Abstract
Coccidioidomycosis is a potentially life-threatening respiratory disease which is endemic to the southwestern United States and arid regions of Central and South America. It is responsible for approximately 150,000 infections annually in the United States alone. Almost every human organ has been reported to harbor parasitic cells of Coccidioides spp. in collective cases of the disseminated form of this mycosis. Current understanding of the mechanisms of protective immunity against lung infection has been largely derived from murine models of pulmonary coccidioidomycosis. However, little is known about the nature of the host response to Coccidioides in extrapulmonary tissue. Primary subcutaneous coccidioidal infection is rare but has been reported to result in disseminated disease. Here, we show that activation of MyD88 and Card9 signal pathways are required for resistance to Coccidioides infection following subcutaneous challenge of C57BL/6 mice, which correlates with earlier findings of the protective response to pulmonary infection. MyD88−/− and Card9−/− mice recruited reduced numbers of T cells, B cells, and neutrophils to the Coccidioides-infected hypodermis compared to wild-type mice; however, neutrophils were dispensable for resistance to skin infection. Further studies have shown that gamma interferon (IFN-γ) production and activation of Th1 cells characterize resistance to subcutaneous infection. Furthermore, activation of a phagosomal enzyme, inducible nitric oxide synthase, which is necessary for NO production, is a requisite for fungal clearance in the hypodermis. Collectively, our data demonstrate that MyD88- and Card9-mediated IFN-γ and nitric oxide production is essential for protection against subcutaneous Coccidioides infection.
INTRODUCTION
Two species of Coccidioides, C. immitis and C. posadasii, are soilborne molds and the etiologic agents of coccidioidomycosis, a potentially life-threatening human respiratory disease that is also known as San Joaquin Valley fever. This pulmonary mycosis is endemic to the southwestern United States and certain arid regions of Mexico, as well as Central and South America (1). Recent evidence has revealed that C. posadasii occurs in Washington State, well north of the previously documented boundary of regions of endemicity for coccidioidal infections in the United States (2). Coccidioides is a diphasic pathogen, characterized by a saprobic phase that produces dry, air-dispersed, infectious spores from vegetative mycelia and a parasitic cycle which is unique among the medically important fungi (3). Inhaled spores grow isotropically in the lungs of the mammalian host to form the first generation of parasitic cells (spherule initials). The spherule initials continue to enlarge and differentiate into multinucleate spherules (>80 μm in diameter). The cytoplasm of these coenocytes undergoes a process of segmentation by invagination of the inner spherule wall layer, followed by the formation of a multitude of endospores, each 2 to 10 μm in diameter. Endospores also undergo isotropic growth while still contained within the maternal spherule and eventually cause the spherule wall to rupture. The released endospores give rise to a second generation of parasitic cells and the cycle continues. First-generation spherule initials and endospores (10 to 20 μm in diameter) can be engulfed by host neutrophils and macrophages, while mature spherules (40 to 100 μm in diameter) are too large to be phagocytosed (3, 4). Interactions between the pathogen and host defense cells thereby results in both an intracellular and extracellular relationship.
Skin involvement in human coccidioidomycosis is typically the result of primary lung infection. Erythema nodosum and erythema multiforme are cutaneous manifestations of Coccidioides infection that can appear in humans at any time from several days to 3 weeks after the onset of the acute primary pulmonary disease (5). These are mild to severe skin rashes and are considered to be the result of delayed-type hypersensitivity reactions to circulating Coccidioides antigen rather than actual cutaneous infections. Acute pulmonary infection in humans, on the other hand, can lead to a chronic form of coccidioidomycosis in which the pathogen may disseminate hematogenously and/or lymphatically to the skin (6–9). We have been unable to replicate the chronic disease and associated skin manifestation in mice following instillation of Coccidioides spores in the lungs, in part because acute infection in the murine model typically results in death of most of the animals within 18 days postchallenge. The mouse model described here simulates a human, primary percutaneous infection that is the result of a puncture wound involving a Coccidioides spore-bearing fomite. Although the skin is rarely the site of infection, agricultural workers and others who labor outdoors in regions where Coccidioides is endemic are at high risk of encountering the environmental pathogen, and primary cutaneous infections among these individuals have been documented (10, 11). Laboratory accidents resulting in skin infections with the cultured pathogen have also been reported. Traumatic cutaneous inoculation of Coccidioides can result in prolonged illness and life-threatening disseminated disease, including coccidioidal meningitis (11–14). The goals of this study were to establish a subcutaneously challenged murine model of coccidioidomycosis for determination of the nature of the host immune response to infection of the skin and to compare the results of histological and immunological examinations of subcutaneously challenged mice to those in reported studies of protective immunity in our pulmonary model of this respiratory disease (15–18). The CD4+ T cell-mediated response, especially Th1 and Th17 immunity, has been shown to be essential for resistance to pulmonary coccidioidal infection (15–19). Vaccine-induced activation of myeloid differentiation factor 88 (MyD88)-mediated and caspase adaptor recruitment domain family member 9 (Card9)-mediated immune responses have also been demonstrated to be necessary for resistance to pulmonary coccidioidomycosis (18, 19). Both MyD88 and Card9 are required for differentiation and development of Th1 and Th17 immunity in Coccidioides-infected lungs (18, 19). MyD88- and Card9-mediated signal pathways also facilitate microbe-induced production of reactive oxygen species (ROS) and nitric oxide (NO) (20–23). However, in the case of pulmonary disease, we and others have shown that inducible NO synthase (iNOS) and NADPH oxidase 2 (NOX2), two enzymes required for ROS and NO production, have limited influence on the control of Coccidioides infection of lung tissue (24–26). In this study, we investigated the role of MyD88- and Card9-mediated signaling pathways in the host response to subcutaneous Coccidioides infection by using mice that are deficient in expression of MyD88, Card9, the gamma interferon (IFN-γ) receptor, interleukin-17A (IL-17A), IL-17 receptor A, iNOS, and NOX2.
MATERIALS AND METHODS
Fungal strains, growth conditions, and spore preparation.
The virulent fungal strain used to challenge mice in this study was a highly virulent, clinical isolate of C. posadasii (C735) that was cultured on glucose-yeast extract (GYE) growth medium (1% glucose, 0.5% yeast extract, 1.5% agar) for 3 to 4 weeks at 30°C to generate a confluent layer of spores on the agar surface. Spores were harvested and suspended in phosphate-buffered saline (PBS) (15). All culturing and preparatory procedures which involved live cells of C. posadasii were conducted in a biosafety level 3 (BSL3) laboratory.
Mouse strains.
Breeding pairs of inbred strains of mice on a C57BL/6 genetic background were obtained from Jackson Laboratory unless otherwise stated. The murine strains included C57BL/6 (stock number 000664), B6.129P2(SJL)-Myd88tm1.1Defr/J (MyD88−/− mice; stock number 009088), and B6.129P2-Nos2tm1Lau/J (iNOS−/− mice; stock number 002609). Breeding pairs of mice lacking expression of Card9 (Card9−/−) were provided by Marcel Wűthrich at the University of Wisconsin—Madison Medical School (Madison, WI) based upon a material transfer agreement with Xin Lin at M. D. Anderson Cancer Center (Houston, TX) (27). B6.129S7-Ifngtm1Ts/J (IFN-γ receptor-deficient mice; IFN-γr−/−) were a gift from Thomas Forsthuber at the University of Texas at San Antonio. Breeder pairs of B6.129P2-Il17atm1Yiw (IL-17A deficient; IL-17a−/− mice) and IL-17 receptor A-deficient mice (IL-17ra−/−) were provided by Marcel Wűthrich based upon material transfer agreements with Yoichiro Iwakura at The University of Tokyo and Amgen, Inc. (Thousand Oaks, CA), respectively (28, 29). Breeding pairs of B6.129S-Cybbtm1Din/J mice (referred to as gp91phox−/− or NOX2−/− mice), which are deficient in NADPH oxidase, were kindly supplied by Joshua Fierer at the University of California at San Diego School of Medicine (San Diego, CA). B6.129S-Cybbtm1Din/J mice, are referred to as NOX2−/− mice in this study (25). Mice were housed in a specific-pathogen-free animal facility at UTSA and handled according to guidelines approved by the University Institutional Animal Care and Use Committee. Mice were relocated to an animal BSL3 (ABSL3) laboratory at UTSA prior to infection.
Subcutaneous challenge and evaluation of fungal burden and survival.
Both male and female mice at age 12 to 16 weeks were used in this study. Hair on an area of the posterior quadrant of the abdomen (approximately 2 by 2 cm) was removed with a depilatory agent (Nair; Church and Dwight, Ewing, NJ) 1 day before inoculation. The area of hair removal was swabbed with 70% ethanol. Mice were challenged subcutaneously (s.c.) with 5 × 104 viable spores of C. posadasii isolate C735 suspended in 100 μl PBS. The spores were delivered using a 27-gauge needle which was inserted at the posterior border of the hairless abdominal region to ensure that the needle did not enter a blood vessel. Spore inoculation resulted in a small raised skin inflammation (<2 mm), which dissipated within 24 h postchallenge. Control mice were injected with PBS following the same inoculation protocol as above. The fungal burden in infected hypodermal tissue, which included visible abscesses and adjacent draining lymph nodes (Absc + dLN group), was determined at the indicated days postchallenge by plating serial dilutions of the tissue homogenates on GYE agar containing 50 μg/ml chloramphenicol as described previously (30). The skin abscesses and adjacent draining lymph nodes were radically enlarged and fused together in most of the infected MyD88−/− and Card9−/− mice at 16 days postchallenge (dpc). Thus, both tissues were combined for CFU determination. The fungal burdens in lung and spleen homogenates were determined in the same manner. The number of CFU of Coccidioides was expressed on a log scale and reported for individual mice of each group as previously described (30). Survival studies of subcutaneously challenged wild-type (WT), MyD88−/−, Card9−/−, NOX2−/−, and iNOS−/− mice were conducted over a period of 75 to 100 days postchallenge.
Histopathology.
Comparative histopathology was conducted with excised subcutaneous abscesses obtained from WT, MyD88−/−, and Card9−/− mice (3 mice per strain) which had been challenged subcutaneously as described above and sacrificed at 16 dpc. Tissue fixation and embedding procedures were performed as previously described (30), and tissue sections (5 μm thick) were stained with hematoxylin and eosin (H&E) or Gomori methenamine silver stain (GMS) by standard procedures. Sections were examined using a Leica DMI6000 microscope equipped with an automated TurboScan stage (Objective Imaging, Ltd., Cambridge, United Kingdom), and microscope images of subcutaneous abscesses were acquired and analyzed using Surveyor software (Objective Imaging) as reported elsewhere (31).
Leukocyte isolation.
Subcutaneous abscesses and adjacent draining (inguinal) lymph nodes were carefully excised from the infected hypodermis (4 mice per group) at 16 dpc for preparation of total leukocytes, which were used for determination of leukocyte subpopulations and ROS- and NO-producing granulocytes. Abscesses and lymph nodes were minced into small pieces (∼2 to 3 mm) with a pair of sterile scissors in ice-cold PBS and digested with 0.3 Wench units of Liberase Blendzyme 3 (Roche Diagnostics, Indianapolis, IN) at 37°C in an incubator set at 5% CO2 for 30 min. To further release host cells, the tissue was mashed with the back of a sterile 3-ml syringe plunger and passed through a cell strainer (70-μm-diameter pore size) into a petri dish (60 by 15 mm) containing 5 ml of RPMI 1640 medium (HyClone, Logan, UT) plus 1% heat-inactivated fetal bovine serum (FBS; HyClone). The dispersed cells were centrifuged (500 × g) for 5 min without use of the centrifuge brake. The cell pellet was resuspended and incubated for 5 min in 3 ml of ACK lysing buffer (Lonza, Inc., Walkersville, MD) for lysis of erythrocytes. Two volumes of RPMI 1640 plus 1% FBS were added to the mixture, which was then filtered through a second cell strainer (40-μm-diameter pore size). The total leukocytes were pelleted as described above and resuspended in 3 ml of Hanks' balanced salt buffer (HBSS) that was layered onto 3 ml Ficoll-Paque Plus (GE Healthcare, Pittsburgh, PA). The samples were then centrifuged at 400 × g for 20 min at 18°C. Both the lymphocyte layer at the interface and the granulocytes above the bottom pellet were harvested. The isolated cells were washed with 3 volumes of RPMI 1640 medium and resuspended in RPMI 1640 medium without phenol red or FBS for subsequent analyses. Total leukocytes prepared from the excised abscess plus dLN group by this method were also used for cytokine intracellular staining of IFN-γ+ and IL-17+ cells.
Quantification of leukocyte subpopulations in subcutaneous tissue.
Flow cytometry was used to determine the percentages and absolute numbers of CD4+ T cells, CD8+ T cells, B cells, natural killer cells (NK), neutrophils (PMN), macrophages (MΦ), eosinophils (EOS), and dendritic cells (DC) in abscess and dLN-derived leukocytes, as previously described (15, 24). All fluorochrome-conjugated antibodies used in this study were purchased from BD Biosciences (Franklin Lakes, NJ). Data were acquired with a FACSCalibur flow cytometer (BD Biosciences) and analyzed using the FlowJo software package (Tree Star, Inc., Ashland, OR). The results were first gated on live cells that had higher forward scatter (FSC) and lower side scatter (SSC) than dead cells. The live cells were subsequently gated on the total CD45+ leukocyte population for further analyses. The gating strategies were the following: CD3+ CD4+ CD8−, CD3+ CD4− CD8+, CD19+, CD161c(NK1.1)+ CD3−, Ly6G+ CD11b+ CD11c−, Mac3+ CD11c− Ly6G−, SiglecF+ CD11c−, and CD11b+ CD11c+ (24). Cell numbers were normalized to the dissected hypodermis tissue weight (measured in grams).
Intracellular cytokine staining.
Percentages and cell numbers of IFN-γ- and IL-17A-producing CD4+ and CD4− subpopulations in abscess and lymph node homogenates derived from Coccidioides-infected WT, MyD88−/−, and Card9−/− mice at 16 dpc were determined as previously reported (15, 16). Leukocytes prepared from draining (inguinal) lymph nodes of naive mice of the same strains were used as negative controls (data not shown). Aliquots of leukocytes were stimulated with anti-CD3 and anti-CD28 antibodies in the presence of GolgiStop in 10% FBS-supplemented RPMI 1640 for 4 h at 37°C. Permeabilized cells were stained with selected fluorochrome-conjugated monoclonal antibodies specific for CD3, CD4, CD11b, CD45, IFN-γ, or IL-17A. The percentages and cell numbers of the specific cytokine-producing CD4+ T cells and CD4− innate cells were determined in the gated, live CD45+ leukocyte population.
Depletion of neutrophils from wild-type mice and selected in vivo neutralization of IL-17A in IFN-γr−/− mice.
Wild-type mice were injected intraperitoneally (i.p.) with 200 μg of anti-Ly6G (clone IA8; Bio-X-cell, West Lebanon, NH) to deplete neutrophils. To neutralize IL-17 in animals, we treated IFN-γr−/− mice with anti-IL-17A monoclonal antibody (MAb; clone M210; generously provided by Amgen, Inc.) as previously reported (17). Mice were injected i.p. with 200 μg MAb, 24 h before subcutaneous challenge with C. posadasii spores. This treatment was repeated every 3 days after challenge until mice were sacrificed at 16 dpc. Each mouse received a total of 6 injections of monoclonal antibody. Control mice received equivalent amounts of normal rat IgG (Sigma Chemical Co., St. Louis, MO). The efficacy of neutrophil or IL-17A depletion in abscesses and adjacent lymph nodes was monitored by flow cytometry (15).
Quantification of intracellular NO and ROS production by flow cytometry.
For detection of NO and ROS production, aliquots of abscess plus dLN-derived leukocytes (2 × 106) obtained from WT, MyD88−/−, or Card9−/− mice were incubated with a NO fluorescent probe following the manufacturer's protocol (Cell BioLabs, Inc., San Diego, CA) or with 10 μM 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate (CM-H2DCFDA; catalog number 6827; Fisher Scientific, Grand Island, NY) in 200 μl colorless RPMI medium without phenol red and FBS at 37°C and 5% CO2 for 2 h. Leukocytes isolated from inguinal lymph nodes of each strain of noninfected (naive) mice were used as negative controls. The NO fluorescent probe is oxidized to a highly fluorescent triazolo-fluorescein analog in the presence of intracellular NO. CM-H2DCFDA, a general oxidative stress indicator, is oxidized by intracellular ROS to CM-DCF, which emits green fluorescence. The labeled leukocytes were blocked with Fc blocker (0.25 μg/50 μl) for 15 min at 4°C and subsequently labeled with allophycocyanin-conjugated anti-CD11b and analyzed by flow cytometry. Percentages and cell numbers per tissue weight of NO+ cells and ROS+ cells were determined for WT, MyD88−/−, and Card9−/− mice at 16 dpc. The gated live CD11b+ cells were analyzed for separate mean of fluorescence intensity (MFI) of triazolo-fluorescein and CM-DCF, which are indicators of NO and ROS production, respectively.
Statistical analyses.
The Mann-Whitney U test was used to compare differences between the fungal burdens of wild-type and knockout mice, measured as CFU as well as percentages and numbers of specific cytokine-producing T cells in abscesses plus draining (inguinal) lymph nodes, lungs, and spleen of mice, as previously reported (15, 24, 30). Survival data were examined by Kaplan-Meier survival analysis and a chi-squared test to compare survival plots, as reported previously (30). A P value of <0.05 was considered statistically significant.
RESULTS
C57BL/6 WT mice developed chronic but self-limited subcutaneous coccidioidomycosis.
WT mice exhibited resistance to subcutaneous infection following challenge with a suspension of 5 × 104 viable spores prepared from a clinical isolate (C735) of C. posadasii (Fig. 1A). Infected mice showed asymptomatic behavior but formed a large abscess at the subcutaneous inoculation site (hypodermis). About 30 to 40% of the infected mice revealed infection of the dermis and developed pustules and ulcers at 2 to 4 weeks postchallenge. Over a period of 4 to 8 weeks after inoculation, most of the pustules had ruptured and the skin had healed. Subcutaneous abscesses plus adjacent draining (inguinal) lymph nodes in the hypodermis were harvested for CFU assays. The mean fungal burden in these tissues was sustained at 4.2 × 104 CFU during the first 16 dpc. A decline of the Coccidioides burden in subcutaneous tissue was subsequently observed over the next 84 days. At 100 days postchallenge (dpc), only 60% of the mice had detectable organisms, which ranged from 20 to 180 CFU in the hypodermis. No viable Coccidioides cells were detected in the lungs or spleen at 100 days after subcutaneous challenge (detection limit, 5 CFU per organ).
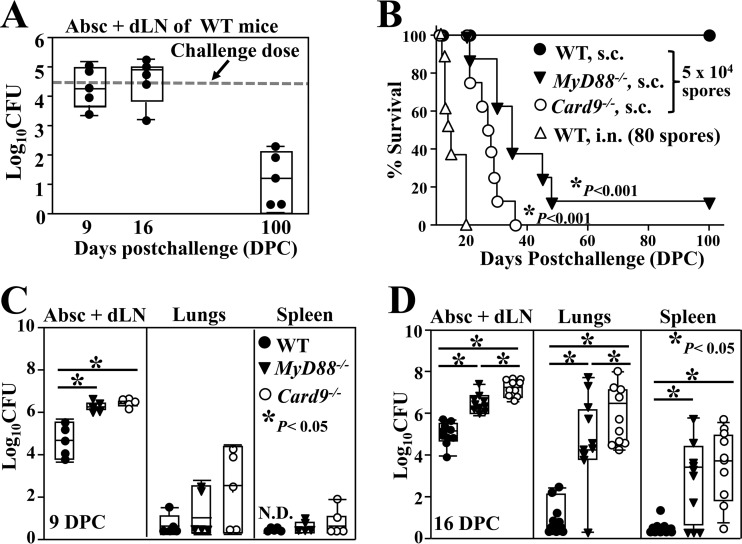
FIG 1.
WT C57BL/6 mice are resistant to subcutaneous infection with Coccidioides, but not MyD88−/− and Card9−/− mice. All three strains of mice were challenged with 5 × 104 viable spores isolated from a clinically virulent isolate (C735) of C. posadasii by subcutaneous injection in the abdominal region. Abscesses and lymph nodes (Abs +dLN) from infected hypodermis were collected, combined, and homogenized for CFU determinations. (A) The CFU of Coccidioides were detected in dilution plate cultures of Abs + dLN homogenates of WT mice at 9, 16, and 100 dpc. The dashed line indicates the inoculation dose. (B) Survival of WT, MyD88−/−, and Card9−/− mice (n = 10) after a subcutaneous challenge was recorded when the mice approached a moribund stage. A separate group of WT mice were challenged with 80 viable spores by the intranasal route as a control. Both MyD88−/− and Card9−/− mice showed significantly reduced survival compared to WT mice (Kaplan-Meier analysis, chi-squared test, P < 0.001). (C and D) CFU of Coccidioides detected in dilution plate cultures of homogenates prepared from Abs + dLN, lungs, and spleen of WT, MyD88−/−, and Card9−/− mice at 9 and 16 dpc, respectively. Asterisks indicate statistically significant differences (Mann-Whitney U test, P < 0.05) in fungal burdens of the compared mouse groups.
MyD88 and Card9 were essential for resistance to subcutaneous infection with Coccidioides.
MyD88−/− and Card9−/− mice were challenged subcutaneously with 5 × 104 viable spores, and their survival and fungal burden were compared to those of WT mice. Both knockout strains of mice succumbed to disseminated lethal disease, while 100% of the WT mice challenged subcutaneously survived for at least 100 days (Kaplan-Meier survival analysis and chi-squared test, P < 0.001 for subcutaneously challenged knockout versus WT mice) (Fig. 1B). WT mice challenged by the intranasal route all died within 20 days postchallenge. Dissemination of Coccidioides from the subcutaneous inoculation site to the lungs and spleen of MyD88−/− and Card9−/− mice had occurred by approximately 9 and 16 dpc, respectively (Fig. 1C and D). The mean CFU in the infected abscess plus dLN samples and lung tissue of Card9−/− mice at 16 dpc (7.21 ± 0.41 and 5.98 ± 1.31 log10, respectively) (mean ± standard error of the mean [SEM]) were significantly higher than that of MyD88−/− mice (6.39 ± 0.56 and 4.39 ± 2.35 log10, respectively; Mann-Whitney U test, P < 0.03) (Fig. 1D).
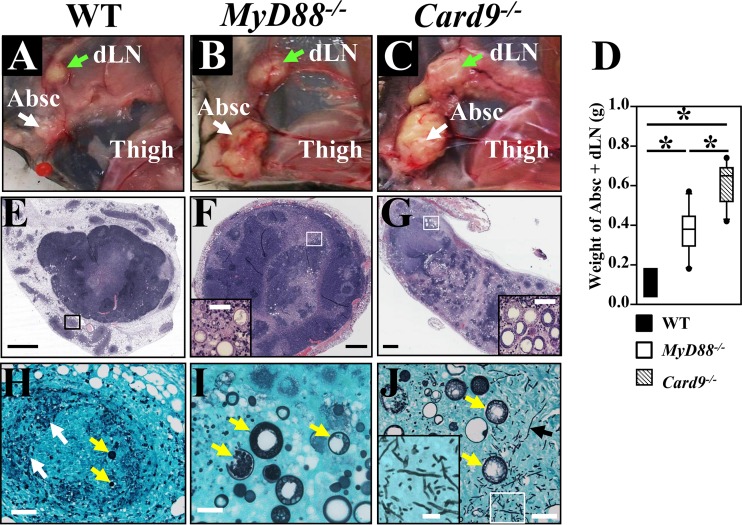
Gross tissue comparison of infected WT, MyD88−/−, and Card9−/− mice revealed that the immunocompromised animals each had significantly higher abscess plus dLN weights than the immunocompetent (WT) mice (Fig. 2A to D). The weights of dissected abscess plus dLN tissues from the infected hypodermis of MyD88−/− and Card9−/− mice were significantly greater than those of WT mice (Fig. 2D; Mann-Whitney U test, P < 0.05) at 16 dpc. Histopathological examinations showed that the inguinal lymph nodes of all three strains of mice contained spherules in various stages of endosporulation. The histopathology of subcutaneous abscesses obtained from WT mice clearly showed more condensed inflammation and consolidation of the infection than seen in the MyD88−/− and Card9−/− mice (Fig. 2E to G). After microscopic examination of an entire sectioned abscess of a representative WT mouse (Fig. 2E), very few spherules were detected. In contrast, clusters of parasitic cells were found in abscesses of both the MyD88−/− and Card9−/− mice, and the density of infection was clearly greatest in the abscesses of Card9−/− mice (c.f. inserts in Fig. 2F and G). When viewed at higher magnification, we observed that there were more GMS-stained spherules in the sectioned abscesses of the two strains of knockout mice than in the WT mice (yellow arrows in Fig. 2H and I). The abscesses of infected WT mice showed abundant fungal debris (white arrows in Fig. 2H). Surprisingly, hyphal elements together with abundant spherules in various stages of maturation were detected in the abscesses of Card9−/− mice (insert in Fig. 2J), which is an atypical finding in Coccidioides-infected tissue. To verify that the hyphal fragments were produced by C. posadasii, DNA was isolated from these tissue sections and used as a template for PCR amplification of both the Coccidioides-specific antigen gene (CSA) and fungal rDNA with a pair of primers that could amplify 18S rDNA for all filamentous fungi, as previously reported (32). Nucleotide sequence analysis of the CSA and rDNA amplicons confirmed that only C. posadasii was detectable in the infected tissue obtained from Card9−/− mice (data not shown).
FIG 2.
Comparative histopathology of subcutaneous abscesses of WT, MyD88−/−, and Card9−/− mice reveals greater consolidation of inflammatory tissue and antifungal clearance in WT mice. All three strains of mice were challenged with 5 ×104 Coccidioides spores by the subcutaneous route. Gross anatomy (A to C) and weights of dissected abscess plus dLN (Absc + dLN) samples are shown (D). Histopathology (E to G) of subcutaneous abscesses of the MyD88−/− and Card9−/− mice showed striking elevated necrosis at the centers (pink areas) compared to WT mice. White and green arrows in panels A to C indicate abscesses and infected inguinal lymph nodes, respectively. Tissue sections of the subcutaneous abscesses were stained with H&E stain (10×) (E to G) or Gomori methenamine sliver stain (40×) (H to J). The corresponding areas enclosed by boxes in panel E to G are shown at higher magnification in panels H to J. White, yellow, and black arrows in panels H to J indicate amorphous fungal debris, spherules at different development stages, and hyphal fragments, respectively. Intact spherules were not detected in the centers of abscess isolated from WT mice, but spherules were present in a satellite granuloma, indicated with a box in panel E. Inserts in panels F, G, and J represent enlarged images of spherules and hyphal fragments, respectively. Bars in panel E to G and H to J represent 1 mm and 100 μm, respectively.
MyD88−/− and Card9−/− mice had reduced percentages and numbers of T cells, B cells, and neutrophils compared to WT mice, but neutrophils were dispensable for resistance.
We employed flow cytometry to compare leukocyte subpopulations of CD4+ and CD8+ T cells, B cells, natural killer cells (NK), neutrophils (polymorphonuclear leukocytes [PMN]), MΦ, eosinophils (EOS), and dendritic cells (DCs) in the gated live CD45+ populations which had infiltrated Coccidioides-infected hypodermis samples of WT, MyD88−/−, and Card9−/− mice at 16 dpc. The numbers of CD4+ T cells, CD8+ T cells, B cells, and neutrophils were normalized to the weight of tissue and were consistently higher in WT mice than in MyD88−/− and Card9−/− mice (Fig. 3A).
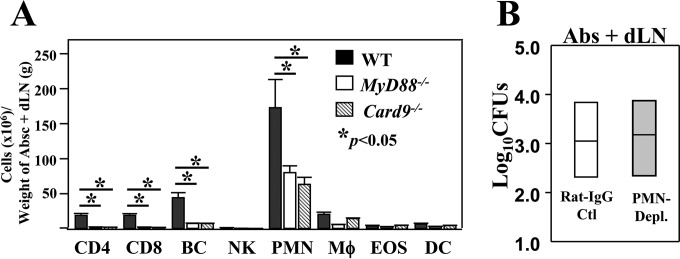
FIG 3.
Neutrophils, B cells, and T cells were significantly reduced in MyD88−/− and Card9−/− mice compared to numbers in WT mice, but neutrophils were not essential for resistance to subcutaneous Coccidioides infection. (A) Cell numbers of the infiltrated leukocyte subpopulations were identified by cytometric analysis of total live leukocytes isolated from the subcutaneous abscesses of groups of infected WT, MyD88−/−, and Card9−/− mice (n = 4) at 16 dpc. Asterisks indicate statistically significant differences between cell numbers of CD4+ and CD8+ T cells, B cells (BC), and neutrophils (PMNs) of MyD88−/− and Card9−/− mice versus numbers in WT mice. The bars represent the means ± SEM. The results shown are representative of two independent experiments. (B) A dose of 200 μg of IA8 MAb was injected i.p. at 24 h prior to challenge and at 2, 5, 8, 11, and 14 dpc to deplete neutrophils. Greater than 90% of neutrophils were depleted, as measured by flow cytometry at 16 dpc. The CFU of C. posadasii detected in dilution plate cultures of abscess (Absc) plus dLN homogenates of mice at 16 dpc are shown. CFU are reported in the box plots for 5 mice per group.
We next asked whether neutrophils, the major infiltrating cells in the hypodermis, play a role in resistance to subcutaneous Coccidioides infection. C57BL/6 mice were treated with anti-Ly6G monoclonal antibody (clone IA8) during the first 16 dpc. Mice intraperitoneally injected with rat IgG served as controls. We confirmed efficient depletion (90% ± 4%) of Ly6G+ neutrophils in the Coccidioides-infected hypodermis. Mice were subjected to fungal burden assays at this time point. CFU in abscess plus dLN samples of the neutrophil-depleted mice were comparable to those of control mice (3.0 ± 0.8 log10 versus 3.1 ± 0.9 log10) (Fig. 3B). There were no detectable CFU in lungs or spleen of either group of mice. Neutrophils appeared to be dispensable in this murine model of subcutaneous Coccidioides infection.
MyD88−/− and Card9−/− mice had reduced percentages and numbers of IFN-γ+ and IL-17A+ cells in Coccidioides-infected subcutaneous tissue compared to WT mice.
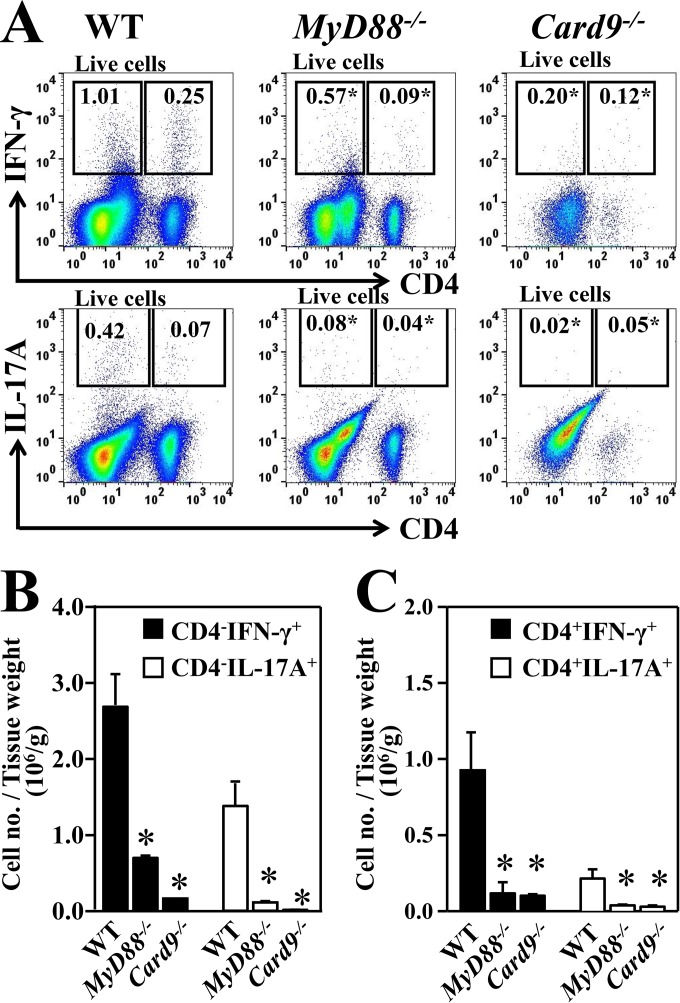
We next determined the percentages and cell numbers of IFN-γ- and IL-17-producing CD4+ T cells and CD4− cells in the gated total CD45+ leukocyte population isolated from abscesses plus dLN of MyD88−/− and Card9−/− mice at 16 dpc compared to data for subcutaneously infected WT mice (Fig. 4A to C). Both the percentages and cell numbers of IFN-γ- and IL-17-producing CD4+ T cells and CD4− leukocytes were significantly lower in MyD88−/− and Card9−/− mice than in WT mice. We confirmed that over 95% of the IFN-γ- and IL-17-producing CD4+ cells were also CD3+ and CD11b−, indicating that they were T helper cells. These data suggest that MyD88 and Card9 are required for development of both IFN-γ- and IL-17-mediated innate and adaptive immunity against subcutaneous Coccidioides infection.
FIG 4.
Reduced acquisition of IFN-γ- and IL-17A-producing CD4− and CD4+ T cells in Coccidioides-infected subcutaneous tissues of MyD88−/− and Card9−/− mice compared to tissues of WT mice. Leukocytes were isolated from the subcutaneous abscesses and adjacent inguinal lymph nodes of groups of WT, MyD88−/−, and Card9−/− mice (n = 4) that were subcutaneously challenged at 16 dpc. (A) Representative flow cytometric dot plots of IFN-γ- and IL-17-producing CD4− leukocytes and CD4+ T cells within the gated CD45+ subpopulation are shown. Numbers inside the gates indicate the mean percentages of the gated subsets. (B and C) Bar plots of the cell number per tissue weight of IFN-γ+ (closed bars) and IL-17A+ (open bars) CD4− leukocytes (B) and CD4+ T cells (C) in the abscesses and lymph nodes of these three strains of mice. The bars represent the means ± SEM. Asterisks indicate a statistically significant difference in cell numbers for the infected MyD88−/− and Card9−/− mice compared to the WT mice (Mann-Whitney U test, P < 0.05).
The IFN-γ receptor was essential for resistance to subcutaneous Coccidioides infection.
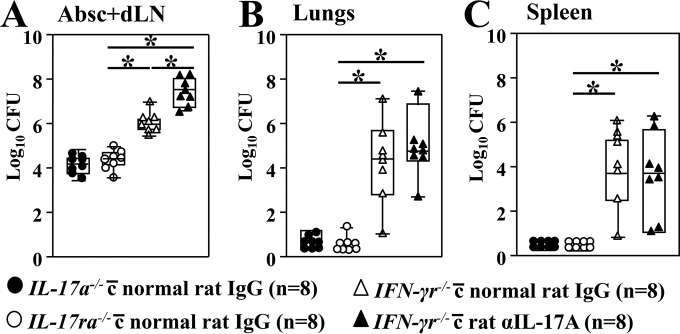
We further determined whether IFN-γ- and IL-17A-mediated immunity play essential roles. A group of IFN-γr−/− mice (n = 8) was treated with anti-IL-17A MAb to neutralize IL-17A during the first 16 days after subcutaneous infection with Coccidioides. Age- and gender-matched IFN-γr−/−, IL-17a−/−, and IL-17ra−/− mice were treated with normal rat IgG using the same regimen. The fungal burden in absess plus dLN samples, lungs, and spleen were determined at 16 dpc. Surprisingly, IL-17a−/− and IL-17ra−/− mice were as resistant to subcutaneous Coccidioides infection as the WT animals, based on comparable CFU in their infected abscess plus dLN homogenates (Fig. 5A; c.f. Fig. 1D). One IL-17ra−/− mouse had detectable CFU in the lungs, while no dissemination of Coccidioides to the spleen of infected IL-17a−/− and IL-17ra−/− mice was detected (Fig. 5B and C). On the contrary, IFN-γr−/− mice had significantly higher numbers of CFU in their abscess plus dLN samples than did IL-17a−/−, IL-17ra−/−, or WT mice and they developed disseminated coccidioidomycosis to the lungs and spleen (Fig. 5B and C) (Mann-Whitney U test, P < 0.05). These data suggest that the IFN-γ receptor is essential for resistance to subcutaneous Coccidioides infection. The IL-17A-depleted IFN-γr−/− mice showed significantly increased CFU in infected hypodermis tissue (abscess plus dLN) compared to the rat IgG-treated IFN-γr−/− mice. Moreover, the IL-17A-depleted mice showed a trend of elevated numbers of CFU in lungs compared to littermates; however, the increase was not statistically significant (Fig. 5B). Taken together, these data suggest that IL-17-mediated immunity might be required for protection against subcutaneous Coccidioides infection when IFN-γ-mediated signaling is absent.
FIG 5.
The IFN-γ receptor, but not the IL-17A or IL-17 receptor, is indispensable for resistance to subcutaneous coccidioidomycosis. A group of IFN-γr−/− mice was injected i.p. with 200 μg of anti-IL-17A MAb 24 h prior to subcutaneous challenge and at 2, 5, 8, 11, and 14 dpc. An additional three groups of IL-17a−/−, IL-17ra−/−, and IFN-γr−/− mice were injected with 200 μg rat IgG as controls. All mice were subcutaneously challenged with 5 ×104 viable Coccidioides spores. The CFU of C. posadasii were detected in dilution plate cultures of the subcutaneous tissue (A), lung (B), and spleen (C) homogenates of mice at 16 dpc. CFU are reported in box plots for 8 mice per group. Asterisks indicate a statistically significant difference between the compared mouse groups (Mann-Whitney U test, P < 0.05).
MyD88- and Card9-mediated NO production is required for resistance to subcutaneous Coccidioides infection.
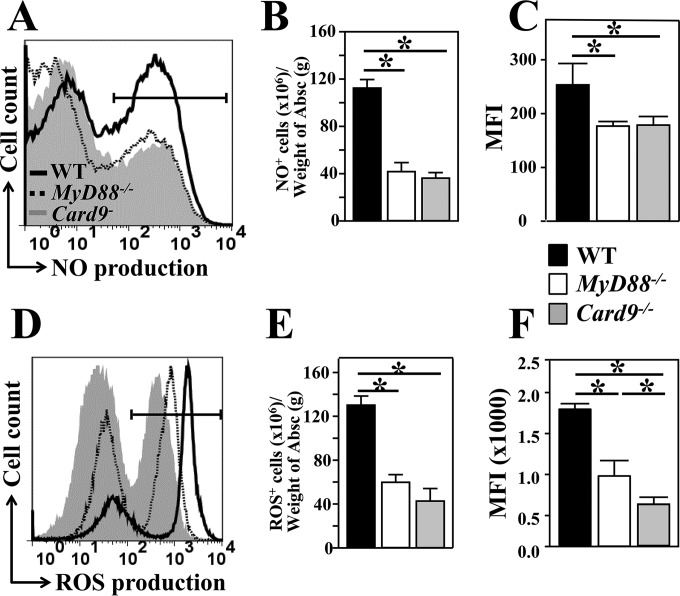
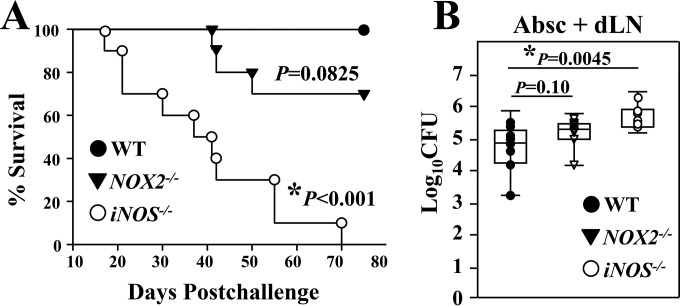
IFN-γ signaling has been shown to modulate NO and ROS production (21–24). These two phagosomal enzymes are indicators of the innate inflammatory response to microbial infection. We compared intracellular production of NO and ROS by CD11b+ cells that were recruited to the subcutaneous abscesses of WT, MyD88−/−, and Card9−/− mice. We measured the percentages, cell numbers, and MFI of labeled NO- and ROS-producing cells (Fig. 6A to C and D to F, respectively). Results of our combined assays revealed that both NO+ and ROS+ cells in the gated CD11b+ subpopulation were significantly reduced in MyD88−/− and Card9−/− mice compared to numbers in WT mice (Mann-Whitney U test, P < 0.01). Our data suggest a correlation between amounts of NO and ROS production by CD11b+ cells and the degree of fungal clearance in the subcutaneous abscesses and lymph nodes of WT, MyD88−/−, and Card9−/− mice (c.f. Fig. 1C and D). Next, we asked whether iNOS−/− and NOX2−/− mice, which are deficient in NO and ROS production, respectively, are more susceptible to subcutaneous infection with Coccidioides than are WT mice. Subcutaneously challenged iNOS−/− mice showed a significantly reduced percent survival (Kaplan-Meier survival analysis and chi-squared test, P < 0.001) (Fig. 7A) and increased CFU in abscess plus dLN samples compared to WT mice at 16 dpc (Fig. 7B). NOX2−/− mice showed trends of reduced survival and increased CFU in abscess plus dLN samples; however, the increase was not statistically significant. At that time point, no detectable CFU in lungs and spleen of these two knockout strains were observed. Our data indicate that MyD88- and Card9-mediated NO production is required for resistance to subcutaneous coccidioidomycosis.
FIG 6.
Both NO and ROS production correlated with reduction of fungal burden in abscess (Absc) plus dLN samples. Percentages, cell numbers, and geometric MFI of NO+ (A to C) and ROS+ (D to F) cells in the gated CD11b+ granulocytes in the subcutaneous abscesses of WT, MyD88−/−, and Card9−/− mice were measured by flow cytometry using NO and CM-H2DCFDA fluorescent probes, respectively. Abscess-derived leukocytes were isolated from the three strains of mice at 16 dpc. The fluorescent intensities of triazolo-fluorescein and CM-DCF were used as indicators of the capacity of NO and ROS production, respectively. Histogram plots of NO (A) and ROS (D) production of WT, MyD88−/−, and Card9−/− mice are shown. The mean ± SEM cell numbers per tissue weight (B and E) and the MFI (C and F) were graphed for each strain of mice. Asterisks indicate statistically significant differences between the compared groups of mice (Mann-Whitney U test, P < 0.05).
FIG 7.
The phagosomal enzyme iNOS was essential for resistance to subcutaneous Coccidioides infection. (A and B) Survival plots and CFU (B) in the dissected abscess (Absc) plus dLN samples are presented for WT mice and iNOS−/− and NOX2−/− mice, which are deficient in NO and ROS production, respectively. All mice (n = 10 per group) were challenged by the subcutaneous route with 5 × 104 viable spores isolated from the virulent C. posadasii C735 isolate. Asterisks represent statistically significant differences between iNOS−/− mice and WT mice (P < 0.05).
DISCUSSION
Immune responses to microbial challenge in various tissues are adapted to maintain organ physiology and host survival (33). The immune system can be divided into three main compartments: the systemic immune system, which surveils the blood and detects blood-borne pathogens; the mucosal immune system, which detects pathogens invading various mucosal surfaces; and the cutaneous immune system, which detects pathogens entering via the skin. Each of the three immune compartments faces distinct challenges in generating effective antimicrobial immunity while maintaining homeostasis and avoiding immunopathogenesis and tissue destruction. As a result, immunity has to operate in a tissue-specific manner (34). A comparison of host responses to subcutaneous and intranasal routes of challenge with Coccidioides spores in C57BL/6 mice underscores these principles. While early activation of Card9 is common to skin and lung infections, downstream differences in signaling pathways are apparent and result in distinct acquired immune responses to infection.
The route of challenge with Coccidioides is a pivotal variable for disease outcome in murine models of fungal infection (35). In most instances, the inoculum should be administered via the natural, physiologically relevant route of infection. Six types of inoculation routes have been explored to establish various forms of murine coccidioidomycosis that include intranasal instillation and intratracheal inoculation to induce pulmonary disease, intraperitoneal and intravenous infection imitating systemic disseminated disease, and intracranial or intrathecal infection resulting in meningeal disease. The dry, air-dispersed Coccidioides spores are routinely used for inoculation and they are highly virulent, with as few as 10 spores delivered to the lungs of susceptible C57BL/6 and BALB/c causing disseminated disease and lethality in 12 to 20 days (36). Injection of 30 to 100 spores by the intravenous, intraperitoneal, or intrathecal route is also lethal (37). However, none of the above inoculation methods could induce cutaneous or subcutaneous coccidioidomycosis, which commonly occurs in patients with chronic, disseminated coccidioidomycosis. In this context, we have established a murine model of subcutaneous Coccidioides infection that can be used to investigate host protective immunity and fungal pathogenesis in cutaneous coccidioidomycosis. The subcutaneous inoculation method delivers a consistent dose of spores and results in significant fungal burden in the hypodermis but survival of C57BL/6 mice for immunological assays. Similar to subcutaneous infection in humans, subcutaneous infection in immunocompetent (C57BL6 WT) mice results in chronic but self-limited disease, while we have shown in this study that mice lacking expression of MyD88, Card9, the IFN-γ receptor, or iNOS fail to control and prevent systemic dissemination of Coccidioides.
The human skin is the most common extrapulmonary site of endemic mycoses and is frequently the first clinical manifestation of disseminated coccidioidomycosis (6, 38). Although the skin is rarely the primary Coccidioides infection site, cutaneous infections have been reported among agricultural workers and laboratory personnel (12). In these cases, a nodule or ulcer occurs at the inoculation site, accompanied by lymphangitis and regional lymphadenopathy, but systemic symptoms are almost always absent in immunocompetent patients. Our murine data are in agreement with human coccidioidomycosis, in that dissemination of this pathogen from the skin is absent in immunocompetent wild-type C57BL/6 mice, while dissemination is observed first to adjacent inguinal dLN and then to lungs and spleen of the infected MyD88−/− and Card9−/− mice by the hematogenous and/or lymphatic routes.
A histopathological study of skin biopsy specimens with detectable fungal cells from 104 coccidioidomycosis patients revealed that inflammation of lesions could be classified as well-organized granulomas, including suppurative (34.7%), sarcoid-like (18.6%), and necrotizing (4.2%) forms, and poorly differentiated granulomas that mainly contained lymphocytes (lymphoplasmacytic, 26.3%) or neutrophils (neutrophilic, 13.7%) (39). Suppurative granulomas were characterized predominantly by infiltration of neutrophils (PMNs) and microabscess formation (39). We have also observed that PMNs are the main type of cell that infiltrates abscesses and dLN of wild-type and tested mutant strains of mice. However, PMNs are dispensable in resistance to subcutaneous Coccidioides infection. We have observed that PMN-depleted mice recruit higher percentages and numbers of Mac3+ macrophages, indicating they may compensate the loss of neutrophils during subcutaneous infection (data not shown).
The most insights into host defense against coccidioidomycosis have been obtained from a murine model of pulmonary coccidioidomycosis. However, immune defense mechanisms against Coccidioides infection in the extrapulmonary organs and tissues are poorly understood. MyD88 and Card9 are two cytosolic immune adaptors that transduce signals from IL-1 receptor/Toll-like receptors (TIR/TLRs) and C-type lectin receptors (CLRs), respectively, to bridge innate and adaptive immunity (20, 40–45). We recently reported that MyD88- and Card9-mediated signaling pathways contribute to the development of vaccine-induced Th17 and Th1 immunity against pulmonary Coccidioides infection (18, 19). MyD88 signaling leads to the activation of protein kinases and the expression of transcription factors that trigger the expression of cytokines and inflammatory factors required for T cell development and differentiation (46). MyD88 signaling is required for efficient innate and adaptive immune responses to dimorphic fungal infections in lungs (17, 41, 42). Our data have also revealed that costimulation of the MyD88- and Card9-mediated signaling pathways are required for a protective inflammatory response against Coccidioides infection in the hypodermis.
Card9 is the critical immune adaptor that transduces signals downstream of several immunoreceptor tyrosine-based activation motif (ITAM)-associated CLRs, including Dectin-1, -2, and -3 and Mincle. Following receptor engagement and spleen tyrosine kinase (Syk) phosphorylation, CARD9 forms a complex with B cell lymphoma protein 10 (Bcl10) and mucosa-associated lymphoid tissue protein 1 (Malt1), which transduces signaling to the NF-κB pathway. This central positioning of Card9 in multiple signaling pathways explains why lack of CARD9 results in severe susceptibility to both pulmonary and subcutaneous coccidioidomycosis. The mannose receptor and Mincle are dispensable for resistance to pulmonary coccidioidomycosis (18, 47). All three Dectin receptors contribute to recognition of spherules (18, 47, 48; unpublished data). However, these receptors may function either alone or in synergy in resistance to pulmonary and subcutaneous infections and their regulatory mechanisms await clarification (18, 43).
Patients with a mutation in the IL-12 or IFN-γ receptors are predisposed to disseminated coccidioidomycosis (49). Numbers of polyfunctional CD4+ T cells that produce IL-2, IFN-γ, and TNF-α in peripheral blood mononuclear cells obtained from naturally immune donors are significantly higher than numbers in nonimmune donors (50). The IL-12/IFN-γ and Th1 axis of immunity appears to be critical for control of disseminated coccidioidomycosis in humans (51). Moreover, patients with HIV-1 infection or neutropenia due to chemotherapy or preparation for solid organ transplantation are at high risk of coccidioidomycosis (52, 53). Many studies of pulmonary coccidioidomycosis have reported IFN-γ production as a correlate of vaccine-induced protection in mice (54, 55). Surprisingly, we have found that the murine IL-17A and Th17 cells are essential for conferring resistance to pulmonary Coccidioides infection, while the IFN-γ receptor and Th1 cells are dispensable (15, 17, 19). These results raise the possibility that there is a differential requirement of IFN-γ- and IL-17A-mediated immunity in defense against pulmonary and cutaneous coccidioidomycosis. Our data show that IFN-γ is required for prevention of systemic dissemination after a subcutaneous inoculation with Coccidioides spores, while IL-17 is essential when IFN-γ signaling is absent. Consistent with this dichotomy in immunity to fungal infections, IL-17A production and Th17 immunity are essential for host defense against chronic mucocutaneous candidiasis, while IFN-γ-mediated oxidative and nonoxidative phagocytic function are essential for control systemic candidiasis (56, 57).
ROS and NO derived from phagocytic NADPH oxidase (NOX2) and iNOS activities, respectively, have been reported to contribute to host defense against numerous microbial pathogens (58, 59). However, we have found that both NOX2−/− and iNOS2−/− mice have comparable fungal burdens and survival rates after an intranasal challenge with approximately 80 Coccidioides spores as WT mice (24, 25). On the contrary, iNOS−/− mice are significantly more susceptible to subcutaneous Coccidioides infection than WT mice. These results suggest that MyD88 and Card9 govern the IFN-γ-dependent innate immune responses, including NO production, to combat subcutaneous Coccidioides infection. These results have important implications in development of effective immunotherapies and a vaccine against diverse forms of coccidioidomycosis. While IFN-γ immunotherapy has been successfully applied to treat several cases of refractory, disseminated coccidioidomycosis (60), it may be more beneficial for patients with chronic pulmonary coccidioidomycosis to receive IL-17A cytokine therapy.
ACKNOWLEDGMENTS
This work was supported by research grants from the National Institutes of Health, NIAID R01 AI-071118 (awarded to G.T.C.) and NIAID R21 AI114762 (awarded to C.-Y.H.). Additional support was provided by the Margaret Batts Tobin Foundation of San Antonio.
REFERENCES
- 1.Brown J, Benedict K, Park BJ, Thompson GR III. 2013. Coccidioidomycosis: epidemiology. Clin Epidemiol 5:185–197. doi: 10.2147/CLEP.S34434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Litvintseva AP, Marsden-Haug N, Hurst S, Hill H, Gade L, Driebe EM, Ralston C, Roe C, Barker BM, Goldoft M, Keim P, Wohrle R, Thompson GR III, Engelthaler DM, Brandt ME, Chiller T. 2015. Valley fever: finding new places for an old disease. Coccidioides immitis found in Washington State soil associated with recent human infection. Clin Infect Dis 60:e1–e3. doi: 10.1093/cid/ciu681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cole G, Xue J, Borra P, KS, Borra R, Tarcha E, Schaller R, Yu J-J, Hung C-Y. 2006. Virulence mechanisms of Coccidioides, p 363–391. In Heitman J, Filler S, Edwards J, Mitchell A (ed), Molecular principles of fungal pathogenesis. ASM Press, Washington, DC. [Google Scholar]
- 4.Cole GT, Xue JM, Okeke CN, Tarcha EJ, Basrur V, Schaller RA, Herr RA, Yu JJ, Hung CY. 2004. A vaccine against coccidioidomycosis is justified and attainable. Med Mycol 42:189–216. doi: 10.1080/13693780410001687349. [DOI] [PubMed] [Google Scholar]
- 5.Kwon-Chung KJ, Bennett JE. 1992. Coccidioidomycosis, p 356–396. In Cann C. (ed), Medical mycology. Lea & Febiger, Philadelphia, PA. [Google Scholar]
- 6.Smith JA, Riddell JT, Kauffman CA. 2013. Cutaneous manifestations of endemic mycoses. Curr Infect Dis Rep 15:440–449. doi: 10.1007/s11908-013-0352-2. [DOI] [PubMed] [Google Scholar]
- 7.Forbus WD, Bestebreurtje AM. 1946. Coccidioidomycosis; a study of 95 cases of the disseminated type with special reference to the pathogenesis of the disease. Mil Surg 99:653–719. [PubMed] [Google Scholar]
- 8.Arsura EL, Kilgore WB, Caldwell JW, Freeman JC, Einstein HE, Johnson RH. 1998. Association between facial cutaneous coccidioidomycosis and meningitis. West J Med 169:13–16. [PMC free article] [PubMed] [Google Scholar]
- 9.Chiller TM, Galgiani JN, Stevens DA. 2003. Coccidioidomycosis. Infect Dis Clin North Am 17:41–57. doi: 10.1016/S0891-5520(02)00040-5. [DOI] [PubMed] [Google Scholar]
- 10.Levan NE, Huntington RW Jr. 1965. Primary cutaneous coccidioidomycosis in agricultural workers. Arch Dermatol 92:215–220. [DOI] [PubMed] [Google Scholar]
- 11.Winn WA. 1965. Primary cutaneous coccidioidomycosis. Reevaluation of its potentiality based on study of three new cases. Arch Dermatol 92:221–228. [DOI] [PubMed] [Google Scholar]
- 12.Gildardo JM, Leobardo VA, Nora MO, Jorge OC. 2006. Primary cutaneous coccidiodomycosis: case report and review of the literature. Int J Dermatol 45:121–123. doi: 10.1111/j.1365-4632.2004.02446.x. [DOI] [PubMed] [Google Scholar]
- 13.Ondo AL, Zlotoff BJ, Mings SM, Rochester LC, Shanler SD. 2010. Primary cutaneous coccidioidomycosis: an incidental finding. Clin Exp Dermatol 35:e42–e43. doi: 10.1111/j.1365-2230.2009.03552.x. [DOI] [PubMed] [Google Scholar]
- 14.Wilson JW, Smith CE, Plunkett OA. 1953. Primary cutaneous coccidioidomycosis; the criteria for diagnosis and a report of a case. Calif Med 79:233–239. [PMC free article] [PubMed] [Google Scholar]
- 15.Hung CY, Gonzalez A, Wuthrich M, Klein BS, Cole GT. 2011. Vaccine immunity to coccidioidomycosis occurs by early activation of three signal pathways of T helper cell response (Th1, Th2, and Th17). Infect Immun 79:4511–4522. doi: 10.1128/IAI.05726-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hung CY, Castro-Lopez N, Cole GT. 2014. Vaccinated C57BL/6 mice develop protective and memory T cell responses to Coccidioides posadasii infection in the absence of interleukin-10. Infect Immun 82:903–913. doi: 10.1128/IAI.01148-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wüthrich M, Gern B, Hung C-Y, Ersland K, Rocco N, Pick-Jacobs J, Galles K, Filutowicz H, Warner T, Evans M, Cole GT, Klein B. 2011. Vaccine-induced protection against 3 systemic mycoses endemic to North America requires Th17 cells in mice. J Clin Invest 121:554–568. doi: 10.1172/JCI43984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang H, LeBert V, Hung CY, Galles K, Saijo S, Lin X, Cole GT, Klein BS, Wuthrich M. 2014. C-type lectin receptors differentially induce Th17 cells and vaccine immunity to the endemic mycosis of North America. J Immunol 192:1107–1119. doi: 10.4049/jimmunol.1302314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hung CY, Jimenez-Alzate Mdel P, Gonzalez A, Wuthrich M, Klein BS, Cole GT. 2014. Interleukin-1 receptor but not Toll-like receptor 2 is essential for MyD88-dependent Th17 immunity to Coccidioides infection. Infect Immun 82:2106–2114. doi: 10.1128/IAI.01579-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu W, Hsu YM, Bi L, Songyang Z, Lin X. 2009. CARD9 facilitates microbe-elicited production of reactive oxygen species by regulating the LyGDI-Rac1 complex. Nat Immunol 10:1208–1214. doi: 10.1038/ni.1788. [DOI] [PubMed] [Google Scholar]
- 21.Drummond RA, Brown GD. 2011. The role of Dectin-1 in the host defence against fungal infections. Curr Opin Microbiol 14:392–399. doi: 10.1016/j.mib.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 22.Ryan KA, Smith MF Jr, Sanders MK, Ernst PB. 2004. Reactive oxygen and nitrogen species differentially regulate Toll-like receptor 4-mediated activation of NF-kappa B and interleukin-8 expression. Infect Immun 72:2123–2130. doi: 10.1128/IAI.72.4.2123-2130.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ichikawa S, Miyake M, Fujii R, Konishi Y. 2012. MyD88 associated ROS generation is crucial for Lactobacillus induced IL-12 production in macrophage. PLoS One 7:e35880. doi: 10.1371/journal.pone.0035880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gonzalez A, Hung C-Y, Cole GT. 2011. Nitric oxide synthase activity has limited influence on the control of Coccidioides infection in mice. Microb Pathog 51:161–168. doi: 10.1016/j.micpath.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gonzalez A, Hung CY, Cole GT. 2011. Absence of phagocyte NADPH oxidase 2 leads to severe inflammatory response in lungs of mice infected with Coccidioides. Microb Pathog 51:432–441. doi: 10.1016/j.micpath.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Margolis DA, Viriyakosol S, Fierer J, Kirkland TN. 2011. The role of reactive oxygen intermediates in experimental coccidioidomycois in mice. BMC Microbiol 11:71. doi: 10.1186/1471-2180-11-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hsu YM, Zhang Y, You Y, Wang D, Li H, Duramad O, Qin XF, Dong C, Lin X. 2007. The adaptor protein CARD9 is required for innate immune responses to intracellular pathogens. Nat Immunol 8:198–205. doi: 10.1038/ni1426. [DOI] [PubMed] [Google Scholar]
- 28.Ye P, Rodriguez FH, Kanaly S, Stocking KL, Schurr J, Schwarzenberger P, Oliver P, Huang W, Zhang P, Zhang J, Shellito JE, Bagby GJ, Nelson S, Charrier K, Peschon JJ, Kolls JK. 2001. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med 194:519–527. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakae S, Komiyama Y, Nambu A, Sudo K, Iwase M, Homma I, Sekikawa K, Asano M, Iwakura Y. 2002. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity 17:375–387. doi: 10.1016/S1074-7613(02)00391-6. [DOI] [PubMed] [Google Scholar]
- 30.Xue J, Chen X, Selby D, Hung CY, Yu JJ, Cole GT. 2009. A genetically engineered live attenuated vaccine of Coccidioides posadasii protects BALB/c mice against coccidioidomycosis. Infect Immun 77:3196–3208. doi: 10.1128/IAI.00459-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hung CY, Hurtgen BJ, Bellecourt M, Sanderson SD, Morgan EL, Cole GT. 2012. An agonist of human complement fragment C5a enhances vaccine immunity against Coccidioides infection. Vaccine 30:4681–4690. doi: 10.1016/j.vaccine.2012.04.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pan S, Cole GT. 1995. Molecular and biochemical characterization of a Coccidioides immitis-specific antigen. Infect Immun 63:3994–4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hohl TM. 2014. Overview of vertebrate animal models of fungal infection. J Immunol Methods 410:100–112. doi: 10.1016/j.jim.2014.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hu W, Pasare C. 2013. Location, location, location: tissue-specific regulation of immune responses. J Leukoc Biol 94:409–421. doi: 10.1189/jlb.0413207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hohl TM. 2014. Overview of vertebrate animal models of fungal infection. J Immunol Methods 40:100–112. doi: 10.1016/j.jim.2014.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muhammed M, Feldmesser M, Shubitz LF, Lionakis MS, Sil A, Wang Y, Glavis-Bloom J, Lewis RE, Galgiani JN, Casadevall A, Kontoyiannis DP, Mylonakis E. 2012. Mouse models for the study of fungal pneumonia: a collection of detailed experimental protocols for the study of Coccidioides, Cryptococcus, Fusarium, Histoplasma and combined infection due to Aspergillus-Rhizopus. Virulence 3:329–338. doi: 10.4161/viru.20142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Clemons KV, Capilla J, Stevens DA. 2007. Experimental animal models of coccidioidomycosis. Ann N Y Acad Sci 1111:208–224. doi: 10.1196/annals.1406.029. [DOI] [PubMed] [Google Scholar]
- 38.Blair JE. 2007. State-of-the-art treatment of coccidioidomycosis: skin and soft-tissue infections. Ann N Y Acad Sci 1111:411–421. doi: 10.1196/annals.1406.010. [DOI] [PubMed] [Google Scholar]
- 39.Carpenter JB, Feldman JS, Leyva WH, DiCaudo DJ. 2010. Clinical and pathologic characteristics of disseminated cutaneous coccidioidomycosis. J Am Acad Dermatol 62:831–837. doi: 10.1016/j.jaad.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 40.Adachi O, Kawai T, Takeda K, Matsumoto M, Tsutsui H, Sakagami M, Nakanishi K, Akira S. 1998. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity 9:143–150. doi: 10.1016/S1074-7613(00)80596-8. [DOI] [PubMed] [Google Scholar]
- 41.Loures FV, Pina A, Felonato M, Feriotti C, de Araujo EF, Calich VL. 2011. MyD88 signaling is required for efficient innate and adaptive immune responses to Paracoccidioides brasiliensis infection. Infect Immun 79:2470–2480. doi: 10.1128/IAI.00375-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Viriyakosol S, Fierer J, Brown GD, Kirkland TN. 2005. Innate immunity to the pathogenic fungus Coccidioides posadasii is dependent on Toll-like receptor 2 and Dectin-1. Infect Immun 73:1553–1560. doi: 10.1128/IAI.73.3.1553-1560.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Drummond RA, Saijo S, Iwakura Y, Brown GD. 2011. The role of Syk/CARD9 coupled C-type lectins in antifungal immunity. Eur J Immunol 41:276–281. doi: 10.1002/eji.201041252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Robinson MJ, Osorio F, Rosas M, Freitas RP, Schweighoffer E, Gross O, Verbeek JS, Ruland J, Tybulewicz V, Brown GD, Moita LF, Taylor PR, Reis e Sousa C. 2009. Dectin-2 is a Syk-coupled pattern recognition receptor crucial for Th17 responses to fungal infection. J Exp Med 206:2037–2051. doi: 10.1084/jem.20082818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saijo S, Iwakura Y. 2011. Dectin-1 and Dectin-2 in innate immunity against fungi. Int Immunol 23:467–472. doi: 10.1093/intimm/dxr046. [DOI] [PubMed] [Google Scholar]
- 46.O'Neill LA, Bowie AG. 2007. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol 7:353–364. doi: 10.1038/nri2079. [DOI] [PubMed] [Google Scholar]
- 47.Viriyakosol S, Jimenez Mdel P, Saijo S, Fierer J. 2014. Neither dectin-2 nor the mannose receptor is required for resistance to Coccidioides immitis in mice. Infect Immun 82:1147–1156. doi: 10.1128/IAI.01355-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Viriyakosol S, Jimenez MP, Gurney MA, Ashbaugh ME, Fierer J. 2013. Dectin-1 is required for resistance to coccidioidomycosis in mice. mBio 4:e00597-12. doi: 10.1128/mBio.00597-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vinh DC, Masannat F, Dzioba RB, Galgiani JN, Holland SM. 2009. Refractory disseminated coccidioidomycosis and mycobacteriosis in interferon-gamma receptor 1 deficiency. Clin Infect Dis 49:e62–e65. doi: 10.1086/605532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nesbit L, Johnson SM, Pappagianis D, Ampel NM. 2010. Polyfunctional T lymphocytes are in the peripheral blood of donors naturally immune to coccidioidomycosis and are not induced by dendritic cells. Infect Immun 78:309–315. doi: 10.1128/IAI.00953-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vinh DC, Schwartz B, Hsu AP, Miranda DJ, Valdez PA, Fink D, Lau KP, Long-Priel D, Kuhns DB, Uzel G, Pittaluga S, Hoover S, Galgiani JN, Holland SM. 2011. Interleukin-12 receptor β1 deficiency predisposing to disseminated coccidioidomycosis. Clin Infect Dis 52:e99–e102. doi: 10.1093/cid/ciq215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ampel NM. 2007. Coccidioidomycosis in persons infected with HIV-1. Ann N Y Acad Sci 1111:336–342. doi: 10.1196/annals.1406.033. [DOI] [PubMed] [Google Scholar]
- 53.Vikram HR, Dosanjh A, Blair JE. 2011. Coccidioidomycosis and lung transplantation. Transplantation 92:717–721. doi: 10.1097/TP.0b013e31822e6e9a. [DOI] [PubMed] [Google Scholar]
- 54.Xue J, Hung CY, Yu JJ, Cole GT. 2005. Immune response of vaccinated and non-vaccinated mice to Coccidioides posadasii infection. Vaccine 23:3535–3544. doi: 10.1016/j.vaccine.2005.01.147. [DOI] [PubMed] [Google Scholar]
- 55.Cox RA, Magee DM. 2004. Coccidioidomycosis: host response and vaccine development. Clin Microbiol Rev 17:804–839. doi: 10.1128/CMR.17.4.804-839.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lionakis MS, Holland SM. 2013. CARD9: at the intersection of mucosal and systemic antifungal immunity. Blood 121:2377–2378. doi: 10.1182/blood-2013-01-480434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Filler SG. 2012. Insights from human studies into the host defense against candidiasis. Cytokine 58:129–132. doi: 10.1016/j.cyto.2011.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gao XP, Standiford TJ, Rahman A, Newstead M, Holland SM, Dinauer MC, Liu QH, Malik AB. 2002. Role of NADPH oxidase in the mechanism of lung neutrophil sequestration and microvessel injury induced by Gram-negative sepsis: studies in p47phox-/- and gp91phox-/- mice. J Immunol 168:3974–3982. doi: 10.4049/jimmunol.168.8.3974. [DOI] [PubMed] [Google Scholar]
- 59.Nathan C, Shiloh MU. 2000. Reactive oxygen and nitrogen intermediates in the relationship between mammalian hosts and microbial pathogens. Proc Natl Acad Sci U S A 97:8841–8848. doi: 10.1073/pnas.97.16.8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Duplessis CA, Tilley D, Bavaro M, Hale B, Holland SM. 2011. Two cases illustrating successful adjunctive interferon-gamma immunotherapy in refractory disseminated coccidioidomycosis. J Infect 63:223–228. doi: 10.1016/j.jinf.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]