for measuring the GGCT activity by a Dug1-coupled method as described above. For activation of affinity-purified Protein A-tagged RipAY from E. coli, the Protein A-tagged fusion protein-immobilized beads were incubated with the total protein lysate from yeast cells in 1 ml of IPP150 buffer at 4 °C for 2 h, washed three times with IPP150 buffer, and then used for measuring the GGCT activity by a Dug1-coupled method as described above.
Immunoblotting Analysis
Yeast cells expressing GFP-taggedRipAY or other ChaC proteins were collected and resuspended in 1 ml of PBS. After the addition of trichloroacetic acid (TCA) to a final concentration of 10%, cells were incubated on ice for 15 min and precipitated by centrifugation. TCA-treated yeast cells were washed twice with 1 ml of ice-cold acetone and dried. Total proteins were extracted from the dried cells by beating cells with glass beads (two pulses of 5 min each at 65 °C) in Urea-SDS cracking buffer (6 m urea, 1% SDS, 50 mm Tris-HCl, pH 7.5). The total protein concentration of the cell extracts was determined using a micro-BCA protein assay kit (Thermo Scientific). Ten-microgram aliquots of total protein were subjected to immunoblotting analysis, which was performed using affinity-purified rabbit anti-GFP antiserum (our laboratory stock) or rabbit anti-glucose-6-phosphate dehydrogenase antibody (Sigma, A9521) and horseradish peroxidase-conjugated anti-rabbit IgG serum (Cell Signaling Technologies). Signals were detected using Immunostar LD Western blotting detection reagent (Wako).
Measurement of Total Cellular Glutathione
Ten A600 of yeast cells expressing RipAY and their mutants under the control of the GAL1 promoter were washed once with 1 ml of KPE (0.1 m potassium phosphate buffer with 5 mm EDTA, pH 7.5) and lysed in 200 μl of 5% sulfosalicylic acid using glass beads. Cells were vortexed for 1 min with 1-min intervals 10 times and centrifuged for 10 min at 4 °C. Total cellular glutathione in yeast was measured as described previously (24). In brief, 10 μl of supernatant was mixed with 60 μl of DTNB (2 mg/3 ml) and 60 μl of glutathione reductase (2.5 units/ml) in a 96-well plate. Sixty microliters of NADPH (2 mg/3 ml) was added in the above mixtures to start the reaction. Absorbance was measured at every 15 s at 415 nm. Absorbance at 2 min was used to compare the glutathione level in the different samples. Total cellular glutathione in yeast is normalized to A600 of yeast cells.
Measurement of Total Cellular Glutathione in Bacteria-inoculated Plants
A 108-cfu/ml suspension of R. solanacearum OE1-1 (WT) (25), RK5081 (T3SS mutant, ΔhrcU-1), or RK7101 (ΔripAY-4) in a ∼50 μl volume was inoculated into fully expanded leaves of 6–8-week-old susceptible eggplant (Solanum melongena cv. Senryo 2-gou) using a needleless disposable syringe, and the bacteria-inoculated plants were maintained in a greenhouse facility. Two leaf disks (0.8 cm2 each) were taken 1 day after inoculation and frozen in liquid nitrogen. The frozen samples were extracted by shaking five times with 5-mm diameter stainless beads for 30 s in a 2.0-ml screw-capped vial using a bead beater device. Three hundred microliters of 5% sulfosalicylic acid in KPE was added to the samples, and then the samples were vortexed and centrifuged at 8,000 × g for 20 min at 4 °C. Total cellular glutathione of the samples extracted from bacteria-inoculated plants was measured by the same method as used for the samples from yeast as described above. Total cellular glutathione in bacteria-inoculated leaf was normalized to mg of leaf material.
Fluorescent Microscopic Analysis
Fluorescence of GFP in the non-fixed yeast cells was observed using an Olympus BX51 microscope (Olympus) with a GFP filter. Images were captured with a Hamamatsu C11440-10C Orca-Flash 2.8 CMOS camera (Hamamatsu Photonics) using Metamorph software (Molecular Devices).
Yeast Two-hybrid Analysis
The yeast two-hybrid analysis was performed using a GAL4-based yeast two-hybrid system according to the manufacturer's instructions (MATCHMAKER Two-Hybrid System 3; Clontech). RipAY with the E216Q active site mutation (RipAYE216Q) was used for yeast two-hybrid analysis to avoid the growth inhibition caused by its expression in yeast. The ripAYE216Q gene was amplified by PCR and cloned into the EcoRI and BamHI sites of pGBKT7 vector to generate a construct of RipAYE216Q fused in-frame to the Gal4 DNA-binding domain as the bait. GatewayTM technology (Life Technologies) was used to construct the prey plasmids. Thioredoxin cDNAs (R. solanacearum TrxA/RSc1188, S. cerevisiae Trx1, and Arabidopsis thaliana Trx-h2, Trx-h3, and Trx-h5) were amplified by PCR and cloned into the gateway donor vector pDONR221 by the BP reaction. The pDONR221-Trx-h5 plasmid was used to introduce the C39S,C42S active site mutation into Trx-h5. The thioredoxin genes on pDONR221 plasmid were cloned into pACT2-based gateway Gal4 activation domain plasmid, pMT731, by the LR reaction to generate the prey plasmids. The bait and prey plasmids were cotransformed into two-hybrid reporter yeast strain AH109 cells. The transformants were spotted on SD (−Trp, −Leu) plates and SD plates with different stringency conditions (low: −Trp, −Leu, −His; medium: −Trp, −Leu, −His, + 1 mm 3-amino-1,2,4-triazole; high: −Trp, −Leu, −His, −Ade), and the interaction of RipAY and thioredoxins was assessed by their growth under the different stringency conditions.
Purification of the RipAY Activator from Yeast
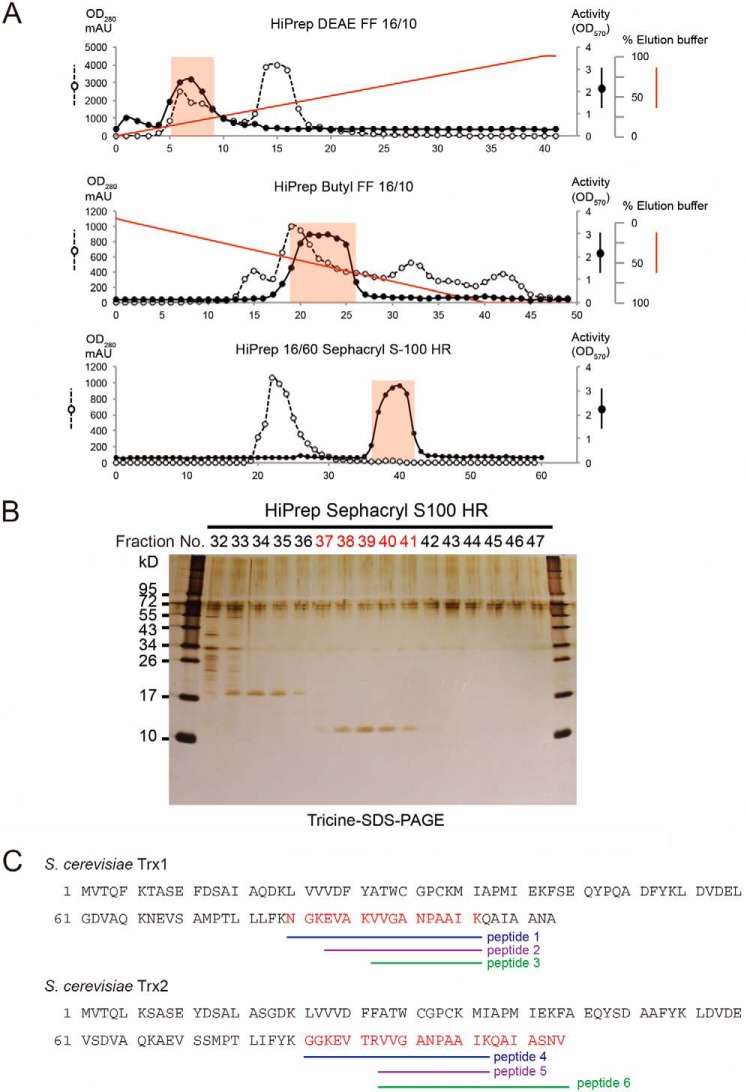
Soluble proteins were extracted from 10 g of S. cerevisiae MTY654 (dug3Δ ecm38Δ gcg1Δ) cells mechanically disrupted by a French press. The extracted S. cerevisiae proteins were precipitated with 100% ammonium sulfate. Precipitated proteins were dialyzed overnight at 4 °C against 6 liters of 20 mm Tris-HCl, pH 7.5. S. cerevisiae proteins were separated over a HiPrep DEAE FF 16/10 ion exchange column with a salt gradient from 0 to 1 m NaCl (GE Healthcare). Individual fractions were assayed for their ability to stimulate GGCT activity of a recombinant RipAY purified from E. coli. Fractions possessing the majority of activity were pooled and dialyzed overnight at 4 °C against 2 liters of 20 mm Tris-HCl, pH 7.5, 1.5 m (NH4)2SO4. After dialysis, S. cerevisiae proteins were separated on a HiPrep Butyl FF 16/10 hydrophobic interaction column with a salt gradient from 1.5 to 0 m (NH4)2SO4 (GE Healthcare). Individual fractions retaining the activity were pooled and concentrated to 1 ml using an Amicon Ultra-2mL 3K centrifugal filter device (Millipore). Positive fractions were then separated over a HiPrep 16/60 Sephacryl 200 high resolution gel filtration column (GE Healthcare). Proteins in this final purification step contained in the positive fraction were separated by Tricine SDS-PAGE and visualized with a Sil-Best Stain One (Nakarai Tesque Inc.) or a Coomassie Brilliant Blue stain.
In-gel digestion and the following LC-MS/MS analysis of the activator protein from yeast were performed as described previously (26). Protein identification was performed in the Agilent Spectrum MILL MS proteomics workbench against the Swiss-Prot protein database search engine.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism version 6 or Microsoft Excel software and specific tests noted throughout.
Results
Expression of a ChaC Domain-containing Effector, RipAY, Causes Growth Inhibition of Yeast
To understand the molecular mechanism of pathogenesis for R. solanacearum, we screened the collection of 36 previously identified effector proteins of R. solanacearum GMI1000 (13, 27) using a yeast galactose-inducible expression system. This yeast expression screen revealed eight effectors that cause growth inhibition of yeast S. cerevisiae. Details of this screen along with the complete set of identified effectors will be described elsewhere.3
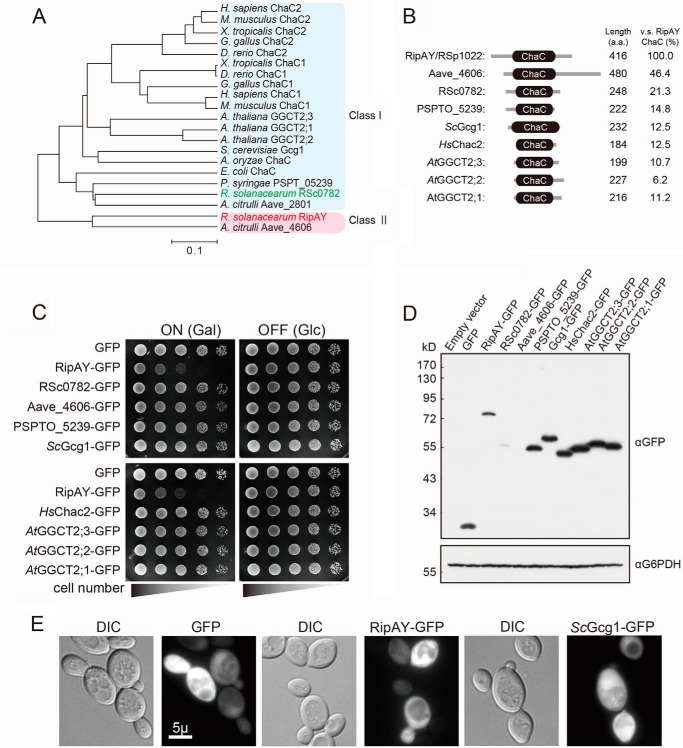
To predict the molecular function of the identified effectors, we first searched for the functional domain or motif using the Pfam search engine based on their primary sequences. One of the identified effectors, RipAY (locus tag RSp1022) contains a ChaC domain, originally identified in a protein encoded by E. coli chaC and assumed to be a possible regulator of the cha operon (Ca2+/H+ antiporter) (28). Orthologues of the ChaC protein are found in all phyla examined. We investigated the phylogenic relationship of the protein with other ChaC protein sequences from various organisms. RipAY and Aave_4606 identified in the genome of the cucurbit pathogenic bacterium Acidovorax citrulli (referred to as the class II ChaC protein family) are clearly an out-group of the ChaC proteins conserved in various organisms (referred to as the class I ChaC protein family) in a phylogenic reconstruction (Fig. 1A). Interestingly, both the R. solanacearum and A. citrulli genome contain another ChaC protein (RSc0782 and Aave_2801, respectively), which belongs to the class I ChaC protein family. RipAY and Aave_4606 have N- and C-terminal extension sequences outside of their ChaC domains and are much larger than the class I ChaC proteins (∼400 amino acids for class II versus ∼200 amino acids for class I) (Fig. 1B). The ChaC domain of RipAY shows lower identity to that of the class I ChaC proteins (6.2–21.3%) but higher identity to that of the class II ChaC protein, Aave_4606 (46.4%) (Fig. 1B). It seems that RipAY and Aave_4606 most probably originated from a duplication of the class I ChaC protein family genes and evolved to acquire a specific function as an effector.
FIGURE 1.
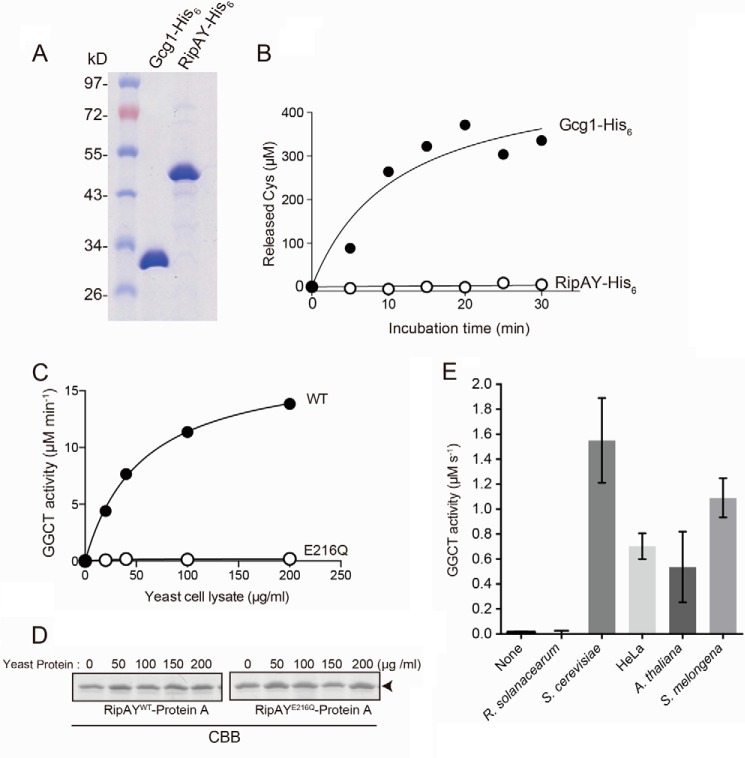
Expression of RipAY, but not other ChaC family proteins from various organisms, causes growth inhibition of yeast. A, phylogenetic analysis of ChaC domain containing proteins from various organisms. The organisms, locus tag/gene name, and GenbankTM accession numbers (in parentheses) are as follows: E. coli ChaC (L28709.1); R. solanacearum RSc0782 (AL646052.1) and RSp1022/RipAY (AL646053.1); A. citrulli Aave_2801 (CP000512.1) and Aave_4606 (CP000512.1); P. syringae PSPT_05239 (AE016853.1); S. cerevisiae YER163c/Gcg1 (NM_001179053.3); Aspergillus oryzae ChaC (XM_001818295.2); Homo sapiens ChaC1 (NM_001142776.1) and ChaC2 (NM_001008708.2); Mus musculus ChaC1 (NM_026929.4) and ChaC2 (NM_026527.3); Xenopus tropicalis ChaC1 (NM_001130284.1) and ChaC2 (NM_001017137.2); Gallus gallus ChaC1 (NM_001199656.1) and ChaC2 (AJ720918.1); Danio rerio ChaC1 (NM_001110126.1) and ChaC2 (BC154137.1); A. thaliana AT5G26220/GGCT2;1 (BT005234.1), AT4G31290/GGCT2;2 (BT006411.1), and AT1G44790/GGCT2;3 (BT006192.1). B, schematic representation of putative domain organization of ChaC domain-containing proteins from various organisms. C, yeast growth inhibition assay showing serial dilutions of S. cerevisiae BY4743 cells grown under inducing (galactose) or noninducing (glucose) conditions that are carrying plasmids expressing the indicated C-terminal GFP-tagged proteins under the control of the GAL1 promoter. The cells were grown at 26 °C for 2 days for SD (−Ura) and 3 days for SGal (−Ura). D and E, detections of the ChaC domain-containing proteins expressed in yeast. Yeast cells carrying the plasmids expressing GFP-tagged RipAY or other ChaC proteins under the control of the GAL1 promoter were grown in SD (−Ura) liquid medium to mid-log phase and then shifted to SGal (−Ura) liquid medium to induce the protein expression, further cultured for 12 h, and analyzed by immunoblotting with anti-GFP antibody (D) or fluorescence microscopy with native GFP fluorescence (E). Glucose 6-phosphate dehydrogenase (G6PDH) was used as a loading control. Scale bar, 5 μm. DIC, differential interference contrast.
We next examined whether heterologous expression of the other ChaC proteins also caused growth inhibition of yeast as observed in that of RipAY (Fig. 1C). Expression of neither GFP alone nor the GFP-tagged C terminus of other ChaC proteins showed any impact on yeast growth (Fig. 1C); nonetheless, the protein expression level, except for RSc0782 and Aave_4606, was even higher than that of RipAY in yeast (Fig. 1D). We failed to detect the expression of the Aave_4606-GFP fusion protein in yeast, probably because the Aave_4606 gene has a high GC content (73%) and many rare codons for yeast (34 rare codons (CGC or CGG) of 38 total codons for arginine) (data not shown). We also examined the localization of the proteins in yeast cells using the native fluorescence of GFP fused at their C termini and found GFP fluorescence of RipAY and most of the other ChaC proteins expressed in the cytoplasm of yeast cells (Fig. 1E).4 These results suggest that RipAY affects the cytoplasmic component(s) with different activities from the other ChaC proteins.
Expression of RipAY in Yeast Causes a Decrease in Intracellular Glutathione via Its GGCT Activity
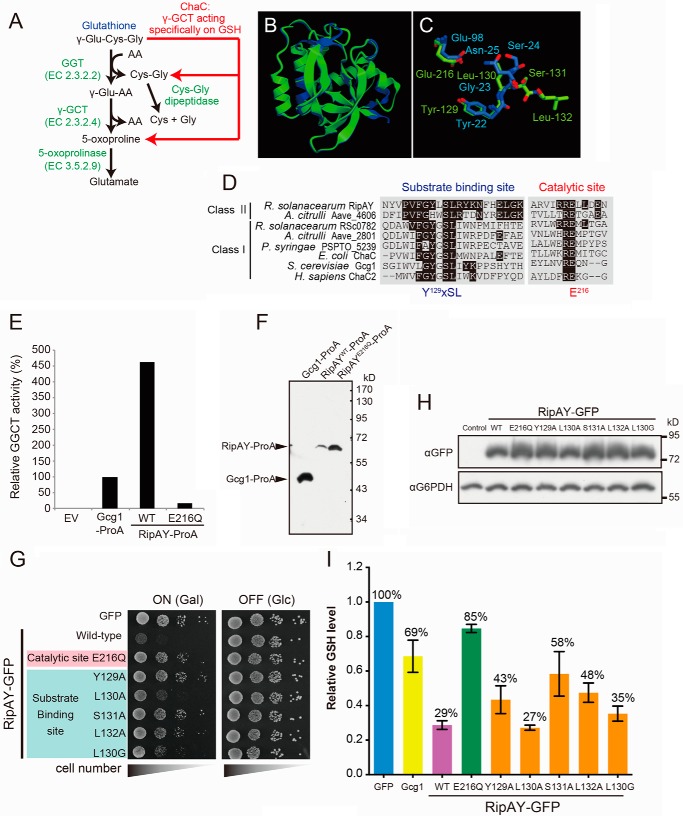
Recently, it has been reported that the class I ChaC proteins from yeast, plants, and mammals function as GGCTs acting specifically to degrade glutathione but not other γ-glutamyl peptides (Fig. 2A) (18, 29). The ChaC domain of RipAY has limited similarity to that of the class I ChaC proteins (Fig. 1B). However, the structure of RipAY modeled on the Phyre2 website indicated that it closely resembles the structure of human GGCT (C7orf24) (30) (Fig. 2, B and C). There is a very high structural similarity between RipAY and GGCT, although there is little amino acid similarity (data not shown). The Phyre2 search showed that most key residues in the catalytic domain of GGCT aligned with corresponding residues of RipAY (Fig. 2C). Furthermore, multiple sequence alignment of the ChaC proteins from various organisms revealed that RipAY contains the signature motifs for the substrate binding site (Y129XSL) and catalytic glutamate residue (Glu216) (Fig. 2D), indicating that it may function in a similar manner.
FIGURE 2.
Expression of RipAY in yeast causes growth inhibition by depletion of intracellular glutathione through its conserved ChaC domain. A, the GSH catabolism by a classical γ-glutamyl transpeptidase- and GGCT-dependent pathway and a novel ChaC family-dependent pathway. GGT, γ-glutamyl transpeptidase. B, the RipAY protein was modeled using the Phyre2 server. The homology model obtained was superimposed on the crystal structure of GGCT (C7orf24, Protein Data Bank codes 2PN7 and 2RBH) using the graphics program CueMol. Shown is superimposition of homology-modeled RipAY structure (green) on GGCT structure (blue). C, putative active site residues of RipAY (Y129LSL and Glu216; green) were superimposed on corresponding active site residues of GGCT (Y22GSN and Glu98; blue). D, multiple alignment of amino acid sequences in the regions of putative substrate binding and catalytic sites of the class I and II ChaC proteins. The conserved amino acids, Y129XSL and Glu216, are the putative substrate binding site and catalytic glutamate residue for GGCT, respectively. E, Protein A-tagged Gcg1, RipAY WT, and active site mutant, E216Q, were expressed in a glutathione-degrading enzyme-deficient strain (dug3Δ ecm38Δ gcg1Δ triple mutant) of yeast cells and immunopurified with IgG-beads. The GGCT activity of the beads immobilized with Gcg1, RipAYWT, or RipAYE216Q proteins was measured by a Dug1-coupled method as described under “Experimental Procedures.” EV, empty vector. F, the proteins extracted from the beads shown in E were resolved on SDS-PAGE followed by immunoblotting using a rabbit IgG. G, mutations in putative substrate binding and catalytic sites of RipAY restore the yeast growth inhibition caused by expression of RipAY. Yeast cells carrying empty vector or GAL1 expression vector of WT or the indicated mutant proteins of RipAY were spotted on SD (−Ura) and SGal (−Ura) plates and cultured for 2 days and 3 days, respectively. H, yeast cells carrying GAL1 expression plasmid of GFP-tagged WT or the indicated mutant proteins of RipAY were grown in SD (−Ura) liquid medium to mid-log phase and then shifted to SGal (−Ura) liquid medium to induce the protein expression, further cultured for 12 h, and analyzed by immunoblotting with anti-GFP antibody. I, mutations in putative substrate binding and catalytic sites of RipAY restore the reduction of intracellular GSH level in yeast caused by expression of RipAY. The total GSH levels of the cell lysates from yeast cells expressing the indicated proteins were measured as described under “Experimental Procedures.” Values represent the mean ± S.E. (error bars) (n ≥ 3).
To ask directly whether RipAY had GGCT activity toward glutathione, we first determined the GGCT activity of RipAY expressed in yeast. In this regard, we employed a Protein A tag to affinity-purify the proteins using IgG-beads directly from the Protein A-tagged RipAY-expressing yeast lysates and then determined GGCT activity of the beads bound with RipAY protein using a Dug1-coupled assay for ChaC family proteins (18). To prevent contamination of endogenous glutathione-degrading enzymes from the yeast lysate, we used a glutathione-degrading enzyme-deficient strain (dug3Δ ecm38Δ gcg1Δ triple mutant) to express the Protein A-tagged ChaC proteins in yeast cells. Consistent with a previous report (18), the beads bound with yeast class I ChaC family protein Gcg1 showed significant GGCT activity (Fig. 2E). Remarkably, we detected robust GGCT activity in the beads bound with wild-type RipAY (RipAYWT) but not with the catalytically inactive mutant RipAY (RipAYE216Q). Interestingly, RipAYWT showed ∼4.5 times higher activity than that of the beads bound with Gcg1, although the amount of the RipAYWT protein was much lower than that of Gcg1 (Fig. 2F). This result demonstrated that RipAY protein purified from yeast possesses GGCT activity toward glutathione in vitro.
To ask whether yeast growth inhibition was caused by the GGCT activity of RipAY, we next performed a mutational analysis of the putative catalytic residues in the ChaC domain of RipAY and then assessed the mutants using the yeast growth inhibition test (Fig. 2G). Substitution of the putative catalytic glutamate residue to glutamine (E216Q) or the conserved substrate-binding site to alanine (Y129A, S131A, and L132A) almost completely restored the yeast growth inhibition caused by expression of RipAY. However, the substitution of non-conserved leucine at position 130 (Leu130) to alanine (L130A) resulted in only weak restoration. Furthermore, the mutation of Leu130 to glycine, L130G, which mimics the substrate-binding site of the class I ChaC protein, could partially restore the growth, indicating that RipAY may have a different substrate binding mechanism from that of the class I ChaC proteins. Immunoblots were probed with anti-GFP (for RipAYs) and anti-glucose-6-phosphate dehydrogenase (as an internal control) antibodies, revealing similar amounts of RipAY-GFP proteins (Fig. 2H).
We next examined whether expression of RipAY proteins affected the intracellular glutathione level in yeast (Fig. 2I). Cells overexpressing Gcg1 showed a modest decrease in glutathione (decreased to 69% relative to the control). Interestingly, cells expressing RipAYWT led to a marked decrease in glutathione (decreased to 29% relative to the control), whereas cells expressing RipAYE216Q did not show a significant glutathione decrease. Together, these data showed that RipAY functions as a GGCT both in vitro and in vivo.
Addition of Exogenous Glutathione Did Not Restore RipAY-dependent Growth Inhibition in Yeast
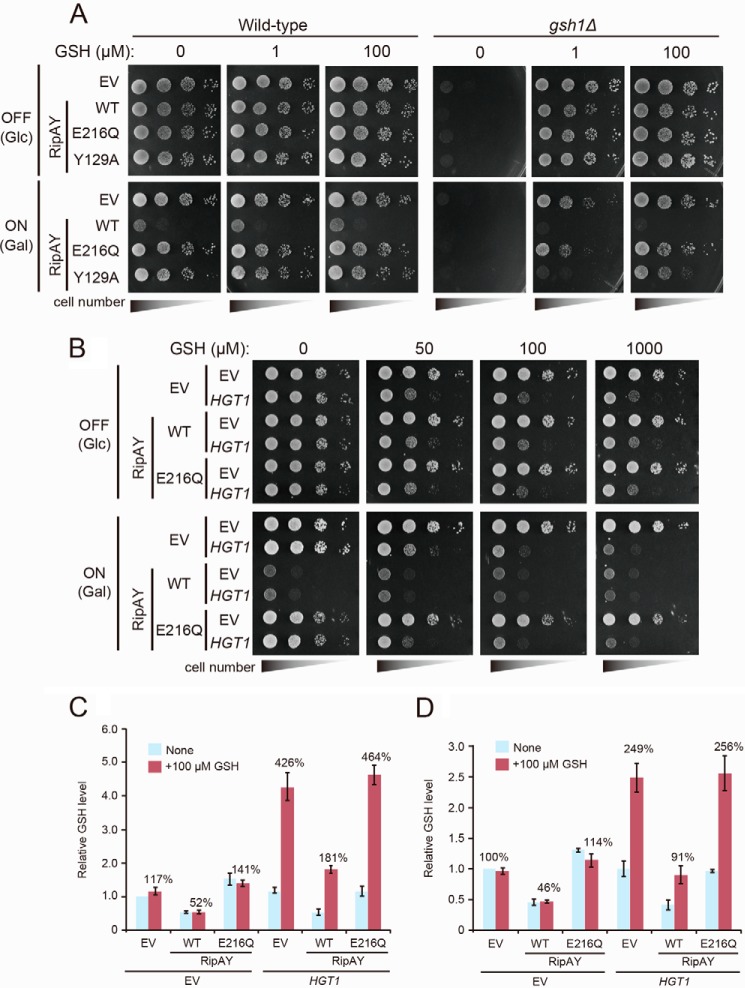
We next investigated the effect of the addition of exogenous glutathione on the growth of RipAY-expressing yeast cells. Unexpectedly, the addition of exogenous glutathione could not restore the RipAY-dependent growth inhibition in both yeast WT and gsh1Δ mutant, which has a defect in endogenous glutathione synthesis (Fig. 3A). Interestingly, expression of the conserved substrate-binding site mutant (Y129A), whose expression does not cause growth inhibition in WT cells, causes growth inhibition in gsh1Δ mutant cells on medium containing 1 μm glutathione, but an excess of glutathione (100 μm) could restore this growth inhibition. Based on this result, we hypothesized that the kinetics of glutathione degradation by RipAY is much faster than that of glutathione uptake by plasma membrane glutathione transporter, Hgt1 (31). As a consequence, available glutathione is limited in yeast cells expressing RipAY, causing growth inhibition. To confirm this hypothesis, we investigated the effect of overexpression of Hgt1 in yeast cells expressing RipAY. Overexpression of Hgt1 using the strong constitutive TEF1 promoter conferred a glutathione-sensitive phenotype to yeast WT cells (Fig. 3B) as reported previously (32) and showed an increased intracellular glutathione level (426 ± 39% for the 30-min culture with glutathione and 249 ± 23% for the 19-h culture with glutathione relative to that of control cells) when cells were cultured in medium containing 100 μm glutathione (Fig. 3, C and D). Surprisingly, we observed that overexpression of Hgt1 could not restore the RipAY-dependent growth inhibition on medium containing 100 μm glutathione (Fig. 3B), although the intracellular glutathione level was restored (181 ± 10% for the 30-min culture with glutathione and 91 ± 15% for the 19-h culture with glutathione relative to that of control cells) in these conditions (Fig. 3, C and D). Collectively, these results indicate that RipAY has another target(s) besides glutathione, at least in yeast.
FIGURE 3.
Increased uptake of GSH does not rescue the growth inhibition effect caused by expression of RipAY. A, yeast wild-type and gsh1Δ/gsh1Δ homozygote cells carrying the indicated plasmids were spotted on SD (−Ura) (OFF) or SGal (−Leu, −Ura) (ON) plates supplemented with the indicated concentration of GSH. EV, empty vector. Cells were incubated at 26 °C for 2 days for SD (−Leu, −Ura) or 3 days for SGal (−Leu, −Ura). B, yeast cells carrying the indicated plasmids were spotted on SD (−Leu, −Ura) (RipAY expression: OFF) or SGal (−Leu, −Ura) (RipAY expression: ON) plates supplemented with the indicated concentration of GSH. Cells were incubated at 26 °C for 2 days for SD (−Leu, −Ura) or 3 days for SGal (−Leu, −Ura). RipAY or HGT1 were expressed under the control of a galactose-inducible GAL1 promoter or a strong constitutive TEF1 promoter, respectively. C, yeast cells carrying the indicated plasmids growing exponentially in SD (−Leu, −Ura) liquid medium were transferred to SGal (−Leu, −Ura) liquid medium and cultured at 26 °C for 19 h, and then GSH (100 μm) was supplemented at 30 min before termination of the culture. D, yeast cells carrying indicated plasmids growing exponentially in SD (−Leu, −Ura) liquid medium were transferred to SGal (−Leu, −Ura) liquid medium supplemented with or without 100 μm GSH and cultured at 26 °C for 19 h. The total GSH levels of the cell lysates from yeast cells expressing indicated proteins were measured. Values represent the mean ± S.E. (error bars) (n = 4 for C and n = 3 for D).
Inoculation of R. solanacearum into Eggplant Leaves Causes a Decrease in Intracellular Glutathione
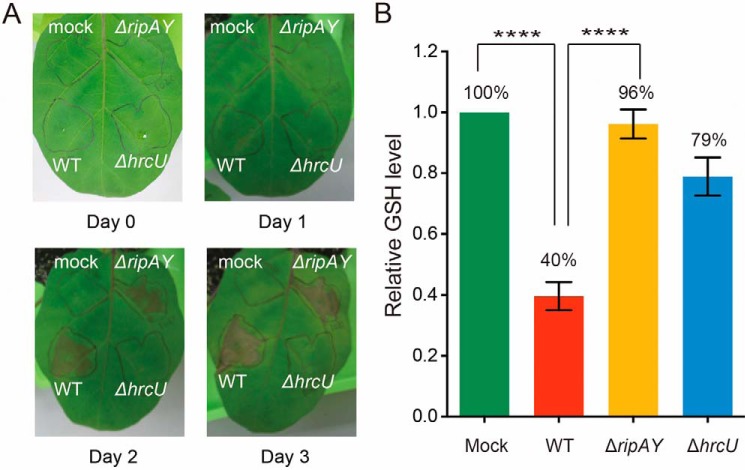
To explore the function of RipAY in host plant cells during R. solanacearum infection, we performed an inoculation test of R. solanacearum WT and ripAY-deficient (ΔripAY) cells into eggplant leaf mesophyll tissues. We observed typical necrotic lesions in the area inoculated with both R. solanacearum WT and ΔripAY cells at 2 days postinoculation but not in the area inoculated with a T3SS mutant, ΔhrcU, and control mock (Fig. 4A), indicating that RipAY is dispensable for virulence of R. solanacearum on eggplant.
FIGURE 4.
The GSH level of eggplant leaves inoculated with R. solanacearum wild-type, RipAY-deficient, and T3SS-deficient strains. A, necrotic lesions on eggplant leaf 3 days postinoculation with R. solanacearum WT strain (WT), RipAY-deficient strain (ΔripAY), or T3SS-deficient strain (ΔhrcU). Mock, inoculation of buffer without bacteria as a control. B, the GSH level of total lysate extracted from eggplant leaves inoculated with the indicated R. solanacearum strains at 1 day postinoculation was measured as described under “Experimental Procedures.” Values represent the mean ± S.E. (error bars) (n ≥ 7). ****, p < 0.0001, one-way analysis of variance post hoc test (Turkey's multiple comparison test).
We next examined glutathione levels in eggplant leaves inoculated with R. solanacearum. Lysates were extracted from the area inoculated with R. solanacearum WT, ΔripAY, and ΔhrcU cells and control mock at 1 day postinoculation, and then the total glutathione level of these lysates was analyzed (Fig. 4B). We observed a modest decrease of glutathione in ΔhrcU-inoculated eggplant leaves (decreased to 79% relative to the control), indicating that a pathogen-associated molecular pattern-triggered immunity response may affect the host glutathione homeostasis. Strikingly, eggplant leaves inoculated with R. solanacearum WT showed a significant decrease in glutathione (decreased to 40% relative to the control), whereas eggplant leaves inoculated with ΔripAY did not show any significant decrease (Fig. 4B), implying that RipAY functions as a GGCT in host plant cells during R. solanacearum infection.
RipAY GGCT Activity Is Triggered by a Eukaryotic Factor
To perform a detailed enzyme kinetic analysis of GGCT activity of RipAY toward glutathione, recombinant RipAY and Gcg1 proteins were purified from E. coli using His6 tag and nickel-nitrilotriacetic acid affinity chromatography (Fig. 5A), and their GGCT activity was determined by a Dug1-coupled assay (18). As shown in Fig. 5B, the recombinant Gcg1 protein showed significant GGCT activity toward glutathione, as described previously (18). However, we failed to detect GGCT activity of recombinant RipAY, although we could detect robust GGCT activity in RipAY expressed in yeast (Fig. 2C). Based on these results, we hypothesized that RipAY might acquire its GGCT activity from an activator harbored in yeast cells but not in E. coli cells. To test this hypothesis, the beads bound with recombinant Protein A-tagged RipAYWT or RipAYE216Q proteins expressed in E. coli were preincubated with a yeast extract, and then GGCT activity of the beads was measured. As shown in Fig. 5C, yeast extract could stimulate the GGCT activity of RipAYWT but not that of catalytically inactive RipAYE216Q in a dose-dependent manner, showing that yeast extract contains the activator(s) for RipAY. We did not observe any size changes in RipAY proteins after treatment with yeast extract (Fig. 5D), indicating that activation of RipAY is not dependent on cleavage or modification of the protein. Furthermore, not only a yeast extract, but also other eukaryotic cell extracts, including those of human cultured cells (HeLa) and two plants, A.thaliana and eggplant (S. melongena), but not that of R. solanacearum, could stimulate GGCT activity of a bacterially expressed RipAY-His6 protein (Fig. 5E). These data demonstrated that RipAY acquires its GGCT activity by a eukaryote-specific factor.
FIGURE 5.
RipAY exhibits GGCT activity in the presence of eukaryotic factor(s). A, purification of recombinant Gcg1 and RipAY proteins. Recombinant His6-tagged Gcg1 and RipAY were expressed in E. coli and purified using nickel-nitrilotriacetic acid affinity chromatography. Purified proteins were analyzed on SDS-PAGE with Coomassie Blue staining. B, measurement of GGCT activity of recombinant Gcg1 and RipAY purified from E. coli. 0.1 μg of Gcg1 and 1 μg of RipAY were incubated with 5 mm glutathione, the reactions were terminated at the indicated time points, the released Cys-Gly dipeptides were digested with Cys-Gly dipeptidase Dug1, and then the released Cys was measured by acidic ninhydrin. C, effect of concentration of the yeast protein extract on activation of GGCT activity of RipAY. The beads bound with RipAYWT or RipAYE216Q proteins expressed in E. coli were preincubated with different concentrations of native yeast protein extracted from a glutathione-degrading enzyme-deficient strain (dug3Δ ecm38Δ gcg1Δ triple mutant) for 2 h at 4 °C and washed with the same buffer three times, and then GGCT activity of the beads was measured by the Dug1-coupled method. D, the Protein A-tagged RipAYWT and RipAYE216Q proteins extracted from the beads shown in C were resolved on SDS-PAGE and visualized by Coomassie Brilliant Blue (CBB) staining. E, the GGCT activity of bacterially expressed RipAY-His6 protein incubated with 10 μg of protein of the cell lysates from R. solanacearum, S. cerevisiae, human cultured cell HeLa, A. thaliana, or eggplant S. melongena cells was measured by a Dug1-coupled method. Values represent the mean ± S.E. (error bars) (n ≥ 3).
Purification and Identification of the Activator for RipAY from Yeast Extract
Heat treatment (95 °C for 5 min) or extensive dialysis of the yeast extract did not eliminate the activation of RipAY, but proteinase K treatment completely eliminated it, suggesting that the activator must be heat-stable and proteinaceous (data not shown). To identify the activator of RipAY from a yeast extract, a biochemical approach was employed. Proteins extracted from yeast cells were sequentially fractionated by anion exchange, hydrophobic interaction, and gel filtration chromatography (Fig. 6A). Fractions were assayed for their ability to stimulate GGCT activity of RipAY. Those GGCT stimulation activities from the final purification step were analyzed by Tricine SDS-PAGE (Fig. 6B). A single 11-kDa protein was correlated with activity (fractions 37–41). Mass spectrometry identified this protein as Trx1 and Trx2 proteins, isoenzymes of yeast cytoplasmic thioredoxin (Trx) (Fig. 6C).
FIGURE 6.
Identification of thioredoxin as a eukaryotic activator for GGCT activity of RipAY. A, biochemical purification of the eukaryotic activator of RipAYfrom yeast protein extracts by HiPrep DEAE FF 16/10 ion exchange, HiPrep Butyl FF 16/10 hydrophobic interaction, and HiPrep 16/60 Sephacryl S-100 HR gel filtration chromatography. B, gel filtration fractions surrounding activity were loaded onto a Tricine SDS-polyacrylamide gel, visualized by silver staining. C, gel bands of fractions 37–41 stained with Coomassie Brilliant Blue were collected and in-gel digested, and following LC-MS/MS analysis, yeast thioredoxin Trx1 and Trx2 proteins were identified as the activator. Amino acid sequences of S. cerevisiae Trx1 and Trx2 and their corresponding tryptic peptides identified by LC-MS/MS analysis are shown.
Yeast Thioredoxins Can Stimulate GGCT Activity of RipAY Both in Vivo and in Vitro
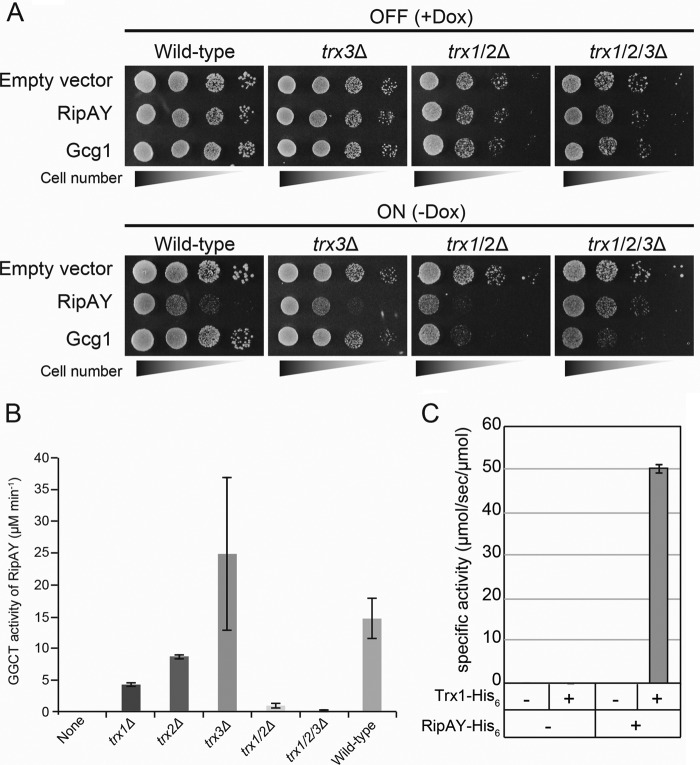
The yeast proteome contains three Trx isoenzymes: two cytoplasmic isoenzymes (Trx1 and Trx2) and one mitochondrial isoenzyme (Trx3). Because RipAY is expressed in the cytoplasm in yeast cells (Fig. 1E), we expected that the growth inhibition effect caused by RipAY must be eliminated by simultaneous deletion of its candidate activators, Trx1 and Trx2. RipAY and Gcg1 proteins were expressed under the control of the doxycycline-repressible Tet-off promoter (33) in yeast WT, trx3Δ single-, trx1/2Δ double-, and trx1/2/3Δ triple-mutant cells, and the growth of yeast was scored by a spot assay (Fig. 7A). Interestingly, we observed that overexpression of Gcg1 also causes growth inhibition in both trx1/2Δ and trx1/2/3Δ mutants (Fig. 7A, bottom), suggesting that a subtle decrease of glutathione in Trx-deficient cells causes growth inhibition because the Trx system shares some redundant functions in cellular redox homeostasis with the glutathione system (34). Contrary to our expectation, we still observed the growth-inhibitory effect by expression of RipAY in trx1/2Δ mutant cells (Fig. 7A, third bottom panel). However, an additional deletion of TRX3 from trx1/2Δ mutant (trx1/2/3Δ) clearly restored the growth inhibition (Fig. 7A, bottom right panel). Consistent with these results, lysate from trx1/2/3Δ cells almost completely lost the activation activity of RipAY, whereas lysate from trx1/2Δ cells still retained weak but significant activation activity (Fig. 7B). In addition, the lysate from trx3Δ cells could activate GGCT activity of RipAY, and also trx3Δ cells expressing RipAY exhibited growth inhibition. These data indicate that the major activators for RipAY in yeast cells must be Trx1 and Trx2. Finally, we demonstrated that a bacterially expressed Trx1-His6 protein could stimulate GGCT activity of RipAY in vitro (Fig. 7C). Taken together, these results demonstrated that yeast Trxs could stimulate GGCT activity of RipAY both in vivo and in vitro.
FIGURE 7.
Yeast thioredoxins can activate a GGCT activity of RipAY both in vivo and in vitro. A, S. cerevisiae WT, trx3Δ single mutant, trx1/2Δ double mutant,and trx1/2/3Δ triple mutant cells carrying plasmid expressing RipAY or Gcg1 under the control of the Tet-off promoter were grown in noninducing (+Dox) or inducing (−Dox) conditions. Yeast cells transformed with corresponding empty vector were used as a control. The cells were grown at 26 °C for 3 days for SD (−Ura, +Dox) and 4 days for SD (−Ura, −Dox). B, the GGCT activity of bacterially expressed RipAY-His6 protein incubated with 10 μg of protein of the cell lysates from the indicated S. cerevisiae thioredoxin mutant cells was measured by the Dug1-coupled method. Values represent the mean ± S.E. (error bars) (n ≥ 3). C, bacterially expressed S. cerevisiae Trx1-His6 protein can activate the GGCT activity of bacterially expressed RipAY-His6 protein.
Plant Trxs Bind to and Activate RipAY in an Isoform-specific Manner
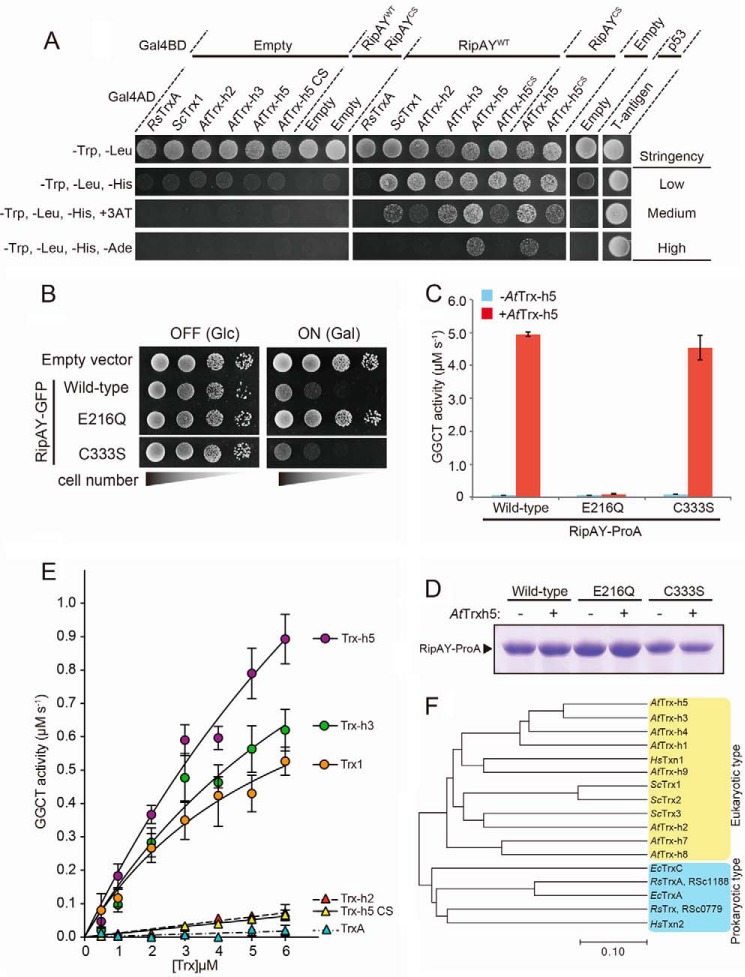
Next, we investigated whether plant Trxs could also activate RipAY. Because Arabidopsis extract could activate RipAY in vitro (Fig. 5E) and Trxs from Arabidopsis are relatively well characterized, we employed the Arabidopsis Trxs for this purpose. The Arabidopsis genome encodes 19 Trxs belonging to six major groups: f, m, h, o, x, and y (35). Whereas most of these Trxs are located in chloroplasts and mitochondria, the h-type Trxs, which consist of eight isoenzymes, are generally thought to be located in the cytoplasm. Among these h-type Trxs in Arabidopsis, AtTrx-h2 has the highest similarity to yeast Trxs (Fig. 8F). Furthermore, AtTrx-h3 is the most highly constitutively expressed cytoplasmic Trx, and AtTrx-h5 is substantially up-regulated upon infection with Pseudomonas syringae (36). We therefore chose these three Arabidopsis Trxs as candidate activators for RipAY. We first tested whether RipAY directly interacted with Trxs using yeast two-hybrid analysis. As shown in Fig. 8A, we could detect the interaction of RipAY with all eukaryotic Trxs tested but not with R. solanacearum Trx, RsTrxA, under both low and medium stringency conditions (−Trp/−Leu/−His and −Trp/−Leu/−His/+3-amino-1,2,4-triazole, respectively). However, we could detect the interaction of RipAY with only AtTrx-h5 under high stringency conditions (−Trp/−Leu/−His/−Ade), indicating that RipAY binds AtTrx-h5 with higher affinity than the other eukaryotic Trxs in yeast two-hybrid analysis. Trxs catalyze disulfide reduction through a redox-active site, WCXPC (34). We also examined whether redox-active disulfide residues of AtTrx-h5 are required for the binding to RipAY. The redox-inactive mutant AtTrx-h5CS, in which active site cysteine residues at positions 39 and 42 were substituted by serine, showed decreased binding and activation activity relative to the WT but still bound to RipAY and exhibited significant activation activity (Fig. 8, A and E). Moreover, the RipAYCS mutant, in which the sole cysteine residue at position 333 was substituted by serine, also bound to both the WT and the redox-inactive mutant of AtTrx-h5. Furthermore, heterologous expression of the RipAYCS mutant caused growth inhibition of yeast (Fig. 8B), and recombinant RipAYCS mutant protein expressed in E. coli exhibited Trx-dependent activation in vitro, which was similar to that of RipAYWT (Fig. 8, C and D). Taken together, these data indicate that the eukaryotic Trxs bind to RipAY without intermolecular disulfide binding-dependent covalent interaction.
FIGURE 8.
Plant thioredoxins can bind to RipAY and activate GGCT activity of RipAY in an isoform-specific manner. A, yeast two-hybrid assay for interaction between RipAY and various thioredoxins on the plate with different stringency conditions. RipAY WT (RipAYWT) and a mutant form of RipAY (RipAYCS), in which the sole cysteine residue at position 333 was substituted by serine, were used as bait. R. solanacearum TrxA (RsTrxA), S. cerevisiae Trx1 (ScTrx1), A. thaliana Trx-h2, -h3, and -h5 (AtTrx-h2, AtTrx-h3, and AtTrx-h5), and a mutant form of Trx-h5 (AtTrx-h5CS), in which active site cysteine residues at position 39 and 42 were substituted by serine, were used as prey. B, yeast cells carrying empty vector or GAL1 expression vector of WT, active site mutant E216Q, or C333S mutant of RipAY were spotted on SD (−Ura) and SGal (−Ura) plates and cultured for 2 days and 3 days, respectively. C, GGCT activity of the beads bound with Protein A-tagged RipAYWT, RipAYE216Q, or RipAYC333S proteins expressed in E. coli incubated with 1.6 μm AtTrx-h5-His6 protein was measured by the Dug1-coupled method. Values represent the mean ± S.E. (error bars) (n = 3). D, Protein A-tagged RipAYWT, RipAYE216Q, and RipAYC333S proteins extracted from the beads shown in C were resolved on SDS-PAGE and visualized by Coomassie Brilliant Blue staining. E, effect of thioredoxin isoforms on activation of GGCT activity of RipAY. The GGCT activity of RipAY incubated with various concentrations of thioredoxin isoforms was measured by the Dug1-coupled method. Values represent the mean ± S.E. (n ≥ 3). F, phylogenetic analysis of thioredoxin isoforms from various organisms. The organisms, locus tag/gene name, and GenbankTM accession numbers (in parentheses) are as follows: R. solanacearum, RSc1188/RsTrxA (AL646052.1) and RSc0779/RsTrx (AL646052.1); E. coli, EcTrxA (M26133.1) and EcTrxC (U8594.1); H. sapiens, HsTxn1 (JQ313905.1) and HsTxn2 (DQ891579.2); S. cerevisiae, YLR043C/ScTrx1 (NM_001181930.1), YGR209C/ScTrx2 (NM_001181338.3), and YCR083C/ScTrx3 (NM_001178789.1); A. thaliana, At3g51030/AtTrx-h1 (NM_114963.4), At5g39950/AtTrx-h2 (AY113052.1), At5g42980/AtTrx-h3 (AY065098.1), At1g19730/AtTrx-h4 (BT004710.1), At1g45145/AtTrx-h5 (AY040028.1), At1g59730/AtTrx-h7 (BT0031540.1), At1g69880/AtTrx-h8 (BT003670.1), and At3g08710/AtTrx-h9 (BT0011728.1).
We next tested whether the plant Trxs stimulated GGCT activity of RipAY in vitro. The GGCT activity of RipAY toward glutathione was measured in the presence of various concentrations of each recombinant Trx isoenzyme. As shown in Fig. 8E, AtTrx-h5 could most effectively stimulate GGCT activity of RipAY (>10-fold compared with AtTrx-h2: 0.790 ± 0.073 μm/s at 5 μm AtTrx-h5 versus 0.066 ± 0.009 μm/s at 5 μm AtTrx-h2). Interestingly, the RipAY activation activity of Trxs was correlated with the RipAY binding ability of Trxs (order of RipAY activation activity: AtTrx-h5 > AtTrx-h3 > ScTrx1 ≫ AtTrx-h2 ≥ AtTrx-h5CS). The addition of RsTrxA to RipAY failed to stimulate GGCT activity, consistent with its inability to bind RipAY. Taken together, these data showed that plant Trxs bind to RipAY and stimulate its GGCT activity in an isoform-specific manner.
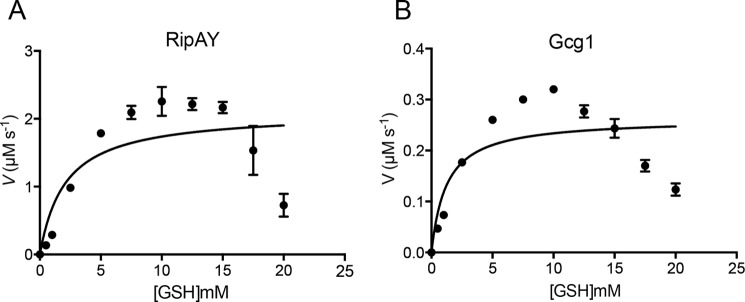
Because heterologous expression of RipAY, but not the other ChaC proteins, caused growth inhibition of yeast (Fig. 1C) and a marked decrease of intracellular glutathione in yeast cells (Fig. 2F), we speculated that the catalytic efficiency of RipAY might be much higher than that of the class I ChaC proteins. To confirm this speculation, we performed kinetic analysis of RipAY and Gcg1 toward glutathione and compared their kinetic parameters. Both RipAY, which was activated by 8 μm AtTrx-h5, and Gcg1 showed Michaelis-Menten kinetics toward glutathione (Fig. 9, A and B). We observed some inhibition at high substrate (glutathione) concentrations. The kinetic parameters revealed similar Km values for both Gcg1 (1.23 ± 0.17 mm) and RipAY (2.11 ± 0.17 mm), although the kcat values were markedly different; the kcat value of RipAY was 160-fold higher than that of Gcg1 (52.8 ± 9.58 s−1 for RipAY versus 0.33 ± 0.02 s−1 for Gcg1) (Table 4). Furthermore, the specificity constant, kcat/Km value of RipAY is 94-fold higher than that of Gcg1 (25.2 ± 1.22 s−1/mm for RipAY versus 0.27 ± 0.03 s−1/mm for Gcg1). Collectively, we clearly demonstrated that the catalytic efficiency of RipAY is much higher than that of the class I ChaC protein.
FIGURE 9.
Enzyme kinetic analysis of activated RipAY and Gcg1. Shown is a Michaelis-Menten plot of Trx-h5-activated RipAY (A) and Gcg1 (B) toward glutathione. Gcg1 (0.8 μm) or RipAY (0.04 μm) in the presence of Trx-h5 (8 μm) was used for determination of kinetic parameters. Different concentrations of glutathione were used ranging from 0.5 to 20 mm. Dug1-coupled assay was used for the study as described under “Experimental Procedures.” Data of three independent experiments were analyzed by non-linear regression using GraphPad prism version 6.0. Error bars, S.E.
TABLE 4.
Kinetic parameters of S. cerevisiae Gcg1 and AtTrx-h5-activated RipAY
| Enzyme | Km | kcat | kcat/Km | Comparison of kcat/Km value versus RipAY |
||
|---|---|---|---|---|---|---|
| Gcg1 | AtGGCT2;2a | AtGGCT2;3a | ||||
| mm | s−1 | s−1 mm−1 | -fold | |||
| Gcg1 | 1.23 ± 0.17 | 0.33 ± 0.02 | 0.27 ± 0.03 | |||
| RipAYb | 2.11 ± 0.17 | 52.8 ± 9.58 | 25.2 ± 1.22 | 94.0 | 66.7 | 1,081 |
a Data from Kumar et al. (29).
b RipAY was activated by 8 μm AtTrx-h5.
Discussion
The T3SS effector proteins exhibit various molecular activities that generally allow bacteria to escape from host immune systems and break down barriers for pathogen growth and dissemination. Uncovering the molecular functions of T3SS effector proteins is therefore one of the most important subjects for a mechanistic understanding of pathogenesis (4). In this study, we revealed that a ChaC domain-containing effector protein, RipAY, from the plant pathogenic bacterium R. solanacearum modulates the abundance of host intracellular glutathione by acting as a eukaryotic thioredoxin-dependent GGCT.
Expression of RipAY Causes Growth Inhibition in Yeast
We initially identified RipAY as one of the R. solanacearum effectors whose expression causes growth inhibition in yeast (Fig. 1C). Subsequent analysis revealed that this growth inhibition effect might be caused by a decrease in glutathione, which is dependent on a ChaC domain in RipAY (Fig. 2, E and F). Kumar et al. (18) reported that expression of human CHAC1 in yeast mutant cells with limited glutathione availability leads to apoptosis through depletion of intracellular glutathione, and this effect is rescued by the addition of exogenous glutathione. Unexpectedly, we found that the addition of exogenous glutathione failed to rescue the growth inhibition caused by expression of RipAY (Fig. 3A). Furthermore, overexpression of Hgt1 in RipAY-expressing cells could not restore the growth inhibition on media containing glutathione, although the intracellular glutathione level was almost restored in this condition (Fig. 3, B and C). Importantly, because mutation in the putative catalytic site completely restored the growth inhibition, this effect is dependent on GGCT activity of RipAY (Fig. 3B). These data clearly indicate that yeast growth inhibition must be a consequence of RipAY-dependent degradation of another yet unidentified γ-glutamyl compound(s) rather than glutathione. Recently, Chi et al. (37) reported that mouse CHAC1/Botch, which promotes embryonic neurogenesis through inhibition of Notch signaling, deglycinates the γ-glutamyl-glycine at position 1,669 of Notch to prevent the S1 furin-like cleavage of Notch. Further analysis will be required to understand the molecular mechanisms underlying the yeast growth inhibition observed in the expression of RipAY.
RipAY Is a Novel Type of Effector That Targets Host Glutathione
To our knowledge, RipAY is the first T3SS effector targeting host intracellular glutathione. Glutathione has been repeatedly reported to play a crucial role in plant immune responses during biotic stresses (20). For instance, the Arabidopsis pad2-1 mutant, which was originally identified as a phytoalexin camalexin-deficient mutant, was shown to be mutated in γ-glutamylcysteine synthetase (Gsh1), a critical enzyme that catalyzes the first committed step in glutathione synthesis (38). Mutant pad2-1 plants fail to accumulate significant levels of glutathione and are rendered highly susceptible to biotrophic pathogens and insects (19). Oppositely, an enhanced level of glutathione by expression of GSH1 in tobacco (Nicotiana tabacum) confers biotic stress tolerance, probably thorough the disease resistance-priming gene, NPR1-dependent salicylic acid-mediated pathway (39). Furthermore, Arabidopsis knock-out mutants for one of two glutathione reductase genes, GR1, which show a constitutive increase in oxidized glutathione, accumulate less plant defense hormone, salicylic acid, than the WT and show increased sensitivity to virulent P. syringae (40). We show that inoculation of R. solanacearum WT into eggplant leaves causes a significant decrease of glutathione, and mutation of RipAY in R. solanacearum restores this glutathione decrease (Fig. 3B). Taken together, these results indicate that RipAY affects the host immune response by decreasing host intracellular glutathione. However, despite the importance of RipAY for host glutathione homeostasis, an R. solanacearum mutant lacking RipAY was not attenuated for virulence toward eggplant (Fig. 4A), arguing for the existence of additional effector proteins that interfere with a host disease-resistant signaling pathway. Indeed, a systematic effector knock-out approach for R. solanacearum failed to identify mutants with reduced pathogenicity on two host plants, indicating a functional overlap among effectors (41).
Regulation of the Abundance of Host Intracellular Glutathione by RipAY
Given that most bacterial pathogens translocate only trace amounts of their effector proteins into host cell cytoplasm (42), it seemed that RipAY must be a GGCT with extremely high catalytic efficiency to decrease host intracellular glutathione during R. solanacearum infection. In fact, our enzyme kinetic analysis revealed that the kcat value of Trx-h5-activated RipAY was much higher than that of a S. cerevisiae class I ChaC protein, Gcg1 (160-fold), although both activated RipAY and Gcg1 show a similar Km value (Table 4). Recently, Kumar et al. (29) reported a detailed enzyme kinetic analysis of Arabidopsis ChaC orthologues, AtGGCT2;2 and AtGGCT2;3. Based on their data, we estimated that the kcat/Km value of RipAY is 66.7- and 1,081-fold higher than that of AtGGCT2;2 and AtGGCT2;3, respectively (Table 4). This extremely high catalytic efficiency of thioredoxin-activated RipAY compared with the intrinsic class I ChaC proteins is logical, because probably very limited amounts of the bacterial effector are efficiently translocated inside the plant host cells. Injection of a very active RipAY could allow depletion of the host intracellular glutathione during R. solanacearum infection.
Activation of RipAY by Eukaryotic Thioredoxins
A truly unexpected finding was that for RipAY to exhibit GGCT activity, it has to be bound and activated by host plant cytoplasmic thioredoxins in an isoform-specific manner (Fig. 8E). Recent findings provide remarkable examples for the emerging concept of spatiotemporal regulation of pathogen effectors by coupling the catalytic activity to the arrival into a host cell's cytoplasm (42–46). The mechanism of activation coupled with T3SS-dependent delivery ensures that eukaryotic cells are specifically and potently targeted and that the bacterium is protected from the toxic effects of its own enzymatic activity (46). Interestingly, the GGCT activity of RipAY is stimulated by thioredoxins, which are relatively well conserved within both prokaryotes and eukaryotes, and the R. solanacearum genome also contains five thioredoxin or thioredoxin-like proteins. Our data suggest that eukaryotic but not prokaryotic thioredoxin(s) can specifically bind to RipAY and stimulate GGCT activity of RipAY (Fig. 8, A and E). Phylogenic tree analysis revealed that eukaryotic-type thioredoxins are clearly divided from prokaryotic-type thioredoxins (Fig. 8F). As one might expect, our results raise two interesting questions regarding how RipAY recognizes eukaryotic thioredoxins in an isoform-specific manner and how thioredoxins stimulate the GGCT activity of RipAY. Further studies will be required for a better understanding of the mechanisms underlying thioredoxin recognition and the enzyme activation of RipAY at the molecular level.
A Working Model for the Role of RipAY during R.solanacearum Infection
Pathogen infection induces the expression of several thioredoxins in Arabidopsis (36, 47). In particular, Trx-h5 plays an important role in plant defense because it was shown that Trx-h5 facilitates the reduction of NPR1 disulfides to catalyze the oligomer-to-monomer switch, which in turn promotes the activation of salicylic acid-dependent plant defense (36). Intriguingly, we found that Trx-h5 could most efficiently stimulate the GGCT activity of RipAY in vitro (Fig. 8E). Based on this observation, we speculate that RipAY is injected into the host plant cell via the T3SS apparatus as an inactive form and then converted to an active form by host eukaryotic thioredoxins, in particular Trx-h5, during R. solanacearum infection and then degrades glutathione and other unknown γ-glutamyl compounds, which together might prevent the activation of the host disease response (Fig. 10). Further analysis will be required to understand the role of RipAY during R. solanacearum infection at the molecular level.
FIGURE 10.
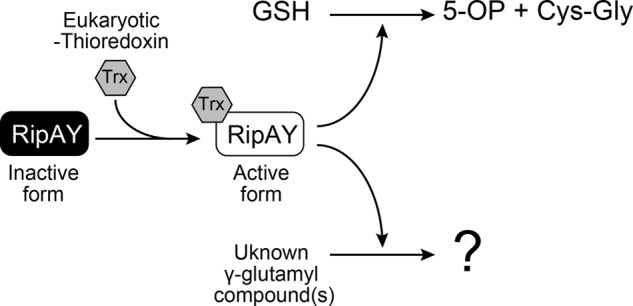
A model depicting how the RipAY T3SS effector is activated by host eukaryotic thioredoxins to trigger GGCT activity. RipAY was injected into a host plant cell as an inactive form, and then its GGCT activity was stimulated by host eukaryotic thioredoxins, such as Trx-h5, to degrade glutathione and other unknown γ-glutamyl compound(s). 5-OP, 5-oxoproline.
Author Contributions
S. F. and M. T. designed the experiments; S. F., K. O., T. Kitagawa, C. P., T. Kawazoe, and M. T. performed the experiments; K. N., Y. K., S. G., and M. V. contributed new reagents/analytic tools; S. F., N. T., and M. T. analyzed data; and M. T. wrote the paper. All authors reviewed the results and approved the final version of the manuscript.
Acknowledgments
We thank Tomoe Nakagawa and Jun-ichi Hasegawa for contributions to the initial experiments. We also thank Dr. Yasuomi Tada and Nodoka Oka (Nagoya University) for valuable advice and for supplying A. thaliana cDNA clones. We are grateful to Drs. Goro Takata and Akihide Yoshihara for technical assistance in operating the ÄKTA Explorer system. We acknowledge the technical expertise of the DNA core facility of the Gene Research Center, Kagawa University. We express our deep appreciation to Prof. Anand Bachhawat for generously providing pTEF-416-HGT1 plasmid and for valuable discussion of the data and critical reading of the manuscript.
This work was supported by Japan Society for the Promotion of Science KAKENHI Grants 22580095 and 25450104 and also, in part, by the General Research Grant of the Institute of Fermentation, Osaka. The authors declare that they have no conflicts of interest with the contents of this article.
J. Hasegawa, T. Nakagawa, S. Fujiwara, N. Tanaka, and M. Tabuchi, manuscript in preparation.
S. Fujiwara and M. Tabuchi, unpublished result.
- T3SS
- type III secretion system
- GGCT
- γ-glutamyl cyclotransferase
- SD
- synthetic dextrose
- SGal
- synthetic galactose
- Tricine
- N-[2-hydroxy-1,1-bis(hydroxymethyl)ethyl]glycine
- Trx
- thioredoxin.
References
- 1. Dean P. (2011) Functional domains and motifs of bacterial type III effector proteins and their roles in infection. FEMS Microbiol. Rev. 35, 1100–1125 [DOI] [PubMed] [Google Scholar]
- 2. Bhavsar A. P., Guttman J. A., and Finlay B. B. (2007) Manipulation of host-cell pathways by bacterial pathogens. Nature 449, 827–834 [DOI] [PubMed] [Google Scholar]
- 3. Galán J. E. (2009) Common themes in the design and function of bacterial effectors. Cell Host Microbe. 5, 571–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Deslandes L., and Rivas S. (2012) Catch me if you can: bacterial effectors and plant targets. Trends Plant Sci. 17, 644–655 [DOI] [PubMed] [Google Scholar]
- 5. Siggers K. A., and Lesser C. F. (2008) The Yeast Saccharomyces cerevisiae: a versatile model system for the identification and characterization of bacterial virulence proteins. Cell Host Microbe. 4, 8–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Valdivia R. H. (2004) Modeling the function of bacterial virulence factors in Saccharomyces cerevisiae. Eukaryot. cell 3, 827–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lesser C. F., and Miller S. I. (2001) Expression of microbial virulence proteins in Saccharomyces cerevisiae models mammalian infection. EMBO J. 20, 1840–1849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Salanoubat M., Genin S., Artiguenave F., Gouzy J., Mangenot S., Arlat M., Billault A., Brottier P., Camus J. C., Cattolico L., Chandler M., Choisne N., Claudel-Renard C., Cunnac S., Demange N., et al. (2002) Genome sequence of the plant pathogen Ralstonia solanacearum. Nature 415, 497–502 [DOI] [PubMed] [Google Scholar]
- 9. Genin S. (2010) Molecular traits controlling host range and adaptation to plants in Ralstonia solanacearum. New Phytol. 187, 920–928 [DOI] [PubMed] [Google Scholar]
- 10. Coll N. S., and Valls M. (2013) Current knowledge on the Ralstonia solanacearum type III secretion system. Microb. Biotechnol. 6, 614–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Genin S., and Denny T. P. (2012) Pathogenomics of the Ralstonia solanacearum species complex. Annu. Rev. Phytopathol. 50, 67–89 [DOI] [PubMed] [Google Scholar]
- 12. Deslandes L., and Genin S. (2014) Opening the Ralstonia solanacearum type III effector tool box: insights into host cell subversion mechanisms. Curr. Opin. Plant Biol. 20, 110–117 [DOI] [PubMed] [Google Scholar]
- 13. Peeters N., Carrère S., Anisimova M., Plener L., Cazalé A. C., and Genin S. (2013) Repertoire, unified nomenclature and evolution of the Type III effector gene set in the Ralstonia solanacearum species complex. BMC Genomics 14, 859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Angot A., Peeters N., Lechner E., Vailleau F., Baud C., Gentzbittel L., Sartorel E., Genschik P., Boucher C., and Genin S. (2006) Ralstonia solanacearum requires F-box-like domain-containing type III effectors to promote disease on several host plants. Proc. Natl. Acad. Sci. U.S.A. 103, 14620–14625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Poueymiro M., Cazalé A. C., François J. M., Parrou J. L., Peeters N., and Genin S. (2014) A Ralstonia solanacearum type III effector directs the production of the plant signal metabolite trehalose-6-phosphate. mBio 10.1128/mBio.02065-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Le Roux C., Huet G., Jauneau A., Camborde L., Trémousaygue D., Kraut A., Zhou B., Levaillant M., Adachi H., Yoshioka H., Raffaele S., Berthomé R., Couté Y., Parker J. E., and Deslandes L. (2015) A receptor pair with an integrated decoy converts pathogen disabling of transcription factors to immunity. Cell 161, 1074–1088 [DOI] [PubMed] [Google Scholar]
- 17. Mukaihara T., Tamura N., and Iwabuchi M. (2010) Genome-wide identification of a large repertoire of Ralstonia solanacearum type III effector proteins by a new functional screen. Mol. Plant Microbe. Interact. 23, 251–262 [DOI] [PubMed] [Google Scholar]
- 18. Kumar A., Tikoo S., Maity S., Sengupta S., Sengupta S., Kaur A., and Bachhawat A. K. (2012) Mammalian proapoptotic factor ChaC1 and its homologues function as γ-glutamyl cyclotransferases acting specifically on glutathione. EMBO Rep. 13, 1095–1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Spoel S. H., and Loake G. J. (2011) Redox-based protein modifications: the missing link in plant immune signalling. Curr. Opin. Plant Biol. 14, 358–364 [DOI] [PubMed] [Google Scholar]
- 20. Dubreuil-Maurizi C., and Poinssot B. (2012) Role of glutathione in plant signaling under biotic stress. Plant Signal. Behav. 7, 210–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ito H., Fukuda Y., Murata K., and Kimura A. (1983) Transformation of intact yeast cells treated with alkali cations. J. Bacteriol. 153, 163–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Imai Y., Matsushima Y., Sugimura T., and Terada M. (1991) A simple and rapid method for generating a deletion by PCR. Nucleic Acids Res. 19, 2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tamura K., Stecher G., Peterson D., Filipski A., and Kumar S. (2013) MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol. Biol. Evol. 30, 2725–2729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rahman I., Kode A., and Biswas S. K. (2006) Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat. Protoc. 1, 3159–3165 [DOI] [PubMed] [Google Scholar]
- 25. Kanda A., Yasukohchi M., Ohnishi K., Kiba A., Okuno T., and Hikichi Y. (2003) Ectopic expression of Ralstonia solanacearum effector protein PopA early in invasion results in loss of virulence. Mol. Plant Microbe. Interact. 16, 447–455 [DOI] [PubMed] [Google Scholar]
- 26. Kuramitsu Y., Miyamoto H., Tanaka T., Zhang X., Fujimoto M., Ueda K., Tanaka T., Hamano K., and Nakamura K. (2009) Proteomic differential display analysis identified upregulated astrocytic phosphoprotein PEA-15 in human malignant pleural mesothelioma cell lines. Proteomics 9, 5078–5089 [DOI] [PubMed] [Google Scholar]
- 27. Poueymiro M., and Genin S. (2009) Secreted proteins from Ralstonia solanacearum: a hundred tricks to kill a plant. Curr. Opin. Microbiol. 12, 44–52 [DOI] [PubMed] [Google Scholar]
- 28. Ivey D. M., Guffanti A. A., Zemsky J., Pinner E., Karpel R., Padan E., Schuldiner S., and Krulwich T. A. (1993) Cloning and characterization of a putative Ca2+/H+ antiporter gene from Escherichia coli upon functional complementation of Na+/H+ antiporter-deficient strains by the overexpressed gene. J. Biol. Chem. 268, 11296–11303 [PubMed] [Google Scholar]
- 29. Kumar S., Kaur A., Chattopadhyay B., and Bachhawat A. K. (2015) Defining the cytosolic pathway of glutathione degradation in Arabidopsis thaliana: role of the ChaC/GCG family of γ-glutamyl cyclotransferases as glutathione-degrading enzymes and AtLAP1 as the Cys-Gly peptidase. Biochem. J. 468, 73–85 [DOI] [PubMed] [Google Scholar]
- 30. Oakley A. J., Yamada T., Liu D., Coggan M., Clark A. G., and Board P. G. (2008) The identification and structural characterization of C7orf24 as γ-glutamyl cyclotransferase: an essential enzyme in the γ-glutamyl cycle. J. Biol. Chem. 283, 22031–22042 [DOI] [PubMed] [Google Scholar]
- 31. Bourbouloux A., Shahi P., Chakladar A., Delrot S., and Bachhawat A. K. (2000) Hgt1p, a high affinity glutathione transporter from the yeast Saccharomyces cerevisiae. J. Biol. Chem. 275, 13259–13265 [DOI] [PubMed] [Google Scholar]
- 32. Kumar C., Igbaria A., D'Autreaux B., Planson A. G., Junot C., Godat E., Bachhawat A. K., Delaunay-Moisan A., and Toledano M. B. (2011) Glutathione revisited: a vital function in iron metabolism and ancillary role in thiol-redox control. EMBO J. 30, 2044–2056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tabuchi M., Kawai Y., Nishie-Fujita M., Akada R., Izumi T., Yanatori I., Miyashita N., Ouchi K., and Kishi F. (2009) Development of a novel functional high-throughput screening system for pathogen effectors in the yeast Saccharomyces cerevisiae. Biosci. Biotechnol. Biochem. 73, 2261–2267 [DOI] [PubMed] [Google Scholar]
- 34. Meyer Y., Buchanan B. B., Vignols F., and Reichheld J. P. (2009) Thioredoxins and glutaredoxins: unifying elements in redox biology. Annu. Rev. Genet. 43, 335–367 [DOI] [PubMed] [Google Scholar]
- 35. Gelhaye E., Rouhier N., Navrot N., and Jacquot J. P. (2005) The plant thioredoxin system. Cell Mol. Life Sci. 62, 24–35 [DOI] [PubMed] [Google Scholar]
- 36. Tada Y., Spoel S. H., Pajerowska-Mukhtar K., Mou Z., Song J., Wang C., Zuo J., and Dong X. (2008) Plant immunity requires conformational changes [corrected] of NPR1 via S-nitrosylation and thioredoxins. Science 321, 952–956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chi Z., Byrne S. T., Dolinko A., Harraz M. M., Kim M. S., Umanah G., Zhong J., Chen R., Zhang J., Xu J., Chen L., Pandey A., Dawson T. M., and Dawson V. L. (2014) Botch is a γ-glutamyl cyclotransferase that deglycinates and antagonizes Notch. Cell Rep. 7, 681–688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Parisy V., Poinssot B., Owsianowski L., Buchala A., Glazebrook J., and Mauch F. (2007) Identification of PAD2 as a γ-glutamylcysteine synthetase highlights the importance of glutathione in disease resistance of Arabidopsis. Plant J. 49, 159–172 [DOI] [PubMed] [Google Scholar]
- 39. Ghanta S., Bhattacharyya D., Sinha R., Banerjee A., and Chattopadhyay S. (2011) Nicotiana tabacum overexpressing γ-ECS exhibits biotic stress tolerance likely through NPR1-dependent salicylic acid-mediated pathway. Planta 233, 895–910 [DOI] [PubMed] [Google Scholar]
- 40. Mhamdi A., Hager J., Chaouch S., Queval G., Han Y., Taconnat L., Saindrenan P., Gouia H., Issakidis-Bourguet E., Renou J. P., and Noctor G. (2010) Arabidopsis GLUTATHIONE REDUCTASE1 plays a crucial role in leaf responses to intracellular hydrogen peroxide and in ensuring appropriate gene expression through both salicylic acid and jasmonic acid signaling pathways. Plant Physiol. 153, 1144–1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cunnac S., Occhialini A., Barberis P., Boucher C., and Genin S. (2004) Inventory and functional analysis of the large Hrp regulon in Ralstonia solanacearum: identification of novel effector proteins translocated to plant host cells through the type III secretion system. Mol. Microbiol. 53, 115–128 [DOI] [PubMed] [Google Scholar]
- 42. Gaspar A. H., and Machner M. P. (2014) VipD is a Rab5-activated phospholipase A1 that protects Legionella pneumophila from endosomal fusion. Proc. Natl. Acad. Sci. U.S.A. 111, 4560–4565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fu H., Coburn J., and Collier R. J. (1993) The eukaryotic host factor that activates exoenzyme S of Pseudomonas aeruginosa is a member of the 14-3-3 protein family. Proc. Natl. Acad. Sci. U.S.A. 90, 2320–2324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Christen M., Coye L. H., Hontz J. S., LaRock D. L., Pfuetzner R. A., Megha, and Miller S. I. (2009) Activation of a bacterial virulence protein by the GTPase RhoA. Sci. Signal. 2, ra71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Coaker G., Falick A., and Staskawicz B. (2005) Activation of a phytopathogenic bacterial effector protein by a eukaryotic cyclophilin. Science 308, 548–550 [DOI] [PubMed] [Google Scholar]
- 46. Anderson D. M., Schmalzer K. M., Sato H., Casey M., Terhune S. S., Haas A. L., Feix J. B., and Frank D. W. (2011) Ubiquitin and ubiquitin-modified proteins activate the Pseudomonas aeruginosa T3SS cytotoxin, ExoU. Mol. Microbiol. 82, 1454–1467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Laloi C., Mestres-Ortega D., Marco Y., Meyer Y., and Reichheld J. P. (2004) The Arabidopsis cytosolic thioredoxin h5 gene induction by oxidative stress and its W-box-mediated response to pathogen elicitor. Plant Physiol. 134, 1006–1016 [DOI] [PMC free article] [PubMed] [Google Scholar]