Abstract
The transient dermal exposure is one where the skin is exposed to chemical for a finite duration, after which the chemical is removed and no residue remains on the skin’s surface. Chemical within the skin at the end of the exposure period can still enter the systemic circulation. If it has some volatility, a portion of it will evaporate from the surface before it has a chance to be absorbed by the body. The fate of this post-exposure “skin depot” is the focus of this theoretical study. Laplace domain solutions for concentration distribution, flux, and cumulative mass absorption and evaporation are presented, and time domain results are obtained through numerical inversion. The Final Value Theorem is applied to obtain the analytical solutions for the total fractional absorption by the body and evaporation from skin at infinite time following a transient exposure. The solutions depend on two dimensionless variables: χ, the ratio of evaporation rate to steady-state dermal permeation rate; and the ratio of exposure time to membrane lag time. Simple closed form algebraic equations are presented that closely approximate the complete analytical solutions. Applications of the theory to the dermal risk assessment of pharmaceutical, occupational, and environmental exposures are presented for four example chemicals.
Keywords: absorption potential, transdermal, passive diffusion/transport, percutaneous, skin, solvent evaporation
INTRODUCTION
Recent analyses have advanced our understanding of the absorption of chemicals in contact with skin from finite dose1–5 and transient exposures.6,7 The former is characterized as an exposure to a small (finite) dose (mass/area) of chemical, the disposition of which has been shown to depend on the relative rates of evaporation and permeation as well as the initial load. The finite dose is a good model for splash-type exposures in the workplace and also for pharmaceutical and cosmetic product applications. The transient exposure is one where the skin is exposed to chemical for a finite duration, after which the chemical is removed and no residue remains atop the surface. Chemical within the skin at the end of the exposure period can still enter the systemic circulation. If it has some volatility, a portion of it will evaporate from the skin surface before it has a chance to be absorbed by the body. As an example that is relevant to dermal risk assessment, consider bathing or showering with contaminated water. Dermal absorption proceeds for the duration of the exposure, but once the bath or shower has ended, contaminant residing within the skin may still be absorbed by the body while some may evaporate into the surrounding air. The fate of this post-exposure “skin depot” is the focus of this theoretical study.
Frasch and Barbero7 provided analytical solutions for total mass absorbed by the body (exposure duration plus post-exposure) for the extreme cases of non-volatile and infinitely volatile chemicals. N’Dri-Stempfer and Bunge6 presented finite difference post-transient exposure solutions for chemicals of varying volatility. Based on the numerical results, they derived a four-parameter empirical equation to predict post-exposure evaporation expressed as a fraction of the mass residing within the membrane at the conclusion of the exposure time. Herein, we derive the complete analytical solutions for fractional absorption by the body and evaporation from skin for variable volatility. In this study, as in previous ones, the skin is considered to be a single pseudo-homogeneous membrane.
THEORY
It is assumed here that the skin is transiently exposed to a (possibly) volatile chemical. At the end of the exposure period, the skin is efficiently decontaminated such that zero residual chemical remains on the surface. We wish to determine the disposition of chemical residing within the skin following this exposure.
With the exception of highly lipophilic chemicals, the main barrier property of the skin is imparted by the stratum corneum (SC). In its simplest form, the SC may be considered to be a uniform effective medium of thickness h, occupying the space between x = 0 (the skin surface) and x = h (bottom of tissue). The permeant has an effective diffusivity D that does not vary with position or time. This implies that neither the permeant nor its vehicle alter the SC permeability. The SC, initially free of chemical, is exposed to a constant concentration in vehicle Cv for a specified duration texp. It is assumed that the chemical does not bind to the SC and that the dermal vasculature acts as a perfect sink at the bottom of the tissue.
With these stipulations, post-exposure (t ≥ texp) permeant transport is governed by the one-dimensional diffusion equation:
| (1) |
with the initial condition:
| (2) |
where Kmv is the membrane–vehicle partition coefficient and the lag time . The boundary conditions are:
| (3) |
where
| (4) |
The parameter χ is the dimensionless ratio of the evaporation rate to the steady-state dermal absorption rate of the permeant, and is discussed in detail elsewhere.2,6 Physically, χ describes the post-exposure conditions at the skin surface and its value, depending on the situation, can vary from zero, representing zero flux from the skin surface, to infinity, which corresponds to zero concentration (sink conditions) on the skin surface. In addition to representing chemicals that evaporate rapidly from the skin surface, χ → ∞ mathematically describes a situation in which a chemical is removed from the skin with a continuous rinse or solvent immersion. In instances where chemical volatility is equated with χ in this manuscript, it is understood that volatility is relative to the dermal absorption rate. Any two chemicals may have similar evaporation rates, but χ will differ if the dermal absorption rates differ.
The initial concentration distribution specified by Eq. (2) is given by Crank.8 Note that the initial mass (per unit area) within the SC, that is, the total mass at the end of the exposure time, is:
| (5) |
where the permeability coefficient kp = KmvD/h.
METHODS
The solution of Eq. (1) with associated initial and boundary conditions was undertaken using the method of Laplace transforms. Solutions for concentration distribution, flux, and cumulative mass absorption and evaporation are presented. Time domain solutions were obtained through numerical inversion of the Laplace domain equations using Scientist (MicroMath Scientific Software, St. Louis, Missouri). The Final Value Theorem was applied to obtain total cumulative mass absorption and evaporation at infinite time postexposure, expressed as fractions of the total mass within the skin at the end of the exposure time. Simple closed form algebraic equations are presented that closely approximate the complete analytical solutions. For practical applications, the time it takes for the body to absorb 90% of the total infinite-time amount was estimated.
RESULTS
The Laplace transform of Eq. (1) is:
| (6) |
with the hat (ˆ) indicating a function of the Laplace variable s, and . The Laplace transform of Eq. (3) is:
| (7) |
The solution of Eq. (6) with specified initial (Eq. (2)) and boundary (Eq. (7)) conditions is:
| (8) |
with
| (9) |
where kn = nπ/h, and
| (10) |
Ĉp (x, s) is the particular solution to Eq. (6). Its value depends on the specific form taken by the nonhomogeneous terms (here, −C0(x)/D). Tables of solutions are available in a number of sources (e.g., the CRC Standard Mathematical Tables9).
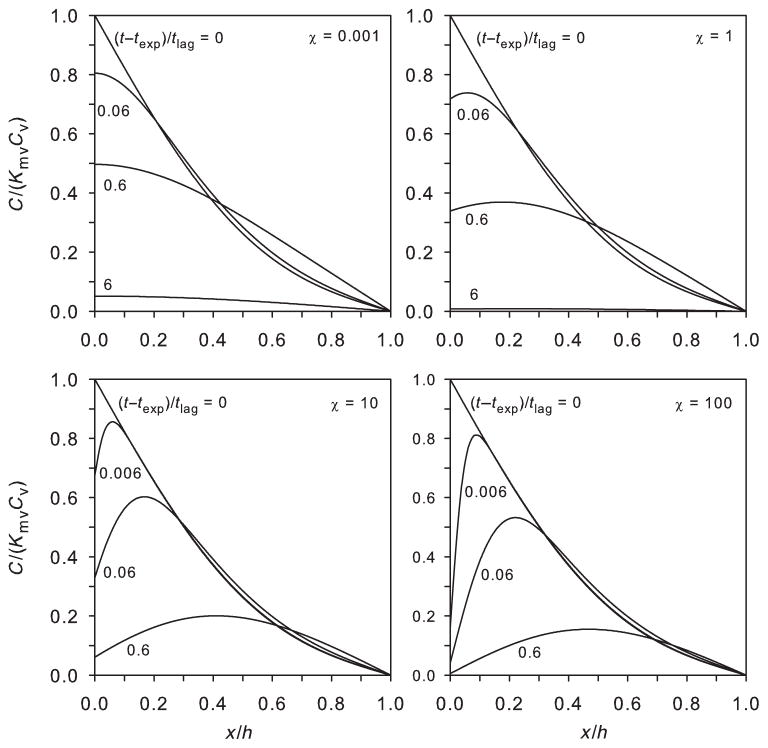
Figure 1 shows plots of C(x, t) within the membrane for various values of χ. The plots represent inverse Laplace transforms of Eq. (8). For small χ, there is little evaporation and the chemical concentration is greatest at the skin surface. The time to clear chemical from the SC is relatively long. For large χ, most of the chemical within the skin at the end of the exposure evaporates. Chemical concentration is greatest in the mid to upper portions of the skin, and the clearance time is relatively short.
Figure 1.
Dimensionless concentration distributions at relative positions within the membrane for varying times after an exposure of texp/tlag = 0.6. Different values of χ are shown, representing non-volatile (χ = 0.001), semivolatile (χ = 1, 10), and highly volatile (χ = 100) compounds. Plots represent inverse Laplace transforms of Eq. (8).
In the Laplace domain, the flux is given by:
| (11) |
with
| (12) |
The cumulative mass absorption by the body (mass/area) is given by:
| (13) |
and the cumulative mass evaporation from the skin surface is given by:
| (14) |
The convention that the x axis points into the skin necessitates the minus sign in Eq. (14) because efflux from the skin by evaporation corresponds to transport in the −x direction.
Figure 2 shows plots of normalized absorption by the body over time (mabs(t)/ m0) and evaporation from the surface (mevap (t) /m0) for several values of χ when texp/tlag = 0.6. Plots were obtained from the inverse Laplace transforms of Eqs. (13) and (14), both divided by Eq. (5). For a poorly volatile permeant (small χ), nearly all of the chemical within the skin at the end of the exposure time is eventually absorbed by the body. For highly volatile compounds (large χ), evaporation is substantial and under the exposure conditions shown in Figure 2, only about 27% of the initial skin amount is absorbed. Compared with a small χ permeant, the time to completely distribute the chemical from the SC is shorter.
Figure 2.
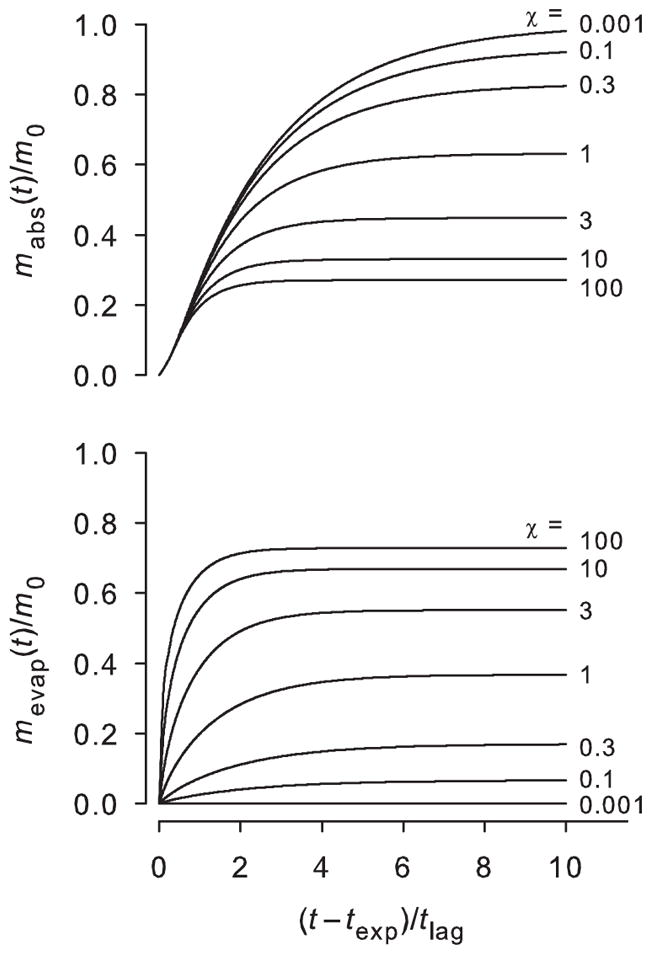
Mass absorption into the body (top) and mass evaporation from the skin surface (bottom) over time following an exposure of texp/tlag = 0.6 for various values of χ. Plots represent inverse Laplace transforms of Eqs. (13) and (14), normalized by Eq. (5).
The total amount absorbed by the body (per area), after infinite time may be calculated from the Final Value Theorem:
| (15) |
The result is given here as the fraction of the initial amount present in the membrane at the end of the exposure duration (Eq. (5)) that absorbs into the body:
| (16) |
At infinite time, all permeant has either been absorbed or evaporated. Therefore, the fraction that evaporates is:
| (17) |
Detailed solutions are available as Supplementary Materials (Part 1). Note that the limits for long exposure times (texp ≫ tlag) are:
| (18) |
| (19) |
Thus, the fraction of what is in the skin following a long exposure that absorbs into the body is between 1/3, for a highly volatile chemical, and one, for a non-volatile chemical.
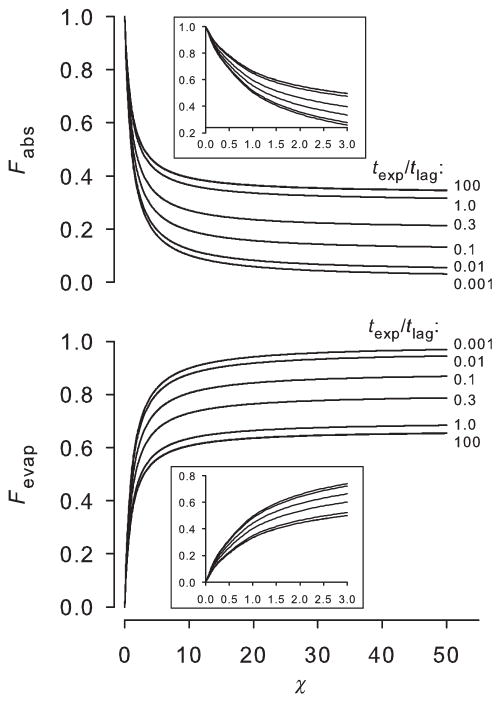
Figure 3 shows the solutions to Eqs. (16) and (17) for the first 100 terms in the series, for which the quantities are independent of the number of terms to at least six significant figures for texp/tlag ≥ 0.001. For a poorly volatile permeant (small χ), nearly all of the chemical within the skin at the end of the exposure time is eventually absorbed by the body; this outcome is independent of exposure time. For χ < 0.1, less than 10% of the amount in the skin evaporates. As χ increases, evaporation becomes important and for highly volatile chemicals, at least 2/3 of the chemical in the skin at the end of the exposure evaporates. For highly volatile compounds, the total absorbed and evaporated fractions depend on the exposure time: Fabs varies from zero for small values of texp/tlag to a maximum of one-third for large values of texp/tlag.
Figure 3.
The fractions of chemical in the skin at the end of exposure that will be absorbed (Fabs) and evaporated (Fevap). Plots represent 100 terms of Eqs. (16) and (17). Insets: Fabs and Fevap for smaller values of χ.
Equations (16) and (17) are somewhat cumbersome, although they may readily be approximated to a finite number of terms using commercially available mathematical software packages (e.g., Mathcad or even Excel). The presence of infinite series in the equations is a consequence of the initial concentration distribution of permeant (Eq. (2)). Because of the complexity of these equations, simple algebraic approximations were sought empirically. The following one-parameter equations were explored:
| (20) |
| (21) |
Nonlinear regression with the 100-term series solutions yielded a value of 2.906 for a1. The correlation coefficient R2 > 0.98, but the differences are largest for small texp/tlag. Numerical comparisons were made to investigate errors of using the simple Fabs equation. The estimate for Fabs provided by the Eq. (20) approximation should be within 10% of the exact value if:
| (22) |
Consequently, Eqs. (20) and (21) (with a1 as specified) may be used with confidence in lieu of the full series solutions, with the proviso implied by Eq. (22). Additional information on the numerical comparisons leading to Eq. (22) is presented in Supplementary Materials (Part 2).
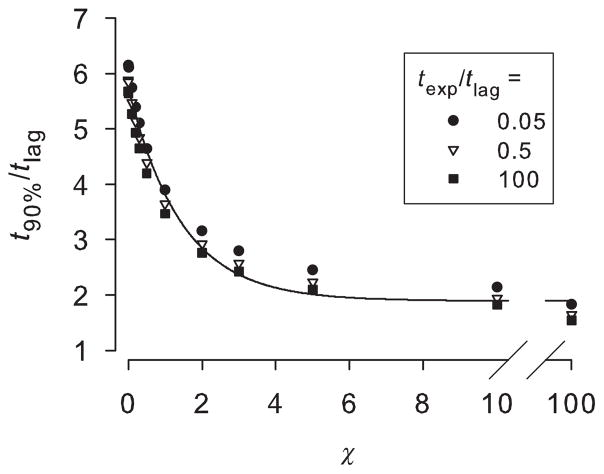
In any realistic setting, infinite time is of course an abstraction. A practical application may require an estimate of the time it takes for the body to absorb most of the final quantity. Figure 4 shows the time after the exposure ends to reach 90% of the final quantity absorbed by the body (t90%). There is a weak dependence on texp/tlag: all intermediate values fell within the displayed values of 0.05 and 100. The time it takes for nearly complete absorption ranges from about 6 × tlag for poorly volatile compounds to about 2 × tlag for highly volatile ones. The pooled data were fitted to the following three-parameter decay curve:
| (23) |
Figure 4.
Time after the exposure ends to reach 90% of the final quantity absorbed by the body (t90%). Solid line is a three-parameter exponential decay curve with parameter values, listed in the text, fitted to the pooled data.
Parameter values for the solid curve displayed in Figure 4 are: a2 = 1.895, b2 = 3.856, c2 = 0.698; the global R2 > 0.97.
The figure confirms and quantifies what is shown in Figure 2: for large χ, the time to distribute almost all of the chemical from the SC is shorter than that for small χ. For a poorly volatile chemical, surface evaporation is insignificant and most chemical within the skin must diffuse inward into the body. The chemical is concentrated at the skin surface (Fig. 1) and so the overall time to complete absorption is relatively longer. For large χ, most of the chemical within the skin at the end of the exposure evaporates. Chemical concentration is greatest in the mid to upper portions of the skin (Fig. 1). Rapid evaporation clears this permeant and the overall time to complete dermal absorption is shorter.
DISCUSSION
The solutions presented here for Fabs and Fevap refer to the post-exposure absorbed and evaporated fractions of the amount of permeant present in the membrane at the end of the exposure time (t = texp). In many instances, it would be desirable to estimate the total amount of permeant that has been absorbed into the body from the entire transient exposure. For example, dermal risk assessments of exposures to occupational chemicals, environmental contaminants, cosmetic and consumer products, as well as pharmaceutical compound applications, require such estimates.
The total mass absorbed (mT, mass/area) is given by:
| (24) |
where mabs(texp) is the mass that has been absorbed into the body at t = texp10:
| (25) |
Fabs is given by Eq. (16), and m0 by Eq. (5). Frasch and Barbero7 derived solutions for mT (m∞ in their terminology) for the special cases of χ = ∞ (their Case 1) and χ = 0 (Case 2). Note that mT may be estimated with quantities commonly measured from standard in vitro diffusion cell experiments, specifically kp and tlag. Other required parameters are chemical concentration, exposure duration and χ. Kasting and Miller2 provide equations to estimate χ based on known or measurable chemical properties including vapor pressure, molecular weight, octanol–water partition coefficient, and water solubility. Alternatively, χ may be measured directly under controlled conditions. Gajjar et al.11 provide data on the evaporation rates of 21 volatile organic compounds from films of neat liquid on human skin, which may be combined with measured or estimated steady-state dermal flux of the compounds from a solution at unit activity (i.e., either neat or in a saturated solution) to obtain χ.
A strategy such as outlined in this paper could be used to identify chemical and exposure situations for which chemical in the SC is or is not likely to be systemically absorbed. Current practices vary on whether chemical in skin at the end of an exposure should or should not be included in estimates of the systemically absorbed.12 For example, risk assessment guidance documents from the USEPA,13 OECD,14–16 and ECETOC17 identify chemical left after washing the exposed skin, including the entire SC, as absorbable but not absorbed. In contrast, the European Commission18 and USEPA in the final test rule for in vitro dermal penetration rate testing issued in 200419 consider chemical in the SC and deeper skin layers as absorbed. European guidance for cosmetics and consumer products exclude chemical in the SC from estimates of the absorbed dose,20–24 whereas the EFSA25 specifies that chemical in all but the first two tape strips should be classified as absorbed unless it can be shown that remaining chemical is not bioavailable or that >75% of the material in the receptor solution (or systemically absorbed in an in vivo study) occurred within half of the duration of the sampling time.
In Tables 1 and 2, we illustrate the use of the method by calculating Fabs, m0, mT, and the fraction of mT that is absorbed postexposure (Fabs × m0/mT). Table 1 contains the chemical and skin permeation properties for four example chemicals, and Table 2 contains calculated values for skin exposures of 5 min, 1 h, and much longer than tlag. The chosen chemicals are important components in dermatological, cosmetic, and consumer products (ethanol, diphenylamine), produced and used in large quantities in the United States (p-nitrophenol and diphenylamine), and a commonly used plasticizer (benzylbutylphthalate). They illustrate a range of evaporation and skin penetration rates, giving small and large estimates for χ. Disposition of chemical in the SC at the end of the exposure was calculated assuming the skin was either left open to the air or immersed in a liquid solution with a large saturation concentration for the chemical. Washout into a liquid solution corresponds to χ approaching infinity, which was approximated with χ = 1000. The absorption fraction (Eq. (16)) and absorbed masses (Eq. (24)) have been calculated using the first 20 terms of the series, which were sufficient for the solutions to be independent of the number of terms to three significant digits. Fractional absorption was also calculated using the simple approximation represented by Eq. (20). The quantities differ most for small values of Fabs, but overall the agreement is excellent. The absorbed masses were calculated assuming an exposed area of 180 cm2, equivalent to the area of the palm of one hand,26 and a vehicle concentration equal to the chemical’s aqueous saturation value; that is, the calculations were made using estimates of maximum flux conditions. The time for 90% of the ultimate absorption of chemical within the skin at the end of exposure (Eq. (23)) is also listed. An Excel spreadsheet was developed to perform these calculations; it is available for use in Supplementary Materials (Part 4).
Table 1.
Chemical and Skin Permeation Properties for Four Example Compounds*
| Compound | CAS No. | MW | Log Kow | Pvap | Sw | tlag | kp | χ |
|---|---|---|---|---|---|---|---|---|
| Ethanol | 64–17–5 | 46.1 | −0.31 | 59.3 | 789 | 0.29 | 0.0008 | 155 |
| Diphenylamine | 122–39–4 | 169.2 | 3.5 | 0.00067 | 0.0636 | 1.34 | 0.0077 | 5.3 |
| Benzylbutyl-phthalate | 85–68–7 | 312.4 | 4.73 | 8.25 × 10−6 | 0.00269 | 8.86 | 0.037 | 0.48 |
| p-Nitroaniline | 100–01–6 | 138.1 | 1.39 | 0.0001 | 0.728 | 1.29 | 0.00844 | 0.060 |
Table 2.
Estimates of the Disposition of Chemical for Skin Exposed to Air or Liquid with a High Saturation Limit at the End of the Exposure for Four Example Compounds at Three Different Exposure Durations and at Maximum Activity (i.e., Saturated in an Aqueous Vehicle)*
| Compound | χ | texp | texp/tlag | Fabs | Fabs (alt) | m0 | mT | Fabs × m0/mT | t90%/tlag | t90% | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ethanol | Air | 155 | 5 min | 0.29 | 0.20 | 0.19 | 48.8 | 9.71 | 1.00 | 1.9 | 0.6 |
| 1 h | 3.4 | 0.34 | 0.34 | 98.6 | 114 | 0.29 | |||||
| 10 × tlag | 10 | 0.34 | 0.34 | 98.8 | 330 | 0.10 | |||||
| Liquid | 1000 | 5 min | 0.29 | 0.19 | 0.19 | 48.8 | 9.51 | 1.00 | 1.9 | 0.6 | |
| 1 h | 3.4 | 0.33 | 0.33 | 98.6 | 114 | 0.29 | |||||
| 10 × tlag | 10 | 0.33 | 0.33 | 98.8 | 330 | 0.10 | |||||
| Diphenylamine | Air | 5.3 | 5 min | 0.062 | 0.24 | 0.21 | 0.081 | 0.019 | 1.00 | 2.0 | 2.7 |
| 1 h | 0.75 | 0.40 | 0.41 | 0.270 | 0.119 | 0.90 | |||||
| 10 × tlag | 10 | 0.44 | 0.44 | 0.354 | 1.22 | 0.13 | |||||
| Liquid | 1000 | 5 min | 0.062 | 0.091 | 0.056 | 0.081 | 0.007 | 1.00 | 1.9 | 2.5 | |
| 1 h | 0.75 | 0.28 | 0.30 | 0.270 | 0.088 | 0.87 | |||||
| 10 × tlag | 10 | 0.33 | 0.33 | 0.354 | 1.18 | 0.10 | |||||
| Benzylbutyl-phthalate | Air | 0.48 | 5 min | 0.0094 | 0.69 | 0.68 | 0.043 | 0.029 | 1.00 | 4.7 | 41 |
| 1 h | 0.11 | 0.72 | 0.71 | 0.147 | 0.105 | 1.00 | |||||
| 10 × tlag | 10 | 0.78 | 0.78 | 0.476 | 1.80 | 0.21 | |||||
| Liquid | 1000 | 5 min | 0.0094 | 0.036 | 0.010 | 0.043 | 0.002 | 1.00 | 1.9 | 17 | |
| 1 h | 0.11 | 0.12 | 0.094 | 0.147 | 0.018 | 1.00 | |||||
| 10 × tlag | 10 | 0.33 | 0.33 | 0.476 | 1.59 | 0.10 | |||||
| p-Nitroaniline | Air | 0.060 | 5 min | 0.065 | 0.95 | 0.95 | 1.00 | 0.95 | 1.00 | 5.6 | 7.2 |
| 1 h | 0.78 | 0.96 | 0.96 | 3.31 | 3.34 | 0.95 | |||||
| 10 × tlag | 10 | 0.96 | 0.96 | 4.28 | 17.0 | 0.24 | |||||
| Liquid | 1000 | 5 min | 0.065 | 0.093 | 0.058 | 1.00 | 0.093 | 1.00 | 1.9 | 2.5 | |
| 1 h | 0.78 | 0.29 | 0.30 | 3.31 | 1.11 | 0.85 | |||||
| 10 × tlag | 10 | 0.33 | 0.33 | 4.28 | 14.3 | 0.10 |
Definitions (in alphabetical order): Fabs, fraction of chemical in the SC at end of the chemical exposure that is predicted to absorb systemically (unitless). Calculated with 1st 20 terms of Eq. (16); Fabs (alt), alternate form of Fabs calculated from Eq. (20); m0, initial mass in membrane at the end of the exposure duration (mg), assuming an exposed area of 180 cm2 and a vehicle concentration equal to the water saturation value; mT, total mass absorbed into the body from the entire transient exposure (mg), both during and after the exposure, assuming the same exposure area and vehicle concentration, t90%, estimated time to 90% of Fabs (h); tlag, lag time for chemical transport through the SC (h), taken from Table 1; texp, length of time that skin is exposed to the chemical; χ, ratio of the evaporation rate from the SC surface to the dermal absorption rate through the SC (unitless), calculated for air exposure as described in Table 1.
The absorption fraction is smallest for volatile chemicals with short exposure times relative to the lag time. Thus, although ethanol is much more volatile than diphenylamine, almost the same fraction of diphenylamine and ethanol absorbs after a 5 min exposure because the ratio of exposure time to lag time is smaller for diphenylamine. However, because its lag time is larger than ethanol’s, 90% absorption of diphenylamine takes four times longer than ethanol (2.7 compared with 0.6 h). Evaporation of compounds with low vapor pressure can occur if the skin permeation rate is slow. Benzylbutylphthalate is a low vapor pressure example in which the estimate for χ = 0.48 and 21% is predicted to evaporate, although 41 h are required.
For the chemicals listed in Table 2, at least 85% of the total absorbed mass is absorbed after the exposure for exposure durations up to 0.78 × tlag. Thus, mT will depend strongly on Fabs (and hence χ). For skin exposed to air following a 5 min exposure to p-nitroaniline, (small χ, large Fabs), nearly all of the skin depot (m0) is eventually absorbed. If the skin is exposed to liquid (large χ, small Fabs), mT is only about 10% that of the air-exposed skin but in both cases, nearly all of the absorption occurs postexposure. As texp/tlag increases, a greater proportion of mT occurs within the exposure duration. For a 1 h ethanol exposure, about 30% of the total absorption occurs postexposure; this percentage would increase for a chemical with less volatility. Even for exposures of 10 × tlag, at least 10% of the total absorption occurs postexposure, and this post-exposure amount may exceed the total absorbed amount for shorter exposures. These results support the consideration of skin depot amounts in estimates of the systemically absorbed amount. Theoretically, two-thirds or more of all the chemicals could be washed out of the skin with a suitable solvent, although, except for ethanol, the time required would be unrealistically large (2.4 or more hours).
Strong chemical binding to skin components and desquamation of the skin would also prevent systemic absorption of chemical from the SC and skin. Because turnover of the SC occurs over many days, it is only important for chemicals that are strongly bound or slowly permeating as shown by diffusion modeling similar to that described here for evaporation.39,40 Based on these model results, the USEPA risk assessment guidance for dermal absorption considers the effect of desquamation.41
This manuscript provides a theoretical framework for transient dermal exposures to volatile chemicals that do not bind to the membrane or substantially impact skin permeability. With the results presented here, combined with previous studies, substantial progress has been made in our understanding of the disposition of chemicals from both finite and transient dose dermal exposures. Analytical expressions for fractional absorption and evaporation have previously been presented for the finite dose case.5 The current work provides corresponding expressions for the transient exposure. A logical next step might be the inclusion of binding of compounds to skin components.42,43 The theory should be tested by controlled in vitro experiments using skin or artificial membranes.7
Supplementary Material
Acknowledgments
Funding for this research was provided by the National Institute for Occupational Safety and Health.
Footnotes
DISCLAIMER
The findings and conclusions of this report are those of the authors and do not necessarily represent the official position of the National Institute for Occupational Safety and Health or the Centers for Disease Control.
This article contains supplementary material available from the authors upon request or via the Internet at http://onlinelibrary.wiley.com/.
References
- 1.Kasting GB. Kinetics of finite dose absorption through skin 1. Vanillylnonanamide. J Pharm Sci. 2001;90(2):202–212. doi: 10.1002/1520-6017(200102)90:2<202::aid-jps11>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 2.Kasting GB, Miller MA. Kinetics of finite dose absorption through skin 2: Volatile compounds. J Pharm Sci. 2006;95(2):268–280. doi: 10.1002/jps.20497. [DOI] [PubMed] [Google Scholar]
- 3.Anissimov YG, Roberts MS. Diffusion modeling of percutaneous absorption kinetics: 2. Finite vehicle volume and solvent deposited solids. J Pharm Sci. 2001;90(4):504–520. doi: 10.1002/1520-6017(200104)90:4<504::aid-jps1008>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 4.Anissimov YG. Mathematical models for different exposure conditions. In: Roberts MS, Walters KA, editors. Dermal absorption and toxicity assessment. New York: Informa Healthcare; 2008. pp. 271–286. [Google Scholar]
- 5.Frasch HF. Dermal absorption of finite doses of volatile compounds. J Pharm Sci. 2012;101(7):2616–2619. doi: 10.1002/jps.23149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.N’Dri-Stempfer B, Bunge AL. How much chemical in the skin will evaporate?. Poster presented at 2nd International Conference on Occupational and Environmental Exposure of Skin to Chemicals; Stockholm, Sweden: Karolinska Institute; 2005. [Accessed June 2005]. [accessed 08/16/2014]. at: https://ia600500.us.archive.org/21/items/OEESC2/AbPost081Bunge.html. [Google Scholar]
- 7.Frasch HF, Barbero AM. The transient dermal exposure: Theory and experimental examples using skin and silicone membranes. J Pharm Sci. 2008;97(4):1578–1592. doi: 10.1002/jps.21035. [DOI] [PubMed] [Google Scholar]
- 8.Crank J. The mathematics of diffusion. 2. Oxford: Oxford University Press; 1975. p. 50. [Google Scholar]
- 9.CRC. CRC standard mathematical tables. In: Beyer William H., editor. Differential Equations Table III. 26. Boca Raton: CRC Press; 1981. p. 376. [Google Scholar]
- 10.Crank, op. cit., p. 51.
- 11.Gajjar RM, Miller MA, Kasting GB. Evaporation of volatile organic compounds from human skin in vitro. Ann Occup Hyg. 2013;57(7):853–865. doi: 10.1093/annhyg/met004. [DOI] [PubMed] [Google Scholar]
- 12.WHO (World Health Organization) Environmental health criteria 235, dermal absorption. Geneva, Switzerland: WHO Press; 2006. [Google Scholar]
- 13.USEPA (United States Environmental Protection Agency) Health effects test guidelines, OPPTS 870.7600 dermal penetration. Washington, DC: USEPA; 1998. Aug, EPA 712-C-98–350. [Google Scholar]
- 14.OECD (Organization for Economic Co-operation and Development) OECD guideline for the testing of chemical, skin absorption: In vitro method, 428, Adopted April 13. Paris, France: OECD; 2004a. [Google Scholar]
- 15.OECD (Organization for Economic Co-operation and Development) OECD guideline for the testing of chemical, skin absorption: In vivo method, No. 427, Adopted April 13. Paris, France: OECD; 2004b. [Google Scholar]
- 16.OECD (Organization for Economic Co-operation and Development) Guidance document for the conduct of skin absorption studies. Paris, France: OECD; 2004c. OECD series on testing and assessment, No. 28, Adopted March 5. [Google Scholar]
- 17.ECETOC (European Centre for Ecotoxicology and Toxicology of Chemicals) Percutaneous absorption. Brussels, Belgium: ECETOC; 1993. Monograph No 20. [Google Scholar]
- 18.EC (European Commission) Guidance document on dermal absorption, health & consumer protection directorate-general, Sanco/222/2000 rev. 7. 2004 Mar 19; [Google Scholar]
- 19.US EPA. Fed Reg. April 26, 2004. Vol. 69. Washington, DC: US EPA; 2004. [Accessed August 29, 2014]. In vitro dermal absorption rate testing of certain chemicals of interest to the Occupational Safety and Health Administration; pp. 22402–22441. DOCID:fr26ap04–21. at: http://www.epa.gov/fedrgstr/EPA-TOX/2004/April/Day-26/t9409.htm. [Google Scholar]
- 20.COLIPA (European Cosmetic Toiletry and Perfumery Association) Cosmetic ingredients: Guidelines for percutaneous absorption/penetration. Brussels, Belgium: COLIPA Publications; 1995. [Google Scholar]
- 21.SCCP (Scientific Committee on Consumer Products) Opinion on basic criteria for the in vitro assessment of dermal absorption of cosmetic ingredients—Updated March 2006, Adopted by the SCCP during the 7th plenary of 28 March 2006, European Commission, Health & Consumer Protection Directorate-General, Directorate C-Public Health and Risk assessment, C7-Risk assessment, SCCP/0970/06 2006 [Google Scholar]
- 22.Steiling W, Kreutz J, Hofer H. Percutaneous penetration/dermal absorption of hair dyes in vitro. Toxicol in Vitro. 2001;15:565–570. doi: 10.1016/s0887-2333(01)00062-5. [DOI] [PubMed] [Google Scholar]
- 23.SCCNFP (The Scientific Committee on Cosmetic Products and Non-food Products Intended for Consumers) The SCCNFP’s notes of guidance for the testing of cosmetic ingredients and their safety evaluation, 5th revision. Adopted by the SCCNFP during the 25th plenary meeting of October 20, 2003, SCCNFP/0690/03 Final.2003. [Google Scholar]
- 24.Diembeck W, Beck H, Benech-Kieffer F, Courtellemont P, Dupuis J, Lovell W, Paye M, Spengler J, Steiling W. Test guidelines for in vitro assessment of dermal absorption and percutaneous penetration of cosmetic ingredients. Food Chem Toxicol. 1999;37:191–205. doi: 10.1016/s0278-6915(98)00114-8. [DOI] [PubMed] [Google Scholar]
- 25.EFSA (European Food Safety Authority) Guidance on dermal absorption, panel on plant protection products and their residues (PPR) EFSA J. 2012;10(4):2665, 30. [Google Scholar]
- 26.NIOSH. Current intelligence bulletin 61: A strategy for assigning new NIOSH skin notations. Cincinnati, Ohio: 2009. [Accessed July 28, 2009]. DHHS (NIOSH) Publication No. 2009–147. at: http://www.cdc.gov/niosh/docs/2009-147/default.html. [Google Scholar]
- 27.Liu Z, Ye W, Little JC. Predicting emissions of volatile and semivolatile organic compounds from building materials: A review. Build Environ. 2013;64:7–25. [Google Scholar]
- 28.Geankoplis CJ. Transport processes and separation process principles. 4. Upper Saddle River, New Jersey: Prentice Hall; 2003. [Google Scholar]
- 29.Wolfram|Alpha. [Accessed August 20, 2014];Wolfram Alpha LLC. 2014 at: http://www.wolframalpha.com/input/?i=air±viscosity±at±25C±and±1±atmosphere±divided±by±air±density±at±25C±and±1±atmosphere.
- 30.Cussler EL. Diffusion-mass transfer in fluid systems. Cambridge: Cambridge University Press; 1984. p. 112. [Google Scholar]
- 31.Perry RH, Green DW, Maloney JO, editors. Perry’s chemical engineers’ handbook. 6. New York: McGraw-Hill Inc; 1997. [Google Scholar]
- 32.Girman JR. Simple modeling to determine appropriate operating conditions for emission testing in small chambers. In: Nagda Niren L., editor. In Modeling of indoor air quality and exposure. Philadelphia: American Society for Testing and Materials; 1993. pp. 145–148. ASTM STP 1205. [Google Scholar]
- 33.Evans M. Housing, climate, and comfort. New York: J Wiley & Sons, Inc; 1980. p. 175. [Google Scholar]
- 34.Knudsen HN, Kjaer UD, Nielsen PA, Wolkoff P. Sensory and chemical characterization of VOC emissions from building products: Impact of concentration and air velocity. Atmos Environ. 1999;33:1217–1230. [Google Scholar]
- 35.VENT-AXIA. Ventilation handbook. Vent-Axia; 2010. [Accessed August 15, 2014]. at http://www.scribd.com/doc/25144576/VENT-AXIA-Ventilation-Handbook. [Google Scholar]
- 36.Chemtura. Determination of the in vitro absorption rate of diphenylamine, ID No. EPA-HQ-OPPT-2003–0006–0330. [Accessed August 20, 2014];Final report to US EPA for TSCA Section 4 (Docket identification number OPPT-2003–006) 2006 at: http://www.regulations.gov/#!documentDetail;D=EPA-HQ-OPPT-2003--0006--0330.
- 37.Scheuplein RJ, Blank IH. Mechanism of percutaneous absorption. IV. Penetration of nonelectrolytes (alcohols) from aqueous solutions and from pure liquids. J Invest Dermatol. 1973;60:286–296. doi: 10.1111/1523-1747.ep12723090. [DOI] [PubMed] [Google Scholar]
- 38.Aceto Corporation. Human percutaneous absorption and cutaneous disposition of [14C]-nitroaniline in vitro, ID No. EPA-HQ-OPPT-2003–0006–0320. [Accessed August 29, 2014];Final report to US EPA for TSCA Section 4 (Docket identification number OPPT-2003–006) 2005 at: http://www.regulations.gov/#!documentDetail;D=EPA-HQ-OPPT-2003--0006--0320.
- 39.Reddy MB, Bunge AL. Does desquamation reduce permeation? In: Bronaugh RL, Maibach HI, editors. In Percutaneous absorption, drugs-cosmetics-mechanisms-methodology. Boca Raton, Florida: Taylor & Francis; 2005. pp. 1414–1419. [Google Scholar]
- 40.Reddy MB, Guy RH, Bunge AL. Does epidermal turnover reduce percutaneous penetration? Pharm Res. 2000;17:1414–1419. doi: 10.1023/a:1007522200422. [DOI] [PubMed] [Google Scholar]
- 41.USEPA. Risk assessment guidance for superfund, volume I: Human health evaluation manual. Washington, DC: Office of Superfund Remediation and Technology Innovation; 2004. [Accessed August 29, 2014]. (Part E, supplemental guidance for dermal risk assessment) Final, EPA/540/R/99/005, OS-WER 9285.7–02EP, PB99–963312, July, 2004. at: http://www.epa.gov/oswer/riskassessment/ragse/index.htm. [Google Scholar]
- 42.Anissimov YG, Roberts MS. Diffusion modelling of percutaneous absorption kinetics: 4. Effects of a slow equilibration process within stratum corneum on absorption and desorption kinetics. J Pharm Sci. 2009;98(2):772–781. doi: 10.1002/jps.21461. [DOI] [PubMed] [Google Scholar]
- 43.Frasch HF, Barbero AM, Hettic JM, Nitsche JM. Tissue binding affects the kinetics of theophylline diffusion through the stratum corneum barrier layer of skin. J Pharm Sci. 2011;100(7):2989–2995. doi: 10.1002/jps.22489. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.