Abstract
The SlyA transcriptional regulator has important roles in the virulence and pathogenesis of several members of the Enterobacteriaceae family, including Salmonella enterica serovar Typhimurium and Escherichia coli. Despite the identification of the slyA gene in Shigella flexneri nearly 2 decades ago, as well as the significant conservation of SlyA among enteric bacteria, the role of SlyA in Shigella remains unknown. The genes regulated by SlyA in closely related organisms often are absent from or mutated in S. flexneri, and consequently many described SlyA-dependent phenotypes are not present. By characterizing the expression of slyA and determining its ultimate effect in this highly virulent organism, we postulated that novel SlyA-regulated virulence phenotypes would be identified. In this study, we report the first analysis of SlyA in Shigella and show that (i) the slyA gene is transcribed and ultimately translated into protein, (ii) slyA promoter activity is maximal during stationary phase and is negatively autoregulated and positively regulated by the PhoP response regulator, (iii) the exogenous expression of slyA rescues transcription and virulence-associated deficiencies during virulence-repressed conditions, and (iv) the absence of slyA significantly decreases acid resistance, demonstrating a novel and important role in Shigella virulence. Cumulatively, our study illustrates unexpected parallels between the less conserved S. flexneri and S. Typhimurium slyA promoters as well as a unique role for SlyA in Shigella virulence that has not been described previously in any closely related organism.
INTRODUCTION
The MarR/SlyA family of transcription factors controls an array of biological functions critical to bacterial physiology and survival (1–5). In the Enterobacteriaceae family, the DNA binding protein SlyA regulates diverse aspects of virulence (reviewed in reference 6). SlyA originally was identified in Salmonella enterica serovar Typhimurium for its ability to induce hemolytic and cytotoxic phenotypes when overexpressed in Escherichia coli (7, 8). Since then, in S. Typhimurium, SlyA has been implicated in facilitating intracellular survival within professional macrophages (7, 9), contributing to cell envelope modification (10), and conferring resistance to antimicrobial peptides and oxidative stress (11, 12). Meanwhile, in E. coli, SlyA contributes to virulence differently by inducing a hemolytic phenotype (13), facilitating the synthesis of a virulence antigen (14, 15), and contributing to type 1 fimbriation (16). The role of SlyA in the human pathogen Shigella, however, has not been described despite the identification of a slyA gene (8) and the high amino acid identity that SlyA shares with SlyA proteins found in closely related organisms (Table 1).
TABLE 1.
S. flexneri SlyA conservation relative to members of the Enterobacteriaceae family
| Organism | Rep straina | Locus name | DNA accession no.b | DNA identity (%) | DNA length (bp) | Protein accession no.b | Amino acid identity (%) | Amino acid length (aa) |
|---|---|---|---|---|---|---|---|---|
| Escherichia coli | K-12 substr. MG1665 | b1642 | U00096.3 | 99 | 435 | NP_416159.2 | 100 | 144 |
| Escherichia coli (EIEC)c | 53638 | NDd | ND | ND | ND | EDU63848 | 100 | 144 |
| Escherichia coli (EHEC)e | O157:H7 Sakai chromosome | ECs2351 | BA000007.2 | 99 | 435 | NP_310378.2 | 100 | 144 |
| Shigella boydii | Sb227 | SBO _1492 | CP000036.1 | 100 | 435 | YP_407937.2 | 100 | 144 |
| Shigella dysenteriae | Sd197 | SDY _1865 | CP000034.1 | 99 | 435 | YP_403465.2 | 100 | 144 |
| Shigella flexneri | 2457T | S1801 | AE014073.1 | 100 | 435 | NP_837330.2 | 100 | 144 |
| Shigella sonnei | Ss046 | SSON _1514 | CP000038.1 | 100 | 435 | YP_310449.2 | 100 | 144 |
| Salmonella Typhimurium | 14028s | STM14 _1742 | CP001363.1 | 82 | 435 | YP_005237314.1 | 95 | 144 |
| Salmonella Typhi | Ty2 | t1312 | AE014613.1 | 81 | 435 | NP_805113.1 | 90 | 144 |
| Klebsiella pneumoniae | NTUH- K2044 | KP1 _3054 | AP006725.1 | 75 | 441 | YP_002919758.1 | 87 | 146 |
| Yersinia pestis | KIM10 | y1961 | AE009952.1 | 77 | 432 | NP_669276.1 | 77 | 143 |
| Yersinia pseudotuberculosis | YPIII | BZ22 _2437 | CP009792 | 77 | 432 | ACA68167 | 77 | 143 |
| Dickeya dadantii | 3937 | Dda3937 _00475 | CP002038.1 | 72 | 438 | ADM98879 | 75 | 145 |
Representative strain.
Accession numbers are from the National Center for Biotechnology Information (NCBI).
Enteroinvasive Escherichia coli.
Not determined. Sequence unavailable.
Enterohemorrhagic Escherichia coli.
Shigella flexneri is closely related to both Salmonella spp. and E. coli. It carries a large (∼220-kb) virulence plasmid responsible for the invasive and virulent nature of this organism. Encoded on the large virulence plasmid is VirB, a transcriptional regulator essential to Shigella virulence. VirB functions to counteract the repression of virulence gene promoters mediated by H-NS (17–19), a histone-like nucleoid structuring protein that prevents the inappropriate expression of horizontally acquired genes (reviewed in reference 20). As a derepressor, VirB binds to promoters to facilitate the rearrangement of the H-NS–DNA nucleoprotein complex to ultimately upregulate transcription. This regulatory activity is a common feature of an emerging group of proteins that do not behave as traditional transcriptional activators; instead, they function solely to alleviate H-NS-mediated repression by remodeling the nucleoprotein complex (20). SlyA is included in this group of proteins, because for most SlyA-regulated promoters characterized so far, SlyA binds to target promoters to counteract repression mediated by H-NS to facilitate the transcription of virulence genes (11, 16, 21–25).
Included in the SlyA regulon is slyA itself, the promoter of which is positively autoregulated in E. coli (14) and negatively autoregulated in S. Typhimurium (26). This promoter also has been shown to be positively regulated by the PhoP response regulator of the PhoP/PhoQ two-component system in S. Typhimurium (10, 11). Meanwhile, H-NS negatively regulates the orthologous rovA promoter in Yersinia pseudotuberculosis (27) but has no effect on the slyA promoter in E. coli (14). The intricate regulation of slyA and its downstream targets is critical to many virulence features identified thus far in E. coli and S. Typhimurium; however, the regulation of slyA in Shigella and its role in virulence remain undetermined.
Here, we provide the first characterization of SlyA in Shigella. We describe three major aspects of slyA: the regulation and activity of the slyA promoter, the effect of exogenous slyA expression on Shigella virulence phenotypes and gene regulation, and a novel SlyA-dependent role in acid resistance. Overall, our study characterizes an important transcriptional regulator in S. flexneri and uncovers a novel role for SlyA in acid resistance, a virulence feature that is essential for the successful pathogenesis of the bacterium.
MATERIALS AND METHODS
Bacterial growth.
Bacterial strains and plasmids used in this study are listed in Table 2. E. coli and S. flexneri strains were grown routinely in Trypticase soy broth (TSB) or Luria broth (LB) overnight at 30°C and then subcultured and grown at 37°C (unless otherwise noted) with aeration at 325 rpm. When necessary, antibiotics were added at the following final concentrations: ampicillin, 100 μg/ml; chloramphenicol, 25 μg/ml; kanamycin, 25 or 50 μg/ml; tetracycline, 12.5 μg/ml; and spectinomycin, 100 μg/ml. To ensure that S. flexneri strains maintained the virulence plasmid, Congo red binding was tested routinely on TSB agar (TSA) plates (1.5% agar [wt/vol]) containing 0.01% (wt/vol) Congo red (referred to as Congo red plates).
TABLE 2.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant description | Source or reference |
|---|---|---|
| Strains | ||
| E. coli | ||
| K-12 (MC4100) | 62 | |
| JW5267 | (BW25113) ΔslyA::Kn | 28 |
| S. flexneri | ||
| 2457T | S. flexneri serotype 2a | 63 |
| AWY3 | (2457T) virB::Tn5 | 36 |
| BS103 | 2457T cured of the virulence plasmid | 49 |
| BS184 | (2457T) mxiC-lacZ | 31 |
| NWG1 | (2457T) ΔslyA::Kn | This study |
| NWG11 | (2457T) ΔslyA::lacZ | This study |
| NWG13 | (2457T) ΔphoP::Kns | This study |
| NWG14 | (2457T) ΔslyA::lacZ hns::Tn10 | This study |
| NWG15 | (2457T) ΔslyA::lacZ ΔphoP::Kns | This study |
| Plasmids | ||
| pBAD18 | Arabinose inducible, pBRori; Ampr | 64 |
| pATM324 | pBAD18-virB; Ampr | 65 |
| pNWG03 | pBAD18-his-slyA; Ampr | This study |
| pAFW04 | pACYC184-PicsP-lacZ; Cmr | 19 |
| pSRG58 | pACYC184-PphoP-phoPQ; Spcr | 33 |
| pNWG07 | pACYC184; Spcr | This study |
| pBR322 | pMB1ori; Ampr | 66 |
| pNWG13 | pBR322-PslyA-slyA; Ampr | This study |
Strain construction.
NWG1 (ΔslyA::Kn) was constructed via P1-mediated transduction of the E. coli ΔslyA::Kn locus (which contains a deletion of the slyA gene between the start and stop codons generated using lambda red recombination; JW5267 from the Keio collection [28]) into 2457T. Transductants were selected on 100 μg/ml kanamycin. The slyA deletion in 2457T was confirmed via PCR with primers W424/W425 (Table 3). NWG11 (ΔslyA::lacZ) was constructed using FLP-mediated site-specific recombination and with the subsequent integration of pKG136 into the FLP recombination target site generated in strain NWG1, as previously described (29). The inserted lacZ and its endogenous start and stop codons create a transcriptional fusion directly controlled by the slyA promoter. NWG13 (ΔphoP::Kns) was constructed by using the FLP recombinase (30) to remove the kanamycin cassette from the S. flexneri 2457T ΔphoP::Kn mutant, which was constructed via P1-mediated transduction of the E. coli ΔphoP::Kn locus (which contains a deletion of the phoP gene between the start and stop codons generated using lambda red recombination; JW1116 from the Keio collection [28]) into 2457T. NWG14 (ΔslyA::lacZ hns::Tn10) was constructed via P1-mediated transduction of the hns::Tn10 locus of BS185 (31) into NWG11. Transductants were selected on 12.5 μg/ml tetracycline. NWG15 (ΔslyA::lacZ phoP::Kns) was constructed via P1-mediated transduction of ΔslyA::lacZ (NWG11) into NWG13. Transductants were selected on 100 μg/ml kanamycin.
TABLE 3.
Primers used in this study
| Primer name | Sequence 5′→3′ |
|---|---|
| W69 | TGAGACGTCGACGGGCACCTCACTTTAGCACTGAAGCC |
| W70 | GTGTCAGTCGACGCGTTTTCAAGGATTAGTCCTTAATCGG |
| W401 | AACGTCAATGAGCAAAGGTATTAA |
| W402 | TACGGGAGGCAGCAGTGG |
| W403 | GGACTAATAGCAGGTTAC |
| W404 | GCAGTTAATCCAATATAAGG |
| W408 | CACTAGGTTCTGATCTGGCACGG |
| W409 | CTCGATGCCAATCGCTTTTGCC |
| W410 | CTTCTGGGAATAGTCCTGACAACC |
| W411 | GAGTTGACTGACTTTTCGGCCTCC |
| W424 | AAAGTAGATTCCTTTACGACCG |
| W425 | AATGAACAAAACGCAGGGTGTC |
| W537 | TCATTCAAGCTTCAAGAACCGCAACTATCGCTACAAT |
| W556 | TCATTCGGATCCCCCCCTTCATTTCACCCTTTGGCCTG |
| W565 | CAAATAGGGGTTCCGCGCACATTTC |
| W566 | GGGGCCTGCCACCATACCCACGCCG |
Plasmid construction.
pNWG03 (pBAD-slyA) was constructed by ligating fragments from EcoRI- and SalI-digested pBAD18 and pCA24N-his-slyA (JW5267 from the ASKA collection [32]), which generated 4,580-bp and 527-bp fragments, respectively. pNWG03 contains the slyA gene from the second codon onwards. pNWG07 (pACYC184-Spcr), an empty vector control to pSRG58, was constructed by self-ligating the SphI-digested pSRG58 (pACYC184-PphoP-phoPQ [33]), which removed the entire phoP locus and the majority of the phoQ locus. The remaining 3.2-kb fragment contains the ori and aadA gene. pNWG13 (pBR322-PslyA-slyA) was constructed by ligating fragments from the HindIII- and BamHI-digested pBR322, which released a 3,739-bp fragment, and pNWG12 (a high-copy-number vector containing the entire slyA coding region and putative promoter [500 bp upstream of the start codon] that was PCR generated using W537/W556 [Table 3]), which released a 946-bp fragment. The sequence of the inserted DNA was verified by Sanger sequencing using primers W565/W566 (Table 3).
Reverse transcription-PCR (RT-PCR).
Wild-type S. flexneri 2457T and E. coli MC4100 cells were grown in TSB at 37°C to late exponential phase. Total RNA was isolated using the TRIzol reagent (Life Technologies) using the manufacturer's specifications. Two micrograms of DNase I-treated RNA was reverse transcribed using a high-capacity cDNA reverse transcription kit (Applied Biosystems). PCR was carried out using 100 ng of cDNA as the template. Specific primer sets (described in Table 3) were used to amplify the following regions of interest: slyA (primers W408/409), rrsA (primers W401/402), icsP (primers W403/404), ompT (primers W410/411), and icsP promoter region (PicsP; primers W69/70). In addition, genomic DNA from S. flexneri 2457T and E. coli MC4100 was used as a positive PCR control for each primer set.
Western blot analysis.
E. coli MC4100 and S. flexneri 2457T wild-type and ΔslyA::Kn cells were cultured in 20 ml LB at 37°C to late exponential phase. Cells were harvested, washed twice with phosphate-buffered saline (PBS), resuspended in 0.5 ml PBS, and lysed via sonication. Total cell protein concentration was determined using a Bradford assay. Equal amounts of total protein preparations were separated on 17.5% SDS-acrylamide gels, and SlyA was detected using a polyclonal anti-SlyA antibody obtained from the Blomfield laboratory as previously described (16) and enhanced using chemiluminescence (ECL; Pierce).
β-Galactosidase assays.
The activity of the slyA promoter was determined by measuring β-galactosidase activity in the S. flexneri 2457T and E. coli MC4100 ΔslyA::lacZ strains using the Miller protocol (34). Promoter activity was routinely analyzed in three independent assays with at least two independent transformations. To measure slyA promoter activity throughout a time course, β-galactosidase activity was determined in the ΔslyA::lacZ strain harboring pBR322 or pBR322-PslyA-slyA. Overnight cultures were subcultured 1:100 in LB and grown at 37°C with aeration to various time points.
To measure the PhoP- and Mg2+-dependent regulation of the slyA promoter, β-galactosidase activity was determined in the ΔslyA::lacZ strain harboring pACYC184 or pACYC184-PphoP-phoPQ (33) using conditions similar to those used previously (24). Cultures were grown in N minimal buffer (35) supplemented with 0.4% glucose, 0.4% Casamino Acids, 0.01 mg/ml nicotinic acid, 0.01 mg/ml tryptophan, 0.01 mg/ml thiamine, and 0.1 mM CaCl2 (collectively termed N minimal medium). Cells were grown overnight in N minimal medium supplemented with 10 mM MgCl2, washed twice with N minimal medium, and subcultured 1:10 into fresh N minimal medium supplemented with 0.01 mM (low) or 10 mM (high) MgCl2. Cells were grown at 37°C to late exponential phase and then harvested to measure β-galactosidase activity.
To measure the SlyA- and VirB-dependent regulation of the promoters controlling mxiC and icsP, β-galactosidase activity was measured in cells bearing either the mxiC-lacZ reporter (BS184 [31]) or PicsP-lacZ (pAFW04a [19]) and carrying either pBAD, pBAD-virB, or pBAD-slyA. Overnight cultures were subcultured 1:100 in LB and grown under derepressed (37°C or wild-type background, respectively) or repressed (30°C or virB::Tn5 mutant background, respectively) conditions. As previously described (36), mid-exponential-phase cultures were supplemented with 0.2% l-arabinose to induce the pBAD promoter. Cells were grown to late exponential phase and then harvested to measure β-galactosidase activity.
Congo red binding assay.
S. flexneri 2457T wild-type and virB::Tn5 cells harboring pBAD, pBAD-virB, or pBAD-slyA were grown overnight in LB supplemented with 0.2% (wt/vol) d-glucose. The next day, cultures were serially diluted (40-fold dilutions) and ∼6 μl of each dilution was dropped onto TSA Congo red plates supplemented with 0.2% (wt/vol) l-arabinose or d-glucose. Plates were incubated overnight at 37°C to determine the Congo red phenotype. To quantify the relative amount of Congo red dye bound by cells, a fixed number of cells (1.3 × 109 cells/ml) was evaluated. Two culture spots for each sample were scraped off the agar plate and resuspended in 0.75 ml 25% ethanol to remove the Congo red bound to cells. The optical density at 600 nm (OD600) of the cell suspension was measured to normalize samples to cell number. Cell suspensions then were centrifuged to pellet cells. The OD498 of the supernatant was measured to quantify the amount of Congo red dye released from the cells. Relative Congo red binding was determined with the following equation: [(OD498/OD600)/(average (OD498/OD600)2457T pBAD)] × 100.
Acid resistance assay.
Acid resistance assays were adapted from previously described assays (37, 38). S. flexneri 2457T wild-type cells harboring pBAD and ΔslyA cells harboring pBAD or pBAD-slyA were grown overnight in LB at 30°C. The next day, cultures were subcultured 1:100 into 5 ml of LB, pH 7.0 (buffered in 50 mM morpholinepropanesulfonic acid), or LB, pH 5.5 (buffered in 50 mM morpholineethanesulfonic acid), and grown overnight at 37°C. Before acid challenge, 0.2% (wt/vol) l-arabinose was added to cultures to induce the pBAD promoter for 2 h at 37°C. After induction, cultures were diluted 1:1,000 into 5 ml LB, pH 2.5 (acidified with HCl), for the acid challenge. Cultures were challenged in the acidic medium at 37°C for 0, 30, 60, and 120 min. The challenge was stopped by diluting cells (1:40) into fresh LB. Cell viability was determined by (i) dropping ∼6 μl of serially diluted (∼10−3 and 10−5) cultures onto Congo red plates and observing growth after overnight incubation at 37°C and (ii) spreading 100 μl of an appropriate dilution and determining CFU after overnight incubation at 37°C. Percent normalized survival was determined by the following equation: [CFU after challenge/CFU of 2457T pBAD (pH 5.5) before challenge (i.e., 0 min)] × 100.
Statistical analyses.
Two-tailed, two-sample Student's t tests, assuming equal variance, were used throughout this work to determine confidence levels.
RESULTS
slyA is transcribed and translated in S. flexneri.
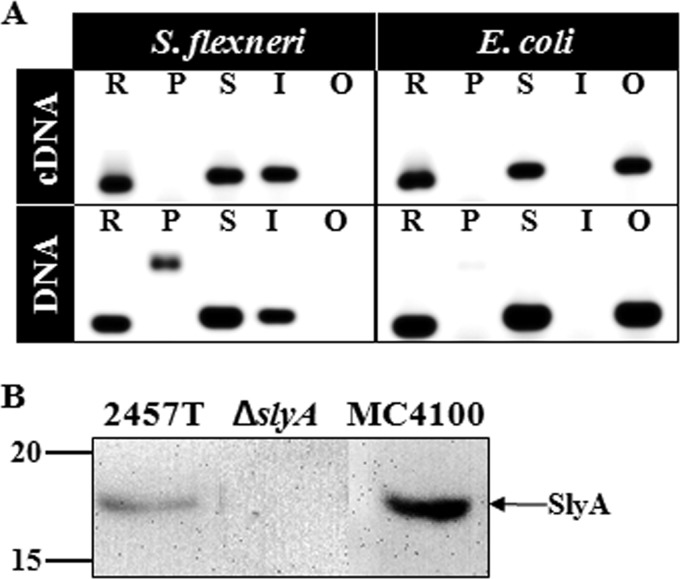
To begin the characterization of SlyA, we chose to determine if its chromosomally encoded gene is expressed in S. flexneri, since this had not been established when this genetic locus was identified (8). The transcription of slyA was determined by reverse transcription-PCR (RT-PCR) using total RNA isolated from S. flexneri 2457T. E. coli MC4100 was used as a positive control for this study, because slyA, which is 99% similar to the slyA gene in S. flexneri (Table 1), is known to be expressed in E. coli (14). Specific primer sets (described in Materials and Methods) were used to amplify the following regions of interest: (i) slyA, gene of interest; (ii) rrsA, positive control; (iii) icsP, S. flexneri positive control and E. coli negative control; (iv) ompT, S. flexneri negative control and E. coli positive control; and (v) icsP promoter region (PicsP), negative nontranscribed control. In addition, each primer set also was used with genomic DNA from S. flexneri 2457T and E. coli MC4100 as a positive control for PCR. As shown in Fig. 1A, slyA was amplified in both the S. flexneri and E. coli cDNA samples. The positive controls were present and the negative controls were absent, as expected. Taken together, these data demonstrate that slyA is transcribed in S. flexneri 2457T.
FIG 1.
slyA is transcribed and translated in S. flexneri 2457T. (A) slyA is expressed. PCR used cDNA (upper) and genomic DNA (lower) from S. flexneri 2457T and E. coli MC4100 grown to late exponential phase in TSB at 37°C. Transcribed sequences were amplified using primers that bind to the coding regions of rrsA (R), slyA (S), icsP (I), and ompT (O), while primers within the icsP promoter (PicsP; P) region were used to amplify nontranscribed sequences. (B) SlyA is produced. Western blot analysis of whole-cell extracts from S. flexneri 2457T, S. flexneri ΔslyA::Kn, and E. coli MC4100 cells grown to late exponential phase using a polyclonal anti-SlyA antibody obtained from the Blomfield laboratory (16).
Since transcription is not always indicative of translation, we determined if SlyA protein is produced in S. flexneri using Western blot analysis. Protein samples obtained from cultures of S. flexneri 2457T wild-type and ΔslyA mutant cultures, as well as E. coli MC4100 wild-type culture, were electrophoresed and probed for the presence of SlyA (which is 100% identical in S. flexneri and E. coli; Table 1) using an affinity-purified polyclonal anti-SlyA antibody (gift from the Blomfield laboratory). The SlyA protein (17.4 kDa) was detected in both wild-type backgrounds but not in the ΔslyA mutant (Fig. 1B), demonstrating SlyA is produced in S. flexneri 2457T.
Transcriptional profile and autoregulation of slyA in S. flexneri.
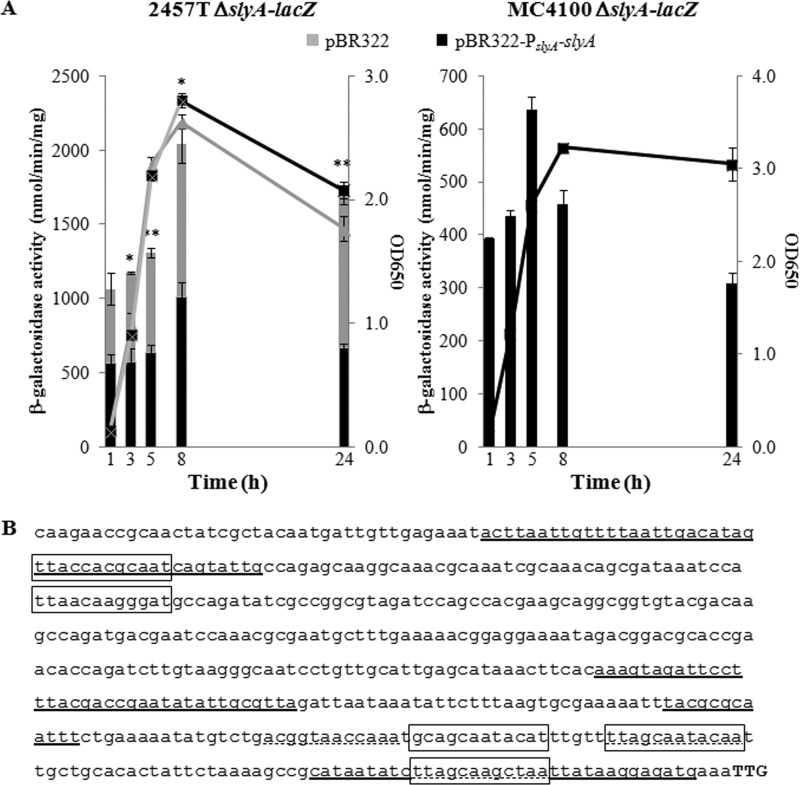
Since we had determined that slyA is expressed in S. flexneri (Fig. 1), we next monitored slyA promoter activity throughout a 24-h growth period using a chromosomal slyA transcriptional lacZ fusion (ΔslyA::lacZ) constructed in the S. flexneri 2457T background, as well as in the E. coli MC4100 background as a control. To approximate physiological levels of SlyA in this reporter strain, a low-copy-number plasmid harboring the slyA gene under the control of its own promoter (pBR322-PslyA-slyA) was introduced. In the presence of SlyA (pBR322-PslyA-slyA), S. flexneri slyA promoter activity peaked during early stationary phase (Fig. 2A). This regulatory pattern is similar to that exhibited by the slyA promoter in S. Typhimurium, whose activity peaks during stationary phase (12), but contrasts with that of the slyA promoter in E. coli, whose activity peaks during late exponential phase and declines upon entry into stationary phase (Fig. 2A) (14). Although it is possible that the LacZ protein is more stably maintained in the S. flexneri background than in E. coli, leading to higher β-galactosidase activity, this seems unlikely because LacZ activity has been shown to decline during stationary phase, when lacZ is expressed from another promoter in S. flexneri (39).
FIG 2.
Transcriptional profile and autoregulation of the slyA promoter in S. flexneri. (A) Growth-dependent activity of the slyA promoter. β-Galactosidase activity of the ΔslyA::lacZ reporter strain in S. flexneri 2457T harboring pBR322 (vector-only control) or pBR322-PslyA-slyA and in E. coli MC4100 harboring pBR322-PslyA-slyA over a 24-h time course. Bars represent slyA promoter activity, and lines represent optical density (650 nm) readings of cultures over time. Standard deviations are shown. Data are representative of three independent trials. A two-tailed Student's t test (assuming equal variance) was used for statistical analysis. *, P < 0.01; **, P < 0.001. (B) Putative SlyA binding sites within the slyA promoter. The slyA promoter sequence (500 bp upstream of the start codon, TTG) with the following sites identified: (i) solid underline, SlyA binding to the E. coli slyA promoter (14); (ii) dashed underline, SlyA binding to the S. Typhimurium slyA promoter (26); (iii) solid box, putative SlyA binding to the S. flexneri slyA promoter.
Moreover, starting at the 3-h time point, slyA promoter activity was found to decrease 2-fold (P < 0.01) in the absence (pBR322) versus the presence of SlyA (Fig. 2A). This suggests that SlyA negatively autoregulates its promoter in S. flexneri, which is similar to the regulation in S. Typhimurium (26) but differs from the regulation in E. coli, where SlyA positively autoregulates the slyA promoter (14). Cumulatively, these observations demonstrate that the regulation of the slyA promoter in S. flexneri is more similar to that found in the more distantly related S. Typhimurium than in its closest relative, E. coli.
Since SlyA has been shown to specifically bind to the slyA promoter in E. coli (14) and S. Typhimurium (11, 26), we searched the S. flexneri slyA promoter sequence for putative SlyA binding sites. Five putative SlyA binding sites (bearing at least a 7/12 match to the consensus sequence [26]) (Fig. 2B) were found within the predicted S. flexneri slyA promoter region (500 bp upstream of the slyA start codon), three of which overlap SlyA binding sites identified in E. coli or S. Typhimurium (Fig. 2B). Taken together, these findings strongly suggest that SlyA directly negatively autoregulates its promoter in S. flexneri.
PhoP-dependent regulation of the slyA promoter in S. flexneri.
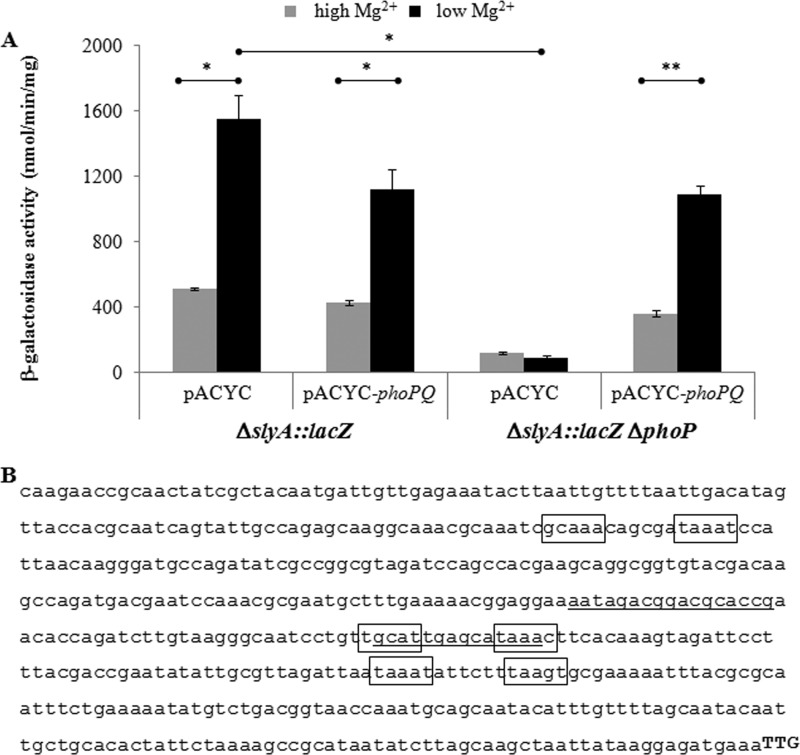
PhoP, the response regulator of the PhoP/PhoQ two-component system, has been shown to positively regulate the slyA promoter in S. Typhimurium (10, 11) and to play a role in Shigella virulence (40). To further characterize the regulation of the S. flexneri slyA promoter, we evaluated the effects of PhoP on slyA promoter activity. β-Galactosidase activity was determined using the ΔslyA::lacZ construct in the wild-type background and an isogenic ΔphoP mutant background under PhoP-activating (i.e., low Mg2+) and PhoP-repressing (i.e., high Mg2+) conditions. In wild-type S. flexneri, a 3-fold (P = 0.006) Mg2+-dependent response was observed between low Mg2+ and high Mg2+ (Fig. 3A). Furthermore, when phoP was absent, slyA promoter activity was abolished (17-fold decrease [P = 0.003] under low-Mg2+ condition) but could be restored to wild-type levels when a wild-type copy of the phoP locus was introduced in trans (pACYC-phoPQ) (Fig. 3A). These data demonstrate that, similar to the slyA promoter in S. Typhimurium, the S. flexneri slyA promoter responds to Mg2+ and is positively regulated by PhoP.
FIG 3.
PhoP-dependent regulation of the slyA promoter in S. flexneri. (A) PhoP- and Mg2+-dependent activity of the slyA promoter. β-Galactosidase activity of a ΔslyA::lacZ reporter strain in S. flexneri 2457T wild-type and isogenic ΔphoP backgrounds harboring pACYC (vector only control) or pACYC-PphoP-phoPQ (pSRG58 [33]). Strains were grown in N minimal medium supplemented with 10 mM Mg2+ (high; gray bars) or 0.01 mM Mg2+ (low; black bars). Each data set is representative of three independent trials. A two-tailed Student's t test (assuming equal variance) was used for statistical analysis. *, P < 0.01; **, P < 0.001. (B) Putative PhoP binding sites within the slyA promoter. The slyA promoter sequence (500 bp upstream of the start codon, TTG) is shown with the following sites identified: (i) solid underline, PhoP binding to the S. Typhimurium slyA promoter (11); (ii) solid box, putative PhoP binding to the S. flexneri slyA promoter.
Three putative PhoP binding sites (minimal 6/10 match to consensus sequence [41–43]) (Fig. 3B) were identified in the S. flexneri slyA promoter region (500 bp upstream of the slyA start codon). One of these sites overlaps a sequence previously shown to be bound by PhoP in S. Typhimurium (11). The presence of these putative sites provides strong evidence that PhoP directly regulates the slyA promoter in S. flexneri.
H-NS does not regulate the slyA promoter in S. flexneri.
The global transcriptional repressor H-NS has been shown to negatively regulate the promoter of the slyA ortholog rovA in Yersinia pseudotuberculosis (27) but has no effect on the slyA promoter in E. coli K-12 (14) (currently, the effect of H-NS on the slyA promoter in S. Typhimurium has not been reported). H-NS is well characterized in S. flexneri as a transcriptional repressor of virulence genes (31, 44). So, to conclude our characterization of the slyA promoter in S. flexneri, the role of H-NS was examined. In Shigella, H-NS-mediated repression is observed at a nonphysiological temperature of 30°C and alleviated at 37°C either directly or indirectly through the production of other thermally responsive transcriptional regulators (31, 44–47). Thus, we used the ΔslyA::lacZ reporter strain to measure slyA promoter activity in isogenic wild-type and hns mutant backgrounds at both 30°C and 37°C. Neither H-NS nor temperature had a significant effect on β-galactosidase activity (data not shown), suggesting that the S. flexneri slyA promoter, similar to the E. coli slyA promoter, is not regulated by H-NS.
Exogenous expression of slyA rescues a virulence-associated phenotype in S. flexneri.
Having characterized the expression of slyA in Shigella, we next focused our attention on the role that SlyA plays in the modulation of Shigella virulence traits. To do this, we initially monitored virulence phenotypes in wild-type S. flexneri and an avirulent virB mutant when slyA was expressed from a high-copy-number plasmid (i.e., pBAD-slyA). We reasoned that this strategy would uncover SlyA-associated virulence phenotypes that may not be so easily detected with physiological levels of SlyA or in the presence of the essential virulence regulator VirB.
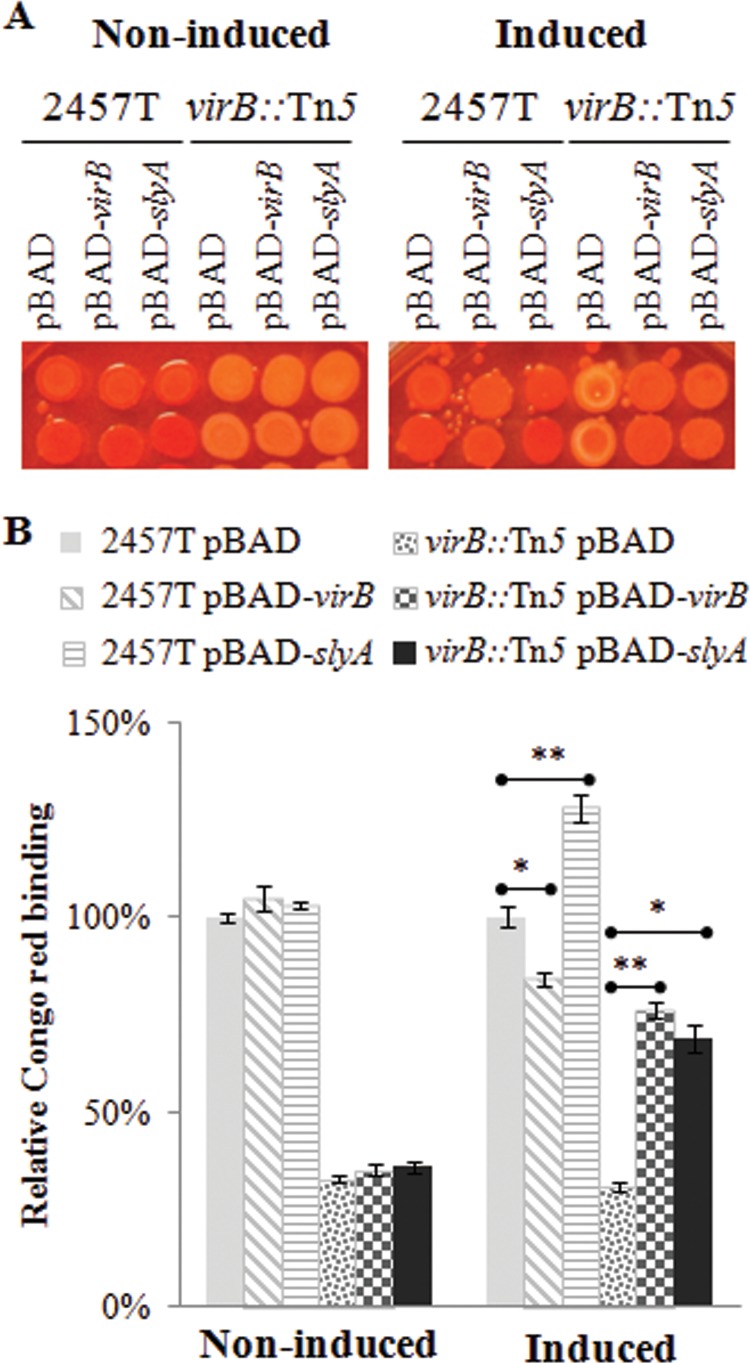
The ability of S. flexneri to bind the organic dye Congo red from agar medium correlates well with the virulence properties of this bacterium, in part due to the presence of a type three secretion system (48, 49). The expression of genes encoding the type three secretion system is regulated by VirB, so the absence of virB S. flexneri produces a Congo red-negative (CR−) phenotype (50, 51). Surprisingly, our initial phenotypic evaluation revealed that the exogenous expression of slyA in a virB mutant background restored Congo red binding. To determine the significance of this restoration, we developed an assay to quantify Congo red binding to cells grown on agar medium (described in Materials and Methods). As expected, under inducing and noninducing conditions, wild-type cells maintained their CR+ phenotype (∼100% Congo red binding) and virB mutant cells harboring pBAD maintained their CR− phenotype (∼28% relative Congo red binding) (Fig. 4). Not surprisingly, under inducing conditions, pBAD-virB restored the CR+ phenotype of the virB mutant (70% relative Congo red binding; P = 0.0004), but strikingly, pBAD-slyA also could restore the CR+ phenotype (68% relative Congo red binding; P = 0.002) in the virB mutant (Fig. 4). These data indicate that the exogenous expression of slyA can restore a CR+ phenotype in the absence of virB.
FIG 4.
Exogenous expression of slyA rescues the Congo red binding deficiency in a virB mutant. (A) Qualitative analysis of Congo red binding. S. flexneri 2457T wild-type and virB::Tn5 strains harboring pBAD, pBAD-virB, or pBAD-slyA were serially diluted (40-fold dilutions) and spotted (approximately 6 μl) onto TSA Congo red plates supplemented with 0.2% (wt/vol) d-glucose (noninduced) or 0.2% (wt/vol) l-arabinose (induced). (B) Quantitative analysis of Congo red binding. Two culture spots (shown in panel A) were scraped off the agar plate and resuspended in 0.75 ml 25% ethanol to remove the Congo red bound to cells. The OD600 was determined to normalize samples against cell number. Cell suspensions were centrifuged to pellet cells, and the OD498 of the supernatant was determined. Relative Congo red binding was calculated as [(OD498/OD600)/(average (OD498/OD600)2457T pBAD)] × 100. Data shown are representative of three independent trials. A two-tailed Student's t test (assuming equal variance) was used for statistical analysis. *, P < 0.01; **, P < 0.001.
Exogenous expression of slyA upregulates virulence gene promoters in S. flexneri.
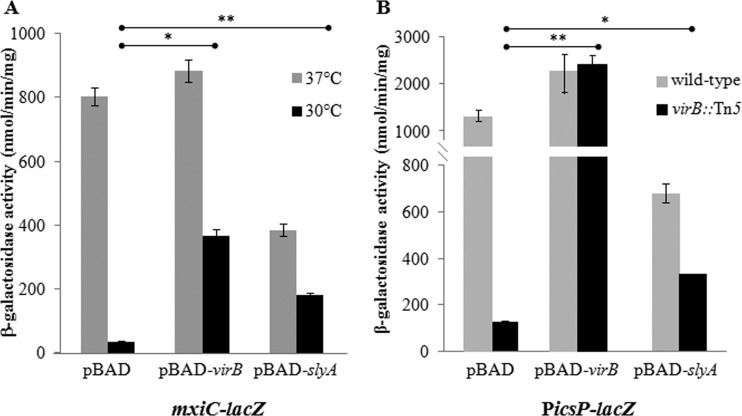
Since the exogenous expression of slyA can compensate for a lack of virB in Congo red binding assays (Fig. 4), we next chose to determine if this SlyA-dependent effect was caused by the upregulation of genes encoding the type three secretion system, which reside in the mxi-spa locus. To test this, β-galactosidase activity was measured in cells carrying the virulence plasmid-encoded mxiC promoter reporter, mxiC-lacZ (BS184 [31]), and harboring pBAD, pBAD-virB, or pBAD-slyA under both repressing conditions (i.e., 30°C) and derepressing conditions (i.e., 37°C). The mxiC promoter activity increased 5-fold (P = 0.0006) when slyA was exogenously expressed (Fig. 5A) and, consistent with previous reports (52), increased 10-fold (P = 0.002) when virB was exogenously expressed at 30°C (considered repressing conditions, because VirB levels are low and cannot counter H-NS). The SlyA-dependent upregulation of this promoter likely explains the restoration of Congo red binding that was observed when slyA was exogenously expressed in a virB mutant (Fig. 4).
FIG 5.
Exogenous expression of slyA upregulates VirB-dependent promoters under repressed conditions. (A) Upregulation of mxiC-lacZ. β-Galactosidase activity of mxiC-lacZ cells harboring pBAD, pBAD-virB, or pBAD-slyA in derepressed (37°C) or repressed (30°C) conditions. (B) Upregulation of PicsP-lacZ. β-Galactosidase activity of cells containing PicsP-lacZ and harboring pBAD, pBAD-virB, or pBAD-slyA in derepressed (wild-type) or repressed (virB::Tn5) conditions is shown. Data are representative of three independent trials conducted in triplicate. A two-tailed Student's t test (assuming equal variance) was used for statistical analysis. *, P < 0.01; **, P < 0.001.
To determine if SlyA-dependent upregulation can extend beyond the mxi-spa locus, we measured the β-galactosidase activity of the icsP promoter, which resides outside the mxi-spa locus and also is regulated by VirB, using the plasmid-borne icsP promoter reporter, PicsP-lacZ (pAFW04a [19]). The icsP promoter activity increased 3-fold (P = 0.003) when slyA was exogenously expressed (Fig. 5B) and, consistent with previous reports (36), increased 7-fold (P = 0.0008) when virB was exogenously expressed in the virB mutant background (considered repressed conditions, because VirB is absent and cannot counter H-NS). This demonstrates that the exogenous expression of slyA also can lead to the upregulation of a gene outside the mxi-spa locus in the absence of virB.
Cumulatively, these data show that superphysiological levels of SlyA can regulate VirB-dependent promoters when VirB is absent. Interestingly, under derepressing conditions where VirB is present and able to counter H-NS-mediated repression, the exogenous expression of slyA led to a modest 2-fold decrease in the activity of both the mxiC (at 37°C; P = 0.004) and icsP (in the wild-type background; P = 0.03) promoters (Fig. 5). This raises the possibility that SlyA does not solely compensate for the lack of VirB but has some additional regulatory role when present at superphysiological levels. Regardless, the effect of SlyA observed in our assays is specific to VirB-dependent promoters, because the exogenous expression of slyA does not increase the activity of the VirB-independent icsA promoter irrespective of whether or not its native regulator, VirF, is present (data not shown).
Acid resistance: a novel role for SlyA in Shigella.
To gain greater insight into the role of SlyA at physiological levels, a literature search was conducted. This revealed that a proteomic analysis in enteroinvasive E. coli (EIEC) had identified over 30 putative SlyA-dependent gene products, several of which had been implicated previously in acid resistance (53). Although the authors did not formally test the role of SlyA in acid resistance in EIEC, we decided to examine the role that SlyA plays in acid resistance in Shigella. To test this, S. flexneri 2457T wild-type and isogenic ΔslyA mutant strains were evaluated using a traditional acid resistance assay (adapted from references 37 and 38).
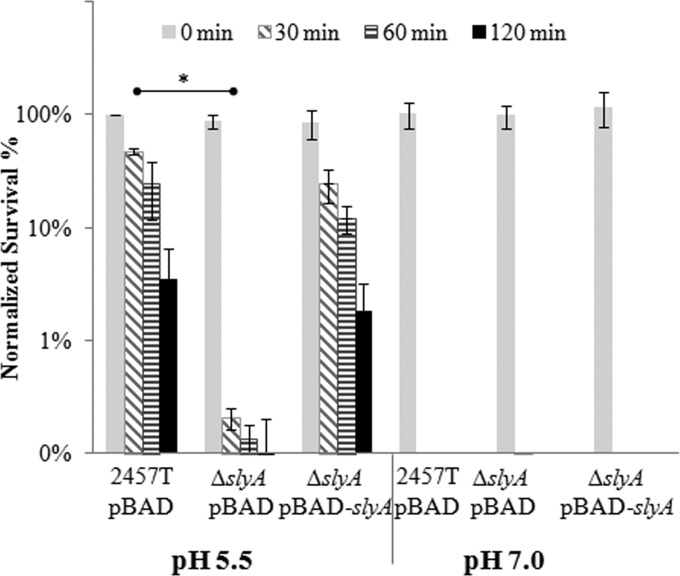
As described previously (54), adaptive conditions “prime” cells to survive an extreme acid challenge at levels several orders of magnitude higher than nonadapted cells. Strikingly, when grown under adaptive conditions (pH 5.5), the survival of ΔslyA cells was decreased approximately 200-fold (P = 0.002) compared to that of wild-type cells after just 30 min of extreme acid challenge (Fig. 6). Importantly, the survival of ΔslyA cells could be restored to wild-type levels when slyA was expressed exogenously (pBAD-slyA) (Fig. 6). As expected, none of the cultures grown under nonadaptive conditions (pH 7.0) were able to survive extreme acid challenge. Consequently, these findings are the first to demonstrate that SlyA has a role in acid resistance in S. flexneri. Since acid resistance has been suggested to be a key factor in the low infectious dose of Shigella spp. (37, 55, 56), these findings elevate the importance of SlyA and SlyA-mediated regulation in Shigella virulence.
FIG 6.
Deletion of slyA decreases S. flexneri acid resistance. S. flexneri 2457T wild-type cells harboring pBAD and ΔslyA cells harboring pBAD or pBAD-slyA grown under adaptive (pH 5.5) or nonadaptive (pH 7.0) conditions were challenged at pH 2.5. The CFU of challenged cells were determined. The percentage of normalized survival was calculated as CFU after challenge divided by CFU of 2457T pBAD (pH 5.5) before challenge (i.e., 0 min) times 100. Data are the averages from three independent trials. A two-tailed Student's t test (assuming equal variance) was used for statistical analysis. *, P < 0.01.
DISCUSSION
The SlyA transcriptional regulator has been demonstrated to be an important virulence factor in several enteric bacteria (reviewed in reference 6); however, prior to this study it had not been studied in the human pathogen Shigella. Here, we describe three significant features of slyA in S. flexneri: (i) the regulation of the slyA promoter, (ii) Shigella virulence phenotypes and promoters regulated by the exogenous expression of slyA, and (iii) the requirement of SlyA in acid resistance. Overall, our characterization of slyA and the regulator it encodes uncovers some interesting parallels between E. coli, Salmonella, and Shigella and also reveals a novel and unique role for SlyA in Shigella virulence.
Our characterization of the S. flexneri slyA promoter revealed that certain aspects of its regulation were more similar to the less conserved S. Typhimurium slyA promoter (68% identical) than the highly conserved E. coli slyA promoter (99% identical). Specifically, this was demonstrated by our observations that the S. flexneri slyA promoter activity is (i) maximal during stationary phase (Fig. 2A), (ii) negatively autoregulated (Fig. 2A), and (iii) positively regulated by PhoP (Fig. 3A). Our finding that PhoP regulates the slyA promoter in S. flexneri adds another member to the PhoP/Q regulon, which previously had consisted of a single virulence plasmid-carried operon (shf-wabB-virK-msbB2) that encodes enzymes involved in bacterial cell wall biosynthesis (33, 57). Interestingly, although the slyA promoter in both S. Typhimurium and S. flexneri has been shown to be regulated by PhoP (10 and 11 and Fig. 3A, respectively), the slyA transcript has not been identified in genomic approaches attempting to characterize the PhoP/Q regulons in S. Typhimurium (22, 58, 59). This apparent discrepancy may be explained by the different experimental conditions and approaches used in these studies (i.e., promoter activity versus mRNA levels) or the possibility that posttranscriptional regulation or modification of slyA mRNA in S. Typhimurium and possibly S. flexneri exists. This will be the subject of future investigations in our laboratory. The observation that the regulation of the slyA promoter in S. flexneri is similar to that in S. Typhimurium but different from that in E. coli is intriguing, since Shigella species diverged from E. coli after E. coli and Salmonella had diverged from their common ancestor. Pondering this evolutionary paradox, one can posit that the similar regulation of slyA in S. flexneri and S. Typhimurium proves advantageous because of their common intracellular lifestyle. Future examination of the S. flexneri SlyA regulon will reveal if there is any support for this idea.
SlyA is a well-characterized transcriptional regulator of virulence-associated promoters in closely related organisms (reviewed in reference 6); therefore, the role of SlyA in Shigella virulence-associated phenotypes was investigated. Surprisingly, our data demonstrated that in the absence of virB, the exogenous expression of slyA could restore Congo red binding to S. flexneri (Fig. 4). We determined that this phenotype was due, at least in part, to SlyA upregulating genes encoding the type three secretion apparatus (i.e., mxiC-lacZ) (Fig. 5A), a process known to lead to Congo red binding. In addition, the exogenous expression of slyA could upregulate the icsP promoter in the absence of virB (Fig. 5B) but not the VirB-independent icsA promoter (data not shown), suggesting SlyA functions to compensate for the lack of VirB when expressed at high levels. The mechanistically intriguing effect of SlyA on VirB-dependent promoters could be caused by (i) SlyA binding to VirB binding sites, (ii) SlyA binding to degenerate SlyA binding sites, (iii) SlyA decreasing intracellular H-NS protein levels, or (iv) some combination thereof. While we acknowledge that high levels of SlyA in these particular assays are responsible for the regulatory effects observed, these findings highlight the relative plasticity of the regulatory networks controlling virulence; if virB is lost or inactivated, simply increasing SlyA levels through the mutation of its promoter could restore some virulence gene expression and phenotypes. This is especially interesting because virB has been shown to be deleted or otherwise inactivated at high frequency when serially passaged at 37°C (60). To our knowledge, our finding that SlyA can compensate for the loss of VirB is the first of its kind to suggest that one derepressor of H-NS-mediated repression compensates for the loss of another. In light of these findings, it is interesting that other derepressors of H-NS-mediated repression have the potential to serve this kind of back-up role when a gene encoding the usual cognate transcriptional regulator is lost or mutated.
Our finding that SlyA is essential for acid resistance in S. flexneri is the first to demonstrate that SlyA is required for this crucial virulence phenotype in enterics. The acid resistance controlled by SlyA may have remained uncharacterized to this point, because attributes of Shigella virulence most commonly are studied in nongastrointestinal (GI) tract models: the Sereny test, which is a conjunctivitis model in mouse; the rabbit ileal loop model, where shigellae are artificially introduced into a closed-off segment of the ileum, thereby bypassing migration through the upper GI tract; and/or in vitro tissue culture assays. None of these assays examine events that lead to the successful passage of shigellae through the stomach, and this may explain why critical factors like SlyA, which promote acid resistance, have not been identified. This is unfortunate, since acid resistance is arguably one of the most critical virulence determinants in Shigella spp., because it has been correlated (32, 50, 51) to the extremely low infectious dose of this pathogen (10 to 100 cells) (61). Therefore, our finding that SlyA is essential for acid resistance in S. flexneri constitutes a novel and significant finding in S. flexneri pathogenesis and raises the possibility that SlyA plays an important role in determining the infectious dose of this highly infectious enteric organism.
In summary, we have characterized three major aspects of slyA in S. flexneri. Our study highlights unexpected similarities between the S. flexneri and S. Typhimurium slyA promoters. We describe an unprecedented relationship between two derepressors of H-NS-mediated repression, SlyA and VirB, and discuss possible implications of our findings. Moreover, we emphasize the importance of SlyA in acid resistance. This is a virulence role not previously demonstrated for SlyA but is one that is likely to be essential for the pathogenesis of this pathogen. Clearly, one important future direction of this work will be to identify genes of the S. flexneri SlyA regulon and specifically those that are responsible for the acid resistance phenotype, which has been described for the first time in this work.
ACKNOWLEDGMENTS
We thank Ian Blomfield for the polyclonal anti-SlyA antibody and Jim Slauch for the pKG137 plasmid used in this work.
Funding Statement
This study was supported by National Institutes of Health (NIH) grant R15 AI090573-02.
REFERENCES
- 1.George AM, Levy SB. 1983. Gene in the major cotransduction gap of the Escherichia coli K-12 linkage map required for the expression of chromosomal resistance to tetracycline and other antibiotics. J Bacteriol 155:541–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Srikumar R, Kon T, Gotoh N, Poole K. 1998. Expression of Pseudomonas aeruginosa multidrug efflux pumps MexA-MexB-OprM and MexC-MexD-OprJ in a multidrug-sensitive Escherichia coli strain. Antimicrob Agents Chemother 42:65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thomson NR, Cox A, Bycroft BW, Stewart GS, Williams P, Salmond GP. 1997. The rap and hor proteins of Erwinia, Serratia and Yersinia: a novel subgroup in a growing superfamily of proteins regulating diverse physiological processes in bacterial pathogens. Mol Microbiol 26:531–544. doi: 10.1046/j.1365-2958.1997.5981976.x. [DOI] [PubMed] [Google Scholar]
- 4.Galan B, Kolb A, Sanz JM, Garcia JL, Prieto MA. 2003. Molecular determinants of the hpa regulatory system of Escherichia coli: the HpaR repressor. Nucleic Acids Res 31:6598–6609. doi: 10.1093/nar/gkg851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roper DI, Fawcett T, Cooper RA. 1993. The Escherichia coli C homoprotocatechuate degradative operon: hpc gene order, direction of transcription and control of expression. Mol Gen Genet 237:241–250. [DOI] [PubMed] [Google Scholar]
- 6.Ellison DW, Miller VL. 2006. Regulation of virulence by members of the MarR/SlyA family. Curr Opin Microbiol 9:153–159. doi: 10.1016/j.mib.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 7.Libby SJ, Goebel W, Ludwig A, Buchmeier N, Bowe F, Fang FC, Guiney DG, Songer JG, Heffron F. 1994. A cytolysin encoded by Salmonella is required for survival within macrophages. Proc Natl Acad Sci U S A 91:489–493. doi: 10.1073/pnas.91.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ludwig A, Tengel C, Bauer S, Bubert A, Benz R, Mollenkopf HJ, Goebel W. 1995. SlyA, a regulatory protein from Salmonella typhimurium, induces a haemolytic and pore-forming protein in Escherichia coli. Mol Gen Genet 249:474–486. doi: 10.1007/BF00290573. [DOI] [PubMed] [Google Scholar]
- 9.Fass E, Groisman EA. 2009. Control of Salmonella pathogenicity island-2 gene expression. Curr Opin Microbiol 12:199–204. doi: 10.1016/j.mib.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Norte VA, Stapleton MR, Green J. 2003. PhoP-responsive expression of the Salmonella enterica serovar typhimurium slyA gene. J Bacteriol 185:3508–3514. doi: 10.1128/JB.185.12.3508-3514.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi Y, Latifi T, Cromie MJ, Groisman EA. 2004. Transcriptional control of the antimicrobial peptide resistance ugtL gene by the Salmonella PhoP and SlyA regulatory proteins. J Biol Chem 279:38618–38625. doi: 10.1074/jbc.M406149200. [DOI] [PubMed] [Google Scholar]
- 12.Buchmeier N, Bossie S, Chen CY, Fang FC, Guiney DG, Libby SJ. 1997. SlyA, a transcriptional regulator of Salmonella typhimurium, is required for resistance to oxidative stress and is expressed in the intracellular environment of macrophages. Infect Immun 65:3725–3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oscarsson J, Mizunoe Y, Uhlin BE, Haydon DJ. 1996. Induction of haemolytic activity in Escherichia coli by the slyA gene product. Mol Microbiol 20:191–199. doi: 10.1111/j.1365-2958.1996.tb02500.x. [DOI] [PubMed] [Google Scholar]
- 14.Corbett D, Bennett HJ, Askar H, Green J, Roberts IS. 2007. SlyA and H-NS regulate transcription of the Escherichia coli K5 capsule gene cluster, and expression of slyA in Escherichia coli is temperature-dependent, positively autoregulated, and independent of H-NS. J Biol Chem 282:33326–33335. doi: 10.1074/jbc.M703465200. [DOI] [PubMed] [Google Scholar]
- 15.Xue P, Corbett D, Goldrick M, Naylor C, Roberts IS. 2009. Regulation of expression of the region 3 promoter of the Escherichia coli K5 capsule gene cluster involves H-NS, SlyA, and a large 5′ untranslated region. J Bacteriol 191:1838–1846. doi: 10.1128/JB.01388-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McVicker G, Sun L, Sohanpal BK, Gashi K, Williamson RA, Plumbridge J, Blomfield IC. 2011. SlyA protein activates fimB gene expression and type 1 fimbriation in Escherichia coli K-12. J Biol Chem 286:32026–32035. doi: 10.1074/jbc.M111.266619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Turner EC, Dorman CJ. 2007. H-NS antagonism in Shigella flexneri by VirB, a virulence gene transcription regulator that is closely related to plasmid partition factors. J Bacteriol 189:3403–3413. doi: 10.1128/JB.01813-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Castellanos MI, Harrison DJ, Smith JM, Labahn SK, Levy KM, Wing HJ. 2009. VirB alleviates H-NS repression of the icsP promoter in Shigella flexneri from sites more than one kilobase upstream of the transcription start site. J Bacteriol 191:4047–4050. doi: 10.1128/JB.00313-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Basta DW, Pew KL, Immak JA, Park HS, Picker MA, Wigley AF, Hensley CT, Pearson JS, Hartland EL, Wing HJ. 2013. Characterization of the ospZ promoter in Shigella flexneri and its regulation by VirB and H-NS. J Bacteriol 195:2562–2572. doi: 10.1128/JB.00212-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stoebel DM, Free A, Dorman CJ. 2008. Anti-silencing: overcoming H-NS-mediated repression of transcription in Gram-negative enteric bacteria. Microbiology 154:2533–2545. doi: 10.1099/mic.0.2008/020693-0. [DOI] [PubMed] [Google Scholar]
- 21.Wyborn NR, Stapleton MR, Norte VA, Roberts RE, Grafton J, Green J. 2004. Regulation of Escherichia coli hemolysin E expression by H-NS and Salmonella SlyA. J Bacteriol 186:1620–1628. doi: 10.1128/JB.186.6.1620-1628.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Navarre WW, Halsey TA, Walthers D, Frye J, McClelland M, Potter JL, Kenney LJ, Gunn JS, Fang FC, Libby SJ. 2005. Co-regulation of Salmonella enterica genes required for virulence and resistance to antimicrobial peptides by SlyA and PhoP/PhoQ. Mol Microbiol 56:492–508. doi: 10.1111/j.1365-2958.2005.04553.x. [DOI] [PubMed] [Google Scholar]
- 23.Lithgow JK, Haider F, Roberts IS, Green J. 2007. Alternate SlyA and H-NS nucleoprotein complexes control hlyE expression in Escherichia coli K-12. Mol Microbiol 66:685–698. doi: 10.1111/j.1365-2958.2007.05950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kong W, Weatherspoon N, Shi Y. 2008. Molecular mechanism for establishment of signal-dependent regulation in the PhoP/PhoQ system. J Biol Chem 283:16612–16621. doi: 10.1074/jbc.M800547200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Song H, Kong W, Weatherspoon N, Qin G, Tyler W, Turk J, Curtiss R III, Shi Y. 2008. Modulation of the regulatory activity of bacterial two-component systems by SlyA. J Biol Chem 283:28158–28168. doi: 10.1074/jbc.M801058200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stapleton MR, Norte VA, Read RC, Green J. 2002. Interaction of the Salmonella typhimurium transcription and virulence factor SlyA with target DNA and identification of members of the SlyA regulon. J Biol Chem 277:17630–17637. doi: 10.1074/jbc.M110178200. [DOI] [PubMed] [Google Scholar]
- 27.Tran HJ, Heroven AK, Winkler L, Spreter T, Beatrix B, Dersch P. 2005. Analysis of RovA, a transcriptional regulator of Yersinia pseudotuberculosis virulence that acts through antirepression and direct transcriptional activation. J Biol Chem 280:42423–42432. doi: 10.1074/jbc.M504464200. [DOI] [PubMed] [Google Scholar]
- 28.Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, Datsenko KA, Tomita M, Wanner BL, Mori H. 2006. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol Syst Biol 2:2006.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ellermeier CD, Janakiraman A, Slauch JM. 2002. Construction of targeted single copy lac fusions using lambda Red and FLP-mediated site-specific recombination in bacteria. Gene 290:153–161. doi: 10.1016/S0378-1119(02)00551-6. [DOI] [PubMed] [Google Scholar]
- 30.Datsenko KA, Wanner BL. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A 97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maurelli AT, Sansonetti PJ. 1988. Identification of a chromosomal gene controlling temperature-regulated expression of Shigella virulence. Proc Natl Acad Sci U S A 85:2820–2824. doi: 10.1073/pnas.85.8.2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kitagawa M, Ara T, Arifuzzaman M, Ioka-Nakamichi T, Inamoto E, Toyonaga H, Mori H. 2005. Complete set of ORF clones of Escherichia coli ASKA library (a complete set of E. coli K-12 ORF archive): unique resources for biological research. DNA Res 12:291–299. [DOI] [PubMed] [Google Scholar]
- 33.Goldman SR, Tu Y, Goldberg MB. 2008. Differential regulation by magnesium of the two MsbB paralogs of Shigella flexneri. J Bacteriol 190:3526–3537. doi: 10.1128/JB.00151-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller J. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 35.Nelson DL, Kennedy EP. 1971. Magnesium transport in Escherichia coli. Inhibition by cobaltous ion. J Biol Chem 246:3042–3049. [PubMed] [Google Scholar]
- 36.Wing HJ, Yan AW, Goldman SR, Goldberg MB. 2004. Regulation of IcsP, the outer membrane protease of the Shigella actin tail assembly protein IcsA, by virulence plasmid regulators VirF and VirB. J Bacteriol 186:699–705. doi: 10.1128/JB.186.3.699-705.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Waterman SR, Small PL. 1996. Identification of sigma S-dependent genes associated with the stationary-phase acid-resistance phenotype of Shigella flexneri. Mol Microbiol 21:925–940. doi: 10.1046/j.1365-2958.1996.00058.x. [DOI] [PubMed] [Google Scholar]
- 38.Lin J, Lee IS, Frey J, Slonczewski JL, Foster JW. 1995. Comparative analysis of extreme acid survival in Salmonella typhimurium, Shigella flexneri, and Escherichia coli. J Bacteriol 177:4097–4104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hensley CT, Kamneva OK, Levy KM, Labahn SK, Africa LA, Wing HJ. 2011. Two promoters and two translation start sites control the expression of the Shigella flexneri outer membrane protease IcsP. Arch Microbiol 193:263–274. doi: 10.1007/s00203-010-0669-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moss JE, Fisher PE, Vick B, Groisman EA, Zychlinsky A. 2000. The regulatory protein PhoP controls susceptibility to the host inflammatory response in Shigella flexneri. Cell Microbiol 2:443–452. doi: 10.1046/j.1462-5822.2000.00065.x. [DOI] [PubMed] [Google Scholar]
- 41.Kato A, Tanabe H, Utsumi R. 1999. Molecular characterization of the PhoP-PhoQ two-component system in Escherichia coli K-12: identification of extracellular Mg2+-responsive promoters. J Bacteriol 181:5516–5520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Groisman EA. 2001. The pleiotropic two-component regulatory system PhoP-PhoQ. J Bacteriol 183:1835–1842. doi: 10.1128/JB.183.6.1835-1842.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yamamoto K, Ogasawara H, Fujita N, Utsumi R, Ishihama A. 2002. Novel mode of transcription regulation of divergently overlapping promoters by PhoP, the regulator of two-component system sensing external magnesium availability. Mol Microbiol 45:423–438. doi: 10.1046/j.1365-2958.2002.03017.x. [DOI] [PubMed] [Google Scholar]
- 44.Hromockyj AE, Tucker SC, Maurelli AT. 1992. Temperature regulation of Shigella virulence: identification of the repressor gene virR, an analogue of hns, and partial complementation by tyrosyl transfer RNA (tRNA1(Tyr)). Mol Microbiol 6:2113–2124. doi: 10.1111/j.1365-2958.1992.tb01385.x. [DOI] [PubMed] [Google Scholar]
- 45.Maurelli AT, Blackmon B, Curtiss R III. 1984. Temperature-dependent expression of virulence genes in Shigella species. Infect Immun 43:195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Porter ME, Dorman CJ. 1994. A role for H-NS in the thermo-osmotic regulation of virulence gene expression in Shigella flexneri. J Bacteriol 176:4187–4191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Porter ME, Dorman CJ. 1997. Differential regulation of the plasmid-encoded genes in the Shigella flexneri virulence regulon. Mol Gen Genet 256:93–103. doi: 10.1007/s004380050550. [DOI] [PubMed] [Google Scholar]
- 48.Sankaran K, Ramachandran V, Subrahmanyam YV, Rajarathnam S, Elango S, Roy RK. 1989. Congo red-mediated regulation of levels of Shigella flexneri 2a membrane proteins. Infect Immun 57:2364–2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maurelli AT, Blackmon B, Curtiss R III. 1984. Loss of pigmentation in Shigella flexneri 2a is correlated with loss of virulence and virulence-associated plasmid. Infect Immun 43:397–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bahrani FK, Sansonetti PJ, Parsot C. 1997. Secretion of Ipa proteins by Shigella flexneri: inducer molecules and kinetics of activation. Infect Immun 65:4005–4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Adler B, Sasakawa C, Tobe T, Makino S, Komatsu K, Yoshikawa M. 1989. A dual transcriptional activation system for the 230 kb plasmid genes coding for virulence-associated antigens of Shigella flexneri. Mol Microbiol 3:627–635. doi: 10.1111/j.1365-2958.1989.tb00210.x. [DOI] [PubMed] [Google Scholar]
- 52.Beloin C, Dorman CJ. 2003. An extended role for the nucleoid structuring protein H-NS in the virulence gene regulatory cascade of Shigella flexneri. Mol Microbiol 47:825–838. doi: 10.1046/j.1365-2958.2003.03347.x. [DOI] [PubMed] [Google Scholar]
- 53.Spory A, Bosserhoff A, von Rhein C, Goebel W, Ludwig A. 2002. Differential regulation of multiple proteins of Escherichia coli and Salmonella enterica serovar Typhimurium by the transcriptional regulator SlyA. J Bacteriol 184:3549–3559. doi: 10.1128/JB.184.13.3549-3559.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Foster JW, Hall HK. 1990. Adaptive acidification tolerance response of Salmonella typhimurium. J Bacteriol 172:771–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gorden J, Small PL. 1993. Acid resistance in enteric bacteria. Infect Immun 61:364–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hong W, Wu YE, Fu X, Chang Z. 2012. Chaperone-dependent mechanisms for acid resistance in enteric bacteria. Trends Microbiol 20:328–335. doi: 10.1016/j.tim.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 57.Kaoukab-Raji A, Biskri L, Bernardini ML, Allaoui A. 2012. Characterization of SfPgdA, a Shigella flexneri peptidoglycan deacetylase required for bacterial persistence within polymorphonuclear neutrophils. Microbes Infect 14:619–627. doi: 10.1016/j.micinf.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 58.Zwir I, Shin D, Kato A, Nishino K, Latifi T, Solomon F, Hare JM, Huang H, Groisman EA. 2005. Dissecting the PhoP regulatory network of Escherichia coli and Salmonella enterica. Proc Natl Acad Sci U S A 102:2862–2867. doi: 10.1073/pnas.0408238102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Monsieurs P, De Keersmaecker S, Navarre WW, Bader MW, De Smet F, McClelland M, Fang FC, De Moor B, Vanderleyden J, Marchal K. 2005. Comparison of the PhoPQ regulon in Escherichia coli and Salmonella typhimurium. J Mol Evol 60:462–474. doi: 10.1007/s00239-004-0212-7. [DOI] [PubMed] [Google Scholar]
- 60.Schuch R, Maurelli AT. 1997. Virulence plasmid instability in Shigella flexneri 2a is induced by virulence gene expression. Infect Immun 65:3686–3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.DuPont HL, Levine MM, Hornick RB, Formal SB. 1989. Inoculum size in shigellosis and implications for expected mode of transmission. J Infect Dis 159:1126–1128. doi: 10.1093/infdis/159.6.1126. [DOI] [PubMed] [Google Scholar]
- 62.Pogliano JA, Beckwith J. 1994. SecD and SecF facilitate protein export in Escherichia coli. EMBO J 13:554–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Formal SB, Dammin GJ, Labrec EH, Schneider H. 1958. Experimental Shigella infections: characteristics of a fatal infection produced in guinea pigs. J Bacteriol 75:604–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Guzman LM, Belin D, Carson MJ, Beckwith J. 1995. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J Bacteriol 177:4121–4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schuch R, Sandlin RC, Maurelli AT. 1999. A system for identifying postinvasion functions of invasion genes: requirements for the Mxi-Spa type III secretion pathway of Shigella flexneri in intercellular dissemination. Mol Microbiol 34:675–689. doi: 10.1046/j.1365-2958.1999.01627.x. [DOI] [PubMed] [Google Scholar]
- 66.Bolivar F, Rodriguez RL, Greene PJ, Betlach MC, Heyneker HL, Boyer HW, Crosa JH, Falkow S. 1977. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene 2:95–113. [PubMed] [Google Scholar]