Abstract
Mental stress can trigger myocardial ischemia, but the prevalence of mental stress–induced ischemia in congestive heart failure (CHF) patients is unknown. We characterized mental stress–induced and adenosine-induced changes in myocardial perfusion and neurohormonal activation in CHF patients with reduced left-ventricular function using SPECT to precisely quantify segment-level myocardial perfusion.
Methods
Thirty-four coronary artery disease patients (mean age ± SD, 62 ± 10 y) with CHF longer than 3 mo and ejection fraction less than 40% underwent both adenosine and mental stress myocardial perfusion SPECT on consecutive days. Mental stress consisted of anger recall (anger-provoking speech) followed by subtraction of serial sevens. The presence and extent of myocardial ischemia was quantified using the conventional 17-segment model.
Results
Sixty-eight percent of patients had 1 ischemic segment or more during mental stress and 81% during adenosine. On segment-by-segment analysis, perfusion with mental stress and adenosine were highly correlated. No significant differences were found between any 2 time points for B-type natriuretic peptide, tumor necrosis factor-α, IL-1b, troponin, vascular endothelin growth factor, IL-17a, matrix metallopeptidase-9, or C-reactive protein. However, endothelin-1 and IL-6 increased, and IL-10 decreased, between the stressor and 30 min after stress. Left-ventricular end diastolic dimension was 179 ± 65 mL at rest and increased to 217 ± 71 after mental stress and 229 ± 86 after adenosine (P < 0.01 for both). Resting end systolic volume was 129 ± 60 mL at rest and increased to 158 ± 66 after mental stress (P < 0.05) and 171 ± 87 after adenosine (P < 0.07), with no significant differences between adenosine and mental stress. Ejection fraction was 30 ± 12 at baseline, 29 ± 11 with mental stress, and 28 ± 10 with adenosine (P = not significant).
Conclusion
There was high concordance between ischemic perfusion defects induced by adenosine and mental stress, suggesting that mental stress is equivalent to pharmacologic stress in eliciting clinically significant myocardial perfusion defects in CHF patients. Cardiac dilatation suggests clinically important changes with both conditions. Psychosocial stressors during daily life may contribute to the ischemic burden of CHF patients with coronary artery disease.
Keywords: heart failure, mental stress, ischemia, myocardial perfusion, adenosine, single-photon emission computed tomography
Mental stress can trigger myocardial perfusion defects and ischemia in a substantial percentage of patients with coronary artery disease (CAD) (1–4). Mental stress ischemia is predictive of an increased incidence of subsequent cardiac events and all-cause mortality (5–7). However, in stable CAD patients, mental stress ischemia is usually less prevalent, occurs at lower heart rates, often elicits smaller ischemic responses, and decreases in myocardial perfusion, compared with both exercise- and adenosine-induced ischemia, (1,4,8–11). Mechanistically, mental stress–induced ischemia has been attributed to increased hemodynamic responses (4,9,12,13), abnormal endothelial function (14–16), increased central nervous system and neuroendocrine activation (4,12,17,18), or increased inflammatory responses (19).
Most of these studies have been performed in patients with normal left-ventricular (LV) function, with the exception of 2 studies of patients with reduced LV function, many without heart failure (20,21). In contrast, the prevalence of mental stress–induced ischemia is unknown in patients with heart failure and CAD. Increased neuroendocrine activation in these patients could significantly influence the effects of mental stress on coronary blood flow and myocardial perfusion. These and other characteristics of heart failure pathophysiology might cause differences in cardiac responses to mental stress compared with the well-characterized effects of adenosine in heart failure patients.
Therefore, one goal of the current study was to characterize mental stress–induced myocardial ischemia in heart failure patients with reduced LV function using SPECT to objectively and precisely quantify segment-level myocardial perfusion. Second, we compared the provocative effects of mental stress to adenosine, a well-characterized pharmacologic stressor whose clinical predictive value is well established. Third, we examined potential hemodynamic and endocrine mechanisms of mental stress–induced decreases in myocardial perfusion.
MATERIALS AND METHODS
Design
As a substudy of the Behavioral Triggers of Heart Failure study of biobehavioral aspects of heart failure exacerbation, subjects were recruited from the Heart Failure Clinic at the University of Maryland Medical Center, Baltimore, Maryland. Patients with a history of congestive heart failure longer than 3 mo, ejection fraction less than 40% in the previous year, and proven CAD (by history of a myocardial infarction or angiography) were included in this study. After written informed consent was obtained from all subjects, participants were scheduled to undergo both mental stress and adenosine stress tests on consecutive days, in counterbalanced order. This study was approved by institutional review boards at the University of Maryland Medical Center and Uniformed Services University of the Health Sciences.
Procedure
On both testing days, patients were asked to fast from midnight the evening before the study and to delay taking their morning β-blocker and calcium channel blocker medications until after the lab testing phase. An intravenous cannula was inserted in the antecubital vein on arrival to permit blood draws during the testing procedure. Blood pressure was continually recorded every 2.5 min from the start of the rest period in both stress conditions (DynaPulse).
Adenosine and Mental Stress Testing
Patients rested for 30 min before mental stress or adenosine administration. For adenosine testing, patients were administered a standard gated 6-min adenosine stress test (Adenoscan; Astellas Pharma US). For mental stress testing, patients rested for 30 min before the mental stress, which consisted of an anger-recall task followed by a mental arithmetic task, both of which reliably elicit increased catecholamines, hemodynamic responses, and myocardial ischemia (1,4,9). For the anger-recall stressor, patients gave a 4-min speech about an anger-provoking incident, and for mental arithmetic, patients verbally subtracted serial sevens from a 4-digit number for 4 min while being urged to improve performance. Participants then rested through a 30-min recovery period (20).
Echocardiography
To evaluate cardiac output and systemic vascular resistance, transthoracic Doppler echocardiography (Vivid Seven: GE-Vingmed Ultrasound AS) was performed at baseline and during both stressors. Stroke volume was assessed by placement of a pulsed wave Doppler sample volume in the LV outflow tract proximate to the aortic valve; recorded systolic velocity integral was multiplied by the LV outflow tract area and calculated using measured LV outflow tract radius (r)′ as 2πr2, to obtain stroke volume. Cardiac output and systemic vascular resistance were determined at the time of recording.
SPECT Acquisition Protocol
Subjects underwent both adenosine and mental stress myocardial perfusion SPECT on consecutive days in randomized order. After a 30-min rest, 201Tl was injected and rest SPECT images acquired approximately 15 min later. After rest image acquisition was completed, patients either were administered adenosine or underwent mental stress induction. For adenosine testing, 99mTc-tetrofosmin was injected 3 min into the infusion protocol. For mental stress, 99mTc-tetrofosmin was injected 3 min into the mental stress task. Upon completion of the adenosine infusion or the mental stress task, SPECT images were acquired approximately 30 min after injection of the radiotracer.
Analysis of Myocardial Perfusion Images
Before analysis, all images were reviewed for technical quality by a technician masked to the study condition. Each scan provided color images of regional perfusion using a 17-segment model (20,22) whereby the segment with maximal mean counts per pixel was identified and designated as the normal reference segment(100% or peak activity). The remaining 16 segments were represented as a percentage of activity measured in the reference segment.
Myocardial Ischemia: Individual Segment Classification
To classify heart segments as being normal, ischemic, or scar tissue, a previously published scoring system was used (23–25). Namely, segments at baseline with 85% perfusion or greater were classified as normal. Segments 85% of peak activity or less were classified as abnormal. Segments that had both less than a 10% difference between rest and stress and less than 50% uptake at rest were classified as scar tissue. Segments with a 10% or greater change in uptake between rest and stress and displaying 50% or greater uptake at baseline were classified as ischemic. The number of normal, ischemic, and scar segments was calculated for each person using this scheme.
Myocardial Ischemia: Whole-Heart Metrics
Whole-heart metrics were calculated to draw more general conclusions about overall cardiac function. During stress, each segment was scored on a 5-point scale: 0, normal, indicated by a greater than 85% uptake during stress; 1, slight reduction of tracer uptake (equivocal), indicated by a greater than 50% uptake at baseline and between 71% and 85% uptake during stress; 2, moderate reduction of uptake (implying significant abnormality), indicated by a greater than 50% uptake at baseline and between 50% and 70% uptake during stress; 3, severe reduction of uptake, indicated by a greater than 50% uptake at baseline and less than 50% uptake during stress; and 4, absence of uptake, indicated by a less than 50% uptake at baseline and less than 50% uptake during stress, or the difference between baseline and stress uptake being less than 10% (23–25). Totaling scores for each segment generated a summed stress score, which was then used to classify patients as being normal or having mild, moderate, or severe reduction in radiotracer uptake; summed stress scores greater than 8 have been found to distinguish ischemic from nonischemic cardiomyopathy (26).
Markers of Vascular Dysfunction and Inflammation
A blood draw was performed before stress at the end of the 30-min rest period. Blood was also drawn 2–3 min after starting mental stress and at the end of the recovery period. Samples were analyzed for plasma B-type natriuretic peptide (BNP), a marker of LV wall stretch associated with heart failure severity (27), and serum C-reactive protein (CRP). Plasma levels of inflammatory markers (IL-6, tumor necrosis factor-α [TNF-α], IL-10, IL-1b, IL-17a, CRP), along with biomarkers of cardiac muscle damage (troponin), angiogenesis (vascular endothelin growth factor), vascular matrix protein degeneration (matrix metallopeptidase-9), and endothelial dysfunction (endothelin-1 [ET-1]), were also assayed.
Data Analysis
Data were analyzed using ANOVA, t tests, or χ2 as appropriate. Data are presented as mean ± SE of the mean or as percentages, unless otherwise noted. A 2-tailed α level of P less than 0.05 was used.
RESULTS
Patient Characteristics
Thirty-five patients were recruited for the study; 34 (31 men; mean age ± SD, 62 ± 10 y; age range, 45–85 y) were included in the analyses. One patient who remained hypertensive after the rest period before baseline readings was withdrawn from the study. Patient demographic and clinical characteristics at baseline are presented in Table 1. Although all patients had known coronary disease based on prior myocardial infarction or angiography, only 1 patient reported ongoing angina.
TABLE 1.
Patient Demographic and Clinical Characteristics
| Characteristic | n |
|---|---|
| Sex | |
| Male | 31 |
| Female | 3 |
| Race* | |
| African American | 15 |
| Caucasian | 19 |
| Age (y) | 62 ± 10 |
| New York Health Association class | |
| 2 | 19 (56%) |
| 3 | 15 (44%) |
| History of smoking | 28 (82%) |
| Diabetic | 23 (68%) |
| History of angina | 14 (41%) |
| History of atrial fibrillation | 7 (21%) |
| History of chronic obstructive pulmonary disease | 5 (15%) |
| History of hypercholesterolemia | 29 (85%) |
| History of hypertension | 28 (82%) |
| History of inducible ventricular tachycardia | 3 (9%) |
| History of valvular disease | 4 (12%) |
| Prior myocardial infarction | 27 (79%) |
| Body mass index | 29.8 ± 6.6 |
| Baseline ejection fraction (%) | 27 ± 9 |
| Baseline cardiac output | 3.30 ± 0.96 |
| Angiotensin-converting enzyme inhibitors | 27 (79%) |
| Angiotension II receptor blocker | 5 (15%) |
| β-blockers | 32 (94%) |
| Calcium channel blocker | 2 (6%) |
| Vasodilator | 7 (21%) |
| Baseline systolic blood pressure (mm Hg) | 118 ± 19 |
| Baseline diastolic blood pressure (mm Hg) | 70 ± 11 |
| Baseline heart rate (bpm) | 68 ± 12 |
Effects of mental stress might differ between races because of endocrine system differences.
Categoric data are expressed as numbers, followed by percentages in parentheses; continuous data are expressed as mean ± SE.
Hemodynamic Responses
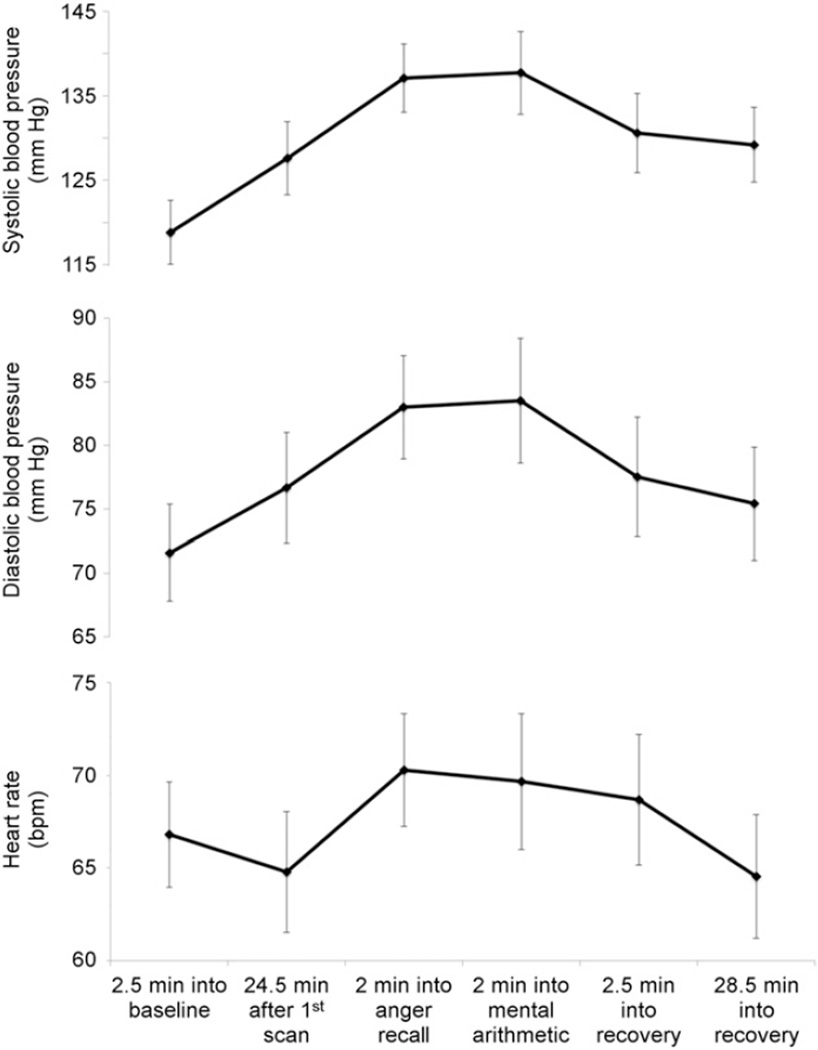
Baseline (resting) hemodynamics did not significantly differ between adenosine and mental stress (adenosine condition: systolic blood pressure [SBP] = 118 ± 19 mm Hg, diastolic blood pressure [DBP] = 70 ± 11 mm Hg, and heart rate [HR] = 68 ± 12 bpm; mental stress condition: SBP = 119 ± 22 mm Hg, DBP = 71 ± 14 mm Hg, and HR = 66 ± 14 bpm). Hemodynamics during the mental stress testing are presented in Figure 1; repeated-measures ANOVA revealed significant changes in SBP (P < 0.001), DBP (P < 0.001), and HR (P < 0.001).
FIGURE 1.
Blood pressure and heart rate during various points in study protocol, including minutes into rest, mental stress, and recovery. Both heart rate and blood pressure increased with stress, P < 0.001.
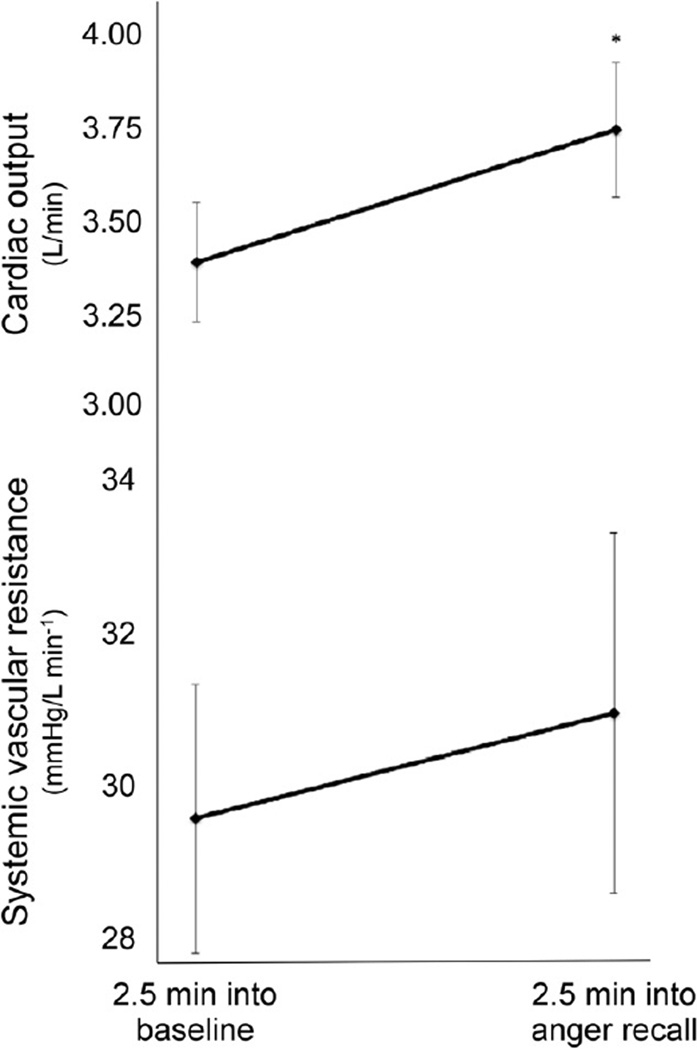
Systemic vascular resistance did not significantly change between baseline (29.8 mm Hg/L min−1) and mental stress (31.0 mm Hg/L min−1, P = 0.569). Cardiac output significantly increased between baseline (3.30 L/min) and mental stress (3.66 L/min, P = 0.048; Fig. 2).
FIGURE 2.
Cardiac output and systemic vascular resistance at baseline and 2 min into mental stress procedure. Increase in cardiac output, but not systemic vascular resistance, was statistically significant. *P < 0.05 as compared with baseline.
Self-Reported Stress
Participants were asked at baseline, during both mental stress tasks, and during recovery to rate their perceived stress on a 5-point Likert-type scale. Perceived stress was significantly higher during both mental stressors, compared with baseline and recovery periods (P < 0.001).
Quantifying Myocardial Ischemia: Whole-Heart Metrics
Using the whole-heart metrics classification for each of the 17 segments to generate a summed stress score to index flow, we classified 32 of 34 (94.1%) patients who completed the mental stress protocol and 29 of 31 (93.5%) patients who completed the adenosine protocol as ischemic with a summed stress score cutoff of 8 or less, indicating no difference in the proportion of ischemic patients between conditions (P = 0.51). By strict criteria evaluating each segment, of 34 patients completing the mental stress protocol, 23 had at least 1 ischemic segment (68%); 25 of 31 patients (81%) had at least 1 ischemic segment during adenosine (P = 0.06).
Cardiac Function
Ejection fraction at baseline was 30 ± 12 at baseline, 29 ± 11 with mental stress, and 28 ± 10 with adenosine (P = not significant). Resting end diastolic volume (EDV) was 179 ± 65 mL, and EDV was 217 ± 71 after mental stress and 229 ± 86 after adenosine. Resting end systolic volume (ESV) was 129 ± 60 mL. ESV was 158 ± 66 after mental stress and 171 ± 87 after adenosine. There were no statistically significant differences between adenosine and mental stress with regard to ESV or EDV. The differences between resting and mental stress were significant for both EDV and ESV (P < 0.01). The difference between resting and adenosine was significant for ESV (P < 0.05, and P = 0.07 for EDV).
Quantifying Myocardial Ischemia: Individual Segment Classification
Each segment was classified as having normal flow, ischemic, or fixed defect. The total number of each classification was then determined for all patients, and adenosine stress was compared with mental stress. The number of segments in each of the 3 segment groups is shown in Figure 3. Paired t tests revealed no significant differences in the number of normal, ischemic, or scarred segments between the 2 types of stressors (normal, P = 0.773; ischemic, P = 0.178; scar, P = 0.167). There was a strong correlation between adenosine and mental stress for the number of normal segments (Spearman ρ = 0.716, P < 0.001), ischemic segments (ρ = 0.743, P < 0.001), and scarred segments (ρ = 0.786, P < 0.001), indicating that segments that were ischemic during adenosine tended to be ischemic during mental stress.
FIGURE 3.
Mean percentage of segments classified as scar, ischemic, or normal during mental stress and after adenosine infusion. There were no differences between mental stress and adenosine interventions.
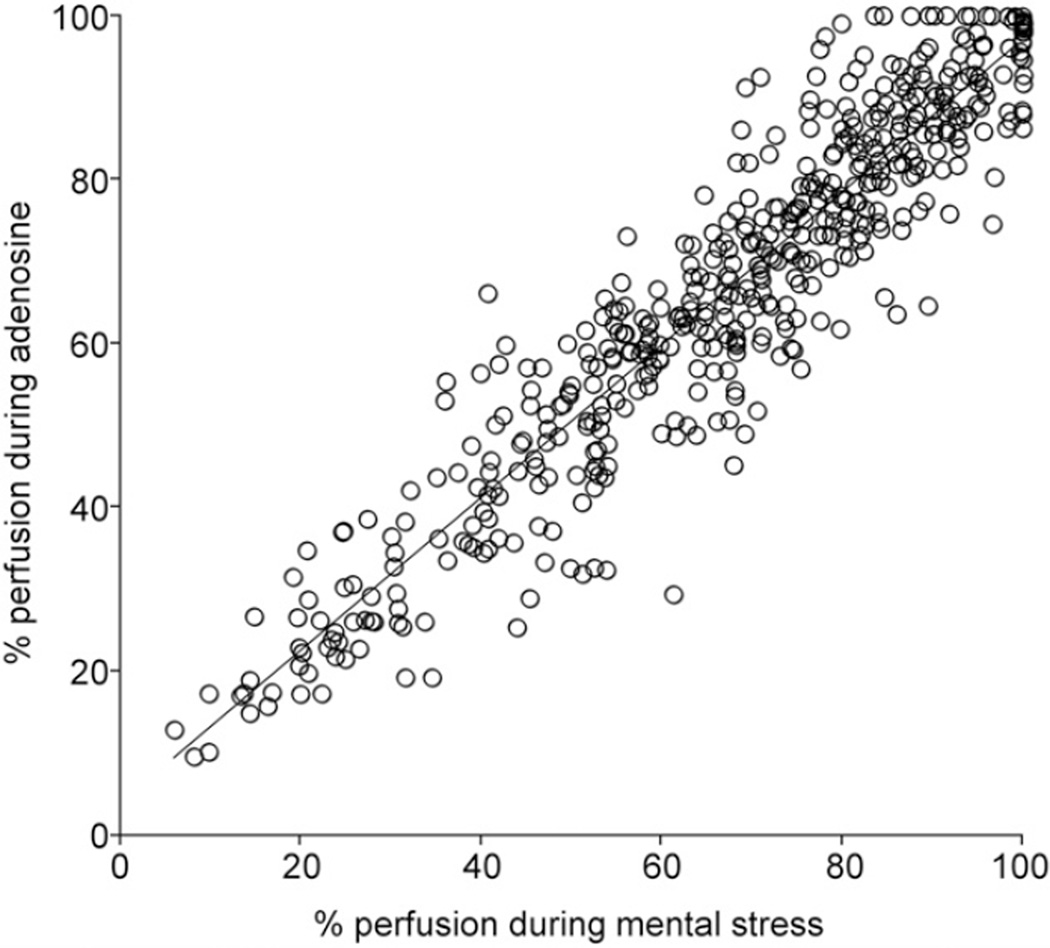
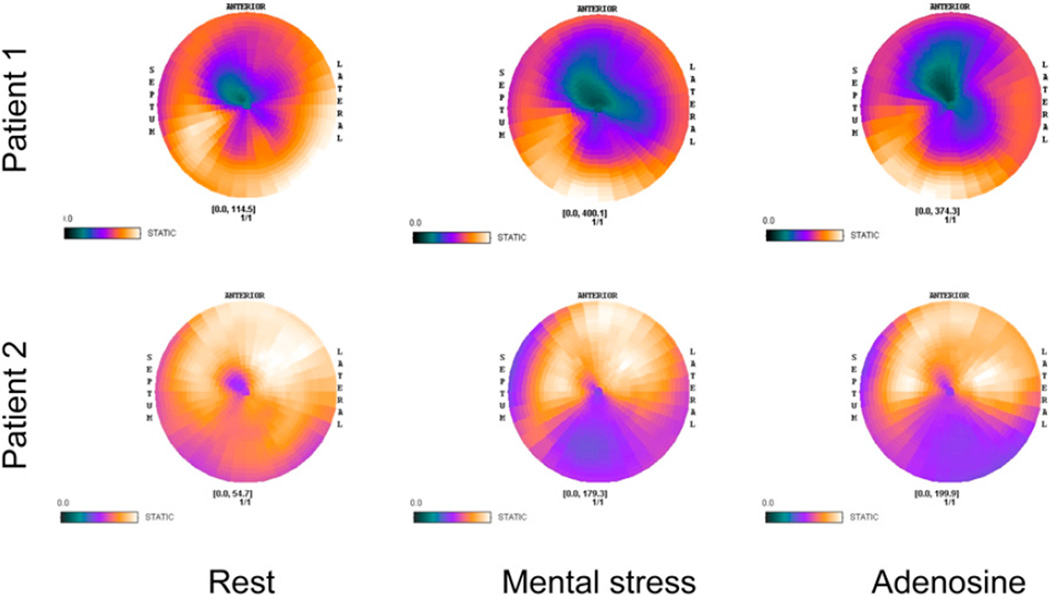
Segment-by-segment comparison between mental stress and adenosine indicated that perfusion was highly correlated for the 2 stress types (ρ range, 0.436–0.915, P < 0.013). Figure 4 presents perfusion concordance for each segment for all participants. Polar maps of rest, mental stress, and adenosine perfusion are shown for 2 patients in Figure 5.
FIGURE 4.
Scatterplot of percentage perfusion for mental stress and adenosine in same segments for each patient (n = 34 patients). There was excellent correlation between percentage perfusion during mental stress and percentage perfusion after adenosine infusion, r2 = 0.88.
FIGURE 5.
Polar maps of perfusion during rest, mental stress, and adenosine are shown for 2 patients. Darker colors indicate decreased perfusion.
Biomarkers During Rest, Stress, and Poststress Portions of Mental Stress Procedure
Mean values for each biomarker at each time point are presented in Table 2. No significant differences were found between any 2 time points for BNP, TNF-α, IL-1b, troponin, vascular endothelin growth factor, IL-17a, matrix metallopeptidase-9, or CRP. However, ET-1 and IL-6 significantly increased between the stressor and 30 min after stress (P = 0.028 and 0.046, respectively); IL-10 significantly decreased between the same time points (P = 0.035).
TABLE 2.
Neurohormonal Values at Rest, During Mental Stress, and After Stress
| Biomarker | Rest | Stress | After stress |
|---|---|---|---|
| BNP (pg/mL) | 410 ± 495 | 390 ± 516 | 412 ± 520 |
| IL-6 (pg/mL) | 5.96 ± 6.12 | 5.75 ± 5.91 | 6.27 ± 5.85* |
| TNF-α (pg/mL) | 6.45 ± 3.57 | 6.38 ± 3.69 | 5.85 ± 3.17 |
| IL-10 (pg/mL) | 1.69 ± 0.94 | 1.81 ± 0.96 | 1.71 ± 1.04* |
| IL-1b (pg/mL) | 0.18 ± 0.15 | 0.22 ± 0.21 | 0.24 ± 0.25 |
| High-sensitivity troponin (pg/mL) | 17.9 ± 28.4 | 16.8 ± 23.7 | 18.2 ± 28.5 |
| Vascular endothelin growth factor (pg/mL) | 55.5 ± 62.8 | 59.1 ± 50.8 | 58.3 ± 53.4 |
| IL-17a (pg/mL) | 0.31 ± 0.26 | 0.33 ± 0.27 | 0.33 ± 0.28 |
| Matrix metallopeptidase-9 (ng/mL) | 1,281 ± 1,094 | 1,326 ± 1,124 | 1,238 ± 1,058 |
| High-sensitivity CRP (mg/L) | 5.9 ± 7.3 | 5.7 ± 7.6 | 5.8 ± 7.8 |
| ET-1 (pg/mL) | 6.4 ± 2.6 | 6.1 ± 2.7 | 6.8 ± 2.8* |
P < 0.05, compared with stress value.
DISCUSSION
Using SPECT perfusion imaging, the present study found a high concordance between ischemic perfusion defects induced by adenosine compared with those induced by mental stress in patients with stable heart failure and CAD. This was true both in number of segments classified as normal or ischemic and in segment-by-segment analyses. Thus, in this sample, mental stress was equivalent to pharmacologic stress testing in eliciting clinically significant defects in myocardial perfusion. These findings suggest that psychosocial stressors during daily life may significantly contribute to the ischemic burden of heart failure patients with CAD.
Previous research has indicated that mental stress ischemia is of lesser severity compared with exercise- or pharmacologically induced ischemia (1,2,4,9). However, none of these studies had compared myocardial perfusion between these 2 types of stress in patients with heart failure and impaired LV function. Moreover, prior studies did not quantitatively examine direct segment-by-segment perfusion, which adds precision and allows a more fine-grained analysis of the effects of mental stress.
The perfusion similarities between these 2 methods for inducing ischemia is particularly notable given the differences in hemodynamic effects of mental stress and adenosine and the different mechanisms by which they produce ischemia. Mental stress increases blood pressure and heart rate, whereas adenosine, a potent vasodilator, is known to produce either few systemic effects or decreases in blood pressure (28,29). It has been suggested that mental stress reduces perfusion in CAD patients via decreases in myocardial blood flow due to endothelial dysfunction (16,30,31). However, the present study and others indicate that mental stress ischemia is also associated with substantial increases in blood pressure (4,9,32,33). One study (4) demonstrated that mental stress–induced wall motion abnormalities were associated with increases in systemic vascular resistance. However, in the present study of heart failure patients, only cardiac output significantly increased because of mental stress, potentially due to an increase in heart rate.
Differences between results of the present study and prior studies may be explained by physiologic characteristics associated with heart failure. Heart failure patients have different hemodynamics, reduced contractile function and reserve capacity, increased neurohormonal activity, and increased peripheral resistance. Medications taken by patients (e.g., diuretics and vasodilators) may also have reduced the effects of mental stress on cardiac output and systemic vascular resistance.
End systolic and diastolic dimensions were increased during the stress conditions, most markedly for mental stress. Cardiac dilatation with stress can reflect ischemia, as the dilatation is reduced after bypass surgery (34). Other possible causes include changes in preload or afterload. Vagal effects, such as after adenosine administration, may also occur and affect cardiac function (35).
Biomarkers
Overall, there were only 2 significant changes in inflammatory markers in response to the acute stress; IL-6 increased and IL-10 decreased between the stress and recovery periods. In a study of healthy young subjects, an increase in IL-6 production was seen with examination stress, and a high-anxiety response to stress was associated with lower IL-10 production (36). The time course seen in the present study was the same as the delayed response of IL-6 to mental stress in healthy men (37). Therefore, the present study findings are in line with the extant literature and extends them to a heart failure patient population.
It is noteworthy that ET-1 also increased with mental stress, which is consistent with previous studies showing increased systemic vascular resistance and blood pressure associated with mental stress ischemia. However, the present study did not observe a significant increase in total peripheral resistance with stress, and there was no demonstrable association between the change in ET-1 and changes in blood pressure. The lack of association between ET-1 and hemodynamics may reflect timing of biomarker measurement and the kinetics of the biomarkers in blood. In addition, actions of the biomarkers at the vascular level may not be adequately reflected in a single measurement at 30 min.
Although there was no overall change in BNP concentrations between baseline, peak stress, and recovery, the difference between baseline and recovery levels of BNP was negatively correlated with the number of normally perfused segments and positively correlated with number of scarred segments during adenosine and mental stress. There was no correlation with the number of ischemic segments, suggesting that mental stress in heart failure patients with LV scarring may be capable of further increasing LV filling pressure and potentiating heart failure exacerbation.
Several limitations of the study should be noted. The small sample size may limit the statistical power to observe changes in endocrine markers or hemodynamic variables. In addition, 31 of 34 subjects were men, and extrapolation to women therefore cannot be made. Furthermore, 68% of subjects had diabetes mellitus (Appendix), and this may affect the results. Regarding biomarkers, the protocol time course for measuring endocrine parameters may not have permitted sufficient time to elapse for changes in biomarker concentration to be assessed in blood. Previous studies have reported significant increases in IL-6 and IL-1Ra only at 2 h after the stressor but not at 45 min after the task (38).
CONCLUSION
The present study demonstrates that in patients with heart failure and CAD, mental stress–induced perfusion defects are remarkably similar, both in severity and in location, to changes induced by adenosine. This is the case despite differences in the hemodynamic profile associated with adenosine and mental stress. Changes in blood pressure due to mental stress were substantial, and heart rate increases were moderate but significant. Although some neurohormonal changes did occur, particularly in ET-1 and inflammatory markers, we did not observe associations between these biomarkers and hemodynamic or perfusion changes. These findings suggest that heart failure patients may be particularly vulnerable to ischemic episodes in the presence of mental stress and that stress-induced perfusion defects can be potent and clinically significant. Further studies are needed to determine the mechanisms associated with mental stress perfusion defects in heart failure patients and determine interventions to reduce the adverse effects of mental stress in these patients.
Acknowledgments
We thank Singulex Inc., Alameda, California, for biomarker analysis.
This study was supported by NHLBI grant 1R01 HL085730. The opinions and assertions expressed herein are those of the authors and do not necessarily express the views of USUHS or the U.S. Department of Defense.
APPENDIX
SPECT Acquisition Protocol
A dual-head SPECT camera was used in the cardiac 90° position of the thorax for 64 projections (20 s/projection). For thallium imaging, two energy windows were used: a 20% window centered at 70-keV peak and a 10% window centered at 167-keV peak. A 15% window centered on the 140 keV was used for the 99mTc-tetrofosmin SPECT study. Images were obtained with a 64 × 64 matrix, and preprocessing was performed using a Butterworth filter of order 5 and cut-off frequency of 40% Nyquist for 201Tl images. For 99mTc, a Butterworth filter of order 2.5 with cut-off at 60% Nyquist was used. Transaxial tomograms were constructed from back projection with a ramp filter and reoriented into short-axis images perpendicular to the long-axis left ventricle. Cardiac dimensions at end systole and diastole were determined, and ejection fraction was calculated. Because of a change in equipment and programs, dimensions and ejection fractions for all conditions (rest, adenosine, and mental stress) were available in only 18 patients.
The mean rest thallium administered dose was 124 MBq (3.35 mCi), mean pharmacologic stress 99mTc-tetrofosmin dose was 1,067 MBq (28.85 mCi), and mean mental stress 99mTc-tetrofosmin dose was 1,013 MBq (27.38 mCi). Estimated effective doses of these commonly performed diagnostic studies, taking into consideration tissue-weighting factors from the most recent International Commission on Radiological Protection publication for radiopharmaceuticals, are 16.2 ± 2.0 mSv for thallium, 6.9 ± 0.6 mSv for pharmacologic stress 99mTc-terofosmin, and 6.2 ± 0.5 mSv for mental stress 99mTc-tetrofosmin (39–41).
Acquisition of Biomarkers
For all blood draws, two 40-mL samples of venous blood were collected in 1 vacuum ethylenediaminetetraacetic acid (4.5 mmol/L) tube and 1 vacuum sodium citrate (32g/L) tube, inverted for 30 s to mix the reagents with the blood sample and kept at 4°C after collection and before separation. Plasma separation was performed by centrifugation at 4°C at 3,000g for 15 min. Aliquots of plasma and serum were stored at −80°C until analysis.
Samples were immediately placed on ice, centrifuged within an hour of collection, aliquoted, and stored at −80°C until analysis as per quality specifications for the specific assays. Samples were analyzed by laboratory personnel who were masked to the purpose of the study. BNP was measured using a commercially available fluorescence immunoassay kit (Beckman Coulter Access II; Beckman Coulter). Serum CRP was assayed with an ELISA kit (Dimension Vista 1500; Siemens). Plasma levels of IL-6, TNF-α, IL-10, IL-1b, IL-17a, troponin, vascular endothelin growth factor, matrix metallopeptidase-9, and ET-1 were assayed using the high-sensitivity Erenna System (Singulex Inc.). For the assays’ controls, the interassay coefficient of variation (%CV) ranged from 6.85% to 26.85%, and the intraassay %CV ranged from 7.35% to 17.71%, indicating high reliability and precision of the assays. The average interassay %CV for the samples was 13.33%. Inflammatory markers were log-transformed prior to analysis to normalize the data for analysis; mean levels of biomarkers are presented as raw values.
Footnotes
DISCLOSURE
No other potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Rozanski A, Bairey CN, Krantz DS, et al. Mental stress and the induction of silent myocardial ischemia in patients with coronary artery disease. N Engl J Med. 1988;318:1005–1012. doi: 10.1056/NEJM198804213181601. [DOI] [PubMed] [Google Scholar]
- 2.Strike PC, Steptoe A. Systematic review of mental stress-induced myocardial ischaemia. Eur Heart J. 2003;24:690–703. doi: 10.1016/s0195-668x(02)00615-2. [DOI] [PubMed] [Google Scholar]
- 3.Jiang W, Samad Z, Boyle S, et al. Prevalence and clinical characteristics of mental stress-induced myocardial ischemia in patients with coronary heart disease. J Am Coll Cardiol. 2013;61:714–722. doi: 10.1016/j.jacc.2012.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldberg AD, Becker LC, Bonsall R, et al. Ischemic, hemodynamic, and neurohormonal responses to mental and exercise stress: experience from the Psychophysiological Investigations of Myocardial Ischemia Study (PIMI) Circulation. 1996;94:2402–2409. doi: 10.1161/01.cir.94.10.2402. [DOI] [PubMed] [Google Scholar]
- 5.Jiang W, Babyak M, Krantz DS, et al. Mental stress-induced myocardial ischemia and cardiac events. JAMA. 1996;275:1651–1656. doi: 10.1001/jama.275.21.1651. [DOI] [PubMed] [Google Scholar]
- 6.Sheps DS, McMahon RP, Becker L, et al. Mental stress-induced ischemia and all-cause mortality in patients with coronary artery disease: results from the Psychophysiological Investigations of Myocardial Ischemia study. Circulation. 2002;105:1780–1784. doi: 10.1161/01.cir.0000014491.90666.06. [DOI] [PubMed] [Google Scholar]
- 7.Jain D, Burg M, Soufer R, Zaret BL. Prognostic implications of mental stress-induced silent left ventricular dysfunction in patients with stable angina pectoris. Am J Cardiol. 1995;76:31–35. doi: 10.1016/s0002-9149(99)80796-6. [DOI] [PubMed] [Google Scholar]
- 8.Krantz DS, Santiago HT, Kop WJ, Bairey Merz CN, Rozanski A, Gottdiener JS. Prognostic value of mental stress testing in coronary artery disease. Am J Cardiol. 1999;84:1292–1297. doi: 10.1016/s0002-9149(99)00560-3. [DOI] [PubMed] [Google Scholar]
- 9.Blumenthal JA, Jiang W, Waugh RA, et al. Mental stress-induced ischemia in the laboratory and ambulatory ischemia during daily life: association and hemodynamic features. Circulation. 1995;92:2102–2108. doi: 10.1161/01.cir.92.8.2102. [DOI] [PubMed] [Google Scholar]
- 10.Kim CK, Bartholomew BA, Mastin ST, Taasan VC, Carson KM, Sheps DS. Detection and reproducibility of mental stress-induced myocardial ischemia with tc-99m sestamibi SPECT in normal and coronary artery disease populations. J Nucl Cardiol. 2003;10:56–62. doi: 10.1067/mnc.2003.26. [DOI] [PubMed] [Google Scholar]
- 11.Hassan M, York KM, Li Q, Lucey DG, Fillingim RB, Sheps DS. Variability of myocardial ischemic responses to mental versus exercise or adenosine stress in patients with coronary artery disease. J Nucl Cardiol. 2008;15:518–525. doi: 10.1016/j.nuclcard.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burg MM, Soufer A, Lampert R, Collins D, Soufer R. Autonomic contribution to endothelin-1 increase during laboratory anger-recall stress in patients with coronary artery disease. Mol Med. 2011;17:495–501. doi: 10.2119/molmed.2010.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jain D, Shaker SM, Burg M, Wackers FJ, Soufer R, Zaret BL. Effects of mental stress on left ventricular and peripheral vascular performance in patients with coronary artery disease. J Am Coll Cardiol. 1998;31:1314–1322. doi: 10.1016/s0735-1097(98)00092-8. [DOI] [PubMed] [Google Scholar]
- 14.Toda N, Nakanishi-Toda M. How mental stress affects endothelial function. Pflugers Arch. 2011;462:779–794. doi: 10.1007/s00424-011-1022-6. [DOI] [PubMed] [Google Scholar]
- 15.Poitras VJ, Pyke KE. The impact of acute mental stress on vascular endothelial function: evidence, mechanisms and importance. Int J Psychophysiol. 2013;88:124–135. doi: 10.1016/j.ijpsycho.2013.03.019. [DOI] [PubMed] [Google Scholar]
- 16.Yeung AC, Vekshtein VI, Krantz DS, et al. The effect of atherosclerosis on the vasomotor response of coronary arteries to mental stress. N Engl J Med. 1991;325:1551–1556. doi: 10.1056/NEJM199111283252205. [DOI] [PubMed] [Google Scholar]
- 17.Soufer R, Jain H, Yoon AJ. Heart-brain interactions in mental stress-induced myocardial ischemia. Curr Cardiol Rep. 2009;11:133–140. doi: 10.1007/s11886-009-0020-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soufer R, Bremner JD, Arrighi JA, et al. Cerebral cortical hyperactivation in response to mental stress in patients with coronary artery disease. Proc Natl Acad Sci USA. 1998;95:6454–6459. doi: 10.1073/pnas.95.11.6454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kop WJ, Weissman NJ, Zhu J, et al. Effects of acute mental stress and exercise on inflammatory markers in patients with coronary artery disease and healthy controls. Am J Cardiol. 2008;101:767–773. doi: 10.1016/j.amjcard.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 20.Akinboboye O, Krantz DS, Kop WJ, et al. Comparison of mental stress-induced myocardial ischemia in coronary artery disease patients with versus without left ventricular dysfunction. Am J Cardiol. 2005;95:322–326. doi: 10.1016/j.amjcard.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 21.Cerqueira MD, Weissman NJ, Dilsizian V, et al. American Heart Association Writing Group on Myocardial Segmentation and Registration for Cardiac Imaging. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. AHA Writing Group on Myocardial Segmentation and Registration for Cardiac Imaging. Circulation. 2002;105:539–542. doi: 10.1161/hc0402.102975. [DOI] [PubMed] [Google Scholar]
- 22.Ramachandruni S, Fillingim RB, McGorray SP, et al. Mental stress provokes ischemia in coronary artery disease subjects without exercise- or adenosine-induced ischemia. J Am Coll Cardiol. 2006;47:987–991. doi: 10.1016/j.jacc.2005.10.051. [DOI] [PubMed] [Google Scholar]
- 23.Bonow RO, Dilsizian V, Cuocolo A, Bacharach SL. Identification of viable myocardium in patients with coronary artery disease and left ventricular dysfunction: comparison of thallium scintigraphy with reinjection and PET imaging with 18F-fluorodeoxyglucose. Circulation. 1991;83:26–37. doi: 10.1161/01.cir.83.1.26. [DOI] [PubMed] [Google Scholar]
- 24.Srinivasan G, Kitsiou AN, Bacharach SL, Bartlett ML, Miller-Davis C, Dilsizian V. 18F-fluorodeoxyglucose single photon emission computed tomography: can it replace PET and thallium SPECT for the assessment of myocardial viability? Circulation. 1998;97:843–850. doi: 10.1161/01.cir.97.9.843. [DOI] [PubMed] [Google Scholar]
- 25.Kitsiou AN, Srinivasan G, Quyyumi AA, Summers RM, Bacharach SL, Dilsizian V. Stress-induced reversible and mild-to-moderate irreversible thallium defects: are they equally accurate for predicting recovery of regional left ventricular function after revascularization? Circulation. 1998;98:501–508. doi: 10.1161/01.cir.98.6.501. [DOI] [PubMed] [Google Scholar]
- 26.Danias PG, Papaioannou GI, Ahlberg AW, et al. Usefulness of electrocardiographic- gated stress technetium-99m sestamibi single-photo emission computed tomography to differentiate ischemic from nonischemic cardiomyopathy. Am J Cardiol. 2004;94:14–19. doi: 10.1016/j.amjcard.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 27.Dao Q, Krishnaswamy P, Kazanegra R, et al. Utility of B-type natriuretic peptide in the diagnosis of congestive heart failure in an urgent-care setting. J Am Coll Cardiol. 2001;37:379–385. doi: 10.1016/s0735-1097(00)01156-6. [DOI] [PubMed] [Google Scholar]
- 28.Giannuzzi P, Shabetai R, Imparato A, et al. Effects of mental exercise in patients with dilated cardiomyopathy and congestive heart failure: an echocardiographic Doppler study. Circulation. 1991;83(4 suppl):II 155–II 165. [PubMed] [Google Scholar]
- 29.Schöder H, Silverman DH, Campisi R, et al. Effect of mental stress on myocardial blood flow and vasomotion in patients with coronary artery disease. J Nucl Med. 2000;41:11–16. [PubMed] [Google Scholar]
- 30.Wilson RF, Wyche K, Christensen BV, Zimmer S, Laxson DD. Effects of adenosine on human coronary arterial circulation. Circulation. 1990;82:1595–1606. doi: 10.1161/01.cir.82.5.1595. [DOI] [PubMed] [Google Scholar]
- 31.Dakak N, Quyyumi AA, Eisenhofer G, Goldstein DS, Cannon RO., III Sympathetically mediated effects of mental stress on the cardiac microcirculation of patients with coronary artery disease. Am J Cardiol. 1995;76:125–130. doi: 10.1016/s0002-9149(99)80043-5. [DOI] [PubMed] [Google Scholar]
- 32.Krantz DS, Helmers KF, Bairey CN, Nebel LE, Hedges SM, Rozanski A. Cardiovascular reactivity and mental stress-induced myocardial ischemia in patients with coronary artery disease. Psychosom Med. 1991;53:1–12. doi: 10.1097/00006842-199101000-00001. [DOI] [PubMed] [Google Scholar]
- 33.Holmes SD, Krantz DS, Kop WJ, Del Negro A, Karasik P, Gottdiener JS. Mental stress hemodynamic responses and myocardial ischemia: does left ventricular dysfunction alter these relationships? Psychosom Med. 2007;69:495–500. doi: 10.1097/PSY.0b013e3180cabc73. [DOI] [PubMed] [Google Scholar]
- 34.Harpole DH, Jr, Jones RH. Left ventricular function under stress before and after myocardial revascularization. Am Heart J. 1992;124:273–279. doi: 10.1016/0002-8703(92)90587-l. [DOI] [PubMed] [Google Scholar]
- 35.Kop WJ, Verdino RJ, Gottdiener JS, O’Leary ST, Bairey Merz CN, Krantz DS. Changes in heart rate and heart rate variability before ambulatory ischemic events. J Am Coll Cardiol. 2001;38:742–749. doi: 10.1016/s0735-1097(01)01451-6. [DOI] [PubMed] [Google Scholar]
- 36.Maes M, Song C, Lin A, et al. The effects of psychological stress on humans: increased production of pro-inflammatory cytokines and a Th1-like response in stress-induced anxiety. Cytokine. 1998;10:313–318. doi: 10.1006/cyto.1997.0290. [DOI] [PubMed] [Google Scholar]
- 37.von Känel R, Kudielka BM, Preckel D, Hanebuth D, Fischer JE. Delayed response and lack of habituation in plasma interleukin-6 to acute mental stress in men. Brain Behav Immun. 2006;20:40–48. doi: 10.1016/j.bbi.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 38.Steptoe A, Willemsen G, Owen N, Flower L, Mohamed-Ali V. Acute mental stress elicits delayed increases in circulating inflammatory cytokine levels. Clin Sci (Lond) 2001;101:185–192. [PubMed] [Google Scholar]
- 39.International Commission on Radiological Protection. Recommendations of the International Commission on Radiological Protection. ICRP publication 103. Ann ICRP. 2007;37:1–332. doi: 10.1016/j.icrp.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 40.Einstein AJ, Moser KW, Thompson RC, Cerqueira MD, Henzlova MJ. Radiation dose to patients from cardiac diagnostic imaging. Circulation. 2007;116:1290–1305. doi: 10.1161/CIRCULATIONAHA.107.688101. [DOI] [PubMed] [Google Scholar]
- 41.Laskey WK, Feinendegen LE, Neumann RD, Dilsizian V. Low-level ionizing radiation from non-invasive cardiac imaging: can we extrapolate estimated risks from epidemiologic data to the clinical setting? JACC Cardiovasc Imaging. 2010;3:517–524. doi: 10.1016/j.jcmg.2009.11.017. [DOI] [PubMed] [Google Scholar]