Abstract
Sepsis caused by Staphylococcus aureus is increasing in incidence. With the alarming use of antibiotics, S. aureus is prone to become methicillin resistant. Antibiotics are the only widely used pharmacological treatment for sepsis. Interestingly, mice fed high-fat diet (HFD) rich in polyunsaturated fatty acids have better survival of S. aureus-induced sepsis than mice fed HFD rich in saturated fatty acids (HFD-S). To investigate what component of polyunsaturated fatty acids, i.e., omega-3 or omega-6 fatty acids, exerts beneficial effects on the survival of S. aureus-induced sepsis, mice were fed HFD rich in omega-3 or omega-6 fatty acids for 8 weeks prior to inoculation with S. aureus. Further, mice fed HFD-S were treated with omega-3 fatty acid metabolites known as resolvins. Mice fed HFD rich in omega-3 fatty acids had increased survival and decreased bacterial loads compared to those for mice fed HFD-S after S. aureus-induced sepsis. Furthermore, the bacterial load was decreased in resolvin-treated mice fed HFD-S after S. aureus-induced sepsis compared with that in mice treated with vehicle. Dietary omega-3 fatty acids increase the survival of S. aureus-induced sepsis by reversing the deleterious effect of HFD-S on mouse survival.
INTRODUCTION
Sepsis is a deadly disease with an increasing incidence worldwide (1). It is the leading cause of death in intensive care units, with a mortality rate of 30 to 70% (2). Today, antibiotics are the only widely used pharmacological treatment for sepsis (3). Staphylococcus aureus and other Gram-positive bacteria account for the major part of its increased incidence (1, 4). Resistance to antibiotics is a growing problem worldwide. S. aureus bacteria are a threat to human health, given that they have obtained a gene that allows them to become methicillin resistant (5). In intensive care units in Europe, more than 50% of the isolates of S. aureus are methicillin resistant (6).
Sepsis is considered a two-phase disease with an initial hyperinflammatory state, followed by a later hypoimmune state, and most deaths occur in the hypoimmune state (2, 7). A large portion of the previous efforts to develop new treatments have been focused on dampening the hyperinflammatory state (8). However, these strategies have led to very minor results. A better understanding of factors that can influence the mortality rates during the different stages of sepsis might lead to new treatments, in addition to antibiotics.
In previous studies by our research group, we found that the immune system was negatively affected by high-fat diet (HFD) rich in saturated fatty acids (HFD-S), resulting in decreased survival of septic S. aureus-induced infection, compared with a low-fat diet (LFD) (9). Interestingly, in a later study, we found that mice fed HFD rich in polyunsaturated fatty acids (HFD-P) had increased survival of septic S. aureus-induced infection and decreased bacterial loads in the kidneys compared with those for mice fed HFD-S (10).
Polyunsaturated fatty acids, such as those found in HFD-P, can be divided mainly into omega-3 and omega-6 fatty acids on the basis of the location of the first double valence bond in relation to the end of the carbon chain (omega carbon). They can further be divided into cis and trans fatty acids on the basis of the configuration of the two hydrogen atoms on either side of the double bond. If the two hydrogen atoms are on the same side of the chain, the fatty acid is said to have a cis configuration, whereas if the two hydrogen atoms are on opposite sides of the chain, the fatty acid is said to have a trans configuration. Omega-6 fatty acids, especially arachidonic acid, are considered to have essentially proinflammatory properties, whereas omega-3 fatty acids are considered to have anti-inflammatory properties (11). For example, patients with inflammatory diseases such as rheumatoid arthritis and atherosclerosis have been reported to benefit from dietary omega-3 fatty acids (12, 13).
The innate immune response, in particular, the activation of neutrophils, is the first line of defense against bacterial infectious diseases (14). We have previously shown that mice fed HFD-P have a higher frequency of neutrophils in their bone marrow than mice fed HFD-S. The omega-3 fatty acid metabolites known as resolvins are a group of biologically active metabolites that have been found to enhance neutrophils' capacity to phagocytose Escherichia coli (15). It has also been shown that the survival of mice was increased by treatment with resolvin D2 (RvD2) after cecal ligation and puncture, an experimental model of multibacterial sepsis (15). Similar results with increased survival by treatment with RvD1 have been shown after inoculation with E. coli (16).
In the present study, we investigated if HFD rich in omega-3 fatty acids (HFD-ω3) or omega-6 fatty acids (HFD-ω6) is most efficient at increasing survival and decreasing bacterial loads in mice with septic S. aureus-induced infection. We found that mice fed HFD-ω3 survived better than mice fed HFD-ω6 or HFD-S and had decreased bacterial loads compared to those for mice fed HFD-S. Also, mice fed HFD-S had a lower frequency of phagocytosing neutrophils in their circulation. Moreover, we found that mice fed HFD-S had decreased bacterial loads when treated with resolvins after inoculation with S. aureus.
MATERIALS AND METHODS
Experimental protocol.
The experimental design used in this study is shown in Fig. 1. In summary, C57BL/6 mice were fed LFD, HFD-S, HFD-ω3, or HFD-ω6 for 8 weeks. Following 8 weeks on the different diets, mice were either intravenously (i.v.) inoculated with S. aureus or terminated for measurements of immune functions by flow cytometry. Bacterial loads were analyzed in infected animals 6 days after inoculation, and survival was monitored for 17 days after inoculation. The animals remained on their respective diets after inoculation. The measurements of immune parameters included determination of the frequencies of different immune cells in bone marrow and the frequency of neutrophils from the circulation that had phagocytosed pHrodo Green S. aureus BioParticles (pHrodo particles; Life Technologies, CA, USA). To investigate if mice fed HFD-S would benefit from treatment with resolvins, mice were once again fed for 8 weeks, but this time only HFD-S. Following 8 weeks on HFD-S, mice were i.v. inoculated with S. aureus. On days 1 to 4, mice were treated i.v. with the vehicle, RvD1 (Cayman Chemical, Ann Arbor, MI, USA), or RvD2 (Cayman Chemical). Bacterial loads and survival were once again analyzed in infected animals 6 days after inoculation and monitored for 17 days after inoculation, respectively. All experiments were approved in advance by the local ethics committee for animal care at the University of Gothenburg.
FIG 1.
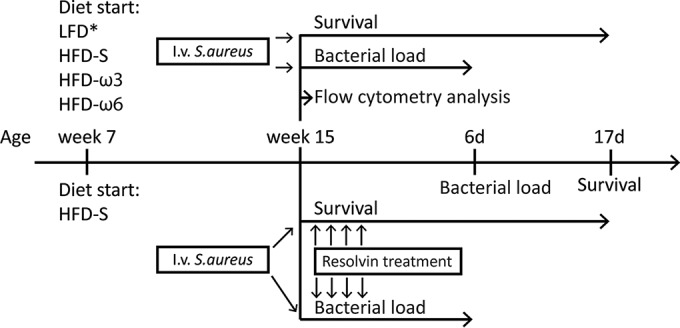
Experimental design. Seven-week-old mice were randomized into different diet groups. After 8 weeks on the diets, mice were either terminated for collection of bone marrow and blood for flow cytometry analysis or inoculated with S. aureus for bacterial load or survival analysis. Mice inoculated with S. aureus were either terminated after 6 days (6d) for bacterial load analysis or monitored continuously for up to 17 days (17d) for survival analysis. Another set of mice were fed only HFD-S. After 8 weeks on the diet, mice were inoculated with S. aureus and randomized to receive i.v. treatment with the vehicle, RvD1, or RvD2. Bacterial load and survival analyses were conducted in the same way as described above. Experimental groups: LFD, HFD-S, HFD-ω3, HFD-ω6, HFD-S and treatment with the vehicle (vehicle), HFD-S and treatment with RvD1, and HFD-S and treatment with RvD2. *, LFD was not included in the first survival study.
Diets.
The compositions of the diets used are shown in Table 1. Mice were randomized to receive LFD (D12450B; 3.9 kcal/g, 10 kcal% fat, 20 kcal% protein, 70 kcal% carbohydrate; Research Diets, New Brunswick, NJ, USA), HFD-S (D12492; 5.2 kcal/g, 60 kcal% fat, 20 kcal% protein, 20 kcal% carbohydrate; Research Diets), HFD-ω3 (D090120501; same fat, protein, and carbohydrate composition as HFD-S but 31% of lard exchanged for ROPUFA 75EE; Research Diets), or HFD-ω6 (D10031504; same fat, protein, and carbohydrate composition as HFD-S but 69% of lard exchanged for safflower oil; Research Diets). The HFDs were matched to have similar carbohydrate and protein contents and to differ only in fat composition and concentration. The concentration of omega-3 fatty acids was almost 21-fold higher in HFD-ω3 (24.7%) than in HFD-ω6 (1.2%). In contrast, the concentration of omega-6 fatty acids was almost 3-fold higher in HFD-ω6 (62.9%) than in HFD-ω3 (23.4%). The amounts of trans fatty acids were similar in HFD-S and HFD-ω3 (0.7 and 0.5%, respectively) and lowest in HFD-ω6 (0.2%).
TABLE 1.
Energy densities and compositions of the experimental diets used in this study
| Parameter | LFD | HFD-S | HFD-ω3 | HFD-ω6 |
|---|---|---|---|---|
| Energy density (kcal/g) | 3.9 | 5.2 | 5.2 | 5.2 |
| Macronutrients (% kcal) | ||||
| Protein | 20 | 20 | 20 | 20 |
| Carbohydrate | 70 | 20 | 20 | 20 |
| Fat | 10 | 60 | 60 | 60 |
| Fat sources (% of total fat) | ||||
| Soybean oil | 55.6 | 9.3 | 9.3 | 9.3 |
| Lard | 44.4 | 90.7 | 62.2 | 27.8 |
| ROPUFA 75EE | 28.5 | |||
| Safflower oil | 63.0 | |||
| Fatty acids (% by wt of total fatty acids) | ||||
| ∑ SFAa | 22.7 | 32.0 | 23.6 | 16.0 |
| ∑ MUFAb | 29.8 | 36.0 | 28.3 | 19.9 |
| ∑ PUFAc | 47.5 | 32.0 | 48.1 | 64.1 |
| ∑ n-3 total fat | 5.2 | 2.1 | 24.7 | 1.2 |
| ∑ n-6 total fat | 42.4 | 29.9 | 23.4 | 62.9 |
| n-6/n-3 | 8.2 | 14.1 | 0.9 | 51.9 |
SFA, saturated fatty acids.
MUFA, monounsaturated fatty acids.
PUFA, polyunsaturated fatty acids.
Bacterial inoculation, survival, and bacterial loads.
Mice were inoculated i.v. through the tail vein with 0.2 ml of S. aureus LS-1 solution containing 3.0 × 107 to 5.4 × 107 CFU. For bacterial load determination, mice were terminated 6 days after inoculation. Both kidneys were harvested aseptically and prepared as previously described (10). During the time the mice were infected, they were continuously examined for signs of severe sickness: motionlessness, isolation, piloerection, dehydration, and hypothermia. If a mouse showed three or more of these five signs, it was euthanized and considered dead by sepsis.
Body weight and composition.
Body weight was determined at the start of the experiment and every second week until inoculation. Fat and lean body masses were analyzed 3 days before inoculation by dual-energy X-ray absorptiometry (DXA; PIXImus2; Lunar GE Medical Systems, Madison, WI, USA). During the measurements, the mice were placed under inhalation anesthesia with isoflurane (Forene; Abbot Scandinavia, Solna, Sweden).
Flow cytometry. (i) Collection and staining of bone barrow.
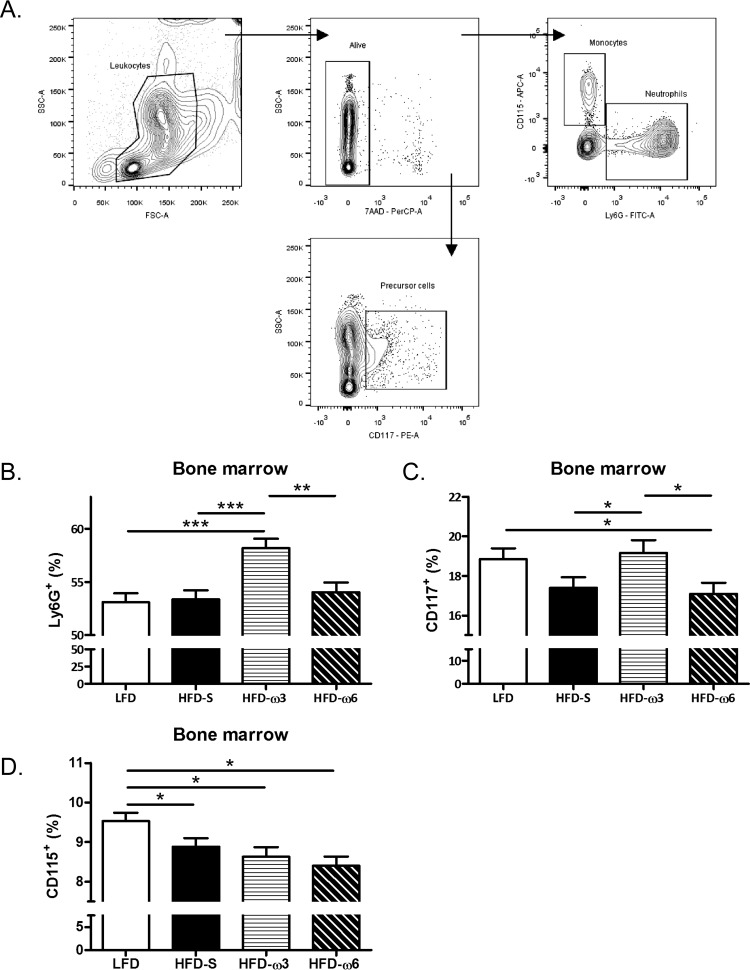
Mice were anesthetized and then perfused with physiological saline at a pressure of 100 mm Hg for 10 min. Bone marrow cells were harvested from the femur and tibia and stored in phosphate-buffered saline (PBS) with 1% fetal calf serum (FCS) on ice until preparation. Bone marrow cell suspensions were centrifuged, incubated with Pharm Lyse (BD Biosciences, Franklin Lakes, NJ, USA) for 6 min on ice to lyse red blood cells, washed, and resuspended in PBS with 1% FCS. Cell concentrations were determined with an automated cell counter (Bio-Rad, Hercules, CA, USA), and 3 × 105 cells from each sample were used for staining. To avoid unspecific binding via Fc-receptor interactions, cells were incubated with Fc block (2.4G2; BD Biosciences, Franklin Lakes, NJ, USA) for 15 min at room temperature. The cells were labeled with the following antibodies: anti-mouse Ly6G-fluorescein isothiocyanate (FITC; clone 1A8; BD Biosciences), anti-mouse CD115-allophycocyanin (APC; clone AFS98; BioLegend, San Diego, CA, USA), anti-mouse CD117-phycoerythrin (PE; clone 2B8; BioLegend), and 7-aminoactinomycin D (7AAD; BD Biosciences) for detection of live/dead cells. Cells were examined with a FACSCantoA flow cytometer (BD Biosciences). Instrument setting was performed with Comp-Beads (BD Biosciences), and data analyses were performed with the FACSDiva software (version 6.1.3; BD Biosciences). Flow cytometry analysis was started with a leukocyte gate and thereafter performed with a live gate with 7AAD. Neutrophils were identified as Ly6G+ CD115− cells (17), hematopoietic precursor cells were identified as CD117+ cells (18), and monocytes were identified as CD115+ cells (19). Flow cytometry results are presented as the frequency of living leukocytes. For the gating strategy used for bone marrow, see Fig. 4A.
FIG 4.
Leukocyte frequency in bone marrow. (A) Gating strategy for neutrophils, monocytes, and hematopoietic precursor cells in bone marrow. SSC, side scatter; FSC, forward scatter. (B) Frequency of Ly6G+ neutrophils in bone marrow (n = 10 mice per group). (C) Frequency of CD117+ precursor cells in bone marrow (n = 10 mice per group). (D) Frequency of CD115+ monocytes in bone marrow (n = 10 mice per group). The data in panels B to D were compared by two-way ANOVA with experiment as a nuisance factor and are the estimated marginal mean plus SEM. *, P < 0.05; **, P <0.01; ***, P < 0.001. The results are presented as the frequency of living leukocytes. Experimental groups: LFD, HFD-S, HFD-ω3, and HFD-ω6.
(ii) Collection and preparation of the blood for phagocytosis measurement.
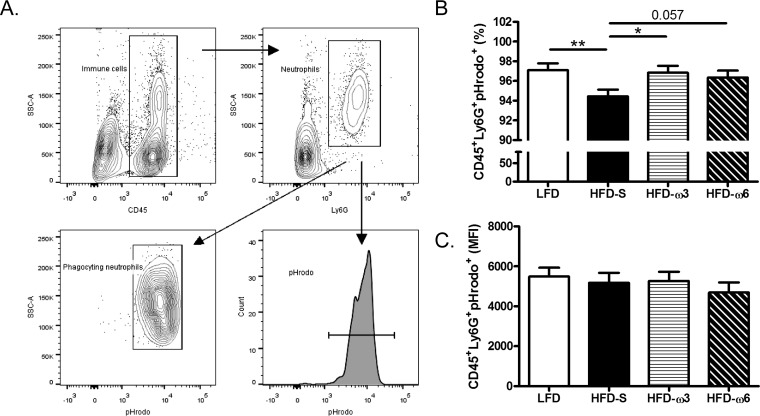
Mice were anesthetized, and their blood was collected transcardially into heparin tubes and stored at room temperature until further preparation. pHrodo Green S. aureus BioParticles (pHrodo particles; Life Technologies, CA, USA) were prepared according to the manufacturer's instructions and incubated with an equal volume of mouse serum at 37°C for 10 min. From each animal, 100 μl of whole blood was used for the sample, as well as for the negative control. Negative-control samples were incubated with 10 μl of 100 μg/ml cytochalasin D (Sigma-Aldrich, MO, USA) dissolved in dimethyl sulfoxide at 37°C for 10 min. The negative control with cytochalasin D was used to determine the background median fluorescence intensity (MFI) when the phagocytosis process had been stopped. A 20-μl volume of the pHrodo-serum suspension was added to the samples, as well as to the negative control, and they were incubated at 37°C for 45 min. All tubes were then transferred to ice to stop the phagocytosis. The red blood cells were then lysed, and the samples were washed twice and resuspended in PBS with 1% FCS. To avoid unspecific binding via Fc-receptor interactions, cells were incubated with Fc block (BD Biosciences) for 15 min at room temperature. The cells were labeled with anti-mouse CD45-peridinin chlorophyll protein (PerCp; clone 30-F11; BD Biosciences) and anti-mouse Ly6G-APC (clone 1A8; BD Biosciences) antibodies. The cells were examined with a FACSCantoA flow cytometer (BD Biosciences). Instrument setting was performed with Comp-Beads (BD Biosciences) for the antibodies and cells for pHrodo particles. Data analyses were performed with the FACSDiva software (version 6.1.3; BD Biosciences). Flow cytometry analysis of phagocytosis capacity was started with a singlet gate, followed by forward and side scatter, gating leukocytes. Phagocytosing neutrophils were identified as CD45+ Ly6G+ pHrodo+. For the gating strategy used for blood, see Fig. 5A. The MFI represents the median amount of pHrodo particles engulfed by neutrophils after subtraction of the MFI of the negative control (MFI sample – MFI negative control).
FIG 5.
Capacity of circulating neutrophils to phagocytose pHrodo particles. (A) Gating strategy for pHrodo-phagocytosing neutrophils in circulation for both frequency and MFI. SSC, side scatter. (B) Frequencies of circulating CD45+ Ly6G+ pHrodo+ neutrophils (n = 10 mice per group). (C) MFI of circulating CD45+ Ly6G+ pHrodo+ neutrophils (n = 10, 8, 9, and 8 mice per group, respectively). The data in panels A and B were compared by two-way ANOVA with the experimental day as a nuisance factor and are the estimated marginal mean plus the SEM. *, P < 0.05; **, P < 0.01. Experimental groups: LFD, HFD-S, HFD-ω3, and HFD-ω6.
Blood hematology analysis.
Blood samples were collected transcardially and stored in EDTA tubes on ice until blood analysis was conducted. Blood was analyzed with a VetH5 blood analyzer (Abaxis, Union City, CA, USA). This device is capable of discriminating between granulocytes and other leukocytes but not among neutrophils, eosinophils, and basophils.
Cytokine analysis of serum.
Serum was collected and stored at −80°C until further preparation. The cytokines included were interleukin-1β (IL-1β), IL-6, IL-10, IL-17A, gamma interferon (IFN-γ), monocyte chemotactic protein 1 (MCP-1), and tumor necrosis factor alpha (TNF-α). The concentrations of the cytokines were determined with a Luminex kit prepared according to the manufacture's specifications (Bio-Rad, Hercules, CA, USA).
Free fatty acid analysis.
Plasma (25 to 50 μl) was spiked with 43.5 nmol of the internal standard (C23:0 fatty acid) and extracted by the automated BUME method (20). After evaporation of the total extracts, the free fatty acids were derivatized to methyl esters with trimethylsilyldiazomethane (2 M hexane solution; Sigma-Aldrich, Sweden). After 2 h of derivatization at room temperature, the samples were evaporated and reconstituted in 200 μl of heptane. The fatty acid methyl esters were analyzed with a 6890N gas chromatograph (Agilent, Santa Clara, CA, USA) coupled to a flame ionization detector. For analysis, 1 μl of the heptane phase was injected (1:10 split) and separated on a DB23 column (60 m by 0.25 mm [inner diameter], 0.15 μm; J&W 122-2361) with a linear gradient of 160 to 230°C at 4°C/min. Quantification of the fatty acid methyl esters was done by relating the endogenous signals to the signal from the internal standard.
Survival and bacterial loads in resolvin experiments.
To investigate if mice fed HFD-S would benefit from treatment with resolvins, mice were fed for 8 weeks, but this time only HFD-S. At 15 weeks of age, mice were i.v. inoculated with S. aureus. For more detailed information about the inoculation and diet, see the previous sections. On days 1 to 4, mice were treated i.v. with the vehicle (0.5% ethanol in saline), RvD1 (1 or 10 ng/dose), or RvD2 (1 or 10 ng/dose). Bacterial loads and survival were once again analyzed in infected animals 6 days after inoculation and monitored for 17 days after inoculation, respectively.
Statistical analysis.
Analysis of the survival of mice fed LFD, HFD-S, HFD-ω3, or HFD-ω6 was done with the log rank test, and the results are illustrated as Kaplan-Meier curves. To investigate the correlation between body weight and survival, Cox regression analysis was performed with body weight as a covariate. Statistical analysis of the bacterial load and flow cytometry analysis consisted of a two-way analysis of variance (ANOVA) with the experiments and experimental days as factors. Each factor was considered a nuisance factor. The bacterial load and flow cytometry data are expressed as the estimated marginal mean plus standard error of the mean (SEM). Body weight and composition were analyzed by ANOVA, followed by Tukey's post hoc test. When Levene's test revealed unequal variance, Dunnett's T3 post hoc test was used for comparisons of numerical data among the four experimental groups. In this case, the data are expressed as the mean plus SEM. Analysis of the survival of mice fed HFD-S and treated with the vehicle, RvD1, or RvD2 was done with the log rank test, and the results are illustrated as Kaplan-Meier curves. Statistical analysis of bacterial loads after resolvin treatment consisted of a two-way ANOVA with contrast and the experiments as a factor. The experiment was considered a nuisance factor. Differences in sample numbers were due to laboratory errors or lack of sample material. All tests were two sided, and P < 0.05 was considered significant. All of the statistical analyses were carried out with the SPSS software (version 18.0.2 for Windows; IBM Corporation, Armonk, NY, USA).
RESULTS
Mice fed HFD-ω3 have better survival and decreased bacterial loads after S. aureus infection compared with mice fed HFD-S.
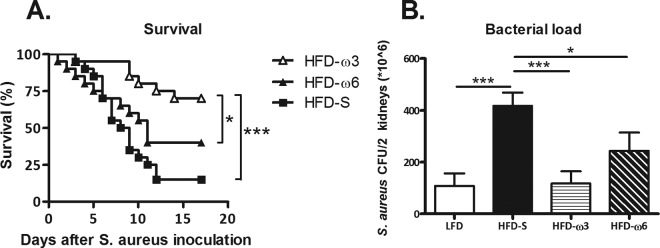
Survival at day 17 after i.v. inoculation with S. aureus was 4.7-fold greater among mice fed HFD-ω3 than among mice fed HFD-S and 2.7-fold greater than among mice fed HFD-ω6 (P < 0.001 and P = 0.04, respectively) (Fig. 2A). However, there was no difference in survival between mice fed HFD-S and those fed HFD-ω6 (Fig. 2A). As shown in Fig. 2B, bacterial loads 6 days after i.v. inoculation mirrored the survival data. Mice fed HFD-S had a 3.5-fold-greater bacterial load than mice fed LFD or HFD-ω3 (P < 0.001 and P < 0.001, respectively) and a 1.5-fold-greater bacterial load than mice fed HFD-ω6 (P = 0.05). There was no difference among the bacterial loads of mice fed LFD, HFD-ω3, or HFD-ω6 (Fig. 2B).
FIG 2.
Survival and bacterial loads. (A) Survival at 0 to 17 days after S. aureus inoculation after 8 weeks on HFD-S, HFD-ω3, or HFD-ω6. The log rank test was used to determine statistically significant differences (n = 20 mice per group). (B) Bacterial loads in the kidneys at day 6 after S. aureus inoculation after 8 weeks on LFD, HFD-S, HFD-ω3, or HFD-ω6. Two-way ANOVA with experiment as a nuisance factor was used to determine statistically significant differences (n = 18, 16, 18, and 10 mice per group, respectively). The data shown are the estimated marginal mean plus SEM. *, P < 0.05; ***, P < 0.001. Experimental groups: LFD, HFD-S, HFD-ω3, and HFD-ω6.
S. aureus may use exogenous fatty acids for incorporation in its cell membrane (21). This energy-saving mechanism can potentially result in enhanced growth of S. aureus. We therefore investigated serum saturated fatty acid content. The saturated fatty acid contents of all of the groups were similar, except for mice fed HFD-ω3, where the saturated fatty acid content was increased (Table 2).
TABLE 2.
Hematology analysis, serum cytokine levels, and free fatty acid contents of serum
| Parameter | LFD | HFD-S | HFD-ω3 | HFD-ω6 |
|---|---|---|---|---|
| WBCa count | 2.1 ± 0.3h | 3.1 ± 0.3 | 3.5 ± 0.2e | 3.3 ± 0.4e |
| LYMb count | 1.6 ± 0.3 | 2.4 ± 0.2 | 2.4 ± 0.2 | 2.3 ± 0.3 |
| GRANc count | 0.4 ± 0.1 | 0.6 ± 0.1 | 0.9 ± 0.1e | 0.8 ± 0.1 |
| Concn of: | ||||
| IL-1β | 928 ± 107 | 951 ± 131 | 997 ± 59 | 801 ± 94 |
| IL-6 | 30 ± 6 | 34 ± 7 | 41 ± 4 | 33 ± 4 |
| IL-10 | 164 ± 25 | 250 ± 45 | 239 ± 23 | 191 ± 30 |
| IL-17 | 983 ± 154 | 1,262 ± 134 | 1,146 ± 136 | 1,098 ± 172 |
| IFN-γ | 281 ± 38 | 335 ± 61 | 316 ± 27 | 259 ± 41 |
| MCP-1 | 1,050 ± 150 | 1,024 ± 152 | 1,063 ± 65 | 890 ± 118 |
| TNF-α | 24,646 ± 3,944 | 28,270 ± 5,428 | 27,118 ± 2,970 | 25,051 ± 4,395 |
| SFAd content (mol%) | 44.6 ± 1.7 | 45.9 ± 1.0 | 54.5 ± 0.9e,f,g | 45.9 ± 1.8 |
WBC, white blood cells.
LYM, lymphocytes.
GRAN, granulocytes.
SFA, saturated fatty acids.
P < 0.05 versus LFD.
P < 0.05 versus HFD-S.
P < 0.05 versus HFD-ω6.
Values are means ± standard errors of the means (SEM).
Uninfected mice fed HFD-ω3 are less obese than mice fed HFD-S but obese compared with mice fed LFD.
Obesity per se may increase susceptibility to infection (22). To investigate possible effects of the different experimental diets on body weight and composition, we performed a DXA analysis. After 8 weeks on the different experimental diets, mice fed LFD had 36% lower body weight (P < 0.001) and 59% lower fat mass (P < 0.001) than mice fed HFD-S, whereas mice fed HFD-ω3 had only 16% lower body weight (Fig. 3A; P = 0.002) and 27% lower fat mass (Fig. 3B; P < 0.001) than mice fed HFD-S. Mice fed HFD-ω6 had a body weight and fat mass similar to those of mice fed HFD-S. At the same time, the lean mass did not differ between any of the experimental groups (Fig. 3A and B). Further, the increased body weight of mice fed HFD-S did not influence survival (P = 0.56).
FIG 3.
Body weight and composition. (A) Body weights of uninfected mice after 8 weeks on a diet. One-way ANOVA with Dunnett's T3 post hoc test was used to determine statistically significant differences (n = 10 mice per group). (B) Lean mass and fat mass of uninfected mice after 8 weeks on a diet, measured by DXA. One-way ANOVA with Dunnett's post hoc test for lean mass and Tukey's test for fat mass were used to determine statistically significant differences. The data shown are the mean plus SEM (n = 10 mice per group). **, P < 0.01; ***, P < 0.001. Experimental groups: LFD, HFD-S, HFD-ω3, and HFD-ω6.
Uninfected mice fed HFD-ω3 have a higher frequency of neutrophils in their bone marrow than mice fed HFD-S.
To investigate the dietary effect on neutrophils, the frequency of neutrophils in the bone marrow of uninfected mice was analyzed. For the gating strategy used, see Fig. 4A. Mice fed HFD-ω3 had 9% higher frequency of Ly6G+ neutrophils in bone marrow (P < 0.001) than mice fed HFD-S, 10% higher (P < 0.001) than mice fed LFD, and 8% higher (P < 0.01) than mice fed HFD-ω6 (Fig. 4B). However, there was no difference among the frequencies of neutrophils in the bone marrow of mice fed LFD, HFD-S, or HFD-ω6.
To investigate if dietary fats can affect the frequency of progenitor cells in uninfected mice, bone marrow cells were stained with the general hematopoietic precursor marker CD117. Mice fed HFD-ω3 had a 10% higher frequency of CD117+ precursor cells in their bone marrow (P < 0.05) than mice fed HFD-S and a 12% higher frequency (P < 0.05) than mice fed HFD-ω6. At the same time, mice fed LFD had a 10% higher frequency of CD117+ precursor cells (P < 0.05) than mice fed HFD-ω6. There was no difference in the frequency of hematopoietic progenitor cells in bone marrow between mice fed HFD-ω3 or LFD, between mice fed LFD or HFD-S, or between mice fed HFD-S or HFD-ω6 (Fig. 4C).
The frequency of CD115+ monocytes in bone marrow was investigated since they also have the capacity to phagocytose bacteria. Mice fed LFD had 7% (P < 0.05), 10% (P ≤ 0.01), and 13% (P ≤ 0.001) higher frequencies of CD115+ monocytes than mice fed HFD-S, HFD-ω3, or HFD-ω6, respectively. There was no difference in the frequency of monocytes in bone marrow among mice fed HFD-S, HFD-ω3, or HFD-ω6 (Fig. 4D).
Mice fed HFD-S have a decreased frequency of neutrophils phagocytosing pHrodo particles compared with that for mice fed LFD or HFD-ω3.
Not only is the frequency of neutrophils important for the survival of sepsis, but neutrophil phagocytosing capacity is crucial for survival during an infection. To investigate if the diet also affects the capacity of neutrophils to phagocytose bacteria, blood from the mice was challenged with pHrodo particles and analyzed by flow cytometry. Circulating neutrophils from mice fed HFD-S had a lower capacity to phagocytose pHrodo particles than those from mice fed LFD (2.7%; P ≤ 0.01) or HFD-ω3 (2.5%; P < 0.05) (Fig. 5B). The neutrophils of mice fed HFD-S had a slightly and nonsignificantly lower (2.0%; P = 0.057) capacity to phagocytose pHrodo particles than those of mice fed HFD-ω6. There was no difference between any of the groups in the MFI of the neutrophils that had phagocytosed pHrodo particles (Fig. 5C).
There was no difference in the white blood cell counts of mice fed HFD-S, HFD-ω3, or HFD-ω6.
To investigate the dietary effect on the immune cells in circulation, the white blood cell, granulocyte, and lymphocyte concentrations in blood were analyzed. Mice fed HFD-ω3 or HFD-ω6 had higher white blood cell concentrations in their circulation than mice fed LFD (Table 2). There was no difference between mice fed LFD and mice fed HFD-S or among mice fed HFD-S, HFD-ω3, or HFD-ω6. Further, mice fed HFD-ω3 had a greater granulocyte concentration in their circulation than mice fed LFD, but there were no differences among mice fed LFD, HFD-S, or HFD-ω6. The diet did not affect the lymphocyte concentration in the circulation in any of the groups.
There was no systemic inflammation in any of the mice fed the different diets.
Since inflammation can affect the concentration of neutrophils both in bone marrow and in circulation, the levels of several inflammatory cytokines (IL-1β, IL-6, IL-10, IL-17A, IFN-γ, MCP-1, and TNF-α) in serum were investigated. There were no differences between the groups in any of these cytokines (Table 2).
Mice fed HFD-S might benefit from treatment with resolvins after inoculation with S. aureus.
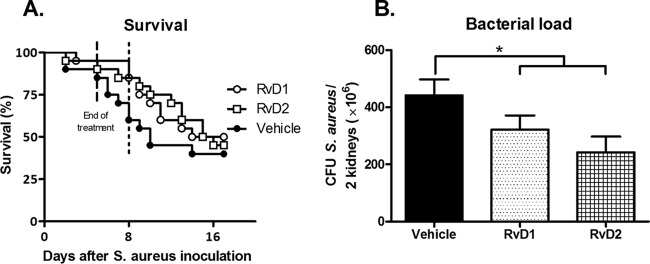
Resolvins are metabolites of omega-3 fatty acids. Given the beneficial effects of dietary omega-3 fatty acids described above, we hypothesized that the negative effects of HFD-S could be reduced by treating mice with i.v. resolvins after inoculation with S. aureus. As shown in Fig. 6, feeding mice HFD-S and treating them with resolvins on days 1 to 4 after inoculation did not influence survival at day 17. However, more resolvin-treated mice than vehicle-treated mice survived to day 8 after inoculation (85 and 60% survival, respectively) (Fig. 6A). Further, treatment with resolvins decreased bacterial loads in the kidneys 6 days after S. aureus inoculation (P < 0.05) (Fig. 6B).
FIG 6.
Survival and bacterial loads after treatment with resolvins. (A) Survival on days 0 to 17 after S. aureus inoculation after 8 weeks on HFD-S. On days 1 to 4, mice received the vehicle (0.5% ethanol in saline), RvD1, or RvD2. The log rank test was used to determine statistically significant differences (n = 20 mice per group). (B) Bacterial loads in the kidneys on day 6 after S. aureus inoculation after 8 weeks on HFD-S. Two-way ANOVA with the experiment as a nuisance factor was used to determine statistically significant differences (n = 23, 28, and 22 mice per group, respectively). The data shown are the estimated marginal mean plus SEM. *, P < 0.05. Experimental groups: vehicle, RvD1, and RvD2.
DISCUSSION
Dietary omega-3 fatty acids increase the survival of mice after S. aureus-induced sepsis.
In this study, we investigated the effects of dietary omega-3 and omega-6 fatty acids on the immune system and survival after S. aureus-induced sepsis. Our results show that addition of dietary omega-3 fatty acids to dietary HFD led to greater mouse survival of S. aureus-induced sepsis than a diet rich in saturated fatty acids or omega-6 fatty acids. Additionally, the results show that mice fed HFD-ω3, HFD-ω6, or LFD had decreased bacterial loads in their kidneys compared to those for mice fed HFD-S, indicating that feeding mice HFD-ω3 can reverse the deleterious effects of saturated fatty acids on survival of S. aureus-induced sepsis. Mice fed HFD-ω6 also had decreased bacterial loads in their kidneys compared to those for mice fed HFD-S, but the difference was not great enough to affect their survival. S. aureus could benefit from using exogenous fatty acids for incorporation in the cell membrane (21). This energy-saving mechanism can potentially result in enhanced growth of S. aureus and could potentially explain the increased bacterial loads and the subsequent mortality rate of mice fed HFD-S. Still, this is not likely since the saturated fatty acids contents of the different diets are quite similar. Further, the saturated fatty acid content in serum of mice fed HFD-S was not increased after 8 weeks. Our findings agree with our previous studies showing that mice fed HFD-S have an impaired immune response compared to that of mice fed LFD (9). The findings of this study are also in line with our recent finding that mice fed HFD-P have greater survival and a higher frequency of neutrophils in their bone marrow than mice fed HFD-S (10). Thus, our results show that omega-3 fatty acids are the most probable fatty acids needed to exert the beneficial properties of a polyunsaturated fatty acid diet.
The effects of dietary fatty acids on immune function are not correlated with body fat mass.
The type of dietary fat seems to be more important for the immune system than the total amount of dietary fat consumed and the resulting obesity. In line with our previous findings, there was no correlation between body weight and survival; hence, the increased body weight of mice fed HFD-S did not influence their survival.
Dietary omega-3 fatty acids increase survival of sepsis and stimulate the immune system.
In the literature, it is debated whether patients die from sepsis because of underactivity of the immune system coupled with enhanced bacterial growth or because of an overactivity of the immune system that impairs physiological functions and decreases the survival of the host (2, 7, 23). The present study shows that the bacterial load 6 days after inoculation was decreased in mice fed HFD-ω3 compared to that in mice fed HFD-S, indicating that the immune systems of mice fed HFD-ω3 were more effective. The 3.5-fold difference in the bacterial loads of mice fed HFD-ω3 and those fed HFD-S may not seem a dramatic one; however, since an infection is a dynamic relationship between the host's immune system and the bacterial growth rate, it is plausible that more dramatic differences could be present at earlier time points. Further, as the difference between the mortality rates of mice fed HFD-ω3 and mice fed HFD-ω6 occurred from about 1 week after inoculation, it seems likely that the decreased bacterial loads of mice fed HFD-ω3 are associated with their greater survival.
Still, we cannot exclude the possibility that other factors, such as bacterial virulence and/or direct antimicrobial effects of omega-3 fatty acids on S. aureus, could contribute to the improved survival of mice fed HFD-ω3. To address whether sera from mice fed HFD-ω3 contained any inhibitory substances influencing bacterial growth, we used the disk diffusion method; however, we found no evidence that the sera of mice fed HFD-ω3 contained any inhibitory substances (data not shown). Hence, we conclude that mice fed HFD-ω3 survive S. aureus-induced sepsis because of a greater capacity to clear bacteria.
Dietary omega-3 fatty acids stimulate neutrophil frequency and function.
The immune response to bacterial infections, especially for defense against S. aureus, is greatly dependent on neutrophils (24, 25). Therefore, the frequency of neutrophils in the bone marrow of uninfected mice after 8 weeks on different diets was analyzed. We have previously shown that mice fed a diet rich in polyunsaturated fatty acids (a mixture of both omega-3 and omega-6 fatty acids) also had a higher frequency of neutrophils in their bone marrow (10). This was confirmed in the present study in mice fed HFD-ω3. In contrast, monocytes in bone marrow were not affected by the type of fat in the diet, indicating that the effect of omega-3 fatty acids on neutrophils may be specific to neutrophils only and not to all phagocytosing innate immune cells. It should be noted that the increased frequency of neutrophils in bone marrow in mice fed HFD-ω3 does not automatically result in an increased frequency of neutrophils in blood; rather, it seems that neutrophils are retained within the bone marrow, ready to be released upon stimulation (26).
The frequency of circulating neutrophils that phagocytosed pHrodo particles was higher in mice fed HFD-ω3 than in mice fed HFD-S and similar to that in mice fed LFD. Thus, it is most likely that the increased survival is attributable not to a single mechanism but rather to a combination of an increased frequency of neutrophils in the bone marrow and improved phagocytosing capacity contributing to the increased survival of mice fed HFD-ω3.
Further, a previous study by our group also indicated that dietary polyunsaturated fatty acids increase the migration capacity of neutrophils (10). Taken together, these findings indicate that polyunsaturated fatty acids, especially omega-3 fatty acids, affect both the frequency of neutrophils in bone marrow and their function in circulation.
Dietary omega-3 fatty acids may both stimulate neutrophil function and inhibit inflammation.
Clinically, a high frequency of neutrophils, especially in blood, is regarded as a sign of an ongoing infection with concomitant inflammation. However, we did not see any differences in circulating granulocytes in the present study. Further, we have previously shown that mice fed a diet rich in polyunsaturated fatty acids (a mixture of omega-3 and omega-6 fatty acids) also had an increased frequency of neutrophils in their bone marrow. Their increased frequency of neutrophils did not seem to be a symptom of inflammation since they did not have greater levels of inflammatory markers in their serum than mice fed HFD-S in the uninfected state (10). This is further confirmed in the present study, where none of the diets affected the levels of the inflammatory cytokines IL-1β, IL-6, IL-10, IL-17A, IFN-γ, MCP-1, and TNF-α in serum. Thus, the increased neutrophil frequency found in bone marrow is not due to ongoing systemic inflammation. Actually, dietary omega-3 fatty acids have been suggested to suppress inflammation (11). Hence, these findings suggest that it is possible to stimulate the capacity to fight infections (e.g., with HFD-ω3) without causing the deleterious effects associated with chronic inflammation, i.e., a type of chronic immune activation in the absence of infection.
Dietary omega-3 fatty acids stimulate hematopoietic precursor cells.
We wanted to investigate if diet affects the frequency of CD117+ hematopoietic precursor cells in bone marrow. Mice fed HFD-ω3 had a higher frequency of hematopoietic precursor cells than mice fed HFD-S or HFD-ω6. Therefore, it might be speculated that mice fed HFD-ω3 are more prepared to quickly mobilize a leukocyte defense in general to fight off bacteria.
Potentially beneficial effects of resolvins on the bacterial load in sepsis.
In vivo, omega-3 fatty acids can be metabolized into a novel substance group named resolvins (15). It has previously been suggested that treatment with RvD1 and RvD2 can increase the survival of sepsis induced by cecal ligation and puncture; however, the mechanism responsible for this remains unclear (15, 27). It has been suggested that resolvins affect the migration capacity of neutrophils (28). Since our previous findings indicate that polyunsaturated fatty acids also affect the migration capacity of neutrophils and our present study shows that omega-3 fatty acids increase the capacity of neutrophils to phagocytose, we decided to investigate if mice fed HFD-S could be saved by treatment with resolvins after S. aureus-induced sepsis. The overall survival of resolvin- and vehicle-treated mice on day 17 after inoculation did not differ. However, more resolvin-treated mice than vehicle-treated mice survived to day 8 after inoculation (85 and 60% survival, respectively). Further, there was a trend toward increased survival of mice treated with resolvins on days 7 and 9 after inoculation. Moreover, on day 6 after inoculation, mice treated with resolvins had decreased bacterial loads in their kidneys compared to those for mice treated with the vehicle. Taken together, these results suggest that mice fed HFD-S can benefit from treatment with resolvins after S. aureus-induced sepsis; however, treatment with the doses used in the present study and only treatment during days 1 to 4 after S. aureus-induced sepsis were not sufficient to affect the overall mortality rate 17 days after inoculation. Further studies are warranted, as resolvins are an interesting potential treatment for S. aureus-induced sepsis. That they can be administered after the infection is manifested by the results of both this study and a previous one (15, 16), and they do not require pretreatment, as diet does.
The differences between the effects of omega-3 and omega-6 fatty acids.
Our finding that a diet containing omega-3 fatty acids, but not omega-6 fatty acids, can stimulate the immune system and produce a mortality rate during infection lower than that of mice fed HFD-S is of interest in relation to earlier studies on the health effects of omega-3 and omega-6 fatty acids. Arachidonic acid, which is an omega-6 fatty acid, is a precursor of both prostaglandins and leukotrienes. Prostaglandins and leukotrienes are believed to be mostly proinflammatory, while omega-3 fatty acid metabolites are mostly anti-inflammatory (11). For example, patients with the inflammatory disease rheumatoid arthritis benefit from dietary omega-3 fatty acids (12). Dietary omega-3 fatty acids are also believed to decrease the risk of cardiovascular disease, possibly thanks to suppressed inflammation, but the beneficial effect of omega-3 fatty acids in this context may be rather small (13). The exact relationship between the various effects of dietary omega-3 and omega-6 fatty acids remains to be elucidated.
For technical reasons, the omega-3 fatty acid diet in the present study contained some omega-6 fatty acids (about as much as the omega-3 fatty acids). In contrast, the omega-6 fatty acid diet only contained 1/50 omega-3 fatty acids and should reflect a pure omega-6 fatty acid effect. Therefore, what distinguishes the omega-3 fatty acid diet from the omega-6 fatty acid diet is the presence of omega-3 fatty acids rather than the absence of omega-6 fatty acids. Moreover, the contents of saturated fatty acids and monounsaturated fatty acids were only moderately lower in the omega-3 fatty acid diet than in the HFD-S diet. Importantly, this means that omega-3 fatty acids themselves are likely to be the reason for the positive effects of an omega-3 fatty acid diet. As omega-3 fatty acids are the precursors of resolvins, this is in line with the beneficial effects of resolvins on infections.
It may be speculated that the protective effect of omega-3 fatty acids is effective only in slightly immunosuppressed states, such as that of mice fed HFD-S. Interestingly, a previous sepsis study demonstrated the effect of resolvins on mice fed standard chow (27). Since resolvins are metabolites of omega-3 fatty acids, it is possible that omega-3 fatty acids can act independently of other dietary components; however, this needs to be investigated further.
Conclusions.
In conclusion, our findings indicate that feeding mice omega-3 fatty acids results in better survival of sepsis than that of mice fed a diet rich in saturated fatty acids. The mechanism responsible for this is most likely a combination of an increased frequency of neutrophils and precursor cells in bone marrow and a restored phagocytosis capacity. These factors, prior to infection, contribute to decreased bacterial growth. From a clinical perspective, these results indicate that people eating a diet rich in saturated fatty acids, i.e., most of the people in the western world, could benefit from an increased intake of omega-3 fatty acids.
ACKNOWLEDGMENTS
We are grateful for the excellent technical assistance of Sansan Hua, Peter Micallef, and Karl Wendt. We thank Stefan Lange for expertise regarding bacterial growth, the disk diffusion analysis, and helpful comments on the manuscript.
We confirm that there are no conflicts of interests.
Funding Statement
This work was supported by grants from the Swedish Research Council (K2013-54X-09894-19-3 [J.-O.J.] and 2667 [M.E.J.]), the Swedish Society for Medical Research (M.E.J.), and the Sahlgrenska Center for Cardiovascular Metabolic Research (CMR, no. A305: 188, J.-O.J.), which is supported by the Swedish Strategic Foundation, EC FP7 funding (Full4Health FP7-KBBE-2010 – 4-266408, J.-O.J.), the Magnus Bergvall Foundation (M.E.J.), Längmanska Kulturfonden (M.E.J.), Stiftelsen Gamla trotjänarinnor (M.E.J.), OE och Edla Johanssons vetenskapliga Stiftelse (M.E.J.), the Lars Hiertas Foundation (M.E.J.), the Åke Wiberg Foundation (M.E.J.), and Stiftelsen Tornspiran (S.L.S.).
REFERENCES
- 1.Martin GS, Mannino DM, Eaton S, Moss M. 2003. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med 348:1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 2.Russell JA. 2006. Management of sepsis. N Engl J Med 355:1699–1713. doi: 10.1056/NEJMra043632. [DOI] [PubMed] [Google Scholar]
- 3.Kotsaki A, Giamarellos-Bourboulis EJ. 2012. Emerging drugs for the treatment of sepsis. Expert Opin Emerg Drugs 17:379–391. doi: 10.1517/14728214.2012.697151. [DOI] [PubMed] [Google Scholar]
- 4.Benfield T, Espersen F, Frimodt-Moller N, Jensen AG, Larsen AR, Pallesen LV, Skov R, Westh H, Skinhoj P. 2007. Increasing incidence but decreasing in-hospital mortality of adult Staphylococcus aureus bacteraemia between 1981 and 2000. Clin Microbiol Infect 13:257–263. doi: 10.1111/j.1469-0691.2006.01589.x. [DOI] [PubMed] [Google Scholar]
- 5.Levy SB, Marshall B. 2004. Antibacterial resistance worldwide: causes, challenges and responses. Nat Med 10:S122–129. doi: 10.1038/nm1145. [DOI] [PubMed] [Google Scholar]
- 6.Bassetti M, Ginocchio F, Giacobbe DR. 2011. New approaches for empiric therapy in Gram-positive sepsis. Minerva Anestesiol 77:821–827. [PubMed] [Google Scholar]
- 7.Hotchkiss RS, Karl IE. 2003. The pathophysiology and treatment of sepsis. N Engl J Med 348:138–150. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 8.Remick DG. 2003. Cytokine therapeutics for the treatment of sepsis: why has nothing worked? Curr Pharm Des 9:75–82. doi: 10.2174/1381612033392567. [DOI] [PubMed] [Google Scholar]
- 9.Strandberg L, Verdrengh M, Enge M, Andersson N, Amu S, Onnheim K, Benrick A, Brisslert M, Bylund J, Bokarewa M, Nilsson S, Jansson JO. 2009. Mice chronically fed high-fat diet have increased mortality and disturbed immune response in sepsis. PLoS One 4:e7605. doi: 10.1371/journal.pone.0007605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Svahn SL, Grahnemo L, Palsdottir V, Nookaew I, Wendt K, Gabrielsson B, Schele E, Benrick A, Andersson N, Nilsson S, Johansson ME, Jansson JO. 2015. Dietary polyunsaturated fatty acids increase survival and decrease bacterial load during septic Staphylococcus aureus infection and improve neutrophil function in mice. Infect Immun 83:514–521. doi: 10.1128/IAI.02349-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calder PC. 2015. Marine omega-3 fatty acids and inflammatory processes: effects, mechanisms and clinical relevance. Biochim Biophys Acta 1851:469–484. doi: 10.1016/j.bbalip.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 12.Miles EA, Calder PC. 2012. Influence of marine n-3 polyunsaturated fatty acids on immune function and a systematic review of their effects on clinical outcomes in rheumatoid arthritis. Br J Nutr 107(Suppl 2):S171–S184. doi: 10.1017/S0007114512001560. [DOI] [PubMed] [Google Scholar]
- 13.Calder PC. 2012. The role of marine omega-3 (n-3) fatty acids in inflammatory processes, atherosclerosis and plaque stability. Mol Nutr Food Res 56:1073–1080. doi: 10.1002/mnfr.201100710. [DOI] [PubMed] [Google Scholar]
- 14.Day RB, Link DC. 2012. Regulation of neutrophil trafficking from the bone marrow. Cell Mol Life Sci 69:1415–1423. doi: 10.1007/s00018-011-0870-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spite M, Norling LV, Summers L, Yang R, Cooper D, Petasis NA, Flower RJ, Perretti M, Serhan CN. 2009. Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Nature 461:1287–1291. doi: 10.1038/nature08541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chiang N, Fredman G, Backhed F, Oh SF, Vickery T, Schmidt BA, Serhan CN. 2012. Infection regulates pro-resolving mediators that lower antibiotic requirements. Nature 484:524–528. doi: 10.1038/nature11042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kitagawa Y, Kikuchi S, Arita Y, Nishimura M, Mizuno K, Ogasawara H, Kawano T, Ochiai T, Otsuji E, Imai T. 2013. Inhibition of CCL20 increases mortality in models of mouse sepsis with intestinal apoptosis. Surgery 154:78–88. doi: 10.1016/j.surg.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 18.Ikuta K, Weissman IL. 1992. Evidence that hematopoietic stem cells express mouse c-kit but do not depend on steel factor for their generation. Proc Natl Acad Sci U S A 89:1502–1506. doi: 10.1073/pnas.89.4.1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Breslin WL, Strohacker K, Carpenter KC, Haviland DL, McFarlin BK. 2013. Mouse blood monocytes: standardizing their identification and analysis using CD115. J Immunol Methods 390:1–8. doi: 10.1016/j.jim.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 20.Löfgren L, Stahlman M, Forsberg GB, Saarinen S, Nilsson R, Hansson GI. 2012. The BUME method: a novel automated chloroform-free 96-well total lipid extraction method for blood plasma. J Lipid Res 53:1690–1700. doi: 10.1194/jlr.D023036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Altenbern RA. 1977. Cerulenin-inhibited cells of Staphylococcus aureus resume growth when supplemented with either a saturated or an unsaturated fatty acid. Antimicrob Agents Chemother 11:574–576. doi: 10.1128/AAC.11.3.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Falagas ME, Kompoti M. 2006. Obesity and infection. Lancet Infect Dis 6:438–446. doi: 10.1016/S1473-3099(06)70523-0. [DOI] [PubMed] [Google Scholar]
- 23.Deitch EA. 1998. Animal models of sepsis and shock: a review and lessons learned. Shock 9:1–11. [DOI] [PubMed] [Google Scholar]
- 24.Phillipson M, Kubes P. 2011. The neutrophil in vascular inflammation. Nat Med 17:1381–1390. doi: 10.1038/nm.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rigby KM, DeLeo FR. 2012. Neutrophils in innate host defense against Staphylococcus aureus infections. Semin Immunopathol 34:237–259. doi: 10.1007/s00281-011-0295-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Strydom N, Rankin SM. 2013. Regulation of circulating neutrophil numbers under homeostasis and in disease. J Innate Immun 5:304–314. doi: 10.1159/000350282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen F, Fan XH, Wu YP, Zhu JL, Wang F, Bo LL, Li JB, Bao R, Deng XM. 2014. Resolvin D1 improves survival in experimental sepsis through reducing bacterial load and preventing excessive activation of inflammatory response. Eur J Clin Microbiol Infect Dis 33:457–464. doi: 10.1007/s10096-013-1978-6. [DOI] [PubMed] [Google Scholar]
- 28.Lee HN, Surh YJ. 2012. Therapeutic potential of resolvins in the prevention and treatment of inflammatory disorders. Biochem Pharmacol 84:1340–1350. doi: 10.1016/j.bcp.2012.08.004. [DOI] [PubMed] [Google Scholar]