Abstract
Trypanosoma cruzi infection, which is the etiological agent of Chagas disease, is associated with intense inflammation during the acute and chronic phases. The pathological progression of Chagas disease is influenced by the infiltration and transmigration of inflammatory cells across the endothelium to infected tissues, which are carefully regulated processes involving several molecular mediators, including adhesion molecules and platelet-activating factor (PAF). We have shown that PAF production is dependent upon calcium-independent group VIA phospholipase A2β (iPLA2β) following infection of human coronary artery endothelial cells (HCAECs) with T. cruzi, suggesting that the absence of iPLA2β may decrease the recruitment of inflammatory cells to the heart to manage parasite accumulation. Cardiac endothelial cells isolated from iPLA2β-knockout (iPLA2β-KO) mice infected with T. cruzi demonstrated decreased PAF production compared to that by cells isolated from wild-type (WT) mice but demonstrated increases in adhesion molecule expression similar to those seen in WT mice. Myocardial inflammation in iPLA2β-KO mice infected with T. cruzi was similar in severity to that in WT mice, but the iPLA2β-KO mouse myocardium contained more parasite pseudocysts. Upon activation, macrophages from iPLA2β-KO mice produced significantly less nitric oxide (NO) and caused less T. cruzi inhibition than macrophages from wild-type mice. Thus, the absence of iPLA2β activity does not influence myocardial inflammation, but iPLA2β is essential for T. cruzi clearance.
INTRODUCTION
Trypanosoma cruzi is a protozoan parasite that results in significant cardiac pathology and is the etiological agent of Chagas disease. It is estimated that over 10 million people worldwide are currently infected with T. cruzi, and of these, about 300,000 reside in the United States (1). Chagas disease is endemic to South and Central America, where people risk acquiring the parasite from the triatomine insect vector. Chagas disease progresses from an acute stage, which may or may not be symptomatic, to a chronic stage, in which 20 to 30% of infected individuals exhibit cardiac involvement that may lead to heart failure, arrhythmias, and death (2). The long asymptomatic period separating the two stages of the disease is known as the indeterminate phase and may last for several decades.
During the acute stage of T. cruzi infection, the parasites infect the myocardium, leading to an intense inflammatory response. Several proinflammatory cytokines and signaling pathways are activated to facilitate the transmigration of inflammatory cells in an attempt to control parasite invasion. Activation of the endothelium and upregulation of endothelial cell adhesion molecules, such as intercellular adhesion molecule 1 (ICAM-1) and vascular cell adhesion molecule 1 (VCAM-1), following T. cruzi infection are critical for these processes (3). Our group has previously demonstrated increased expression of platelet-activating factor (PAF), in addition to the upregulation of adhesion molecules, in human coronary artery endothelial cells (HCAECs) acutely infected with T. cruzi (4). The role of PAF in the recruitment, transmigration, and activation of inflammatory cells is well established (5–8).
PAF is an acetylated alkyl ether glycerophospholipid that can elicit biological effects at concentrations as low as 10−12 M (9). Mice treated with a PAF receptor antagonist demonstrate earlier mortality and increased parasitemia, suggesting that PAF is necessary for resistance to Chagas disease (10). Further, PAF-deficient mice have increased parasitemia, increased tissue parasitism, a more intense inflammatory response in the heart, and increased mortality following infection with T. cruzi (11). Thus, PAF production may be a critical host defense response to T. cruzi infection that serves to retard the progression of Chagas disease. Earlier studies have suggested that PAF can induce nitric oxide (NO) production in macrophages infected with T. cruzi (10).
Although studies have described the role of PAF in T. cruzi infection, much less information concerning the mechanism underlying PAF accumulation is available. We recently demonstrated that PAF production requires calcium-independent group VIA phospholipase A2β (iPLA2β) and is greatly blunted in iPLA2β-knockout (iPLA2β-KO) mice (4). Although we have focused on iPLA2β-mediated PAF production in the cardiovascular system, the enzyme is also involved in modulating arachidonic acid release from vascular cells and vasomotor tone (12). We have shown that the absence of endothelial cell iPLA2β activity is associated with a decrease in prostacyclin release. The predominant iPLA2 isoform in the myocardium is the calcium-independent group VIB PLA2 (iPLA2γ), which is responsible for the production of arachidonic acid-derived eicosanoids. Although few studies to date have addressed the role of phospholipase A2 (PLA2) in myocarditis, several inflammatory metabolites produced following PLA2-catalyzed hydrolysis of membrane phospholipids have been implicated in Chagas disease (10, 11, 13). Finally, previous studies have suggested that iPLA2β may be required for inducible nitric oxide synthase (iNOS) upregulation, increased NADP oxidase 4 (Nox4) expression, and chemotaxis in macrophages (14, 15). Here, we compared wild-type (WT) and iPLA2β-KO mice to determine whether iPLA2β deficiency influences cardiac inflammation and parasite accumulation following T. cruzi infection.
MATERIALS AND METHODS
Parasitology.
Tissue culture trypomastigotes (TCTs) from the Brazil strain of T. cruzi were propagated in NIH 3T3 mouse embryonic fibroblasts grown in Dulbecco's modified Eagle medium (DMEM) supplemented with 2% neonatal calf serum. NIH 3T3 cells were infected with T. cruzi when 60% confluence was reached. Infected cells ruptured following parasite multiplication, releasing an abundant number of parasites. The supernatant containing the parasites was collected, and parasite numbers were determined using a Neubauer hemocytometer.
Mice and infections.
Animal protocols were in strict accordance with the National Institutes of Health guidelines for the humane treatment of animals and were reviewed and approved by the Animal Care and Use Committee of Saint Louis University. C57BL/6 WT or iPLA2β-KO mice were used for in vitro and in vivo studies. Mice were infected subcutaneously with 5,000 Brazil strain blood-form trypomastigotes (BFTs) and sacrificed at different time points postinfection. The hearts were collected in paraformaldehyde fixative or frozen in liquid nitrogen before processing for histological analysis. The hearts were trimmed, processed, and embedded in paraffin. Five-micrometer tissue sections were cut, and sections were stained with hematoxylin and eosin (H&E). The investigators were blind to the identity of the samples, as numerical identifiers specific to each individual mouse were used, and the samples were evaluated microscopically using a 4-point system as described previously (16, 17). The parasite pseudocysts in 20 separate bright-field regions of the ventricle were counted.
Murine endothelial cell isolation.
Endothelial cells were isolated from mouse heart by collagenase digestion. The diced heart muscle was incubated in 2 mg/ml collagenase for 1 h at 37°C, and the digested tissue was passed through a cell strainer. Cells were incubated with murine immunoglobulins to block Fc receptors and then incubated with anti-mouse platelet endothelial cell adhesion molecule 1 (PECAM-1) coupled to magnetic beads. The cells obtained were cultured until they reached confluence and sorted again using ICAM-2 antibodies coupled with magnetic beads. The eluted cells were washed, resuspended in cell culture medium, and plated. Nonadherent cells were removed on the next day, and cells were grown to confluence and passaged at a 1-to-3 dilution.
PAF assay.
Murine cardiac endothelial cells grown in 12-well culture dishes were washed twice with Hanks' balanced salt solution containing 135 mM NaCl, 0.8 mM MgSO4, 10 mM HEPES (pH 7.4), 1.2 mM CaCl2, 5.4 mM KCl, 0.4 mM KH2PO4, 0.3 mM Na2HPO4, and 6.6 mM glucose and incubated with 50 μCi [3H]acetic acid for 20 min. After the selected time interval for incubation with the appropriate agents, lipids were extracted from the cells by the method of Bligh and Dyer. The chloroform layer was concentrated by evaporation under N2, applied to a silica gel 60 thin-layer chromatography plate, and developed in chloroform-methanol-acetic acid-water (50:25:8:4, vol/vol). The region corresponding to PAF was scraped, and the radioactivity was quantified using liquid scintillation spectrometry. The loss of PAF during extraction and chromatography was corrected for by adding a known amount of [14C]PAF as an internal standard. [14C]PAF was synthesized by acetylating the sn-2 position of lyso-PAF with [14C]acetic anhydride using 0.33 M dimethylaminopyridine as a catalyst. The synthesized [14C]PAF was purified by high-pressure liquid chromatography.
Endothelial cell surface expression of adhesion molecules.
Murine cardiac endothelial cells were grown to confluence in 16-mm culture dishes and were incubated with T. cruzi (multiplicity of infection [MOI], 0.2) for up to 96 h. At the end of the incubation, the cells were fixed with 1% paraformaldehyde and incubated overnight at 4°C. The cells were then washed three times with phosphate-buffered saline (PBS) and then blocked with Tris-buffered saline–Tween 20 supplemented with 0.8% (wt/vol) bovine serum albumin and 0.5% (wt/vol) fish gelatin for 1 h at 24°C. Appropriate primary antibody (1:50; Santa Cruz Biotechnology, Santa Cruz, CA) was used before treatment with horseradish peroxidase-conjugated rabbit anti-goat immunoglobulin secondary antibody (1:5,000; Santa Cruz Biotechnology, Santa Cruz, CA). Subsequently, each well was incubated in the dark with a 3,3′,5,5′-tetramethylbenzidine liquid substrate system, and color development was measured at 450 nm.
Isolation of murine BMDMs.
Bone marrow-derived macrophages (BMDMs) were isolated from the femurs of WT and iPLA2β-KO mice. Briefly, mice were sacrificed, and the femurs were removed and cleansed of tissue. The marrows were flushed from the femurs using DMEM, disrupted by passage through a 23-gauge needle, and collected by centrifugation. The pellets were resuspended in ACK lysis buffer (0.15 M NH4Cl, 10 mM KHCO3, 0.10 mM EDTA), and incubated for 10 min on ice to lyse the red blood cells. The cells were centrifuged and resuspended in bone marrow conditioned medium supplemented with 20% fetal bovine serum (FBS) and 1% penicillin-streptomycin devoid of macrophage colony-stimulating factor (M-CSF). Following 2 to 3 h of incubation at 37°C with 5% CO2, nonadherent cells were decanted and resuspended in fully supplemented bone marrow conditioned medium (with M-CSF). Cells were plated in 100-mm culture dishes for 5 to 7 days, following which they were used for experiments.
Nitrite release from murine macrophages.
The nitrite released into the culture supernatants obtained from RAW 264.7 cells or murine bone marrow-derived macrophages was measured. Cells were grown to confluence and infected with T. cruzi (MOI, 0.2) with or without interferon gamma (IFN-γ; 100 units/ml) for 24 h. The nitrite concentration was measured by mixing 50 μl of the culture supernatant with the Griess reagent system. The absorbance at 550 nm of each sample was measured, and the concentration of nitrite was determined with reference to a nitrite standard curve with nitrite concentrations ranging from 0 to 100 μM.
T. cruzi inhibition assay.
BMDMs were plated in 8-well tissue culture slide chambers (Nunc Lab-Tek; Thermo Scientific, Waltham, MA) at 1 × 105 cells/well in DMEM with 10% FBS. After overnight culture at 37°C, nonadherent cells were washed away and macrophages were infected with T. cruzi culture-derived metacyclic trypomastigotes (CMTs) at an MOI of 5 (5 × 105 parasites/well) for 3 h. The generation of CMTs was described previously (18). Extracellular parasites were then washed away, and fresh medium or medium containing purified IFN-γ (100 U/ml; Sigma Chemical Co., St. Louis, MO) was added. Two days later, the slide chambers were removed from the slides, and the slides were washed, dried, fixed, and stained with a modified Wright Giemsa stain (Diff-Quik; IMEB, Inc., San Marcos, CA). The number of infected macrophages was determined microscopically. Percent inhibition was calculated by use of the following: {1 − [(number of infected cells per 200 cells treated with IFN-γ)/(number of infected cells per 200 cells treated with medium alone)]} × 100.
Statistical analysis.
Comparison of the values for statistically significant differences was performed by Student's t test or one-way analysis of variance with post hoc analysis, performed using Dunnett's test. All results are expressed as the mean + standard error of the mean (SEM). Statistical significance was considered to be a P value of <0.05.
RESULTS
PAF production and adhesion molecule expression in cardiac endothelial cells.
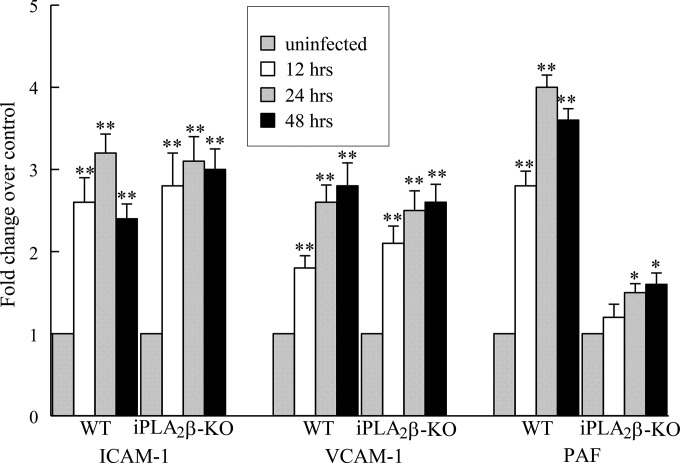
Our previous in vitro studies indicated that iPLA2β is critical for recruiting inflammatory cells to the endothelium via the production of PAF (4, 6). Thus, we measured PAF production and the expression of adhesion molecules in cardiac endothelial cells isolated from WT and iPLA2β-KO mice (Fig. 1). Cardiac endothelial cells isolated from WT mice demonstrated a significant increase in cell surface expression of intercellular adhesion molecule 1 (ICAM-1) and vascular cell adhesion molecule 1 (VCAM-1) when incubated with T. cruzi for up to 48 h (Fig. 1). The increase in adhesion molecules was accompanied by a significant increase in PAF accumulation in T. cruzi-infected WT endothelial cells (Fig. 1). When endothelial cells isolated from iPLA2β-KO mice were incubated with T. cruzi, a significant increase in cell surface expression of ICAM-1 and VCAM-1 similar to that seen with cells isolated from WT mice was observed (Fig. 1). However, PAF production in T. cruzi-infected endothelial cells isolated from iPLA2β-KO mouse heart was significantly reduced compared to that in endothelial cells isolated from WT mouse heart (Fig. 1). Thus, the absence of iPLA2β is associated with impaired PAF production but does not affect the cell surface expression of adhesion molecules in response to T. cruzi infection.
FIG 1.
Expression of ICAM-1 and VCAM-1 and PAF production in endothelial cells isolated from the hearts of WT and iPLA2β-KO mice infected with T. cruzi (MOI, 0.2) for up to 48 h. Values are normalized to those observed in the absence of infection. The values shown are means + SEMs for 4 separate cell cultures. *, P < 0.05 compared to untreated controls; **, P < 0.01 compared to untreated controls.
Studies in iPLA2β-KO mice.
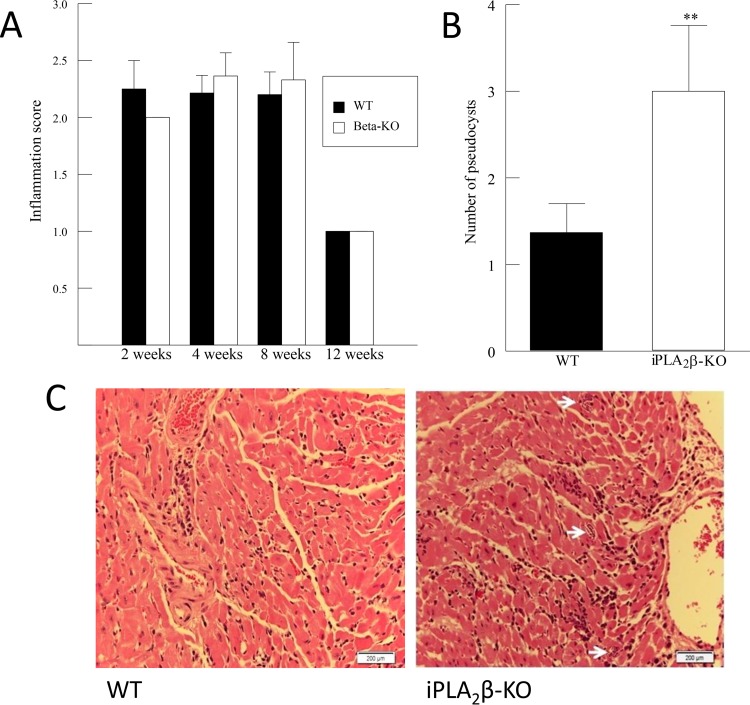
WT and iPLA2β-KO mice were infected with the Brazil strain of T. cruzi (5,000 parasites), and cardiac inflammation was measured by histopathological evaluation, as described in Materials and Methods, and assigned a numerical value on the basis of the size and severity of the inflammatory foci. Cardiac inflammation was observed in WT and iPLA2β-KO mice at 2, 4, and 8 weeks postinfection but resolved by week 12 (Fig. 2A). No difference in the inflammation score between WT and iPLA2β-KO mice was observed at any time point at which the score was determined following infection with T. cruzi (Fig. 2A), but the number of parasite pseudocysts was increased in the hearts of iPLA2β-KO mice compared to the hearts of WT mice at 4 weeks postinfection (Fig. 2B). These data suggest that iPLA2β deficiency does not significantly affect the transmigration of inflammatory cells following T. cruzi infection but that parasite clearance is impaired in iPLA2β-deficient mice and that iPLA2β could play a protective role in acute infection.
FIG 2.
(A) Myocardial inflammation score for WT and iPLA2β-KO mice following T. cruzi infection for up to 12 weeks. (B) Number of parasites in the myocardium per field for WT and iPLA2β-KO mice following T. cruzi infection for 4 weeks. **, P < 0.01 compared to WT. The results in panels A and B are means + SEMs for at least 6 separate samples. (C) Hematoxylin-and-eosin-stained sections of hearts from WT and iPLA2β-KO mice following 4 weeks of T. cruzi infection. Arrows, parasite pseudocysts in the myocardium.
Nitrite production by RAW 264.7 macrophages.
Macrophages play a critical role in clearing T. cruzi via the release of nitric oxide (NO). The myocardium of iPLA2β-KO mice had an inflammation score comparable to that of the myocardium of WT mice, but the myocardium of iPLA2β-KO mice had an increased number of pseudocysts, which suggests that macrophages from iPLA2β-KO mice may be deficient in their parasite clearance ability.
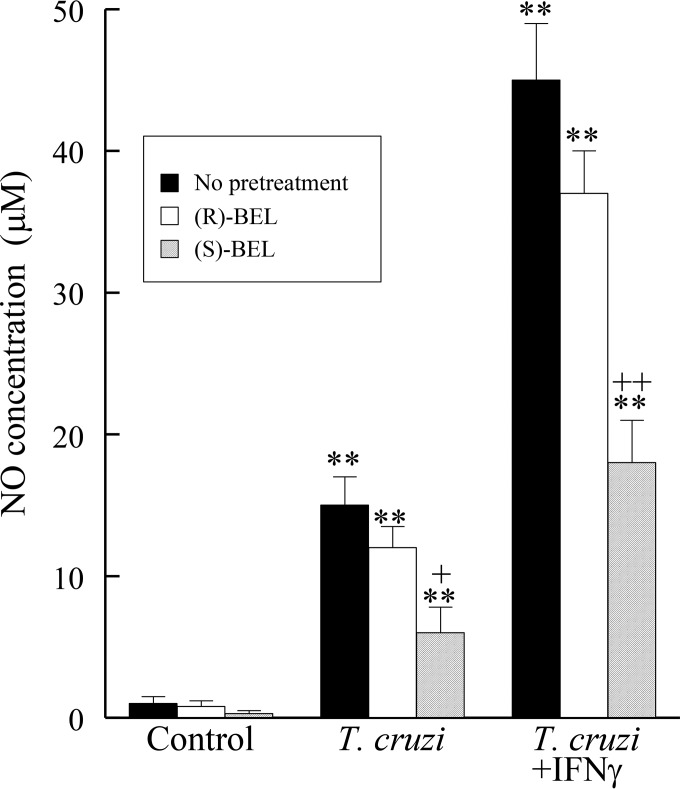
To examine the role of iPLA2β in macrophage responses to T. cruzi infection, RAW 264.7 cells were infected (MOI, 0.2; 48 h) in the absence or presence of the (R)- or (S)-enantiomer of the iPLA2 inhibitor bromoenol lactone (BEL; 0.5 μM). NO release was measured by colorimetric determination of the amount of nitrite, a stable breakdown product of NO, produced. Infection with T. cruzi resulted in a significant increase in NO release (Fig. 3), and this was significantly augmented by coincubation with interferon gamma (IFN-γ), a known activator of the macrophage iNOS pathway (Fig. 3). Pretreatment of RAW 264.7 cells with the calcium-independent group VIB PLA2 (iPLA2γ) inhibitor (R)-BEL did not significantly affect NO release following T. cruzi infection with or without IFN-γ (Fig. 3), but pretreatment with the iPLA2β inhibitor (S)-BEL significantly reduced NO release in response to T. cruzi infection in the presence or absence of IFN-γ (Fig. 3).
FIG 3.
NO release from cells of the murine macrophage cell line RAW 264.7 following infection with T. cruzi (MOI, 0.2) with or without IFN-γ (100 units) for 24 h. **, P < 0.01 compared to untreated cells; +, P < 0.05 compared to treated or infected cells in the presence or absence of BEL (0.5 μM); ++, P < 0.01 compared to treated or infected cells in the presence or absence of BEL (0.5 μM). The values shown are means + SEMs for 4 separate cell cultures.
Nitrite production and parasite intracellular inhibition by mouse BMDMs.
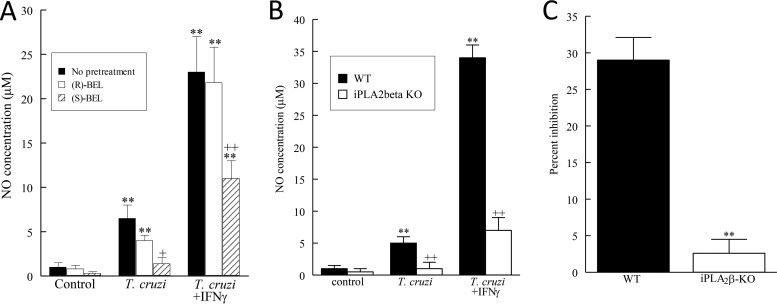
Bone marrow-derived macrophages (BMDMs) were isolated from WT and iPLA2β-KO mice, and their NO production was determined by measurement of the amount of nitrite after incubation with T. cruzi with or without IFN-γ. As with RAW 264.7 cells (Fig. 4), T. cruzi infection stimulated NO production by WT mouse BMDMs, and this was amplified by coincubation with IFN-γ (Fig. 4A). Pretreatment with (S)-BEL but not (R)-BEL significantly inhibited the WT mouse BMDM NO production stimulated by T. cruzi infection with or without IFN-γ (Fig. 4A). BMDMs isolated from iPLA2β-KO mice released negligible amounts of NO compared to the amount released by WT mouse macrophages (Fig. 4B). These data indicate that iPLA2β is important for NO production by macrophages, which is critical for parasite clearance in the acute stage of T. cruzi infection. Finally, inhibition of intracellular replication of T. cruzi was significantly greater in BMDMs derived from WT mice than BMDMs derived from iPLA2β-KO mice (Fig. 4C). Thus, we conclude that iPLA2β deficiency impairs macrophage NO production in acute T. cruzi infection and that this results in an increased parasite content in the hearts of infected mice.
FIG 4.
NO release from murine BMDMs and inhibition of T. cruzi intracellular parasites. (A) NO release from WT mouse BMDMs incubated with T. cruzi (MOI, 0.2) with or without IFN-γ (100 units) for 24 h. **, P < 0.01 compared to untreated cells; +, P < 0.05 compared to treated or infected cells in the presence or absence of BEL (10 μM); ++, P < 0.01 compared to treated or infected cells in the presence or absence of BEL (10 μM). (B) BMDMs isolated from WT or iPLA2β-KO mice were treated with T. cruzi (MOI, 0.2) or IFN-γ (100 units) for 24 h. **, P < 0.01 compared to untreated cells; ++, P < 0.01 compared to NO release between WT and iPLA2β-KO mice. (C) T. cruzi inhibition in BMDMs isolated from WT or iPLA2β-KO mice infected with T. cruzi (MOI, 5). **, P < 0.01 compared to data between WT and iPLA2β-KO mice. The values shown are means + SEMs for at least 4 separate cell cultures.
DISCUSSION
A characteristic pathological feature in Chagas disease is cardiac inflammation during both the acute and chronic stages. Acute parasitemia elicits the transmigration of inflammatory cells across the endothelial cell barrier to the myocardium, and this process involves the increased expression of adhesion molecules and proinflammatory cytokines when T. cruzi infects endothelial cells (19, 20). We and others have demonstrated previously that infection of endothelial cells with T. cruzi results in significant PAF production and the upregulation of cell surface adhesion molecules that can directly contribute to inflammatory cell recruitment to infected sites (4, 19, 20). We have also recently shown that endothelial cell PAF production following T. cruzi infection requires iPLA2β (4), which suggested that prolonged endothelial cell activation in chronic Chagas disease may be associated with iPLA2β activation and PAF production, which facilitates the recruitment and transmigration of inflammatory cells to sites of infection. However, the absence of iPLA2β did not result in a significant decrease in inflammatory cells in the myocardium, despite the significant decrease in PAF production in T. cruzi-infected endothelial cells from the iPLA2β-KO mouse heart (Fig. 1). In this study, we observed a significant increase in cell surface expression of adhesion molecules in response to T. cruzi infection that was not significantly affected by the absence of iPLA2β. Since we did not observe a difference in inflammatory cells in the hearts of WT or iPLA2β-KO mice infected with T. cruzi, our data suggest that the upregulation of adhesion molecules is sufficient to recruit inflammatory cells across the endothelium in the presence of decreased PAF production.
Macrophages comprise key immune cells found in chagasic lesions and play an important role in parasite clearance via NO production (21, 22). Previous studies have suggested that iPLA2β may be required for iNOS upregulation in macrophages through activation of cyclic AMP response element-binding protein (CREB) via iPLA2-derived lysoplasmenylcholine (23). iPLA2β has been demonstrated to be important in macrophage chemotaxis (14), and macrophage iPLA2β has recently been shown to generate lysophosphatidic acid, which is required for increased NADP oxidase 4 (Nox4) expression and H2O2 generation (15). These studies suggest a possible mechanism by which NO production in iPLA2β-deficient macrophages and tissue parasite clearance are impaired. We demonstrated here that pretreatment of RAW 264.7 cells with the iPLA2β inhibitor (S)-BEL [but not with its (R)-BEL enantiomer] blunted the NO production induced by T. cruzi infection and its amplification by coincubation with IFN-γ, which indicates that iPLA2β contributes to NO production. Further studies were performed using bone marrow-derived macrophages obtained from T. cruzi-infected WT and iPLA2β-KO mice with or without IFN-γ. In agreement with previously published data (21, 22), macrophages from iPLA2β-KO mice released less NO than cells from WT mice. Thus, the absence of iPLA2β results in impaired parasite clearing by murine macrophages attributable to their diminished NO production, and this could explain the increased cardiac parasite load in iPLA2β-KO mice infected with T. cruzi. An increased tissue parasite load could eventually result in greater myocardial damage and more adverse clinical outcomes in patients with chronic Chagas disease. The knowledge that parasite clearance by macrophages requires iPLA2β-dependent NO release may lead to new avenues for therapeutic interventions in Chagas disease.
In conclusion, these data indicate that iPLA2β plays an important role in host defense against trypanosome infection by contributing significantly to macrophage NO production. The significance of endothelial cell iPLA2β-dependent PAF production in cardiac inflammation during acute Chagas disease remains uncertain, but recruitment of inflammatory cells is enhanced in T. cruzi-infected endothelial cells. Because inflammation is a carefully regulated process involving several mediators with some redundancy in function, impaired PAF production may only minimally affect the recruitment of inflammatory cells across the endothelium following T. cruzi infection in vivo, and this might be offset in part by the concomitant increase in cell surface expression of endothelial adhesion molecules.
REFERENCES
- 1.Bern C, Verastegui M, Gilman RH, Lafuente C, Galdos-Cardenas G, Calderon M, Pacori J, Del Carmen Abastoflor M, Aparicio H, Brady MF, Ferrufino L, Angulo N, Marcus S, Sterling C, Maguire JH. 2009. Congenital Trypanosoma cruzi transmission in Santa Cruz, Bolivia. Clin Infect Dis 49:1667–1674. doi: 10.1086/648070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rassi A, Little WC. 2000. Chagas' heart disease. Clin Cardiol 23:883–889. doi: 10.1002/clc.4960231205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lannes-Vieira J, Silverio JC, Pereira IR, Vinagre NF, Carvalho CM, Paiva CN, da Silva AA. 2009. Chronic Trypanosoma cruzi-elicited cardiomyopathy: from the discovery to the proposal of rational therapeutic interventions targeting cell adhesion molecules and chemokine receptors—how to make a dream come true. Mem Inst Oswaldo Cruz 104(Suppl 1):S226–S235. [DOI] [PubMed] [Google Scholar]
- 4.Sharma J, Eickhoff CS, Hoft DF, Marentette JO, Turk J, McHowat J. 2014. Absence of calcium-independent phospholipase A2 beta impairs platelet-activating factor production and inflammatory cell recruitment in Trypanosoma cruzi-infected endothelial cells. Physiol Rep 2:e00196. doi: 10.1002/phy2.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prescott SM, McIntyre TM, Zimmerman GA, Stafforini DM. 2002. Sol Sherry lecture in thrombosis: molecular events in acute inflammation. Arterioscler Thromb Vasc Biol 22:727–733. doi: 10.1161/01.ATV.0000016153.47693.B2. [DOI] [PubMed] [Google Scholar]
- 6.Sharma J, Young DM, Marentette JO, Rastogi P, Turk J, McHowat J. 2012. Lung endothelial cell platelet-activating factor production and inflammatory cell adherence are increased in response to cigarette smoke component exposure. Am J Physiol Lung Cell Mol Physiol 302:L47–L55. doi: 10.1152/ajplung.00179.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stafforini DM, McIntyre TM, Zimmerman GA, Prescott SM. 2003. Platelet-activating factor, a pleiotrophic mediator of physiological and pathological processes. Crit Rev Clin Lab Sci 40:643–672. doi: 10.1080/714037693. [DOI] [PubMed] [Google Scholar]
- 8.Prescott SM, Zimmerman GA, Stafforini DM, McIntyre TM. 2000. Platelet-activating factor and related lipid mediators. Annu Rev Biochem 69:419–445. doi: 10.1146/annurev.biochem.69.1.419. [DOI] [PubMed] [Google Scholar]
- 9.Montrucchio G, Alloatti G, Camussi G. 2000. Role of platelet-activating factor in cardiovascular pathophysiology. Physiol Rev 80:1669–1699. [DOI] [PubMed] [Google Scholar]
- 10.Aliberti JC, Machado FS, Gazzinelli RT, Teixeira MM, Silva JS. 1999. Platelet-activating factor induces nitric oxide synthesis in Trypanosoma cruzi-infected macrophages and mediates resistance to parasite infection in mice. Infect Immun 67:2810–2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Talvani A, Santana G, Barcelos LS, Ishii S, Shimizu T, Romanha AJ, Silva JS, Soares MB, Teixeira MM. 2003. Experimental Trypanosoma cruzi infection in platelet-activating factor receptor-deficient mice. Microbes Infect 5:789–796. doi: 10.1016/S1286-4579(03)00146-1. [DOI] [PubMed] [Google Scholar]
- 12.White MC, McHowat J. 2007. The therapeutic potential of phospholipase A2 inhibitors in cardiovascular disease. Cardiovasc Hematol Agents Med Chem 5:91–95. doi: 10.2174/187152507779315859. [DOI] [PubMed] [Google Scholar]
- 13.Michelin MA, Silva JS, Cunha FQ. 2005. Inducible cyclooxygenase released prostaglandin mediates immunosuppression in acute phase of experimental Trypanosoma cruzi infection. Exp Parasitol 111:71–79. doi: 10.1016/j.exppara.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 14.Mishra RS, Carnevale KA, Cathcart MK. 2008. iPLA2beta: front and center in human monocyte chemotaxis to MCP-1. J Exp Med 205:347–359. doi: 10.1084/jem.20071243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tan C, Day R, Bao S, Turk J, Zhao QD. 2014. Group VIA phospholipase A2 mediates enhanced macrophage migration in diabetes mellitus by increasing expression of nicotinamide adenine dinucleotide phosphate oxidase 4. Arterioscler Thromb Vasc Biol 34:768–778. doi: 10.1161/ATVBAHA.113.302847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eickhoff CS, Lawrence CT, Sagartz JE, Bryant LA, Labovitz AJ, Gala SS, Hoft DF. 2010. ECG detection of murine chagasic cardiomyopathy. J Parasitol 96:758–764. doi: 10.1645/GE-2396.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Postan M, Cheever AW, Dvorak JA, McDaniel JP. 1986. A histopathological analysis of the course of myocarditis in C3H/He mice infected with Trypanosoma cruzi clone Sylvio-X10/4. Trans R Soc Trop Med Hyg 80:50–55. doi: 10.1016/0035-9203(86)90193-8. [DOI] [PubMed] [Google Scholar]
- 18.Giddings OK, Eickhoff CS, Smith TJ, Bryant LA, Hoft DF. 2006. Anatomical route of invasion and protective mucosal immunity in Trypanosoma cruzi conjunctival infection. Infect Immun 74:5549–5560. doi: 10.1128/IAI.00319-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang H, Calderon TM, Berman JW, Braunstein VL, Weiss LM, Wittner M, Tanowitz HB. 1999. Infection of endothelial cells with Trypanosoma cruzi activates NF-kappaB and induces vascular adhesion molecule expression. Infect Immun 67:5434–5440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Michailowsky V, Celes MR, Marino AP, Silva AA, Vieira LQ, Rossi MA, Gazzinelli RT, Lannes-Vieira J, Silva JS. 2004. Intercellular adhesion molecule 1 deficiency leads to impaired recruitment of T lymphocytes and enhanced host susceptibility to infection with Trypanosoma cruzi. J Immunol 173:463–470. doi: 10.4049/jimmunol.173.1.463. [DOI] [PubMed] [Google Scholar]
- 21.Moran JM, Buller RM, McHowat J, Turk J, Wohltmann M, Gross RW, Corbett JA. 2005. Genetic and pharmacologic evidence that calcium-independent phospholipase A2beta regulates virus-induced inducible nitric-oxide synthase expression by macrophages. J Biol Chem 280:28162–28168. doi: 10.1074/jbc.M500013200. [DOI] [PubMed] [Google Scholar]
- 22.Maggi LB Jr, Moran JM, Scarim AL, Ford DA, Yoon JW, McHowat J, Buller RM, Corbett JA. 2002. Novel role for calcium-independent phospholipase A2 in the macrophage antiviral response of inducible nitric-oxide synthase expression. J Biol Chem 277:38449–38455. doi: 10.1074/jbc.M206247200. [DOI] [PubMed] [Google Scholar]
- 23.Williams SD, Ford DA. 2001. Calcium-independent phospholipase A2 mediates CREB phosphorylation and c-fos expression during ischemia. Am J Physiol Heart Circ Physiol 281:H168–H176. [DOI] [PubMed] [Google Scholar]