Abstract
Alzheimer’s disease (AD) is an age-related neurodegenerative disease distinguished by progressive memory loss and cognitive decline. It is accompanied by classical neuropathological changes, including cerebral deposits of amyloid-beta peptide (Aβ) containing senile plaques, neurofibrillary tangles (NFTs) of phosphorylated tau (p-tau), and clusters of activated glial cells. Postmortem studies strongly support a critical role for neuroinflammation in the pathogenesis of AD, with activated microglia and reactive astrocytes surrounding senile plaques and NFTs. These are accompanied by an elevated expression of inflammatory mediators that further drives Ab and p-tau generation. Although epidemiological and experimental studies suggested that long-term use of non-steroidal anti-inflammatory drugs (NSAIDs) may lessen AD risk by mitigating inflammatory responses, primary NSAID treatment trials of AD have not proved successful. Elevated systemic butyrylcholinesterase (BuChE) levels have been considered a marker of low-grade systemic inflammation, and BuChE levels are reported elevated in AD brain. Recent research indicates that selective brain inhibition of BuChE elevates acetylcholine (ACh) and augments cognition in rodents free of the characteristic undesirable actions of acetylcholinesterase-inhibitors (AChE-Is). Hence, centrally active BuChE-selective-inhibitors, cymserine analogs, have been developed to test the hypothesis that BuChE-Is would be efficacious and better tolerated than AChE-Is in AD. The focus of the current study was to undertake an in-silico evaluation of an agent to assess its potential to halt the self-propagating interaction between inflammation, Ab and p-tau generation. Molecular docking studies were performed between the novel BuChE-I, N1-p-fluorobenzyl-cymserine (FBC) and inflammatory targets to evaluate the potential of FBC as an inhibitor of p38, JNK kinases and TNF-α with respect to putative binding free energy and IC50 values. Our in-silico studies support the ability of FBC to bind these targets in a manner supportive of anti-inflammatory action that is subject to molecular dynamics and physiochemical studies for auxiliary confirmation.
Keywords: Alzheimer’s disease, Anticholinesterase, Docking, Fluorobenzylcymserine, TNF-α, MAP3 kinases, Butyrylcholinesterase, Neuroinflammation
INTRODUCTION
Inflammation is a regular and imperative response to physiological challenge by harmful stimuli, such as contagious agents, antigen-antibody reactions, thermal, chemical, physical agents, and ischemia [1]. In response to atypical invasion, the activation of different intracellular signaling mechanisms may be a retort of cells to such an incursion [1,2]. Inflammation occurs as a result of activation of cellular elements and the presence of various biochemical mediators that have been reviewed [2]. For an acute inflammatory reaction against all invading microorganism, human neutrophils, being the major cellular component, constitute the first defense barrier [3–5]. Human neutrophils show a rapid response to changing chemotactic factors and cytokines [6–9]. Neutrophil activation initially involves intracellular mechanistic processes that increase and induce the sudden stimulation of protein phosphorylation, which has key roles in the regulation of many neutrophil functions (9–15) and of other cell types. Inflammation within the brain - neuroinflammation - is an invariable feature that accompanies most, if not all, neurodegenerative and cerebrovascular disorders and, as reported for the leading cause of dementia in the elderly, Alzheimer’s disease (AD), exacerbates the pathogenesis. As detailed in reviews by Cuello [16] and Selkoe [17], the classical pathological features present at the time of AD clinical diagnosis are intracellular neurofibrillary tangles (NFTs) of paired filaments of abnormally phosphorylated tau protein (p-tau) along with cerebrovascular and extracellular deposits of amyloid plaques comprised of aggregated amyloid-beta peptide (Aβ). Postmortem studies strongly endorse a fundamental role for neuroinflammation in AD, with the presence of activated microglia and reactive astrocytes surrounding amyloid deposits and NFTs accompanied by an elevated expression of proinflammatory mediators [18]. Furthermore, elevation in proinflammatory cytokines have been associated with presymptomatic and promordal AD stages that together may last a decade or more prior to the onset of dementia [19]. Epidemiological, observational as well as preclinical studies support the concept that long-term use of non-steroidal anti-inflammatory drugs (NSAIDs) [20–23] and other experimental agents [24,25] may potentially reduce AD risk by inhibiting inflammatory responses. However, no such association has been found with exposure to NSAIDs shortly before onset of dementia [23,26–28]. Indeed, results have been disappointing, as NSAID trials in patients with established dementia or milder cognitive symptoms have shown no mitigation of symptom progression [23,26–28]. Albeit those asymptomatic subjects treated with anti-inflammatory agents may experience a reduced AD incidence [23].
Pathophysiological studies of AD subjects and animal models indicate the presence of chemical mediators, such as tumor necrotic factor-α (TNF-α), interleukins (IL-1, 6 and 8), together with mitogen activated protein kinases (MAP kinase), transcription factors and zinc dependent matrix metalloproteinases (MMP) [29–37]. MAP3 kinases, for example, play a vital role in regulating cellular functions and mediating intracellular signal transduction as well as extracellular stimuli responses [38–40]. In this regard, three discrete mammalian MAP kinases have been identified that are classified into (i) intracellular; the stress-activated protein kinases or c-Jun kinases that regulates stress-activated signal transduction (ii) extracellular signal-regulated kinases (ERKs or p44/42 MAP kinase), and (iii) p38 MAP kinase (a mammalian homologue of HOG1, also known as CSBP, RK, or mpk2), each of which possess apparently unique signaling pathways [29–33]. Many of these proteins have key functions in the progression of inflammation and the inhibition of such inflammatory mediators may potentially mitigate inflammatory disorders and, thereby, offer new targets for anti-inflammatory drugs. [32–37].
In the present study we used N1-p-fluorobenzyl-cymserine (FBC) as a ligand for targets associated with inflammation. Our chosen targets included TNF-α, MAP kinases, matrix metalloproteinase’s (MMP), IL-1 and NFKB. FBC was originally synthesized on the pharmacophore of cymserine [41] as butyrylcholinesterase (BuChE) ligand and inhibitor. Elevated levels of BuChE have been described in AD brain, particularly within the hippocampus, attributed to an increase in the prevalence of reactive astrocytes and microglia cells and to Aβ plaques, to which this enzyme is localized [42–46]. In light of the lack of classical acetylcholinesterase (AChE) inhibitor-mediated adverse events following the use of a highly selective BuChE inhibitor [41], within the current study, to potentially halt the self-propagating interaction between inflammation, Aβ and p-tau generation, molecular docking studies were performed between the BuChE inhibitor FBC and inflammatory targets. These studies are performed to evaluate the potential of FBC as an inhibitor of TNF-α, NFKB, MMP, IL-1, p38 and JNK kinase with respect to putative binding free energy and IC50 values.
MATERIALS AND METHODS
Receptor & Ligand Dataset
FBC was used as ligand in the current study. A dataset of anti-inflammatory targets was created that includes, NFKB, MMP, IL-1, TNF-α and two Map kinases (p38 kinase & JNK kinase) as receptors. The molecular structure of FBC was drawn using Chemoffice [47], later geometry optimization along with energy minimization was corrected by MO-PAC [48] using the RM1 semi-empirical method. The three-dimensional (3D) structures of NFKB, MMP, IL-1, TNF-α, p38 Kinase and c-JNK Kinase were available within the Protein Data Bank (PDB) [49], and the structures that we used in our study included 6 receptors: i) 2O61 (NFKB), ii) 3O2X (MMP13), iii) 3O4O (IL-1) iv) 2AZ5 (TNF-α), v) 1W84 (p38 kinase) vi) 3V6R (JNK kinase). Binding site residues that may be involved in inhibition were identified by checking the interactions of these receptors with inhibitor complexes reported within the PDB as result of experimental proofs. After performing docking experiments, these were viewed and analyzed by using Discovery Studio [50].
Docking Studies
To evaluate whether FBC could act as a potential anti-inflammatory drug, we performed docking studies of FBC interactions with the collected anti inflammatory targets using Autodock 4.2 [51]. Each docking experiment consisted of 100 runs, yielding 100 docked conformations. The program AutoGrid was used to generate the grid maps. Each grid was centered at the structure of the corresponding receptor. The grid dimensions were 80 × 80 × 80 Å3 with points separated by 0.375 Å. For all ligands, random starting positions, random orientations and torsions were used. The translation, quaternion and torsion steps were taken from default values in AutoDock. The Lamarckian genetic algorithm method was used for minimization using default parameters. Simulations were performed with a maximum of 2.5 million energy evaluations and a maximum of 27,000 generations. Parameters for the docking included: a population size of 150; a random starting position and conformation; a maximal mutation of 2Å in translation and 50° in rotations; an elitism of 1; a mutation rate of 0.02 and a crossover rate of 0.8; and finally a local search rate of 0.06.
RESULTS
Docking Results
FBC and the six receptors having PDB structures (2O61, 3O2X, 3O4O, 2AZ5, 1W84, and 3V6R) were subjected to molecular docking using Autodock 4.2 [51]. A careful analysis of dockings was undertaken using the binding site information residues that may be important for inhibition. After performing docking experiments, these were viewed and analyzed using Discovery Studio [50]. Amongst all the evaluated targets, the three receptors 2AZ5 (TNF-α), 1W84 (p38 kinase) and 3V6R (JNK kinase) showed significant interactions with critical amino acids whereas no significant bindings were seen with 2O61(NFKB), 3O2X (MMP13) and 3O4O (IL-1). As we have focused on results of molecular dockings, so the receptors that showed significant bindings with critical amino acids were studied in more detail. Table 1 shows the binding residues for TNF-α, p38 kinase and JNK kinase that may be involved in inhibition which can further be verified through experimental studies.
Table 1.
Binding site residues for i) TNF-α, ii) p38 kinase and iii) JNK kinase.
| Receptor Name and PDB ID | Hydrogen Bond Interacting Residues | Hydrophobic interacting Residues |
|---|---|---|
| TNF-α, 2AZ5 | Gly121 B | Leu55 D, Tyr59 A, Tyr119A, Leu120A, Gly121A, Tyr151 A, Leu57B, Tyr59 B, Tyr119B, Leu120B, Tyr151 B. |
| p38 kinase, 1W84 | Ala51 A, Met109 A, Gly110 A | Lys53A, Tyr35A, Gly110A, His107A, Val38A, Thr106A, Ile 84A, Leu167A, Leu104A, Met109A |
| JNK kinase, 3V6R | Met149 A, Cys154 A, | Met146 A, Leu206A, Val78A, Asn152A, Cys154A, Gln155A, Ser193A |
As occupancy of interacting residues as; (Tyrosine and Leucine; where 6 no of Tyr residues are interacting in FBC-TNF-α complex and 1 in FBC-p38 Kinase complex; 4 no of Leu residues are interacting in FBC-TNF-α complex, 2 no. of residues in FBC-p38 Kinase complex and 1 in FBC-JNK kinase complex). This shows that Leu involvement in the interacting groove is important in all three different kinases.
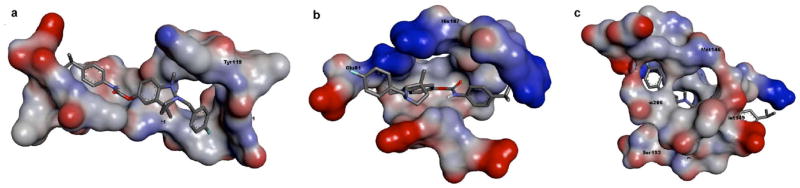
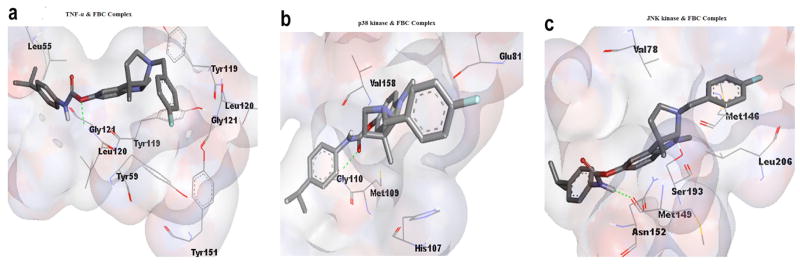
Computational calculations to determine the best inhibitory conformation, at a temperature of 298K, yielded that FBC has inhibitory effects when it interacts with TNF-α, p38 Kinase and JNK Kinase with a putative binding free energy of −7.77, −7.83 and −7.84 kcal/mol, respectively, with the inhibition constants of 2.03, 1.82 and 1.78 μM, respectively. The docked conformations were evaluated in detail and the best binding conformations were extracted. Details of different in-silico parameters recorded during FBC interactions with TNF-α, p38 kinase and JNK kinase such as energy profile value for an individual ligand and receptor interactions summarized in Table 2. From Fig. (1) it can be inferred that FBC bound within a deep narrow groove available on all the three targets and that the respective binding sites provide a sufficiently adequate surface complementary for supporting good docking. In Fig (2a), FBC is shown docked within the binding site of TNF-α that involves a series of favorable interactions. In this regard, the FBC ‘-Oxygen-’ atom is Hydrogen-bonded with Gly121 chain B of TNF-α (Oxygen-2.2Å..HN, atomic distance; between oxygen and hydrogen atom), whereas hydrophobic interactions of FBC were found with the residues Leu55D, Leu120A, Leu120B, Tyr59A, Tyr119A, Tyr119B, Tyr151A and Gly121A. In the case of interaction with p38 kinase, FBC was assessed to have one hydrogen bond between the FBC ‘-Oxygen-’ atom and the Gly110 of p38 kinase (Oxygen-2.2Å..HN, atomic distance; between oxygen and hydrogen atom;) and other hydrophobic interactions were determined with the residues Met109, His107, Val158 and Glu81 Fig (2b). Similarly, for the remaining chosen target, JNK kinase, strong interactions with FBC were determined to involve one hydrogen bond between residues Met149 and the FBC ‘-Hydrogen-’ atom (-H..1.9Å..O,) and other hydrophobic interactions with the residues Val78, Leu206, Met176, Asn152 and Ser193 Fig (2c). The presence of the hydrogen bond and multiple hydrophobic interactions are important in holding the proposed inhibitor FBC within the binding sites of the chosen targets and making binding possible.
Table 2.
Computational profile of different in-silico parameters recorded during FBC interactions with TNF-α, p38 kinase and JNK kinase.
| Properties | FBC Interactions | ||
|---|---|---|---|
| TNF-α | P38 Kinase | JNK Kinase | |
| Binding Energy (kcal/mol) | −7.77 | −7.83 | −7.84 |
| Ki (μM) | 2.03 | 1.82 | 1.78 |
| Intermolecular Energy (kcal/mol) | −9.55 | −9.62 | −9.63 |
| vdW + Hbond + desolv Energy (kcal/mol) | −9.87 | −8.33 | −9.86 |
| Electrostatic Energy (kcal/mol) | 0.31 | −1.29 | 0.23 |
| Final Total Internal Energy (kcal/mol) | −0.62 | 0.76 | −0.58 |
| Torsional Free Energy (kcal/mol) | 1.79 | 1.79 | 1.79 |
| Unbound System’s Energy (kcal/mol) | −0.62 | −0.76 | −0.58 |
| Ref RMS (Å) | 69.49 | 40.58 | 37.81 |
| Temperature (K) | 298.5 | 298.5 | 298.5 |
Fig. 1. FBC within the binding cavity of (a) TNF-α binding site, (b) p38 kinase binding site, (c) JNK kinase.
The structures are viewed using Discovery Studio (Accelrys Inc., San Diego, CA). The receptor binding sites are shown as opaque spheres colored according to heteroatoms, where as FBC molecules are viewed in sticks. Hydrogen bonding interactions between FBC and respective receptors are highlighted in a green color.
Fig. 2. Binding pattern of FBC with (a) TNF-α binding site, (b) p38 kinase binding site, (c) JNK kinase.
The binding sites are shown in white spheres with a degree of 80% transparency, the interacting residues are marked in a pink color on the spheres and also presented as lines with labels, and FBC molecules are displayed as sticks. The green lines exhibit hydrogen bonding interactions between FBC and respective receptors.
CONCLUSION
The most interactive residues between the target proteins (p38, JNK kinases and TNF-α) and FBC proved to be hydrophobic ones present within the deep narrow groove occupying a main interacting core of the active site. Site specific inhibition of p38, JNK kinases and TNF-α through important interacting residues(Tyrosine and Leucine; where 6 number of Tyr residues are interacting in FBC-TNF-α complex and 1 in FBC-p38 Kinase complex; 4 number of Leu residues are interacting in FBC-TNF-α complex, 2 number of residues in FBC-p38 Kinase complex and 1 in FBC-JNK kinase complex) by FBC may be useful if the surrounded residues are tagged by a phosphate group, which may result in cellular signal transduction after phosphorylation. As MAP kinases are involved in the cascade leading to inflammation, they play a key role in the progression of inflammation and inhibition of such inflammatory mediators may potentially mitigate inflammatory diseases; thereby offering new targets for anti inflammatory drugs are worthy of experimental assessment.
DISCUSSION
Inflammation is a complex pathological condition associated with an exaggerated human immune system response involving a complex symphony of activated immune cells and bio-molecules. Treatment of inflammatory diseases, particularly chronic inflammatory diseases such as rheumatoid arthritis, inflammatory bowel disease [52,53], as well as neurodegenerative disorders that are invariably accompanied by neuroinflammation [54–56], has become a huge challenge for both scientists and clinicians as there are few safe drugs available for effective long-term therapy. In treating inflammatory diseases, NSAIDs and selective COX-2 inhibitors have been conventionally the most extensively used drugs to date. However, their chronic use has been demonstrated to have highly adverse effects and the use of these inhibitors might even lead to fatalities, consequent to cardiovascular and thrombotic events [57]. As a consequence, attention is currently turning to support the design, synthesis and lead optimization of inhibitors for signal transduction and anti-cytokine drugs rather than to more conventional NSAIDS and COX proteins [1].
There is hence a developing unmet need for ligands/inhibitors that potentially bind to and interact with anti-inflammatory targets without adverse effects. In this regard cymserine analogues, structurally related to physostigmine and phenserine [58, 59], act as reversible cholinesterase inhibitors with a preference for BuChE [60,61], from which more BuChE-selective FBC was generated. Cymserine analogues have demonstrated the ability to augment cognitive performance in elderly healthy rodents [41] and those with A β-induced learning deficits involving neuroinflammation [62]. BuChE is elevated in both AD brains [42, 62,63], where it co-localizes with the classical hallmarks of AD [43–46], as well as in low grade systemic inflammation that has been linked to the pathogenesis of insulin resistance, type 2 diabetes mellitus, hyperlipidemias, age-related macular degeneration and other conditions [64,65]. In this study, we for the first time evaluated FBC as a direct potential anti-inflammatory drug supported by in-silico studies. The three proteins, TNF-α, p38 and MAP kinases, appear to be targets worth pursuing, from our analysis of 6 proteins involved in inflammatory signaling cascades [66–70]. As docking data portray, FBC exhibited interactions with critical amino acids that may be required for inhibition of these three key anti-inflammatory targets. On the basis of these experimental results and interaction data, we propose FBC as an interesting inhibitor for anti inflammatory targets p38, JNK kinases and TNF-α with putative binding free energy values that ranged from −7.77 kcal/mol to −7.84 kcal/mol and inhibition constant values that ranged from 1.78 μM to 2.03 μM. These values appear to be potentially achievable in vivo, based on a structural analogue that has been developed into animals and humans [71], and hence support further analyses of FBC in preclinical cellular and in vivo models.
Acknowledgments
This research was supported in part by the COMSATS Institute of Information Technology, Islamabad, Pakistan, the King Fahd Medical Research Center, King Abdulaziz University, Jeddah, Saudi Arabia, and the Intramural Research Program, National Institute on Aging, NIH, Baltimore, Maryland, USA.
ABBREVIATIONS
- AChE
Acetylcholinesterase
- AChE-Is
Acetylcholinesterase inhibitors
- AD
Alzheimer’s disease
- APP
Amyloid-β precursor protein
- Aβ
amyloid-β peptide
- BuChE
Butyrylcholinesterase
- BuSCh
Butyrylthiocholine iodide
- BuChE-Is
butyrylcholinesterase inhibitors
- ChEs
Cholinesterases
- ChE-Is
Cholinesterase inhibitors
- CNS
Central nervous system
- CSBP
Cytokine-stimulated anti-inflammatory drug binding protein
- FBC
Fluorobenzylcymserine
- HOG1
High-osmolarity glycerol response
- NFTs
Neurofibrillary tangles
- RK
Response kinases
- TNF-α
Tumor Necrosis Factor-Alpha
- MAP kinase
Mitogen activated protein kinase
- JNK
c-Jun N-terminal kinase
- PDB
Protein Data Bank
Footnotes
CONFLICT OF INTEREST
The authors confirm that this article content has no conflicts of interest.
PATIENT CONSENT
Declared none.
Send Orders of Reprints at reprints@benthamscience.net
References
- 1.Kulkarni RG, Achaiah G, Narahari Sastry G. Novel targets for antiinflammatory and antiarthritic agents. Curr Pharm Des. 2006;12:2437–2454. doi: 10.2174/138161206777698945. [DOI] [PubMed] [Google Scholar]
- 2.Goldyne ME, Burrish GF, Poubelle P, Borgeat P. Arachidonic acid metabolism among human mononuclear leukocytes. Lipoxygenase-related pathways. J Biol Chem. 1984;259(14):8815–8819. [PubMed] [Google Scholar]
- 3.Haslett C, Savill JS, Meagher L. The neutrophil. Curr Opin Immunol. 1989;2:10–18. doi: 10.1016/0952-7915(89)90091-5. [DOI] [PubMed] [Google Scholar]
- 4.Pillinger MH, Abramson SB. The neutrophil in rheumatoid arthritis. Rheum Dis Clin North Am. 1995;21:691. [PubMed] [Google Scholar]
- 5.Bogomolski-Yahalom V, Matzner Y. Disorders of neutrophil function. Blood Rev. 1995;9:183. doi: 10.1016/0268-960x(95)90024-1. [DOI] [PubMed] [Google Scholar]
- 6.Membrane Activation in Immunologically Relevant Cells. Progress in Allergy. 1988;42:1–312. [PubMed] [Google Scholar]
- 7.Pike MC. Chapter 2 Chemoattractant receptors as regulators of phagocytic cell function. Curr Topics Membran Transport. 1990;35:19–43. [Google Scholar]
- 8.Gomez-Cambronero J, Sha’afi RI. Granulocyte-macrophage colony-stimulating factor and the neutrophil: mechanisms of action. Adv Exp Med Biol. 1991;314:35. doi: 10.1007/978-1-4684-6024-7_3. [DOI] [PubMed] [Google Scholar]
- 9.Buhl AM, Avdi N, Worthen GS, Johnson GL. Mapping of theC5a receptor signal transduction network in human neutrophils. Proc Natl Acad Sci USA. 1994;91:9190. doi: 10.1073/pnas.91.19.9190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crowley JJ, Raffin TA. Tumor necrosis factor-induced protein phosphorylation in human neutrophils. Am J Respir Cell Mol Biol. 1991;284:91. doi: 10.1165/ajrcmb/5.3.284. [DOI] [PubMed] [Google Scholar]
- 11.Gomez-Cambronero J, Wang E, Johnson G, Huang CK, Sha’afi RI. Platelet-activating factor induces tyrosine phosphorylation in human neutrophils. J Biol Chem. 1991;266:6240. [PubMed] [Google Scholar]
- 12.Grinstein S, Furuya W. Chemoattractant-induced tyrosine phosphorylationand activation of microtubule-associated protein kinase in human neutrophils. J Biol Chem. 1992;267:18122. [PubMed] [Google Scholar]
- 13.Downey GP, Chan CK, Lea P, Takai A, Grinstein S. Phorbol ester-induced actin assembly in neutrophils: role of protein kinase C. J Cell Biol. 1992;116:695. doi: 10.1083/jcb.116.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Katz DA, Haimovich B, Greco RS. Neutrophil activation by expanded polytetrafluoroethylene is dependent on the induction of protein phosphorylation. Surgery. 1994;116:446. [PubMed] [Google Scholar]
- 15.Durstin M, Durstin S, Molski TFP, Becker EL, Sha’afi RI. Cytoplasmic phospholipase A2 translocates to membrane fraction in human neutrophils activated by stimuli that phosphorylate mitogen-activated protein kinase. Proc Natl Acad Sci USA. 1994;91:3142. doi: 10.1073/pnas.91.8.3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cuello AC. Overview of the alzheimer’s disease pathology and potential therapeutic targets. Pharmacol Mech Alzheimer’s Ther. 2007:1–27. [Google Scholar]
- 17.Selkoe DJ. Alzheimer’s disease: genes, proteins, and therapy. Physiol Rev. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- 18.Ferretti MT, Bruno MA, Ducatenzeiler A, Klein WL, Cuello AC. Intracellular Aβ-oligomers and early inflammation in a model of Alzheimer’s disease. Neurobiol Aging. 2012;33(7):1329–1342. doi: 10.1016/j.neurobiolaging.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 19.Azizi G, Mirshafiey A. The potential role of proinflammatory and antiinflammatory cytokines in Alzheimer disease pathogenesis. Immunopharmacol Immunotoxicol. 2012;34:881–895. doi: 10.3109/08923973.2012.705292. [DOI] [PubMed] [Google Scholar]
- 20.Andersen K, Launer LJ, Ott A, Hoes AW, Breteler MM, Hofman A. Do nonsteroidal anti-inflammatory drugs decrease the risk for Alzheimer’s disease? The Rotterdam Study Neurology. 1995;45:1441–1445. doi: 10.1212/wnl.45.8.1441. [DOI] [PubMed] [Google Scholar]
- 21.McGeer PL, McGeer E, Rogers J, Sibley J. Anti-inflammatory drugs and Alzheimer disease. Lancet. 1990;335:1037. doi: 10.1016/0140-6736(90)91101-f. [DOI] [PubMed] [Google Scholar]
- 22.Klegeris A, McGeer PL. Non-steroidal anti-inflammatory drugs (NSAIDs) and other anti-inflammatory agents in the treatment of neurodegenerative disease. Curr Alzheimer Res. 2005;2:355–365. doi: 10.2174/1567205054367883. [DOI] [PubMed] [Google Scholar]
- 23.Breitner JC, Baker LD, Montine TJ, Meinert CL, Lyketsos CG, Ashe KH, Brandt J, Craft S, Evans DE, Green RC, Ismail MS, Martin BK, Mullan MJ, Sabbagh M, Tariot PN ADAPT Research Group. Extended results of the Alzheimer’s disease anti-inflammatory prevention trial. Alzheimers Dement. 2011;7:402–411. doi: 10.1016/j.jalz.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leea HE, Kima DH, Parka SJ, Kima JM, Leea YW, Junga JM, Leea CH, Honga JH, Liua X, Caia M, Parkb KJ, Janga DS, Ryu JH. Neuroprotective effect of sinapic acid in a mouse model of amyloid β1-42 protein-induced Alzheimer’s disease. Pharmacol Biochem Behavior. 2012;103(2):260–266. doi: 10.1016/j.pbb.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 25.Jin MN, Yao Z, Takaishi Y, Duan HQ. Lignans from Schisandra propinqua with inhibitory effects on lymphocyte proliferation. Planta Medica. 2012;78(8):807–813. doi: 10.1055/s-0031-1298439. [DOI] [PubMed] [Google Scholar]
- 26.Aisen PS, Schafer KA, Grundman M, Pfeiffer E, Sano M, Davis KL, Farlow MR, Jin S, Thomas RG, Thal LJ. Effects of rofecoxib or naproxen vs placebo on Alzheimer disease progression: a randomized controlled trial. JAMA. 2003;289:2819–2826. doi: 10.1001/jama.289.21.2819. [DOI] [PubMed] [Google Scholar]
- 27.Martin BK, Szekely C, Brandt J, Piantadosi S, Breitner JC, Craft S, Evans D, Green R, Mullan M. Cognitive function over time in the Alzheimer’s Disease Anti-inflammatory Prevention Trial (ADAPT): results of a randomized, controlled trial of naproxen and celecoxib. Arch Neurol. 2008;65:896–905. doi: 10.1001/archneur.2008.65.7.nct70006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thal LJ, Ferris SH, Kirby L, Block GA, Lines CR, Yuen E, Assaid C, Nessly ML, Norman BA, Baranak CC, Reines SA. A randomized, double-blind, study of rofecoxib in patients with mild cognitive impairment. Neuropsychopharmacology. 2005;30:1204–1215. doi: 10.1038/sj.npp.1300690. [DOI] [PubMed] [Google Scholar]
- 29.Nahas N, Waterman WH, Sha’afi RI. Granulocyte-macrophagecolony-stimulating factor (GM-CSF) promotes phosphorylation and an increase in the activity of cytosolic phospholipase A2 in human neutrophils. Biochem J. 1996;313:503. doi: 10.1042/bj3130503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choy EH, Panayi GS. Cytokine pathways and joint inflammation in rheumatoid arthritis. N Eng J Med. 2001;344(12):907–916. doi: 10.1056/NEJM200103223441207. [DOI] [PubMed] [Google Scholar]
- 31.Thornton S, Duwel LE, Boivin GP, Ma Y, Hirsch R. Association of the course of collagen-induced arthritis with distinct patterns of cytokine and chemokine messenger RNA expression. Arthritis Rheumat. 1999;42(6):1109–1118. doi: 10.1002/1529-0131(199906)42:6<1109::AID-ANR7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 32.Herlaar E, Brown Z. p38 MAPK signalling cascades in inflammatory disease. Mol Med Today. 1999;5(10):439–447. doi: 10.1016/s1357-4310(99)01544-0. [DOI] [PubMed] [Google Scholar]
- 33.Ono K, Han J. The p38 signal transduction pathway: activation and function. Cell Signal. 2000;12(1):1–13. doi: 10.1016/s0898-6568(99)00071-6. [DOI] [PubMed] [Google Scholar]
- 34.Sweeney SE, Firestein GS. Signal transduction in rheumatoid arthritis. Curr Opin Rheumatol. 2004;16(3):231–237. doi: 10.1097/00002281-200405000-00011. [DOI] [PubMed] [Google Scholar]
- 35.Zarubin T, Han J. Activation and signaling of the p38 MAP kinase pathway. Cell Res. 2005;15:11–18. doi: 10.1038/sj.cr.7290257. [DOI] [PubMed] [Google Scholar]
- 36.Morris GM, Goodsell DS, Halliday RS, Huey R, Hart WE, Belew RK, Olson AJ. Automated docking using a lamarckian genetic algorithm and empirical binding free energy function. J Comput Chem. 1998;19:1639–1662. [Google Scholar]
- 37.Mussener A, Litton MJ, Lindroos E, Klareskog L. Cytokine production in synovial tissue of mice with collagen-induced arthritis (CIA) Clin Exp Immunol. 1997;107(3):485–493. doi: 10.1046/j.1365-2249.1997.3181214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anderson NG. MAP kinases: ubiquitous signal transducers and potentially important components of the cell cycling machinery in eukaryotes. Cell Signal. 1992;4:239. doi: 10.1016/0898-6568(92)90063-e. [DOI] [PubMed] [Google Scholar]
- 39.Blenis J. Signal transduction via the MAP kinases: proceed at your own RSK. Proc Natl Acad Sci USA. 1993;90:5889. doi: 10.1073/pnas.90.13.5889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Davis RJ. The mitogen-activated protein kinase signal transduction pathway. J Biol Chem. 1993;268:14553. [PubMed] [Google Scholar]
- 41.Greig NH, Utsuki T, Ingram DK, Wang Y, Pepeu G, Scali C, Yu QS, Mamczarz J, Holloway HW, Giordano T, Chen D, Furukawa K, Sambamurti K, Brossi A, Lahiri DK. Selective butyrylcholinesterase inhibition elevates brain acetylcholine, augments learning and lowers Alzheimer beta-amyloid peptide in rodent. Proc Natl Acad Sci USA. 2005;102:17213–17218. doi: 10.1073/pnas.0508575102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perry EK, Perry RH, Blessed G, Tomlinson BE. Changes in brain cholinesterases in senile dementia of Alzheimer type. Neuropathol Appl Neurobiol. 1978;4:273–277. doi: 10.1111/j.1365-2990.1978.tb00545.x. [DOI] [PubMed] [Google Scholar]
- 43.Geula C, Mesulam MM. Cholinesterases and the pathology of Alzheimer disease. Alzheimer Dis Assoc Disord. 1995;9(Suppl 2):23–28. doi: 10.1097/00002093-199501002-00005. [DOI] [PubMed] [Google Scholar]
- 44.Kadir A, Marutle A, Gonzalez D, Schöll M, Almkvist O, Mousavi M, Mustafiz T, Darreh-Shori T, Nennesmo I, Nordberg A. Positron emission tomography imaging and clinical progression in relation to molecular pathology in the first Pittsburgh Compound B positron emission tomography patient with Alzheimer’s disease. Brain. 2011;34(1):301–317. doi: 10.1093/brain/awq349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Darvesh S, Cash MK, Reid GA, Martin E, Mitnitski A, Geula C. Butyrylcholinesterase is associated with β-amyloid plaques in the transgenic APPSWE/PSEN1dE9 mouse model of Alzheimer disease. J Neuropathol Exp Neurol. 2012;71:2–14. doi: 10.1097/NEN.0b013e31823cc7a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Darvesh S. Butyrylcholinesterase radioligands to image Alzheimer’s disease brain. Chem Biol Interact. 2012 doi: 10.1016/j.cbi.2012.08.009. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 47.Cambridge Soft Corporation. Chemoffice. USA: 2010. [Google Scholar]
- 48.Stewart JJ. MOPAC: A semiempirical molecular orbital program. J Computer-Aided Mol Des. 1990;4(1):1–103. doi: 10.1007/BF00128336. [DOI] [PubMed] [Google Scholar]
- 49.Berman H, Henrick K, Nakamura H, Markley JL. The worldwide Protein Data Bank (wwPDB): ensuring a single, uniform archive of PDB data. Nucl Acids Res. 2007:D301–D303. doi: 10.1093/nar/gkl971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Accelrys Inc., San Diego, CA.
- 51.Morris GM, Goodsell DS, Halliday RS, Huey R, Hart WE, Belew RK, Olson AJ. Automated docking using a lamarckian genetic algorithm and and empirical binding free energy function. J Comput Chem. 1998;19:1639–1662. [Google Scholar]
- 52.Bradley JR. TNF-mediated inflammatory disease. J Pathol. 2008;214:149–160. doi: 10.1002/path.2287. [DOI] [PubMed] [Google Scholar]
- 53.Huston JM, Tracey KJ. The pulse of inflammation: heart rate variability, the cholinergic anti-inflammatory pathway and implications for therapy. J Intern Med. 2011;269:45–53. doi: 10.1111/j.1365-2796.2010.02321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zilka N, Kazmerova Z, Jadhav S, Neradil P, Madari A, Obetkova D, Bugos O, Novak M. Who fans the flames of Alzheimer’s disease brains? Misfolded tau on the crossroad of neurodegenerative and inflammatory pathways. J Neuroinflam. 2012;9:47. doi: 10.1186/1742-2094-9-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Frankola KA, Greig NH, Luo W, Tweedie D. Targeting TNF-α to elucidate and ameliorate neuroinflammation in neurodegenerative diseases. CNS Neurol Disord Drug Targets. 2011;10:391–403. doi: 10.2174/187152711794653751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Frank-Cannon TC, Alto LT, McAlpine FE, Tansey MG. Does neuroinflammation fan the flame in neurodegenerative diseases? Mol Neurodegener. 2009;16(4):47. doi: 10.1186/1750-1326-4-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bäck M, Yin L, Ingelsson E. Cyclooxygenase-2 inhibitors and cardiovascular risk in a nation-wide cohort study after the withdrawal of rofecoxib. Eur Heart J. 2012;33:1928–33. doi: 10.1093/eurheartj/ehr421. [DOI] [PubMed] [Google Scholar]
- 58.Greig NH, Pei XF, Soncrant TT, Ingram DK, Brossi A. Phenserine and ring C hetero-analogues: drug candidates for the treatment of Alzheimer’s disease. Med Res Rev. 1995;15:3–3. doi: 10.1002/med.2610150103. [DOI] [PubMed] [Google Scholar]
- 59.Greig NH, Sambamurti K, Yu QS, Brossi A, Bruinsma GB, Lahiri DK. An overview of phenserine tartrate, a novel acetylcholinesterase inhibitor for the treatment of Alzheimer’s disease. Curr Alzheimer Res. 2005;2:281–290. doi: 10.2174/1567205054367829. [DOI] [PubMed] [Google Scholar]
- 60.Yu Q, Holloway HW, Utsuki T, Brossi A, Greig NH. Synthesis of novel phenserine-based-selective inhibitors of butyrylcholinesterase for Alzheimer’s disease. J Med Chem. 1999;42:1855–1861. doi: 10.1021/jm980459s. [DOI] [PubMed] [Google Scholar]
- 61.Greig NH, Yu QS, Brossi A, Matin E, Lahiri DK, Darvesh S. Butyrylcholinesterase, the Cinderella cholinesterase, as a drug target for Alzheimer’s diseases and related dementias. In: Martinez A, editor. Medicinal Chemistry Of Alzheimer’s Disease. Transworld Research Network; Research Signpost, Kerala, India: 2008. pp. 79–109. [Google Scholar]
- 62.Furukawa-Hibi Y, Alkam T, Nitta A, Matsuyama A, Mizoguchi H, Suzuki K, Moussaoui S, Yu QS, Greig NH, Nagai T, Yamada K. Butyrylcholinesterase inhibitors ameliorate cognitive dysfunction induced by amyloid-β peptide in mice. Behav Brain Res. 2011;225:222–229. doi: 10.1016/j.bbr.2011.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Greig NH, Utsuki T, Yu Q, Zhu X, Holloway HW, Perry T, Lee B, Ingram DK, Lahiri DK. A new therapeutic target in Alzheimer’s disease treatment: attention to butyrylcholinesterase. Curr Med Res Opin. 2001;17(3):159–165. doi: 10.1185/0300799039117057. [DOI] [PubMed] [Google Scholar]
- 64.Das UN. Acetylcholinesterase and butyrylcholinesterase as markers of low-grade systemic inflammation. Ann Hepatol. 2012;11:409–411. [PubMed] [Google Scholar]
- 65.Sridhar GR, Rao AA, Srinivas K, Nirmala G, Lakshmi G, Suryanarayna D, Rao PV, Kaladhar DG, Kumar SV, Devi TU, Nitesh T, Hanuman T. Butyrylcholinesterase in metabolic syndrome. Med Hypotheses. 2010;75:648–651. doi: 10.1016/j.mehy.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 66.Marshall CJ. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995;80:179. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- 67.Hunter T. Protein kinases and phosphatases: the yin and yang of proteinphosphorylation and signaling. Cell. 1995;80:225. doi: 10.1016/0092-8674(95)90405-0. [DOI] [PubMed] [Google Scholar]
- 68.Derijard D, Raingeaud J, Barrett T, Wu IH, Han J, Ulevitch RJ, Davis RJ. Independent human MAP kinase signal transduction pathways defined by MEK and MKK isoforms. Science. 1995;267:682. doi: 10.1126/science.7839144. [DOI] [PubMed] [Google Scholar]
- 69.Sanchez I, Hughes RT, Mayer BJ, Yee K, Woodgett JR, Avruch J, Kyriakis JM, Zon LI. Role of SAPK/ERK kinase-1 in the stress activated pathway regulating transcription factor c-Jun. Nature. 1994;372:794. doi: 10.1038/372794a0. [DOI] [PubMed] [Google Scholar]
- 70.Han J, Lee JD, Bibbs L, Ulevitch RJ. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science. 1994;265:808. doi: 10.1126/science.7914033. [DOI] [PubMed] [Google Scholar]
- 71.Maccecchini ML, Chang MY, Pan C, John V, Zetterberg H, Greig NH. Posiphen as a candidate drug to lower CSF amyloid precursor protein, amyloid-β peptide and τ levels: target engagement, tolerability and pharmacokinetics in humans. J Neurol Neurosurg Psychiatr. 2012;83:894–902. doi: 10.1136/jnnp-2012-302589. [DOI] [PMC free article] [PubMed] [Google Scholar]