Abstract
Objective
To determine the extent to which prospectively identified responders to cognitive therapy (CT) for recurrent major depressive disorder (MDD) hypothesized to be lower risk show significantly less relapse/recurrence than treated higher risk counterparts across 32 months.
Method
Outpatients (N = 523), aged 18–70, with recurrent MDD received 12–14 weeks of CT. The last seven consecutive scores from the Hamilton Rating Scale for Depression (HRSD-17), were used to stratify/define responders (n = 290) into lower (seven HRSD-17 scores of ≤ 6; n = 49; 17%) and higher risk (n = 241; 83%). The lower risk entered the 32-month follow-up. Higher risk patients were randomized to 8 months of continuation-phase CT or clinical management plus double-blind fluoxetine or pill placebo, with a 24-month follow-up.
Results
Lower risk patients were significantly less likely to relapse over the first 8 months compared to higher risk (Kaplan-Meier [KM] estimates (i.e., 4.9%=lower risk; 22.1%= higher risk; log-rank χ2 = 6.83, p = .009). This increased risk was attenuated, but not completely neutralized, by active continuation-phase therapy. Over the subsequent 24 months, the lower and higher risk groups did not differ in relapse/recurrence risk.
Conclusions
Rapid and sustained acute-phase CT remission identifies responders who do not require continuation-phase treatment to prevent relapse (i.e., return of an index episode). To prevent recurrence (i.e., new episodes), however, strategic allocation and more frequent “dosing” of CT and/or targeted maintenance-phase treatments may be required. Longitudinal follow-up is recommended.
Keywords: depression, cognitive therapy, relapse, recurrence, predictors of outcomes
There are effective treatments for Major Depressive Disorder (MDD), but none are curative, so relapse and recurrence after response to acute-phase treatment are major public-health problems. Most people with MDD have more than one episode, and 85% of those who recover from one episode will experience another within 15 years (Mueller et al., 1999). The high prevalence of MDD, its recurring course, risk for intergenerational transmission, increased mortality rates, and emotional, social, and financial costs are well established (Keller, 2003).
Developing and disseminating treatments that prevent relapse and recurrence remain high, international priorities. Among treatments with established efficacy, cognitive therapy (CT) is distinguished by its potential to delay or prevent relapse (with mixed results for recurrence prevention) for some patients who respond (i.e., show reduced depressive symptoms) to acute-phase treatment (Hollon et al., 2005). Because this preventive effect is documented after discontinuation of acute-phase CT (A-CT), and a parallel effect is not found after discontinuation of pharmacotherapy, using CT for relapse prevention may improve public health and conserve resources. A meta-analysis of 28 studies of cognitive behavioral therapy with 1,880 adults found that (1) 29% of acute-phase responders relapse or recur within 1 year, (2) 54% relapse or recur within 2 years, and (3) compared to assessment-only controls, continuation-phase CT (C-CT) improved long-term outcome by reducing relapse and recurrence 21% while provided and 29% over follow-up (Vittengl, Clark, Dunn, & Jarrett, 2007; replicated by Cuijpers et al., 2013).
Although A-CT can reduce relapse and recurrence for some patients, its preventive effects are not uniform. Because A-CT prevents relapse and recurrence for about half its responders over 2 years and C-CT offers additional protection, we need to know which patients need these different “doses” of CT. If investigators can identify risk factors for relapse/recurrence and convert these to usable tools, then treatment length can be tailored to individuals to change their course of illness, thereby benefitting public health.
We showed previously (Jarrett et al., 2001; Thase et al., 1992) that incomplete remission (i.e., having residual symptoms) during A-CT predicts greater relapse and recurrence, and that C-CT is preventive for some responders. Jarrett et al. (2001) retrospectively developed a formula for stable remission during A-CT—having Hamilton Rating Scale for Depression-17 (HRSD-17; Hamilton, 1960) scores of 6 or less for the last seven measurements. This marker of lower risk for relapse separated patients with recurrent MDD into higher and lower risk groups, who did versus did not require C-CT immediately after response to A-CT. We later simplified the formula and translated it into a practical criterion—the final symptom-severity score—to assess need for C-CT in clinical settings (Jarrett, Vittengl, & Clark, 2008; see Segal et al., 2010, for an example of its use. If replicable, patients with MDD, clinicians, and health-care policy makers can use such tools to make informed decisions for maximizing positive outcomes and minimizing costs.
The formulae we developed were motivated by one of the most robust findings in the depression literature: Incomplete remission predicts relapse (e.g., Paykel et al., 1999; Thase et al., 1992; Jarrett et al. 2001). Consequently, continuing treatment until patients receive an adequate dose (Lutz et al., 2002) to reach “good enough” outcomes (Baldwin et al., 2009) such as full remission (Zimmerman et al., 2007) is recommended broadly. The purpose of the current analysis was to determine the extent to which the original, retrospectively identified formula predicted relapse and recurrence over the first 8 months post-acute-phase response in adults with recurrent MDD. Higher risk patients were randomized to 8 months of continuation-phase cognitive therapy (C-CT) or continuation-phase clinical management plus double-blinded fluoxetine (FLX) or matched pill placebo (PBO). Lower risk patients received assessment-only follow-up every 4 months (i.e., no further protocol treatment). To estimate durability of effects and/or recurrence, we repeated the analysis (1) over the first 20 and 32 months of post-acute observation, after the lower risk responders had completed the acute phase treatments and the higher risk responders the continuation-phase treatments; and (2) during months 9–32 (i.e., after higher risk responders’ continuation treatments had ended) among patients who survived months 0–8 without relapse.
In summary, we report on a prospective test of the formula’s predictive values. We ask, “How long can lower risk CT responders avoid or delay further treatment without relapse or recurrence?” We hypothesized that if lower risk CT responders (who are not treated) relapse at a lower rate than higher risk responders (who are treated), then lower risk responders may be able to participate in “watchful waiting” rather than continuation-phase treatments. We also sought to identify clinical indicators of risk before the final weeks of A-CT to provide practitioners with early signals of which patients may need continuation-phase treatment to avoid relapse.1
We predicted that lower risk patients would relapse significantly less than higher risk patients. We were particularly interested in determining the extent to which C-CT (for higher risk responders) “neutralized” risk to a level comparable to that of lower risk responders (with no further treatment). Further, we predicted that, better outcomes of lower versus higher risk patients over 32 months; we emphasize that prospective evaluation is necessary for this test.
Method
The study received annual approval by the Institutional Review Boards at each data collection site (The University of Texas Southwestern Medical Center and The University of Pittsburgh), as well as ongoing oversight and annual approval by a Data Safety and Monitoring Board. Informed consent was obtained after review of written, verbal, and/or videotaped materials for patients who entered the acute phase of the protocol. Consent was reconfirmed at each “phase change” (i.e., after A-CT, before randomization to the continuation phase, and before entry into follow-up). Additional methodological detail regarding this Continuation-Phase Cognitive Therapy Relapse Prevention [C-CT-RP] Trial, registered at ClinicalTrials.gov [NCT00118404, NCT00183664, and NCT00218764]) including patients, recruitment, inclusion and exclusion criteria, power estimates, and randomization by a statistician, is available in Jarrett and Thase (2010). See Jarrett, Minhajuddin, Gershenfeld, Friedman, & Thase (2013) for the primary results from the randomized trial (i.e., higher risk responders).
Participants
Participants were recruited through the Internet, local media, printed announcements, and self- and practitioner referrals. Included were those who a) were diagnosed with DSM-IV non-psychotic, recurrent MDD using the Structured Clinical Interview for Depression (SCID-I; First, Spitzer, Gibbon, & Williams, 1996), b) scored ≥14 on the 17-item Hamilton Rating Scale for Depression (HRSD-17; Hamilton, 1960) at the initial and second interview, c) were aged 18–70, and d) provided informed consent. Excluded individuals a) had concurrent medical disorders or treatments associated with depressive symptoms, b) had psychotic or organic mental disorders, bipolar disorder, active substance dependence, predominant obsessive-compulsive or eating disorders, c) could not speak or write English, d) planned to become pregnant in the first 11 months after intake, e) had previously failed to respond to either an 8-week trial of CT by a certified therapist or 6 weeks of 40 mg. of fluoxetine, f) required hospitalization for suicidal ideation, or g) were unable or unwilling to comply with the treatment protocol.
Procedure
Design
A total of 523 individuals met inclusion criteria and consented to A-CT. Responders (defined below) who consented for further study attended assessments with an independent evaluator (blinded to strata and cell) every 4 months for 32 months after completion of A-CT. The pattern of remission during the final 6 weeks of A-CT was used to delineate prospectively those patients at lower and higher risk for depression (defined below). Those judged to be at higher risk were randomized to an 8-month experimental continuation phase comparing C-CT versus double-blinded, matched FLX versus PBO; those at lower risk entered the follow-up at the end of A-CT.
Patient Flow
Figure 1 illustrates the numbers of patients who were evaluated, began acute-phase treatment, responded, were denoted as at lower or higher risk, entered continuation-phase treatment, and entered longitudinal follow-up.
Figure 1.
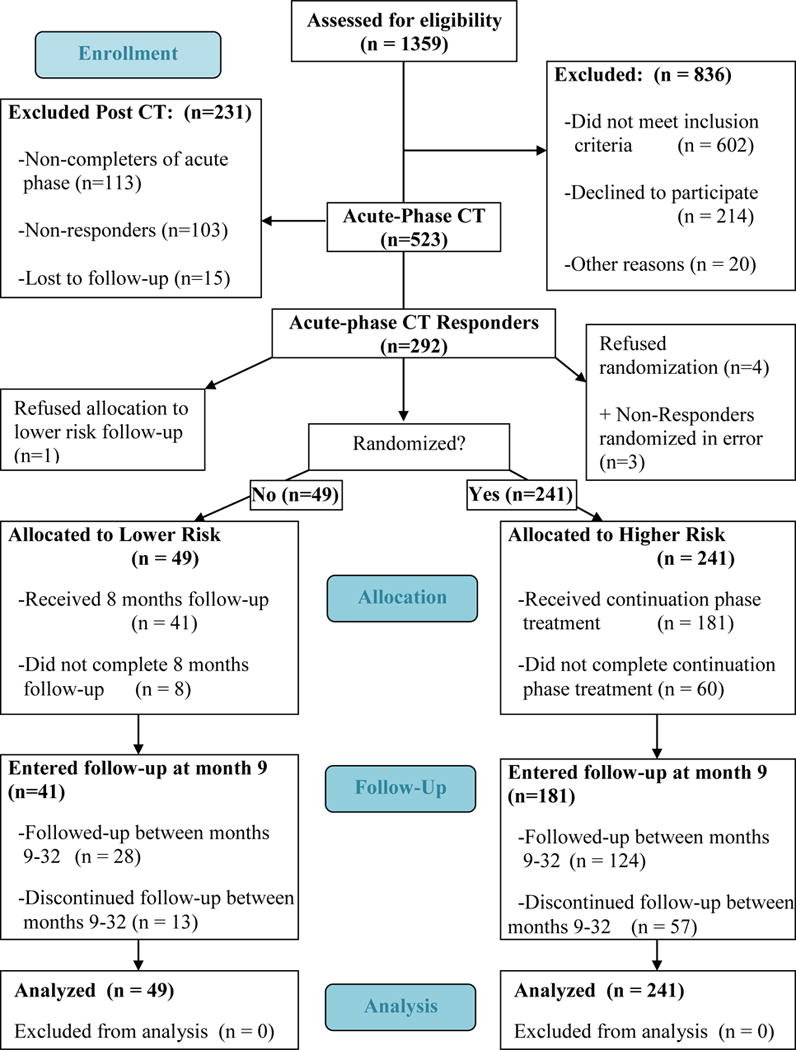
The Consort 2010 Flow Diagram For Division by Strata
Acute-Phase CT2 (Beck, Rush, Shaw, & Emery, 1979) included 16–20 individual sessions conducted within a 12–14 week protocol by 16 experienced therapists. Sessions 1–8 were twice weekly; then patients with < 40% reduction in HRSD-17 scores continued bi-weekly sessions for 4 more weeks, whereas all others began weekly sessions. The goal of A-CT is to teach patients to reduce depressive symptoms by identifying, evaluating, and addressing negative thoughts cognitively and behaviorally. Therapist competence was evaluated by peer therapists and supervisors using the Cognitive Therapy Scale (CTS; Young & Beck, 1980), and scores indicated competence in 93% of 368 sessions evaluated. Pharmacotherapy was not provided nor permitted during A-CT protocol.
Of 523 patients, 113 (22%) did not complete A-CT. From the intention-to-treat sample, 55.8% responded to A-CT, defined as the absence of MDD and an HRSD-17 ≤ 12 rated by an independent evaluator. A relatively inclusive threshold for response was used to maximize the number of patients eligible for randomization (see Jarrett & Thase, 2010). Five eligible responders refused further participation; three non-responders were randomized in error3. Non-responders were referred for community care.
Delineation of risk strata
Using the last seven consecutive HRSD-17 scores of A-CT, responders were stratified into lower and higher risk groups (i.e., all seven HRSD-17 scores ≤ 6 vs. any of the seven scores > 6)4. Thirteen patients were missing 1 to 3 of these scores, so their last seven available HRSD-17 scores were used, spanning weeks 4–12. Of 290 classified CT responders, 241 and 49 (83 vs. 17%) were categorized as at higher and lower risk, respectively.
Higher risk stratum randomization
A statistician oversaw design of stratification and blocking, and used a computer generator to randomize patient to cells. Study pharmacists housed and implemented the randomization when site coordinators requested assignments. Four higher risk responders completed CT but refused randomization3. Of the 241 randomized to the experimental/continuation phase, 86 entered C-CT (Jarrett, 1989 Jarrett, 1992; Jarrett et al., 2008), 86 entered FLX, and 69 entered double blinded PBO. The C-CT protocol consisted of 10 sessions provided by the patient’s A-CT therapist over 8 months; the first four sessions were biweekly, the remaining six monthly. The goal of C-CT is to prevent relapse/recurrence by reducing residual symptoms, improving learned skills, enhancing strengths, decreasing the probability of stressful events and anticipating relapse/recurrence risks. Responders randomized to the medication clinic attended 10 evaluation visits on the same schedule as C-CT patients. (See Jarrett & Thase 2010; Jarrett et al., 2013 for pharmacotherapy protocol details). Treating psychiatrists were prohibited from using psychosocial interventions.
Completion/attrition during continuation phase
Sixty of 241 patients (25%) dropped due to clinician (n = 7; 12%) or patient (n = 53; 88%) decisions. Clinician decisions included withdrawal due to side effects or use of nonprotocol treatment (n = 3), clinician error (n = 3), and non-compliance with protocol (n = 1). Patient decisions included refused protocol medication (n = 6), withdrew consent for contact (n = 5), moved (n = 4), lacked efficacy (n = 2), and other reasons (n = 1); in addition, 33 patients were lost to follow-up.
Lower risk stratum
Of 50 lower risk patients eligible for follow-up, 49 (98%) consented to enter the lower risk stratum and to assessments over the full 32-month follow-up period3.
Protocol-treatment-free follow-up phase
Follow-up consisted of six assessments over 24 months after the experimental phase. No protocol treatment was provided; when patients relapsed or recurred, we assisted them in finding treatment in the community. Of the 241 higher risk patients randomized in the experimental phase, 181 (75%) entered long term follow-up, during which 57 higher risk patients dropped due to clinician error (n = 3) or patient decision (n = 54), including withdrew consent to contact (n = 3), moved (n = 4), and other reasons (n = 46, including 42 participants lost to follow-up. Of the 49 lower risk patients, 41 (84%) entered the follow-up phase; eight dropped during the continuation phase, including five lost to follow-up. Thirteen lower risk patients withdrew from long-term follow-up, including 11 lost to follow-up.
Measures
Longitudinal Interval Follow-Up Evaluation (LIFE)
The LIFE (Keller et al., 1987) was used to assess DSM-IV psychiatric disorders, psychosocial functioning, and treatment consumption retrospectively. Our dependent variables were relapse and/or recurrence of DSM-IV major depressive disorder defined by weekly Psychiatric Status Ratings (PSRs) collected by one of multiple experienced evaluators blinded to strata and treatment assignment. If an evaluator became unblinded, a different evaluator collected the remaining outcomes for that patient. The 6-point PSR scale corresponds to clusters of DSM-IV MDD symptoms. Post-A-CT, recovery was defined as PSR scores ≤ 2 for 35 consecutive weeks. Relapse and recurrence were defined as scores ≥ 5 for 2 or more consecutive weeks before and after recovery, respectively. Interviews were conducted at 4, 8, 12, 16, 20, 24, 28, and 32 months post-A-CT, when a patient left the study, and when patients, therapists, or evaluators suspected MDD relapse or recurrence. Interrater reliabilities are typically > .70 (Keller et al., 1987). In this study, the median lag-1 autocorrelation was .87, indicating high week-to-week consistency.
LIFE Psychiatric Treatment History (LIFE TXHX)
An independent evaluator (who did not collect the PSR data) completed the LIFE TXHX. The evaluator recorded any non-protocol psychosocial and/or psychopharmacological treatments patients received. Psychosocial treatments included any individual, group, family, or self-help therapy sessions attended. The evaluator recorded the problem or diagnosis addressed by the therapy, the frequency of the therapy, and the index patient (e.g., patient, spouse, child, couple, or family). The evaluator also recorded any psychopharmacological treatments including type of medication, the problem or diagnosis, and the daily dosage in milligrams. Non-protocol treatments consumed for at least 2 weeks any time before relapse/recurrence were independently coded as either mood-altering or not by three clinicians. A mood-altering non-protocol treatment was operationalized as a treatment that: (a) had the potential to affect mood during the observational period, (b) targeted the patient, and (c) was provided at a therapeutic dose. After rating the non-protocol treatments independently, the three clinicians discussed any discrepancies in the coding and came to a consensus for each treatment. The final mood-altering outcome (yes/no) and number of weeks patients received the treatment was recorded and used as a covariate in data analyses.
Diagnostic interview
Experienced evaluators conducted an expanded SCID-I in which DSM-IV diagnoses and history of illness were collected for the patient’s lifetime. The patients provided demographic information by answering a questionnaire.
Hamilton Rating Scale for Depression (HRSD-17)
The HRSD-17 (Hamilton, 1960) is a widely used 17-item clinician rating scale of depressive symptom severity in individuals diagnosed with a mood disorder. Scores < 6 indicate negligible depressive symptoms. The HRSD-17 has high interrater reliability (r = .85) and converges (rs = .70 – .83) with self-report depression measures (Clark & Watson, 1991). The current report used 19 assessments from pre-A-CT to randomization; internal consistency (α) was acceptable (M = .75).
Attributional Style Questionnaire (ASQ)
We used a shortened version of the ASQ that maintains its original psychometric features (Dykema, Bergbower, Doctora, & Peterson, 1996) and yield scores for stability and globality that have been shown to have good reliability and validity (Dykema et al., 1996; Peterson et al., 1982). Alphas for the stable and global scales we used were .81 and .79, respectively.
Dysfunctional Attitudes Scale (DAS)
The DAS (Form A; Weissman, 1979) is a 40-item self-report measure of attitudes hypothesized to relate to depression with established reliability and concurrent validity with measures of depressive symptoms and negative cognitive content (e.g., Haeffel et al., 2005; Ilardi & Craighead, 1999). Alpha internal consistency for the DAS in this study was .93.
Beck Hopelessness Scale (BHS)
The BHS uses 20 true/false items to assess negative expectancies for the future (Beck & Steer, 1988). It correlates with suicidality and depression severity (Beck, Kovacs, & Weissman, 1975; Beck, Steer, Kovacs, & Garrison, 1985). Alpha internal consistency for the BHS in this study was .89.
Self-Control Schedule (SCS)
The 36-item SCS (Rosenbaum, 1980) measures learned resourcefulness (e.g., problem-solving strategies). It correlates with higher confidence, fewer depressive symptoms (Akgun, 2004; Slessareva & Muraven, 2004), and CT response. Alpha internal consistency for the SCS in this study was .85.
Inventory of Interpersonal Problems (IIP)
The 127-item IIP (Horowitz, Rosenberg, Baer, Ureno, & Villasenor, 1988) measures the extent to which behaviors, thoughts, and feelings have caused problems in personal relationships. It correlates moderately with psychiatric symptoms and scores decrease with psychotherapy. Test-retest and internal consistency are high. Alpha of the IIP total score in this study was .97.
Social Adjustment Scale—Self Report (SAS-SR)
The SAS-SR (M. M. Weissman & Bothwell, 1976) measures self-reported functioning across multiple social domains. Reliability is moderate to good (rs = .74 to .80; Edwards, Yarvis, Mueller, Zingale, & Wagman, 1978). It correlates with interviewer ratings of social functioning and is sensitive to change after 4 weeks of treatment (Weissman & Bothwell, 1976; Weissman, Prusoff, Thompson, Harding, & Myers, 1978). Alpha internal consistency in the current study was .80.
Dyadic Adjustment Scale (DYS)
The 32-item DYS (Spanier, 1976) measures positive adjustment and satisfaction in marital/committed dyads. The measure has high reliability, and both content and criterion validity. Alpha internal consistency in this study was .94.
Working Alliance Inventory (WAI)
The 36-item WAI (Horvath & Greenberg, 1989) assesses the therapist-patient relationship. We used both the client (WAI-C) and therapist (WAI T) versions of the measure which has shown good reliability and convergent validity between patient and clinician ratings, and moderate predictive validity with diverse psychotherapy outcome measures (Martin, Garske, & Davis, 2000). At mid-A-CT in this study, alpha internal consistency was .94 for both the client and therapist forms.
Analyses
We report on an intention-to-treat sample from all patients who: consented to A-CT (N = 523), entered the lower risk stratum (n = 49), and were randomized to the experimental/continuation-phase treatments (n = 241). Patients who dropped out were censored at their last available data point. The data analyzed here were collected from March 30, 2000 through June 1, 2011, (i.e., end of study). All analyses followed the intention-to-treat principle including A-CT responders who agreed to the longer term study phases. In the primary analyses, we estimated and compared Kaplan-Meier survival curves for time to relapse/recurrence for the lower and higher risk strata using log-rank χ2 tests over the first 8 and over 20, and 32 months.
Background context and primary analyses
The primary aim of the parent randomized clinical trial (from which current data are drawn) focused exclusively on comparing the relapse/recurrence rates of higher risk patients randomized to C-CT, FLX, or PBO (Jarrett & Thase, 2010). Therein, Kaplan-Meier analyses over the 8-month experimental continuation phase showed that the C-CT (18%) and FLX (18%) groups showed significantly less relapse than PBO (33%), but C-CT and FLX did not differ. The CCT, FLX, and PBO groups did not differ significantly in relapse/recurrence over 20 (35, 35, and 43%, respectively) or 32 (45, 41, and 56%, respectively) months post-randomization (Jarrett et al., 2013). The C-CT, FLX, and PBO groups did not differ in stable remission or recovery at 8, 20, or 32 months (Vittengl, Clark, Thase, & Jarrett, 2014). Cox regression estimates were that the large majority of the higher risk patients who did not relapse experienced stable remission (97%) and recovery (94%) within 32 months (Vittengl et al., 2014). Because C-CT and FLX did not differ significantly in relapse/recurrence, we collapsed the C-CT and FLX cells into an “active treatment” group to contrast with the lower risk patients.
Follow-up analyses
We found risk-group based differences, so we repeated each log-rank test, comparing relapse/recurrence of lower risk patients separately for the pill placebo and active continuation-treatment groups. In secondary analyses, we also compared relapse/recurrence rates from months 9–32 of the active treatments versus PBO for responders who survived the continuation phase.
Exploratory analyses
We sought to identify early indicators of risk strata among CT responders from the diagnostic or A-CT phases; we compared the characteristics of the higher and lower risk strata using t-tests or χ2 tests, as appropriate. To promote discovery and generate hypotheses, we used a liberal alpha level (p < .10) to select potentially important predictors of each stratum. We then entered these possibly significant variables into a multivariate logistic regression analysis. We used stepwise selection and retained predictors with alphas < .50 to enter and < .05 to stay in the model. Forward and backward variable selection methods (with alpha < .05 to enter or stay in the model, respectively) yielded the same results as the reported stepwise procedure.
Results
Preliminary Results
Non-protocol treatments
There were no differences between the higher and lower risk strata in the proportion of patients consuming mood-altering non-protocol treatment by 8 months (6.1% for lower risk versus 6.2% for higher risk patients), 20 months (8.2% for lower risk versus 12.0% for higher risk patients), or 32 months (10.2% for lower risk versus 14.9% for higher risk patients) after A-CT, Fisher exact test ps > .50. Based on these null findings, non-protocol treatment consumption was ignored in the remaining analyses.
Overall Sample Characteristics and Strata Differences
Pretreatment and mid-treatment process characteristics of the full sample and the two risk strata appear in Table 1. The risk-strata groups did not differ significantly on sociodemographic measures or prior clinical course. Lower (vs. higher) risk responders showed significantly lower pretreatment depressive severity (HRSD-17; effect size d = 0.61) and less pretreatment impairment in cognitive and interpersonal functioning (ASQ stable d = 0.33, ASQ global d = 0.52, DAS d = 0.37, SCS d = 0.39, BHS d = 0.38, IIP d = 0.53, SAS-SR d = 0.38, and DYS d = 0.41). By the midpoint of A-CT, therapists of lower (vs. higher) risk patients reported better WAI-T alliances (d = 0.40). Patients’ scores on the WAI-C, however, did not differ significantly between risk strata. By approximately the midpoint (A-CT session 9), significantly more lower risk patients showed an early response to A-CT (≥ 40% reduction in pretreatment HRSD-17 score; 89.8% vs. 47.7%; effect size r = .32).
Table 1.
Pretreatment Characteristics of Full Sample and Higher versus Lower Risk Patients
| Characteristic | Total (n = 290) | Higher risk (n = 241)a | Lower risk (n = 49)b | Test Statistic | p |
|---|---|---|---|---|---|
| Demographic | |||||
| Female sex: n (%) | 196 (67.6) | 162 (67.2) | 34 (69.4) | .868c | |
| White race: n (%) | 249 (85.9) | 205 (85.1) | 44 (89.8) | .502c | |
| Age (years): M (SD) | 42.6 (12.1) | 42.7 (11.8) | 42.2 (13.6) | t = 0.27d | .789 |
| Marital status: n (%) | .637c | ||||
| Single | 164 (56.6) | 138 (57.3) | 26 (53.1) | ||
| Partnered | 126 (43.4) | 103 (42.7) | 23 (46.9) | ||
| Education: M (SD) | 15.6 (2.8) | 15.7 (2.9) | 14.9 (2.3) | t = 1.86d | .064 |
| Employment: n (%) | χ2 = 3.22e | .780 | |||
| Full-time | 145 (50.0) | 120 (49.8) | 25 (51.0) | ||
| Part-time | 33 (11.4) | 28 (11.6) | 5 (10.2) | ||
| Homemaker/caregiver | 19 (6.6) | 18 (7.5) | 1 (2.0) | ||
| Student | 17 (5.9) | 14 (5.8) | 3 (6.1) | ||
| Retired | 10 (3.5) | 9 (3.7) | 1 (2.0) | ||
| Other | 17 (5.9) | 13 (5.4) | 4 (8.2) | ||
| Unemployed | 49 (16.9) | 39 (16.2) | 10 (20.4) | ||
| Clinical | |||||
| HRSD-17 Score: M (SD) | 19.6 (3.9) | 20.0 (3.9) | 17.7 (3.0) | t = 3.91d | <.001 |
| Age of onset (years): Mdn (IQR) | 18.0 (12.0) | 18.0 (12.0) | 20.0 (14.0) | χ2 = 0.61f | .434 |
| Current episode length (months): Mdn (IQR) | 8.0 (19.0) | 9.0 (23.0) | 7.0 (14.0) | χ2 = 1.14f | .286 |
| Length of illness (years): Mdn (IQR) | 20.0 (18.0) | 20.0 (18.0) | 17.0 (25.0) | χ2 = 1.42f | .234 |
| Number of episodes: Mdn (IQR) | 4.0 (2.0) | 4.0 (2.0) | 4.0 (2.0) | χ2 = 0.01f | .908 |
| Comorbid DSM-IV diagnoses - n (%) | |||||
| Current | 114 (39.3) | 96 (39.8) | 18 (36.7) | .750c | |
| Lifetime | 213 (73.5) | 181 (75.1) | 32 (65.3) | .160c | |
| Current double depression: n (%) | 14 (4.8) | 11 (4.6) | 3 (6.1) | .713c | |
| Depressive subtype: n (%) | |||||
| RDC endogenous, definite | 99 (34.4) | 84 (35.1) | 15 (30.6) | .622c | |
| DSM-IV melancholia | 101 (35.1) | 85 (35.6) | 16 (32.7) | .745c | |
| Cognitive | |||||
| ASQ Stable: M (SD) | 1.1 (0.9) | 1.2 (0.9) | 0.9 (0.8) | t = 2.08d | .038 |
| ASQ Global: M (SD) | 1.1 (1.0) | 1.2 (0.9) | 0.7 (1.0) | t = 3.31d | .001 |
| DAS: M (SD) | 149.7 (34.6) | 151.9 (33.5) | 139.3 (38.0) | t = 2.31d | .022 |
| BHS: M (SD) | 11.6 (5.3) | 11.9 (5.1) | 10.0 (5.6) | t = 2.39d | .018 |
| SCS: M (SD) | −4.8 (26.8) | −6.7 (25.5) | 3.8 (30.9) | t = 2.29d | .013 |
| Interpersonal | |||||
| IIP: M (SD) | 1.6 (0.5) | 1.7 (0.5) | 1.4 (0.5) | t = 3.39d | <.001 |
| SAS-SR: M (SD) | 2.5 (0.4) | 2.5 (0.4) | 2.4 (0.5) | t = 2.40d | .017 |
| DYS: M (SD) | 89.5 (22.9) | 87.7 (22.6) | 97.0 (23.3) | t = 2.08d | .039 |
| Severity and clinical | |||||
| Early Responders: n (%) | 159 (54.8) | 115 (47.7) | 44 (89.8) | <.001c | |
| Late Responders: n (%) | 131 (45.2) | 126 (52.3) | 5 (10.2) | ||
| Process: M (SD) | |||||
| Working Alliance – Therapist | 5.9 (0.6) | 5.9 (0.6) | 6.1 (0.6) | t = 2.52d | .012 |
| Working Alliance – Client | 6.2 (0.6) | 6.2 (0.6) | 6.3 (0.6) | t = 1.25d | .214 |
Note. N = Number; M = Mean; Mdn = Median; SD = Standard Deviation; IQR = interquartile range. HRSD-17 = Hamilton Rating Scale for Depression; RDC = Research Diagnostic Criteria; ASQ = Attributional Style Questionnaire; DAS = Dysfunctional Attitudes Scale; BHS = Beck Hopelessness Scale. SCS = Self-control Schedule. IIP = Inventory of Interpersonal Problems; SAS-SR = Social Adjustment Scale –Self Report. DYS = Dyadic Adjustment Scale.
N reduced due to missing data: DSM-IV melancholia (n = 239) and RDC endogenous definite (n = 239); ASQ (n = 228); DAS (n = 232); BHS (n = 227); SCS (n = 227); IIP (n = 237); SAS-SR (n = 234); DYS (n = 136, completed only by patients in marital or similar relationships); Working Alliance – Therapist (n = 230); Working Alliance – Client (n = 222).
N reduced due to missing data: DAS (n = 48); SAS-SR (n = 48); DYS (n = 32); Working Alliance – Client (n = 45).
Exact p values are from Fisher’s exact test.
t statistics are from t-tests for independent samples; p values are two-tailed.
χ2 statistics for contingency tables.
χ2 statistics are from Kruskal-Wallis test for medians.
Adverse events
No lower risk patients had adverse events. Two higher risk patients were hospitalized for worsening symptoms.
Primary Research Questions
Were lower risk responders less likely to relapse across the first 8 post-A-CT months compared to higher risk responders (a) overall and (b) randomized to either pill placebo or active treatment?
Yes; see Figure 2 and Table 2. Lower (vs. higher) risk responders were significantly less likely to relapse within 8 months (2 of 49 vs. 43 of 241; χ2[1] = 6.83, p < .01, R2 = .023). Kaplan-Meier [KM] estimates were 4.9% for lower risk and 22.1% for higher risk.5
Yes, lower risk responders were less likely to relapse compared to either group. We first compared the lower risk group with the higher risk patients in PBO. As 17 of 69 those patients relapsed (KM = 32.7%), the magnitude of the risk reduction for lower risk classification was about 6 fold; χ2(1) = 12.0, p < .01, R2 = .10. The relapse rate of the active treatment (i.e., C-CT or FLX) higher risk patients was intermediate (26 of 172; KM = 18.3%), but still significantly greater than that of the lower risk group (χ2(1) = 4.77, p = .03, R2 = .021).
Figure 2.
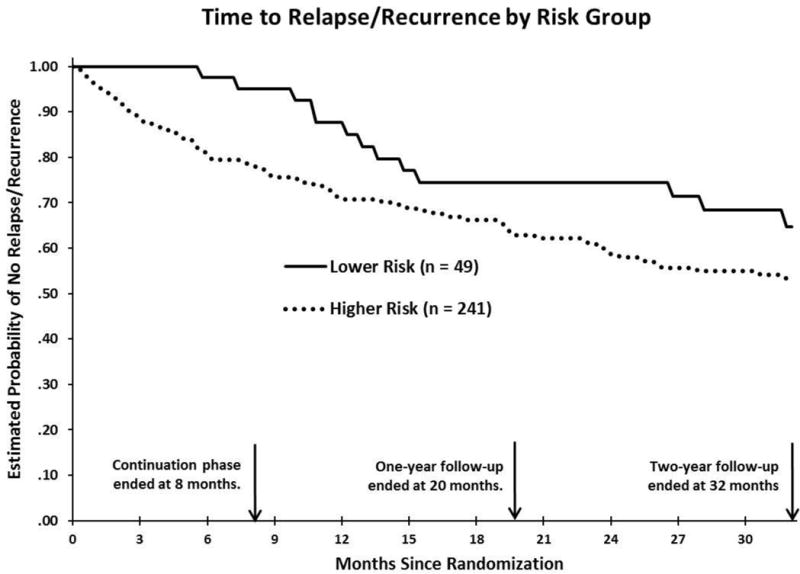
Kaplan-Meier time-to-event curves for relapse or recurrence (DSM-IV major depressive disorder) diagnosed by a blind evaluator over 32 months (139 weeks) in lower and higher risk strata. Log-rank tests showed significant differences between risk strata through 8 months, χ2(1) = 6.83, p < .01, but not 20 months, χ2(1) = 2.89, p = .09, or 32 months, χ2(1) = 3.00, p = .08.
Table 2.
Estimated Percentages of Patients Reaching Target Events by Time and Risk Group
| Time since randomization | Lower Risk Group (n = 49) | Higher Risk Group
|
||
|---|---|---|---|---|
| Full higher risk sample (n = 241) | Active treatment (n = 172): FLX or C-CT | Non-specific treatment (n = 69): PBO | ||
| Patients with relapse or recurrence | ||||
| 8 Months | 4.9 | 22.1* | 18.3* | 32.7* |
| 20 Months | 25.6 | 37.1 | 35.1 | 42.7* |
| 32 Months | 35.4 | 46.6 | 43.2 | 56.3* |
Note. Tabled values are estimated percentages of patients experiencing outcomes from Kaplan-Meier analyses. Patients in the higher risk group were randomized to 8 months of: C-CT = Continuation-phase Cognitive Therapy; FLX= Continuation Fluoxetine; or PBO = Pill Placebo. Within each row, time-to-event curves marked with * differ at p < .05 from the lower risk group by log-rank test.
Were lower risk responders less likely to relapse/recur across the first 20 months after completing A-CT than higher risk responders (a) overall and (b) randomized to either pill placebo or active treatment?
(a) No, they did not differ significantly; see Figure 2 and Table 2. Over the first 20 months after the end of acute phase CT (which also is 1 year after higher risk patients completed the continuation-phase treatments), the lower risk patients showed a trend to relapse or recur (10 of 49; KM = 25.6%) less often than higher risk patients (67 of 241; KM = 37.1%), but the difference was not statistically significant (χ2[1] = 2.89, p = .09, R2 = .010). (b) Lower risk responders did not differ from higher risk responders who received active treatment (χ2[1] = 1.99, p = .16, R2 = .009; 10 of 49 vs. 46 of 172; KM = 25.6 vs. 35.1%), but showed less relapse/recurrence than those in PBO (21 of 69 KM = 42.7%; χ2[1] = 4.56, p = .03, R2 = .038.
Were lower risk responders less likely to relapse/recur across the full 32 months after completing A-CT than higher risk responders (a) overall and (b) randomized to either pill placebo or active treatment?
No, they did not differ significantly. Over the 32 months after the end of acute phase CT (i.e., 2 years after higher risk patients completed the continuation-phase treatments), the lower risk patients showed a trend to relapse or recur (13 of 49; KM = 35.4%) less often than patients of higher risk (80 of 241; KM = 46.6%): χ2(1) = 3.00, p = .08, R2 = .010. Estimated mean time to relapse/recurrence for the lower (vs. higher) risk patients were 114.3 and 96.6 weeks (SEs = 6.4 and 3.8), respectively after discontinuation of acute phase CT.
Lower risk responders did not differ from higher risk responders receiving active treatment, but showed less relapse/recurrence than responders receiving pill placebo; see Figure 2 and Table 2. Specifically, 13 of 49 (KM = 35.4%) lower risk responders and 54 of 172 (KM = 43.2%) of higher risk responders who received active continuation-phase treatment relapsed/recurred over the first 32 months post-acute phase CT (χ2[1] = 1.75, p = .19, R2 = .008). However, patients randomized to PBO experienced more relapse/recurrence over 32 months (26 of 69; KM = 56.3%) compared to lower risk patients: χ2(1) = 5.80, p = .02, R2 = .048.
After all protocol treatment ended, did surviving lower-risk responders differ in relapse/recurrence estimates during months 9–32 from higher responders (a) overall and (b) randomized to either pill placebo or active treatment?
No, there were no differences; (see Supplemental Figure 1). We next compared relapse/recurrence rates during the follow-up phase (i.e., the 2 years after completion of continuation-phase-treatment), To clarify the durability of risk differences between groups, we limited these analyses to patients who did not relapse or drop during the first 8 months after A-CT (N = 181; 38 lower and 143 higher risk patients).
Kaplan-Meier analysis indicated that the lower and higher risk responders did not differ in relapse/recurrence risk over these 2 years: lower (vs. higher) risk group KM = 32.0% and 31.5%; 11 of 38 and 37 of 143, respectively (log-rank χ2[1] = 0.00, p = .95, R2 = .000).
The relapse/recurrence rate for lower risk responders did not differ significantly from those of higher risk responders who were randomized either to 8 months of active continuation-phase treatment or to PBO over months 9–32 post A-CT. Specifically, 11 of 38 (KM = 32.0%) lower risk patients, 28 of 108 patients (KM = 30.5%) in active treatment and 9 of 35 patients (KM = 35.1%) randomized to PBO relapsed/recurred: χ2(1) = 0.01, p = .94, R2 = .000 for the comparison with active treatment and χ2(1) = 0.00, p = .99, R2 = .000 for that with PBO.
Secondary Analyses
What is/are the best pretreatment predictor(s) of higher and lower risk?
In multivariate analyses, final pre-treatment HRSD-17, ASQ global subscale, and years of education were the best collective predictors of risk strata. To reach this solution, all pretreatment univariate predictors that were statistically significant at p < .10 (education in years, final pretreatment HRSD-17, ASQ global and stable subscales, DAS, BHS, SCS, IIP, and SAS-SR scores, except the DYS [completed only by married or cohabiting participants, and excluded so as not to reduce sample size]) were included in a multivariate logistic regression analysis. Using a variable retention p ≤ .05 criterion in a backward-selection method, pre-treatment HRSD-17 (χ2(1) = 11.92, p < .01; odds ratio = 0.84), the ASQ-global subscale (χ2(1) = 7.64, p < .01; odds ratio = 0.63), and years of education (χ2(1) = 5.40, p = .02; odds ratio = 0.86) each significantly predicted risk, model R2 = .10 and area under the curve = .71. On average, the odds of being in the lower risk stratum is reduced by about 16% with each one point increase in HRSD-17 (or 63% for a 1 SD increase in HRSD-17, based on the pre-treatment sample in Table 1), 37% for each point increase in ASQ-global subscale score (36% for a 1 SD increase in ASQ-global), and 14% for each additional year of education (39% for a 1 SD increase in education).
What is/are the best mid-treatment predictor(s) of higher and lower risk?
Of the mid-treatment predictors tested, early versus late response to A-CT is the single best predictor of risk strata. All mid-treatment univariate predictors that were statistically significant at p < .10 (WAI-T scores and early/late response to A-CT) were included in a multivariate logistic regression analysis. Using a backward selection method, patients’ rate of response by session 9 of A-CT (out of 16–20 sessions) was the most significant predictor of risk strata, χ2(1) = 21.45, p < .01, odds ratio = 9.64, R2 = .11, AUC = .71. Among early responders, 44 of 159 (27.6%) were in the lower risk strata, whereas only 5 of 131 (3.8%) late responders were lower risk. This last finding is not surprising, because both early/late response and risk strata are based on depressive symptom levels, but it is important nonetheless, as it provides clinicians with a mid-treatment indicator of whether patients are likely to need therapeutic revisions or additions to a traditional 16- to 20-session A-CT protocol.
Discussion
In summary, the lower higher risk formula paired with these results provide clinicians with signals to use before, during, and after A-CT to decide which A-CT responders can safely discontinue CT with a lower risk of relapse. The marker of positive outcomes, (i.e., the definition of lower risk in our analyses), was reaching a low symptom level by roughly the middle of A-CT, and maintaining few residual symptoms during the remaining weeks of acute-phase treatment. These prospective findings, from a large sample with two sites, strongly confirm that rapid and stable remission during the final weeks of A-CT identifies the majority of responders who do not need additional immediate continuation-phase treatment to prevent relapse. These responders appear to have positive outcomes that are sustained through approximately the first year after remission during A-CT. Thereafter, these lower risk patients do experience recurrence or new episodes of depression, albeit at a lower rate than might be expected based on the natural course of illness (Mueller et al., 1999). In addition, the finding that over the first year the relapse/recurrence rates of lower risk responders do not differ from higher risk responders on active treatment, but are significantly lower than those on pill placebo, reinforces both the positive effects of continuation-phase treatment and the validity of the higher and lower risk distinction.
This report builds on the emerging literature showing that different forms of preventive CT have robust effects and are aptly named (Bockting, Hollon, Jarrett, Kuyken, & Dobson et al., in press; Clarke, Mayo-Wilson, Kenny, & Pilling, 2015). The primary contributions of this report are to identify which CT responders need continuation-phase treatment to avoid relapse and to offer hypotheses on how to prevent recurrence. We prospectively evaluated the extent to which the lower and higher classification of risk predicted relapse and recurrence in a sample that is quite large within psychotherapy trials. In previous reports (Jarrett et al., 2001; Jarrett et al., 2008; Thase et al., 1992), the classification was based on post-hoc analyses. Results here prospectively confirm that unstable acute-phase remission among responders foreshadows depressive relapse and recurrence. These data suggest that lowered risk for relapse is marked in A-CT responders by the last seven consecutive HRSD-17 scores of A-CT being 6 or less.
The lower risk patients (17% of the responders) prospectively showed a significantly lower rate of relapse over the first 8 months post-A-CT compared to higher risk responders; however, as the time from the end of A-CT increased, so did their rate of depressive recurrence. We underscore the importance of teaching patients diagnosed with recurrent depression to recognize their symptoms and suggest routine diagnostic evaluations for those not receiving continuation- or maintenance-phase therapy. We hypothesize that lower risk patients might benefit from maintenance-phase treatment that is strategically tied to the onset of sub-threshold depressive symptoms that begin to emerge as the time away from A-CT increases, which in this sample was about 16 months post A-CT.
Over the first year after A-CT response, approximately 12.4% of the lower risk patients had relapsed or recurred, again suggesting that these patients generally did not require immediate post-acute-phase treatment. This conclusion was confirmed by the finding that over the first 8 months after A-CT, lower risk responders experienced less relapse without continuation-phase treatment compared to the higher risk responders who were on C-CT or FLX. In addition, these findings show that the greater relapse among the higher risk responders cannot be attributed exclusively to those on pill placebo plus clinical management. Importantly, for those responders judged to be at lower risk, the benefits of a 12-week course of A-CT were typically sustained for more than 1 year. This is a new and exciting finding because it means that almost all of an identifiable subgroup of patients can discontinue treatment after 12 weeks and expect sustained prevention for about 1 year. At the same time, the findings also inform us that after the first year, patients in the lower and higher risk strata did not differ in their risk of recurrence, highlighting the importance of longitudinal follow-up for patients with recurrent depression to ensure rapid recognition and promptly reinitiating treatment when clinically indicated.
In contrast to the 12.4% of lower risk patients who relapsed over the first year post A-CT, 29.2% of higher risk patients had relapsed or recurred, even despite receiving 8 months of C-CT. In multivariate analyses, compared to the lower risk patients, the higher risk patients had significantly more pathological pre-treatment scores on the HRSD-17 and ASQ global subscale, and, unexpectedly, more years of education. Higher risk patients also responded more slowly to A-CT. The fact that greater symptom severity, more depressive cognitive content, and a slow A-CT response can be measured early in CT affords patients and clinicians more time to prepare for probable need for C-CT among A-CT responders. In summary, patients who initially were “healthier” on symptom and psychosocial measures and faster to respond were less likely to relapse/recur.
While there is some temporal overlap between the mid-treatment predictors and the definition of early versus late response, there is no temporal overlap with the definition of relapse and recurrence. The importance of these analyses is practical and underscores the need for corrective therapeutic actions (e.g., better specifying targets, increasing session frequency, adding antidepressant medication) when there is little early response and thus risk for later relapse or recurrence is increased.
The current report has limitations, most of which affect generalizability. The findings pertain to adults with recurrent MDD treated by experienced cognitive therapists who received ongoing supervision. The proportion of patients who were classified as lower risk was both lower than anticipated (17%; 49/290) and while similar to (Nordberg, Castonguay, Fisher, Boswell, & Kraus, 2014) was substantially smaller than in earlier studies by our groups (Jarrett et al., 2001; Thase et al., 1992). Clearly, additional investigation is needed to determine the extent to which differences in the study protocols or sample characteristics across several decades of research or other variables accounted for these differences. In addition, although the LIFE assessment of MDD has demonstrated inter-rater reliability in past studies, and showed test-retest reliability in the current study, resources prohibited verifying inter-rater reliability among the current patients.
The findings underscore that although most patients with recurrent MDD are at increased risk for relapse and/or recurrence following CT, the care of responders can be “personalized” using fairly inexpensive and straightforward clinician and patient ratings before and during A-CT. Specifically, higher risk responders characterized by (a) more symptoms and depressive cognitive content, (b) poorer social-interpersonal functioning, (c) a slower rate of acute-phase response and (d) worse therapeutic alliance at mid-treatment, needed immediate continuation-phase treatment to prevent relapse. In contrast, fewer of their lower risk counterparts experienced relapse and most did not appear to need continuation-phase treatment. However, across the subsequent 24 months of follow-up (i.e., months 9–32), the lower risk group experienced recurrence of depressive episodes at a rate that did not differ statistically from that of the higher risk responders who had completed the continuation phase without relapsing.
This finding raises a new question: What can be done to reduce the risk of recurrent depression following successful CT even further? The study results highlight the need for treatment allocation strategically tied to risk markers. We recommend longitudinal diagnostic follow-up as standard clinical practice for patients with recurrent MDD. Future research is needed to determine how frequently and for what durations patients with recurrent depression require follow-up evaluation and/or maintenance-phase treatment to prevent recurrence.
Supplementary Material
Public Health Significance.
These findings strongly confirm that lower risk responders, with stable remission, do not need continuation-phase treatment to prevent relapse. In contrast, the higher risk responders, with unstable remission, do benefit from continuation phase treatment.
Acknowledgments
This report was supported by Grants Number K24 MH001571, R01 MH058397, R01 MH069619 (to Robin B. Jarrett, Ph.D.) and R01 MH058356, R01 MH069618 (to Michael E. Thase, M.D.) from the National Institute of Mental Health (NIMH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIMH or the National Institutes of Health. We also wish to acknowledge the unrestricted support of Eli Lilly and Company, who provided fluoxetine and matched pill placebo for the first 6 years of the study. Thereafter, study materials were purchased and prepared to appear identical for both sites by the pharmacy at The University of Texas Southwestern Medical Center. We are grateful to our patients, research teams, and colleagues at The University of Texas Southwestern Medical Center, The University of Pittsburgh, and The University of Pennsylvania who made this trial possible. We appreciate the assistance of Lauren Singer, M.S. and Joanne Sanders, M.S., for their research support.
Financial Disclosure:
During the past three years Dr. Thase has consulted with and/or served on advisory boards for Advir, Alkermes, Allergan, AstraZeneca, Avenir, Bristol-Myers Squibb Company, Cerecor, Cerenex, Eli Lilly and Company, Forest Laboratories, Janssen Pharmaceutica, Johnson & Johnson, Lundbeck, MedAvante, Merck, Moksha8, Naurex, Neuronetics, Novartis, Otsuka, Nestlé (formerly Pamlab), Pfizer Pharmaceuticals, Roche, Shire, Sunovion, Takeda, and Teva. During this time, he has received grant support from Alkermes, Assurerx, AstraZeneca, Avenir, Eli Lilly and Company, Forest Laboratories, Janssen/Johnson & Johnson, Otsuka, and Roche, as well as funding from the Agency for Healthcare Research and Quality and the NIMH. He has equity holdings for MedAvante, Inc. and has received royalties from American Psychiatric Publishing, Inc. (APPI), Guilford Publications, Herald House, and W.W. Norton & Company, Inc. Two books currently promoted by the APPI specifically pertain to cognitive therapy. Dr. Thase also discloses that his spouse is an employee of Peloton Advantage, which does business with several pharmaceutical companies that market medications used to treat depression.
Dr. Jarrett’s medical center collects the payments from the cognitive therapy she provides to patients. Dr. Jarrett is a paid consultant to the NIMH and NIH. Dr. Jarrett and Dr. Vittengl are paid reviewers for UpToDate.
Footnotes
The primary goal of this paper was not to predict risk strata (although secondary analyses were conducted to examine this issue); rather the strata were factors embedded in the study design. Thus, tests of the predictive validity of the risk strata were confounded with the absence/presence of continuation-phase treatment. However, if the lower risk responders relapse less without continuation-phase treatment compared to the higher risk responders (who were on continuation-phase treatment), then it is was a strong test of the hypothesis that lower risk responders can delay treatment without risk of immediate relapse.
The following significant protocol violations occurred during the 10 years of data collection: Two patients entered A-CT with HRSD-17 = 13 at one of the two diagnostic visits; of these during A-CT, 1 responded and 1 dropped out. Four (1 early and 3 late) responders were misclassified as late and early responders, respectively. As recommended by Lachin (2000) and the Data Safety and Monitoring Board (DSMB), they are analyzed here as they were treated during data collection.
The following significant protocol violations occurred during the 10 years of data collection: Three A-CT nonresponders were randomized to the continuation/experimental phase in error and are included in the intention to treat analysis, as recommended by Lachin (2000) and the DSMB.
The following significant protocol violations occurred during the 10 years of data collection: One lower risk responder was misclassified as higher risk in error, and randomized to C-CT; this patient was analyzed as a higher risk patient.
Risk group predicted relapse over 8 months months more strongly than did the pre-treatment variables that differed significantly between strata (see Online Supplement 1 for regression analysis results).
Contributor Information
Robin B. Jarrett, Email: Robin.Jarrett@UTSouthwestern.edu, Professor of Psychiatry, Elizabeth H. Penn Professor in Clinical Psychology.
Abu Minhajuddin, Email: Abu.Minhajuddin@UTSouthwestern.edu, Associate Professor; Biostatistician.
Jeffrey R. Vittengl, Email: vittengl@truman.edu, Professor and Chair.
Lee Anna Clark, Email: lclark6@nd.edu, William J. and Dorothy K. O’Neill Professor of Psychology.
Michael E. Thase, Email: thase@mail.med.upenn.edu, Professor of Psychiatry.
References
- Akgun S. The effects of situation and learned resourcefulness on coping responses. Social Behavior and Personality. 2004;32:441–448. [Google Scholar]
- Baldwin SA, Berkeljon A, Atkins DC, Olsen JA, Nielsen SL. Rates of change in naturalistic psychotherapy: Contrasting dose–effect and good-enough level models of change. Journal of Consulting and Clinical Psychology. 2009;77:203–211. doi: 10.1037/a0015235. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA. Manual for the Beck Hopelessness Scale. San Antonio, TX: The Psychological Corporation; 1988. [Google Scholar]
- Beck AT, Kovacs M, Weissman A. Hopelessness and suicidal behavior. An overview. JAMA. 1975;234(11):1146–1149. [PubMed] [Google Scholar]
- Beck AT, Rush AJ, Shaw BF, Emery G. Cognitive therapy of depression. New York: Guilford Press; 1979. [Google Scholar]
- Beck AT, Steer RA, Kovacs M, Garrison B. Hopelessness and eventual suicide: a 10-year prospective study of patients hospitalized with suicidal ideation. American Journal of Psychiatry. 1985;142(5):559–563. doi: 10.1176/ajp.142.5.559. [DOI] [PubMed] [Google Scholar]
- Bockting C, Hollon SD, Jarrett RB, Kuyken W, Dobson K. A Lifetime Approach to Major Depressive Disorder: The Contributions of Psychological Interventions in Preventing Relapse and Recurrence. Clinical Psychology Review’s special issue “Major Depressive Disorder”. doi: 10.1016/j.cpr.2015.02.003. (In Press) [DOI] [PubMed] [Google Scholar]
- Clark LA, Watson D. Tripartite model of anxiety and depression: Psychometric evidence and taxonomic implications. Journal of Abnormal Psychology. 1991;100:316–336. doi: 10.1037//0021-843x.100.3.316. [DOI] [PubMed] [Google Scholar]
- Clarke K, Mayo-Wilson E, Kenny J, Pilling S. Can non-pharmacological interventions prevent relapse in adults who have recovered from depression? A systematic review and meta-analysis of randomised controlled trials. Clinical Psycholy Review. 2015;39:58–70. doi: 10.1016/j.cpr.2015.04.002.. [DOI] [PubMed] [Google Scholar]
- Cuijpers P, Hollon SD, van Straten A, Bockting C, Berking M, Andersson G. Does cognitive behaviour therapy have an enduring effect that is superior to keeping patients on continuation pharmacotherapy? A meta-analysis. BMJ Open. 2013;3(4) doi: 10.1136/bmjopen-2012-002542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykema J, Bergbower K, Doctora JD, Peterson C. An Attributional Style Questionnaire for general use. Journal of Psychoeducational Assessment. 1996;14(2):100–108. [Google Scholar]
- Edwards DW, Yarvis RM, Mueller DP, Zingale HC, Wagman WJ. Test-taking and the stability of adjustment scales: Can we assess patient deterioration? Evaluation Quarterly. 1978;2:275–291. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV Axis I Disorders-Patient Edition (SCID-I/P, Version 2.0) New York: New York State Psychiatric Institute, Biometrics Research Department; 1996. [Google Scholar]
- Haeffel GJ, Abramson LY, Voelz ZR, Metalsky GI, Halberstadt L, Dykman BM, Alloy LB. Negative cognitive styles, dysfunctional attitudes, and the remitted depression paradigm: A search for the elusive cognitive vulnerability to depression factor among remitted depressives. Emotion. 2005;5(3):343–348. doi: 10.1037/1528-3542.5.3.343. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery & Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollon SD, Jarrett RB, Nierenberg AA, Thase ME, Trivedi M, Rush AJ. Psychotherapy and medication in the treatment of adult and geriatric depression: Which monotherapy or combined treatment? Journal of Clinical Psychiatry. 2005;66(4):455–468. doi: 10.4088/jcp.v66n0408. [DOI] [PubMed] [Google Scholar]
- Horowitz LM, Rosenberg SE, Baer BA, Ureno G, Villasenor VS. Inventory of Interpersonal Problems: Psychometric properties and clinical applications. Journal of Consulting & Clinical Psychology. 1988;56(6):885–892. doi: 10.1037//0022-006x.56.6.885. [DOI] [PubMed] [Google Scholar]
- Horvath AO, Greenberg LS. Development and validation of the Working Alliance Inventory. Journal of Counseling Psychology. 1989;36:223–233. [Google Scholar]
- Ilardi SS, Craighead WE. The relationship between personality pathology and dysfunctional cognitions in previously depressed adults. Journal of Abnormal Psychology. 1999;108(1):51–57. doi: 10.1037//0021-843x.108.1.51. [DOI] [PubMed] [Google Scholar]
- Jarrett RB. Cognitive therapy for recurrent unipolar major depressive disorder The continuation/maintenance phase Unpublished treatment manuals 1989; 1992 [Google Scholar]
- Jarrett RB, Kraft D, Doyle J, Foster BM, Eaves G, Silver PC. Preventing recurrent depression using cognitive therapy with and without a continuation phase. Archives of General Psychiatry. 2001;58(4):381–388. doi: 10.1001/archpsyc.58.4.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrett RB, Minhajuddin A, Gershenfeld H, Friedman ES, Thase ME. Preventing depressive relapse and recurrence in higher-risk cognitive therapy responders: a randomized trial of continuation phase cognitive therapy, fluoxetine, or matched pill placebo. JAMA Psychiatry. 2013;70(11):1152–1160. doi: 10.1001/jamapsychiatry.2013.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrett RB, Thase ME. Comparative efficacy and durability of continuation phase cognitive therapy for preventing recurrent depression: Design of a double-blinded, Fluoxetine- and pill-placebo–controlled, randomized trial with 2-Year follow-up. Contemporary Clinical Trials. 2010;31:355–377. doi: 10.1016/j.cct.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrett RB, Vittengl JR, Clark LA. How much cognitive therapy, for which patients will prevent depressive relapse? Journal of Affective Disorders. 2008;111:185–192. doi: 10.1016/j.jad.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller MB. Past, present, and future directions for defining optimal treatment outcome in depression: remission and beyond. JAMA. 2003;289(23):3152–3160. doi: 10.1001/jama.289.23.3152. [DOI] [PubMed] [Google Scholar]
- Keller MB, Lavori PW, Friedman B, Nielson E, Endicott J, McDonald-Scott P, Andreasen N. The Longitudinal Interval Follow-up Evaluation: A comprehensive method for assessing outcome in prospective longitudinal studies. Archives of General Psychiatry. 1987;44(6):540–548. doi: 10.1001/archpsyc.1987.01800180050009. [DOI] [PubMed] [Google Scholar]
- Lachin JM. Statistical considerations in the Intent-to-Treat Principle. Controlled Clinical Trials. 2000;21:167–189. doi: 10.1016/s0197-2456(00)00046-5. [DOI] [PubMed] [Google Scholar]
- Lutz W, Martinovich Z, Howard KI, Leon SC. Outcomes management, expected treatment response, and severity-adjusted provider profiling in outpatient psychotherapy. Journal of Clinical Psychology. 2002;58:1291–1304. doi: 10.1002/jclp.10070. [DOI] [PubMed] [Google Scholar]
- Martin DJ, Garske JP, Davis MK. Relation of the therapeutic alliance with outcome and other variables: A meta-analytic review. Journal of Consulting and Clinical Psychology. 2000;68(3):438–450. [PubMed] [Google Scholar]
- Mueller TI, Leon AC, Keller MB, Solomon DA, Endicott J, Coryell W, Maser JD. Recurrence after recovery from major depressive disorder during 15 years of observational follow-up. American Journal of Psychiatry. 1999;156(7):1000–1006. doi: 10.1176/ajp.156.7.1000. [DOI] [PubMed] [Google Scholar]
- Nordberg SS, Castonguay LG, Fisher AJ, Boswell JF, Kraus D. Validating the rapid responder construct within a practice research network. Journal of Clinical Psychology. 2014;70(9):886–903. doi: 10.1002/jclp.22077. [DOI] [PubMed] [Google Scholar]
- Paykel ES, Scott J, Teasdale JD, Johnson AL, Garland A, Moore R, Pope M. Prevention of relapse in residual depression by cognitive therapy: A controlled trial. Archives of General Psychiatry. 1999;56(9):829–835. doi: 10.1001/archpsyc.56.9.829. [DOI] [PubMed] [Google Scholar]
- Peterson C, Semmel A, von Baeyer C, Abramson LY, Matalsky GI, Seligman MP. The Attributional Style Questionnaire. Cognitive Therapy and Research. 1982;6:287–300. [Google Scholar]
- Rosenbaum M. A schedule for assessing self-control behaviors: Preliminary findings. Behavior Therapy. 1980;11:109–121. doi: 10.1016/S0005-7894(80)80040-2. [DOI] [Google Scholar]
- Segal ZV, Bieling P, Young T, MacQueen G, Cooke R, Martin L, Levitan RD. Antidepressant monotherapy vs sequential pharmacotherapy and mindfulness-based cognitive therapy, or placebo, for relapse prophylaxis in recurrent depression. Arch Gen Psychiatry. 2010;67(12):1256–1264. doi: 10.1001/archgenpsychiatry.2010.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slessareva E, Muraven M. Sensitivity to punishment and self-control: The mediating role of emotion. Personality and Individual Differences. 2004;36:307–319. [Google Scholar]
- Spanier GB. Measuring dyadic adjustment: New scales for assessing the quality of marriage and similar dyads. Journal of Marriage and the Family. 1976;38:15–28. [Google Scholar]
- Thase ME, Simons AD, McGeary J, Cahalane JF, Hughes C, Harden T, Friedman E. Relapse after cognitive behavior therapy of depression: Potential implications for longer courses of treatment. American Journal of Psychiatry. 1992;149(8):1046–1052. doi: 10.1176/ajp.149.8.1046. [DOI] [PubMed] [Google Scholar]
- Vittengl JR, Clark LA, Dunn TW, Jarrett RB. Reducing relapse and recurrence in unipolar depression: A comparative meta-analysis of cognitive-behavioral therapy’s effects. Journal of Consulting and Clinical Psychology. 2007;75(3):475–488. doi: 10.1037/0022-006X.75.3.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vittengl JR, Clark LA, Thase ME, Jarrett RB. Stable remission and recovery after acute-phase cognitive therapy for recurrent major depressive disorder. Journal of Consulting & Clinical Psychology. 2014 doi: 10.1037/a0037401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman AN. The Dysfunctional Attitudes Scale: A validation study (Doctoral dissertation, U Pennsylvania, 1979) Diss Abstracts International. 1979;40:1389B–1390B. [Google Scholar]
- Weissman MM, Bothwell S. Assessment of social adjustment by patient self-report. Archives of General Psychiatry. 1976;33(9):1111–1115. doi: 10.1001/archpsyc.1976.01770090101010. [DOI] [PubMed] [Google Scholar]
- Weissman MM, Prusoff BA, Thompson D, Harding PS, Myers JK. Social adjustment by self-report in a community sample and in psychiatric outpatients. Journal of Nervous and Mental Disease. 1978;166:317–326. doi: 10.1097/00005053-197805000-00002. [DOI] [PubMed] [Google Scholar]
- Young J, Beck AT. Cognitive Therapy Scale: Rating manual. Center for Cognitive Therapy; 1980. [Google Scholar]
- Zimmerman M, Posternak MA, Chelminski I. Heterogeneity among depressed outpatients considered to be in remission. Comprehensive Psychiatry. 2007;48(2):113–117. doi: 10.1016/j.comppsych.2006.10.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


