Abstract
AIM: To identify the typical shape of the rise and fall curve of troponin (Tn) following the different types of myocardial infarction (MI).
METHODS: We conducted a systematic search in PubMed and Embase including all studies which focused on the kinetics of Tn in MI type 1, type 4 and type 5. Tn levels were standardized using the 99th percentile, a pooled mean with 95%CI was calculated from the weighted means for each time point until 72 h.
RESULTS: A total of 34 of the 2528 studies identified in the systematic search were included. The maximum peak level of the Tn was seen after 6 h after successful reperfusion of an acute MI, after 12 h for type 1 MI and after 72 h for type 5 MI. In type 1 MI there were additional smaller peaks at 1 h and at 24 h. After successful reperfusion of an acute MI there was a second peak at 24 h. There was not enough data available to analyze the Tn release after MI associated with percutaneous coronary intervention (type 4).
CONCLUSION: The typical rise and fall of Tn is different for type 1 MI, successful reperfusion of an acute MI and type 5 MI, with different timing of the peak levels and different slopes of the fall phase.
Keywords: Troponin, Myocardial infarction, Systematic review, Reperfusion, Coronary artery bypass grafting
Core tip: In this systematic review we aimed to identify the typical rise and fall of cardiac troponin (Tn) in the different types of myocardial infarction (MI). A total of 34 of the 2528 studies identified in the systematic search were included. The typical rise and fall of Tn is different for type 1 MI, successful reperfusion of an acute MI and type 5 MI, with different timing of the peak levels and different slopes of the fall phase.
INTRODUCTION
Myocardial infarction (MI) is the collective term for myocardial necrosis in the setting of myocardial ischemia[1]. There are many different conditions which can result in myocardial ischemia and subsequent MI. Currently, there are five distinct types of MI defined: Type 1 spontaneous MI related to atherosclerotic plaque rupture, type 2 MI secondary to an imbalance between oxygen supply and oxygen demand, type 3 MI resulting in death when biomarkers are not available, type 4a MI related to percutaneous coronary intervention (PCI), type 4b MI related to stent thrombosis, and type 5 MI related to coronary artery bypass grafting (CABG)[1].
For all different types of MI, excluding type 3, cardiac biomarkers are the cornerstone for diagnosing its occurrence. The preferred cardiac biomarker for the detection of myocardial damage is troponin (Tn)[1]. Tn (subtypes I en T) is part of the contractile apparatus of myocardial cells only and is therefore a highly specific biomarker for myocardial damage[1]. Elevated levels of Tn can be detected within 3-12 h after the start of ischemia and they reach a peak after 12-48 h[2]. However, as Tn is a structural component of myocardial cells, Tn levels will be elevated in patients with chronic heart conditions such as heart failure as well. Therefore, to distinguish between an acute MI and chronic cardiac disease, elevation of Tn alone is not specific enough. There needs to be a significant change in the level of Tn, i.e., a rise and/or a fall. In spontaneous MI a relative difference of more than 20% is considered a significant change[1]. More specifically, in spontaneous MI any level above the 99th percentile is considered a rise[1]. The cut off levels according to the third universal definition for a typical rise in PCI associated MI (> 5 times 99th percentile) and CABG associated MI (> 10 times 99th percentile) are consensus based and not evidence based[1].
The typical rise and/or fall of Tn is thus crucial for the diagnosis of MI[1]. However, the exact shape of the rise and fall curve is largely unknown. Nevertheless, understanding the shape of the rise and fall curve would allow for better timing of Tn blood sampling in clinical practice and would improve diagnostic criteria per type of MI. The aim of this systematic review was to identify the typical shape of the rise and fall curve of Tn following the different types of MI.
MATERIALS AND METHODS
Literature search
Medline (PubMed) and Embase were searched from 1966 through October 2013 for publications. We used synonyms and abbreviations for “rising”, “falling”, “changing”, “troponin” and “myocardial infarction” as keywords (See supplementary 1 for search strategies). Based on titles and abstracts, all studies evaluating Tn in MI were included. Different types of studies were eligible, for example cross sectional studies of patients with MI, cohort studies including patients with symptoms of cardiac ischemia, randomized controlled trials concerning treatment or diagnosis of MI and case control studies where the cases had MI. We included studies in patients with MI that focused on cardiac Tn, both Tn-I and Tn-T, and that reported at least two different Tn-values with at least one sample above the cut off level. Abstracts from conference proceedings, non-human studies, non-English studies, and studies on animals, children, chronic conditions and cardiomyopathy were excluded.
First, all titles and abstracts were screened for eligibility. Second, screening was extended to full text for all studies that where either marked as relevant or when the eligibility was unclear from screening titles and abstracts. Eligibility was determined using a standardized form containing the above mentioned criteria.
The methodological quality of included studies was assessed by two observers (DvB and ML) and in case of doubt by a third observer (BvZ) using an adjusted QUADAS-tool[3] (see supplementary 2 for quality criteria). The selected items of the QUADAS-tool enabled us to examine potential sources of bias and variation[4]. The defined quality domains were; representativeness of the spectrum (i.e., the representativeness of the patients in the study for clinical practice), acceptable reference standard, acceptable delay between tests, partial verification avoided, relevant clinical information, uninterpretable results reported, and withdrawals explained. We did not calculate summary scores estimating the overall-quality of included studies since it has been shown that their interpretation is problematic and may be misleading[5].
Data extraction took place using a specifically designed data extraction form. The two observers independently extracted raw data from the included studies to obtain information on Tn levels at different time points. Other elements that were extracted included the year of publication, the type of study, the research question, any subgroups, inclusion and exclusion criteria, the setting (e.g., emergency department, in hospital, post-surgery) and sample size. In addition, the proportion of patients with MI, the mean or median age of patients with MI, the proportion of males with MI, any comorbidities and the diagnostic criteria used for MI were obtained. Finally, test characteristics were extracted such as the type of Tn test, the 99th percentile/upper reference limit/cut off level of the Tn test, limit of detection, number of samples per patient and the sample time points in relation to the event (e.g., admission, surgery).
Data were considered missing if not explicitly mentioned in the text and if impossible to deduct the information directly from other information in the text. Discrepancies between the two observers were resolved by discussion.
Statistical analysis
Studies were divided into four subgroups based on the focus of the articles: Studies on type 1 spontaneous MI, studies that focused on successful reperfusion in the setting of an acute MI (where reperfusion was not initiated or its effect not evaluated), studies on MI associated with PCI (type 4a MI), and studies on MI associated with CABG (type 5 MI). Type 2 MI studies were not included in this systematic review as the etiology behind this type of MI is distinctly different.
In this review we aimed to address the general rise and fall of Tn and not the rise and fall of specific Tn tests. Therefore, all Tn levels that were obtained within 72 h were included in our analysis. If the timing of the samples was not specified, the study was excluded from analysis. If only one data source was available for a given point in time, we excluded this time-point from our analysis.
For each time point up till 72 h we conducted the following procedure. For each study, we first determined the mean and standard deviation (SD) of the Tn values. If available, mean and SD as presented in the article was used. Alternatively, when only a median was available the mean was approximated. For articles with less than 25 patients with MI, we used the formula of Hozo et al[6] to approximate the mean, for articles with 25 or more patients with MI, the median was used as the best estimate of the mean. Articles for which the mean could not be approximated were excluded from analysis. When the standard error (SE) was not available from the articles directly, it was calculated from SD, confidence interval (CI), or median absolute deviation. Articles for which the SE was not available nor could be calculated were excluded from the analysis.
Subsequently, in order to make the Tn levels from different studies comparable, all Tn levels were standardized. Standardization was achieved by dividing the Tn levels by the 99th percentile of that particular Tn test. If the 99th percentile was not available, we used the upper reference limit (URL) or the cut off value for standardization. Studies that did not mention a 99th percentile or an URL or a cut off value for their Tn test were excluded from analysis.
After standardization, results over studies were pooled as follows. Every study was assigned a weight according to the inverse of the variance (1/SE2). The weighted mean per article was calculated by multiplying the mean with the weight. The sum of all weighted means was divided by the sum of all weights to calculate a pooled mean for every timepoint. The SE per timepoint was calculated as follows: 1/(sum of the weights)0.5. From the pooled SE the 95%CI was calculated.
The pooled mean of the standardized Tn levels with the corresponding CI at different time points were analyzed and summarized using a graph.
RESULTS
Search results
Our search resulted in 2528 potentially eligible studies (Figure 1). After screening titles and abstracts 2189 studies were excluded. After reviewing and applying the in- and exclusion criteria to the full text of the remaining 339 studies, 34 studies remained for analysis. There were 17 studies on type 1 spontaneous MI, 8 on successful reperfusion, 1 on MI associated with PCI (type 4), and 9 studies on MI associated with CABG (type 5). One study could be included in the analyses for both type 1 MI and reperfusion. The baseline characteristics of the included studies are summarized in Table 1.
Figure 1.
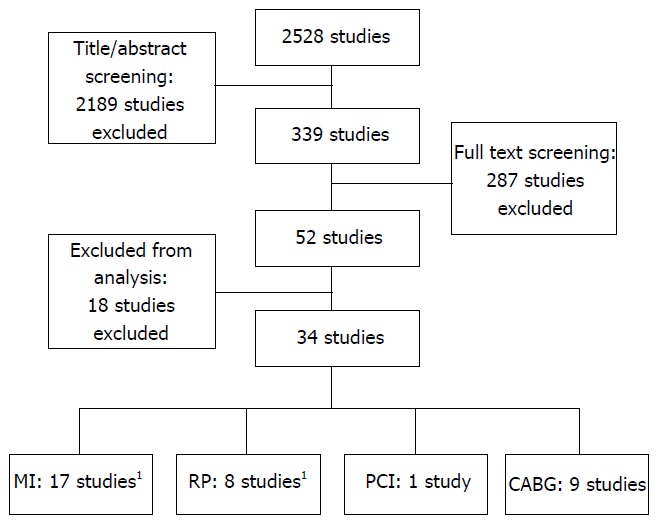
Flow chart. 1Different data from one study has been included in both the MI and RP analysis. MI: Type 1 spontaneous myocardial infarction; RP: Successful reperfusion during an acute myocardial infarction; PCI: Type 4 myocardial infarction associated with percutaneous coronary intervention; CABG: Type 5 myocardial infarction associated with coronary artery bypass surgery.
Table 1.
Baseline characteristics of included studies
| Ref. | Year of publication | NO. of patients | Prevalence MI n (%) | Males with MI n (%) | Diagnostic criteria MI | Tn test | Cut off level | Type of cut off level | Time points measured from |
| Type 1: Spontaneous MI | |||||||||
| Aldous et al[12] | 2011 | 939 | 200 (21) | NA | Biomarkers | HS-TnT (T) | (T) 0.014 μg/L | (T): 99th | Admission |
| ECG | HS-TnI (I) | (I) 0.028 μg/L | (I): 99th | ||||||
| Imaging | |||||||||
| Symptoms | |||||||||
| Aldous et al[13] | 2012 | 385 | 82 (21) | 59 (72) | Biomarkers | TnI (I) | (T): 0.014 μg/L | (T): 99th | Admission |
| ECG | HS-TnT (T) | (I): 0.028 μg/L | (I): 99th | ||||||
| Imaging | |||||||||
| Symptoms | |||||||||
| al-Harbi et al[14] | 2002 | 86 | 51 (59) | 46 (90) | ECG | TnI | 0.05 ng/mL | 99th | Admission |
| Symptoms | |||||||||
| Apple et al[15] | 2009 | 381 | 52 (13) | NA | ESC | TnI | 0.034 μg/ L | 99th | Admission |
| ACC | |||||||||
| Bahrmann et al[16] | 2013 | 306 | 38 (12) | 23 (61) | Biomarkers | HS-TnT | 14 ng/L | 99th | Admission |
| ECG | |||||||||
| Imaging | |||||||||
| Symptoms | |||||||||
| Bertinchant et al[17] | 1996 | 682 | 48 (7) | 41 (85) | WHO | TnI | 0.1 μg/L | Cut off | Admission |
| Biener et al[18] | 2013 | 459 | 111 (3) | 82 (74) | WHO | HS-TnT | 14 ng/mL | 99th | Admission |
| UD | |||||||||
| Bjurman et al[19] | 2013 | 1504 | 1178 (75) | 716 (61) | Biomarkers | HS-TnT | 40 ng/L | 99th | Admission |
| ECG | |||||||||
| Imaging | |||||||||
| Symptoms | |||||||||
| de Winter et al[20] | 2000 | 131 | 131 (100) | NA | Biomarkers | TnT | 0.1 μg/L | URL | Symptoms |
| Symptoms | |||||||||
| Falahati et al[21] | 1999 | 327 | 62 (19) | NA | WHO | TnT | 0.20 μg/L | Cut off | Symptoms |
| Haaf et al[22] | 2012 | 887 | 127 (14) | 87 (69) | Biomarkers | HS-TnT (HT) | (HT): 0.014 μg/L | (HT): 99th | Admission |
| ECG | HS-TnI (HI) | (HI): 0.009 μg/L | (HI): 99th | ||||||
| Imaging | TnI (I) | (I:) 0.009 μg/L | (I:) 99th | ||||||
| Symptoms | |||||||||
| Lucia et al[23] | 2001 | 82 | 42 (51) | 32 (76) | Biomarkers | TnI | 1.5 ng/mL | URL | Admission |
| ECG | |||||||||
| Symptoms | |||||||||
| Mohler et al[24] | 1998 | 100 | 21 (21) | NA | Biomarkers | TnT | 0.1 mg/L | Cut off | Admission |
| ECG | |||||||||
| Symptoms | |||||||||
| Mueller et al[25] | 2012 | 863 | 165 (21) | 121 (73) | UD | HS-TnT | 14 ng/L | 99th | Admission |
| Reichlin et al[26] | 2011 | 836 | 108 (13) | 73 (68) | Biomarkers | Hs-TnT (T) | (T): 0.014 μg/L | (T): 99th | Admission |
| ECG | TnI-ultra (I) | (I): 0.04 μg/L | (I): 99th | ||||||
| Imaging | |||||||||
| Symptoms | |||||||||
| Reichlin et al[27] | 2013 | 840 | 120 (14) | 81 (68) | Biomarkers | Hs-TnT (T) | (T): 14 ng/L | (T): 99th | Admission |
| ECG | HS-TnI (I) | (I): 9 ng/L | (I): 99th | ||||||
| Imaging | (I) 9 ng/L | (I): 99th | |||||||
| Symptoms | |||||||||
| Wu[28] | 2009 | 14 | 4 (29) | 4 (100) | NA | TnI-ultra | 0.04 μg/L | 99th | Admission |
| Successful reperfusion during acute MI | |||||||||
| Abe et al[29] | 1994 | 38 | 26 (68) | 20 (77) | ECG | TnT | 0.2 ng/mL | URL | Start treatment |
| Symptoms | |||||||||
| Apple et al[30] | 1996 | 25 | 17 (68) | NA | ECG | TnI | 3.1 μg/L | URL | Start treatment |
| Symptoms | |||||||||
| Ferraro et al[9] | 2012 | 87 | 87 (100) | 68 (78) | NA | TnI-ultra | 0.04 μg/L | Cut off | Before and after PCI |
| Ferraro et al[31] | 2013 | 856 | 360 (42) | 253 (70) | Biomarkers | TnI-ultra | 40 ng/L | 99th | Before and after PCI |
| ECG | |||||||||
| Symptoms | |||||||||
| Mair et al[32] | 1991 | 172 | 33 (18) | NA | WHO | TnT | 0.5 μg/L | 99th | NA |
| Ricchiuti et al[33] | 2000 | 83 | 23 (28) | 17 (74) | WHO | TnI | 0.8 μg/L | URL | End of treatment |
| Tanasijevic et al[34] | 1997 | 30 | 19 (63) | 15 (79) | NA | TnI | 0.6 ng/mL | URL | Admission |
| Tanasijevic et al[35] | 1999 | 442 | 344 (78) | 258 (75) | NA | TnI | 0.4 ng/mL | Cut off | Before and after treatment |
| Type 4: MI associated with percutaneous coronary intervention | |||||||||
| Reimers et al[36] | 1997 | 80 | 5 (6) | NA | Biomarkers | TnT | 0.1 μg/L | URL | Before PCI and after |
| ECG | |||||||||
| Imaging | |||||||||
| Type 5: MI associated with coronary artery bypass grafting | |||||||||
| Abdel Aziz et al[37] | 2000 | 50 | 14 (28) | 14 (100) | Biomarkers | TnT | 10 μg/L | Cut off | Declamping |
| ECG | |||||||||
| Alyanakian et al[38] | 1998 | 41 | 5 (12) | NA | ECG | TnI | 0.6 μg/L | URL | Start CPB |
| Imaging | |||||||||
| Benoit et al[39] | 2001 | 260 | 8 (3) | NA | Biomarkers | TnI | 0.6 μg/L | URL | Before OR, end of ECC |
| ECG | |||||||||
| Imaging | |||||||||
| Fellahi et al[40] | 1999 | 102 | 7 (7) | 4 (57) | ECG | TnI | 0.6 ng/mL | Cut off | Admission ICU |
| Katus et al[41] | 1991 | 45 | 5 (11) | NA | ECG | TnI | 0.5 mg/L | URL | After surgery |
| Lim et al[42] | 2011 | 28 | 9 (32) | 7 (78) | UD | TnI-ultra | 0.06 μg/L | 99th | End of surgery |
| Mair et al[43] | 1994 | 119 | 10 (8) | 9 | ECG | TnI (I) | (I): 0.10 μg/L | (I): URL | Declamping |
| TnT (T) | (T): 0.10 μg/L | (T): Cut off | |||||||
| Thielmann et al[44] | 2004 | 55 | 55 (100) | 26 (74) | Biomarkers | TnI | 0.5 ng/mL | Cut off | Declamping |
| ECG | |||||||||
| Thielmann et al[45] | 2005 | 94 | 94 (100) | 67 (71) | Biomarkers | TnI | 20 ng/mL | Cut off | Declamping |
| ECG | |||||||||
99th: 99th percentile; ACC: American College of Cardiology; CPB: Cardiopulmonary bypass; ECC: Extracorporeal circulation; ESC: European Society of Cardiology Criteria for MI; HS-TnI: High sensitive TnI; HS-TnT: High sensitive TnT; MI: Myocardial infarction; NA: Not available; PCI: Percutaneous coronary intervention; Tn: Troponin; UD: Universal definition of MI; URL: Upper reference limit; WHO: World Health Organization Criteria for MI.
Quality of the included studies
Table 2 describes the results of the quality assessment. Almost all studies avoided partial verification, worked with relevant clinical information and a representative spectrum of patients with MI. Very few studies reported uninterpretable results or explained withdrawals.
Table 2.
Quality of the included articles based on a modified QUADAS tool
| Ref. | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| Type 1: Spontaneous MI | |||||||
| Aldous et al[12] | + | ? | - | + | + | ? | - |
| Aldous et al[13] | + | + | + | + | + | ? | ? |
| al-Harbi et al[14] | + | ? | + | + | + | ? | ? |
| Apple et al[15] | + | + | + | + | + | ? | ? |
| Bahrmann et al[16] | + | + | - | + | - | ? | + |
| Bertinchant et al[17] | + | + | + | + | + | ? | ? |
| Biener et al[18] | + | + | + | + | + | ? | ? |
| Bjurman et al[19] | + | + | ? | + | + | - | ? |
| de Winter et al[20] | + | - | + | + | + | ? | + |
| Falahati et al[21] | + | + | ? | + | + | ? | ? |
| Haaf et al[22] | + | + | - | + | + | ? | + |
| Lucia et al[23] | + | - | ? | + | + | ? | ? |
| Mohler et al[24] | + | + | + | + | + | ? | ? |
| Mueller et al[25] | + | + | + | + | + | ? | ? |
| Reichlin et al[26] | + | + | - | + | + | ? | ? |
| Reichlin et al[27] | + | + | + | + | + | + | + |
| Wu[28] | + | + | + | + | + | ? | ? |
| Successful reperfusion during acute MI | |||||||
| Abe et al[29] | - | + | - | + | - | ? | - |
| Apple et al[30] | ? | ? | - | ? | ? | ? | ? |
| Ferraro et al[9] | - | ? | + | + | - | - | ? |
| Ferraro et al[31] | + | - | ? | + | + | ? | ? |
| Mair et al[32] | + | + | + | + | + | ? | - |
| Ricchiuti et al[33] | + | + | + | + | ? | ? | ? |
| Tanasijevic et al[34] | ? | ? | ? | - | ? | - | ? |
| Tanasijevic et al[35] | - | - | - | ? | - | + | ? |
| Type 4: MI associated with percutaneous coronary intervention | |||||||
| Reimers et al[36] | - | + | + | + | ? | ? | ? |
| Type 5: MI associated with coronary artery bypass grafting | |||||||
| Abdel Aziz et al[37] | + | - | + | + | - | ? | ? |
| Alyanakian et al[38] | + | + | + | + | - | ? | ? |
| Benoit et al[39] | + | + | + | + | - | ? | ? |
| Fellahi et al[40] | + | - | + | + | - | ? | + |
| Katus et al[41] | + | - | + | + | - | ? | ? |
| Lim et al[42] | + | + | + | + | - | + | + |
| Mair et al[43] | - | + | + | + | + | ? | ? |
| Thielmann et al[44] | + | + | + | + | + | ? | ? |
| Thielmann et al[45] | + | + | + | + | + | ? | ? |
MI: Myocardial infarction. 1: Representativeness of the spectrum; 2: Acceptable reference standard; 3: Acceptable delay between tests; 4: Partial verification avoided; 5: Relevant clinical; 6: Uninterpretable results reported; 7: Withdrawals explained information.
Typical rise and fall of Tn
The pooled mean Tn level in type 1 MI showed an early first peak of 7.0 (95%CI: 6.0-8.0) at 1 h. This initial peak was followed by a maximum pooled mean Tn level of 84 (95%CI: 82-86) at 12 h. A third small peak followed at 24 h (2.7; 95%CI: 2.6-2.9) (Figure 2). Finally, there was a gradual fall of Tn.
Figure 2.
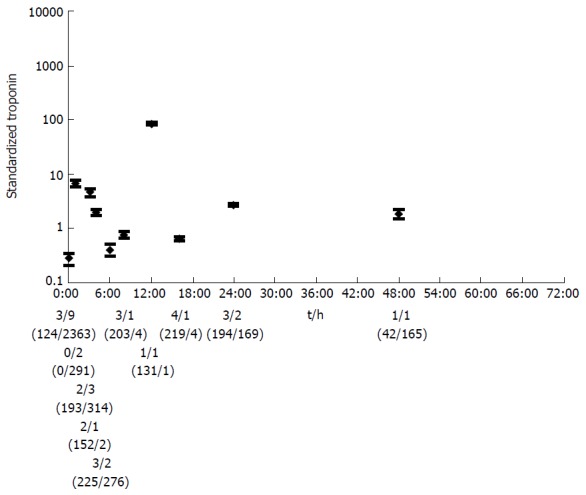
The pooled mean with confidence interval of standardized troponin for the different time points for type 1 spontaneous myocardial infarction. The number of articles per time point with a conventional Tn test/the number of articles with a HS-Tn test, and the number of test values (conventional Tn tests/HS-tests) are shown below the graph. Tn: Troponin; HS: High sensitive.
The maximum pooled mean of Tn after successful reperfusion was at 6 h (1853; 95%CI: 1851-1855), another high peak followed at 24 h (1006; 95%CI: 1004-1007) (Figure 3). Subsequently, there was a pronounced fall in Tn. The pooled mean Tn in type 5 MI associated with CABG raised the first 24 h, after which the Tn levels stabilized (Figure 4). The maximum pooled mean level of Tn was at 72 h (2.2; 95%CI: 1.8-2.6).
Figure 3.
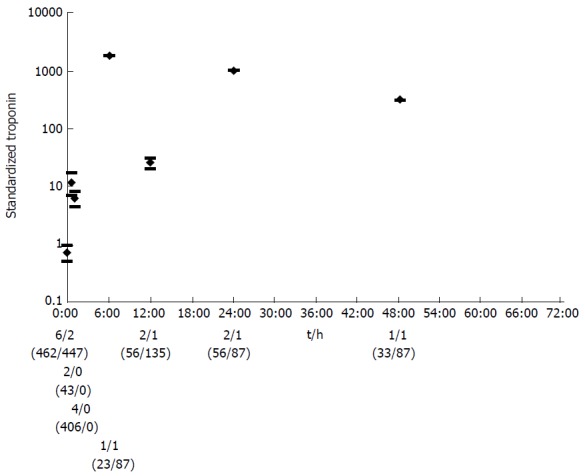
The pooled mean with confidence interval of standardized troponin for the different time points for successful reperfusion after acute myocardial infarction. The number of articles per time point with a conventional Tn test/the number of articles with a HS-Tn test, and the number of test values (conventional Tn tests/HS-tests) are shown below the graph. Tn: Troponin; HS: High sensitive.
Figure 4.
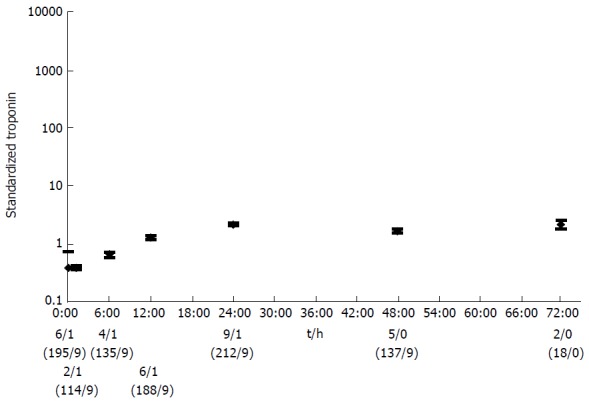
The pooled mean with confidence interval of standardized troponin for type 5 myocardial infarction associated with coronary artery bypass grafting. Time points with only one data source were excluded. The number of articles per time point with a conventional Tn test/the number of articles with a HS-Tn test, and the number of test values (conventional Tn tests/HS-tests) are shown below the graph. Tn: Troponin; HS: High sensitive.
DISCUSSION
In this systematic review we identified the typical shape of the rise and fall curve of Tn following type 1 spontaneous MI, after successful reperfusion of a spontaneous MI, and after type 5 MI associated with CABG. The different types of MI resulted in a different peak level of Tn at different time points followed by distinct fall phases. Understanding these variations of Tn kinetics could result in improvement of the specific diagnostic criteria per type of MI.
It is remarkable that for type 5 MI we found the lowest pooled mean peak level of the different types of MI (2.2 compared to 84 in type 1 MI). This is in contrast with what one should expect when applying the third universal definition. In this definition for type 1 MI the recommended cut off level is defined as the 99th percentile and for type 5 MI 10 times the 99th percentile is recommended[1]. First, the relative high levels of Tn that we found for type 1 MI may be the result of the use of high-sensitive Tn tests. Second, the peak level that we have found in our review for type 5 MI is considerably lower than the optimal cut off level for diagnosing type 5 MI according to a previous published study (266 times the URL)[7]. This could be due to the fact that many of the CABG studies included in our review used a cutoff point instead of a 99th percentile. Likely, these cut off points already take into account the expected higher levels of Tn after CABG. Since we used the cut off level for standardization of Tn if the 99th percentile was not available, this could explain the lower levels of standardized Tn in type 5 MI. In this systematic review we did not include patients without MI from the included studies; therefore we cannot make any claims regarding the optimal diagnostic cut off point.
The recommended interval between two samples to rule MI in or out is 3-6 h[1]. Our results do not support this time interval. For type 1 we found an early first peak after 1 h, followed by a short fall phase. The second rise started at 6 h. This could mean that sampling at 3-6 h might be less optimal than sampling earlier. In type 5 MI the maximum level was at 72 h. Since we did not include any time points after 72 h, we do not know whether this is a peak level or that Tn will rise further. This could mean that Tn should be monitored for more than 72 h postoperatively.
Only one study fulfilled the inclusion criteria focused on type 4 MI. We were therefore unable to analyze the typical rise and fall of Tn after type 4 MI. A review that focused on creatine-kinase M band (CK-MB) in type 4 MI found high levels of CK-MB with a CK-MB level above 10 × URL in 24% of the patients[8].
We found a very large mean peak level of Tn after successful reperfusion in acute MI at 6 h (1853), which is due to one study using a TnI-ultra test in combination with a low cut off level (0.04 μg/L)[9]. It is known that the high sensitive tests require a more pronounced change for the diagnosis MI. While the third universal definition defines a 20% change as significant[1], a rise of > 100% is needed for the high sensitive Tn test[10]. A different cut off level may also be needed for the high-sensitive tests.
This study has several limitations. First, our analysis of the typical rise and fall of Tn is not based on pooling individual patient data from different studies, which would allow for modeling entire biomarker trajectories, but on pooled estimates at different time points used in different studies. To take this into account we refrained from connecting estimates over time. It should however be noted that using individual patient data would be complex as well, given that the studies use a variety of time points; furthermore, the CIs around the pooled estimates are small, so it is rather unlikely that in a substantial number of patients the Tn pattern would be different. Second, we standardized the Tn levels preferentially by using the 99th percentile of Tn. However, the procedure of obtaining a 99th percentile of Tn tests is not uniform[11]. This could result in incorrect standardization and thus restriction of the generalizability. In addition, when the 99th percentile was not available we used the cutoff level. The argumentation for the chosen cutoff level was not always clear. However, the effect of this limitation seems minimal as it may affect the absolute levels of the standardized Tn, but not the Tn rise or fall. Third, the different studies used different criteria to define the baseline time point (0:00 h). These differences were more pronounced in type 1 MI than in type 5 MI articles. This makes the results of type 1 more difficult to interpret. Fourth, we only included studies that focused primarily on Tn levels during MI. This limited the number of included studies. However, the focus of this review was the typical rise and fall of Tn. The excluded studies measured Tn for a different purpose; the timing of the blood sampling and inclusion of the patients was therefore probably not optimal to evaluate the typical rise and fall of Tn. Fourth, Tn levels can be influenced by several patient related factors. For instance, impaired renal function is associated with higher Tn levels. Insufficient patient specific data was available to correct for such patient related factors. However, these factors are likely affecting the absolute levels of Tn and not the shape of the rise-and-fall curve. Finally, we did not scan the reference lists or related studies identified by Medline from the retrieved studies, nor did we hand-search topic specific journals or conference proceedings. However, our study was not a systematic review focusing on diagnostic accuracy or a therapeutic effect, but merely on the kinetics of Tn. Since only studies that focused on the kinetics of Tn were included we considered that the risk of publication bias was low.
The results of this systematic review give insight in the typical rise and fall of Tn in different types of MI. This systematic review is a first step in understanding the similarities and differences in the Tn kinetics between the different types of MI. The different types of MI each seem to result in a unique rise and fall pattern of Tn. In the future this may allow for optimization of the diagnostic criteria per type of MI. Potentially, understanding the kinetics of Tn can also help in monitoring treatment effectiveness.
COMMENTS
Background
An important diagnostic tool for diagnosing myocardial infarction (MI) is monitoring for dynamic cardiac troponin (Tn) levels. Tn levels are expected to rise and fall in MI. However, the exact shape of the Tn curve in MI is unknown. It is also unknown whether the shape of this curve differs for different types of MI. The aim of this systematic review was to identify the typical shape of the rise and fall curve of Tn following the different types of MI.
Research frontiers
The use of Tn in diagnosing the different types of MI was described by Thygesen et al in 2012. For every type of of MI a cut off level and/or the minimum required change in Tn level is suggested for the diagnoses of that particular MI.
Innovations and breakthroughs
An extensive systematic search was conducted to identify all articles concerning the kinetics of Tn in MI type 1, type 4 and type 5. Articles were screened for eligibility and data was extracted in a standardized matter independently by two of the authors. The Tn levels were standardized using the 99th percentile and a pooled mean with 95%CI was calculated for analysis of the results.
Applications
This review suggests that there are important differences in the kinetics of Tn in the different types of MI. Understanding these differences is important for optimizing the diagnostic criteria for these unique types of MI.
Terminology
Myocardial ischemia resulting in myocardial necrosis is called MI. In addition to type 1 spontaneous MI related to atherosclerotic plaque rupture, type 4 MI related to percutaneous coronary intervention and type 5 MI related to coronary artery bypass grafting are classified as distinct types of MI. Cardiac Tn is a sensitive and specific biomarker for myocardial ischemia.
Peer-review
In this systematic review, the authors presented an overview of the typical rise and fall of Tn stratified for the different types of MI.
Footnotes
Conflict-of-interest statement: All the authors declare that they have no competing interests.
Data sharing statement: The technical appendix, statistical code, and dataset are available from the corresponding author at diannevanbeek@hotmail.com.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: October 8, 2015
First decision: November 4, 2015
Article in press: January 7, 2016
P- Reviewer: Armstrong EJ, Chang ST, Kusmic C S- Editor: Qiu S L- Editor: A E- Editor: Li D
References
- 1.Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD. Third universal definition of myocardial infarction. Glob Heart. 2012;7:275–279. doi: 10.1016/j.gheart.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 2.Tiwari RP, Jain A, Khan Z, Kohli V, Bharmal RN, Kartikeyan S, Bisen PS. Cardiac troponins I and T: molecular markers for early diagnosis, prognosis, and accurate triaging of patients with acute myocardial infarction. Mol Diagn Ther. 2012;16:371–381. doi: 10.1007/s40291-012-0011-6. [DOI] [PubMed] [Google Scholar]
- 3.Whiting P, Rutjes AW, Reitsma JB, Bossuyt PM, Kleijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol. 2003;3:25. doi: 10.1186/1471-2288-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whiting P, Rutjes AW, Reitsma JB, Glas AS, Bossuyt PM, Kleijnen J. Sources of variation and bias in studies of diagnostic accuracy: a systematic review. Ann Intern Med. 2004;140:189–202. doi: 10.7326/0003-4819-140-3-200402030-00010. [DOI] [PubMed] [Google Scholar]
- 5.Whiting P, Harbord R, Kleijnen J. No role for quality scores in systematic reviews of diagnostic accuracy studies. BMC Med Res Methodol. 2005;5:19. doi: 10.1186/1471-2288-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jørgensen PH, Nybo M, Jensen MK, Mortensen PE, Poulsen TS, Diederichsen AC, Mickley H. Optimal cut-off value for cardiac troponin I in ruling out Type 5 myocardial infarction. Interact Cardiovasc Thorac Surg. 2014;18:544–550. doi: 10.1093/icvts/ivt558. [DOI] [PubMed] [Google Scholar]
- 8.Park DW, Kim YH, Yun SC, Ahn JM, Lee JY, Kim WJ, Kang SJ, Lee SW, Lee CW, Park SW, et al. Frequency, causes, predictors, and clinical significance of peri-procedural myocardial infarction following percutaneous coronary intervention. Eur Heart J. 2013;34:1662–1669. doi: 10.1093/eurheartj/eht048. [DOI] [PubMed] [Google Scholar]
- 9.Ferraro S, Ardoino I, Boracchi P, Santagostino M, Ciardi L, Antonini G, Braga F, Biganzoli E, Panteghini M, Bongo AS. Inside ST-elevation myocardial infarction by monitoring concentrations of cardiovascular risk biomarkers in blood. Clin Chim Acta. 2012;413:888–893. doi: 10.1016/j.cca.2012.01.034. [DOI] [PubMed] [Google Scholar]
- 10.Mair J. High-sensitivity cardiac troponins in everyday clinical practice. World J Cardiol. 2014;6:175–182. doi: 10.4330/wjc.v6.i4.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sandoval Y, Apple FS. The global need to define normality: the 99th percentile value of cardiac troponin. Clin Chem. 2014;60:455–462. doi: 10.1373/clinchem.2013.211706. [DOI] [PubMed] [Google Scholar]
- 12.Aldous SJ, Richards AM, Cullen L, Than MP. Early dynamic change in high-sensitivity cardiac troponin T in the investigation of acute myocardial infarction. Clin Chem. 2011;57:1154–1160. doi: 10.1373/clinchem.2010.161166. [DOI] [PubMed] [Google Scholar]
- 13.Aldous S, Pemberton C, Richards AM, Troughton R, Than M. High-sensitivity troponin T for early rule-out of myocardial infarction in recent onset chest pain. Emerg Med J. 2012;29:805–810. doi: 10.1136/emermed-2011-200222. [DOI] [PubMed] [Google Scholar]
- 14.al-Harbi K, Suresh CG, Zubaid M, Akanji AO. Establishing a gradient of risk in patients with acute coronary syndromes using troponin I measurements. Med Princ Pract. 2002;11:18–22. doi: 10.1159/000048655. [DOI] [PubMed] [Google Scholar]
- 15.Apple FS, Pearce LA, Smith SW, Kaczmarek JM, Murakami MM. Role of monitoring changes in sensitive cardiac troponin I assay results for early diagnosis of myocardial infarction and prediction of risk of adverse events. Clin Chem. 2009;55:930–937. doi: 10.1373/clinchem.2008.114728. [DOI] [PubMed] [Google Scholar]
- 16.Bahrmann P, Christ M, Bahrmann A, Rittger H, Heppner HJ, Achenbach S, Bertsch T, Sieber CC. A 3-hour diagnostic algorithm for non-ST-elevation myocardial infarction using high-sensitivity cardiac troponin T in unselected older patients presenting to the emergency department. J Am Med Dir Assoc. 2013;14:409–416. doi: 10.1016/j.jamda.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 17.Bertinchant JP, Larue C, Pernel I, Ledermann B, Fabbro-Peray P, Beck L, Calzolari C, Trinquier S, Nigond J, Pau B. Release kinetics of serum cardiac troponin I in ischemic myocardial injury. Clin Biochem. 1996;29:587–594. doi: 10.1016/s0009-9120(96)00105-1. [DOI] [PubMed] [Google Scholar]
- 18.Biener M, Mueller M, Vafaie M, Keller T, Blankenberg S, White HD, Katus HA, Giannitsis E. Comparison of a 3-hour versus a 6-hour sampling-protocol using high-sensitivity cardiac troponin T for rule-out and rule-in of non-STEMI in an unselected emergency department population. Int J Cardiol. 2013;167:1134–1140. doi: 10.1016/j.ijcard.2012.09.122. [DOI] [PubMed] [Google Scholar]
- 19.Bjurman C, Larsson M, Johanson P, Petzold M, Lindahl B, Fu ML, Hammarsten O. Small changes in troponin T levels are common in patients with non-ST-segment elevation myocardial infarction and are linked to higher mortality. J Am Coll Cardiol. 2013;62:1231–1238. doi: 10.1016/j.jacc.2013.06.050. [DOI] [PubMed] [Google Scholar]
- 20.de Winter RJ, Fischer JC, de Jongh T, van Straalen JP, Bholasingh R, Sanders GT. Different time frames for the occurrence of elevated levels of cardiac troponin T and C-reactive protein in patients with acute myocardial infarction. Clin Chem Lab Med. 2000;38:1151–1153. doi: 10.1515/CCLM.2000.175. [DOI] [PubMed] [Google Scholar]
- 21.Falahati A, Sharkey SW, Christensen D, McCoy M, Miller EA, Murakami MA, Apple FS. Implementation of serum cardiac troponin I as marker for detection of acute myocardial infarction. Am Heart J. 1999;137:332–337. doi: 10.1053/hj.1999.v137.92412. [DOI] [PubMed] [Google Scholar]
- 22.Haaf P, Drexler B, Reichlin T, Twerenbold R, Reiter M, Meissner J, Schaub N, Stelzig C, Freese M, Heinzelmann A, et al. High-sensitivity cardiac troponin in the distinction of acute myocardial infarction from acute cardiac noncoronary artery disease. Circulation. 2012;126:31–40. doi: 10.1161/CIRCULATIONAHA.112.100867. [DOI] [PubMed] [Google Scholar]
- 23.Lucia P, Coppola A, Manetti LL, Sebastiani ML, Colliardo A, Cerroni F, De Martinis C, Strappini PM. Cardiac troponin I in acute coronary ischemic syndromes. Epidemiological and clinical correlates. Int J Cardiol. 2001;77:215–222. doi: 10.1016/s0167-5273(00)00429-0. [DOI] [PubMed] [Google Scholar]
- 24.Mohler ER, Ryan T, Segar DS, Sawada SG, Sonel AF, Perkins L, Fineberg N, Feigenbaum H, Wilensky RL. Clinical utility of troponin T levels and echocardiography in the emergency department. Am Heart J. 1998;135:253–260. doi: 10.1016/s0002-8703(98)70090-0. [DOI] [PubMed] [Google Scholar]
- 25.Mueller M, Biener M, Vafaie M, Doerr S, Keller T, Blankenberg S, Katus HA, Giannitsis E. Absolute and relative kinetic changes of high-sensitivity cardiac troponin T in acute coronary syndrome and in patients with increased troponin in the absence of acute coronary syndrome. Clin Chem. 2012;58:209–218. doi: 10.1373/clinchem.2011.171827. [DOI] [PubMed] [Google Scholar]
- 26.Reichlin T, Irfan A, Twerenbold R, Reiter M, Hochholzer W, Burkhalter H, Bassetti S, Steuer S, Winkler K, Peter F, et al. Utility of absolute and relative changes in cardiac troponin concentrations in the early diagnosis of acute myocardial infarction. Circulation. 2011;124:136–145. doi: 10.1161/CIRCULATIONAHA.111.023937. [DOI] [PubMed] [Google Scholar]
- 27.Reichlin T, Twerenbold R, Maushart C, Reiter M, Moehring B, Schaub N, Balmelli C, Rubini Gimenez M, Hoeller R, Sakarikos K, et al. Risk stratification in patients with unstable angina using absolute serial changes of 3 high-sensitive troponin assays. Am Heart J. 2013;165:371–378.e3. doi: 10.1016/j.ahj.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 28.Wu AH. Interpretation of high sensitivity cardiac troponin I results: reference to biological variability in patients who present to the emergency room with chest pain: case report series. Clin Chim Acta. 2009;401:170–174. doi: 10.1016/j.cca.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 29.Abe S, Arima S, Yamashita T, Miyata M, Okino H, Toda H, Nomoto K, Ueno M, Tahara M, Kiyonaga K. Early assessment of reperfusion therapy using cardiac troponin T. J Am Coll Cardiol. 1994;23:1382–1389. doi: 10.1016/0735-1097(94)90381-6. [DOI] [PubMed] [Google Scholar]
- 30.Apple FS, Henry TD, Berger CR, Landt YA. Early monitoring of serum cardiac troponin I for assessment of coronary reperfusion following thrombolytic therapy. Am J Clin Pathol. 1996;105:6–10. doi: 10.1093/ajcp/105.1.6. [DOI] [PubMed] [Google Scholar]
- 31.Ferraro S, Biganzoli E, Marano G, Santagostino M, Boracchi P, Panteghini M, Bongo AS. New insights in the pathophysiology of acute myocardial infarction detectable by a contemporary troponin assay. Clin Biochem. 2013;46:999–1006. doi: 10.1016/j.clinbiochem.2013.03.026. [DOI] [PubMed] [Google Scholar]
- 32.Mair J, Artner-Dworzak E, Lechleitner P, Smidt J, Wagner I, Dienstl F, Puschendorf B. Cardiac troponin T in diagnosis of acute myocardial infarction. Clin Chem. 1991;37:845–852. [PubMed] [Google Scholar]
- 33.Ricchiuti V, Shear WS, Henry TD, Paulsen PR, Miller EA, Apple FS. Monitoring plasma cardiac troponin I for the detection of myocardial injury after percutaneous transluminal coronary angioplasty. Clin Chim Acta. 2000;302:161–170. doi: 10.1016/s0009-8981(00)00365-x. [DOI] [PubMed] [Google Scholar]
- 34.Tanasijevic MJ, Cannon CP, Wybenga DR, Fischer GA, Grudzien C, Gibson CM, Winkelman JW, Antman EM, Braunwald E. Myoglobin, creatine kinase MB, and cardiac troponin-I to assess reperfusion after thrombolysis for acute myocardial infarction: results from TIMI 10A. Am Heart J. 1997;134:622–630. doi: 10.1016/s0002-8703(97)70044-9. [DOI] [PubMed] [Google Scholar]
- 35.Tanasijevic MJ, Cannon CP, Antman EM, Wybenga DR, Fischer GA, Grudzien C, Gibson CM, Winkelman JW, Braunwald E. Myoglobin, creatine-kinase-MB and cardiac troponin-I 60-minute ratios predict infarct-related artery patency after thrombolysis for acute myocardial infarction: results from the Thrombolysis in Myocardial Infarction study (TIMI) 10B. J Am Coll Cardiol. 1999;34:739–747. doi: 10.1016/s0735-1097(99)00274-0. [DOI] [PubMed] [Google Scholar]
- 36.Reimers B, Lachin M, Cacciavillani L, Secchiero S, Ramondo A, Isabella G, Marzari A, Zaninotto M, Plebani M, Chioin R, et al. Troponin T, creatine kinase MB mass, and creatine kinase MB isoform ratio in the detection of myocardial damage during non-surgical coronary revascularization. Int J Cardiol. 1997;60:7–13. doi: 10.1016/s0167-5273(97)02958-6. [DOI] [PubMed] [Google Scholar]
- 37.Abdel Aziz TA, Ali MA, Roberts DG, Al Khaja N. Troponin T as a marker of infarction during coronary bypass surgery. Asian Cardiovasc. 2000;8:19–23. [Google Scholar]
- 38.Alyanakian MA, Dehoux M, Chatel D, Seguret C, Desmonts JM, Durand G, Philip I. Cardiac troponin I in diagnosis of perioperative myocardial infarction after cardiac surgery. J Cardiothorac Vasc Anesth. 1998;12:288–294. doi: 10.1016/s1053-0770(98)90008-8. [DOI] [PubMed] [Google Scholar]
- 39.Benoit MO, Paris M, Silleran J, Fiemeyer A, Moatti N. Cardiac troponin I: its contribution to the diagnosis of perioperative myocardial infarction and various complications of cardiac surgery. Crit Care Med. 2001;29:1880–1886. doi: 10.1097/00003246-200110000-00005. [DOI] [PubMed] [Google Scholar]
- 40.Fellahi JL, Léger P, Philippe E, Arthaud M, Riou B, Gandjbakhch I, Coriat P. Pericardial cardiac troponin I release after coronary artery bypass grafting. Anesth Analg. 1999;89:829–834. doi: 10.1097/00000539-199910000-00004. [DOI] [PubMed] [Google Scholar]
- 41.Katus HA, Schoeppenthau M, Tanzeem A, Bauer HG, Saggau W, Diederich KW, Hagl S, Kuebler W. Non-invasive assessment of perioperative myocardial cell damage by circulating cardiac troponin T. Br Heart J. 1991;65:259–264. doi: 10.1136/hrt.65.5.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lim CC, Cuculi F, van Gaal WJ, Testa L, Arnold JR, Karamitsos T, Francis JM, Digby JE, Antoniades C, Kharbanda RK, et al. Early diagnosis of perioperative myocardial infarction after coronary bypass grafting: a study using biomarkers and cardiac magnetic resonance imaging. Ann Thorac Surg. 2011;92:2046–2053. doi: 10.1016/j.athoracsur.2011.05.019. [DOI] [PubMed] [Google Scholar]
- 43.Mair J, Larue C, Mair P, Balogh D, Calzolari C, Puschendorf B. Use of cardiac troponin I to diagnose perioperative myocardial infarction in coronary artery bypass grafting. Clin Chem. 1994;40:2066–2070. [PubMed] [Google Scholar]
- 44.Thielmann M, Massoudy P, Marggraf G, Knipp S, Schmermund A, Piotrowski J, Erbel R, Jakob H. Role of troponin I, myoglobin, and creatine kinase for the detection of early graft failure following coronary artery bypass grafting. Eur J Cardiothorac Surg. 2004;26:102–109. doi: 10.1016/j.ejcts.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 45.Thielmann M, Massoudy P, Schmermund A, Neuhäuser M, Marggraf G, Kamler M, Herold U, Aleksic I, Mann K, Haude M, et al. Diagnostic discrimination between graft-related and non-graft-related perioperative myocardial infarction with cardiac troponin I after coronary artery bypass surgery. Eur Heart J. 2005;26:2440–2447. doi: 10.1093/eurheartj/ehi437. [DOI] [PubMed] [Google Scholar]


