Abstract
Duodenal adenocarcinoma is a rare but aggressive malignancy. Given its rarity, previous studies have traditionally combined duodenal adenocarcinoma (DA) with either other periampullary cancers or small bowel adenocarcinomas, limiting the available data to guide treatment decisions. Nevertheless, management primarily involves complete surgical resection when technically feasible. Surgery may require pancreaticoduodenectomy or segmental duodenal resection; either are acceptable options as long as negative margins are achievable and an adequate lymphadenectomy can be performed. Adjuvant chemotherapy and radiation are important components of multi-modality treatment for patients at high risk of recurrence. Further research would benefit from multi-institutional trials that do not combine DA with other periampullary or small bowel malignancies. The purpose of this article is to perform a comprehensive review of DA with special focus on the surgical management and principles.
Keywords: Duodenal cancer, Duodenal adenocarcinoma, Periampullary, Whipple, Pancreaticoduodenectomy, Segmental resection, Small bowel
Core tip: Duodenal adenocarcinoma is a rare but aggressive malignancy. Complete surgical resection is recommended when technically feasible. Pancreaticoduodenectomy or segmental duodenal resection may be employed, depending on the tumor location, and either are acceptable options as long as negative margins and adequate lymphadenectomy can be achieved. Although specific data are limited, adjuvant chemotherapy and radiation should be considered for patients at high risk of recurrence.
INTRODUCTION
Although the majority of small bowel adenocarcinomas arise in the duodenum, duodenal adenocarcinoma (DA) still represents less than 1% of all gastrointestinal cancers[1,2]. Not surprisingly, given the rarity of the disease, there is limited data to guide treatment decisions. Early studies grouped DA with other periampullary tumors (pancreatic, ampullary, distal bile duct) when discussing their management options. However, in general, DA has a more favorable outcome. For example, compared to some other periampullary malignancies, DA is more likely to be amenable to curative resection and has more favorable long term outcomes[3]. As a result, treatment strategies have tended to favor aggressive surgical resection. The purpose of this article is to provide a comprehensive review of the epidemiology, presentation, diagnosis, management and prognosis of DA with a special emphasis on surgical principles.
Epidemiology
Small bowel malignancies are relatively rare, accounting for only 2% of all gastrointestinal cancers in the United States[4]. Among small bowel tumors, most malignancies arise from the ileum, followed by the duodenum and lastly the jejunum. While most tumors of the ileum are neuroendocrine in origin, adenocarcinoma is the most common duodenal cancer[4-6]. One large population-based analysis found the duodenum to be the location of 55.7% of adenocarcinomas of the small bowel[5]. The majority of DA arise in the second portion of the duodenum, followed by D3/D4, with cancers of the first portion of the duodenum, especially the duodenal bulb, extremely rare[7,8].
The causative factors for DA have not been clearly identified. Dietary factors, such as increased intake of bread, pasta, sugar and red meat or reduced intake of fruits and vegetables, are risk factors for small bowel adenocarcinoma (SBA) as they are for colorectal cancer[9]. Ingestion of alcohol, coffee and use of tobacco also seem to be risk factors[10]. Nevertheless, the strength of these associations are small and the majority of cases of DA are not associated with any known causative agents. However, duodenal adenomas, such as those that occur in familial adenomatous polyposis (FAP) and Gardner syndrome, are associated with elevated risk of DA[11,12]. Similarly, patients with duodenal polyps are also at increased risk[13]. Although less investigated than in colon cancer, the adenoma-carcinoma sequence is still largely accepted in SBA as well[14,15].
CLINICAL PRESENTATION
Since patients do not typically present until tumors have grown to sufficient size to cause symptoms, the diagnosis of DA is difficult and often delayed. When symptoms do appear they are nonspecific and include abdominal pain, nausea, vomiting, fatigue, weakness, and weight loss. Anemia, gastrointestinal obstruction and jaundice are symptoms associated with advanced disease. Abdominal pain is the most common presenting symptom, associated with 56% of cases[16]. As a result of these delays in diagnosis, many cases of DA are not resectable at presentation due to local and distant invasion. Less often, patients undergoing screening programs may be found to have early DA or even adenoma with dysplasia before symptoms begin[17].
DIAGNOSIS
Imaging
Since early symptoms are typically vague, most patients initially undergo either esophagogastroduodenoscopy or cross sectional imaging. Endoscopy is the preferred diagnostic modality as it allows simultaneous visualization and biopsy. Evaluation by an experienced endoscopist is critical as examination of the entire duodenum is required. While lesions in the third or fourth portion of the duodenum can be technically challenging to view endoscopically, the use of extra-long fiber optic scopes may be helpful[18]. Lesions in the distal duodenum may be missed on initial endoscopic evaluation, resulting in further diagnostic delays. Careful attention to proximity of pertinent structures such as the ampulla of Vater should be given. Endoscopic ultrasound may be performed simultaneously to evaluate local extension or lymphadenopathy. In addition, it may facilitate tissue diagnosis when attempts at luminal biopsy are not successful. Upper gastrointestinal series with oral contrast may facilitate precise localization, evaluate for obstruction and rule out other causes of patients’ symptoms. Contrast-enhanced computed tomography is important for assessing involvement of nearby structures, determining resectability and planning surgery. In cases without a confirmed diagnosis, sensitive but non-specific radiographic features suggestive of malignancy include an exophytic or intramural mass, central necrosis and ulceration[19]. While the role of conventional abdominal ultrasound is limited, especially for tumors < 2 cm in size, lesions appear as irregularly marginated hypoechoic masses[20].
Pathology
Diagnosis of DA requires a thorough histopathologic examination of tissue specimens. Adenocarcinoma of gastric, pancreas, distal bile duct and ampullary origin must be ruled out. The degree of associated dysplasia should be assessed. Among extra-ampullary DA, several distinct subtypes have been described: intestinal, gastric, pancreaticobiliary and indeterminate (Table 1)[21,22]. Interestingly, intestinal type DA has been associated with more favorable prognosis compared to other histological subtype[22-24]. Variable expression of the classic cytokeratin markers CK7 and CK20 have made them largely unhelpful in diagnosing DA[25,26]. However, CDX2, a sensitive marker for colorectal carcinoma, is more often expressed in DA and SBA[25,27]. Expression of Her2 in DA has been inconsistently reported in the literature[25,28], perhaps because expression may be limited to gastric subtypes of DA[22]. Conversely, Overman et al[25] found EGFR and VEGF expression rates of 71% and 91%, respectively, in a large series of SBA which was primarily comprised of DA.
Table 1.
Histopathologic subtypes of duodenal adenocarcinoma
| Phenotype | Histological characteristics | Histologically similar | Immunophenotype markers | Prognosis |
| Intestinal | Tubular/cribiforming glands lined by columnar neoplastic cells | Colonic adenocarcinoma | MUC2, CD10, CDX2 | + |
| Gastric | Tubular/papillary proliferation with foveolar or pyloric-type differentiation | Gastric adenocarcinoma | MUC5AC, MUC6 | - |
| Pancreaticobiliary | Simple glands of cuboidal/columnar cells with rounded pleomorphic nuclei; prominent desmoplastic stroma | Pancreatic and Extrahepatic bile duct adenocarcinoma | MUC1 | - |
| Indeterminate | Poor differentiation | None | MUC1 | - |
Adapted from Ushiku et al[22].
Staging
Staging of DA is based on the 7th edition of the American Joint Committee on Cancer’s TNM staging system that was published in 2010 (Table 2)[29]. Accurate nodal staging depends on adequate lymphadenectomy at the time of surgery[30,31].
Table 2.
7th edition of the American Joint Committee on Cancer’s staging system for small bowel adenocarcinoma
| Primary tumor (T) | Regional lymph nodes (N) | Distant metastases (M) | ||
| Tx - Primary tumor cannot be assessed | Nx - Regional lymph nodes cannot be assessed | Mx - Distant metastases not assessed | ||
| Tis - Carcinoma in situ | N0 - No regional node metastasis | M0 - Distant metastases not present | ||
| T1a - Tumor invades lamina propria | N1 - Metastasis in 1-3 regional nodes | M1 - Distant metastases present | ||
| T1b - Tumor invades submucosa | N2 - Metastasis in 4 or more regional nodes | |||
| T2 - Tumor invades muscularis propria | Stage grouping | |||
| T3 - Tumor invades into the subserosa | Stage 0 | Tis | N0 | M0 |
| Stage I | T1-T2 | N0 | M0 | |
| T4 - Tumor perforates visceral peritoneum; or invades pancreas/bile duct | Stage IIA | T3 | N0 | M0 |
| Stage IIB | T4 | N0 | M0 | |
| Stage IIIA | Any T | N1 | M0 | |
| Stage IIIB | Any T | N2 | M0 | |
| Stage IV | Any T | Any N | M1 | |
SURGICAL MANAGEMENT
Relevant anatomy
The duodenum is the first of portion of the small intestine and functions as a conduit between the stomach and the jejunum while regulating the emptying of gastric contents and enzymatically breaking down the chyme received from the stomach. The surgical management of duodenal cancers varies by the portion of the duodenum involved, and hence the basic anatomic divisions merit review. The first segment of the duodenum is suspended by the hepatoduodenal ligament, lies intraperitoneally, begins caudal to the pylorus and extends 5 cm to the duodenal flexure. Moving retroperitoneally, the second segment spans approximately 7.5 cm and is fixed to and curves around the head of the pancreas to travel medially at the level of L3. The transverse, or third, portion of the duodenum is 10 cm in length and lies anterior to the aorta and inferior vena cava but posterior to the superior mesenteric vein and artery. The ascending, or fourth, segment of the duodenum is approximately 2.5 cm in length and heads superior and laterally to become intraperitoneal again as it reaches the ligament of Treitz at the anatomical boundary of the duodenojejunal junction.
Surgical approach
Tumors located in the second portion of the duodenum typically require pancreaticoduodenectomy (PD) because of proximity to head of the pancreas, distal bile duct and ampulla of Vater. Conversely, tumors occurring in the first, third or fourth portion of the duodenum may be managed by either PD or segmental resection (SR). Some will argue that PD should be used for all DAs, regardless of location, to ensure wide margins and adequate regional lymphadenectomy. This opinion is based on the results of early series reporting few long-term survivors of SR[32-39]. Still others will argue for SR of tumors in either the very proximal or very distal duodenum provided that wide margins can be achieved[40-42] in order to avoid the morbidity of PD. Most studies that compared outcomes of two approaches found no statistically significant difference in outcomes, but were limited by small sample sizes and retrospective design[13,42-48]. Cloyd et al[49] recently utilized the surveilance, epidemiology and end results database to retrospectively compare the outcomes of radical resection (defined as a resection of the primary duodenal tumor en bloc with an adjacent organ, as is performed in PD) vs SR across a population-based cohort of patients with DA. In this study of 1611 patients from 1988 to 2010, radical resection was associated with a greater number of LNs excised but not improved survival (Figure 1). Although PD may be required for technical reasons in some situations, the study suggests that SR is an appropriate strategy as long as negative margins can be obtained[49].
Figure 1.
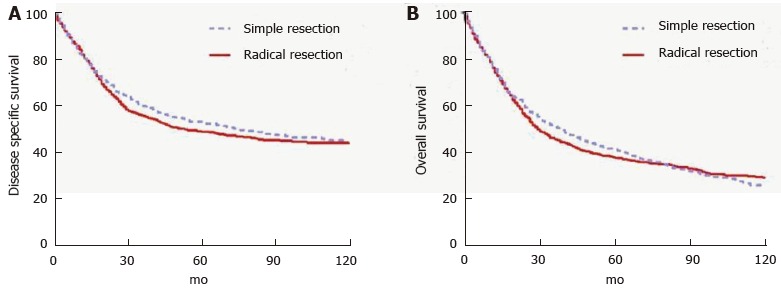
Outcomes of surgery for duodenal adenocarcinoma based on type of surgery. Used with permission: Cloyd et al[49].
Regardless of the approach, an R0 resection remains the most important goal for surgery with curative attempt. Margin status directly impacts outcomes. Sohn et al[35] reported the Johns Hopkins experience and showed a 5 year OS of 58% in margin negative patients vs 0% in margin positive patients. Similarly, Poultsides et al[50] reviewed the Memorial Sloan Kettering Cancer Center (MSKCC) experience and found 5 year OS rates of 55% and 0% among R0 and R1 patients, respectively.
Lymphadenectomy
The importance of an adequate lymphadenectomy cannot be underscored. Sarela et al[51] were among the first to report improved prognostic abilities of the N staging system with higher number of lymph nodes retrieved. In fact, a greater lymph node retrieval has independently been associated with improved survival for patients with DA[2,31,49]. Although the American Joint Committee on Cancer has recommended a minimum pathologic evaluation of 6 lymph nodes, several authors have questioned whether this minimum number should be raised[50,52]. Intuitively, one might expect operations that enable a better lymphadenectomy, such as a classic PD vs a pylorus-preserving PD or PD vs SR, would therefore be associated with improved survival. However, this has not been found to be the case, either in randomized controlled trials[53] or population-based analyses[49]. Although the reasons behind why greater lymph node retrieval is associated with improved survival may be complex and multifactorial, it is likely primarily secondary to improved stage stratification and prognostication.
Palliative surgery
Among patients with localized DA, approximately 43%-87% will have resectable disease[54]. Of the remainder, some will require palliation. The goals of palliative surgery for DA may include relief of gastric outlet obstruction, relief of biliary obstruction and/or pain relief. Operative interventions for gastroduodenal obstruction may include gastrojejunostomy or duodenojejunostomy; either may be constructed in a roux-en-y or loop fashion. Minimally invasive approaches are possible in the correct context. Surgery for biliary obstruction typically involves a roux-en-y hepaticojejunostomy. A 13-year prospective study from the United Kingdom examining surgery for DA found that of the 178 patients included in the study, 150 underwent surgery with curative intention and 28 underwent surgery for palliation. Of those who received palliation, 15 had a gastrojejunostomy, 9 had a double bypass and 4 underwent an exploratory laparotomy without further intervention. Median survival in the palliative surgery group was 8 mo. Not surprisingly, those who undergo palliative surgery are more likely to have a larger tumor, greater degree of invasiveness, as well as regional and distant metastases[55]. For patients who are not already undergoing surgical exploration and require palliation for enteral or biliary obstruction, endoscopically placed duodenal and biliary stents, when technically feasible, are preferable to avoid laparotomy given the limited prognosis.
Pancreas-preserving total duodenectomy
Although a comprehensive discussion is outside the scope of this review article, pancreas-preserving total duodenectomy (PPTD) has emerged as an alternative to PD or SR for patients with benign or pre-malignant conditions of the duodenum, most commonly in the setting of FAP. After total proctocolectomy, upper gastrointestinal cancers are the most common cause of death in patients with FAP[56]. Intense screening programs utilizing duodenoscopy with endoscopic polypectomy have proven effective in reducing the incidence of DA in this high risk population[57]. In patients with diffuse polyposis or Spigelman stage IV disease, however, prophylactic duodenectomy may be indicated[56,58,59]. Several techniques of PPTD have been described[60-63] including minimally invasive options[64]. Despite the advantages of organ preservation, short term morbidity and mortality rates remain high[65]. It is important to note that invasive carcinoma in FAP patients should be treated similarly to sporadic DA with either PD or SR (as described above) in order to ensure adequate margins and lymphadenectomy. Pylorus-preserving PD should be avoided in patients with FAP as the residual duodenal bulb remains at risk for new polyp and carcinoma formation[66].
ADJUVANT THERAPY
Chemotherapy
Unfortunately, little data is currently available to inform the choice of adjuvant chemotherapy following complete surgical resection. The ESPAC-3 trial was a phase 3, multi-institutional, randomized controlled trial comparing observation vs adjuvant fluorouracil vs adjuvant gemcitabine in patients with periampullary cancers (ampullary, bile duct, duodenal or other) who underwent PD with R0 or R1 resection status. Although median survival was not significantly different between the observation and adjuvant therapy groups in the primary analysis (35 mo vs 43 mo), adjuvant chemotherapy was associated with improved OS after multivariable regression (HR = 0.75, 95%CI: 0.57-0.98)[67]. Importantly, periampullary DA comprised a small subset of this study’s population and extra-ampullary DA was not included.
Given its rarity, most therapeutic studies have traditionally combined DA with either other periampullary cancers or small bowel adenocarcinomas. For this reason, chemotherapeutic regimens are not standardized, but increasingly DA is being treated similar to colorectal adenocarcinoma with oxaliplatin-based chemotherapy. Given the tendency of this disease to recur systemically, the role of adjuvant chemotherapy warrants further investigation. Current practice at many centers is to treat patients with high risk features (e.g., nodal metastasis) with oxaliplatin-based chemotherapy[50].
Definitive, or palliative, chemotherapy should be offered to all eligible patients with metastatic or unresectable disease. A phase II prospective trial studied 30 patients with metastatic or unresectable small bowel or ampullary adenocarcinoma who received capecitabine and oxaliplatin and noted a 50% overall response rate, 10% complete response. Median time to progression was 11 mo with median overall survival 20 mo[68,69]. Patients should also be considered for clinical trials as appropriate.
Chemoradiation
The role of adjuvant radiotherapy in the treatment of DA is not well defined. No studies have demonstrated an effect on OS with the use of chemoradiotherapy (CRT). One small study of 14 patients from Johns Hopkins with node-positive DA treated with PD and adjuvant CRT (median dose of 50 Gy, concurrent 5-FU) resulted in improved local control compared with surgery alone (93% vs 67%)[70]. Similarly, a retrospective study of 32 patients from Duke University Medical Center was able to show modest improvement in local control (70% vs 49%) with adjuvant CRT[71]. Unfortunately, neither study showed that adjuvant chemoradiation contributed to improved overall survival: 5-year survival 44% vs 43%[70] and 44% vs 57%[71], respectively. Other retrospective series have shown similar results with improvements in locoregional control but not OS[72]. Nevertheless, this approach targeting improved locoregional control may make CRT particularly useful in patients with lymph node metastases. In a study of 122 patients at a single institution who underwent curative resection for DA, adjuvant CRT in patients with a higher prevalence of regional lymph node metastases was associated with a similar overall survival to that of a group of patients with limited or no nodal metastases who did not receive adjuvant therapy[50].
OUTCOMES
Short term
Surgery for DA can be associated with significant morbidity and mortality. Poultsides et al[50] in their contemporary series of PD at MSKCC, reported a postoperative morbidity rate of 35% and 30-d mortality rate of 2.4%. Solaini et al[48] published a postoperative complication rate of 40% and in-hospital mortality rate of 3.3% for all patients undergoing surgery for DA. In these studies, postoperative pancreatic fistulae (POPF) developed following PD in 14.0% and 10.6% of patients, respectively[48,50]. The impact of the type of resection on postoperative outcomes is controversial. Some have suggested that SR is associated with improved outcomes as it avoids the opportunity for POPF. Tocchi et al[13] reviewed their series of 47 patients undergoing surgery for DA and found SR to be associated with less postoperative morbidity, mortality and length of hospital stay. Bakaeen et al[44] found similar complication rates but shorter LOS in the patients undergoing SR. Other studies have failed to find an effect of surgery type on complication rates[43,48]. The occurrence of a postoperative complication may be associated with worse long term survival[73].
Long term
DA represents an aggressive cancer but in patients with resectable disease, long term outcomes are better than with other periampullary malignancies. In a retrospective study of 122 patients who underwent PD for DA over a 22 year period at MSKCC, ten-year OS was 41%[50]. A prospective cohort study of 150 patients from six United Kingdom hepatopancreaticobiliary centers undergoing curative intent surgery for DA from 2000-2013 found 1-, 3- and 5-year OS rates of 83.9%, 66.7% and 51.2%, respectively. Median disease-free survival was 53 mo[48]. A recent population-based study suggested worse outcomes with 5-year OS rates of 65.9%, 50.4%, 31.4%, and 11.9% for Stage I, II, III and IV, respectively (Figure 2)[49]. Patients with metastatic or unresectable disease have median survival that ranges from 2-8 mo[68,69,74,75].
Figure 2.
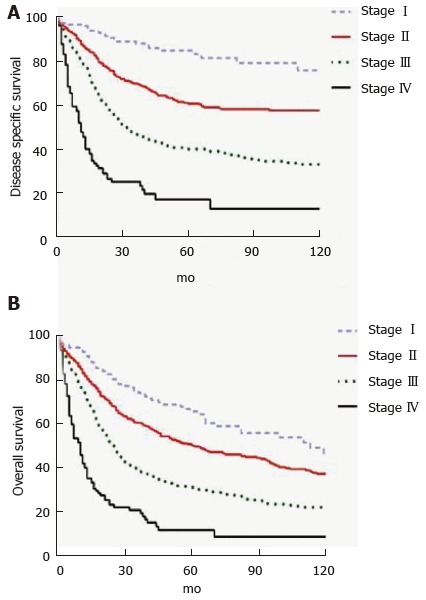
Stage-based disease free (A) and overall (B) survival for patients undergoing surgery for duodenal cancer based on seer data. Used with permission: Cloyd et al[49].
Prognostic factors
Factors associated with worse outcome in DA include patient age, distant metastasis, lymph node metastasis, lymph node ratio, number of lymph nodes harvested, high tumor grade, tumor (T) stage, margin status, lymphovascular or perineural invasion, and overall cancer stage (Table 3). Lymph node metastasis remains one of the most important prognostic determinants[41,43,44,49-51,74,76-78]. In the largest single institution series of 122 patients who underwent PD for DA, the presence of lymph node metastases was the only independent predictor of decreased survival in multivariate analysis. Five-year survival for node negative (N0) patients was 68% compared to 17% in patients with N2 disease[50]. Another study calculated 3-year survival for node negative patients to be 87.5% compared to only 21% in patients with nodal disease[74]. LNR, the ratio of positive LNs to number of LNs excised, may be even a more accurate predictor of prognosis[2,31,49].
Table 3.
Series reporting factors associated with worse survival in duodenal adenocarcinoma
| Ref. | Study period | Total No. of patients | No. of patients resected (%) | PD | 5-year overall survival after resection (%) |
Negative predictors of survival |
||
| Non-predictor | Univariate | Multivariate | ||||||
| Solaini et al[48] | 2000-2013 | 178 | 150 (84.2) | 132 | 43 | T stage, grade, AJCC stage, perineural invasion, size, age | - | Lymphovascular invasion, nodal metastasis |
| Poultsides et al[50] | 1984-2006 | 122 | 122 (100) | 122 | 48 | T stage, tumor grade | Tumor grade, positive margins, perineural invasion, nodal metastasis, vascular invasion | Nodal metastasis |
| Onkendi et al[79] | 1994-2009 | 124 | 99 (79.8) | 70 | 37 | Tumor size, positive nodes, surgical approach, adjuvant therapy | - | T stage and pathologic grade |
| Cecchini et al[80] | 1982-2010 | 169 | 103 (60.9) | 87 | 42 | T stage, nodal metastasis, grade, AJCC stage, lymphovascular invasion, size, age | - | Perineural invasion |
| Liang et al[77] | 1993-2010 | 36 | 36 (100) | 31 | NA | T stage, grade, AJCC stage, lymphovascular invasion, perineural invasion, size | Age > 75, body weight loss, nodal metastasis | Nodal metastasis |
| Malleo et al[73] | 2000-2009 | 37 | 25 (67) | 25 | 711 | T stage, nodal metastasis, AJCC stage, lymphovascular invasion, perineural invasion, size, age | - | Tumor grade, lack of post-operative complications |
| Zhang et al[16] | 1995-2008 | 91 | 59 (65) | NA | 491 | T stage, grade, AJCC stage, lymphovascular invasion, perineural invasion, size, age | - | Nodal metastasis, positive margins |
| Han et al[81] | 1990-2006 | 32 | 28 (88) | 18 | 30 | - | Positive margins | - |
| Struck et al[78] | 1989-2006 | 30 | 30 (100) | 25 | 332 | Positive margins, T stage, adjuvant therapy | Nodal metastasis, stage | |
| Lee et al[74] | 1995-2007 | 53 | 28 (53) | 26 | 44 | Age, gender, weight loss, CA19-9, grade, tumor size | T stage, nodal metastasis, AJCC stage | Nodal metastasis |
| Hurtuk et al[82] | 1984-2005 | 52 | 35 (67) | 24 | NA | Grade, positive margins, nodal metastasis, venous or perineural invasion | Stage T4, tumor size < 3.5 cm | - |
| Hu et al[47] | NA | 43 | 28 (65) | 11 | 27 | - | Positive margins | - |
| Sarela et al[51] | 1983-2000 | 137 | 72 (52.5) | 56 | 711 | Gender, grade, T stage | Age, nodal metastasis | Age, nodal metastasis |
| Tocchi et al[13] | 1980-2000 | 47 | 25 (53) | 9 | 23 | T stage, grade, AJCC stage, lymphovascular invasion, perineural invasion, positive margins, size, age | - | Nodal metastasis |
| Ryder et al[83] | 1957-1998 | 49 | 31 (63) | 27 | 43 | Nodal metastases, location in duodenum, type of resection, adjuvant chemoradiation | - | Tumor size, histologic grade, transmural invasion |
| Kaklamanos et al[43] | 1978-1998 | 63 | 37 (59) | 26 | 30 | Age, gender, grade, T stage | Nodal metastasis | Nodal metastasis |
| Bakaeen et al[44] | 1976-1996 | 101 | 68 (67) | 50 | 54 | Histologic grade, tumor size, location in duodenum, adjuvant chemoradiation | Age, weight loss, T stage, nodal metastasis, AJCC stage | Weight loss, positive margins, nodal metastasis, AJCC stage |
| Sohn et al[35] | 1984-1996 | 55 | 48 (87) | 35 | 53 | Nodal metastasis, adjuvant chemoradiation, tumor size, histologic grade | Positive margins, segmental resection, tumor in third/fourth portion of duodenum | - |
| Sexe et al[76] | 1987-1991 | 85 | 34 (40) | 31 | 23 | AJCC Stage | - | - |
| Rotman et al[84] | 1978-1988 | 66 | 46 (70) | 38 | 45 | Gender, age, weight loss, jaundice, T stage, tumor size, pancreatic invasion nodal metastasis, location of metastatic nodes | - | - |
| Delcore et al[85] | 1960-1990 | 35 | 28 (80) | 21 | 60 | - | GI bleeding, symptomatic > 4 mo, nodal metastasis | - |
| Barnes et al[40] | 1967-1991 | 67 | 36 (54) | 27 | 54 | Nodal metastasis | Stage | |
| Lowell et al[42] | 1970-1991 | 17 | 17 (100) | 8 | 45 | - | First/second portion of the duodeum | - |
| Ouriel et al[33] | 1950-1981 | 65 | 19 (29) | 1 | 30 | - | Histologic grade, nodal metastasis | - |
Values in parentheses are percentages.
R0 resection only;
Three-year survival. PD: Pancreaticoduodenectomy; AJCC: American Joint Committee on Cancer; NA: Not available.
CONCLUSION
Duodenal adenocarcinoma is a rare but aggressive malignancy. Because of the nonspecific symptoms it presents with and the difficulty in confirming a diagnosis, patients may often present with advanced disease. Nonetheless, aggressive surgical resection, when possible, affords the best chance at survival. The decision of whether to perform pancreaticoduodenectomy vs segmental resection depends on the location of the primary tumor as both are acceptable options as long as negative margins can be safely obtained. Lymph node positivity is one of the most important prognostic indicators and a wide lymphadenectomy should be routinely performed. Although data are limited guiding adjuvant therapy options, oxaliplatin-based chemotherapy is typically offered to high risk patients, such as those with positive lymph nodes. In some series, adjuvant radiation is associated with improved local control but no difference in overall survival. Previous research on DA has been limited by small sample sizes and single institutional design. Further research would benefit from multi-institutional trials that do not combined DA with other periampullary or small bowel malignancies.
Footnotes
Conflict-of-interest statement: The authors report no relevant conflicts of interest.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: August 30, 2015
First decision: October 27, 2015
Article in press: December 15, 2015
P- Reviewer: Stift A, Tonelli F S- Editor: Qi Y L- Editor: A E- Editor: Wu HL
References
- 1.Overman MJ, Hu CY, Kopetz S, Abbruzzese JL, Wolff RA, Chang GJ. A population-based comparison of adenocarcinoma of the large and small intestine: insights into a rare disease. Ann Surg Oncol. 2012;19:1439–1445. doi: 10.1245/s10434-011-2173-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Overman MJ, Hu CY, Wolff RA, Chang GJ. Prognostic value of lymph node evaluation in small bowel adenocarcinoma: analysis of the surveillance, epidemiology, and end results database. Cancer. 2010;116:5374–5382. doi: 10.1002/cncr.25324. [DOI] [PubMed] [Google Scholar]
- 3.Yeo CJ, Sohn TA, Cameron JL, Hruban RH, Lillemoe KD, Pitt HA. Periampullary adenocarcinoma: analysis of 5-year survivors. Ann Surg. 1998;227:821–831. doi: 10.1097/00000658-199806000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hatzaras I, Palesty JA, Abir F, Sullivan P, Kozol RA, Dudrick SJ, Longo WE. Small-bowel tumors: epidemiologic and clinical characteristics of 1260 cases from the connecticut tumor registry. Arch Surg. 2007;142:229–235. doi: 10.1001/archsurg.142.3.229. [DOI] [PubMed] [Google Scholar]
- 5.Bilimoria KY, Bentrem DJ, Wayne JD, Ko CY, Bennett CL, Talamonti MS. Small bowel cancer in the United States: changes in epidemiology, treatment, and survival over the last 20 years. Ann Surg. 2009;249:63–71. doi: 10.1097/SLA.0b013e31818e4641. [DOI] [PubMed] [Google Scholar]
- 6.Cunningham JD, Aleali R, Aleali M, Brower ST, Aufses AH. Malignant small bowel neoplasms: histopathologic determinants of recurrence and survival. Ann Surg. 1997;225:300–306. doi: 10.1097/00000658-199703000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ross RK, Hartnett NM, Bernstein L, Henderson BE. Epidemiology of adenocarcinomas of the small intestine: is bile a small bowel carcinogen? Br J Cancer. 1991;63:143–145. doi: 10.1038/bjc.1991.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldner B, Stabile BE. Duodenal adenocarcinoma: why the extreme rarity of duodenal bulb primary tumors? Am Surg. 2014;80:956–959. [PubMed] [Google Scholar]
- 9.Negri E, Bosetti C, La Vecchia C, Fioretti F, Conti E, Franceschi S. Risk factors for adenocarcinoma of the small intestine. Int J Cancer. 1999;82:171–174. doi: 10.1002/(sici)1097-0215(19990719)82:2<171::aid-ijc3>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 10.Neugut AI, Jacobson JS, Suh S, Mukherjee R, Arber N. The epidemiology of cancer of the small bowel. Cancer Epidemiol Biomarkers Prev. 1998;7:243–251. [PubMed] [Google Scholar]
- 11.Yao T, Ida M, Ohsato K, Watanabe H, Omae T. Duodenal lesions in familial polyposis of the colon. Gastroenterology. 1977;73:1086–1092. [PubMed] [Google Scholar]
- 12.Schnur PL, David E, Brown PW, Beahrs OH, ReMine WH, Harrison EG. Adenocarcinoma of the duodenum and the Gardner syndrome. JAMA. 1973;223:1229–1232. [PubMed] [Google Scholar]
- 13.Tocchi A, Mazzoni G, Puma F, Miccini M, Cassini D, Bettelli E, Tagliacozzo S. Adenocarcinoma of the third and fourth portions of the duodenum: results of surgical treatment. Arch Surg. 2003;138:80–85. doi: 10.1001/archsurg.138.1.80. [DOI] [PubMed] [Google Scholar]
- 14.Sellner F. Investigations on the significance of the adenoma-carcinoma sequence in the small bowel. Cancer. 1990;66:702–715. doi: 10.1002/1097-0142(19900815)66:4<702::aid-cncr2820660419>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 15.Nakano Y, Adachi Y, Okamoto H, Kiyama Y, Koyama T, Nakamura SI, Li Q, Sakaida N, Uemura Y, Ikehara S. Adenocarcinoma with adenoma in the jejunum suggesting an adenoma-carcinoma sequence in the small bowel: A case report. Oncol Lett. 2014;8:633–636. doi: 10.3892/ol.2014.2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang S, Cui Y, Zhong B, Xiao W, Gong X, Chao K, Chen M. Clinicopathological characteristics and survival analysis of primary duodenal cancers: a 14-year experience in a tertiary centre in South China. Int J Colorectal Dis. 2011;26:219–226. doi: 10.1007/s00384-010-1063-x. [DOI] [PubMed] [Google Scholar]
- 17.Jaganmohan S, Lynch PM, Raju RP, Ross WA, Lee JE, Raju GS, Bhutani MS, Fleming JB, Lee JH. Endoscopic management of duodenal adenomas in familial adenomatous polyposis--a single-center experience. Dig Dis Sci. 2012;57:732–737. doi: 10.1007/s10620-011-1917-2. [DOI] [PubMed] [Google Scholar]
- 18.Markogiannakis H, Theodorou D, Toutouzas KG, Gloustianou G, Katsaragakis S, Bramis I. Adenocarcinoma of the third and fourth portion of the duodenum: a case report and review of the literature. Cases J. 2008;1:98. doi: 10.1186/1757-1626-1-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kazerooni EA, Quint LE, Francis IR. Duodenal neoplasms: predictive value of CT for determining malignancy and tumor resectability. AJR Am J Roentgenol. 1992;159:303–309. doi: 10.2214/ajr.159.2.1632344. [DOI] [PubMed] [Google Scholar]
- 20.Ishida H, Konno K, Sato M, Naganuma H, Komatsuda T, Yamada N, Hamashima Y, Ishida J, Segawa D, Watanabe S. Duodenal carcinoma: sonographic findings. Abdom Imaging. 2001;26:469–473. doi: 10.1007/s002610000187. [DOI] [PubMed] [Google Scholar]
- 21.Albores-Saavedra J, Hruban R, Klimstra D. In WHO Classification of Tumours of the Digestive System 87-91. Lyon: IARC Press; 2010. [Google Scholar]
- 22.Ushiku T, Arnason T, Fukayama M, Lauwers GY. Extra-ampullary duodenal adenocarcinoma. Am J Surg Pathol. 2014;38:1484–1493. doi: 10.1097/PAS.0000000000000278. [DOI] [PubMed] [Google Scholar]
- 23.Overman MJ, Zhang J, Kopetz S, Davies M, Jiang ZQ, Stemke-Hale K, Rümmele P, Pilarsky C, Grützmann R, Hamilton S, et al. Gene expression profiling of ampullary carcinomas classifies ampullary carcinomas into biliary-like and intestinal-like subtypes that are prognostic of outcome. PLoS One. 2013;8:e65144. doi: 10.1371/journal.pone.0065144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Westgaard A, Tafjord S, Farstad IN, Cvancarova M, Eide TJ, Mathisen O, Clausen OP, Gladhaug IP. Pancreatobiliary versus intestinal histologic type of differentiation is an independent prognostic factor in resected periampullary adenocarcinoma. BMC Cancer. 2008;8:170. doi: 10.1186/1471-2407-8-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Overman MJ, Pozadzides J, Kopetz S, Wen S, Abbruzzese JL, Wolff RA, Wang H. Immunophenotype and molecular characterisation of adenocarcinoma of the small intestine. Br J Cancer. 2010;102:144–150. doi: 10.1038/sj.bjc.6605449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee MJ, Lee HS, Kim WH, Choi Y, Yang M. Expression of mucins and cytokeratins in primary carcinomas of the digestive system. Mod Pathol. 2003;16:403–410. doi: 10.1097/01.MP.0000067683.84284.66. [DOI] [PubMed] [Google Scholar]
- 27.Werling RW, Yaziji H, Bacchi CE, Gown AM. CDX2, a highly sensitive and specific marker of adenocarcinomas of intestinal origin: an immunohistochemical survey of 476 primary and metastatic carcinomas. Am J Surg Pathol. 2003;27:303–310. doi: 10.1097/00000478-200303000-00003. [DOI] [PubMed] [Google Scholar]
- 28.Zhu L, Kim K, Domenico DR, Appert HE, Howard JM. Adenocarcinoma of duodenum and ampulla of Vater: clinicopathology study and expression of p53, c-neu, TGF-alpha, CEA, and EMA. J Surg Oncol. 1996;61:100–105. doi: 10.1002/(SICI)1096-9098(199602)61:2<100::AID-JSO3>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 29.Edge S, Byrd D, Compton C. In: AJCC Cancer Staging Manual. New York, NY: Springer; 2010. pp. 127–132. [Google Scholar]
- 30.Nicholl MB, Ahuja V, Conway WC, Vu VD, Sim MS, Singh G. Small bowel adenocarcinoma: understaged and undertreated? Ann Surg Oncol. 2010;17:2728–2732. doi: 10.1245/s10434-010-1109-x. [DOI] [PubMed] [Google Scholar]
- 31.Tran TB, Qadan M, Dua MM, Norton JA, Poultsides GA, Visser BC. Prognostic relevance of lymph node ratio and total lymph node count for small bowel adenocarcinoma. Surgery. 2015;158:486–493. doi: 10.1016/j.surg.2015.03.048. [DOI] [PubMed] [Google Scholar]
- 32.Moss WM, McCart PM, Juler G, Miller DR. Primary adenocarcinoma of the duodenum. Arch Surg. 1974;108:805–807. doi: 10.1001/archsurg.1974.01350300047013. [DOI] [PubMed] [Google Scholar]
- 33.Ouriel K, Adams JT. Adenocarcinoma of the small intestine. Am J Surg. 1984;147:66–71. doi: 10.1016/0002-9610(84)90036-9. [DOI] [PubMed] [Google Scholar]
- 34.Cortese AF, Cornell GN. Carcinoma of the duodenum. Cancer. 1972;29:1010–1015. doi: 10.1002/1097-0142(197204)29:4<1010::aid-cncr2820290448>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 35.Sohn TA, Lillemoe KD, Cameron JL, Pitt HA, Kaufman HS, Hruban RH, Yeo CJ. Adenocarcinoma of the duodenum: factors influencing long-term survival. J Gastrointest Surg. 1998;2:79–87. doi: 10.1016/s1091-255x(98)80107-8. [DOI] [PubMed] [Google Scholar]
- 36.Brenner RL, Brown CH. Primary carcinoma of the duodenum; report of 15 cases. Gastroenterology. 1955;29:189–198. [PubMed] [Google Scholar]
- 37.Berger L, Koppelman H. Primary carcinoma of the duodenum. Ann Surg. 1942;116:738–750. doi: 10.1097/00000658-194211650-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Newton WT. Mortality and morbidity associated with resection of pancreaticoduodenal cancers. Am Surg. 1961;27:74–79. [PubMed] [Google Scholar]
- 39.Lieber MM, Stewart HL, Lund H. Carcinoma of the peripapillary portion of the duodenum. Ann Surg. 1939;109:383–429. doi: 10.1097/00000658-193903000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barnes G, Romero L, Hess KR, Curley SA. Primary adenocarcinoma of the duodenum: management and survival in 67 patients. Ann Surg Oncol. 1994;1:73–78. doi: 10.1007/BF02303544. [DOI] [PubMed] [Google Scholar]
- 41.Joesting DR, Beart RW, van Heerden JA, Weiland LH. Improving survival in adenocarcinoma of the duodenum. Am J Surg. 1981;141:228–231. doi: 10.1016/0002-9610(81)90163-x. [DOI] [PubMed] [Google Scholar]
- 42.Lowell JA, Rossi RL, Munson JL, Braasch JW. Primary adenocarcinoma of third and fourth portions of duodenum. Favorable prognosis after resection. Arch Surg. 1992;127:557–560. doi: 10.1001/archsurg.1992.01420050081010. [DOI] [PubMed] [Google Scholar]
- 43.Kaklamanos IG, Bathe OF, Franceschi D, Camarda C, Levi J, Livingstone AS. Extent of resection in the management of duodenal adenocarcinoma. Am J Surg. 2000;179:37–41. doi: 10.1016/s0002-9610(99)00269-x. [DOI] [PubMed] [Google Scholar]
- 44.Bakaeen FG, Murr MM, Sarr MG, Thompson GB, Farnell MB, Nagorney DM, Farley DR, van Heerden JA, Wiersema LM, Schleck CD, et al. What prognostic factors are important in duodenal adenocarcinoma? Arch Surg. 2000;135:635–641; discussion 641-642. doi: 10.1001/archsurg.135.6.635. [DOI] [PubMed] [Google Scholar]
- 45.Rose DM, Hochwald SN, Klimstra DS, Brennan MF. Primary duodenal adenocarcinoma: a ten-year experience with 79 patients. J Am Coll Surg. 1996;183:89–96. [PubMed] [Google Scholar]
- 46.van Ooijen B, Kalsbeek HL. Carcinoma of the duodenum. Surg Gynecol Obstet. 1988;166:343–347. [PubMed] [Google Scholar]
- 47.Hu JX, Miao XY, Zhong DW, Dai WD, Liu W, Hu W. Surgical treatment of primary duodenal adenocarcinoma. Hepatogastroenterology. 2006;53:858–862. [PubMed] [Google Scholar]
- 48.Solaini L, Jamieson NB, Metcalfe M, Abu Hilal M, Soonawalla Z, Davidson BR, McKay C, Kocher HM. Outcome after surgical resection for duodenal adenocarcinoma in the UK. Br J Surg. 2015;102:676–681. doi: 10.1002/bjs.9791. [DOI] [PubMed] [Google Scholar]
- 49.Cloyd JM, Norton JA, Visser BC, Poultsides GA. Does the extent of resection impact survival for duodenal adenocarcinoma? Analysis of 1,611 cases. Ann Surg Oncol. 2015;22:573–580. doi: 10.1245/s10434-014-4020-z. [DOI] [PubMed] [Google Scholar]
- 50.Poultsides GA, Huang LC, Cameron JL, Tuli R, Lan L, Hruban RH, Pawlik TM, Herman JM, Edil BH, Ahuja N, et al. Duodenal adenocarcinoma: clinicopathologic analysis and implications for treatment. Ann Surg Oncol. 2012;19:1928–1935. doi: 10.1245/s10434-011-2168-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sarela AI, Brennan MF, Karpeh MS, Klimstra D, Conlon KC. Adenocarcinoma of the duodenum: importance of accurate lymph node staging and similarity in outcome to gastric cancer. Ann Surg Oncol. 2004;11:380–386. doi: 10.1245/ASO.2004.05.021. [DOI] [PubMed] [Google Scholar]
- 52.Gibbs JF. Duodenal adenocarcinoma: is total lymph node sampling predictive of outcome? Ann Surg Oncol. 2004;11:354–355. doi: 10.1245/ASO.2004.02.914. [DOI] [PubMed] [Google Scholar]
- 53.Yeo CJ, Cameron JL, Lillemoe KD, Sohn TA, Campbell KA, Sauter PK, Coleman J, Abrams RA, Hruban RH. Pancreaticoduodenectomy with or without distal gastrectomy and extended retroperitoneal lymphadenectomy for periampullary adenocarcinoma, part 2: randomized controlled trial evaluating survival, morbidity, and mortality. Ann Surg. 2002;236:355–366; discussion 366-368. doi: 10.1097/00000658-200209000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Solej M, D’Amico S, Brondino G, Ferronato M, Nano M. Primary duodenal adenocarcinoma. Tumori. 2008;94:779–786. doi: 10.1177/030089160809400601. [DOI] [PubMed] [Google Scholar]
- 55.Kawahira H, Miura F, Saigo K, Matsunaga A, Natsume T, Akai T, Horibe D, Suzuki K, Nabeya Y, Hayashi H, et al. Survival predictors of patients with primary duodenal adenocarcinoma. Int Surg. 2011;96:111–116. doi: 10.9738/1381.1. [DOI] [PubMed] [Google Scholar]
- 56.Johnson JC, DiSario JA, Grady WM. Surveillance and Treatment of Periampullary and Duodenal Adenomas in Familial Adenomatous Polyposis. Curr Treat Options Gastroenterol. 2004;7:79–89. doi: 10.1007/s11938-004-0028-y. [DOI] [PubMed] [Google Scholar]
- 57.Campos FG, Sulbaran M, Safatle-Ribeiro AV, Martinez CA. Duodenal adenoma surveillance in patients with familial adenomatous polyposis. World J Gastrointest Endosc. 2015;7:950–959. doi: 10.4253/wjge.v7.i10.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Skipworth JR, Morkane C, Raptis DA, Vyas S, Olde Damink SW, Imber CJ, Pereira SP, Malago M, West N, Phillips RK, et al. Pancreaticoduodenectomy for advanced duodenal and ampullary adenomatosis in familial adenomatous polyposis. HPB (Oxford) 2011;13:342–349. doi: 10.1111/j.1477-2574.2011.00292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Groves CJ, Saunders BP, Spigelman AD, Phillips RK. Duodenal cancer in patients with familial adenomatous polyposis (FAP): results of a 10 year prospective study. Gut. 2002;50:636–641. doi: 10.1136/gut.50.5.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kalady MF, Clary BM, Tyler DS, Pappas TN. Pancreas-preserving duodenectomy in the management of duodenal familial adenomatous polyposis. J Gastrointest Surg. 2002;6:82–87. doi: 10.1016/s1091-255x(01)00005-1. [DOI] [PubMed] [Google Scholar]
- 61.Köninger J, Friess H, Wagner M, Kadmon M, Büchler MW. Technique of pancreas-preserving duodenectomy. Chirurg. 2005;76:273–281. doi: 10.1007/s00104-004-0992-8. [DOI] [PubMed] [Google Scholar]
- 62.Imamura M, Komoto I, Doi R, Onodera H, Kobayashi H, Kawai Y. New pancreas-preserving total duodenectomy technique. World J Surg. 2005;29:203–207. doi: 10.1007/s00268-004-7585-z. [DOI] [PubMed] [Google Scholar]
- 63.Koshariya M, Jagad RB, Kawamoto J, Papastratis P, Kefalourous H, Porfiris T, Gevrielidis P, Tzouma C, Lygidakis NJ. Pancreas-preserving total duodenectomy without pancreato-enteric anastomosis. Hepatogastroenterology. 2007;54:2123–2128. [PubMed] [Google Scholar]
- 64.Benetatos N, Ammori MB, Ammori BJ. Laparoscopic pancreas-preserving total duodenectomy for familial adenomatous polyposis. Surg Laparosc Endosc Percutan Tech. 2011;21:e332–e335. doi: 10.1097/SLE.0b013e3182397771. [DOI] [PubMed] [Google Scholar]
- 65.de Castro SM, van Eijck CH, Rutten JP, Dejong CH, van Goor H, Busch OR, Gouma DJ. Pancreas-preserving total duodenectomy versus standard pancreatoduodenectomy for patients with familial adenomatous polyposis and polyps in the duodenum. Br J Surg. 2008;95:1380–1386. doi: 10.1002/bjs.6308. [DOI] [PubMed] [Google Scholar]
- 66.Murakami Y, Uemura K, Sasaki M, Morifuji M, Hayashidani Y, Sudo T, Sueda T. Duodenal cancer arising from the remaining duodenum after pylorus-preserving pancreatoduodenectomy for ampullary cancer in familial adenomatous polyposis. J Gastrointest Surg. 2005;9:389–392. doi: 10.1016/j.gassur.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 67.Neoptolemos JP, Moore MJ, Cox TF, Valle JW, Palmer DH, McDonald AC, Carter R, Tebbutt NC, Dervenis C, Smith D, et al. Effect of adjuvant chemotherapy with fluorouracil plus folinic acid or gemcitabine vs observation on survival in patients with resected periampullary adenocarcinoma: the ESPAC-3 periampullary cancer randomized trial. JAMA. 2012;308:147–156. doi: 10.1001/jama.2012.7352. [DOI] [PubMed] [Google Scholar]
- 68.Overman MJ, Kopetz S, Wen S, Hoff PM, Fogelman D, Morris J, Abbruzzese JL, Ajani JA, Wolff RA. Chemotherapy with 5-fluorouracil and a platinum compound improves outcomes in metastatic small bowel adenocarcinoma. Cancer. 2008;113:2038–2045. doi: 10.1002/cncr.23822. [DOI] [PubMed] [Google Scholar]
- 69.Overman MJ, Varadhachary GR, Kopetz S, Adinin R, Lin E, Morris JS, Eng C, Abbruzzese JL, Wolff RA. Phase II study of capecitabine and oxaliplatin for advanced adenocarcinoma of the small bowel and ampulla of Vater. J Clin Oncol. 2009;27:2598–2603. doi: 10.1200/JCO.2008.19.7145. [DOI] [PubMed] [Google Scholar]
- 70.Swartz MJ, Hughes MA, Frassica DA, Herman J, Yeo CJ, Riall TS, Lillemoe KD, Cameron JL, Donehower RC, Laheru DA, et al. Adjuvant concurrent chemoradiation for node-positive adenocarcinoma of the duodenum. Arch Surg. 2007;142:285–288. doi: 10.1001/archsurg.142.3.285. [DOI] [PubMed] [Google Scholar]
- 71.Kelsey CR, Nelson JW, Willett CG, Chino JP, Clough RW, Bendell JC, Tyler DS, Hurwitz HI, Morse MA, Clary BM, et al. Duodenal adenocarcinoma: patterns of failure after resection and the role of chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2007;69:1436–1441. doi: 10.1016/j.ijrobp.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 72.Kim K, Chie EK, Jang JY, Kim SW, Oh DY, Im SA, Kim TY, Bang YJ, Ha SW. Role of adjuvant chemoradiotherapy for duodenal cancer: a single center experience. Am J Clin Oncol. 2012;35:533–536. doi: 10.1097/COC.0b013e31821dee31. [DOI] [PubMed] [Google Scholar]
- 73.Malleo G, Tonsi A, Marchegiani G, Casarotto A, Paiella S, Butturini G, Salvia R, Bassi C. Postoperative morbidity is an additional prognostic factor after potentially curative pancreaticoduodenectomy for primary duodenal adenocarcinoma. Langenbecks Arch Surg. 2013;398:287–294. doi: 10.1007/s00423-012-0978-9. [DOI] [PubMed] [Google Scholar]
- 74.Lee HG, You DD, Paik KY, Heo JS, Choi SH, Choi DW. Prognostic factors for primary duodenal adenocarcinoma. World J Surg. 2008;32:2246–2252. doi: 10.1007/s00268-008-9678-6. [DOI] [PubMed] [Google Scholar]
- 75.Gibson MK, Holcroft CA, Kvols LK, Haller D. Phase II study of 5-fluorouracil, doxorubicin, and mitomycin C for metastatic small bowel adenocarcinoma. Oncologist. 2005;10:132–137. doi: 10.1634/theoncologist.10-2-132. [DOI] [PubMed] [Google Scholar]
- 76.Sexe RB, Wade TP, Virgo KS, Johnson FE. Incidence and treatment of periampullary duodenal cancer in the U.S. veteran patient population. Cancer. 1996;77:251–254. doi: 10.1002/(SICI)1097-0142(19960115)77:2<251::AID-CNCR5>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 77.Liang TJ, Wang BW, Liu SI, Chou NH, Tsai CC, Chen IS, Yeh MH, Chen YC, Chang PM, Mok KT. Number of involved lymph nodes is important in the prediction of prognosis for primary duodenal adenocarcinoma. J Chin Med Assoc. 2012;75:573–580. doi: 10.1016/j.jcma.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 78.Struck A, Howard T, Chiorean EG, Clarke JM, Riffenburgh R, Cardenes HR. Non-ampullary duodenal adenocarcinoma: factors important for relapse and survival. J Surg Oncol. 2009;100:144–148. doi: 10.1002/jso.21319. [DOI] [PubMed] [Google Scholar]
- 79.Onkendi EO, Boostrom SY, Sarr MG, Farnell MB, Nagorney DM, Donohue JH, Kendrick ML, Reid-Lombardo KM, Harmsen WS, Que FG. 15-year experience with surgical treatment of duodenal carcinoma: a comparison of periampullary and extra-ampullary duodenal carcinomas. J Gastrointest Surg. 2012;16:682–691. doi: 10.1007/s11605-011-1808-z. [DOI] [PubMed] [Google Scholar]
- 80.Cecchini S, Correa-Gallego C, Desphande V, Ligorio M, Dursun A, Wargo J, Fernàndez-del Castillo C, Warshaw AL, Ferrone CR. Superior prognostic importance of perineural invasion vs. lymph node involvement after curative resection of duodenal adenocarcinoma. J Gastrointest Surg. 2012;16:113–120; discussion 120. doi: 10.1007/s11605-011-1704-6. [DOI] [PubMed] [Google Scholar]
- 81.Han SL, Cheng J, Zhou HZ, Zeng QQ, Lan SH. The surgical treatment and outcome for primary duodenal adenocarcinoma. J Gastrointest Cancer. 2009;40:33–37. doi: 10.1007/s12029-009-9073-z. [DOI] [PubMed] [Google Scholar]
- 82.Hurtuk MG, Devata S, Brown KM, Oshima K, Aranha GV, Pickleman J, Shoup M. Should all patients with duodenal adenocarcinoma be considered for aggressive surgical resection? Am J Surg. 2007;193:319–324; discussion 324-325. doi: 10.1016/j.amjsurg.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 83.Ryder NM, Ko CY, Hines OJ, Gloor B, Reber HA. Primary duodenal adenocarcinoma: a 40-year experience. Arch Surg. 2000;135:1070–1074; discussion 1074-1075. doi: 10.1001/archsurg.135.9.1070. [DOI] [PubMed] [Google Scholar]
- 84.Rotman N, Pezet D, Fagniez PL, Cherqui D, Celicout B, Lointier P. Adenocarcinoma of the duodenum: factors influencing survival. French Association for Surgical Research. Br J Surg. 1994;81:83–85. doi: 10.1002/bjs.1800810128. [DOI] [PubMed] [Google Scholar]
- 85.Delcore R, Thomas JH, Forster J, Hermreck AS. Improving resectability and survival in patients with primary duodenal carcinoma. Am J Surg. 1993;166:626–630; discussion 630-631. doi: 10.1016/s0002-9610(05)80668-3. [DOI] [PubMed] [Google Scholar]


