Abstract
Pathogenic Yersinia species utilize a type III secretion system to translocate Yop effectors into infected host cells. Yop effectors inhibit innate immune responses in infected macrophages to promote Yersinia pathogenesis. In turn, Yersinia-infected macrophages respond to translocation of Yops by activating caspase-1, but different mechanisms of caspase-1 activation occur, depending on the bacterial genotype and the state of phagocyte activation. In macrophages activated with lipopolysaccharide (LPS) prior to Yersinia pseudotuberculosis infection, caspase-1 is activated by a rapid inflammasome-dependent mechanism that is inhibited by translocated YopM. The possibility that other effectors cooperate with YopM to inhibit caspase-1 activation in LPS-activated macrophages has not been investigated. Toward this aim, epistasis analysis was carried out in which the phenotype of a Y. pseudotuberculosis yopM mutant was compared to that of a yopJ yopM, yopE yopM, yopH yopM, yopT yopM, or ypkA yopM mutant. Activation of caspase-1 was measured by cleavage of the enzyme, release of interleukin-1β (IL-1β), and pyroptosis in LPS-activated macrophages infected with wild-type or mutant Y. pseudotuberculosis strains. Results show enhanced activation of caspase-1 after infection with the yopJ yopM mutant relative to infection by any other single or double mutant. Similar results were obtained with the yopJ, yopM, and yopJ yopM mutants of Yersinia pestis. Following intravenous infection of mice, the Y. pseudotuberculosis yopJ mutant was as virulent as the wild type, while the yopJ yopM mutant was significantly more attenuated than the yopM mutant. In summary, through epistasis analysis this work uncovered an important role for YopJ in inhibiting caspase-1 in activated macrophages and in promoting Yersinia virulence.
INTRODUCTION
Being the first line of defense against invading pathogens, the innate immune system has evolved to respond to general patterns of infection, termed pathogen-associated molecular patterns (PAMPs), via pattern recognition receptors (PRRs) (1, 2). Toll-like receptors (TLRs), acting as PRRs, detect distinct PAMPs from invading bacteria, such as flagellin (TLR5) and lipopolysaccharide (LPS; TLR4). Upon PAMP recognition, TLRs activate mitogen-activated protein kinase (MAPK) and nuclear factor-κB (NF-κB) pathways, which direct production of host-protective factors such as proinflammatory cytokines (3, 4). Bacterial pathogens produce virulence factors that inhibit PRR-directed innate immune responses in order to promote infection (5, 6). For example, numerous Gram-negative bacterial pathogens encode type III secretion systems (T3SSs) that are used to counteract host innate immune responses (7). During bacterium-host cell contact, T3SSs are activated and translocate effectors across the plasma membrane and into the eukaryotic cytosol. Translocated effectors inhibit innate immune responses and promote pathogenesis (7, 8). In order to counteract infection by virulent pathogens, host cells can sense perturbations caused by T3SSs and/or effectors and produce heightened innate immune responses (9, 10).
Disruptions induced by T3SSs and/or effectors in macrophages infected with bacterial pathogens commonly result in the activation of caspase-1 (11–14). Active caspase-1 promotes maturation and secretion of cytokines such as interleukin-1β (IL-1β) and IL-18, which are produced as inactive precursors. Caspase-1 is also produced as a proenzyme, and its activation typically occurs in an inflammasome, a multioligomeric complex that serves as a molecular platform for the recruitment and auto-proteolytic cleavage of procaspase-1 (11–13, 15). Active caspase-1 also induces lysosome exocytosis and a form of inflammatory cell death, termed pyroptosis, which is characterized by osmotic swelling of the macrophage and subsequent rupture of the plasma membrane, resulting in the release of proinflammatory molecules (16). Several inflammasomes have been identified, and all but one (that formed by AIM2) contain proteins that are part of the nucleotide-binding domain leucine-rich repeat (NLR) family. These NLRs serve as cytosolic PRRs and are activated upon recognition of cytosolic PAMPs or danger signals (2, 11, 15). For some NLRs, such as NLRP3, the adaptor protein apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC) mediates inflammasome assembly by interacting with capase-1 and the NLR (11, 13, 15). Complexes of NLRs and ASC form large structures in macrophages that can be detected as foci by microscopic techniques (17–19). In cases where inflammasome assembly is triggered in a T3SS-dependent manner upon infection of macrophages with pathogens, specific PAMPs identified to activate caspase-1 upon contaminating the cytosol include ectopically translocated flagellin, rod proteins, needle proteins, and translocated effector proteins (2, 11–13, 15). The NLRP3 inflammasome requires an initial priming signal (also referred to as signal 1), which can be achieved through TLR signaling, and induces expression of NLRP3 and pro-IL-1β via the MAPK and NF-κB pathways (2, 11, 15). This was originally thought to be the purpose of priming; however, recent studies have shown that new protein synthesis and upregulation of NLRP3 are not essential for subsequent NLRP3 inflammasome activation (20, 21). Cytosolic PAMPs or other perturbations and danger signals serve as a second signal (signal 2) for activation of the NLRP3 inflammasome (2, 11–13, 15).
A T3SS that is essential for virulence in pathogenic Yersinia species (Yersinia pestis, Yersinia pseudotuberculosis, and Yersinia enterocolitica) functions to counteract phagocytosis, inhibit cytokine production, and induce cell death in infected macrophages (22–25). This system secretes multiple proteins, including YopB and YopD, which insert into the plasma membrane and are thought to create a conduit called a translocon for delivery of the Yop effectors. Seven Yop effectors that are translocated into host cells have been characterized to date (22, 25–28). The translocon and several of the effectors, including YopJ, YopK, and YopM, have been implicated in activating or inhibiting caspase-1 in macrophages infected with Yersinia (29–37). However, different mechanisms of caspase-1 activation or inhibition occur in Yersinia-infected macrophages, depending on the activation state of the phagocyte (38) and other conditions of the interaction (23).
During infection of naive murine macrophages, YopJ induces a proinflammatory mode of cell death that is associated with delayed caspase-1 activation. YopJ inhibits activation of MAPKs (extracellular signal-regulated kinase 1 and 2 [ERK1/2], Jun N-terminal protein kinase [JNK], and p38) and NF-κB via acetylation of serine and threonine residues on MAPK kinases (MAPKKs) and IKKβ, respectively (35, 39–42). TAK1, a MAPK kinase kinase, has also been identified as a target of YopJ acetyltransferase activity (35, 41). Blockage of MAPKs and of NF-κB activation by YopJ prevents production of cytokines and survival factors in macrophages, resulting in the induction of a cell-extrinsic, TLR4-dependent mechanism of cell death (24, 43). Caspase-8 is required for processing and activation of caspase-1 during YopJ-induced cell death in Yersinia-infected macrophages (44, 45). This mechanism of caspase-1 activation is independent of known inflammasome components.
There are several amino acid substitutions between YopJ isoforms from different Yersinia backgrounds, and these variations have been linked to different levels of YopJ-dependent cytotoxicity and caspase-1 activation in naive macrophages infected with Yersinia (34, 37, 46, 47). For instance, the isoform of YopJ in Y. pestis KIM (molecular group 2.MED; YopJKIM) has a Leu at residue 177 and a Glu at residue 206, while the YopJ isoform in Y. pestis CO92 (molecular group ORI.1; YopJCO92) contains a Phe at position 177 and a Lys at position 206 (34). YopJKIM exhibits high cytotoxicity and causes high caspase-1 cleavage, as opposed to YopJCO92, which has low cytotoxicity and induces no measurable caspase-1 cleavage in naive macrophages (34). The YopJ isoform from Y. pseudotuberculosis (YopJYPTB), which causes intermediate cytotoxicity and caspase-1 cleavage, contains a Phe at residue 177 and a Glu at residue 206 (34). The increased activity of YopJKIM is associated with an apparent higher affinity than that of YopJCO92 for IKKβ, and YopJKIM inhibits IkBα and MAPK phosphorylation more efficiently than YopJCO92 (34).
Priming macrophages with LPS before Yersinia infection renders them resistant to YopJ-induced cell death and redirects them to undergo a rapid caspase-1-dependent pyroptotic cell death (38). This rapid inflammasome-dependent caspase-1 activation mechanism requires insertion of the translocon into the plasma membrane of Yersinia-infected macrophages (30, 31, 33, 36, 38). Caspase-1 activation occurs with increased kinetics in LPS-primed macrophages infected with either a yopK or yopM mutant, indicating that either effector is important for counteracting inflammasome assembly (31, 33, 36). YopK and YopM inhibit caspase-1 activation by distinct mechanisms. YopK regulates the rate and fidelity of translocation (48) to prevent inappropriate hyperinjection of substrates. Zwack and colleagues recently showed that hyperinjection of YopD and YopB is important for activation of caspase-1 in macrophages infected with a Y. pseudotuberculosis yopK mutant (30). YopM appears to inhibit a final step in inflammasome assembly by preventing procaspase-1 from localizing to ASC foci, or preinflammasomes, and by inhibiting caspase-1 activation (36).
YopM contains a central domain comprised of leucine-rich repeats (LRRs) (49). The LRRs vary in number and sequence among distinct YopM isoforms encoded by different Yersinia strains (31). In some YopM isoforms the LRRs have been shown to be important for inhibition of caspase-1 activation (31, 36). The 15-LRR isoform in Y. pseudotuberculosis YPIII (YopMYPIII) contains a YLTD sequence in LRR 10 that binds to caspase-1 and acts as a pseudosubstrate inhibitor (36). However, a 15-LRR isoform from Y. pestis KIM (YopMKIM) in which the YLTD sequence was inactivated retained the ability to inhibit activation of caspase-1 (31). In addition, a YopM isoform in Y. pseudotuberculosis 32777 that naturally lacks the YLTD sequence (YopM32777) inhibits activation of caspase-1 (31). These data suggest that all YopM isoforms can inhibit activation of caspase-1, likely by preventing the proform of the enzyme from localizing to preinflammasomes, and additionally, certain isoforms, e.g., YopMYPIII, exhibit an additional pseudosubstrate inhibitor function.
In addition to an LRR domain, all YopM isoforms contain a conserved C-terminal tail, which binds to ribosomal S6 protein kinase 1 (RSK1) (50–52). The YopM C-terminal tail is required for Yersinia virulence (51, 52) and inhibition of caspase-1 activation (31). Binding of YopM to RSK1 induces activation of its kinase activity (50, 53). Activation of RSK1 occurs independently of the upstream kinases ERK1/2 and is sustained because dephosphorylation of RSK1 is prevented in the presence of YopM (50, 53). Binding of RSK1 to YopM in Y. pseudotuberculosis-infected macrophages also stimulates the formation of high-molecular-weight complexes of RSK1 and YopM (51). The role of RSK1 in inhibition of caspase-1 activation by YopM is unknown.
In this study, we investigated if other effectors cooperate with YopM to inhibit activation of caspase-1 in LPS-primed macrophages. For this purpose, epistasis analysis was carried out in which the phenotypes of Yersinia yopM mutants were compared to those of mutants defective for yopM and an additional effector (yopJ, yopE, yopH, yopT, or ypkA). Activation of caspase-1 was measured after infection of LPS-activated macrophages with mutant Yersinia strains. Our results show that in the absence of YopM, an important role for YopJ in the inhibition of caspase-1 activation is uncovered. Thus, surprisingly, in naive macrophages YopJ activates caspase-1, and in LPS-primed macrophages YopJ cooperates with YopM to inhibit caspase-1. Additionally, based upon the known substrate specificity of YopJ, these results suggest that a member of the MAPK kinase family plays a role in the activation of caspase-1 in LPS-primed macrophages infected with Yersinia.
MATERIALS AND METHODS
Bacterial strains and plasmids.
All Y. pseudotuberculosis and Y. pestis strains used in this study are listed in Table 1. We introduced an in-frame deletion of yopM in Y. pestis strains expressing different yopJ alleles (34) as previously described (52), generating KIM5 yopJL177FΔyopM (where yopJL177F indicates the L-to-F change at position 177 encoded by yopJ), KIM5 yopJL177F/E206K ΔyopM, and KIM5 yopJC172AΔyopM. We replaced the coding region of yopM with a neomycin phosphotransferase cassette (npt or Kan) in the Y. pseudotuberculosis 32777 yopJC172A, yopER144A, yopHR409A, yopTC139A, or yopJC172A yopER144A mutant (54) to make each of the corresponding double or triple mutants. A Kan insertion in the ypkA gene (55) was introduced into the 32777 ΔyopM mutant to make the ΔypkA ΔyopM double mutant. For the 32777 yopER144AΔyopM, yopJC172AΔyopM, and yopER144A yopJC172AΔyopM mutants, we excised the Kan cassette using Flp recognition target (FRT) recombination as described previously (52). For infections of bone marrow-derived macrophages (BMDMs), we grew yersiniae overnight in Luria broth (LB) at 28°C in the presence (Y. pestis) or absence (Y. pseudotuberculosis) of 25 μg/ml ampicillin. The following day, we diluted cultures 1:40 in LB containing 20 mM MgCl2 and 20 mM sodium oxalate and grew them at 28°C for 1 h, followed by a temperature shift to 37°C for 2 h.
TABLE 1.
Yersinia strains used in this study
| Strain | Relevant characteristic(s)a | Reference or source |
|---|---|---|
| 32777 | Y. pseudotuberculosis wild-type serogroup O1 strain, pYV+ | 65 |
| 32777 ΔyopM | ΔyopM | 52 |
| 32777 yopJC172A | yopJC172A | 54 |
| 32777 yopJC172A ΔyopM | yopJC172A, ΔyopM | This study |
| 32777 yopER144A ΔyopM | yopER144A, ΔyopM | This study |
| 32777 yopHR409A ΔyopM | yopHR409A, yopM::Kan | This study |
| 32777 yopTC139A ΔyopM | yopTC139A, yopM::Kan | This study |
| 32777 ΔypkA ΔyopM | ypkA::Kan, ΔyopM | This study |
| 32777 ΔyopB ΔyopM | ΔyopM, in-frame deletion of yopB (nucleotides 496–774) | 31 |
| 32777 yopER144A yopJC172A ΔyopM | yopER144A, yopJC172A, ΔyopM | This study |
| 32777 yopJC172A ΔyopM pYopM | Transformed with pMMB67EH expressing yopM from native promoter, Ampr | This study |
| KIM5 | Y. pestis KIM6/pCD1Ap/pMT+/pPCP1+ Δpgm, Ampr | 29 |
| KIM5 ΔyopM | ΔyopM, Ampr | 52 |
| KIM5 yopJC172A | yopJC172A, Ampr | 29 |
| KIM5 yopJC172A ΔyopM | yopJC172A, ΔyopM, Ampr | This study |
| KIM5 yopJYPTB ΔyopM | yopJL177F, ΔyopM, Ampr | This study |
| KIM5 yopJCO92 ΔyopM | yopJL177F/E206K, ΔyopM, Ampr | This study |
yopJC172A, Cys-to-Ala change at position 172 encoded by yopJ; yopER144A, Arg-to-Ala change at position 144 encoded by yopE; yopHR409A, Arg-to-Ala change at position 409 encoded by yopH; yopTC139A, Cys-to-Ala change at position 139 encoded by yopT; yopJL177F, Leu-to-Phe change at position 177 encoded by yopJ; yopJL177F/E206K, Leu-to-Phe change at position 177 and Glu-to-Lys change at position 206 encoded by yopJ.
Mouse infections.
For analysis of time to death and organ colonization, we grew Y. pseudotuberculosis cultures in LB overnight at 28°C, washed the cultures twice with phosphate-buffered saline (PBS), and resuspended them at a density of approximately 1 × 104 CFU/ml. From this suspension, we injected a volume of 100 μl (∼1,000 CFU) into tail veins of C57BL/6 mice (Jackson Laboratories). We monitored mice daily for 14 days for survival analysis, at which point we euthanized the remaining mice. At 4 or 6 days postinfection, we euthanized mice by CO2 asphyxiation and collected spleens and livers in cold PBS. We homogenized spleens and livers with a Stomacher Biomasher and serially diluted and plated organ homogenates onto LB agar plates. We handled all mice according to the guidelines for humane care, and the procedures used were approved by the Stony Brook University Institutional Animal Care and Use Committee.
Bone marrow isolation and culture conditions.
We isolated bone marrow from femurs of 6- to 8-week-old female C57BL/6 mice as previously described (56). At 18 h prior to infection, we seeded BMDMs into six-well tissue culture plates in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (HyClone), 10% L929-cell-conditioned medium, 1 mM sodium pyruvate, 10 mM HEPES, 2 mM glutamate, and 100 ng/ml O26:B6 Escherichia coli LPS (Sigma) at a density of 0.8 × 106 cells/well.
Macrophage infection conditions.
We infected LPS-primed BMDMs with Y. pseudotuberculosis or Y. pestis grown under the conditions described above at a multiplicity of infection (MOI) of 30. We centrifuged tissue culture plates for 5 min at 95 × g to promote contact of yersiniae with BMDMs and subsequently incubated infected BMDMs at 37°C with 5% CO2. Unless otherwise noted, we collected lysates for Western blotting of host and bacterial proteins and supernatants for analysis of secreted IL-1α, IL-1β, and IL-18 and lactate dehydrogenase (LDH) release at 90 min postinfection.
Western blotting of macrophage cell lysates.
We washed BMDMs twice with cold Hanks' balanced salt solution prior to lysing cells with lysis buffer as previously described (31). We quantified total protein using a bicinchoninic acid (BCA) protein assay kit (Pierce) and loaded equivalent amounts of protein prior to resolving proteins by SDS-PAGE using 4 to 12% Bis-Tris gels (Invitrogen). We transferred gels onto 0.22-μm-pore-size polyvinylidene difluoride (PVDF) membranes prior to Western blotting with rabbit polyclonal anti-caspase-1 (Santa Cruz Biotechnology), goat polyclonal anti-IL-1β (R&D Systems), and mouse monoclonal anti-YopE antibodies. We used horseradish peroxidase (HRP)-conjugated anti-rabbit (Cell Signaling), anti-goat, and anti-mouse (Jackson ImmunoResearch) antibodies as secondary reagents. We stripped and reprobed blots using a β-actin antibody conjugated to HRP to control for loading. To detect signals in Western blot assays, we used Amersham ECL Prime Western blotting detection reagent (GE Healthcare). The resulting exposed films were scanned, and processed caspase-1 band signals on the corresponding images were quantified using the gel analysis procedure of ImageJ, version 1.39.
ELISA.
We measured levels of secreted IL-1α, IL-1β, and IL-18 in supernatants from infected or uninfected BMDMs using commercially available enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems for IL-1α and IL-1β and MBL International Corporation for IL-18) according to the manufacturers' instructions.
Cytotoxicity assay.
We measured the LDH released into supernatants from infected and uninfected BMDMs using a CytoTox 96 NonRadioactive Cytotoxicity Assay kit (Promega) according to the manufacturer's instructions.
Statistical analysis.
All statistical analysis was performed using GraphPad Prism, version 6.0, and results from at least three independent experiments. Probability (P) values from mouse survival experiments were calculated using a log rank test while significance of CFU data from spleens and livers was determined by Mann-Whitney analysis. CFU values were analyzed by Grubb's test to identify significant outliers, and all data from any mouse found to have an outlying value were removed prior to calculation of significance. P values from ELISAs and LDH experiments were calculated using one-way analysis of variance (ANOVA) with Tukey's multiple-comparison posttest. P values of <0.05 were considered significant.
RESULTS
Epistasis identifies YopJ as important for inhibition of release of caspase-1-dependent proinflammatory cytokines in LPS-primed macrophages infected with a Y. pseudotuberculosis yopM mutant.
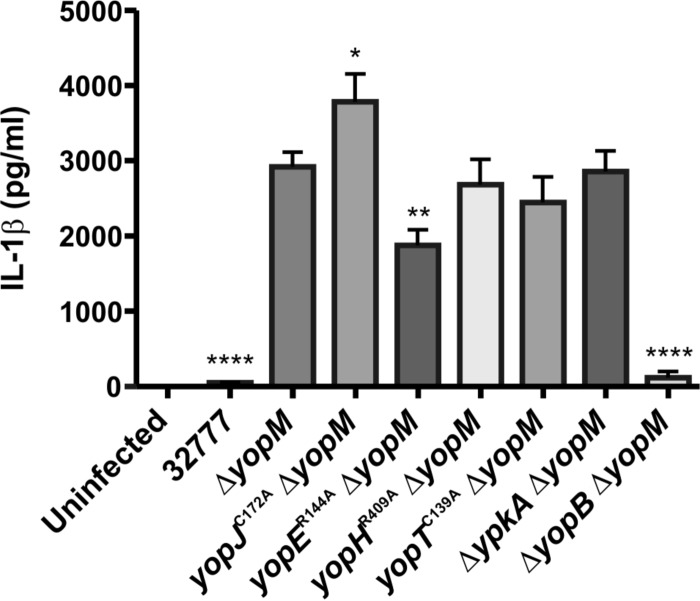
To determine if other effectors cooperate with YopM to inhibit caspase-1-dependent responses in LPS-primed BMDMs, an epistasis experiment was carried out with Y. pseudotuberculosis 32777 (wild type), a yopM mutant (ΔyopM), and a double yopJC172AΔyopM, yopER144AΔyopM, yopHR409AΔyopM, yopTC139AΔyopM, or ΔypkA ΔyopM mutant (Table 1). The translocation-deficient ΔyopB ΔyopM strain (Table 1) was used as a control. BMDMs were treated with purified E. coli LPS for 18 h, conditions that have been shown previously to reproducibly prime these cells to undergo rapid caspase-1-dependent responses to Yersinia infection (31, 36, 38). LPS-primed BMDMs were infected with the above strains for 90 min at an MOI of 30, followed by quantification of secreted IL-1β by ELISA. As shown in Fig. 1, BMDMs infected with the yopJC172AΔyopM mutant secreted significantly larger amounts of IL-1β than those infected with the ΔyopM mutant or the other double mutants, suggesting that YopJ cooperates with YopM to inhibit this caspase-1-dependent host response. Interestingly, BMDMs infected with the yopER144AΔyopM mutant secreted significantly smaller amounts of IL-1β than the ΔyopM mutant (Fig. 1), possibly as a result of increased YopJ translocation in the yopER144A mutant background. We compared the amounts of IL-1β secreted from BMDMs infected with a yopER144A yopJC172AΔyopM triple mutant (Table 1) versus the amounts with the yopJC172A ΔyopM mutant as this comparison allowed us to isolate the effect of the yopER144A mutation in the absence of YopJ activity. Significantly smaller amounts of IL-1β were secreted from BMDMs infected with the yopER144A yopJC172AΔyopM mutant than from infection with the yopJC172AΔyopM mutant (see Fig. S1 in the supplemental material), indicating that the reduced secretion of IL-1β associated with the yopER144A mutation was not due to hypertranslocation of active YopJ.
FIG 1.
Measurement of IL-1β in supernatants of BMDMs infected with Y. pseudotuberculosis. BMDMs from C57BL/6 mice were primed with 100 ng/ml LPS for 18 h and left uninfected or infected with strain 32777 (wild type), a yopM mutant (ΔyopM), or yopJC172AΔyopM, yopER144AΔyopM, yopHR409AΔyopM, yopTC139A ΔyopM, ΔypkA ΔyopM, or ΔyopB ΔyopM double mutant at an MOI of 30. Concentrations of IL-1β in supernatants collected at 90 min postinfection were quantified by ELISA. Data represent average values ± the standard errors of the means from three independent experiments. *, P < 0.05; **, P < 0.01; ****, P < 0.0001, as determined by one-way analysis of variance compared to results for ΔyopM strain-infected macrophages.
We next investigated if YopJ cooperates with YopM to inhibit lysosome exocytosis, which is a caspase-1-dependent antimicrobial response that occurs independent of IL-1β secretion (16). LPS-primed BMDMs were infected with the wild type, the ΔyopM mutant, or the double yopJC172AΔyopM, yopER144AΔyopM, or ΔypkA ΔyopM mutant for 60 min at an MOI of 30, followed by quantification of lysosomal exocytosis by immunofluorescence microscopy. This assay measures surface exposure of LAMP-1 resulting from fusion of lysosomes with the plasma membrane. A trend toward higher lysosome exocytosis was observed in BMDMs infected with the yopJC172AΔyopM mutant than in those infected with the ΔyopM mutant or the other double mutants (X. Wang, A. Ouyang, and J. B. Bliska, unpublished data), confirming that YopJ cooperates with YopM to inhibit caspase-1-dependent host responses.
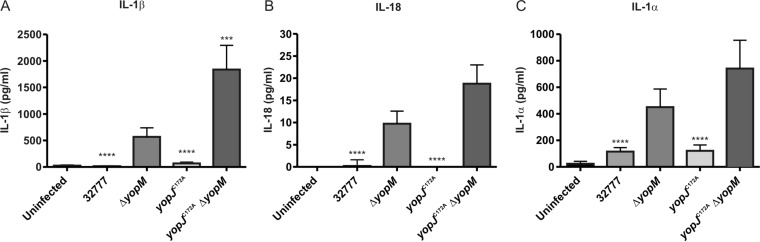
To determine if YopJ cooperates with YopM to inhibit release of multiple caspase-1-dependent cytokines, LPS-primed BMDMs were infected with the wild type, a yopJC172A mutant (Table 1), the ΔyopM mutant, or the double yopJC172AΔyopM mutant as described above, after which medium was collected for quantification of released IL-1β, IL-18, and IL-1α by ELISA. IL-1α is not processed by caspase-1 but is released from infected BMDMs during pyroptosis. Infection with the yopJC172AΔyopM mutant provoked a significantly higher level of IL-1β release than that from infection with the ΔyopM mutant (Fig. 2A). It appeared that larger amounts of IL-18 and IL-1α were also released by BMDMs infected with the yopJC172AΔyopM mutant than by BMDMs infected with the ΔyopM mutant although these differences were not significant (P values of 0.1038 and 0.4372, respectively) (Fig. 2B and C). These data suggest that in the absence of YopM, Y. pseudotuberculosis YopJ inhibits release of IL-1β, and possibly other caspase-1-dependent proinflammatory cytokines, in LPS-primed BMDMs.
FIG 2.
Measurement of caspase-1-dependent proinflammatory cytokines in supernatants of BMDMs infected with Y. pseudotuberculosis. BMDMs from C57BL/6 mice were primed with 100 ng/ml LPS for 18 h and left uninfected or infected with the 32777, ΔyopM, yopJC172A, or yopJC172A ΔyopM strain at an MOI of 30. At 90 min postinfection, supernatants were collected, and concentrations of secreted IL-1β, IL-18, or IL-1α were measured by ELISA. Data represent average values ± the standard errors of the means from three independent experiments. ***, P < 0.001; ****, P < 0.0001, as determined by one-way analysis of variance compared to values for 32777 ΔyopM-infected macrophages.
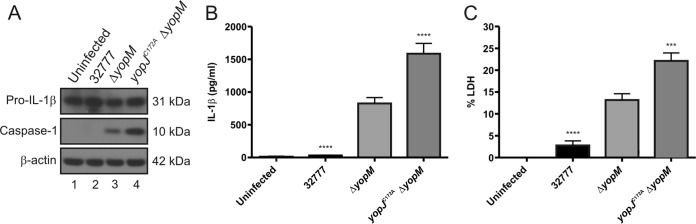
Because pro-IL-1β was produced in the BMDMs prior to infection due to LPS priming, we did not expect that YopJ could inhibit production of the cytokine during infection. To confirm this, steady-state levels of the proform of the cytokine were measured by immunoblotting. As shown in Fig. 3A, equivalent levels of pro-IL-1β were detected in lysates of LPS-primed BMDMs left uninfected or infected with wild-type, ΔyopM, or yopJC172AΔyopM strain as described above. Quantification of mature IL-1β in the medium of the BMDMs used for immunoblotting demonstrated significantly increased release of the cytokine after infection with the yopJC172AΔyopM mutant compared to that with the ΔyopM mutant (Fig. 3B). These data suggest that in the absence of YopM, Y. pseudotuberculosis YopJ inhibits the processing and release, but not production, of pro-IL-1β in LPS-primed BMDMs.
FIG 3.
Measurement of processed caspase-1, pro-IL-1β, mature IL-1β, and pyroptosis in BMDMs infected with Y. pseudotuberculosis. LPS-primed BMDMs from C57BL/6 mice were left uninfected or infected with the 32777, ΔyopM, or yopJC172AΔyopM strain at an MOI of 30. (A) After a 90-min infection, lysates were prepared and subjected to Western blotting with antibodies recognizing pro-IL-1β, caspase-1, or β-actin. Molecular masses of corresponding proteins are shown on the right. Quantification of the processed caspase-1 bands showed that the signal for the yopJC172AΔyopM strain was 4.7 times greater than that for the ΔyopM strain. Supernatants were harvested at 90 min postinfection, and secreted IL-1β was measured by ELISA (B); pyroptosis was measured by quantification of percent LDH release (C). The data in panels B and C represent average values ± the standard errors of the means from seven independent experiments. ***, P < 0.001; ****, P < 0.0001, as determined by one-way analysis of variance compared to results for ΔyopM mutant-infected macrophages.
YopJ inhibits activation of caspase-1 and pyroptosis in LPS-primed macrophages infected with a Y. pseudotuberculosis yopM mutant.
To investigate if YopJ cooperates with YopM to inhibit activation of caspase-1, LPS-primed BMDMs were left uninfected or infected as described above, and cell lysates were subjected to immunoblotting to measure production of processed caspase-1 (p10 subunit). As shown previously, BMDMs infected with strain 32777 did not have any measurable cleaved caspase-1, similar to results in the uninfected control, while ΔyopM mutant-infected BMDMs contained processed caspase-1 (Fig. 3A). However, the yopJC172AΔyopM mutant induced a larger amount of processed caspase-1 than the ΔyopM strain. Equal loading was confirmed by immunoblotting for β-actin (Fig. 3A).
To determine if YopJ cooperates with YopM to inhibit caspase-1-dependent pyroptosis, we assayed release of LDH by LPS-primed BMDMs left uninfected or infected as described above. As shown in Fig. 3C, LDH release was significantly increased in BMDMs infected with the yopJC172AΔyopM mutant compared to the level in the ΔyopM strain. Together, these data show that in the absence of YopM, Y. pseudotuberculosis YopJ inhibits caspase-1 processing and pyroptosis in LPS-primed BMDMs.
YopJ is important for virulence in mice infected with a Y. pseudotuberculosis yopM mutant.
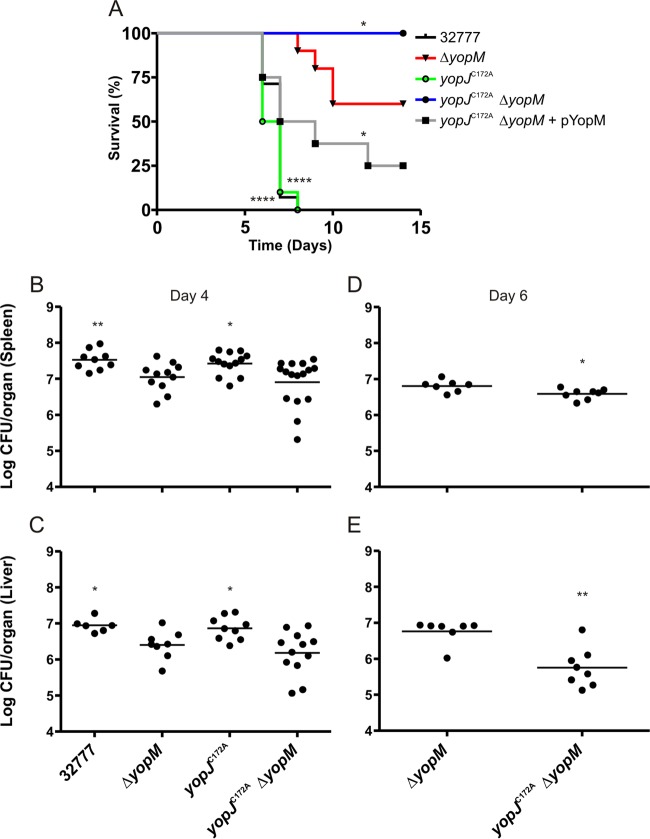
A Y. pseudotuberculosis yopM mutant is attenuated for virulence during intravenous (i.v.) infection of mice (36, 52), while a yopJ mutant is fully virulent under the same conditions (Fig. 4A). To investigate if YopJ promotes Y. pseudotuberculosis virulence in the absence of YopM, C57BL/6 mice were infected i.v. with 1,000 CFU of the 32777, ΔyopM, yopJC172A, or yopJC172AΔyopM strain. Mouse survival was monitored over 14 days. None of the 32777-infected mice or the yopJC172A strain-infected mice survived past day 8, while 60% of the mice infected with the ΔyopM strain survived to day 14 (Fig. 4A). Interestingly, 100% of mice infected with the yopJC172AΔyopM mutant survived to day 14, a survival rate significantly different from that of mice infected with the ΔyopM mutant (Fig. 4A). Introduction of a plasmid expressing yopM in the yopJC172AΔyopM mutant (Table 1) restored virulence, with only 25% of mice surviving to day 14 (Fig. 4A), demonstrating partial complementation.
FIG 4.
Determination of survival and organ colonization of mice infected with Y. pseudotuberculosis. C57BL/6 mice were infected i.v. with ∼1,000 CFU of the indicated Y. pseudotuberculosis strain. (A) Time to death was monitored for 14 days [n = 14 for 32777 and the yopJC172AΔyopM strains; n = 10 for the ΔyopM and yopJC172A strains; and n = 8 for the yopJC172AΔyopM(pYopM) strain]. (B and C) At 4 days postinfection, mice were euthanized, and organs were collected and processed for CFU assay (n = 11 for 32777 and the ΔyopM strains; n = 14 for the yopJC172A strain; and n = 17 for yopJC172AΔyopM strain). Data from all spleens and the subset of livers analyzed are shown, with horizontal bars indicating arithmetic means. Data in panels A, B, and C represent values from four or five independent experiments. (D and E) At 6 days postinfection, mice were euthanized, and organs were collected and processed for CFU assay (n = 8 for the ΔyopM strain, and n = 8 for the yopJC172AΔyopM strain). Data in panels D and E represent values from two independent experiments. Significance of differences between survival curves in panel A was determined by log rank test, while the significance of differences in organ colonization in panels B and C was calculated using a Mann-Whitney test, in all cases compared to values for ΔyopM mutant-infected mice. *, P < 0.05; **, P < 0.01; ****, P < 0.0001. All CFU values were analyzed by Grubb's test to identify significant outliers, and all data from any mouse found to have an outlying value are not shown and were removed prior to the calculation of significance.
To study if YopJ promotes bacterial colonization of organs in the absence of YopM, CFU assays were performed on spleens and livers of mice infected as described above with the 32777, ΔyopM, yopJC172A, or yopJC172AΔyopM strain for 4 days. As shown in Fig. 4, the 32777 and yopJC172A strains colonized spleen (Fig. 4B) and liver (Fig. 4C) at significantly higher levels than the ΔyopM strain. In addition, there was a trend toward reduced colonization of spleen and liver by the yopJC172AΔyopM mutant compared to that of the ΔyopM mutant, but the differences were not statistically significant at 4 days postinfection. However, when CFU assays were performed on spleens and livers of mice infected as described above with the ΔyopM or yopJC172AΔyopM mutant for 6 days, there was a significant reduction in colonization of both organs by the yopJC172AΔyopM mutant compared to the level with the ΔyopM mutant (Fig. 4D and E). Together, these data indicate that YopJ promotes Y. pseudotuberculosis virulence in the absence of YopM.
YopJ inhibits activation of caspase-1, release of IL-1β, and pyroptosis in LPS-primed macrophages infected with a Y. pestis yopM mutant.
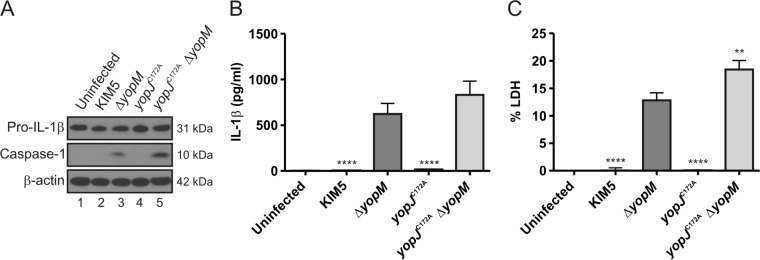
Infection with KIM5 or KIM5 ΔyopM (Table 1) showed that YopM is important for Y. pestis to inhibit caspase-1 processing, IL-1β release, and pyroptosis in LPS-primed BMDMs (Fig. 5A, B, C). To test if YopJ in Y. pestis inhibits caspase-1 activation in the absence of YopM, we infected LPS-primed BMDMs with KIM5, KIM5 ΔyopM, KIM5 yopJC172A, or KIM5 yopJC172AΔyopM (Table 1). No processed caspase-1 could be detected in BMDMs infected with KIM5 or KIM5 yopJC172A, similar to the results with the uninfected control (Fig. 6A). While infection of BMDMs with KIM5 ΔyopM induced measurable amounts of processed caspase-1, BMDMs infected with KIM5 yopJC172AΔyopM contained higher levels of cleaved caspase-1 (Fig. 6A). KIM5 yopJC172AΔyopM-infected BMDMs released increased levels of IL-1β and LDH compared to levels in BMDMs infected with KIM5 ΔyopM (Fig. 6B and C, respectively) although the difference with IL-1β was not significant (P = 0.0969). Higher IL-1β release in LPS-primed BMDMs infected with KIM5 yopJC172AΔyopM was not associated with an increase in pro-IL-1β protein levels (Fig. 6A). These data show that Y. pestis YopJ, similar to Y. pseudotuberculosis YopJ, inhibits caspase-1 activation in the absence of YopM.
FIG 5.
Measurement of processed caspase-1, pro-IL-1β, mature IL-1β, and pyroptosis in BMDMs infected with Y. pseudotuberculosis or Y. pestis. LPS-primed BMDMs were left uninfected or infected with 32777, 32777 ΔyopM, KIM5, or KIM5 ΔyopM for 90 min at an MOI of 30. (A) After infection, lysates were collected, processed, and subjected to Western blotting using antibodies to pro-IL-1β, cleaved caspase-1, or β-actin. Supernatants were collected and analyzed for secreted IL-1β (B) or pyroptosis by measurement of LDH release (C). Data represent average values ± the standard errors of the means from three independent experiments. ****, P < 0.0001, as determined by one-way analysis of variance comparing values in BMDMs infected with 32777 ΔyopM versus those with 32777 infection or BMDMs infected with KIM5 ΔyopM versus those infected with KIM5.
FIG 6.
Measurement of processed caspase-1, pro-IL-1β, mature IL-1β, and pyroptosis in BMDMs infected with Y. pestis. BMDMs from C57BL/6 mice were primed with 100 ng/ml LPS for 18 h and left uninfected or infected with KIM5, KIM5 ΔyopM, KIM5 yopJC172A, or KIM5 yopJC172AΔyopM at an MOI of 30. (A) At 90 min postinfection, lysates were collected, processed, and subjected to Western blotting using antibodies against pro-IL-1β and p10, the 10-kDa subunit of caspase-1. β-Actin was blotted as a loading control. Quantification of the processed caspase-1 bands showed that the signal for KIM5 yopJC172AΔyopM was 2.3 times greater than that for KIM5 ΔyopM. At 90 min postinfection, supernatants were collected, and secreted IL-1β was measured by ELISA (B); pyroptosis was quantified as percent LDH release (C). The data in panel B and C represent average values ± the standard error of the means from three independent experiments. **, P < 0.01; ****, P < 0.0001, as determined by one-way analysis of variance compared to results for KIM5 ΔyopM-infected macrophages.
Different YopJ isoforms in Y. pestis inhibit caspase-1 activation equally in the absence of YopM.
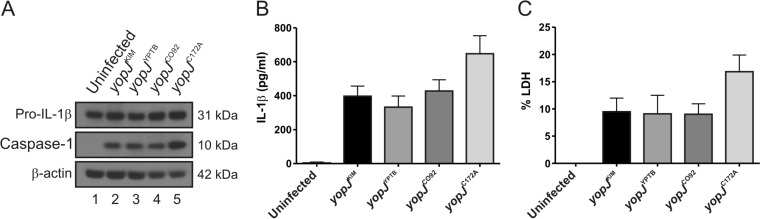
The YopJKIM, YopJYPTB, and YopJCO92 isoforms differentially activate caspase-1 in naive macrophages infected with Y. pestis (34). To study if these distinct isoforms differentially inhibit activation of caspase-1 in LPS-primed BMDMs, infections were carried out as described above with the Y. pestis yopJKIMΔyopM, yopJYPTBΔyopM, and yopJCO92ΔyopM strains (Table 1). LPS-primed BMDMs were also left uninfected or infected with the yopJC172AΔyopM strain as controls. Interestingly, we did not observe a difference between the activity of YopJKIM, YopJYPTB, or YopJCO92 in the inhibition of caspase-1 cleavage, IL-1β release, or pyroptosis (Fig. 7A, B, C). As expected, infection of LPS-primed BMDMs with the yopJC172AΔyopM mutant resulted in a trend toward increased caspase-1 cleavage, IL-1β secretion, and pyroptosis compared to the levels for the other strains (Fig. 7A, B, and C). These data suggest that amino acid variations at residues 177 and 206 do not alter the activity of YopJ in LPS-primed BMDMs with respect to inhibition of caspase-1 activation in the absence of YopM.
FIG 7.
Measurement of processed caspase-1, pro-IL-1β, mature IL-1β, and pyroptosis in BMDMs infected with Y. pestis strains expressing different YopJ isoforms. LPS-primed BMDMs from C57BL/6 mice were left uninfected or infected with KIM5 ΔyopM strains expressing yopJKIM, yopJYPTB, yopJCO92, or yopJC172A at an MOI of 30. (A) Lysates were collected and analyzed at 90 min postinfection using antibodies against pro-IL-1β, cleaved caspase-1, or β-actin. Quantification of the processed caspase-1 bands showed that the signal for KIM5 yopJC172A ΔyopM was approximately 1.4 times greater than that for KIM5 ΔyopM expressing the different isoforms. Supernatants were collected at 90 min postinfection and analyzed for secreted IL-1β by ELISA (B); pyroptosis was quantified by measuring LDH release (C). Data represent average values ± the standard errors of the means from three independent experiments. By one-way analysis of variance there were no significant differences between results for the KIM5 yopJKIM ΔyopM strain and those for the other strains tested.
DISCUSSION
TLRs can work in conjunction with inflammasomes to detect PAMPs during microbial infection. These signaling events are essential for innate immune cells, such as macrophages, to properly respond to invading pathogens (1–4, 11, 13–15). By blocking TLR and/or inflammasome signaling, pathogens can create an initial “silent” infection, preventing the innate immune system from inducing inflammation and reducing activation of adaptive immunity (5, 8, 12). Therefore, studying how pathogens evade host detection via TLR and inflammasome modulation will create a richer understanding of the complex interaction between pathogens and the innate immune system, likely leading to the discovery of novel antimicrobial treatments.
Our goal was to uncover effectors that cooperate with YopM to inhibit activation of caspase-1 in LPS-primed macrophages. By epistasis analysis of yop mutants, our results uncovered a role for YopJ in inhibiting caspase-1 activation and subsequent IL-1β secretion and pyroptosis in LPS-primed BMDMs infected with Y. pseudotuberculosis or Y. pestis yopM mutants (Fig. 1 to 3 and 6). Our data also show that, in the absence of YopM, YopJ promotes virulence of Y. pseudotuberculosis in mice infected intravenously (Fig. 4), presumably by inhibiting caspase-1 cleavage in macrophages that are activated in vivo.
These results were surprising as, in naive macrophages, YopJ activity stimulates the cleavage of caspase-1 and processing of IL-1β and IL-18 (29, 34, 35, 37, 44, 57). This occurs via activation of caspase-8 since blocking MAPK signaling and NF-κB activation by YopJ prevents expression of antiapoptotic proteins, resulting in TLR4-dependent activation of proapoptotic caspases (44, 45). Activation of macrophages via TLR signaling prior to Yersinia infection renders them refractory to YopJ-induced cytotoxicity and provides signal 1 to prime them for inflammasome-dependent caspase-1 activation (38, 58). In LPS-primed BMDMs infected with Yersinia, injection of PAMPs or the generation of danger signals subsequent to translocon insertion provides signal 2, leading to formation of ASC-NLRP3 foci or preinflammasomes (36). YopM inhibits formation of mature inflammasomes in LPS-primed BMDMs, likely by sequestering procaspase-1 away from ASC-NLRP3 foci (36). Our results suggest that YopJ can cooperate with YopM to inhibit mature inflammasome formation in LPS-primed BMDMs. One possibility is that in LPS-primed BMDMs, YopM inhibits a terminal step in a pathway that regulates recruitment of procaspase-1 to preinflammasomes, and YopJ inhibits an upstream step in the same pathway in a redundant fashion.
Both YopJ and YopM target the ERK signaling pathway but at different steps. YopJ inactivates the MAPK kinases in the ERK pathway (MEK1/2 or MAPKK1/2) (39, 42), and YopM interacts with RSK1 (50), which is normally regulated by ERK phosphorylation (42, 53). Although there is no direct evidence that YopM targets RSK1 to inhibit activation of caspase-1, ERK signaling has been implicated in regulation of inflammasome activation. For example, activation of ERK was recently shown to be important for LPS-induced priming of the NLRP3 inflammasome in human monocytes treated with ATP (20). Ghonime et al. showed that inhibition of ERK phosphorylation before LPS priming of human monocytes substantially reduced the cleavage and release of IL-18; however, inhibition of ERK phosphorylation after LPS priming did not alter release of active IL-1β (20). Additionally, in murine macrophages primed with LPS, the ERK pathway was shown to be important for expression of breast cancer cell 3 (BRCC3), a deubiquitinase that acts on NLRP3 to positively regulate inflammasome activation in response to ATP treatment (59, 60). In the above cases, ERK signaling seems to be important for LPS-induced priming, while our data show that YopJ inhibits activation of caspase-1 in LPS-primed BMDMs. If YopJ is inhibiting caspase-1 activation by targeting the ERK pathway, it would indicate that ERK signaling plays different roles in inflammasome signaling in LPS-primed monocytes than in BMDMs or in response to different second signals, e.g., ATP versus translocon insertion during Yersinia infection.
The p38 signaling pathway is an additional potential target for YopJ to inhibit inflammasome activation (61). Yu et al. demonstrated that treatment with a ribosome inhibitor (anisomycin) activated the ASC-pyrin inflammasome in human THP-1 cells through a p38-dependent pathway (61). However, treatment of mouse macrophages with anisomycin did not activate caspase-1, suggesting that the response of the ASC-pyrin inflammasome to “ribotoxic stress” may be human specific (61). It remains to be determined if a p38 signaling pathway stimulated during Yersinia infection of LPS-primed BMDMs could be important for inflammasome activation.
In our epistasis experiments, we observed that LPS-primed BMDMs secreted significantly smaller amounts of IL-1β when they were infected with the yopER144AΔyopM mutant than when they were infected with the ΔyopM mutant (Fig. 1). This was unexpected because previously Schotte et al. had obtained evidence that YopE inhibits activation of caspase-1 in macrophages infected with Yersinia (32). Additionally, Thinwa et al. showed that YopE inhibits an integrin-dependent priming signal (signal 1) that is important for production of IL-18 in epithelial cells infected with Yersinia (62). Schotte et al. used naive murine macrophage-like cells (Mf4/4 line), while Thinwa et al. used human intestinal epithelial cells (Caco-2 line), which could explain why their results differed from ours. Yersinia yopE mutants are known to hypertranslocate effectors into infected host cells (63, 64), and thus initially we thought that the reduced activation of caspase-1 that we observed in LPS-primed BMDMs infected with the yopER144AΔyopM mutant could result from hypertranslocation of YopJ. However, LPS-primed BMDMs infected with a yopER144A yopJC172AΔyopM triple mutant secreted significantly smaller amounts of IL-1β than those infected with the yopJC172AΔyopM double mutant (Fig. S1 in the supplemental material), showing that hypertranslocation of active YopJ is not responsible for the reduction in caspase-1 activation. Additional experiments are needed to understand why loss of YopE GTPase-activating protein (GAP) activity is associated with reduced caspase-1 activation in LPS-primed BMDMs.
In a previous study, higher levels of caspase-1 activation were observed in naive BMDMs infected with Y. pestis expressing the YopJKIM isoform than with a strain expressing the YopJYPTB or YopJCO92 isoform (34, 37). Increased caspase-1 activation in naive BMDMs infected with Y. pestis was correlated with decreased phosphorylation of IkBα, p38, and JNK, suggesting more effective inhibition of the respective upstream kinase, IKKβ, MKK3/6, or MKK4/7, by YopJKIM than by YopJCO92 (34). In addition, YopJKIM displayed increased binding to IKKβ compared to the level with YopJCO92, suggesting that the Leu at residue 177 and/or the Glu at residue 206 increases binding affinity in YopJKIM for MAPK kinase family members (34). Here, we did not observe a significant difference between the three distinct YopJ isoforms with respect to their abilities to inhibit caspase-1 activation in LPS-primed BMDMs infected with a Y. pestis yopM mutant (Fig. 7). This may indicate that YopJ polymorphisms do not affect targeting of the MAPK family member important for caspase-1 activation or, alternatively, that YopJ acetylates a novel substrate to counteract inflammasome signaling. Future studies that distinguish between the above possibilities will provide a better understanding of the target specificity of YopJ and yield insights into how translocon insertion during Yersinia infection of LPS-primed BMDMs activates caspase-1.
Supplementary Material
ACKNOWLEDGMENTS
We thank Yue Zhang and Maya Ivanov for help with construction of bacterial strains, Xiaoying Wang and Andrea Ouyang for performing the lysosome exocytosis assays, Patricio Mena and Jean Rooney for assistance with mouse infections, Galina Romanov for preparation of BMDMs, and Igor Brodsky for providing caspase-1 antibody.
This research was supported by awards from the NIH to J.B.B. (R01AI099222), L.K.C. (T32AI007539 and F31AI118220), and T.J.S. (K12GM102778) and by a Grant-In-Aid of Research (G201503151030819) from Sigma Xi, The Scientific Research Society, to L.K.C.
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.00843-15.
REFERENCES
- 1.Medzhitov R. 2007. Recognition of microorganisms and activation of the immune response. Nature 449:819–826. doi: 10.1038/nature06246. [DOI] [PubMed] [Google Scholar]
- 2.Motta V, Soares F, Sun T, Philpott DJ. 2015. NOD-like receptors: versatile cytosolic sentinels. Physiol Rev 95:149–178. doi: 10.1152/physrev.00009.2014. [DOI] [PubMed] [Google Scholar]
- 3.Lawrence T. 2009. The nuclear factor NF-κB pathway in inflammation. Cold Spring Harb Perspect Biol 1:a001651. doi: 10.1101/cshperspect.a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kawai T, Akira S. 2007. Signaling to NF-κB by Toll-like receptors. Trends Mol Med 13:460–469. doi: 10.1016/j.molmed.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 5.Finlay BB, McFadden G. 2006. Anti-immunology: evasion of the host immune system by bacterial and viral pathogens. Cell 124:767–782. doi: 10.1016/j.cell.2006.01.034. [DOI] [PubMed] [Google Scholar]
- 6.Tournier JN, Quesnel-Hellmann A. 2006. Host-pathogen interactions: a biological rendez-vous of the infectious nonself and danger models? PLoS Pathog 2:e44. doi: 10.1371/journal.ppat.0020044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Troisfontaines P, Cornelis GR. 2005. Type III secretion: more systems than you think. Physiology (Bethesda) 20:326–339. doi: 10.1152/physiol.00011.2005. [DOI] [PubMed] [Google Scholar]
- 8.Raymond B, Young JC, Pallett M, Endres RG, Clements A, Frankel G. 2013. Subversion of trafficking, apoptosis, and innate immunity by type III secretion system effectors. Trends Microbiol 21:430–441. doi: 10.1016/j.tim.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 9.Blander JM, Sander LE. 2012. Beyond pattern recognition: five immune checkpoints for scaling the microbial threat. Nat Rev Immunol 12:215–225. doi: 10.1038/nri3167. [DOI] [PubMed] [Google Scholar]
- 10.Vance RE, Isberg RR, Portnoy DA. 2009. Patterns of pathogenesis: discrimination of pathogenic and nonpathogenic microbes by the innate immune system. Cell Host Microbe 6:10–21. doi: 10.1016/j.chom.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sollberger G, Strittmatter GE, Garstkiewicz M, Sand J, Beer HD. 2014. Caspase-1: the inflammasome and beyond. Innate Immun 20:115–125. doi: 10.1177/1753425913484374. [DOI] [PubMed] [Google Scholar]
- 12.Shin S, Brodsky IE. 2015. The inflammasome: learning from bacterial evasion strategies. Semin Immunol 27:102–110. doi: 10.1016/j.smim.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 13.von Moltke J, Ayres JS, Kofoed EM, Chavarria-Smith J, Vance RE. 2013. Recognition of bacteria by inflammasomes. Annu Rev Immunol 31:73–106. doi: 10.1146/annurev-immunol-032712-095944. [DOI] [PubMed] [Google Scholar]
- 14.Vladimer GI, Marty-Roix R, Ghosh S, Weng D, Lien E. 2013. Inflammasomes and host defenses against bacterial infections. Curr Opin Microbiol 16:23–31. doi: 10.1016/j.mib.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Franchi L, Munoz-Planillo R, Nunez G. 2012. Sensing and reacting to microbes through the inflammasomes. Nat Immunol 13:325–332. doi: 10.1038/ni.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bergsbaken T, Fink SL, den Hartigh AB, Loomis WP, Cookson BT. 2011. Coordinated host responses during pyroptosis: caspase-1-dependent lysosome exocytosis and inflammatory cytokine maturation. J Immunol 187:2748–2754. doi: 10.4049/jimmunol.1100477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Proell M, Gerlic M, Mace PD, Reed JC, Riedl SJ. 2013. The CARD plays a critical role in ASC foci formation and inflammasome signalling. Biochem J 449:613–621. doi: 10.1042/BJ20121198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng J, Waite AL, Tkaczyk ER, Ke K, Richards N, Hunt AJ, Gumucio DL. 2010. Kinetic properties of ASC protein aggregation in epithelial cells. J Cell Physiol 222:738–747. doi: 10.1002/jcp.22005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fernandes-Alnemri T, Wu J, Yu JW, Datta P, Miller B, Jankowski W, Rosenberg S, Zhang J, Alnemri ES. 2007. The pyroptosome: a supramolecular assembly of ASC dimers mediating inflammatory cell death via caspase-1 activation. Cell Death Differ 14:1590–1604. doi: 10.1038/sj.cdd.4402194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghonime MG, Shamaa OR, Das S, Eldomany RA, Fernandes-Alnemri T, Alnemri ES, Gavrilin MA, Wewers MD. 2014. Inflammasome priming by lipopolysaccharide is dependent upon ERK signaling and proteasome function. J Immunol 192:3881–3888. doi: 10.4049/jimmunol.1301974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Juliana C, Fernandes-Alnemri T, Kang S, Farias A, Qin F, Alnemri ES. 2012. Non-transcriptional priming and deubiquitination regulate NLRP3 inflammasome activation. J Biol Chem 287:36617–36622. doi: 10.1074/jbc.M112.407130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shao F. 2008. Biochemical functions of Yersinia type III effectors. Curr Opin Microbiol 11:21–29. doi: 10.1016/j.mib.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 23.Bliska JB, Wang X, Viboud GI, Brodsky IE. 2013. Modulation of innate immune responses by Yersinia type III secretion system translocators and effectors. Cell Microbiol 15:1622–1631. doi: 10.1111/cmi.12164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aepfelbacher M, Trasak C, Ruckdeschel K. 2007. Effector functions of pathogenic Yersinia species. Thromb Haemost 98:521–529. [PubMed] [Google Scholar]
- 25.Viboud GI, Bliska JB. 2005. Yersinia outer proteins: role in modulation of host cell signaling responses and pathogenesis. Annu Rev Microbiol 59:69–89. doi: 10.1146/annurev.micro.59.030804.121320. [DOI] [PubMed] [Google Scholar]
- 26.Li B, Yang R. 2008. Interaction between Yersinia pestis and the host immune system. Infect Immun 76:1804–1811. doi: 10.1128/IAI.01517-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Navarro L, Alto NM, Dixon JE. 2005. Functions of the Yersinia effector proteins in inhibiting host immune responses. Curr Opin Microbiol 8:21–27. doi: 10.1016/j.mib.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 28.Plano GV, Schesser K. 2013. The Yersinia pestis type III secretion system: expression, assembly and role in the evasion of host defenses. Immunol Res 57:237–245. doi: 10.1007/s12026-013-8454-3. [DOI] [PubMed] [Google Scholar]
- 29.Lilo S, Zheng Y, Bliska JB. 2008. Caspase-1 activation in macrophages infected with Yersinia pestis KIM requires the type III secretion system effector YopJ. Infect Immun 76:3911–3923. doi: 10.1128/IAI.01695-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zwack EE, Snyder AG, Wynosky-Dolfi MA, Ruthel G, Philip NH, Marketon MM, Francis MS, Bliska JB, Brodsky IE. 2015. Inflammasome activation in response to the Yersinia type III secretion system requires hyperinjection of translocon proteins YopB and YopD. mBio 6:e02095-14. doi: 10.1128/mBio.02095-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chung LK, Philip NH, Schmidt VA, Koller A, Strowig T, Flavell RA, Brodsky IE, Bliska JB. 2014. IQGAP1 is important for activation of caspase-1 in macrophages and is targeted by Yersinia pestis type III effector YopM. mBio 5:e01402-14. doi: 10.1128/mBio.01402-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schotte P, Denecker G, Van Den Broeke A, Vandenabeele P, Cornelis GR, Beyaert R. 2004. Targeting Rac1 by the Yersinia effector protein YopE inhibits caspase-1-mediated maturation and release of interleukin-1beta. J Biol Chem 279:25134–25142. doi: 10.1074/jbc.M401245200. [DOI] [PubMed] [Google Scholar]
- 33.Brodsky IE, Palm NW, Sadanand S, Ryndak MB, Sutterwala FS, Flavell RA, Bliska JB, Medzhitov R. 2010. A Yersinia effector protein promotes virulence by preventing inflammasome recognition of the type III secretion system. Cell Host Microbe 7:376–387. doi: 10.1016/j.chom.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zheng Y, Lilo S, Brodsky IE, Zhang Y, Medzhitov R, Marcu KB, Bliska JB. 2011. A Yersinia effector with enhanced inhibitory activity on the NF-κB pathway activates the NLRP3/ASC/caspase-1 inflammasome in macrophages. PLoS Pathog 7:e1002026. doi: 10.1371/journal.ppat.1002026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meinzer U, Barreau F, Esmiol-Welterlin S, Jung C, Villard C, Leger T, Ben-Mkaddem S, Berrebi D, Dussaillant M, Alnabhani Z, Roy M, Bonacorsi S, Wolf-Watz H, Perroy J, Ollendorff V, Hugot JP. 2012. Yersinia pseudotuberculosis effector YopJ subverts the Nod2/RICK/TAK1 pathway and activates caspase-1 to induce intestinal barrier dysfunction. Cell Host Microbe 11:337–351. doi: 10.1016/j.chom.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 36.LaRock CN, Cookson BT. 2012. The Yersinia virulence effector YopM binds caspase-1 to arrest inflammasome assembly and processing. Cell Host Microbe 12:799–805. doi: 10.1016/j.chom.2012.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zheng Y, Lilo S, Mena P, Bliska JB. 2012. YopJ-induced caspase-1 activation in Yersinia-infected macrophages: independent of apoptosis, linked to necrosis, dispensable for innate host defense. PLoS One 7:e36019. doi: 10.1371/journal.pone.0036019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bergsbaken T, Cookson BT. 2007. Macrophage activation redirects Yersinia-infected host cell death from apoptosis to caspase-1-dependent pyroptosis. PLoS Pathog 3:e161. doi: 10.1371/journal.ppat.0030161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mittal R, Peak-Chew SY, McMahon HT. 2006. Acetylation of MEK2 and IκB kinase (IKK) activation loop residues by YopJ inhibits signaling. Proc Natl Acad Sci U S A 103:18574–18579. doi: 10.1073/pnas.0608995103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mittal R, Peak-Chew SY, Sade RS, Vallis Y, McMahon HT. 2010. The acetyltransferase activity of the bacterial toxin YopJ of Yersinia is activated by eukaryotic host cell inositol hexakisphosphate. J Biol Chem 285:19927–19934. doi: 10.1074/jbc.M110.126581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paquette N, Conlon J, Sweet C, Rus F, Wilson L, Pereira A, Rosadini CV, Goutagny N, Weber AN, Lane WS, Shaffer SA, Maniatis S, Fitzgerald KA, Stuart L, Silverman N. 2012. Serine/threonine acetylation of TGFβ-activated kinase (TAK1) by Yersinia pestis YopJ inhibits innate immune signaling. Proc Natl Acad Sci U S A 109:12710–12715. doi: 10.1073/pnas.1008203109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mukherjee S, Keitany G, Li Y, Wang Y, Ball HL, Goldsmith EJ, Orth K. 2006. Yersinia YopJ acetylates and inhibits kinase activation by blocking phosphorylation. Science 312:1211–1214. doi: 10.1126/science.1126867. [DOI] [PubMed] [Google Scholar]
- 43.Philip NH, Brodsky IE. 2012. Cell death programs in Yersinia immunity and pathogenesis. Front Cell Infect Microbiol 2:149. doi: 10.3389/fcimb.2012.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Philip NH, Dillon CP, Snyder AG, Fitzgerald P, Wynosky-Dolfi MA, Zwack EE, Hu B, Fitzgerald L, Mauldin EA, Copenhaver AM, Shin S, Wei L, Parker M, Zhang J, Oberst A, Green DR, Brodsky IE. 2014. Caspase-8 mediates caspase-1 processing and innate immune defense in response to bacterial blockade of NF-κB and MAPK signaling. Proc Natl Acad Sci U S A 111:7385–7390. doi: 10.1073/pnas.1403252111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weng D, Marty-Roix R, Ganesan S, Proulx MK, Vladimer GI, Kaiser WJ, Mocarski ES, Pouliot K, Chan FK, Kelliher MA, Harris PA, Bertin J, Gough PJ, Shayakhmetov DM, Goguen JD, Fitzgerald KA, Silverman N, Lien E. 2014. Caspase-8 and RIP kinases regulate bacteria-induced innate immune responses and cell death. Proc Natl Acad Sci U S A 111:7391–7396. doi: 10.1073/pnas.1403477111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zauberman A, Cohen S, Mamroud E, Flashner Y, Tidhar A, Ber R, Elhanany E, Shafferman A, Velan B. 2006. Interaction of Yersinia pestis with macrophages: limitations in YopJ-dependent apoptosis. Infect Immun 74:3239–3250. doi: 10.1128/IAI.00097-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brodsky IE, Medzhitov R. 2008. Reduced secretion of YopJ by Yersinia limits in vivo cell death but enhances bacterial virulence. PLoS Pathog 4:e1000067. doi: 10.1371/journal.ppat.1000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dewoody R, Merritt PM, Marketon MM. 2013. YopK controls both rate and fidelity of Yop translocation. Mol Microbiol 87:301–317. doi: 10.1111/mmi.12099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kerschen EJ, Cohen DA, Kaplan AM, Straley SC. 2004. The plague virulence protein YopM targets the innate immune response by causing a global depletion of NK cells. Infect Immun 72:4589–4602. doi: 10.1128/IAI.72.8.4589-4602.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McDonald C, Vacratsis PO, Bliska JB, Dixon JE. 2003. The Yersinia virulence factor YopM forms a novel protein complex with two cellular kinases. J Biol Chem 278:18514–18523. doi: 10.1074/jbc.M301226200. [DOI] [PubMed] [Google Scholar]
- 51.McCoy MW, Marre ML, Lesser CF, Mecsas J. 2010. The C-terminal tail of Yersinia pseudotuberculosis YopM is critical for interacting with RSK1 and for virulence. Infect Immun 78:2584–2598. doi: 10.1128/IAI.00141-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McPhee JB, Mena P, Bliska JB. 2010. Delineation of regions of the Yersinia YopM protein required for interaction with the RSK1 and PRK2 host kinases and their requirement for interleukin-10 production and virulence. Infect Immun 78:3529–3539. doi: 10.1128/IAI.00269-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hentschke M, Berneking L, Belmar Campos C, Buck F, Ruckdeschel K, Aepfelbacher M. 2010. Yersinia virulence factor YopM induces sustained RSK activation by interfering with dephosphorylation. PLoS One 5:e13165. doi: 10.1371/journal.pone.0013165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang Y, Murtha J, Roberts MA, Siegel RM, Bliska JB. 2008. Type III secretion decreases bacterial and host survival following phagocytosis of Yersinia pseudotuberculosis by macrophages. Infect Immun 76:4299–4310. doi: 10.1128/IAI.00183-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Prehna G, Ivanov MI, Bliska JB, Stebbins CE. 2006. Yersinia virulence depends on mimicry of host Rho-family nucleotide dissociation inhibitors. Cell 126:869–880. doi: 10.1016/j.cell.2006.06.056. [DOI] [PubMed] [Google Scholar]
- 56.Celada A, Gray PW, Rinderknecht E, Schreiber RD. 1984. Evidence for a gamma-interferon receptor that regulates macrophage tumoricidal activity. J Exp Med 160:55–74. doi: 10.1084/jem.160.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang Y, Bliska JB. 2010. YopJ-promoted cytotoxicity and systemic colonization are associated with high levels of murine interleukin-18, gamma interferon, and neutrophils in a live vaccine model of Yersinia pseudotuberculosis infection. Infect Immun 78:2329–2341. doi: 10.1128/IAI.00094-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bergsbaken T, Cookson BT. 2009. Innate immune response during Yersinia infection: critical modulation of cell death mechanisms through phagocyte activation. J Leukoc Biol 86:1153–1158. doi: 10.1189/jlb.0309146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Boudreau HE, Broustas CG, Gokhale PC, Kumar D, Mewani RR, Rone JD, Haddad BR, Kasid U. 2007. Expression of BRCC3, a novel cell cycle regulated molecule, is associated with increased phospho-ERK and cell proliferation. Int J Mol Med 19:29–39. [PubMed] [Google Scholar]
- 60.Py BF, Kim MS, Vakifahmetoglu-Norberg H, Yuan J. 2013. Deubiquitination of NLRP3 by BRCC3 critically regulates inflammasome activity. Mol Cell 49:331–338. doi: 10.1016/j.molcel.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 61.Yu JW, Farias A, Hwang I, Fernandes-Alnemri T, Alnemri ES. 2013. Ribotoxic stress through p38 mitogen-activated protein kinase activates in vitro the human pyrin inflammasome. J Biol Chem 288:11378–11383. doi: 10.1074/jbc.M112.448795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thinwa J, Segovia JA, Bose S, Dube PH. 2014. Integrin-mediated first signal for inflammasome activation in intestinal epithelial cells. J Immunol 193:1373–1382. doi: 10.4049/jimmunol.1400145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Black DS, Bliska JB. 2000. The RhoGAP activity of the Yersinia pseudotuberculosis cytotoxin YopE is required for antiphagocytic function and virulence. Mol Microbiol 37:515–527. [DOI] [PubMed] [Google Scholar]
- 64.Aili M, Isaksson EL, Carlsson SE, Wolf-Watz H, Rosqvist R, Francis MS. 2008. Regulation of Yersinia Yop-effector delivery by translocated YopE. Int J Med Microbiol 298:183–192. doi: 10.1016/j.ijmm.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 65.Simonet M, Falkow S. 1992. Invasin expression in Yersinia pseudotuberculosis. Infect Immun 60:4414–4417. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.