Abstract
Developing a single selective ligand to a target relevant to two mechanistically interlinked diseases, such as type 2 diabetes mellitus (T2DM) and a neurodegenerative disorder, like Parkinson's disease or Alzheimer's disease, provides the potential for an effective treatment that may impact both. The enzyme 5-lipoxygenase (5-LOX) has been revealed responsible for producing fatty acid molecules, leukotrienes. These leukotrienes are known to produce inflammatory responses in asthma and allergic reactions, to induce a reduction of tyrosine hydroxylase in brain, and are involved in the development of cardiac strokes, obesity and type 2 diabetes. N1-p-fluorobenzyl-cymserine (FBC), an analogue of cymserine and a known cholineterase inhibitor, was evaluated for inhibition of pleiotropic 5-LOX in our study. The stable 3D structure of 5-LOX was obtained from the Protein Data Bank (PDB) database and was implied for homology modeling of four reported mutant models. Each generated model was submitted to the Protein Model Database (PMDB) and employed for measuring inhibition and ligand efficiency of FBC with support of molecular docking. For each model, normal as well as mutant, FBC yielded remarkable inhibition constant values, with exothermic free binding energies. The current study revealed a highly reactive narrow fissure near the non-heme iron binding pocket of 5-LOX that contains residues crucial for 5-LOX stability and FBC binding. Investigating the binding of FBC with stabilized and destabilized 5-LOX structures confirmed it as a candidate therapeutic inhibitor worthy of assessment in preclinical models of T2DM and neurodegeneration.
Keywords: -lipoxygenase, docking, fluorobenzylcymserine, 5type 2 diabetes
INTRODUCTION
Various genome wide studies have focused on the possible reasons underpinning mechanistic links between the occurrences of type 2 diabetes mellitus (T2DM) and neurodegenerative disorders, such as Parkinson's disease (PD) or Alzheimer's disease (AD). The increased prevalence of both T2DM and impaired glucose regulation has been widely reported in both PD and AD [1, 2]. Another common feature of T2DM, insulin resistance has also been related to central nervous system dysfunction and ageing [3]. Indeed, multiple potential mechanisms are involved in the association between T2DM and neurodegeneration. These include glucose toxicity, direct effects of insulin, amyloid-β peptide (Aβ) induced inhibitory actions on neuronal insulin receptor signaling, inflammatory cytokines and oxidative stress [4-11]. A number of new therapeutic strategies have been proposed to offset neurodegenerative disorders. Some of these include incretins [12], TNF-alpha inhibition [13-15], antioxidants [16-18] non-steroidal anti-inflammatory drugs and inhibition of protein misfolding by inflammatory mediators [19-21]. Many of these approaches possess the potential to impact pathways of relevance in multiple diseases and, as advised by Collins [22], a “focus on targets that may be relevant to multiple diseases” provides a way forward to generate greater opportunity for clinical translation and success.
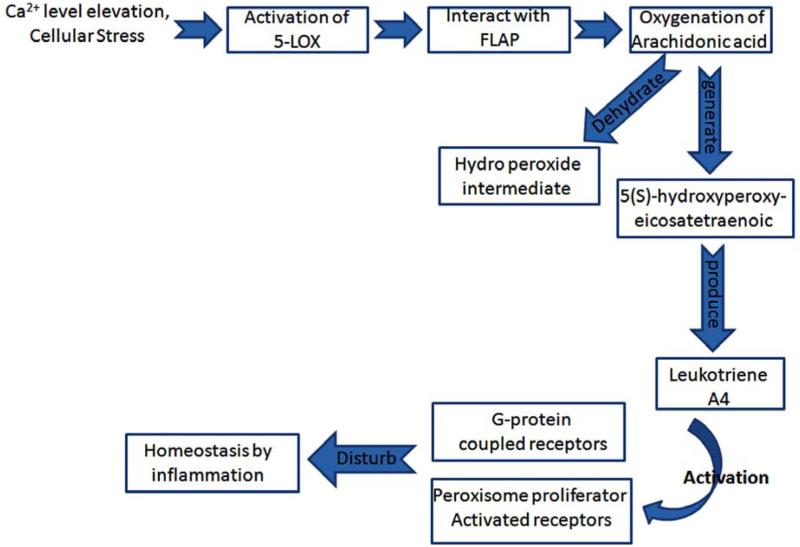
Recently, the 5-LOX gene (ALOX5) has been reported involved across a number of physiological actions [23]. 5-LOX is a member of a family of dioxygenases that share up to 60% sequence similarity in humans. It is a calcium-requiring, ATP-requiring and non-heme iron-requiring enzyme. However, the best characterized enzyme action is its ability to catalyze the initial step in the oxygenation of arachidonic acid at the 5-position to generate 5(S)-hydroxyperoxyeicosatetraenoic and, thereafter, the dehydration of the hydroperoxide intermediate to produce the epoxide, 5(S),6(S)-oxidoeicosatetraenoic acid (leukotriene A4). 5-LOX is expressed primarily within inflammatory cells, specifically polymorphonuclear leukocytes, monocytes, eosinophils, B-lymphocytes and mast cells. Following its activation, primarily consequent to intracellular Ca2+ elevation and cellular stress, 5-LOX translocates to the nuclear compartment where it interacts with 5-LOX-activating protein (FLAP) to convert arachidonic acid into leukotrienes [23, 24]. Released leukotrienes exert their biological actions through activation of defined G-protein-coupled receptors and potentially via peroxisome proliferator-activated receptors (Fig. 1).
Fig. (1).
Schematic diagram of the mechanism depicting the disturbed homeostasis response caused by activation of 5-LOX. When 5-LOX enzyme becomes activated consequent to different environmental factors, this leads to the production of leukotrienes and subsequent receptor activation that can cause an inflammatory response and the induction of cascades leading ultimately to neurodegenerative disorders.
The inhibition and knockout of 5-LOX, FLAP or leukotriene receptors have provided insight into the physiological actions mediated by these and their awry regulation in disease development [23, 24]. 5-LOX and associated chronic overproduction of leukotrienes can cause inflammation, which can provide a beneficial part of homeostatis but its dysregulation can be harmful to human health. This can lead not only to asthma and allergic reactions but can have a role in cardiovascular disease, stroke, myocardial infarction, cancer, obesity and T2DM [25-27]. Mammalian 5-LOX are monomeric enzymes of 672 to 673 amino acids. A model structure of human 5-LOX has been generated on the known crystal structure of the ferrous form of rabbit reticulocyte 15-LOX [28]. This model, supported by mutagenesis studies [23], comprises of an N-terminal β-sandwich (comprising residues 1-114) and a larger C-terminal catalytic domain that contains the iron moiety (residues 121-673). The catalytic binding pocket of 5-LOX is composed of several α-helices and contains a non-heme iron group, which is a major prosthetic group involved in redox reactions [29]. Investigation of this 5-LOX iron binding pocket has revealed that the active site is more spacious and flexible than any other paralog of LOX (e.g., 12-LOX, 15-LOX). Along with the iron binding pocket, a substrate binding cleft contains various residues having acidic and basic moieties, hydrophobic residues and additional amino acids which aid the interactions of LOX inhibitors inside the pocket. In the current study, we evaluated a potential interaction of 5-LOX with a specific inhibitor, FBC. FBC is an analogue of cymserine, a known cholinesterase inhibitor, with a preference for the butyryl form of the enzyme [30, 31], a drug class being developed for the treatment of AD [32]. Additionally molecular docking studies were performed between normal and mutant LOX proteins, in order to characterize the mode of inhibition of FBC by focusing interactions between the two.
MATERIALS AND METHODS
The molecular structure of FBC was generated using ChemOffice 8.0 Ultra, and energy was minimized using MOPAC2009 and RMI semi-empirical methods. The tertiary structure of stable 5-LOX was available at RCSB PDB, pdb id: 3O8Y [33], as a dimeric oxidoreductase having a molecular weight of 158986 kDa, a resolution of about 2.39 Å and containing iron as a prosthetic group. In order to perform flexible docking, a monomer of 5-LOX and FBC were used in AUTODOCK 4.2. We generated 3-dimensional models of a total of four reported mutants of 5-LOX by using homology modeling in the automated mode of a Swiss Modeler [34] and submitted these to PMDB [35] (PM0078252, PM0078253, PM0078254, PM0078255). Sequence similarity and mutant sites are shown in Fig. (2). Each model was subjected to docking for a maximum of 150 populations. Iron was added to the LOX protein, as a prosthetic group, thereby, adding additional charges to the gasteric charges. A grid box was set for each normal and mutant enzyme around the core/iron pocket, with measuring of the X, Y, Z co-ordinates as 126°, 90° and 80°, respectively. While setting the grid, various docking experiments were performed, including the peripheral helical motifs. However, the core iron binding pocket was always contemplated carefully. A hybrid of Lamarckian and Genetic Algorithm is used by AUTODOCK 4.2, with maximum energy evaluations equal to 2,500,000, RMSD cut off of 2.0 Å, cross-over and mutation rates (recombination) equal to 0.8 and 0.02 respectively. A total of 100 runs were performed for each docking experiment. Flexible docking was performed between the ligand and receptor, with the exclusion of non-interacting water molecules. Only eleven water molecules, which were found consistent in the binding pocket, were present to maintain their role for interaction.
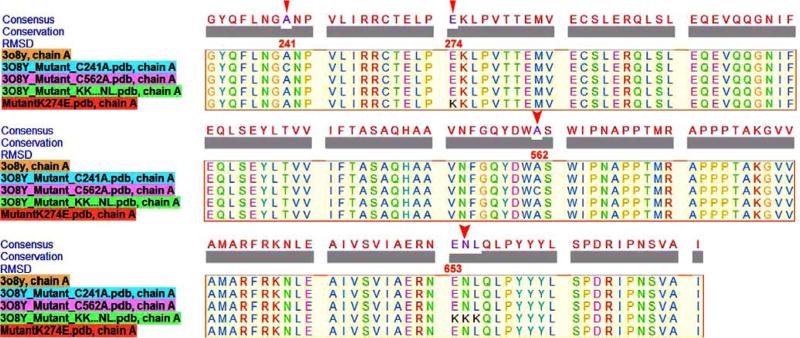
Fig. (2). Multiple sequence alignment of normal and mutant 5-LOX sequences.
Structural superimposition of normal protein sequence (3O8Y) with four generated mutants is performed. Sequence conservation and mutant residues are highlighted. Red triangles indicate the mutant residue along with their position.
RESULTS & DISCUSSION
The stabilized structure of the 5-LOX enzyme was subjected to docking interactions with FBC as an inhibitor. Gilbert and colleagues [33] have described specific residues within the 5-LOX structure from which mutants can be generated to inhibit 5-LOX activity at various points. Among these mutants, the most important, which could induce destabilization of the 5-LOX enzyme, was replacement of GluAsnLeu (ENL) 653-655 with a lysine rich region, LysLysLys (KKK) 653-655 [36]. The second and third generated mutant involved the substitution of Ala in alpha-helices by Cys at position 562 and 241 respectively (Fig. 2). The presence of Cys establishes more disulphide bridges than hydrogen bonding, hence disturbing the structural fidelity of alpha-helices, leading to an awry protein folded structure. The fourth mutation, taken into account, was a novel polymorphism in which Lys replaced Glu at position 274. This polymorphism was first reported by Bai and colleagues [37], and is involved in a disease phenotype of bronchial asthma.
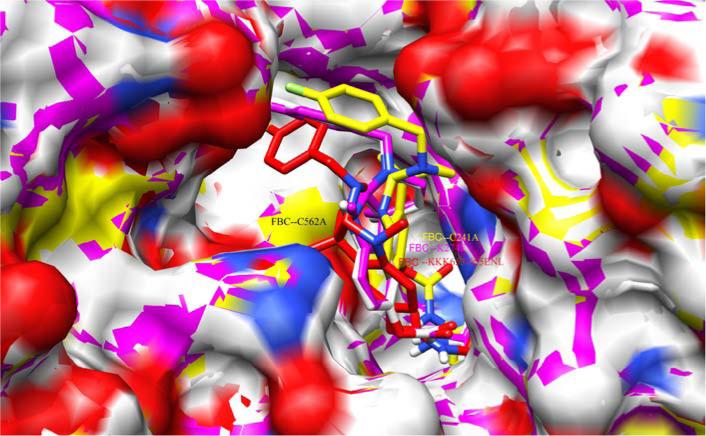
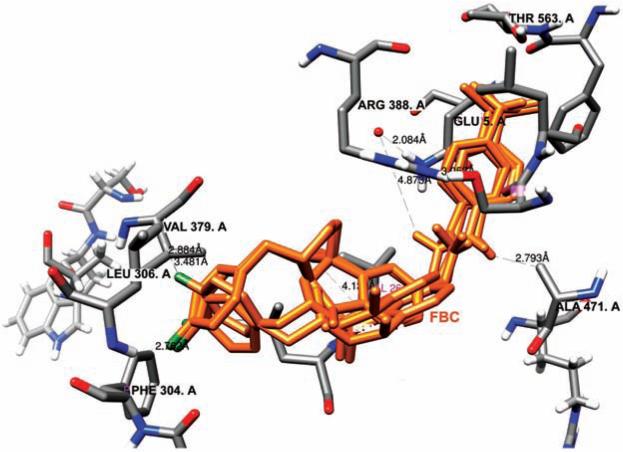
Hence in our study, we exploited five structures of 5-LOX, including one normal stabilized enzyme and four mutants, interacting with FBC. Our aim was to assess the inhibition constant and ligand efficiency of FBC for normal as well as mutant protein. The interaction of FBC was tested with each mutant in order to check the stability of loss of function mutations after docking with the inhibitor. The binding pocket of 5-LOX contains iron, bounded with three His and an Ile. Parallel to this active site there is a strong binding groove in the form of a fissure, leading to a central pocket (Fig. 3). The FBC inhibitor intercalated into this narrow groove and made strong van der Waal interactions with the neighboring residues. Arg 388, Gln 567, Thr 563, Ser 465, Leu 306, Val 261, Ala 471 and Val 379 were found to be the most conserved and crucial residues of the narrow groove. One interesting observation was that all these interacting residues lie within the range of <5.0 Å (Fig. 4). As FBC comprises of highly electronegative fluorine, nitrogen and oxygen as its functional groups, its likelihood of successfully reaching the central pocket is high. Due to three conserved histidines (His 367, His 372, His 550), the active site's iron exists with a valency of Fe3+. However, in the event that a mutant is generated with substitution of any of these histidines, FBC may be in a position to make possible interactions with iron. The flourobenzyl moiety within FBC provides electro negativity to its structure and makes it more interactive with the neighboring residues. A comparison of docking results was made by two important parameters (binding energy and inhibition constant), shown in Table 1. The binding energy values revealed that the Cys mutant has lowest binding energy (ΔG −7.91 Kcal/mol) as compared to other mutants. Similarly FBC's lowest inhibition constant was also found for the Cys mutant (Ki 0.37 μ M). However, a slight variation was observed in the parameter of all mutant variants. The values of Ki and ΔG for the normal enzyme are also worthy of noting, specifically 0.545 μM and −8.54 Kcal/mol, respectively. These results support the fact that FBC can be used as a potentially active inhibitor against normal/active 5-LOX as well as its mutant variants.
Fig. (3). Docked result of FBC in complex with novel identified narrow fissure near the non-heme iron binding pocket of 5-LOX.
Superimposition of mutants docked complexes are shown in different colors, Cys241Ala mutant: yellow, Lys274Glu: pink, LysLysLys653-656GluAsnLys: red, Cys562Ala: white.
Fig. (4). Atomic distances between reactive functional groups of FBC and neighboring residues of 5-LOX.
The electronegative oxygen, nitrogen and fluorine showed interaction with Val 379, Leu 306, Phe 304, Ala 471, Arg 388, Ser 475 and Thr 563. The criterion for atomic distances was set ≤ 4.5 Å. Atomic distances were measured using Chimera 1.5.3.
Table 1.
Docking Result Parameters of FBC with Normal 5-LOX Structure and its Four Homology Modeled Mutants. The Interacting Residues in All Proteins were Found to be Conserved with Atomic Distances ≤4.5 Å
| Protein | Binding Free Energy Kcal/mol |
Inhibition Constant Ki, μM |
Ligand Efficiency |
Intermolecular Energy |
Reference for Mutation |
Interacting Residues | Atomic Distance with FBC (Å) |
|---|---|---|---|---|---|---|---|
| 3O8Y | −8.54 | 0.545 | −0.24 | −10.33 | [33] | Arg246, Val361, Ala453, Val243, Leu244 | 2.2, 2.9, 2.4, 3.6, 4.05 |
| Cys562Ala | −7.91 | 1.58 | −0.23 | −9.7 | [33] | Arg264, Ala471, Arg264, Thr563, Val379, Leu306 | 2.25,2.79, 3.7, 3.2, 2.88, 2.88 |
| Cys241Ala | −8.76 | 0.376 | −0.26 | −10.07 | [33] | Val261, Val379, Leu466, Gln567, Arg388 | 3.3, 4.5, 3.5, 3.7, 3.43 |
| LysLysLys653-655GluAsnLeu | −8.38 | 0.725 | −0.24 | −10.17 | [33] | Leu306, Gln305, HOH733, Val261, Arg388, Gln567 | 4.9,3.87,2.7, 3.5, 3.7, 4.5 |
| Lys274Glu | −8.58 | 0.515 | −0.25 | −10.37 | [35] | Arg264, Leu262, Arg388, Gln567, Ala471 | 3.2, 3.0, 4.5, 4.5, 3.5 |
CONCLUSIONS
From our docking data values, we can deduce that whereas FBC may be a potent inhibitor against normal 5-LOX, it would be yet more effective with the mutant structures. As the substitution of Glu with Lys is highly important for stability, FBC can inhibit this mutant with decidedly agreeable energy parameters. Among the eleven water molecules within the binding pocket, only one showed interaction with the Lys rich mutant, supporting the notion of instability of 5-LOX by Lys substitution. In synopsis, the current work supports the appraisal of FBC in preclinical cellular and in vivo models as a potentially interesting inhibitor of 5-LOX to evaluate this drug target in the development of T2DM and neurodegenerative disorders.
ACKNOWLEDGEMENTS
This research was supported in part by the COMSATS Institute of Information Technology, Islamabad, Pakistan, the King Fahd Medical Research Center, King Abdulaziz University, Jeddah, Saudi Arabia, and the Intramural Research Program, National Institute on Aging, NIH, Baltimore, Maryland, USA.
LIST OF ABBREVIATIONS
- AD
Alzheimer's disease
- Aβ
Amyloid-β peptide
- FBC
N1-p-fluorobenzyl-cymserine
- T2DM
Type 2 diabetes mellitus
- PD
Parkinson's disease
- 5-LOX
5-lipoxygenase
- PDB
Protein Data Bank
- PMDB
Protein Model Database
Footnotes
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Arvanitakis Z, Wilson RS, Bienias JL, Evans DA, Bennett DA. Diabetes mellitus and risk of Alzheimer disease and decline in cognitive function. Arch Neurol. 2004;61:661–6. doi: 10.1001/archneur.61.5.661. [DOI] [PubMed] [Google Scholar]
- 2.Hu G, Jousilahti P, Bidel S, Antikainen R, Tuomilehto J. Type 2 diabetes and the risk of Parkinson's disease. Diabetes Care. 2007;30:842–7. doi: 10.2337/dc06-2011. [DOI] [PubMed] [Google Scholar]
- 3.Cholerton B, Baker LD, Craft S. Insulin resistance and pathological brain ageing. Diabet Med. 2011;28:1463–75. doi: 10.1111/j.1464-5491.2011.03464.x. [DOI] [PubMed] [Google Scholar]
- 4.Schmidt R, Launer LJ, Nilsson LG, et al. Magnetic resonance imaging of the brain in diabetes: the Cardiovascular Determinants of Dementia (CASCADE) Study. Diabetes. 2004;53:687–92. doi: 10.2337/diabetes.53.3.687. [DOI] [PubMed] [Google Scholar]
- 5.Biessels GJ, Kappelle AC, Bravenboer B, Erkelens DW, Gispen WH. Cerebral function in diabetes mellitus. Diabetologia. 1994;37:643–50. doi: 10.1007/BF00417687. [DOI] [PubMed] [Google Scholar]
- 6.Kamal A, Biessels GJ, Urban IJ, Gispen WH. Hippocampal synaptic plasticity in streptozotocin-diabetic rats: impairment of long-term potentiation and facilitation of long-term depression. Neuroscience. 1999;90:737–45. doi: 10.1016/s0306-4522(98)00485-0. [DOI] [PubMed] [Google Scholar]
- 7.Magarinos AM, McEwen BS. Experimental diabetes in rats causes hippocampal dendritic and synaptic reorganization and increased glucocorticoid reactivity to stress. Proc Natl Acad Sci USA. 2000;97:11056–61. doi: 10.1073/pnas.97.20.11056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li ZG, Zhang W, Grunberger G, Sima AA. Hippocampal neuronal apoptosis in type 1 diabetes. Brain Res. 2002;946:221–31. doi: 10.1016/s0006-8993(02)02887-1. [DOI] [PubMed] [Google Scholar]
- 9.Haan MN. Therapy insight: type 2 diabetes mellitus and the risk of late-onset Alzheimer's disease. Nat Clin Pract Neurol. 2006;2(3):159–66. doi: 10.1038/ncpneuro0124. [DOI] [PubMed] [Google Scholar]
- 10.Abbatecola AM, Olivieri F, Corsonello A, et al. Genome-wide association studies: is there a genotype for cognitive decline in older persons with type 2 diabetes? Curr Pharm Des. 2011;17(4):347–56. doi: 10.2174/138161211795164239. [DOI] [PubMed] [Google Scholar]
- 11.Priyadarshini M, Kamal MA, Greig NH, et al. Alzheimer disease and type 2 diabetes: Exploring the association to obesity and tyrosine hydroxylase. CNS Neurol Disord Drug Targets. 2012;11(4):482–9. doi: 10.2174/187152712800792767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salcedo I, Tweedie D, Li Y, Greig NH. Neuroprotective and neurotrophic actions of glucagon-like peptide-1: an emerging opportunity to treat neurodegenerative and cerebrovascular disorders. Br J Pharmacol. 2012;166:1586–99. doi: 10.1111/j.1476-5381.2012.01971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lipton SA, Gu Z, Nakamura T. Inflammatory mediators leading to protein misfolding and uncompetitive/fast off-rate drug therapy for neurodegenerative disorders. Int Rev Neurobiol. 2007;82:1–27. doi: 10.1016/S0074-7742(07)82001-0. [DOI] [PubMed] [Google Scholar]
- 14.Frankola KA, Greig NH, Luo W, Tweedie D. Targeting TNF-α to elucidate and ameliorate neuroinflammation in neurodegenerative diseases. CNS Neurol Disord Drug Targets. 2011;10:391–403. doi: 10.2174/187152711794653751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tweedie D, Ferguson RA, Fishman K, et al. Tumor necrosis factor-α synthesis inhibitor 3:6’-dithiothalidomide attenuates markers of inflammation, Alzheimer pathology and behavioral deficits in animal models of neuroinflammation and Alzheimer's disease. J Neuroinflammation. 2012;9:106. doi: 10.1186/1742-2094-9-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ho YS, Poon DC, Chan TF, Chang RC. From small to big molecules: how do we prevent and delay the progression of age-related neurodegeneration? Curr Pharm Des. 2012;18:15–26. doi: 10.2174/138161212798919039. [DOI] [PubMed] [Google Scholar]
- 17.Mandel SA, Weinreb O, Amit T, Youdim MB. Molecular mechanisms of the neuroprotective/neurorescue action of multi-target green tea polyphenols. Front Biosci. 2012;4:581–98. doi: 10.2741/S286. [DOI] [PubMed] [Google Scholar]
- 18.Reale M, Pesce M, Priyadarshini M, Kamal MA, Patruno A. Mitochondria as an easy target to oxidative stress events in Parkinson's disease. CNS Neurol Disord Drug Targets. 2012;11:430–8. doi: 10.2174/187152712800792875. [DOI] [PubMed] [Google Scholar]
- 19.Lipton SA, Gu Z, Nakamura T. Inflammatory mediators leading to protein misfolding and uncompetitive/fast off-rate drug therapy for neurodegenerative disorders. Int Rev Neurobiol. 2007;82:1–27. doi: 10.1016/S0074-7742(07)82001-0. [DOI] [PubMed] [Google Scholar]
- 20.Lleo A, Galea E, Sastre M. Molecular targets of non-steroidal anti-inflammatory drugs in neurodegenerative diseases. Cell Mol Life Sci. 2007;64:1403–18. doi: 10.1007/s00018-007-6516-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doherty GH. Nitric oxide in neurodegeneration: potential benefits of non-steroidal anti-inflammatories. Neurosci Bull. 2011;27:366–82. doi: 10.1007/s12264-011-1530-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Collins FS. Reengineering translational science: the time is right. Sci Transl Med. 2011;3::90cm17. doi: 10.1126/scitranslmed.3002747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rådmark O, Werz O, Steinhilber D, Samuelsson B. 5-Lipoxygenase: regulation of expression and enzyme activity. Trends Biochem Sci. 2007;32:332–41. doi: 10.1016/j.tibs.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 24.Rådmark O, Samuelsson B. Regulation of the activity of 5-lipoxygenase, a key enzyme in leukotriene biosynthesis. Biochem Biophys Res Commun. 2010;396:105–10. doi: 10.1016/j.bbrc.2010.02.173. [DOI] [PubMed] [Google Scholar]
- 25.Mehrabian M, Schulthess FT, Nebohacova M, et al. Identification of ALOX5 as a gene regulating adiposity and pancreatic function. Diabetologia. 2008;51(6):987–8. doi: 10.1007/s00125-008-1002-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ben-Asher E, Lancet D. 5-Lipoxygenase activating protein (ALOX5AP): association with cardiovascular infarction and stroke. Isr Med Assoc J. 2004;6(5):318–9. [PubMed] [Google Scholar]
- 27.Yoshida T, Kato K, Yokoi K, et al. Association of genetic variants with chronic kidney disease in Japanese individuals with type 2 diabetes mellitus. Int J Mol Med. 2009;23(4):529–37. doi: 10.3892/ijmm_00000161. [DOI] [PubMed] [Google Scholar]
- 28.Gillmor SA, Villasenor A, Fletterick R, Sigal E, Browner MF. The structure of mammalian 15-lipoxygenase reveals similarity to the lipases and the determinants of substrate specificity. Nat Struct Biol. 1997;4:1003–9. doi: 10.1038/nsb1297-1003. [DOI] [PubMed] [Google Scholar]
- 29.Charlier C, Hénichart JP, Durant F, Wouters J. Structural insights into human 5-lipozygenase inhibition: combined ligand-based and target-based approach. J Med Chem. 2006;49(1):186–95. doi: 10.1021/jm050870x. [DOI] [PubMed] [Google Scholar]
- 30.Greig NH, Yu QS, Brossi A, Matin E, Lahiri DK, Darvesh S. Butyrylcholinesterase, the Cinderella cholinesterase, as a drug target for Alzheimer's diseases and related dementias. Med Chem Alzheimers Dis. 2008:79–109. [Google Scholar]
- 31.Kamal MA, Al-Jafari AA, Yu QS, Greig NH. A Kinetic Analysis of the Inhibition of Cymserine with Human Butyrylcholinesterase. Biochem Biophys Acta. 2006;1760(2):200–6. doi: 10.1016/j.bbagen.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 32.Greig NH, Utsuki T, Ingram DK, et al. Selective butyrylcholinesterase inhibition elevates brain acetylcholine, augments learning and lowers Alzheimer β-amyloid peptide in rodent. Proc Natl Acad Sci USA. 2005;102(47):17213–8. doi: 10.1073/pnas.0508575102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gilbert NC, Bartlett SG, Waight MT, et al. The structure of human 5-lipoxygenase. Science. 2011;331(6014):217–9. doi: 10.1126/science.1197203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arnold K, Bordoli L, Kopp J, Schwede T. The SWISS-MODEL workspace: a web-based environment for protein structure homology modeling. Bioinformatics. 2006;22(2):195–201. doi: 10.1093/bioinformatics/bti770. [DOI] [PubMed] [Google Scholar]
- 35.Castrignanò T, De Meo PD, Cozzetto D, Talamo IG, Tramontano A. The PMDB Protein Model Database. Nucleic Acid Res. 2006:D306–9. doi: 10.1093/nar/gkj105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuhn H, Anton M, Gerth C, Habenicht A. Amino acid differences in the deduced 5-lipoxygenase sequence of CAST atherosclerosis-resistance mice confer impaired activity when introduced into the human ortholog. Arterioscler Thromb Vasc Biol. 2003;23(6):1072–6. doi: 10.1161/01.ATV.0000074167.01184.48. [DOI] [PubMed] [Google Scholar]
- 37.Bai C, Matsui E, Ohnishi H, et al. A novel polymorphism, E254K, in the 5-lipoxygenase gene associated with bronchial asthma. Int J Mol Med. 2008;21(2):139–44. [PubMed] [Google Scholar]