Abstract
Nematode infection upregulates interleukin-4 (IL-4) and IL-13 and induces STAT6-dependent changes in gut function that promote worm clearance. IL-4 and IL-13 activate the type 2 IL-4 receptor (IL-4R), which contains the IL-13Rα1 and IL-4Rα chains. We used mice deficient in IL-13Rα1 (IL-13Rα1−/−) to examine the contribution of IL-13 acting at the type 2 IL-4R to immune and functional responses to primary (Hb1) and secondary (Hb2) infections with the gastrointestinal nematode parasite Heligmosomoides bakeri. There were differences between strains in the IL-4 and IL-13 expression responses to Hb1 but not Hb2 infection. Following Hb2 infection, deficient mice had impaired worm expulsion and higher worm fecundity despite normal production of Th2-derived cytokines. The upregulation of IL-25 and IL-13Rα2 in Hb1- and Hb2-infected wild-type (WT) mice was absent in IL-13Rα1−/− mice. Goblet cell numbers and resistin-like molecule beta (RELM-β) expression were attenuated significantly in IL-13Rα1−/− mice following Hb2 infections. IL-13Rα1 contributes to the development of alternatively activated macrophages, but the type 1 IL-4R is also important. Hb1 infection had no effects on smooth muscle function or epithelial permeability in either strain, while the enhanced mucosal permeability and changes in smooth muscle function and morphology observed in response to Hb2 infection in WT mice were absent in IL-13Rα1−/− mice. Notably, the contribution of claudin-2, which has been linked to IL-13, does not mediate the increased mucosal permeability following Hb2 infection. These results show that activation of IL-13Rα1 is critical for key aspects of the immune and functional responses to Hb2 infection that facilitate expulsion.
INTRODUCTION
Gastrointestinal nematodes infect approximately one billion people, primarily in underdeveloped areas, and are a major cause of childhood morbidity and mortality. In industrialized regions, the well-established inverse correlation between the incidence of enteric nematode infection and that of inflammatory bowel diseases (IBD) has fueled investigations into the therapeutic potential of nematodes and/or their products (1). Enteric nematode infection generates a polarized upregulation of the Th2 cytokines interleukin-4 (IL-4) and IL-13, which promotes infiltration of immune cells and induction of alternatively activated macrophages (M2) in the intestine. The dependency of the immune response on IL-4 versus IL-13, however, varies somewhat among nematodes. Mice without functional or adequate levels of IL-13 have delayed expulsion of Nippostrongylus brasiliensis and failed to expel Trichuris muris (2). Deficiency in IL-13 gene expression or administration of an IL-13 antagonist, however, does not suppress the ability of IL-4 to expel Trichinella spiralis (2); while mice deficient in IL-4 do not clear Heligmosomoides bakeri (3). These differences may be explained, in part, by the binding of IL-4 and IL-13 to the type 1 and type 2 IL-4 receptor (IL-4R). IL-4 binds with high affinity to the type-1 IL-4R, which consists of a heterodimer of the IL-4Rα and -γC chains. IL-4, but not IL-13, binds to the type-1 IL-4 receptor that is expressed primarily on immune cells, including T cells and macrophages. The type 2 IL-4R, also called the type 1 IL-13R (4), binds both IL-13 and IL-4 via a heterodimer formed by IL-4Rα and IL-13Rα1. The IL-13Rα1 chain has a low affinity for IL-13; however, binding of IL-13 with IL-13Rα1 rapidly associates with the IL-4Rα chain to induce downstream signaling. IL-13 also binds with high affinity to the decoy receptor IL-13Rα2, which removes IL-13 from the milieu (5, 6). The expression of the decoy receptor is upregulated by IL-13 during nematode infection, acting to control the biological activity of IL-13. The shared effects of IL-4 and IL-13 on gut function are thought to be mediated by their binding to the type 2 IL-4R that is expressed by structural cells, including epithelial cells and some immune cells such as macrophages (7). Despite their immunologic and biologic redundancy, IL-4 and IL-13 are considered to have distinct roles in the gut in vivo that have not been fully elucidated.
The precise contribution of each of the type 1 and type 2 IL-4Rs in the lung is central to the role of IL-4 and IL-13 in pulmonary pathologies such as asthma and allergy (8, 9). In the gut, interest in IL-13-mediated effects is motivated by the proposed role for IL-13 in the increased permeability in patients with IBD (10, 11). Mice with an increased constitutive expression of IL-13 in small intestine also had increased intestinal permeability (4). In addition, the increased availability of IL-13 in mice deficient in IL-13Rα2 (IL-13Rα2−/−) results in enhanced permeability and a hypercontractility of smooth muscle in the small intestine (5, 12). There is also evidence that IL-13 can signal through IL-13Rα2 (13, 14) in chronic inflammation and that IL-13 can signal independently of the IL-4Rα chain (15). Thus, the control of the effects of IL-13 working at the type 2 IL-4R has clinical importance in both pulmonary and gastrointestinal pathologies. The specific contribution of IL-13 working through IL-13Rα1 to parasitic nematode infection is inferred from studies using IL-13−/− or STAT6−/− mice, but these mice do not address the specific effects of IL-13 working through the type 2 IL-4R. One study showed that IL-13Rα1−/− mice had impaired intestinal clearance of a primary infection with N. brasiliensis. (8)
Infection with H. bakeri recapitulates many of the critical features of infection in humans. This strictly enteric nematode establishes a chronic primary (Hb1) infection despite the induction of a polarized type 2 response, while a secondary (Hb2) challenge infection elicits a strong immune memory response and worm clearance. Of interest is that IL-4, rather than IL-13, is critical for protection against Hb1 and Hb2 infections (16), and mice treated with exogenous IL-4 clear H. bakeri more effectively (17). There is no information on the contribution of the type 2 IL-4R to Hb1 versus Hb2 infections.
The aims of the present study were to determine the contribution of IL-13Rα1 to nematode infection-induced (i) development and maintenance of the type 2 immune response to Hb1 versus Hb2 infections, (ii) development of the associated changes in smooth muscle function and epithelial cell secretion, absorption, and permeability, and (iii) upregulation of STAT6-dependent genes. We show that IL-13, working through IL-13Rα1 on the type 2 IL-4R, plays a key role in both primary and secondary type 2 responses. The downstream effects result in the local development of the M2 phenotype as well as the associated changes in smooth muscle and epithelial morphology and function needed for worm expulsion. In addition, we discuss the importance of specific mechanisms responsible for the impaired mucosal barrier function during nematode infection.
MATERIALS AND METHODS
Mouse model.
IL-13Rα1−/− mice on a BALB/c genetic background were obtained from the National Institutes of Health (Bethesda, MD). Appropriate age-matched female wild-type (WT) BALB/c mice were purchased from Jackson Laboratory (Bar Harbor, ME). These mice are not from the same source, and we recognize that the microbiome may be different. Our previously published data, however, show that the kinetics of the Th2 response, including changes in gut function that facilitate worm expulsion, are highly reproducible in C57BL/6 mice obtained from different commercial vendors or bred in our facility (18–20). The animal experiments were conducted in accordance with the principles set forth in the Guide for the Care and Use of Laboratory Animals (21) and reviewed and approved by the Beltsville Area Animal Care and Use Committee (protocol 10-03).
Nematode infection.
WT and knockout mice were inoculated orally with 200 infective third-stage larvae (L3) of H. bakeri for 2 weeks. Five mice from each group were euthanized for the primary (Hb1) infection study, while the other five mice from each group were administered an anthelmintic drug to clear the infection. The drug-cured mice were allowed to rest for 4 weeks and then were reinfected with 200 L3 H. bakeri larvae and euthanized 14 days after the secondary (Hb2) infection.
Egg and worm counts.
Feces were collected from WT and IL-13Rα1−/− mice on day 14 post-Hb1 and -Hb2 infection and placed in phosphate-buffered saline (PBS), and the number of eggs was counted using a light microscope. For adult worm burdens, the small intestine from WT and IL-13Rα1−/− mice was collected at euthanasia, and worms were counted using a dissecting scope.
Ussing chamber.
Segments of muscle-free jejunum were mounted in Ussing chambers that exposed 0.126 cm2 of tissue to 5 ml of Krebs buffer and allowed to equilibrate for 15 min. Electrodes connected to agar-salt bridges were used to measure the potential difference. Every 50 s, the tissues were short-circuited at 1 V (DVC 1000 voltage clamp; World Precision Instruments, Sarasota, FL) to allow calculation of tissue resistance utilizing Ohm's law (V = IR), where V is voltage, I is the current through the tissue, and R is the resistance of the tissue. In addition, the short circuit current (Isc) was monitored continuously over the length of the experiment. To test the tissue secretory response to acetylcholine, increasing doses (10 nM to 1 μM) were added sequentially to the serosal side of the mounted tissue to determine concentration-dependent changes in Isc. In separate tissues, glucose (0.625 to 40 mM) was added to the mucosal side of the mounted tissue to assess sodium-linked glucose absorption. As a measure of resistance, muscle-free segments of jejunum were taken from uninfected and H. bakeri-infected WT and IL13Rα1−/− mice and mounted in micro-Snapwells. Transepithelial electrical resistance (TEER) was measured at 30-min intervals using a planar electrode (Endohm Snap electrode) connected to an EVOM-G WPI analyzer (World Precision Instruments) and expressed as ohms per square centimeter.
Smooth muscle contractility.
Sections (1 cm) of jejuna were flushed of contents and suspended longitudinally in individual 8-ml organ baths that were maintained in Krebs buffer at 37°C. One end of the tissue was attached to an isometric tension transducer (model FT03; Grass Medical Instruments, Quincy, MA), and the other end was attached to the bottom of the bath. Before starting treatment, tissues were stretched to a load of 9.8 mN (1 g), and tension was recorded using Grass model 79 polygraph (Grass Medical Instruments) and expressed as force per cross-sectional area. After a 20-min equilibration period, spontaneous contractions were measured, followed by measurement of concentration-dependent responses to acetylcholine and 5-hydroxytryptamine (5-HT), as well as frequency-dependent responses to electric field stimulation (EFS [1 to 20 Hz]). At the end of the treatment, the tissues were weighed and the final length was recorded to allow normalization of responses to tissue mass and volume.
Quantitative real-time PCR.
Total RNA was prepared from whole tissue of jejunum (5, 19, 22). Real-time quantitative PCR was performed on an iCycler detection system (Bio-Rad, Hercules, CA). Primer sequences were designed using Beacon Designer 4.0 (Premier Biosoft International, Palo Alto, CA) and were synthesized by the Biopolymer Laboratory of the University of Maryland. PCR was performed in a 25-μl volume with SYBR green supermix (Bio-Rad). The amplification conditions were 95°C for 3 min followed by 50 cycles of 95°C for 15s, 60°C for 15s, and 72°C for 20s. The fold changes in mRNA expression were relative to the respective control treatment group after normalization to the 18S rRNA housekeeping gene.
Histology.
To determine the intestinal thickness of the smooth muscle layer, sections of jejunum were cut open along the mesenteric border, rinsed, and fixed in 4% paraformaldehyde for 2 h. Fixed tissues were embedded in paraffin, sectioned, and stained with Giemsa. Smooth muscle thickness and the number of goblet cells per high-powered field (hpf) were determined in well-oriented Giemsa-stained sections (Nikon Eclipse TE2000E) with the computer software MetaVue (Universal Imaging Corp.) by two investigators who were unaware of the treatment.
Immunohistochemistry.
Unstained sections of previously fixed tissues were processed to remove paraffin and placed in preheated (95°C) Tris-EDTA buffer for 40 min for epitope exposure. The slides were incubated next with 3% hydrogen peroxide in water for 5 min. An avidin-biotin kit (Vector Laboratories, Burlingame, CA) was used to detect claudin-2 expression using the primary anti-claudin-2 antibody (1/100 [Invitrogen, Carlsbad, CA]) followed by a secondary biotinylated anti-mouse IgG reagent according to the kit instructions. Diaminobenzine (DAB [Vector Laboratories]) was then applied to visualize claudin-2.
Western blotting.
Sections of mid-jejunum (1 cm) were homogenized in 1 ml of tissue protein extraction reagent (T-PER [Thermo Scientific]). Samples were then centrifuged, and supernatants were collected for further analysis. The Pierce bicinchoninic acid (BCA) protein assay kit (Thermo Scientific, Waltham, MA) was used to measure the protein concentration for each sample. Samples (60 μg) were loaded into wells of the 12% Tris-glycine gel (Invitrogen) and electrophoresed. The membrane used to transfer the separated protein bands was stained with Ponceau S solution for determination of equal loading. The membrane was then blocked with 5% milk and incubated with anti-claudin-2 antibody (2 μg/ml [Invitrogen]) overnight followed by an anti-mouse IgG horseradish peroxidase (HRP)-labeled secondary antibody (1/100,000 [KPL, Gaithersburg, MD]), with the chemiluminescence signal measured by a FujiFilm Luminescent image analyzer and Darkbox (FujiFilm Medical Systems, Stamford, CT).
Statistics.
Agonists and stimulation responses were fitted to spline/locally weighted scatterplot smoothing (LOWESS) curves (GraphPad, San Diego, CA). Statistical analysis was performed using one-way analysis of variance (ANOVA) followed by Bonferroni test to compare the responses and levels of mRNA expression among the different groups.
RESULTS
IL-13Rα1 modulates the type 2 immune response to infection with H. bakeri.
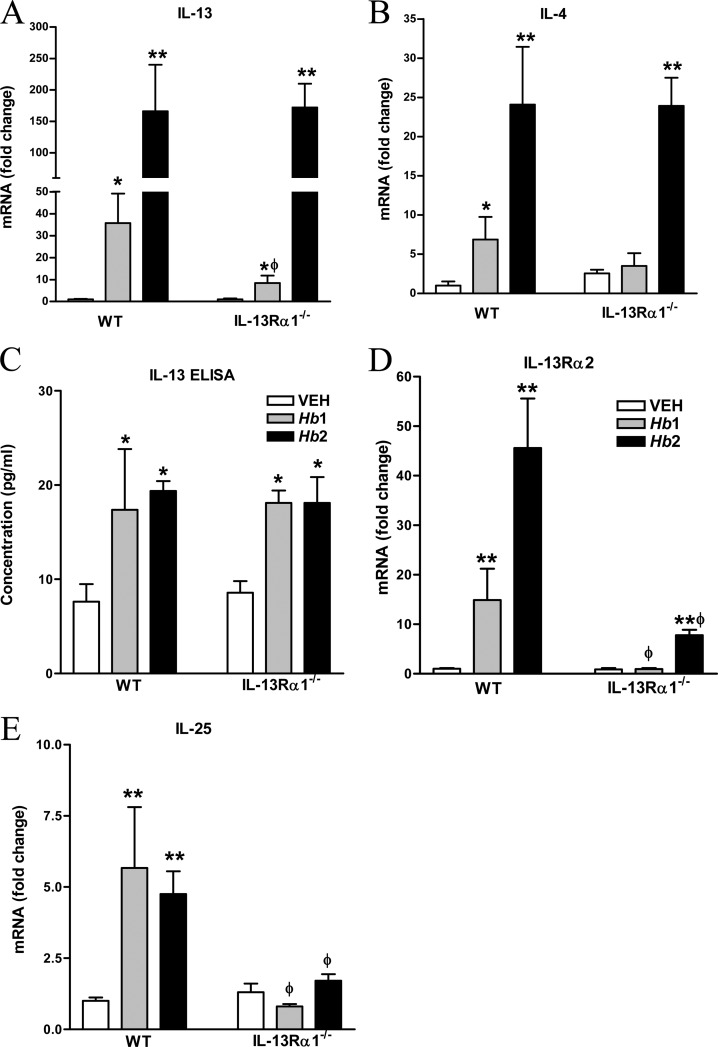
In WT mice, there was a modest increase in IL-13, IL-4, and IL-25 mRNA in Hb1 infection, with a more robust elevation in the expression of these cytokines in Hb2 infection (Fig. 1A to E). In contrast, there was a modest upregulation of IL-13, but not IL-4 or IL-25, mRNA in IL-13Rα1−/− mice in Hb1 infection that was attenuated compared to that in WT mice. In IL-13Rα1−/− mice, Hb2 infection increased the expression of IL-4 and IL-13, but not IL-25, mRNA to levels comparable to that in WT Hb2 infection. Surprisingly, there was comparable upregulation in IL-13 protein in both strains during infection in both Hb1 and Hb2 infections (Fig. 1C). In WT mice, changes in the expression of the decoy receptor, IL-13Rα2, in Hb1 and Hb2 infections paralleled those in IL-13 and IL-4 (Fig. 1E). In contrast, there was no change in IL-13Rα2 expression in Hb1-infected mice and a modest, but significant, upregulation in Hb2-infected IL-13Rα1−/− mice that was significantly lower than in WT mice. These data demonstrate the primary dependence of expression of the decoy receptor on the type 2 IL-4R.
FIG 1.
Effect of IL-13Rα1 deficiency on Hb1 and Hb2 infection-induced changes in cytokine and cytokine receptor expression. (A) IL-13 mRNA expression. (B) IL-4 mRNA expression. (C) Intestinal IL-13 protein levels. ELISA, enzyme-linked immunosorbent assay. (D) IL-13Rα2 mRNA expression. (E) IL-25 mRNA expression. *, P < 0.05, and **, P < 0.01, compared to WT vehicle (VEH); ϕ, P < 0.05, compared to the respective WT Hb1 or Hb2 infection. n = 5 to 7 per group.
Our previous study showed that IL-25 is critical for host protective immunity in nematode infection, with IL-13 being the major downstream Th2 cytokine (20). In this study, IL-25 was upregulated significantly in both Hb1- and Hb2-infected mice in WT, but not IL-13Rα1−/−, mice (Fig. 1E), implicating a positive-feedback loop between IL-13 and IL-25. Expression of the Th1 cytokines IL-12 and IFN-γ was unaltered by infection in either strain (see Fig. S1 in the supplemental material), showing that the lack of upregulation of Th2 cytokines could not be linked to an inappropriate Th1 response.
IL-13Rα1- and H. bakeri-induced changes in host protective immunity.
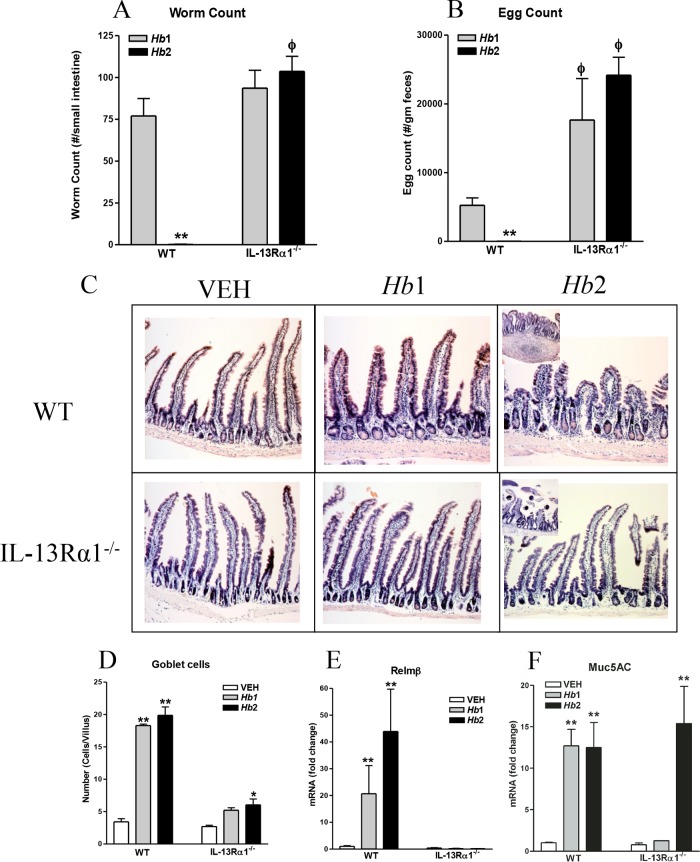
Hb1 infection was chronic, with adult worms visible in the small intestine at day 12 postinoculation in both WT and IL-13Rα1−/− mice (Fig. 2A). Despite similar numbers of worms in both Hb1-infected strains, fecal egg counts in IL-13Rα1−/− mice were significantly higher than in WT mice (Fig. 2B). As average fecal egg production is inversely proportional to worm burden in healthy mice, the higher fecundity in IL-13Rα1−/− mice is consistent with a reduced protective Th2 immune response. By day 12 post-Hb2 infection in WT mice, worms were cleared, and there were no eggs in the feces. In contrast, adult worms and high egg counts persisted in Hb2-infected IL-13Rα1−/− mice. Adult worms were visible adjacent to the mucosa in proximal sections of small intestine (Fig. 2C, inset for IL-13Rα1 Hb2 infection) in Hb2-infected IL-13Rα1−/− mice, but not in WT mice. These data demonstrate that only WT mice were able to mount an effective protective memory type 2 response.
FIG 2.
Effect of IL-13Rα1 deficiency on Hb1 and Hb2 infection-induced host protective immunity. (A) Intestinal worm counts. (B) Fecal egg counts. (C) Infection-induced alterations in intestinal morphology. (D) Goblet cell counts. (E) RELM-β expression. (F) Muc5ac mRNA expression. *, P < 0.05, and **, P < 0.01, compared to WT VEH; ϕ, P < 0.05, compared to WT Hb1 infection. n = 5 to 7 per group.
The differences in intestinal morphology were most prominent in Hb2-infected WT versus IL-13Rα1−/− mice. Hb2 infection produced a slight blunting of villi in WT mice that was not observed in IL-13Rα1−/− mice at day 12 postinoculation (Fig. 2C, right panels). The Hb2-infected WT mice had cysts in the intestinal submucosa (Fig. 2C inset for WT mice with Hb2 infection) in which larvae mature prior to reentry into the lumen as adult worms (23). These structures were absent in IL-13Rα1−/− mice. Increased numbers of goblet cells are a characteristic feature of Hb2 infection and are a source of protective factors, such as RELM-β, that are critical for worm expulsion (24). A marked increase in the number of goblet cells was observed in WT mice following both Hb1 and Hb2 infections. In contrast, Hb1 infection did not alter the number of goblet cells in IL-13Rα1−/− mice, while Hb2 infection induced a modest, but significant, increase in goblet cell number that was still reduced compared to that in WT mice (Fig. 2D). RELM-β expression was elevated in Hb1 infection and enhanced following Hb2 infection in WT, but not in IL-13Rα1−/−, mice (Fig. 2E). These results indicate that IL-13, working through IL-13Rα1, is associated with reduced RELM-β and impaired worm clearance. In contrast, the level of Muc5ac gene expression was elevated following both Hb1 and Hb2 infections in WT mice and during Hb2 infection in IL-13Rα1−/− mice (Fig. 2F), showing that Muc5ac is independent of the type 2 IL-4R.
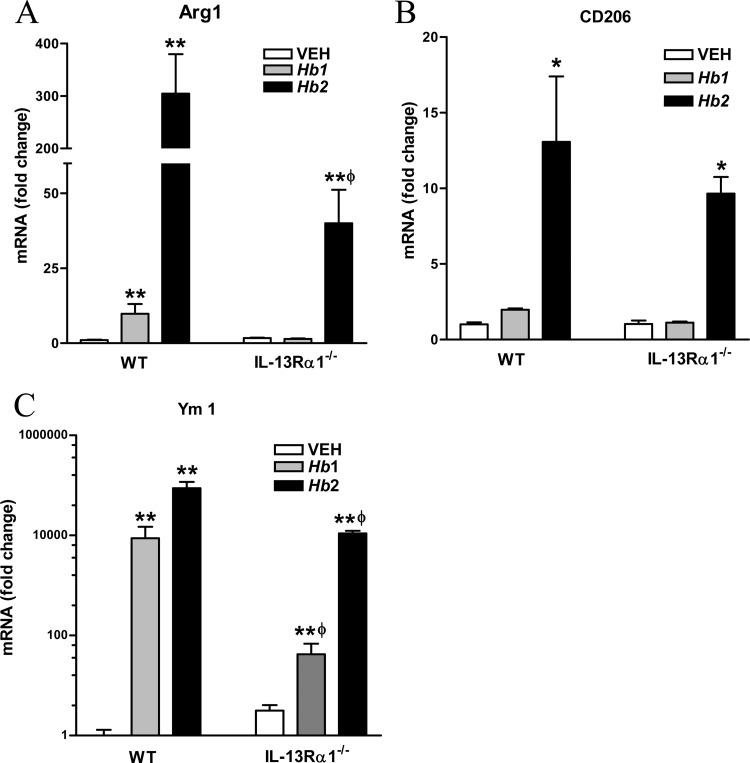
IL-13Rα1 contributes to the development of alternatively activated macrophages (M2).
In WT mice, Hb1 infection significantly increased the expression of the M2 markers arginase-1 and Ym1, while Hb2 infection significantly upregulated the expression of arginase-1, Ym1, and CD206 (Fig. 3A to C). In IL-13Rα1−/− mice, Hb1 infection had no effect on the expression of arginase-1 or CD206 but upregulated Ym1. Arginase-1 expression was upregulated in Hb2-infected IL-13Rα1−/− mice, but at levels significantly below those observed in WT mice. In contrast, levels of CD206 and Ym1 expression were comparable following Hb2 infection in both strains (Fig. 3A to D). These data indicate that IL-13, working through IL-13Rα1, contributes to, but is not absolutely required for, the development of the M2 phenotype.
FIG 3.
Effect of IL-13Rα1 deficiency on Hb1 and Hb2 infection-induced development of M2. (A) Arginase-1 (Arg1) mRNA expression. (B) CD206 mRNA expression. (C) Ym1 mRNA expression. *, P < 0.05, and **, P < 0.01, compared to WT VEH; ϕ, P < 0.05, compared to respective WT Hb1 or Hb2 infection. n = 4 to 7 per group.
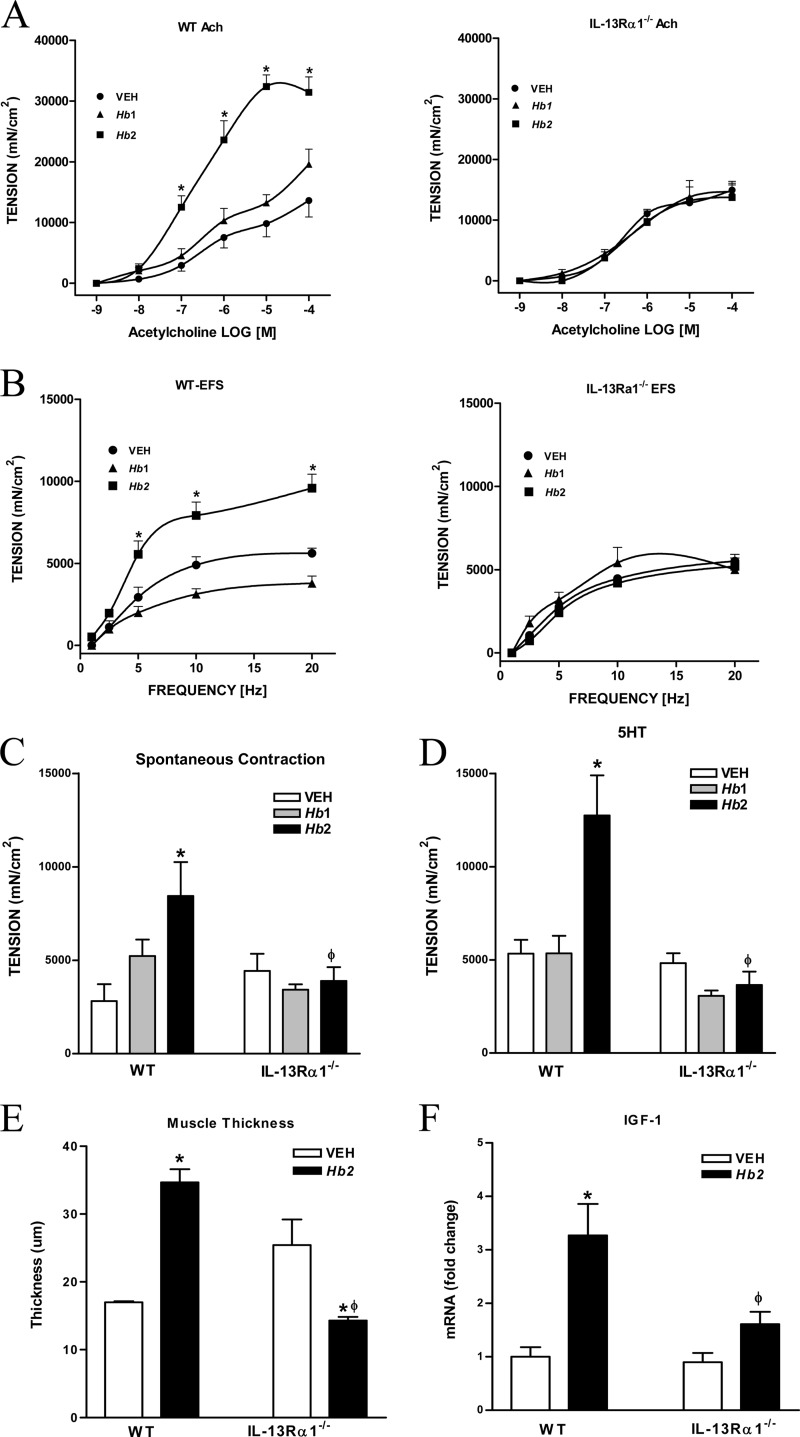
IL-13Rα1 is critical for H. bakeri infection-induced changes in smooth muscle function and morphology.
Smooth muscle responses to acetylcholine, 5-HT, and EFS, as well as the amplitude of spontaneous contractions were similar in uninfected WT and IL-13Rα1−/− mice (Fig. 4A to D), demonstrating a lack of a constitutive role for IL-13 working through the type 2 IL-4R in smooth muscle function. Hb1 infection had no effect on smooth muscle contractility (Fig. 4A to D) in either WT or IL-13Rα1−/− mice. Hb2 infection in WT mice induced the expected hypercontractility to acetylcholine (Fig. 4A), EFS (Fig. 4B), and 5-HT (Fig. 4D) and increased the amplitude of spontaneous contractions (Fig. 4C), all of which were abrogated in Hb2-infected IL-13Rα1−/− mice. The Hb2-induced effects of acetylcholine on smooth muscle are due, in part, to interactions between acetylcholine and increased muscarinic M3 receptor (M3R) expression; while the effects of 5-HT and protease activated receptor 1 (PAR-1) agonists are mediated by an IL-13/STAT6-dependent upregulation of the 5-HT2A receptor and PAR-1 (25, 26). The upregulation of M3R, 5-HT2A, and PAR-1 in Hb2-infected WT mice was attenuated or blocked significantly in IL-13Rα1−/− mice (see Table S1 in the supplemental material). These data indicate that worm clearance depends on the IL-13-induced muscle hypercontractility that is mediated, in part, by upregulation of the expression of specific receptors via the type 2 IL-4R and STAT6.
FIG 4.
Effect of IL-13Rα1 deficiency on Hb1 and Hb2 infection-induced changes in smooth muscle morphology and function. (A) Concentration-dependent response to acetylcholine (Ach). (B) Frequency-dependent nerve stimulation (EFS). (C) Smooth muscle spontaneous contractions. (D) Contractile responses to 5-HT (10 μM). (E) Smooth muscle thickness. (F) IGF-1 mRNA expression. *, P < 0.05, compared to WT VEH; ϕ, P < 0.05, compared to WT Hb2 infection. n = 5 to 7 per group.
In addition to changes in function, M2 cells also contribute to infection-induced increases in smooth muscle thickness that are associated with upregulation of growth factors such as insulin-like growth factor 1 (IGF-1) (22). Hb2 infection increased smooth muscle thickness (Fig. 4E) and significantly enhanced IGF-1 gene expression in WT, but not in IL13Rα1−/−, mice (Fig. 4F). These data show that IL-13, working through the type 2 IL-4R, also plays a key role in infection-induced changes in smooth muscle morphology.
IL-13Rα1 contributes to specific H. bakeri-induced alterations in epithelial function.
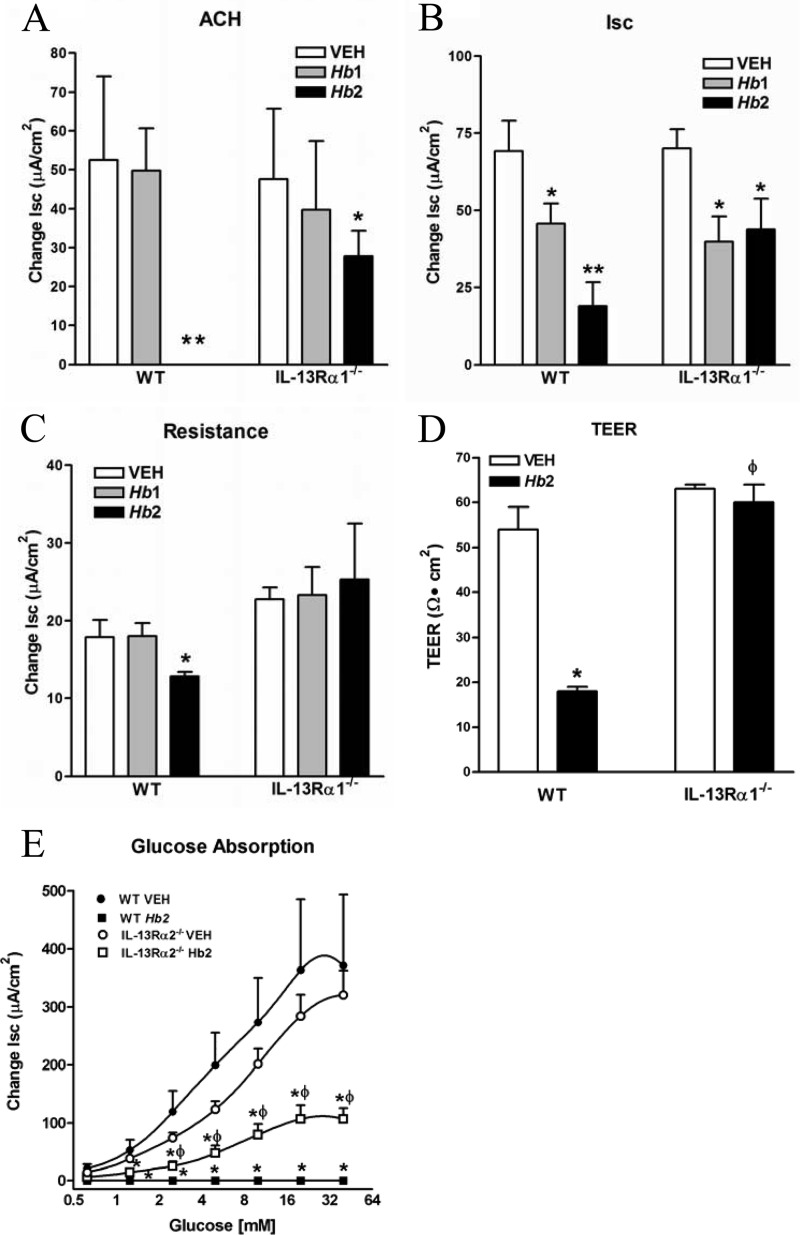
Intestinal secretory responses to acetylcholine were unaffected by Hb1 infection in either strain, but were reduced significantly by Hb2 infection in WT and IL13Rα1−/− mice (Fig. 5A). Basal ion flux across the tissue was decreased in both Hb1 and Hb2 infections in WT and IL13Rα1−/− mice (Fig. 5B). Epithelial permeability was unchanged by Hb1 infection in either strain but was increased significantly (decreased resistance in Ussing chambers) in Hb2-infected WT mice but not in IL13Rα1−/− mice (5C). The strain-specific changes in resistance in Hb2 infection were confirmed using TEER (Fig. 5D).
FIG 5.
Effect of IL-13Rα1 deficiency on Hb2 infection-induced changes in epithelial function. Shown are secretory responses to acetylcholine (100 μM) (A) and basal short circuit current (Isc) (B), mucosal resistance in mucosae mounted in Ussing chambers (C), transepithelial electrical resistance (TEER) in mucosae mounted in Snapwells (D), and glucose absorption (E). *, P < 0.05, and **, P < 0.01, compared to WT VEH; ϕ, P < 0.05, compared to WT Hb2 infection. n = 5 to 7 per group.
The cystic fibrosis transmembrane regulator (CFTR) is the major chloride channel in epithelial cell. In the present study, CFTR expression was unaltered by Hb2 infection in WT mice, but was increased in IL13Rα1−/− mice (see Table S1 in the supplemental material). In contrast, glucose absorption was decreased significantly following Hb2 infection in IL-13Rα1−/− mice compared to uninfected controls, but not to the levels observed in WT mice (Fig. 5E), consistent with a observed role for both IL-4 and IL-13 in this effect (18, 27, 28). These data show that the infection-induced antisecretory and antiabsorptive effects, as well as the upregulation of CFTR expression in response to Hb2, can occur independently of the type 2 IL-4R. In contrast, the infection-induced changes in intestinal permeability are linked exclusively to the type 2 IL-4R.
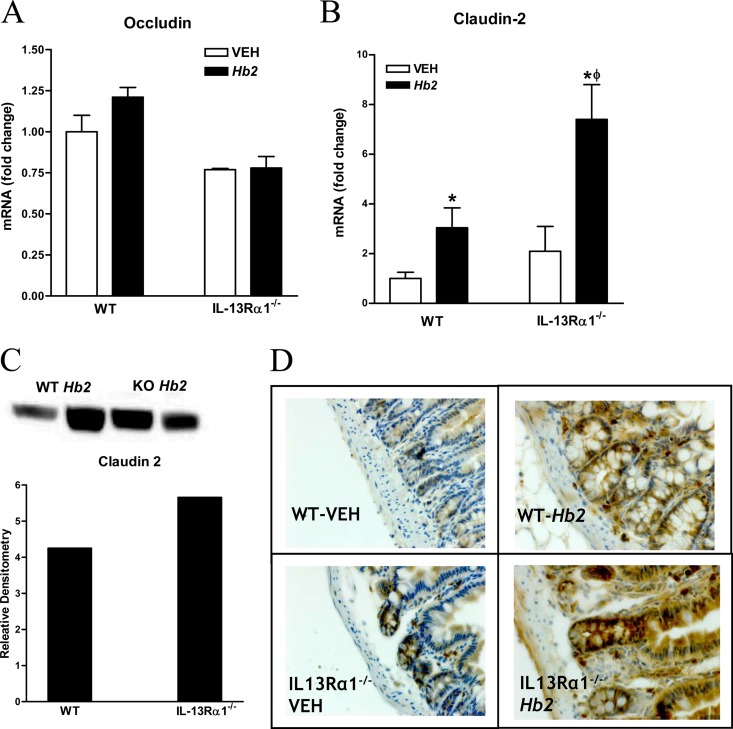
There are a number of mechanisms that may contribute to this effect, including changes in the expression of tight junction proteins (TJPs), release of mast cell mediators, and increased expression of STAT6-dependent genes that may be linked to permeability. Expression of the TJP occludin was unchanged following Hb2 infection (Fig. 6A) in either strain of mice. In contrast, expression of mRNA and the pore-forming protein claudin-2 was upregulated significantly following Hb2 infection in both WT and IL-13Rα1−/− mice (Fig. 6B and C). Immunohistochemistry revealed that claudin-2 was expressed predominantly in the crypt region, with an increase in the number of cells stained in response to Hb2 infection in both strains (Fig. 6D). Hb2 infection had no effect on expression of the other pore-forming TJPs, claudin-15 and claudin-12 (see Table S2 in the supplemental material).
FIG 6.
Changes in tight junction protein in Hb2-infected WT and IL-13Rα1−/− mice. (A) Occludin mRNA expression. (B) Claudin-2 mRNA expression. (C) Claudin-2 protein expression. Lanes 1 and 2, WT Hb2 infection; lanes 3 and 4, IL-13Rα1 knockout (KO) Hb2 infection; lanes 5 and 6, WT VEH; lanes 7 and 8, IL-13Rα1 KO VEH. (D) Immunohistochemistry using claudin-2 antibody. *, P < 0.05, compared to WT VEH; ϕ, P < 0.05, compared to WT Hb1 or Hb2 infection. n = 5 to 7 per group for mRNA and n = 2 per group for protein.
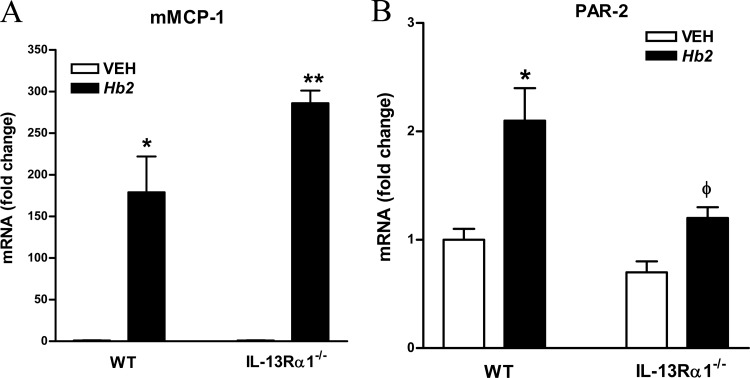
H. bakeri infection is associated with an increase in mucosal mast cell (MMC) numbers (29). IL-13 increased expression of PAR-2, a receptor for tryptase and trypsin that is linked to increased epithelial permeability (30). In WT mice, Hb1 infection increased murine mast cell protease-1 (mMCP-1) expression, with a further elevation following Hb2 infection, consistent with previous reports (3, 27, 28). In IL-13Rα1−/− mice, there was also a robust upregulation of mMCP-1 in both Hb1 and Hb2 infections (Fig. 7A). These findings are consistent with our previous finding that infection-induced influx of MMCs is an IL-13-independent event (28). It was notable that Hb2 infection increased PAR-2 expression in WT mice, but not in IL-13Rα1−/− mice (Fig. 7B), suggesting that the maintenance of mucosal resistance in a secondary H. bakeri infection of IL-13Rα1−/− mice may be due, in part, to the lack of upregulation of PAR-2 on epithelial cells, despite a greater expression of mMCP-1 activity in the mucosa.
FIG 7.
Effects of IL-13Rα1 deficiency on factors that contribute to Hb2 infection-induced changes in mucosal barrier function. (A) mMCP-1 expression. (B) Protease-activated receptor 2 (PAR-2) mRNA expression. *, P < 0.05, and **, P < 0.01, compared to WT VEH; ϕ, P < 0.05, compared to WT Hb2 infection. n = 5 to 7 per group.
DISCUSSION
In the present study, we showed that the type-2 IL-4R has unique and critical functions in regulating the host type 2 immune responses to Hb1 and Hb2 infections. In Hb1 infection, IL-4 expression was upregulated in WT, but not in IL-13Rα1−/−, mice, while expression of the IL-13 gene and protein was elevated in both strains. In Hb2 infection, the levels of upregulation of both IL-13 and IL-4 gene expression, as well the level of IL-13 protein, were comparable in both strains. As the chronic nature of Hb1 infection can be cleared by administration of exogenous IL-4 or IL-13 in WT mice (31), the levels of IL-13 in Hb1 infection in both strains indicate that elevated IL-13 alone is insufficient to clear the worms. This confirms the documented importance of IL-4 in clearance of H. bakeri (3). A second factor to consider is that the biological activity of IL-13 is controlled by levels of the decoy receptor (5). In WT mice, IL-13Rα2 expression was increased in response to Hb1 infection and further increased by Hb2 infection, but these effects were abrogated or attenuated in IL-13Rα1−/− mice. These data indicate that in the chronic Hb1 infection, the Th2 response is limited in WT mice, and levels of IL-13 induce a comparably modest expression of the decoy receptor and may not be high enough to lower IL-13 levels. In contrast, in Hb2 infection, the stronger memory Th2 response leads to elevated levels of IL-13 and IL-13Rα2 expression, which in turn effectively control IL-13 levels. The importance of this regulatory feedback loop can be appreciated by studies showing that mice deficient in the decoy receptor have exaggerated IL-13-mediated effects, both constitutively and in response to nematode infection (5, 12). The impaired upregulation of the decoy receptor in IL-13Rα1−/− mice indicates a major role for the type 2 IL-4R and STAT6 in the expression of the decoy receptor and control biological effects of IL-13. We cannot; however, completely exclude the contribution of other receptors, including the type 1 IL-4R.
The present study establishes several features important to type 2 IL-4R (IL-13Rα1)-dependent worm expulsion. Intestinal cysts induced by parasitic H. bakeri larvae penetrating the submucosal layer are a focal point for infiltrating CD4+ T cells and other immune cells, which increase local Th2 cytokines and induce alternatively activated macrophages (32). This microenvironment is muted during Hb1 infection (32) and also was absent during Hb2 infection in IL-13Rα1−/− mice, indicating that IL-13 is needed for the type 2 memory response that drives this tissue response. We did not investigate the possible changes in the outcomes based on differences in the microbiome in mice from different sources. Our previous data, however, demonstrate the reproducibility of the type 2 response in a number of mouse strains from different sources. Of interest is that in 2 separate studies, H. bakeri was associated with increased abundance of bacteria in the ileum (33, 34), indicating that this may be another characteristic feature of infection observed in different mouse strains.
Goblet cell mucus production, RELM-β, and Muc5ac are critical for the host defense against nematode infection (24, 35, 36). There was a significant increase in the number of goblet cells as well as in the expression of RELM-β and Muc5ac in WT mice during both Hb1 and Hb2 infections. Since Hb1 infection is chronic, increased levels of goblet cell RELM-β and Muc5ac alone are insufficient for effective worm clearance. In IL-13Rα1−/− mice, the upregulation of RELM-β expression was absent completely during Hb2 infection, despite a significant, but small, increase in the number of goblet cells. These data suggest that IL-13 binding to IL-13Rα1 is critical for goblet cell proliferation, and the increased RELM-β expression can be linked to the increase in goblet cell number. In contrast, Muc5ac expression was upregulated in IL-13Rα1−/− mice during Hb2 infection to levels similar to those in WT mice, indicating that changes in mucus composition are not linked inexorably to an increase in goblet cell number or to IL-13.
In the present study, only the expression of arginase-1 was attenuated significantly in IL-13Rα1−/− mice during Hb2 infection. In both strains, CD206 was unchanged in Hb1 infection but increased in Hb2 infection. These data are consistent with previous data from our laboratory indicating that both IL-4 and IL-13 promote M2 development through the type 1 and type2 IL-4Rs (22). These data also suggest that lack of polarization toward the M2 phenotype, as a result of the lower expression of Th2 cytokines, may contribute to the chronicity of Hb1 infection in both strains. CD206, FIZZ1, and YM1 are also considered to be STAT6-dependent M2 markers. We showed previously that the N. brasiliensis-induced upregulation of FIZZ1 and YM1 in the intestine was unchanged by macrophage depletion (22), indicating that infection also upregulates the expression of these markers in cells other than macrophages.
Other characteristic features of enteric nematode infection are a thickening of the muscularis externa and a hypercontractility of intestinal smooth muscle that is associated with worm clearance (19, 27). We previously demonstrated that infection-induced changes in intestinal smooth muscle contractility are STAT6 and M2 dependent (19, 22), but the contribution of the type 1 versus type 2 IL-4R has not been established. In the present study, Hb1 infection had no effect on smooth muscle responses in either strain, consistent with the modest upregulation of IL-4 and IL-13. In contrast, Hb2 infection induced a hypercontractility to all stimuli in WT mice that was completely absent during Hb2 infection in IL-13Rα1−/− mice. This may be due, in part, to the inhibition of the infection-induced increase in M3R and 5-HT2A expression. M3R is one of a growing number of receptors, including 5-HT2A and PAR-1, that impact smooth muscle function and are dependent on IL-13/STAT6 but not on IL-4 (25, 26). These receptors are expressed on both smooth muscle and macrophages and highlight the importance of IL-13 to the interaction between macrophages and smooth muscle that results in the hypercontractility observed in nematode infection. Our previous data showed the infection-induced hypercontractile responses to PAR-2 and acetylcholine were normalized in the absence of neural input (19, 27). The reduced responses to EFS observed in IL-13Rα1−/− mice during Hb2 infection confirm a role for the type 2 IL-4R in the neural hypersensitivity of nematode infection.
M2 markers play a vital role in smooth muscle function and morphology during a nematode infection, (22), and depletion of M2 markers with clodronate-containing liposomes inhibited smooth muscle contractility and proliferation and impaired worm expulsion (22, 23). In the present study, however, there was a complete loss of nematode-induced hypercontractility and increased muscle thickness in IL-13Rα1−/− mice during Hb2 infection, even in the presence of significant M2 marker expression. The failed upregulation of IGF-1 in smooth muscle during Hb2 infection in IL-13Rα1−/− mice is consistent with the inhibition of smooth muscle hypertrophy and hyperplasia. The residual expression of these markers in infected IL-13Rα1−/− mice may be attributed to IL-4 activation of type 1 IL-4R that contributes to M2 development during Hb2 infection.
Among the dominant features of nematode infection are decreased glucose absorption, hyposecretion, and increased mucosal permeability (18, 27, 28). The net result of these effects is increased intraluminal fluid in vivo that facilitates worm expulsion. The inhibition of glucose absorption in the present study appeared to involve both IL-4 and IL-13, consistent with our earlier data showing the dependence of these effects on STAT6 (28). In a recent study, overexpression of IL-13 was associated with enhanced secretory responses that were linked to an IL-13Rα1-dependent upregulation of CFTR (4). We showed that Hb2 infection inhibited fluid secretion in WT and IL-13Rα1−/− mice, despite a modest upregulation of the CFTR. These data show that increased fluid secretion mediated by IL-13-induced increase in CFTR expression is not part of the type 2 response against nematode infection. We showed previously that both nematode infection as well as exogenous IL-4/IL-13-induced alterations in epithelial secretion were STAT6 dependent (18, 28). The present study demonstrates that IL-4 may play a greater role than IL-13 in the nematode-induced hyposecretion. It should also be noted that the effects of exogenous IL-4 on epithelial secretion were dependent on mast cells (28). These data clearly indicate that the effects of IL-4 and IL-13 on epithelial secretion during nematode infection are mediated through different mechanisms.
The lack of an effect of Hb1 infection on intestine permeability and smooth muscle contractility is consistent with a diminished type 2 response. Hb2 infection-induced decrease in resistance (increased permeability) was evident when measured by Ussing chambers or TEER and is consistent with previous studies with mice treated with exogenous IL-4 or IL-13 or transgenic mice that overexpress IL-13 in the small intestine (4). We evaluated several factors known to control epithelial permeability, including TJPs, MMCs, and PARs. Much of the evidence for IL-13-mediated changes in epithelial barrier function is derived from studies in epithelial cell lines showing that IL-13 increases permeability, in part, through the upregulation of claudin-2 (37), one of several pore-forming TJPs in the claudin family. The clinical importance of this observation is that both IL-13 and claudin-2 are elevated in inflammatory pathologies such as ulcerative colitis (10, 11, 38). In the present study, we showed that Hb2 infection increased claudin-2 expression in WT mice, coincident with increased permeability, but had no effect on occludin or other pore-forming claudins. Surprisingly, Hb2 infection also induced upregulated claudin-2 expression in IL-13Ra1−/− mice. This observation indicated that claudin-2, like CFTR, can be upregulated without a functional type 2 IL-4R and that the increased permeability during Hb2 infection is independent of claudin-2 expression. These data are consistent with recent studies, which showed that the effects of IL-13 on permeability in epithelial cultures could not be attributed entirely to claudin-2 (37).
In the present study, the enhanced permeability during Hb2 infection in WT mice was not due to MMC activity, as mMCP-1 levels were similar in both infected WT and IL-13Rα1−/− mice. Previous reports showed mastocytosis in response to infection was not blocked in STAT6−/− mice (3), in mice with global deficiency in IL-4Rα (39), or in IL-13/4/5−/− mice (40). Our data confirm that the type2 IL-4R does not play a major role in the influx of MMC during Hb2 infection. Proteolytic cleavage of PAR-2 by mast-cell-derived serine proteases enhances epithelial permeability (30). We showed previously that nematode-induced upregulation of PAR-2 expression was STAT6 dependent (41). This observation was supported by the absence of PAR-2 expression in IL-13Rα1−/− mice during Hb2 infection. While PAR-2 may not be the sole mechanism of the IL-13-mediated changes in barrier function, it is likely that this effect is linked to upregulation of unknown STAT6-dependent genes, including the gene coding for PAR-2.
In conclusion, these data show the importance of IL-13, working primarily through IL-13Rα1, to the host type 2 responses to nematode infection. IL-13 contributed to the influx and development of M2, but the development also required the type 1 IL-4R. We firmly established the importance of IL-13Rα1 on nonhematopoietic cells for worm clearance, including H. bakeri-induced changes in smooth muscle contractility and morphology, goblet cell mucus production, and RELM-β expression. Of interest is that IL-13 is not required for the upregulation of Muc5ac, further delineating the contribution of the type 2 IL-4R to goblet cell function. Finally, Hb infection induced changes in epithelial permeability during a secondary infection by a mechanism that was independent of claudin-2 expression but may involve STAT-6-dependent genes such as PAR-2. The dissociation of IL-13 and upregulation of claudin-2 expression may have important ramifications for the proposed relationship between increased permeability and IL-13 in the context of clinical and experimental colitis.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by NIH grants R01-AI/DK49316 (T.S.-D.) and R01-DK083418 (A.Z.) and USDA CRIS project 1235-51000-055 (J.F.U.).
The views expressed in this article are those of the authors and do not reflect the official policy or position of the Department of Defense (K.B.M.) or the U.S. Department of Agriculture (J.F.U.). This work was prepared as part of our official duties (K.B.M. and J.F.U.).
The authors have no conflicts of interest to disclose.
Funding Statement
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.00990-15.
REFERENCES
- 1.Elliott DE, Summers RW, Weinstock JV. 2005. Helminths and the modulation of mucosal inflammation. Curr Opin Gastroenterol 21:51–58. [PubMed] [Google Scholar]
- 2.Finkelman FD, Wynn TA, Donaldson DD, Urban JF. 1999. The role of IL-13 in helminth-induced inflammation and protective immunity against nematode infections. Curr Opin Immunol 11:420–426. doi: 10.1016/S0952-7915(99)80070-3. [DOI] [PubMed] [Google Scholar]
- 3.Finkelman FD, Shea-Donohue T, Morris SC, Gildea L, Strait R, Madden KB, Schopf L, Urban JF Jr. 2004. Interleukin-4- and interleukin-13-mediated host protection against intestinal nematode parasites. Immunol Rev 201:139–155. doi: 10.1111/j.0105-2896.2004.00192.x. [DOI] [PubMed] [Google Scholar]
- 4.Wu D, Ahrens R, Osterfeld H, Noah TK, Groschwitz K, Foster PS, Steinbrecher KA, Rothenberg ME, Shroyer NF, Matthaei KI, Finkelman FD, Hogan SP. 2011. Interleukin-13 (IL-13)/IL-13 receptor alpha1 (IL-13Ralpha1) signaling regulates intestinal epithelial cystic fibrosis transmembrane conductance regulator channel-dependent Cl− secretion. J Biol Chem 286:13357–13369. doi: 10.1074/jbc.M110.214965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morimoto M, Zhao A, Sun R, Stiltz J, Madden KB, Mentink-Kane M, Ramalingam T, Wynn TA, Urban JF Jr, Shea-Donohue T. 2009. IL-13 receptor α2 regulates the immune and functional response to Nippostrongylus brasiliensis infection. J Immunol 183:1934–1939. doi: 10.4049/jimmunol.0804299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wynn TA. 2003. IL-13 effector functions. Annu Rev Immunol 21:425–456. doi: 10.1146/annurev.immunol.21.120601.141142. [DOI] [PubMed] [Google Scholar]
- 7.Van Dyken SJ, Locksley RM. 2013. Interleukin-4- and interleukin-13-mediated alternatively activated macrophages: roles in homeostasis and disease. Annu Rev Immunol 31:317–343. doi: 10.1146/annurev-immunol-032712-095906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramalingam TR, Pesce JT, Sheikh F, Cheever AW, Mentink-Kane MM, Wilson MS, Stevens S, Valenzuela DM, Murphy AJ, Yancopoulos GD, Urban JF, Donnelly RP, Wynn TA. 2008. Unique functions of the type II interleukin 4 receptor identified in mice lacking the interleukin 13 receptor α1 chain. Nat Immunol 9:25–33. doi: 10.1038/ni1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rothenberg ME, Wen T, Shik D, Cole ET, Mingler MM, Munitz A. 2011. IL-13 receptor alpha 1 differentially regulates aeroallergen-induced lung responses. J Immunol 187:4873–4880. doi: 10.4049/jimmunol.1004159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heller F, Florian P, Bojarski C, Richter J, Christ M, Hillenbrand B, Mankertz J, Gitter AH, Burgel N, Fromm M, Zeitz M, Fuss I, Strober W, Schulzke JD. 2005. Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology 129:550–564. [DOI] [PubMed] [Google Scholar]
- 11.Rosen MJ, Frey MR, Washington MK, Chaturvedi R, Kuhnhein LA, Matta P, Revetta FL, Wilson KT, Polk DB. 2011. STAT6 activation in ulcerative colitis: a new target for prevention of IL-13-induced colon epithelial cell dysfunction. Inflamm Bowel Dis 17:2224–2234. doi: 10.1002/ibd.21628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morimoto M, Morimoto M, Zhao A, Madden KB, Dawson H, Finkelman FD, Mentink-Kane M, Urban JF Jr, Wynn TA, Shea-Donohue T. 2006. Functional importance of regional differences in localized gene expression of receptors for IL-13 in murine gut. J Immunol 176:491–495. doi: 10.4049/jimmunol.176.1.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fichtner-Feigl S, Fuss IJ, Young CA, Watanabe T, Geissler EK, Schlitt HJ, Kitani A, Strober W. 2007. Induction of IL-13 triggers TGF-beta1-dependent tissue fibrosis in chronic 2,4,6-trinitrobenzene sulfonic acid colitis. J Immunol 178:5859–5870. doi: 10.4049/jimmunol.178.9.5859. [DOI] [PubMed] [Google Scholar]
- 14.Fichtner-Feigl S, Strober W, Kawakami K, Puri RK, Kitani A. 2006. IL-13 signaling through the IL-13[alpha]2 receptor is involved in induction of TGF-β1 production and fibrosis. Nat Med 12:99–106. doi: 10.1038/nm1332. [DOI] [PubMed] [Google Scholar]
- 15.Mattes J, Yang M, Siqueira A, Clark K, MacKenzie J, McKenzie ANJ, Webb DC, Matthaei KI, Foster PS. 2001. IL-13 induces airways hyperreactivity independently of the IL-4Rα chain in the allergic lung. J Immunol 167:1683–1692. doi: 10.4049/jimmunol.167.3.1683. [DOI] [PubMed] [Google Scholar]
- 16.Reynolds L, Filbey K, Maizels R. 2012. Immunity to the model intestinal helminth parasite Heligmosomoides polygyrus. Semin Immunopathol 34:829–846. doi: 10.1007/s00281-012-0347-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Urban JF, Maliszewski CR, Madden KB, Katona IM, Finkelman FD. 1995. IL-4 treatment can cure established gastrointestinal nematode infections in immunocompetent and immunodeficient mice. J Immunol 154:4675–4684. [PubMed] [Google Scholar]
- 18.Madden KB, Yeung KA, Zhao A, Gause WC, Finkelman FD, Katona IM, Urban JF Jr, Shea-Donohue T. 2004. Enteric nematodes induce stereotypic STAT6-dependent alterations in intestinal epithelial cell function. J Immunol 172:5616–5621. doi: 10.4049/jimmunol.172.9.5616. [DOI] [PubMed] [Google Scholar]
- 19.Zhao A, McDermott J, Urban JF Jr, Gause W, Madden KB, Yeung KA, Morris SC, Finkelman FD, Shea-Donohue T. 2003. Dependence of IL-4, IL-13, and nematode-induced alterations in murine small intestinal smooth muscle contractility on Stat6 and enteric nerves. J Immunol 171:948–954. doi: 10.4049/jimmunol.171.2.948. [DOI] [PubMed] [Google Scholar]
- 20.Zhao A, Urban JF, Sun R, Stiltz J, Morimoto M, Notari L, Madden KB, Yang Z, Grinchuk V, Ramalingam TR, Wynn TA, Shea-Donohue T. 2010. Critical role of IL-25 in nematode infection-induced alterations in intestinal function. J Immunol 185:6921–6929. doi: 10.4049/jimmunol.1000450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Research Council. 2011. Guide for the care and use of laboratory animals, 8th ed National Academies Press, Washington, DC. [Google Scholar]
- 22.Zhao A, Urban JF Jr, Anthony RM, Sun R, Stiltz J, van Rooijen N, Wynn TA, Gause WC, Shea-Donohue T. 2008. Th2 cytokine-induced alterations in intestinal smooth muscle function depend on alternatively activated macrophages. Gastroenterology 135:217–225. doi: 10.1053/j.gastro.2008.03.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anthony RM, Urban JF Jr, Alem F, Hamed HA, Rozo CT, Boucher JL, Van RN, Gause WC. 2006. Memory T(H)2 cells induce alternatively activated macrophages to mediate protection against nematode parasites. Nat Med 12:955–960. doi: 10.1038/nm1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herbert DBR, Yang JQ, Hogan SP, Groschwitz K, Khodoun M, Munitz A, Orekov T, Perkins C, Wang Q, Brombacher F, Urban JF, Rothenberg ME, Finkelman FD. 2009. Intestinal epithelial cell secretion of RELM-beta protects against gastrointestinal worm infection. J Exp Med 206:2947–2957. doi: 10.1084/jem.20091268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao A, Urban JF Jr, Morimoto M, Elfrey JE, Madden KB, Finkelman FD, Shea-Donohue T. 2006. Contribution of 5-HT2A receptor in nematode infection-induced murine intestinal smooth muscle hypercontractility. Gastroenterology 131:568–578. doi: 10.1053/j.gastro.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 26.Zhao A, Morimoto M, Dawson H, Elfrey JE, Madden KB, Gause WC, Min B, Finkelman FD, Urban JF Jr, Shea-Donohue T. 2005. Immune regulation of protease-activated receptor-1 expression in murine small intestine during Nippostrongylus brasiliensis infection. J Immunol 175:2563–2569. doi: 10.4049/jimmunol.175.4.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shea-Donohue T, Sullivan C, Finkelman FD, Madden KB, Morris SC, Goldhill J, Pineiro-Carrero V, Urban JF Jr. 2001. The role of IL-4 in Heligmosomoides polygyrus-induced alterations in murine intestinal epithelial cell function. J Immunol 167:2234–2239. doi: 10.4049/jimmunol.167.4.2234. [DOI] [PubMed] [Google Scholar]
- 28.Madden KB, Whitman L, Sullivan C, Gause WC, Urban JF Jr, Katona IM, Finkelman FD, Shea-Donohue T. 2002. Role of STAT6 and mast cells in IL-4- and IL-13-induced alterations in murine intestinal epithelial cell function. J Immunol 169:4417–4422. doi: 10.4049/jimmunol.169.8.4417. [DOI] [PubMed] [Google Scholar]
- 29.Madden KB, Urban JF Jr, Ziltener HJ, Schrader JW, Finkelman FD, Katona IM. 1991. Antibodies to IL-3 and IL-4 suppress helminth-induced intestinal mastocytosis. J Immunol 147:1387–1391. [PubMed] [Google Scholar]
- 30.Jacob SS, Shastry P, Sudhakaran PR. 2002. Monocyte-macrophage differentiation in vitro: modulation by extracellular matrix protein substratum. Mol Cell Biochem 233:9–17. doi: 10.1023/A:1015593232347. [DOI] [PubMed] [Google Scholar]
- 31.Finkelman FD, Shea-Donohue T, Goldhill J, Sullivan CA, Morris SC, Madden KB, Gause WC, Urban JF. 1997. Cytokine regulation of host defense against parasitic gastrointestinal nematodes: lessons from studies with rodent models. Annu Rev Immunol 15:505–533. doi: 10.1146/annurev.immunol.15.1.505. [DOI] [PubMed] [Google Scholar]
- 32.Morimoto M, Morimoto M, Whitmire J, Xiao S, Anthony RM, Mirakami H, Star RA, Urban JF, Gause WC. 2004. Peripheral CD4 T cells rapidly accumulate at the host:parasite interface during an inflammatory Th2 memory response. J Immunol 172:2424–2430. doi: 10.4049/jimmunol.172.4.2424. [DOI] [PubMed] [Google Scholar]
- 33.Rausch S, Held J, Fischer A, Heimesaat MM, Kühl AA, Bereswill S, Hartmann S. 2013. Small intestinal nematode infection of mice is associated with increased enterobacterial loads alongside the intestinal tract. PLoS One 8:e74026. doi: 10.1371/journal.pone.0074026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walk ST, Blum AM, Ewing SA-S, Weinstock JV, Young VB. 2010. Alteration of the murine gut microbiota during infection with the parasitic helminth, Heligmosomoides polygyrus. Inflamm Bowel Dis 16:1841–1849. doi: 10.1002/ibd.21299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hasnain SZ, Wang H, Ghia JE, Haq N, Deng Y, Velcich A, Grencis RK, Thornton DJ, Khan WI. 2010. Mucin gene deficiency in mice impairs host resistance to an enteric parasitic infection. Gastroenterology 138:1763–1771. doi: 10.1053/j.gastro.2010.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hasnain SZ, Evans CM, Roy M, Gallagher AL, Kindrachuk KN, Barron L, Dickey BF, Wilson MS, Wynn TA, Grencis RK, Thornton DJ. 2011. Muc5ac: a critical component mediating the rejection of enteric nematodes. J Exp Med 208:893–900. doi: 10.1084/jem.20102057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weber CR, Raleigh DR, Su L, Shen L, Sullivan EA, Wang Y, Turner JR. 2010. Epithelial myosin light chain kinase activation induces mucosal interleukin-13 expression to alter tight junction ion selectivity. J Biol Chem 285:12037–12046. doi: 10.1074/jbc.M109.064808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prasad S, Mingrino R, Kaukinen K, Hayes KL, Powell RM, MacDonald TT, Collins JE. 2005. Inflammatory processes have differential effects on claudins 2, 3 and 4 in colonic epithelial cells. Lab Invest 85:1139–1162. doi: 10.1038/labinvest.3700316. [DOI] [PubMed] [Google Scholar]
- 39.Michels CE, Scales HE, Saunders KA, McGowan S, Brombracher F, Alexander J, Lawrence CE. 2009. Neither interleukin-4 receptor alpha expression on CD4+ T cells, or macrophages and neutrophils is required for protective immunity to Trichinella spiralis. Immunology 128:e385–e394. doi: 10.1111/j.1365-2567.2008.02987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fallon PG, Jolin HE, Smith P, Emson CL, Townsend MJ, Fallon R, Smith P, McKenzie ANJ. 2002. IL-4 induces characteristic Th2 responses even in the combined absence of IL-5, IL-9, and IL-13. Immunity 17:7–17. doi: 10.1016/S1074-7613(02)00332-1. [DOI] [PubMed] [Google Scholar]
- 41.Shea-Donohue T, Notari L, Stiltz J, Sun R, Madden KB, Urban JF Jr, Zhao A. 2010. Role of enteric nerves in immune-mediated changes in protease-activated receptor 2 effects on gut function. Neurogastroenterol Motil 22:1138–e1291. doi: 10.1111/j.1365-2982.2010.01557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.