Abstract
For almost 2 decades, results from Chlamydia pathogenesis investigations have been conceptualized using a cytokine polarization narrative. Recent viral immunity studies identifying protective tissue-resident memory T cells (Trm) suggest an alternative paradigm based on localized immune networks. As Chlamydia vaccines enter the preclinical pipeline and, in the case of an attenuated trachoma vaccine, are given to human subjects, it may be useful to ask whether cytokine polarization is the appropriate framework for understanding and evaluating vaccine efficacy. In this review, we revisit C. trachomatis pathogenesis data from mice and humans using a Trm narrative and note a comfortable concordance with the Chlamydia pathogenesis literature.
INTRODUCTION
Chlamydia trachomatis infections of ocular and genital tract mucosal epithelia elicit host responses that include formation of submucosal lymphoid aggregates that persist at the site of infection after infectious bacteria are no longer present. In trachoma, these aggregates, called follicles, are easily visible without magnification on routine physical examination and are used to make the clinical diagnosis. Lymphoid aggregates have also been documented in uterine and cervical samples of women with C. trachomatis genital tract infections and are seen in the C. muridarum mouse model of genital tract infections (detailed below). Investigators have speculated about a role for local lymphoid aggregates in protective immunity to Chlamydia. Most presciently, Morrison and Morrison concluded that the “persistence of CD4+-T-cell clusters long after infection had resolved (day 70) may provide for a readily mobilizable T-cell response by which previously infected animals can quickly respond to and control a secondary infectious challenge” (1). Strikingly, this concept is not featured in working models of Chlamydia immunopathogenesis. Instead, CD4 T cell cytokine polarization (Th1, Th2, Th17, …) has served as theoretical scaffolding for describing immunoprotection and immunopathology associated with infection. It is unclear whether cytokine polarization is the appropriate framework for understanding Chlamydia pathogenesis. Recent work in viral pathogenesis has identified semiautonomous tissue-localized lymphoid structures containing memory T cells as being critical for protective antiviral immunity (2, 3). The available data suggest that the tissue-resident memory T cells (Trm) in lymphoid aggregates formed in response to C. trachomatis infection play a central role in protective and pathological immune responses in the eye and genital tract.
TISSUE-RESIDENT MEMORY T CELLS
Trm were originally described for CD8 T cells in the setting of recurrent herpes simplex virus (HSV) reactivations. HSV infects peripheral sensory nerves at the site of infection and travels in a retrograde manner up nerve axons to set up a latent infection in the associated sensory ganglion. When HSV reactivates due to UV exposure or stress, it travels back down the nerve axon and infects the stromal cells adjacent to nerve endings at the site of the original infection. This reactivation event can happen multiple times but typically becomes less and less frequent over time, suggesting the slow acquisition of acquired immunity. In 2007, Zhu et al. elegantly showed that HSV-specific CD8 T cells appeared in the skin during clearance of HSV lesions and then persisted in the skin adjacent to nerve endings for months after the HSV lesion had healed (4). A more complete description of the unique tissue-resident memory CD4 and CD8 T cell immunobiology followed, including local expansion in nonlymphoid tissue dependent on recruitment of monocytes from blood (5), lack of circulation of CD8 Trm through blood and conventional lymphoid compartments (6), and partial phenotypic definition of the subset (7, 8). It was recently shown that the mechanism underlying attenuated HSV (tk- mutant virus) infections and providing protection to mice from subsequent lethal virulent HSV challenge depends on the presence of CD4 Trm in vaginal tissues. The protective CD4 Trm were found in macrophage-organized “memory lymphocyte clusters” (MLC) that developed after vaginal but not systemic attenuated infection. Interestingly, parabiotic experiments performed in the same study showed some communication of the circulating CD4 T cell pool with the preformed MLC in vaginal tissue but also showed that MLC structures were stable without access to circulating CD4 T cells. MLC preservation was dependent on macrophage production by the Ccl5 gene likely driven by low-level basal secretion of gamma interferon (IFN-γ) by CD4 Trm (9). Despite the elegance of these experiments, it remains unclear whether macrophages need to present a persistent cognate antigen to maintain the MLC and retain CD4 Trm. This information is essential to vaccine design.
CHLAMYDIA Trm
While not specifically using the term “Trm,” literature in the Chlamydia immunology field has described tissue-resident memory T cells and a variant of MLC since the mid-1980s, when Kiviat et al. described lymphoid follicles with transformed lymphocytes and plasma cells in cervical and endometrial samples from women with C. trachomatis infections (10–12). Shortly thereafter, Reacher et al. described CD4 and CD8 infiltrates in chronic trachoma conjunctival biopsy specimens (13), and compelling trachoma follicle immunohistochemistry done by Abu el-Asrar et al. showed conjunctival follicle architecture consisting of a prominent B cell component with addition of CD4/CD8 T cells and macrophages (14). Similarly, baboons transcervically infected with C. trachomatis serovar E and macaque salpingeal pockets repeatedly infected with C. trachomatis serovar D showed germinal center formation (follicles) that included plasma cells on routine histology of the cervix and salpingeal tissue, respectively (15, 16). An immunohistochemistry study in the C. muridarum mouse model by Morrison and Morrison is a virtual case study in Trm immunobiology (1). In that study, more than 1 month postresolution of a genital tract infection, there was a new (not seen in naive mice) CD8 intraepithelial lymphocyte population lining the epithelium in the vagina and there were new CD4 T cell clusters predominantly in the endometrium and localized in the stroma immediately beneath the epithelium. The CD4 clusters in the endometrium had a significant B cell component early and some macrophages with rare CD8 T cells. B cells were no longer detectable in clusters at late time points based on staining for B220 and CD19, which do not detect plasma cells. The authors concluded that the CD4 T cell clusters induced by infection “may provide for a readily mobilizable T-cell response by which previously infected animals can quickly respond to and control a secondary infectious challenge” (1). It is notable that B cells are a prominent feature of Chlamydia infection-induced lymphoid follicles in trachoma and genital tract infections of humans and that their morphology is like that of plasma cells, suggesting that they are antigen-experienced memory B cells. Using transgenic B cell approaches, it is well-established in mice that B cells activated with T cell help lose B220 expression (17) and that B220-negative B cells exist in the peritoneum in much higher levels than seen in peripheral blood (PB) (18, 19). These data suggest that there are tissue-resident B cells, likely plasma cells, as well as Trm in mucosal compartments, including the genital tract. The B cell-centered architecture documented in human Chlamydia infections, and inferred in mice, differs significantly from the macrophage-centered architecture associated with protection from experimental HSV infections of the genital tract and may represent an alternative memory lymphocyte architecture specific to intracellular bacterial pathogens such as C. trachomatis.
CYTOKINE POLARIZATION
Chlamydia pathogenesis research has shown that the IFN-γ pathway plays an important role in protective immunity. Mice deficient in interleukin-12 (IL-12) or IFN-γ action have prolonged shedding of bacteria from the genital tract and dissemination to organs outside the genital tract (20). In the mid-1990s, it was shown that IFN-γ and T cell contact resulted in upregulated epithelial inducible nitric oxide synthase (iNOS) genes and nitric oxide production sufficient to kill intracellular mouse and human Chlamydia (21–23). These results fit naturally into a Th1/Th2 paradigm, with protective immunity ascribed to CD4 T cells making IFN-γ. This narrative was reinforced by the findings that adoptive transfer of Chlamydia-specific Th2 cells (24) and Chlamydia infections experimentally skewed toward Th2 responses were accompanied by prolonged and disseminated infection (25–28). As the cytokine polarization paradigm evolved, these results were extended to include detrimental effects of Th17-skewed responses (29). A major finding suggesting that Th1 responses may not be sufficient to understand Chlamydia pathogenesis was that iNOS gene knockout mice cleared C. muridarum genital tract infections (30, 31). This result suggested that clearance of Chlamydia from the genital tract might not be adequately explained by IFN-γ results and, by extension, the Th1/Th2 paradigm.
We subsequently showed that there was a second CD4 T cell mechanism independent of nitric oxide and relatively independent of IFN-γ capable of terminating Chlamydia replication in reproductive tract epithelial cells (32). Following up those results, we showed that a mechanism dependent on the Plac8 gene was sufficient to clear the mouse genital tract in the absence of nitric oxide production (33). The Plac8 gene-dependent mechanism does not appear to depend on IFN-γ beyond its role in upregulating major histocompatibility complex (MHC) class II expression on reproductive tract epithelial cells (antigen presentation). iNOS gene knockout mice clear C. muridarum because they still have the Plac8 gene-dependent mechanism and vice versa. Other independent lines of investigation also raised questions about whether Th1 is a sufficient descriptor for protective immunity. The earliest came from adoptive transfer of Chlamydia-specific T cell clones, where a Chlamydia-specific CD4 T cell clone producing only IFN-γ was not protective whereas a Chlamydia-specific CD4 T cell clone producing tumor necrosis factor alpha (TNF-α) and IFN-γ was capable of clearing chronic C. muridarum infections in nude mice (34). In our investigations of vaccine-induced immunity, we found that antigens and adjuvants that generated multifunctional cytokine responses (IFN-γ and TNF-α) were protective whereas those generating a conventional Th1 response (IFN-γ only) were not (35–37), in agreement with studies done in Leishmania (38) and Mycobacterium tuberculosis (39). These results changed the vocabulary of protection from “Th1” to “multifunctional Th1,” and at that point the Th1/Th2/Th17 model appeared to be transforming itself to explain existing data.
HUMAN STUDIES, IFN-γ, AND IL-13
Early human clinical investigations supported a protective Th1 narrative for Chlamydia infections. In a study comparing women with histories of pelvic inflammatory disease (PID) or multiple Chlamydia infections to seropositive women with neither, protection correlated with enhanced peripheral blood mononuclear (PBM) IFN-γ responses to Chlamydia hsp60, consistent with protective Th1 immunity (40). Although early trachoma immunoepidemiology suggested the importance of the Th1/Th2 framework (41), later studies failed to support the concept (42, 43). Strikingly, IL-13 production as measured by transcriptomics was epidemiologically correlated with protection in the latter study. In 2005, we did a clinical investigation of incident Chlamydia genital tract infections in a high-risk sex worker cohort. In that study, we found two independent immune correlates for remaining free of incident infection (protection) (44). The first was peripheral blood mononuclear cell (PBMC) production of IFN-γ when activated by Chlamydia hsp60, consistent with a protective Th1 response. The second predictor of protection was PBMC production of IL-13 when activated by Chlamydia elementary bodies. IL-13 is a quintessential Th2 cytokine. At that time, we were unable to reconcile those results.
CHLAMYDIA Trm AS AN ALTERNATIVE PARADIGM
We now propose to reconcile the results by considering an alternative narrative not centered on Th1/Th2/Th17 cytokine polarization. We propose that Chlamydia-specific lymphoid aggregates persisting after clearance of bacteria are central to Chlamydia immunobiology and that Chlamydia pathogenesis data can be comfortably explained by the presence of tissue-resident memory T cells unconstrained by the cytokine polarization vocabulary. There are two major predictions that come directly from a Trm model for Chlamydia pathogenesis. The first is demonstrable effective local versus systemic immunity. The second is challenge-induced expansion of the numbers of Chlamydia-specific T cells within genital tract tissues, a recognized feature of Trm during protective immune responses.
Older studies in the guinea pig inclusion conjunctivitis (GPIC; Chlamydia caviae) model of trachoma demonstrated a local immunity effect. Guinea pigs that clear a GPIC ocular infection cannot be reinfected ocularly and have no immunopathology on rechallenge. Guinea pigs originally infected at non-ocular sites develop ocular infections on rechallenge that are cleared more quickly but with significant immunopathology (45). The most straightforward interpretation of these data is that the primary ocular infections left behind a local memory response that was very effective at preventing reinfection, consistent with the presence of Trm.
A characteristic feature of Trm is expansion in their nonlymphoid tissue residence during recall responses. This phenomenon was seen in a C. muridarum mouse model during an investigation focused on homing. α4β7, the lymphocyte receptor for the mucosal addressin MadCam-1, is expressed by a large majority of Chlamydia-specific CD4 T cell clones isolated in the C. muridarum mouse model (46) (our unpublished data), even though those clones came from immune splenocytes, which represent a central immune compartment. Cell surface expression of α4β7 by T cells in mucosal immune compartments is upregulated during activation (47). In 1997, Kelly and Rank found increased α4β7 intensity and numbers of α4β7 CD4 T cells in the genital tract but not in iliac lymph nodes or spleen during secondary infectious genital challenge (48). Although these findings were interpreted as representing recruitment to the genital tract during secondary infections, in retrospect they more likely represent expansion of the numbers of Chlamydia-specific α4β7 CD4 Trm in the genital tract. In support of this conjecture, Stary et al. (49) showed that mucosal but not systemic immunization with an experimental Chlamydia vaccine resulted in the seeding of the mucosa with effector T cells that established Trm and protective immunity.
We propose a working model for protective immunity to Chlamydia infections of the eyes and genital mucosal surfaces based on Trm. A central feature of the model is infection-induced formation of lymphoid aggregates based on immune B cells rather than on the macrophages described in the experimental HSV vaginal infections. This is based on memory lymphocyte clusters (MLC) containing plasma cells in endometrial and cervical samples from infected women and specific immunohistochemistry of trachoma follicles showing a B cell-predominant MLC infrastructure. In the C. muridarum mouse model, Morrison and Morrison demonstrated an infection-induced CD8 intraepithelial lymphocyte population as well as endometrial MLC-containing B cells midinfection but not late in infection. We attribute the latter discrepancy to staining with B220 and CD19, which are not reliable markers for plasma cells. In support of that conjecture, Kelly and Rank found ∼4% B cells (and 0% macrophages) in genital tract single-cell suspensions 49 days post-primary infection using an Ig kappa chain-specific antibody (which stains plasma cells) and flow cytometry rather than B220 immunohistochemistry. This model resolves several contradictory observations that haunt Th1/Th2 cytokine polarization determinism.
First among the resolved inconvenient truths is that we can believe our data. In a Trm model, it is acceptable for CD4 T cells to make IFN-γ and IL-13. We hypothesize that dual IFN-γ/IL-13 CD4 T cells specific for Chlamydia explain the protection seen in our sex worker cohort study in 2005. The alternative cytokine polarization narrative would be that protected individuals harbor separate Chlamydia-specific Th1 and Th2 responses. Instead, we favor a model in which protective immunity is mediated by Trm and IL-13 is simply a biomarker for CD4 Trm in peripheral blood. (We have unpublished mouse data demonstrating a subset of CD4γ13 T cells that localize to the genital tract mucosa). A Trm model comfortably explains why multiple infections and/or a prolonged time course may be required to establish protective immunity in humans. The surface area of the human reproductive tract is immense. Trm represent local immunity with limited circulation and require a supporting infrastructure that develops as a consequence of local infection. The experimental-mouse analogy is the difference between cellular immunity generated by C. muridarum and that generated by human C. trachomatis strains. C. muridarum causes a very productive genital tract infection with extensive surface area involvement and a robust immune response that sets up a protective immune state after a single infection. In contrast, C. trachomatis strains inoculated into mice at very high levels of inclusion-forming units (IFU) caused a minimally productive (minimal surface area involvement) infection, with repeated infections required to generate demonstrable T cell-mediated protection. The length of time that it takes for C. trachomatis infection to result in expansion of Trm numbers under the mucosal surface of the reproductive tract could explain the epidemiological observations indicating that early antimicrobial treatment arrests immunity (50).
THE c-MLC
The ontogeny of Chlamydia-specific MLC is entirely unknown and likely differs in the eye versus the genital tract. CD4 T cells are the dominant T cell subset, with CD8 T cells as a significant minority subset, in human trachoma follicles; the most abundant cell type is B cells (51). T cell subset data for human genital tract Chlamydia memory lymphocyte clusters (c-MLC) are nonexistent, but uterine c-MLC contain almost exclusively CD4 T cells in the C. muridarum mouse model (1). Before dismissing this difference as a mouse anomaly, note that Chlamydia pathogenesis in the eye and that in the genital tract differ. Clinical trachoma isolates (serovars A to C) have nonfunctional tryptophan synthesis operons (52), suggesting that the presence of IFN-γ-induced indoleamine 2,3-dioxygenase (tryptophan starvation defense pathway) is not consequential to Chlamydia replication in the eye. Furthermore, while CD8 T cells are not critical for clearing C. muridarum genital tract infections (53), they appeared to play an important role in protective eye immunity in a nonhuman primate trachoma model (54). Speculation about how c-MLC assemble is beyond the scope of this review, with little published data to inform a discussion. In broad terms, the genital tract consists of nonpermissive tissue to which circulating central memory T cells have limited access (9). For HSV and Chlamydia, it is clear that MLC formation requires a local immune response in the genital tract. Based on data from other systems (see the excellent recent reviews in references 3, 55, and 56), c-MLC formation likely requires recruitment (specific chemokines), activation, immobilization (MadCam-1 and CD69 downregulation of S1PR1, a promigratory receptor), and then maintenance of antigen-presenting cells (APC) (specifically, immune B cells) and effector T cells that may or may not require antigenic stimulation. Our untested bias based on the persistence of PmpG1 gene antigen in splenic APC long after clearance of genital tract infections (57) is that antigen stimulation will be central to c-MLC maintenance. Viable aberrant chlamydial forms in APC could serve as a source for persistent presentation of a subset of chlamydial antigens that include that encoded by the PmpG gene. A unique feature of c-MLC is that immune B cells may be a (or the) critical APC.
A B cell-centered c-MLC model (see Fig. 1) would predict that B cell-deficient mice would have impaired recall immune responses due to an altered MLC. This is the case. It was the defect in the secondary response of B cell-deficient mice to C. muridarum challenge that suggested a role for antibody in adaptive immunity (58), with subsequent investigations identifying the antibody-dependent T cell-independent protective immune response (59). The antibody-dependent protection mechanism requires CD4 T cells during the primary infection, consistent with a B cell-centered model and also with T cell help for establishing adaptive B cell immunity.
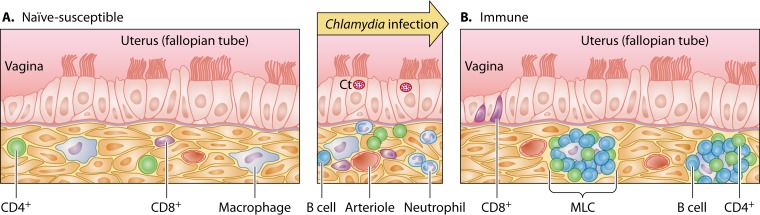
FIG 1.
Trm model for Chlamydia trachomatis immunity. (A) The susceptible genital tract epithelium (independently of host's systemic immune status) has scattered lymphocytes and macrophages in stroma underlying the epithelium. In the setting of infection (middle panel), defense of this epithelial surface requires recruitment of distant immune effectors, providing an opportunity for Chlamydia to replicate. (B) In the immune-protected state, CD8 intraepithelial lymphocytes (IEL) line the vagina and CD4 B cell-based memory lymphocyte clusters (MLC) line the uterus (and likely the fallopian tubes), affording Chlamydia little time or opportunity to replicate. Protective immunity occurs when the epithelial surface area protected by Trm reaches critical mass as a result of prolonged or repeated infections. Ct, Chlamydia trachomatis replicating within parasitophorous vacuoles (inclusions).
SUMMARY
A Trm model for protective immunity does not exclude a role for MLC in immunopathology, nor does it dictate a CD4 or CD8 T cell response. Based on recent data in the macaque model for trachoma showing a protective role for CD8 T cells (54), it is possible that the Trm within MLC induced by trachoma and genital tract infections are different, possibly mirroring different microenvironments, as reflected by the presence of tryptophan synthesis operons in all clinical isolates from the genital tract (used to escape IFN-γ-induced tryptophan depletion) and the absence of tryptophan operons in clinical trachoma isolates, suggesting that the IFN-γ-induced immunity is less relevant in the eye (52). This model raises several interesting theoretical challenges for vaccine development. There are little to no data regarding c-MLC ontogeny and an incomplete understanding of B cells in the balance between immunoprotection and immunopathology. High Chlamydia-specific antibody titers are associated with detrimental reproductive outcomes in women, while in mouse vaccine studies, antibodies (importantly, when specific for a protective T cell epitope-source protein, i.e., major outer membrane protein [MOMP]) contribute to protection. It is not immediately clear how vaccines other than attenuated strains delivered to the eye or genital tract would be able to establish Chlamydia-specific Trm/MLC using conventional vaccination techniques. Perhaps mucosal delivery of subunit vaccines with novel mucosal adjuvants could elicit the protective Trm/MLC response (60).
Biographies

Raymond M. Johnson is an Associate Professor of Internal Medicine/Infectious Diseases and Microbial Pathogenesis at the Yale University School of Medicine. He earned his B.A. (chemistry interdisciplinary) at Lawrence University, Appleton, WI and his M.D. and Ph.D. degrees in the Medical Scientist Training Program (MSTP) at the University of Chicago, Pritzker School of Medicine, studying herpes simplex virus immunobiology under the mentorship of Frank W. Fitch and Patricia G. Spear. His internal medicine training and infectious diseases fellowship were at Washington University, St. Louis, MO. For roughly 15 years, his principal research interest has been cellular immunity and immunopathogenesis within the reproductive tract, using the knowledge gained to facilitate Chlamydia vaccine development.

Robert C. Brunham is the head of the Vaccine Research Laboratory at the British Columbia Centre for Disease Control (BCCDC). Until 2014, he was the Executive and Scientific Director of BCCDC. He is also a Professor of Medicine at The University of British Columbia, where he obtained his M.D. in 1972. He has over 350 publications and is internationally regarded as an expert on infectious diseases, including his research on Chlamydia, severe acute respiratory syndrome (SARS), and HIV. In Chlamydia research, he has made seminal contributions to defining the clinical features of infection in women, evaluating the impact of screening and treatment control programs, determining the underlying mechanisms of immunity, and discovering protective antigens suitable for vaccine development. He has analyzed the impact of public health efforts to control Chlamydia, deduced that the strategy is arresting the development of immunity, and developed the rationale that a vaccine will be essential to Chlamydia control.
REFERENCES
- 1.Morrison SG, Morrison RP. 2000. In situ analysis of the evolution of the primary immune response in murine Chlamydia trachomatis genital tract infection. Infect Immun 68:2870–2879. doi: 10.1128/IAI.68.5.2870-2879.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carbone FR. 2015. Tissue-resident memory T cells and fixed immune surveillance in nonlymphoid organs. J Immunol 195:17–22. doi: 10.4049/jimmunol.1500515. [DOI] [PubMed] [Google Scholar]
- 3.Iijima N, Iwasaki A. 2015. Tissue instruction for migration and retention of TRM cells. Trends Immunol 36:556–564. doi: 10.1016/j.it.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu J, Koelle DM, Cao J, Vazquez J, Huang ML, Hladik F, Wald A, Corey L. 2007. Virus-specific CD8+ T cells accumulate near sensory nerve endings in genital skin during subclinical HSV-2 reactivation. J Exp Med 204:595–603. doi: 10.1084/jem.20061792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wakim LM, Waithman J, van Rooijen N, Heath WR, Carbone FR. 2008. Dendritic cell-induced memory T cell activation in nonlymphoid tissues. Science 319:198–202. doi: 10.1126/science.1151869. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization. 1987. Infections, pregnancies, and infertility: perspectives on prevention. Fertil Steril 47:964–968. [PubMed] [Google Scholar]
- 7.Mackay LK, Stock AT, Ma JZ, Jones CM, Kent SJ, Mueller SN, Heath WR, Carbone FR, Gebhardt T. 2012. Long-lived epithelial immunity by tissue-resident memory T (TRM) cells in the absence of persisting local antigen presentation. Proc Natl Acad Sci U S A 109:7037–7042. doi: 10.1073/pnas.1202288109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Teijaro JR, Turner D, Pham Q, Wherry EJ, Lefrancois L, Farber DL. 2011. Cutting edge: tissue-retentive lung memory CD4 T cells mediate optimal protection to respiratory virus infection. J Immunol 187:5510–5514. doi: 10.4049/jimmunol.1102243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iijima N, Iwasaki A. 2014. T cell memory. A local macrophage chemokine network sustains protective tissue-resident memory CD4 T cells. Science 346:93–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kiviat NB, Wolner-Hanssen P, Eschenbach DA, Wasserheit JN, Paavonen JA, Bell TA, Critchlow CW, Stamm WE, Moore DE, Holmes KK. 1990. Endometrial histopathology in patients with culture-proved upper genital tract infection and laparoscopically diagnosed acute salpingitis. Am J Surg Pathol 14:167–175. doi: 10.1097/00000478-199002000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Kiviat NB, Paavonen JA, Brockway J, Critchlow CW, Brunham RC, Stevens CE, Stamm WE, Kuo CC, DeRouen T, Holmes KK. 1985. Cytologic manifestations of cervical and vaginal infections. I. Epithelial and inflammatory cellular changes. JAMA 253:989–996. [PubMed] [Google Scholar]
- 12.Kiviat NB, Paavonen JA, Wolner-Hanssen P, Critchlow CW, Stamm WE, Douglas J, Eschenbach DA, Corey LA, Holmes KK. 1990. Histopathology of endocervical infection caused by Chlamydia trachomatis, herpes simplex virus, Trichomonas vaginalis, and Neisseria gonorrhoeae. Hum Pathol 21:831–837. doi: 10.1016/0046-8177(90)90052-7. [DOI] [PubMed] [Google Scholar]
- 13.Reacher MH, Pe'er J, Rapoza PA, Whittum-Hudson JA, Taylor HR. 1991. T cells and trachoma. Their role in cicatricial disease. Ophthalmology 98:334–341. [DOI] [PubMed] [Google Scholar]
- 14.Abu el-Asrar AM, Geboes K, Tabbara KF, al-Kharashi SA, Missotten L, Desmet V. 1998. Immunopathogenesis of conjunctival scarring in trachoma. Eye 12(Pt 3a):453–460. doi: 10.1038/eye.1998.104. [DOI] [PubMed] [Google Scholar]
- 15.Bell JD, Bergin IL, Harris LH, Chai D, Mullei I, Mwenda J, Dalton VK, Vahratian A, Lebar W, Zochowski MK, Kiulia N, Aronoff DM, Patton DL. 2011. The effects of a single cervical inoculation of Chlamydia trachomatis on the female reproductive tract of the baboon (Papio anubis). J Infect Dis 204:1305–1312. doi: 10.1093/infdis/jir541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Voorhis WC, Barrett LK, Sweeney YT, Kuo CC, Patton DL. 1997. Repeated Chlamydia trachomatis infection of Macaca nemestrina fallopian tubes produces a Th1-like cytokine response associated with fibrosis and scarring. Infect Immun 65:2175–2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dustin LB, Bullock ED, Hamada Y, Azuma T, Loh DY. 1995. Antigen-driven differentiation of naive Ig-transgenic B cells in vitro. J Immunol 154:4936–4949. [PubMed] [Google Scholar]
- 18.Cascalho M, Wong J, Brown J, Jack HM, Steinberg C, Wabl M. 2000. A B220(-), CD19(-) population of B cells in the peripheral blood of quasimonoclonal mice. Int Immunol 12:29–35. doi: 10.1093/intimm/12.1.29. [DOI] [PubMed] [Google Scholar]
- 19.Cascalho M, Wong J, Wabl M. 1997. VH gene replacement in hyperselected B cells of the quasimonoclonal mouse. J Immunol 159:5795–5801. [PubMed] [Google Scholar]
- 20.Morrison RP, Caldwell HD. 2002. Immunity to murine chlamydial genital infection. Infect Immun 70:2741–2751. doi: 10.1128/IAI.70.6.2741-2751.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Igietseme JU. 1996. The molecular mechanism of T-cell control of Chlamydia in mice: role of nitric oxide. Immunology 87:1–8. [PMC free article] [PubMed] [Google Scholar]
- 22.Igietseme JU, Uriri IM, Hawkins R, Rank RG. 1996. Integrin-mediated epithelial-T cell interaction enhances nitric oxide production and increased intracellular inhibition of Chlamydia. J Leukoc Biol 59:656–662. [DOI] [PubMed] [Google Scholar]
- 23.Igietseme JU, Uriri IM, Chow M, Abe E, Rank RG. 1997. Inhibition of intracellular multiplication of human strains of Chlamydia trachomatis by nitric oxide. Biochem Biophys Res Commun 232:595–601. doi: 10.1006/bbrc.1997.6335. [DOI] [PubMed] [Google Scholar]
- 24.Hawkins RA, Rank RG, Kelly KA. 2002. A Chlamydia trachomatis-specific Th2 clone does not provide protection against a genital infection and displays reduced trafficking to the infected genital mucosa. Infect Immun 70:5132–5139. doi: 10.1128/IAI.70.9.5132-5139.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang S, Fan Y, Brunham RC, Yang X. 1999. IFN-gamma knockout mice show Th2-associated delayed-type hypersensitivity and the inflammatory cells fail to localize and control chlamydial infection. Eur J Immunol 29:3782–3792. doi:. [DOI] [PubMed] [Google Scholar]
- 26.Cotter TW, Ramsey KH, Miranpuri GS, Poulsen CE, Byrne GI. 1997. Dissemination of Chlamydia trachomatis chronic genital tract infection in gamma interferon gene knockout mice. Infect Immun 65:2145–2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perry LL, Feilzer K, Caldwell HD. 1997. Immunity to Chlamydia trachomatis is mediated by T helper 1 cells through IFN-gamma-dependent and -independent pathways. J Immunol 158:3344–3352. [PubMed] [Google Scholar]
- 28.Ito JI, Lyons JM. 1999. Role of gamma interferon in controlling murine chlamydial genital tract infection. Infect Immun 67:5518–5521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gondek DC, Roan NR, Starnbach MN. 2009. T cell responses in the absence of IFN-gamma exacerbate uterine infection with Chlamydia trachomatis. J Immunol 183:1313–1319. doi: 10.4049/jimmunol.0900295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Igietseme JU, Perry LL, Ananaba GA, Uriri IM, Ojior OO, Kumar SN, Caldwell HD. 1998. Chlamydial infection in inducible nitric oxide synthase knockout mice. Infect Immun 66:1282–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramsey KH, Miranpuri GS, Poulsen CE, Marthakis NB, Braune LM, Byrne GI. 1998. Inducible nitric oxide synthase does not affect resolution of murine chlamydial genital tract infections or eradication of chlamydiae in primary murine cell culture. Infect Immun 66:835–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jayarapu K, Kerr M, Ofner S, Johnson RM. 2010. Chlamydia-specific CD4 T cell clones control Chlamydia muridarum replication in epithelial cells by nitric oxide-dependent and -independent mechanisms. J Immunol 185:6911–6920. doi: 10.4049/jimmunol.1002596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnson RM, Kerr MS, Slaven JE. 2012. Plac8-dependent and inducible NO synthase-dependent mechanisms clear Chlamydia muridarum infections from the genital tract. J Immunol 188:1896–1904. doi: 10.4049/jimmunol.1102764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Igietseme JU, Ramsey KH, Magee DM, Williams DM, Kincy TJ, Rank RG. 1993. Resolution of murine chlamydial genital infection by the adoptive transfer of a biovar-specific, Th1 lymphocyte clone. Reg Immunol 5:317–324. [PubMed] [Google Scholar]
- 35.Yu H, Jiang X, Shen C, Karunakaran KP, Jiang J, Rosin NL, Brunham RC. 2010. Chlamydia muridarum T-cell antigens formulated with the adjuvant DDA/TDB induce immunity against infection that correlates with a high frequency of gamma interferon (IFN-gamma)/tumor necrosis factor alpha and IFN-gamma/interleukin-17 double-positive CD4+ T cells. Infect Immun 78:2272–2282. doi: 10.1128/IAI.01374-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu H, Karunakaran KP, Jiang X, Shen C, Andersen P, Brunham RC. 2012. Chlamydia muridarum T cell antigens and adjuvants that induce protective immunity in mice. Infect Immun 80:1510–1518. doi: 10.1128/IAI.06338-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu H, Karunakaran KP, Kelly I, Shen C, Jiang X, Foster LJ, Brunham RC. 2011. Immunization with live and dead Chlamydia muridarum induces different levels of protective immunity in a murine genital tract model: correlation with MHC class II peptide presentation and multifunctional Th1 cells. J Immunol 186:3615–3621. doi: 10.4049/jimmunol.1002952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Darrah PA, Patel DT, De Luca PM, Lindsay RW, Davey DF, Flynn BJ, Hoff ST, Andersen P, Reed SG, Morris SL, Roederer M, Seder RA. 2007. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med 13:843–850. doi: 10.1038/nm1592. [DOI] [PubMed] [Google Scholar]
- 39.Forbes EK, Sander C, Ronan EO, McShane H, Hill AV, Beverley PC, Tchilian EZ. 2008. Multifunctional, high-level cytokine-producing Th1 cells in the lung, but not spleen, correlate with protection against Mycobacterium tuberculosis aerosol challenge in mice. J Immunol 181:4955–4964. doi: 10.4049/jimmunol.181.7.4955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Debattista J, Timms P, Allan J, Allan J. 2002. Reduced levels of gamma-interferon secretion in response to chlamydial 60 kDa heat shock protein amongst women with pelvic inflammatory disease and a history of repeated Chlamydia trachomatis infections. Immunol Lett 81:205–210. doi: 10.1016/S0165-2478(02)00036-6. [DOI] [PubMed] [Google Scholar]
- 41.Holland MJ, Bailey RL, Conway DJ, Culley F, Miranpuri G, Byrne GI, Whittle HC, Mabey DC. 1996. T helper type-1 (Th1)/Th2 profiles of peripheral blood mononuclear cells (PBMC); responses to antigens of Chlamydia trachomatis in subjects with severe trachomatous scarring. Clin Exp Immunol 105:429–435. doi: 10.1046/j.1365-2249.1996.d01-792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bailey RL, Holland MJ, Whittle HC, Mabey DC. 1995. Subjects recovering from human ocular chlamydial infection have enhanced lymphoproliferative responses to chlamydial antigens compared with those of persistently diseased controls. Infect Immun 63:389–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Burton MJ, Rajak SN, Bauer J, Weiss HA, Tolbert SB, Shoo A, Habtamu E, Manjurano A, Emerson PM, Mabey DC, Holland MJ, Bailey RL. 2011. Conjunctival transcriptome in scarring trachoma. Infect Immun 79:499–511. doi: 10.1128/IAI.00888-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cohen CR, Koochesfahani KM, Meier AS, Shen C, Karunakaran K, Ondondo B, Kinyari T, Mugo NR, Nguti R, Brunham RC. 2005. Immunoepidemiologic profile of Chlamydia trachomatis infection: importance of heat-shock protein 60 and interferon-gamma. J Infect Dis 192:591–599. doi: 10.1086/432070. [DOI] [PubMed] [Google Scholar]
- 45.Watkins NG, Hadlow WJ, Moos AB, Caldwell HD. 1986. Ocular delayed hypersensitivity: a pathogenetic mechanism of chlamydial-conjunctivitis in guinea pigs. Proc Natl Acad Sci U S A 83:7480–7484. doi: 10.1073/pnas.83.19.7480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hawkins RA, Rank RG, Kelly KA. 2000. Expression of mucosal homing receptor alpha4beta7 is associated with enhanced migration to the Chlamydia-infected murine genital mucosa in vivo. Infect Immun 68:5587–5594. doi: 10.1128/IAI.68.10.5587-5594.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Do JS, Visperas A, Freeman ML, Iwakura Y, Oukka M, Min B. 2014. Colitogenic effector T cells: roles of gut-homing integrin, gut antigen specificity and gammadelta T cells. Immunol Cell Biol 92:90–98. doi: 10.1038/icb.2013.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kelly KA, Rank RG. 1997. Identification of homing receptors that mediate the recruitment of CD4 T cells to the genital tract following intravaginal infection with Chlamydia trachomatis. Infect Immun 65:5198–5208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stary G, Olive A, Radovic-Moreno AF, Gondek D, Alvarez D, Basto PA, Perro M, Vrbanac VD, Tager AM, Shi J, Yethon JA, Farokhzad OC, Langer R, Starnbach MN, von Andrian UH. 2015. VACCINES. A mucosal vaccine against Chlamydia trachomatis generates two waves of protective memory T cells. Science 348:aaa8205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brunham RC, Rekart ML. 2008. The arrested immunity hypothesis and the epidemiology of chlamydia control. Sex Transm Dis 35:53–54. doi: 10.1097/OLQ.0b013e31815e41a3. [DOI] [PubMed] [Google Scholar]
- 51.Abu el-Asrar AM, Geboes K, Missotten L. 2001. Immunology of trachomatous conjunctivitis. Bull Soc Belge Ophtalmol 2001:73–96. [PubMed] [Google Scholar]
- 52.Caldwell HD, Wood H, Crane D, Bailey R, Jones RB, Mabey D, Maclean I, Mohammed Z, Peeling R, Roshick C, Schachter J, Solomon AW, Stamm WE, Suchland RJ, Taylor L, West SK, Quinn TC, Belland RJ, McClarty G. 2003. Polymorphisms in Chlamydia trachomatis tryptophan synthase genes differentiate between genital and ocular isolates. J Clin Invest 111:1757–1769. doi: 10.1172/JCI17993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morrison RP, Feilzer K, Tumas DB. 1995. Gene knockout mice establish a primary protective role for major histocompatibility complex class II-restricted responses in Chlamydia trachomatis genital tract infection. Infect Immun 63:4661–4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Olivares-Zavaleta N, Whitmire WM, Kari L, Sturdevant GL, Caldwell HD. 2014. CD8+ T cells define an unexpected role in live-attenuated vaccine protective immunity against Chlamydia trachomatis infection in macaques. J Immunol 192:4648–4654. doi: 10.4049/jimmunol.1400120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Park CO, Kupper TS. 2015. The emerging role of resident memory T cells in protective immunity and inflammatory disease. Nat Med 21:688–697. doi: 10.1038/nm.3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schenkel JM, Masopust D. 2014. Tissue-resident memory T cells. Immunity 41:886–897. doi: 10.1016/j.immuni.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Johnson RM, Yu H, Kerr MS, Slaven JE, Karunakaran KP, Brunham RC. 2012. PmpG303-311, a protective vaccine epitope that elicits persistent cellular immune responses in Chlamydia muridarum-immune mice. Infect Immun 80:2204–2211. doi: 10.1128/IAI.06339-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang X, Brunham RC. 1998. Gene knockout B cell-deficient mice demonstrate that B cells play an important role in the initiation of T cell responses to Chlamydia trachomatis (mouse pneumonitis) lung infection. J Immunol 161:1439–1446. [PubMed] [Google Scholar]
- 59.Morrison SG, Morrison RP. 2001. Resolution of secondary Chlamydia trachomatis genital tract infection in immune mice with depletion of both CD4+ and CD8+ T cells. Infect Immun 69:2643–2649. doi: 10.1128/IAI.69.4.2643-2649.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brunham RC. 2015. IMMUNOLOGY. A Chlamydia vaccine on the horizon. Science 348:1322–1323. [DOI] [PubMed] [Google Scholar]