Abstract
The management of gynaecological malignancies has undergone a significant change in recent years with our improved understanding of cancer biogenetics, development of new treatment regimens and enhanced screening. Due to the rapid blooming of newer methods and techniques in gynaecology, surgery and oncology the scope and the role of imaging has also widened. Functional imaging in the form of diffusion weighted imaging (DWI) has been recently found to be very useful in assessing various tumours. Its ability to identify changes in the molecular level has dramatically changed the diagnostic approach of radiologists which was solely based on morphological criteria. It can improve the diagnostic accuracy of conventional magnetic resonance imaging, lend a hand in assessing tumour response to treatment regimens and detect tumour recurrence with better spatial resolution, negative radiation and diagnostic accuracy compared to positron emission tomography scan. The ability to quantify the diffusion has also lead to potential prediction of tumour aggressiveness and grade which directly correlate with the patient prognosis and management. Hence, it has become imperative for a radiologist to understand the concepts of DWI and its present and evolving role. In this article we present a brief description of the basics of DWI followed by its role in evaluation of female gynaecological malignancies.
Keywords: Diffusion weighted imaging, Female pelvis, Magnetic resonance imaging, Gynaecology, Malignancy
Core tip: With rapidly evolving strategies in the management of the gynaecological malignancies today there is an increasing role of imaging to keep up with the pace of development and develop newer techniques which help in better management of the patient. Diffusion weighted imaging (DWI) is one such modality which helps in detecting changes at the molecular level and hence can help in better diagnosis of early cancers as well as in assessing response to treatment. The purpose of this article is to make the reader familiar with the basic concepts of DWI and discuss the latest development in this field in evaluation of female gynaecological malignancies.
INTRODUCTION
Diffusion weighted imaging (DWI) has become an integral part of neuroimaging since the 1980’s. However its application in other areas apart from the brain has bloomed recently with the advent of faster imaging techniques and newer technologies like echo planar imaging, multichannel coils, parallel imaging and coils which can produce higher gradient amplitudes.
Its ability to assess changes in the molecular level in the brain has paved way for research in other areas such as oncologic imaging to try and further characterize these lesions and to augment morphological information derived from conventional imaging sequences. In this article we review briefly the basic principles of DWI and discuss its role in imaging of gynaecologic tumours.
Principle of diffusion weighted imaging
At molecular level in biologic tissues water molecules are in random motion called Brownian movement. When these water molecules are placed inside a magnetic field they acquire phase and precession frequency shift as time goes on when there is no restriction to their free movement.
However, the free movement of water molecules (Brownian movement) is dependent on various factors like cellular density, cell membrane integrity, intravascular space and the extracellular space. These factors may hinder or facilitate the movement of water molecules thus indirectly affecting their phase and precession frequencies.
DWI is basically a T2 weighted sequence in which we use two equal and opposite motion probing gradients before and after the 180 degree refocusing pulse. When the water molecules which are freely diffusing are exposed to the first pulse they acquire a phase shift which gradually changes and by the time the other equal and opposite gradient is applied they will not regain the signal; as because of free movement they are in different phases to time just after the first pulse hence no signal is produced at the time of acquisition, however water molecules which are static (diffusion restricted) regain signal as no significant phase shift has occurred by the time of second gradient and the signal loss from the first gradient is regained by the second opposite gradient.
The amplitude, duration and temporal spacing of the gradients are expressed as b value. At low b values back ground signal suppression is less due to T2 effects and at high b values there is good background signal suppression however at the expense of inherent signal loss.
Multiple b values images can be taken and the signal intensity at each value can be plotted on a graph and the log of the slope gives the Apparent Diffusion Coefficient (ADC) values. The ADC value is a quantitative measure of the diffusion in each pixel and it can also be represented as an image which can used in visual assessment of the diffusion value. The number of b values taken vary, generally more the b values more accurate is the calculated ADC value. In our institution we take three b values viz; 0, 400 and 800.
Protocol
DWI of the pelvis is usually single shot Echo Planar Imaging (EPI) sequence which gives excellent contrast to noise ratio (CNR) in spite of poor signal to noise ratio (SNR) due to very good background signal suppression and high tumour intensity. However they are very prone to spatial field distortions and susceptibility artefacts due to bowel movements. To minimize susceptibility artefacts a short TR and various parallel imaging techniques like sensitivity encoding, Generalized Autocalibrating Partial Parallelized Acquisitions are used. To increase the SNR; higher field strength MRI (3 T vs 1.5 T), reducing the echo time, increasing the signal averages, section thickness and a field of view can help. In our institution we perform scans using a 3 T system (Achieva 3T, Phillips Medical System); standard imaging parameters used are given in Table 1.
Table 1.
Parameters used for 3T magnetic resonance imaging
| Parameters | Values |
| Coil | Six channel SENSE body coil |
| TR | 3000 ms |
| TE | 50 ms |
| Flip angle | 90 deg |
| FOV | 35 cm × 21 cm × 21 cm |
| Number of excitations | 3 |
| Slice thickness | 5 |
| Interstice gap | 1 |
| Matrix | 128 × 128 |
| Bandwidth | 3018 Hz/pixel |
| Parallel imaging | SENSE ( factor 2) |
SENSE: Sensitivity encoding; FOV: Field of view.
Cervical cancer
Cervical cancer is the second most common cancer in woman and most common gynaecologic cancer in women worldwide. At present the management of cervical cancer is based on International Federation of Gynaecology and obstetrics (FIGO) clinical staging system because most of the disease burden falls on the developing countries where advanced imaging facilities are sparse and most of the patients present in a locally advanced stage[1]. However, with improved healthcare facilities for screening of early cancer and advanced treatment options; more and more early cervical cancers are diagnosed and fertility preserving surgeries are being done[2,3].
Currently clinical FIGO staging is used to assess these tumours. The clinical FIGO staging has an error rate of 20%-64%. Size of tumor, parametrial involvement, pelvic side wall invasion, bowel and bladder involvement and distant metastases which form important prognostic factors are not adequately evaluated clinically[4,5]. Moreover assessing bowel and bladder involvement by rectosigmoidoscopy and cystoscopy respectively may prove fallacious as only mucosal involvement can be picked up by these methods. Hence there is a need for advanced imaging techniques to detect and stage them accurately.
Cervical cancer is a solid tumour and like many of the malignancies has atypical cells with high nuclear cytoplasmic ratio which are closely packed inhibiting the free movement of water molecules at the microscopic level[6]. Consequently, diffusion weighted imaging has been found to be very helping in detection of cervical cancer (Figure 1). A meta-analysis of the available studies conducted by Hou et al[7] shows that cervical cancer tissue shows restricted diffusion and lower ADC as compared to normal cervical tissue. Other studies have also shown that diffusion weighted MRI helps in precise demarcation of the tumour margins with reduced rates of overestimating the tumour volume (as in T2w in which both edema and mass are hyper intense)[8-15].
Figure 1.
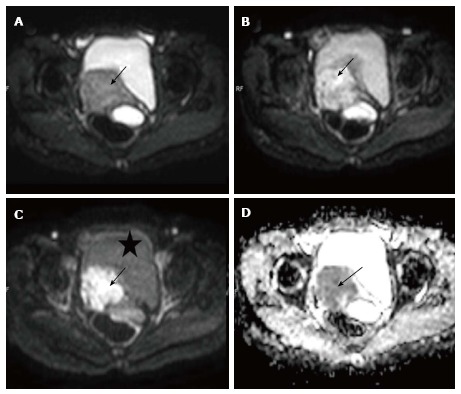
Carcinoma cervix. Axial diffusion weighted imagings of a biopsy proven case of carcinoma cervix show a mass (arrow) involving the cervix having hyper intense signal on b 0 (A), b 400 (B) and b 800 (C) images; Note the progressive loss of signal of the urinary bladder (marked as asterisk) as the B value increases; the mass is also hypointense on corresponding apparent diffusion coefficient image (D) confirming true diffusion restriction.
On conventional MRI the tumour is well delineated on T2 weighted sequence where it appears intermediate in signal intensity in the background T2 hypo intense cervical stroma. However in young patients where the cervical stroma is not very T2 hypo intense, the distinction is not very apparent. Also when there is diffusely infiltrating carcinoma or in early stages of tumour, conventional MRI fails to delineate the exact tumour extent[16,17]. Hence staging in such cases can be difficult and in such cases diffusion weighted MRI may help.
Diffusion weighted imaging is also being evaluated in trying to predict response to chemoradiotheraphy. Tumours with higher ADC at baseline are likely to be more necrotic and cystic and hence more hypoxic; due to these factors they may less responsive to chemoradiotherapy. A study by Liu et al[11] in 17 patients showed that pre-treatment ADC for complete response group was significantly lower than the partial response group (P = 0.005). Another study by Heo et al[18] showed mean ADC and 75th percentile ADC to be significantly higher in patients showing tumour recurrence (P = 0.043 and P = 0.008, respectively). The 75th percentile ADC was also a significant predictor of tumour recurrence in their study (P = 0.009; HR = 1.319). They also found that when the cut-off value of the 75th percentile ADC (0.936 × 10-3 mm2/s) was used, the overall recurrence free survival rate above the cut-off value was significantly lower than that below the cut-off value (51.9% vs 91.7%, P = 0.003, log-rank test)[18]. However studies in this regard are very few and more studies are needed to define the exact ADC cut off.
Chemo radiotherapy induces cell necrosis, apoptosis which in turn leads to cell lysis and increase in extracellular space in turn leading to increased water diffusion[19,20]. Hence, Tumour response especially early post radiation/chemotherapy can be potentially assessed by diffusion weighted imaging even when there is no visible morphological change in size of the tumour. Currently the Response Evaluation Criteria In Solid Tumours (RECIST) criteria which is used for treatment response is based on the reduction in size which takes time and dear time could be wasted when there is a immediate need to change the chemotherapy regimen. Various studies have shown the same and in such cases higher values of ADC can be due to tumour lysis and necrosis which suggests response and lower values suggest incomplete response[10,11,17,21-24]. However, Therapies targeted against tumour vasculature will reduce ADC values as perfusion component of the DWI calculation at low b values may be significant in the tumour, also contribution of the inflammatory component which can increase ADC values is unaccounted for in the studies done so far.
In the post chemoradiotherapy setting the detection of recurrent or residual tumour is of pristine importance. It is very difficult to differentiate on conventional MRI as both edema/inflammation as well as tumour appears alike on convention MR sequences (T1 and T2). PET which is commonly used in post treatment setting can be fallacious as it shows activity in residual tumour as well as inflammatory tissue. However recurrent/residual tumour shows lower ADC values as compared to the edematous/inflammatory tissue and can be used to diagnose recurrence or residual tumour[25].
Endometrial carcinoma
Endometrial carcinoma is one of the common gynaecological malignancy and is the most common gynaecological malignancy in Western countries[26]. Depth of tumour invasion into the myometrium and lymph node spread are two important parameters determining the management and prognosis of the patient[17,27]. Patients having greater depth of myometrial invasion and lymph node metastases have a higher incidence of recurrence and worse prognosis.
They are staged as 1a when the tumour is limited to the endometrium with the intact junctional zone. When there is breech of the junctional zone and the tumour invades into less than 50% of the myometrium it is staged as 1b and 1c when the depth of penetration into myometrium is greater than 50% (Figure 2)[27]. This differentiation can also be done on MRI with T2W and post gadolinium T1 weighted sequences. A systematic review and metaanalysis of nine studies done by Andreano et al[28] showed that DWI and dynamic Contrast Enhanced (DCE) imaging showed similar high sensitivity of 86% in predicting the preoperative stage of the disease. In cases where junctional zone is indistinct or thinned out as in post-menopausal females or in cases of adenomyosis when the size of the junctional zone is increased it may be difficult on DCE and T2W images alone to predict the depth of myometrial invasion. In such cases of diagnostic dilemma DWI can increase the diagnostic confidence[28,29].
Figure 2.
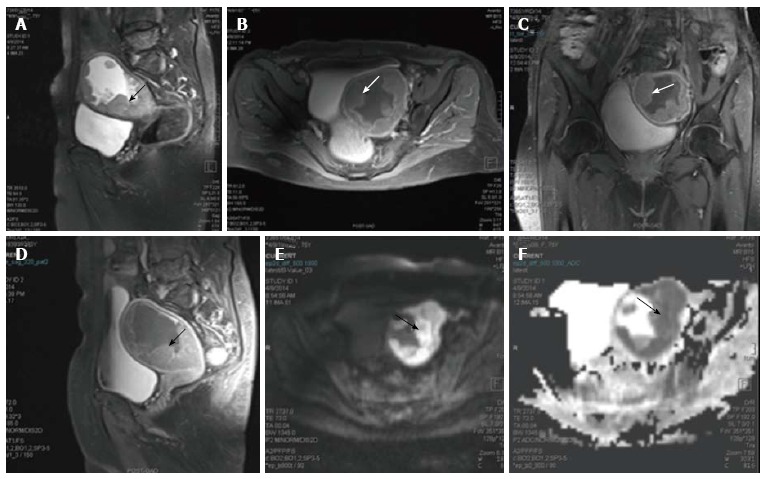
Endometrial carcinoma. Sagittal T2 weighted magnetic resonance image of a female who presented with menorrhagia. It shows irregular nodular thickening of the endometrial (arrow in A). The lesion on post contrast axial (B), coronal (C) and sagittal (D) images shows enhancement. On diffusion weighted imaging (E) and corresponding apparent diffusion coefficient (F) images, the lesion showed significant restriction of diffusion. The tumour is limited to the outer myometrium and no parametrial invasion noted suggesting a T1c disease. This lesion on biopsy turned out to be carcinoma endometrium.
In case of additional presence of benign lesions in myometrium, determining the depth of invasion is problem due to heterogenous appearance of the myometrium. Also in cases when there are large polypoidal tumours there occurs stretching of the junctional zone leading to under staging of the carcinoma of endometrium. In all such cases DWI acts as an important ancillary study to correctly stage the tumour. Correct staging is important as it determines management [type of hysterectomy- Radical vs total abdominal hysterectomy (TAH)] and also in prognosis of the carcinoma[17,27-29].
Another role of DWI is to differentiate residual tumour or recurrence from post-surgical/radiotherapy changes which leads to distortion of the normal anatomy. Since both the residual tumour and post-operative inflammatory/post radiotherapy changes look alike on conventional imaging (both are T2 hyper intense), it is the diffusion weighted imaging which makes a distinction. Any tumour recurrence or residual tumour will show restriction of diffusion with lower ADC values whereas inflammatory tissue will not show any restriction.
Uterine sarcomas
Uterine sarcomas are broadly divided into two groups as non-epithelial and mixed epithelial - non epithelial neoplasms according to their histological characteristics. There are four types which fall into these broad groups; they are leiomyosarcoma, endometrial stromal tumour (ESS), undifferentiated endometrial sarcoma (UES) and adenosarcoma.
It is of pristine importance to differentiate these lesions from benign lesions as their management differs and they are often equal on clinical manifestations and on routine ultrasound imaging[30]. A study showed that the mean ADC value of sarcomas was lower than that of normal myometrium and degenerated leiomyomas; also sarcomas showed higher signal intensity on DWI images; however ordinary leiomyomas and cellular leiomyomas also showed overlapping ADC values and hyper intensity on DWI. This can be due by “T2 black out effect”, since ordinary leiomyomas contain high hyalinized collagen they are hypointense on T2 weighted and DWI images leading to low ADC values.
Another study by Namimoto et al[31] showed similar findings however DWI combined with T2 weighted imaging showed improved diagnostic accuracy in their differentiation.
Thomassin-Naggara et al[32] in a retrospective study proposed a recursive partitioning model, using b 1000 signal intensity, T2 signal intensity, mean ADC, and patient age which correctly classified benign and malignant lesions in 92.4 % of cases.
Adenosarcomas are usually low grade tumours and typically present as polyps. They have been shown to have low ADC values in the limited studies available[33,34]. Overall studies trying to differentiate leiomyoma from sarcomas in terms of ADC values alone have been inconsistent with considerable overlap in values, ADC could be used as a adjuvant tool along with other parameters to increase the diagnostic confidence to characterize benign from malignant lesions[31-37].
Pelvic lymph node assessment
Pelvic lymph node involvement can also be easily assessed with diffusion weighted imaging. The most commonly used criteria for metastatic involvement on conventional MR sequences are morphology based which are node size > 10 mm, irregularly margins, contrast enhancement same as parent tumour tissue, extra nodal soft tissue and necrosis[17,21]. However various studies done shows that this is not an accurate method to detect metastasis[38-41]. Kitajima et al[41] found that in patients of endometrial and cervical cancer patients showed that DWI was better in detecting metastatic nodes compared to even PET CT (P < 0.005). However none were significantly adequate to replace lymphadenectomy. Hence DWI may serve as an important adjuvant method for diagnosis of lymph node involvement along with conventional imaging.
Ovarian cancer
Ovarian cancer is the fifth most common cancer in women worldwide. Very frequently women are diagnosed in late stages as it is most often asymptomatic in early stages[42]. Currently Ultrasound forms the initial imaging investigation of choice for the diagnosis of ovarian cancer; if the ultrasound is highly suspicious of ovarian cancer then patient directly undergoes abdominal CT scan followed by staging laprotomy. Features on ultrasound (US) that are suggestive of ovarian malignancy include an heteroechoic and irregular solid mass, an irregular multilocular cystic mass, presence of solid components or papillary projections in the cyst, high flow on colour doppler, ascitis, peritoneal nodules, and other evidence of metastatic disease in other organs[43]. However some of these masses remain indeterminate on USG and contrast enhanced MRI forms the investigation of choice in these patients. DWI can not only help in identifying the primary lesion (Figure 3) but also helps in identifying the deposits (Figures 3 and 4). Moreover, DWI can identify metastasis to ovary from other primaries (Figure 5).
Figure 3.
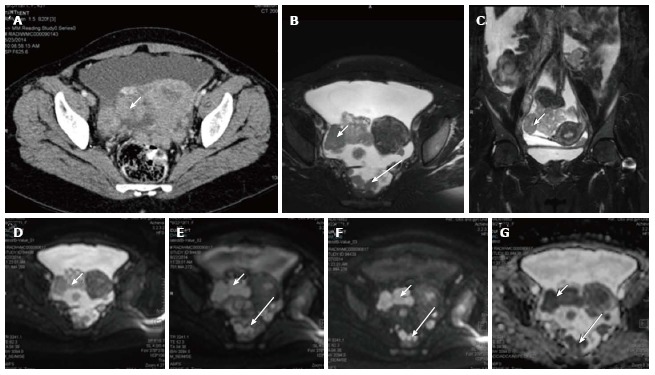
Carcinoma ovary. Axial contrast enhanced computed tomography image (A), T2 weighted axial (B) and coronal (C) images of a patient with known ovarian cancer shows right adnexal mass (arrows) multiple deposits in peritoneum of the pelvis (long arrows); On DWI, b 0 (D), b 400 (E), b 800 (F) and ADC (G) images show significant diffusion restriction in the primary (arrow) as well as peritoneal deposits (long arrow). These lesions were proven to be malignant deposits on fine needle aspiration cytology. ADC: Apparent diffusion coefficient; DWI: Diffusion weighted imaging.
Figure 4.
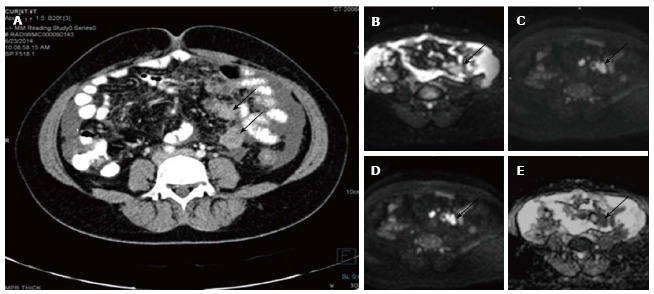
Serosal deposits in carcinoma ovary. Axial contrast enhanced computed tomography image (A) of a patient with known ovarian cancer shows enhancing soft tissues (arrows) over the surface of small bowel suggestive of serosal deposits. On diffusion weighted imaging, b 0 (B), b 400 (C), b 800 (D) and ADC (E) images show significant diffusion restriction in the serosal deposits. These lesions were proven to be malignant deposits on fine needle aspiration cytology. ADC: Apparent diffusion coefficient.
Figure 5.
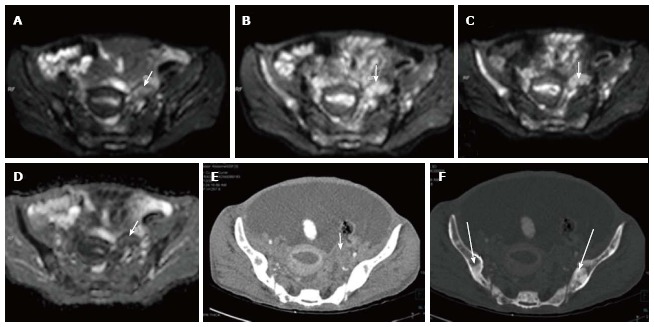
Left ovarian metastasis. Axial diffusion weighted imaging b 0 (A), b 400 (B), b 800 (C) and ADC (D) images of known case of carcinoma breast shows significant restricted diffusion of the left ovarian mass (arrow) suggesting left ovarian metastasis. Note is also made of multiple hypertense diffusion restricting lesions in the b/lilliac bone which were metastatic deposits. Axial contrast enhanced CT (E) showing the left adnexal mass (arrow) with multiple sclerotic metastases (thick arrows) in bilateral illiac bones better seen on CT bone window image (F). ADC: Apparent diffusion coefficient; CT: Computed tomography.
Studies on the utility of DWI to differentiate benign from malignant ovarian lesions have been conflicting. Study done by Katayama et al[44] showed DWI provided no additional advantage over conventional MRI sequences. Another study by Moteki et al[45] showed that in small and medium sized lesions sized less than 12 cm ADC values were significantly lesser in malignant lesions(P < 0.03).In this study the incorrectly higher ADC values in larger lesions > 12 cm were attributed to the sloshing effect intermittent compression of large ovarian lesions by abdominal breathing before the breath-hold scan[45]. In another study Nakayama et al[46] found that diffusion can be useful in fat poor teratomas as the keratinised content showed low ADC values. The problems in characterizing cystic lesions are further compounded by the susceptibility artifacts induced by the blood products in the hemorrhagic cysts and the endometrial cysts[44,45].
Peritoneal deposits
Gynaecological malignancies especially ovarian cancer is known to present with peritoneal and serosal deposits (Figures 3 and 4). This transcoelomic spread of cancer is many of the times first presentation and presence of such implants determine further management. Fujii et al[47] showed sensitivity and specificity of 90% and 95.5% respectively for diffusion weighted imaging in detecting such deposits. In another study done by Low et al[48] combined conventional and DWI imaging showed better sensitivity and accuracy than DWI alone in detecting peritoneal implants. DWI significantly increases accuracy in detecting deposits and can be done routinely to detect the same.
Diffusion-weighted whole-body imaging with background body signal suppression
Whole body magnetic resonance diffusion-weighted imaging with background signal suppression (MR-DWIBS) background signal suppression (MR-DWIBS) is an exciting new technique that has come into vogue. This technique has the potential to detect distant metastasis and can be used as a part of staging work up, look for treatment response and detect recurrence[49,50]. Three-dimensional (3D) display of DWI can produce positron emission tomography (PET) like images.
In a study involving mixed cancer patients; Komori et al[51] found that a larger number of malignant tumors were detected visually with whole-body DWI than with PET/CT. However, it was difficult to differentiate between benign and malignant lesions. This has been one of the drawbacks of DWIBS, many benign lesions as well as normal structures such as abscesses, brain, salivary glands, tonsils, spleen, gallbladder, small intestine/small intestinal contents, colon, adrenal glands, prostate, testes etc may all exhibit high signal intensity showing diffusion restriction and is very difficult to differentiate from malignant involvement[50]. Sugita et al[52] found PET/CT to be more reliable than DWI and contrast-enhanced CT (CE-CT) in the detection of peritoneal dissemination. The sensitivity of PET/CT, DWI, and CE-CT were 94%, 85%, and 83% and the specificity were 94%, 89% and 87%, respectively. Current research in this area is largely limited to mixed tumour group; more studies in this area and research on individual gynaecological tumours are needed to determine the exact utility.
Pitfalls of DWI
Tissues with long relaxation time may have high signal intensity on DWI images especially on low b value images due to a phenomenon known as T2 shine through. If a lesion is bright on both DWI and ADC images, then it’s likely due to shine through effect. This is a problem especially encountered in cystic lesions. In that situation, true diffusion can be found by correlating with the corresponding ADC map image to avoid T2 shine through[52].
On the other hand well-differentiated tumours may exhibit less restriction of diffusion due to their low cellularity. Other benign conditions such as blood (Figure 6), fat, abscesses, lymph nodes, and melanin may show restricted diffusion. Noting the baseline T1 and T2 images may help in arriving at the correct diagnosis.
Figure 6.
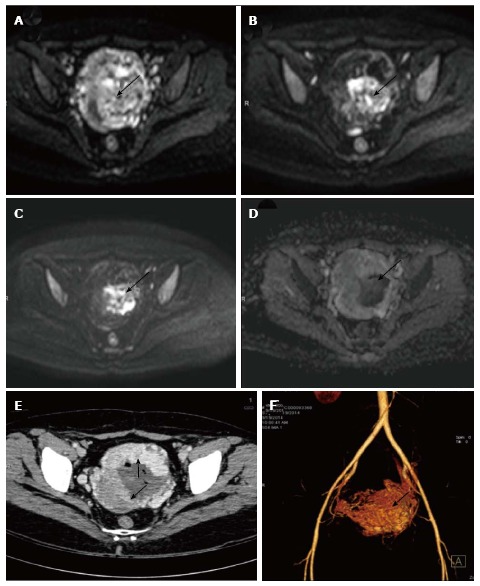
Gestational trophoblastic tumor. Axial diffusion weighted imaging b 0 (A), b 400 (B) and b 800 (C) and images of known case of gestational trophoblastic tumor shows significant restricted diffusion in the center of the mass (arrow). Note there is spurious restriction in ADC image (D). Axial contrast enhanced CT (E) showing the enhancing uterine mass (arrows) with nonenhancing central area likely hemorrhage which is responsible for the spurious diffusion restriction on DWI. CT angiography image (F) showing marked vascularity of the mass leading to intratumoral hemorrhage. ADC: Apparent diffusion coefficient; DWI: Diffusion weighted imaging; CT: Computed tomography.
The normal endometrium especially in secretory phase which is hyperplastic may show diffusion restriction due to very tightly packed cells and consequently will have low ADC values.
T2 shine-through effect may be produced by retained mucus and desmoplastic reaction post radiotheraphy may also show diffusion restriction however on high b value images (b1000 and above) they are no longer hyper intense.
Future direction
Although it has been used for evaluation of uterocervical as well as ovarian malignancies with inspiring results, paucity of studies is noted in the evaluation of vulvovaginal malignancies where deep spread can necessitate the need for cross sectional imaging. In ovarian cystic lesions haemorrhage often complicates the picture by causing susceptibility artifacts because of the EPI method used to acquire the DWI sequence. However with more and more advanced imaging techniques the artefacts can be reduced and it may pave the way for better characterization of ovarian cysts. The role of advanced diffusion imaging techniques such as diffusion tensor imaging, diffusion spectral imaging, diffusion weighted whole body imaging with background signal suppression (DWIBS) needs to be discovered in evaluating pelvic floor muscles and uterine musculatures before fertility preserving surgeries. These techniques have the potential to map the nerve fibre preoperatively which can guide the surgeons during surgery and thus can reduce post-operative complications.
CONCLUSION
In the last few years, DWI is gaining popularity and has been an important addition to the armamentarium of MRI. DWI has emerged as a robust tool for the evaluation of gynaecologic malignancies because of its ability to evaluate the functional status of the tumour similar to PET scan but without the risk of ionizing radiation. As the sequence takes very less time to acquire and is available in most of the 1.5 T and 3 T scanners, it is strongly recommended to be acquired along with routine MR sequences in evaluation of gynaecological malignancies.
Footnotes
Conflict-of-interest statement: There is no conflict of interest associated with any of the senior author or other coauthors contributed their efforts in this manuscript.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: May 25, 2015
First decision: September 23, 2015
Article in press: December 4, 2015
P- Reviewer: Cuce F, Erich C S- Editor: Qiu S L- Editor: A E- Editor: Lu YJ
References
- 1.Pecorelli S, Zigliani L, Odicino F. Revised FIGO staging for carcinoma of the cervix. Int J Gynaecol Obstet. 2009;105:107–108. doi: 10.1016/j.ijgo.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 2.Sankaranarayanan R, Esmy PO, Rajkumar R, Muwonge R, Swaminathan R, Shanthakumari S, Fayette JM, Cherian J. Effect of visual screening on cervical cancer incidence and mortality in Tamil Nadu, India: a cluster-randomised trial. Lancet. 2007;370:398–406. doi: 10.1016/S0140-6736(07)61195-7. [DOI] [PubMed] [Google Scholar]
- 3.Thulaseedharan JV, Malila N, Hakama M, Esmy PO, Cherian M, Swaminathan R, Muwonge R, Sankaranarayanan R. Effect of screening on the risk estimates of socio demographic factors on cervical cancer - a large cohort study from rural India. Asian Pac J Cancer Prev. 2013;14:589–594. doi: 10.7314/apjcp.2013.14.1.589. [DOI] [PubMed] [Google Scholar]
- 4.Creasman WT. New gynecologic cancer staging. Gynecol Oncol. 1995;58:157–158. doi: 10.1006/gyno.1995.1203. [DOI] [PubMed] [Google Scholar]
- 5.Piver MS, Chung WS. Prognostic significance of cervical lesion size and pelvic node metastases in cervical carcinoma. Obstet Gynecol. 1975;46:507–510. [PubMed] [Google Scholar]
- 6.Saslow D, Solomon D, Lawson HW, Killackey M, Kulasingam SL, Cain J, Garcia FA, Moriarty AT, Waxman AG, Wilbur DC, et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin. 2012;62:147–172. doi: 10.3322/caac.21139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hou B, Xiang SF, Yao GD, Yang SJ, Wang YF, Zhang YX, Wang JW. Diagnostic significance of diffusion-weighted MRI in patients with cervical cancer: a meta-analysis. Tumour Biol. 2014;35:11761–11769. doi: 10.1007/s13277-014-2290-5. [DOI] [PubMed] [Google Scholar]
- 8.Hoogendam JP, Klerkx WM, de Kort GA, Bipat S, Zweemer RP, Sie-Go DM, Verheijen RH, Mali WP, Veldhuis WB. The influence of the b-value combination on apparent diffusion coefficient based differentiation between malignant and benign tissue in cervical cancer. J Magn Reson Imaging. 2010;32:376–382. doi: 10.1002/jmri.22236. [DOI] [PubMed] [Google Scholar]
- 9.Charles-Edwards E, Morgan V, Attygalle AD, Giles SL, Ind TE, Davis M, Shepherd J, McWhinney N, deSouza NM. Endovaginal magnetic resonance imaging of stage 1A/1B cervical cancer with A T2- and diffusion-weighted magnetic resonance technique: effect of lesion size and previous cone biopsy on tumor detectability. Gynecol Oncol. 2011;120:368–373. doi: 10.1016/j.ygyno.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 10.Chen J, Zhang Y, Liang B, Yang Z. The utility of diffusion-weighted MR imaging in cervical cancer. Eur J Radiol. 2010;74:e101–e106. doi: 10.1016/j.ejrad.2009.04.025. [DOI] [PubMed] [Google Scholar]
- 11.Liu Y, Bai R, Sun H, Liu H, Wang D. Diffusion-weighted magnetic resonance imaging of uterine cervical cancer. J Comput Assist Tomogr. 2009;33:858–862. doi: 10.1097/RCT.0b013e31819e93af. [DOI] [PubMed] [Google Scholar]
- 12.Kilickesmez O, Bayramoglu S, Inci E, Cimilli T, Kayhan A. Quantitative diffusion-weighted magnetic resonance imaging of normal and diseased uterine zones. Acta Radiol. 2009;50:340–347. doi: 10.1080/02841850902735858. [DOI] [PubMed] [Google Scholar]
- 13.McVeigh PZ, Syed AM, Milosevic M, Fyles A, Haider MA. Diffusion-weighted MRI in cervical cancer. Eur Radiol. 2008;18:1058–1064. doi: 10.1007/s00330-007-0843-3. [DOI] [PubMed] [Google Scholar]
- 14.Naganawa S, Sato C, Kumada H, Ishigaki T, Miura S, Takizawa O. Apparent diffusion coefficient in cervical cancer of the uterus: comparison with the normal uterine cervix. Eur Radiol. 2005;15:71–78. doi: 10.1007/s00330-004-2529-4. [DOI] [PubMed] [Google Scholar]
- 15.Kuang F, Yan Z, Li H, Feng H. Diagnostic accuracy of diffusion-weighted MRI for differentiation of cervical cancer and benign cervical lesions at 3.0T: Comparison with routine MRI and dynamic contrast-enhanced MRI. J Magn Reson Imaging. 2015;42:1094–1099. doi: 10.1002/jmri.24894. [DOI] [PubMed] [Google Scholar]
- 16.Nougaret S, Tirumani SH, Addley H, Pandey H, Sala E, Reinhold C. Pearls and pitfalls in MRI of gynecologic malignancy with diffusion-weighted technique. AJR Am J Roentgenol. 2013;200:261–276. doi: 10.2214/AJR.12.9713. [DOI] [PubMed] [Google Scholar]
- 17.Sala E, Rockall A, Rangarajan D, Kubik-Huch RA. The role of dynamic contrast-enhanced and diffusion weighted magnetic resonance imaging in the female pelvis. Eur J Radiol. 2010;76:367–385. doi: 10.1016/j.ejrad.2010.01.026. [DOI] [PubMed] [Google Scholar]
- 18.Heo SH, Shin SS, Kim JW, Lim HS, Jeong YY, Kang WD, Kim SM, Kang HK. Pre-treatment diffusion-weighted MR imaging for predicting tumor recurrence in uterine cervical cancer treated with concurrent chemoradiation: value of histogram analysis of apparent diffusion coefficients. Korean J Radiol. 2013;14:616–625. doi: 10.3348/kjr.2013.14.4.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moffat BA, Chenevert TL, Meyer CR, McKeever PE, Hall DE, Hoff BA, Johnson TD, Rehemtulla A, Ross BD. The functional diffusion map: an imaging biomarker for the early prediction of cancer treatment outcome. Neoplasia. 2006;8:259–267. doi: 10.1593/neo.05844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moffat BA, Hall DE, Stojanovska J, McConville PJ, Moody JB, Chenevert TL, Rehemtulla A, Ross BD. Diffusion imaging for evaluation of tumor therapies in preclinical animal models. MAGMA. 2004;17:249–259. doi: 10.1007/s10334-004-0079-z. [DOI] [PubMed] [Google Scholar]
- 21.Whittaker CS, Coady A, Culver L, Rustin G, Padwick M, Padhani AR. Diffusion-weighted MR imaging of female pelvic tumors: a pictorial review. Radiographics. 2009;29:759–774; discussion 774-778. doi: 10.1148/rg.293085130. [DOI] [PubMed] [Google Scholar]
- 22.Punwani S. Diffusion weighted imaging of female pelvic cancers: concepts and clinical applications. Eur J Radiol. 2011;78:21–29. doi: 10.1016/j.ejrad.2010.07.028. [DOI] [PubMed] [Google Scholar]
- 23.Harry VN, Semple SI, Gilbert FJ, Parkin DE. Diffusion-weighted magnetic resonance imaging in the early detection of response to chemoradiation in cervical cancer. Gynecol Oncol. 2008;111:213–220. doi: 10.1016/j.ygyno.2008.07.048. [DOI] [PubMed] [Google Scholar]
- 24.Schreuder SM, Lensing R, Stoker J, Bipat S. Monitoring treatment response in patients undergoing chemoradiotherapy for locally advanced uterine cervical cancer by additional diffusion-weighted imaging: A systematic review. J Magn Reson Imaging. 2015;42:572–594. doi: 10.1002/jmri.24784. [DOI] [PubMed] [Google Scholar]
- 25.Nicolet V, Carignan L, Bourdon F, Prosmanne O. MR imaging of cervical carcinoma: a practical staging approach. Radiographics. 2000;20:1539–1549. doi: 10.1148/radiographics.20.6.g00nv111539. [DOI] [PubMed] [Google Scholar]
- 26.Cancer of the Endometrium. SEER Stat Fact Sheets online. Available from: http//seer.cancer.gov/statfacts/html/corp.html.
- 27.Freeman SJ, Aly AM, Kataoka MY, Addley HC, Reinhold C, Sala E. The revised FIGO staging system for uterine malignancies: implications for MR imaging. Radiographics. 2012;32:1805–1827. doi: 10.1148/rg.326125519. [DOI] [PubMed] [Google Scholar]
- 28.Andreano A, Rechichi G, Rebora P, Sironi S, Valsecchi MG, Galimberti S. MR diffusion imaging for preoperative staging of myometrial invasion in patients with endometrial cancer: a systematic review and meta-analysis. Eur Radiol. 2014;24:1327–1338. doi: 10.1007/s00330-014-3139-4. [DOI] [PubMed] [Google Scholar]
- 29.Hori M, Kim T, Onishi H, Imaoka I, Kagawa Y, Murakami T, Nakamoto A, Ueguchi T, Tatsumi M, Enomoto T, et al. Endometrial cancer: preoperative staging using three-dimensional T2-weighted turbo spin-echo and diffusion-weighted MR imaging at 3.0 T: a prospective comparative study. Eur Radiol. 2013;23:2296–2305. doi: 10.1007/s00330-013-2815-0. [DOI] [PubMed] [Google Scholar]
- 30.Wu TI, Yen TC, Lai CH. Clinical presentation and diagnosis of uterine sarcoma, including imaging. Best Pract Res Clin Obstet Gynaecol. 2011;25:681–689. doi: 10.1016/j.bpobgyn.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 31.Namimoto T, Yamashita Y, Awai K, Nakaura T, Yanaga Y, Hirai T, Saito T, Katabuchi H. Combined use of T2-weighted and diffusion-weighted 3-T MR imaging for differentiating uterine sarcomas from benign leiomyomas. Eur Radiol. 2009;19:2756–2764. doi: 10.1007/s00330-009-1471-x. [DOI] [PubMed] [Google Scholar]
- 32.Thomassin-Naggara I, Dechoux S, Bonneau C, Morel A, Rouzier R, Carette MF, Daraï E, Bazot M. How to differentiate benign from malignant myometrial tumours using MR imaging. Eur Radiol. 2013;23:2306–2314. doi: 10.1007/s00330-013-2819-9. [DOI] [PubMed] [Google Scholar]
- 33.Takeuchi M, Matsuzaki K, Yoshida S, Kudo E, Bando Y, Hasebe H, Kamada M, Nishitani H. Adenosarcoma of the uterus: magnetic resonance imaging characteristics. Clin Imaging. 2009;33:244–247. doi: 10.1016/j.clinimag.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 34.Yoshizako T, Wada A, Kitagaki H, Ishikawa N, Miyazaki K. MR imaging of uterine adenosarcoma: case report and literature review. Magn Reson Med Sci. 2011;10:251–254. doi: 10.2463/mrms.10.251. [DOI] [PubMed] [Google Scholar]
- 35.Saremi F, Knoll AN, Bendavid OJ, Schultze-Haakh H, Narula N, Sarlati F. Characterization of genitourinary lesions with diffusion-weighted imaging. Radiographics. 2009;29:1295–1317. doi: 10.1148/rg.295095003. [DOI] [PubMed] [Google Scholar]
- 36.Tamai K, Koyama T, Saga T, Morisawa N, Fujimoto K, Mikami Y, Togashi K. The utility of diffusion-weighted MR imaging for differentiating uterine sarcomas from benign leiomyomas. Eur Radiol. 2008;18:723–730. doi: 10.1007/s00330-007-0787-7. [DOI] [PubMed] [Google Scholar]
- 37.Zhang GF, Zhang H, Tian XM, Zhang H. Magnetic resonance and diffusion-weighted imaging in categorization of uterine sarcomas: correlation with pathological findings. Clin Imaging. 2014;38:836–844. doi: 10.1016/j.clinimag.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 38.Chen YB, Hu CM, Chen GL, Hu D, Liao J. Staging of uterine cervical carcinoma: whole-body diffusion-weighted magnetic resonance imaging. Abdom Imaging. 2011;36:619–626. doi: 10.1007/s00261-010-9642-4. [DOI] [PubMed] [Google Scholar]
- 39.Klerkx WM, Veldhuis WB, Spijkerboer AM, van den Bosch MA, Mali WP, Heintz AP, Bipat S, Sie-Go DM, van der Velden J, Schreuder HW, et al. The value of 3.0Tesla diffusion-weighted MRI for pelvic nodal staging in patients with early stage cervical cancer. Eur J Cancer. 2012;48:3414–3421. doi: 10.1016/j.ejca.2012.06.022. [DOI] [PubMed] [Google Scholar]
- 40.Lee SI, Catalano OA, Dehdashti F. Evaluation of gynecologic cancer with MR imaging, 18F-FDG PET/CT, and PET/MR imaging. J Nucl Med. 2015;56:436–443. doi: 10.2967/jnumed.114.145011. [DOI] [PubMed] [Google Scholar]
- 41.Kitajima K, Yamasaki E, Kaji Y, Murakami K, Sugimura K. Comparison of DWI and PET/CT in evaluation of lymph node metastasis in uterine cancer. World J Radiol. 2012;4:207–214. doi: 10.4329/wjr.v4.i5.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Trandafilovski P. [The intensity of bronchial hyperreactivity and the evolution of pulmonary function] Plucne Bolesti. 2011;42:36–37. [PubMed] [Google Scholar]
- 43.Mohaghegh P, Rockall AG. Imaging strategy for early ovarian cancer: characterization of adnexal masses with conventional and advanced imaging techniques. Radiographics. 2012;32:1751–1773. doi: 10.1148/rg.326125520. [DOI] [PubMed] [Google Scholar]
- 44.Katayama M, Masui T, Kobayashi S, Ito T, Sakahara H, Nozaki A, Kabasawa H. Diffusion-weighted echo planar imaging of ovarian tumors: is it useful to measure apparent diffusion coefficients? J Comput Assist Tomogr. 2002;26:250–256. doi: 10.1097/00004728-200203000-00015. [DOI] [PubMed] [Google Scholar]
- 45.Moteki T, Ishizaka H. Diffusion-weighted EPI of cystic ovarian lesions: evaluation of cystic contents using apparent diffusion coefficients. J Magn Reson Imaging. 2000;12:1014–1019. doi: 10.1002/1522-2586(200012)12:6<1014::aid-jmri29>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 46.Nakayama T, Yoshimitsu K, Irie H, Aibe H, Tajima T, Nishie A, Asayama Y, Matake K, Kakihara D, Matsuura S, et al. Diffusion-weighted echo-planar MR imaging and ADC mapping in the differential diagnosis of ovarian cystic masses: usefulness of detecting keratinoid substances in mature cystic teratomas. J Magn Reson Imaging. 2005;22:271–278. doi: 10.1002/jmri.20369. [DOI] [PubMed] [Google Scholar]
- 47.Fujii S, Matsusue E, Kanasaki Y, Kanamori Y, Nakanishi J, Sugihara S, Kigawa J, Terakawa N, Ogawa T. Detection of peritoneal dissemination in gynecological malignancy: evaluation by diffusion-weighted MR imaging. Eur Radiol. 2008;18:18–23. doi: 10.1007/s00330-007-0732-9. [DOI] [PubMed] [Google Scholar]
- 48.Low RN, Sebrechts CP, Barone RM, Muller W. Diffusion-weighted MRI of peritoneal tumors: comparison with conventional MRI and surgical and histopathologic findings--a feasibility study. AJR Am J Roentgenol. 2009;193:461–470. doi: 10.2214/AJR.08.1753. [DOI] [PubMed] [Google Scholar]
- 49.Ho KC, Lin G, Wang JJ, Lai CH, Chang CJ, Yen TC. Correlation of apparent diffusion coefficients measured by 3T diffusion-weighted MRI and SUV from FDG PET/CT in primary cervical cancer. Eur J Nucl Med Mol Imaging. 2009;36:200–208. doi: 10.1007/s00259-008-0936-5. [DOI] [PubMed] [Google Scholar]
- 50.Kwee TC, Takahara T, Ochiai R, Nievelstein RA, Luijten PR. Diffusion-weighted whole-body imaging with background body signal suppression (DWIBS): features and potential applications in oncology. Eur Radiol. 2008;18:1937–1952. doi: 10.1007/s00330-008-0968-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Komori T, Narabayashi I, Matsumura K, Matsuki M, Akagi H, Ogura Y, Aga F, Adachi I. 2-[Fluorine-18]-fluoro-2-deoxy-D-glucose positron emission tomography/computed tomography versus whole-body diffusion-weighted MRI for detection of malignant lesions: initial experience. Ann Nucl Med. 2007;21:209–215. doi: 10.1007/s12149-007-0010-6. [DOI] [PubMed] [Google Scholar]
- 52.Sugita R, Ito K, Fujita N, Takahashi S. Diffusion-weighted MRI in abdominal oncology: clinical applications. World J Gastroenterol. 2010;16:832–836. doi: 10.3748/wjg.v16.i7.832. [DOI] [PMC free article] [PubMed] [Google Scholar]


