Abstract
Resistin-like molecules (RELMs) are highly expressed following helminth infection, where they impact both the host and helminth. While RELMα (Retnla) impairs helminth expulsion by inhibiting protective Th2 immunity, RELMβ (Retnlb) can promote its expulsion. We employed Retnla−/− and Retnlb−/− mice to delineate the function of both proteins following infection with Nippostrongylus brasiliensis, a hookworm that infects the lung and intestine. Whereas wild-type (WT) and Retnlb−/− mice exhibited equivalent infection-induced inflammation, Retnla−/− mice suffered a heightened inflammatory response, including increased mortality, weight loss, and lung inflammation. In the intestine, Retnla−/− mice had low parasite egg burdens compared to those of WT mice, while Retnlb−/− mice exhibited high egg burdens, suggesting that RELMα and RELMβ have functionally distinct effects on immunity and inflammation to N. brasiliensis. To test the importance of both proteins, we generated Retnla−/− Retnlb−/− mice. Infected Retnla−/− Retnlb−/− mice exhibited similar responses to Retnla−/− mice, including increased mortality and lung inflammation. This inflammatory response in Retnla−/− Retnlb−/− mice negatively impacted N. brasiliensis fitness, as demonstrated by significantly lower worm ATP levels and decreased intestinal worm burden and fecundity. Lung cytokine analysis revealed that Retnla−/− and Retnla−/− Retnlb−/− mice expressed significantly increased levels of interleukin-4 (IL-4). Finally, we generated Retnla−/− mice on the Rag−/− background and observed that the effects of RELMα were abrogated in the absence of adaptive immunity. Together, these data demonstrate that RELMα but not RELMβ significantly impacts the immune response to N. brasiliensis infection by downregulating the Th2 adaptive immune response in the lung, which protects the host but allows improved parasite fitness.
INTRODUCTION
Soil-transmitted helminths (STHs) afflict over 2 billion individuals worldwide and can cause anemia and debilitating symptoms in the lung and intestine (1). Host protection against STHs is dependent on the balance between effector immune responses that promote worm expulsion and immunoregulatory pathways that control excessive infection-induced inflammation (2). Resistin-like molecules (RELMs) are secreted proteins with putative effector and immunoregulatory functions against helminth infections (3, 4). In humans, significant increases in resistin expression occur in both filarial nematode and gastrointestinal nematode infections (5, 6). In mice, RELMα and RELMβ, which share sequence homology with human RELMβ, are induced in response to Schistosoma mansoni and the gastrointestinal helminths Trichuris muris, Heligmosomoides polygyrus, and Nippostrongylus brasiliensis (7–11).
Despite sharing sequence identity and expression patterns, the putative functions of RELMα and RELMβ in helminth infection are different and involve both host-specific and parasite-specific effects. S. mansoni and N. brasiliensis infection led to increased RELMα expression in infected tissue by epithelial cells and innate immune cells, including macrophages and eosinophils, which acted to suppress Th2 immunity (12, 13). Additionally, in ovalbumin-induced allergic airway inflammation, RELMα overexpression led to reduced levels of Th2 cytokines in the lung (14). RELMα is also expressed by dendritic cells in response to interleukin-4 (IL-4), where it is important for the early priming of CD4+ Th2 cells (15). In contrast, RELMβ is expressed by intestinal epithelial cells following helminth infection. In chronic T. muris infection, RELMβ promoted macrophage and T cell activation, leading to increased intestinal inflammation and impaired Th2 immune responses (10). RELMβ also exerts direct effects on helminth parasites. Indeed, RELMβ treatment of N. brasiliensis and H. polygyrus impacted the ability of the parasites to feed, leading to decreased viability and fecundity (11, 16). These studies suggest distinct functions for RELMα and RELMβ in helminth infection: RELMα suppresses Th2-mediated immune responses and impairs helminth expulsion, while RELMβ promotes expulsion. Given these contrasting roles, the functional outcome of the high levels of expression of both proteins in helminth infection is unknown.
We sought to compare the roles of RELMα and RELMβ in infection with N. brasiliensis, which infects the lung and small intestine of mice. N. brasiliensis infection of wild-type (WT) C57BL/6 mice induced significant increases in both RELMα and RELMβ protein levels in the serum and in infected lungs and jejunum, with maximal expression immediately following exposure to the parasite. Although both proteins were significantly upregulated in response to N. brasiliensis, infection of Retnla−/− and Retnlb−/− mice revealed that they had functionally distinct effects on helminth immunity and inflammation. Retnla−/− mice suffered from severe infection-induced inflammation following parasite invasion of the lung tissue, leading to increased weight loss, infection-induced mortality, and increased lung hemorrhaging and eosinophil infiltration compared to WT mice. Histological examination of infected lung sections in Retnla−/− mice revealed heightened perivascular inflammation and larger inflammatory foci with foreign-body giant cells, indicative of an abnormally increased local inflammatory response with evidence of increased tissue damage. In contrast, Retnlb−/− mice did not suffer from exacerbated infection-induced mortality or acute weight loss but exhibited increased lung inflammation. Retnla−/− mice also had reduced intestinal worm burdens and parasite egg production compared to those of WT or Retnlb−/− mice. We tested the importance of both RELMα and RELMβ by generating and validating Retnla−/− Retnlb−/− mice. Following infection with N. brasiliensis, Retnla−/− Retnlb−/− mice suffered from severe acute lung inflammation. This heightened immune response in the lungs of Retnla−/− Retnlb−/− mice resulted in significantly reduced parasite fitness as assessed by worm ATP levels. This reduced worm viability impaired the ability of the worms to infect the intestine and led to accelerated worm expulsion in Retnla−/− Retnlb−/− mice. Given that worm expulsion is promoted by Th2 immune responses, we evaluated the cytokine response in the lungs of infected WT, single-gene-knockout (KO), and double-gene KO mice. Although Retnlb−/− mice exhibited Th2 cytokine expression equivalent to that of WT mice, Retnla−/− and Retnla−/− Retnlb−/− mice had significantly increased IL-4 levels in the lungs compared to WT mice. Additionally, real-time reverse transcription-PCR (RT-PCR) analysis of CD4+ T cells purified from WT or Retnla−/− Retnlb−/− mice revealed significantly elevated CD4+ T cell-derived IL-4 levels. These results suggest that RELMα effects on lung inflammation and parasite expulsion occur through the promotion of adaptive CD4+ Th2 cells. To test this, we generated Rag1−/− Retnla−/− mice, which are deficient in adaptive immune cells, including CD4+ T cells. Rag1−/− Retnla−/− mice exhibited lung inflammation, parasite burdens, and Th2 cytokine levels equivalent to those of Rag1−/− mice, suggesting that the functional effects of RELMα are dependent on adaptive immunity. Taken together, these studies demonstrate the importance of RELMα but not RELMβ in the immune response to hookworm infection, where it downregulates CD4+ Th2 cells, leading to delayed worm expulsion, but provides a critical function in protecting the host from excessive and potentially fatal lung inflammation.
MATERIALS AND METHODS
Mice.
WT C57BL/6 mice purchased from the Jackson Laboratory were bred in-house. Retnla−/− and Retnlb−/− mice were generated and genotyped as previously described (12, 17). Retnla−/− Retnlb−/− mice were generated with VelociGene technology (18), where all exons for RELMα and RELMβ were replaced by a lacZ reporter gene and a neomycin selection cassette. For genotyping of Retnla−/− Retnlb−/− mice, genomic DNA was prepared from ear clips by using a Bioland Scientific Quick Genotyping DNA preparation kit, followed by PCR for the insert with primers LacInF (GGTAAACTGGCTCGGATTAGGG) and LacInR (TTGACTGTAGCGGCTGATGTTG) or quantitative PCR (qPCR) for the WT gene with the TaqMan MGB probe 6-carboxyfluorescein (FAM)-ACCATCCCTGCAAGATCA from Bio-Rad and primers 608TUF (GTCCAAGGGCTCTGACATCTG) and 608TUR (AGCCATAGCCACAAGCACATC). All genetically deficient mice were backcrossed with C57BL/6 mice (11 generations for Retnla−/− and Retnlb−/− mice and 12 generations for Retnla−/− Retnlb−/− mice), and successful backcrossing was validated by using DartMouse genotyping services. Rag1−/− Retnla−/− mice were generated by crossing Retnla−/− mice with Rag1−/− C57BL/6 mice purchased from the Jackson Laboratory. Mice were age matched (6 to 10 weeks old), gender matched, and housed five per cage under an ambient temperature with a 12-h-light/12-h-dark cycle. All protocols for animal use and euthanasia were approved by the University of California—Riverside Institutional Animal Care and Use Committee (protocols A-20120023B and A-20120024E [see http://or.ucr.edu/ori/committees/iacuc.aspx]) and were in accordance with National Institutes of Health guidelines, the Animal Welfare Act, and Public Health Service Policy on Humane Care and Use of Laboratory Animals (19).
Parasites.
N. brasiliensis was obtained from the laboratory of Graham Le Gros (Malaghan Institute, New Zealand), and the life cycle was maintained in Sprague-Dawley rats as previously described (20). Mice were injected subcutaneously with 500 N. brasiliensis third-stage larvae (L3). Following infection, mice were monitored every 1 to 2 days and euthanized in the case of severe infection-induced morbidity as defined by approved IACUC protocols in the laboratory. In our laboratory, with this N. brasiliensis strain, infection of C57BL/6 mice leads to maximal numbers of larvae in the lung by 48 to 72 h (∼50% original inoculum), followed by adult worms reaching the intestine at between days 3 and 4 (∼25% original inoculum). Fecal egg production is observed between days 6 and 9, and parasite expulsion occurs by day 10. Parasites in the lung and small intestine were enumerated after the entire lung or small intestine of infected mice was cut and incubated in phosphate-buffered saline (PBS) at 37°C for >1 h to allow worms to migrate out of the tissue. Eggs in the feces of infected mice were counted by using a McMaster counting chamber on days 6 to 9 following infection.
Parasites collected from the lungs and small intestines were washed with PBS, and the concentration of ATP was measured as previously described (20). Briefly, 10 larvae in 150 μl PBS were mixed with 150 μl CellTiter-Glo 2.0 luminescent reagent (Promega) and homogenized at 4°C. The homogenates were centrifuged at 1,000 × g for 2 min, and 100-μl supernatants in duplicates were transferred to a black 96-well clear-bottom plate and incubated at room temperature for 10 min to stabilize the luminescence signal. An ATP standard curve was included by using ATP disodium salt (Sigma). The PBS wash was used as a negative control. Luminescence was recorded by using a Glomax Multi detection system (Promega).
Sample collection, processing, and flow cytometry.
Bronchoalveolar lavage (BAL) fluid and cells were recovered through washing twice with 1 ml of ice-cold PBS. Cells were recovered by centrifugation, and red blood cells (RBCs) and white blood cells (WBCs) were enumerated by using the Bio-Rad cell counter pre- and post-RBC lysis. For flow cytometry, BAL fluid cells were blocked with 0.6 μg rat IgG and 0.6 μg anti-CD16/32 (2.4G2) and stained for 25 min with antibodies for SiglecF (E50-2440) and Ly6G (1A8) (all from BD Biosciences); F4/80 (BM8), CD11b (M1/70), and CD11c (N418) (all from eBioscience, Affymetrix); and CD4 (RM4-5; Invitrogen). Cells were then washed and analyzed on an LSRII instrument (BD Bioscience), followed by data analysis using FlowJo v10 (Tree Star Inc.). To analyze CD11c− SiglecF+ eosinophils, CD11b+ Ly6G+ neutrophils, and CD4+ T cells, a lineage-negative gate (autofluorescence positive and CD11c+ F4/80+) to gate out alveolar macrophages and dendritic cells was first applied.
Lung and jejunum (1 cm) were weighed and homogenized in 0.5 ml PBS with a Mini-Beadbeater-96 instrument (BioSpec Products) at 4°C, and the supernatant was collected after centrifugation at 4,000 × g for 15 min at 4°C.
For hematoxylin and eosin (H&E) staining, lungs were first inflated with 10% neutral buffered formalin. Jejunum and inflated lungs were fixed with 10% neutral buffered formalin and embedded in paraffin wax. Sections (5 μm) were obtained and stained with hematoxylin and eosin.
Enzyme-linked immunosorbent assay (ELISA) for RELM protein quantification.
Greiner 96-well plates were coated with primary antibodies to RELMα or RELMβ (1 μg/ml; Peprotech) overnight at room temperature. After blocking the plates with 5% newborn calf serum in PBS for 1 h, sera, BAL fluid, or tissue homogenates were added at various dilutions and incubated at room temperature for 2 h. Detection of RELMα and RELMβ was done with biotinylated antibodies (0.5 μg/ml; Peprotech) for 1 h, followed by incubation with streptavidin-peroxidase (Jackson Immunobiology) for 1 h in the dark. The peroxidase substrate 3,3′,5,5′-tetramethylbenzidine (TMB) (BD) was added, followed by the addition of 2 N H2SO4 as a substrate stop, and the optical density (OD) was captured at 450 nm. Samples were compared to a serial fold dilution of recombinant protein.
CD4+ cell purification.
Lungs were removed, cut into small pieces, and incubated with 2 mg/ml collagenase D (Roche) in PBS for 45 min at 37°C with vortexing every 10 to 15 min. The digested tissues were passed through 70-μm cell strainers and washed with Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 1% l-glutamine (Gibco), and 1% penicillin-streptomycin (Gibco). Red blood cells were lysed by using ammonium chloride-potassium (ACK) red blood cell lysis buffer (Gibco). To remove macrophages, cells were cultured in a 6-well plate for 2 h. Nonadherent cells were magnetically labeled with mouse CD4 (L3T4) MicroBeads (Miltenyi Biotec) and then loaded onto a magnetically activated cell sorter (MACS) column, according to the manufacturer's instructions.
Real-time PCR.
RNA from lung tissue was extracted with TRIzol, and RNA from cells was extracted by using an RNeasy minikit (Qiagen). iScript reverse transcriptase (Bio-Rad) was used for cDNA synthesis. RT-qPCR was performed with the Bio-Rad CFX Connect system using Bio-Rad CFX Manager 3.1 software. Primer sequences (from IDT) for real-time PCR are Il4 forward (F) primer CTCAGTACTACGAGTAATCCA, Il4 reverse (R) primer GAATGTACCAGCAAGCCATATC, Il10 F primer AGTGGAGCAGGTGAAGAGTG, Il10 R primer TTCGGAGAGAGGTACAAACG, Il12b F primer GGAAGCACGGCAGCAGAATA, and Il12b R primer AACTTGAGGGAGAAGTAGGAATGG. All other primers (for Gapdh, Ifng, Il13, and Il17a) were purchased from Qiagen.
Statistical analysis.
Data were expressed as means ± standard errors of the means of data for 3 to 6 animals per group. Results are representative of data from 3 to 6 experiments. Gaussian distribution was determined by the D'Agostino-Pearson normality test. For data with a Gaussian distribution, the following parametric analyses were used: unpaired Student's t test (two-group comparisons), one-way analysis of variance (ANOVA) (comparisons of three or more groups with one variable), and two-way ANOVA (comparisons of three or more group with two variables) followed by post hoc multiple-comparison tests (Dunnett or Bonferroni test for parametric data and Dunn's test for nonparametric data). Log base 10 transformation or Kruskal-Wallis ANOVA was used for data without a Gaussian distribution. Comparison of survival curves was done by using a log rank (Mantel-Cox) test. All statistical analyses were performed and graphs were drawn by using GraphPad Prism version 6.02.
RESULTS
Kinetics of RELMα and RELMβ expression following N. brasiliensis infection.
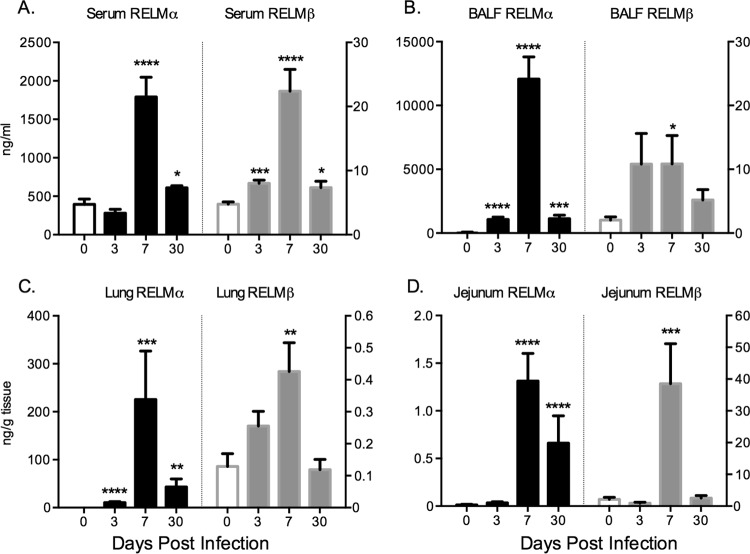
Previous studies have shown that N. brasiliensis infection of the lung and small intestine drives the potent upregulation of RELMα and RELMβ (7, 13); however, a systematic analysis of RELMα and RELMβ protein levels locally in infected tissue and in serum has not been performed and may give important insight into the function and persistence of these highly expressed genes. WT C57BL/6 mice were untreated (day 0) or infected subcutaneously with 500 N. brasiliensis L3 followed by sacrifice at acute (day 3 and day 7) and chronic (day 30) time points. N. brasiliensis infection led to significant increases in serum RELMα and RELMβ levels at day 7 postinfection (Fig. 1A), the time point at which adult parasites are present in the small intestine. RELMα and RELMβ protein levels were also increased in infected tissue but with tissue-specific differences. Specifically, RELMα expression was induced at higher levels in the lung (Fig. 1B and C), coincident with parasite invasion of the lung tissue, while RELMβ expression was dominant in the infected jejunum at day 7 postinfection, when adult worms are present (Fig. 1D). Although infection-induced increases in the levels of both proteins were observed in all the tissues and in serum, there was a striking compartmentalization of RELMα and RELMβ expression: RELMα was expressed at ∼100-, 500-, and 1,000-fold-higher levels than RELMβ in serum, lung, and BAL fluid, respectively, while RELMβ was expressed at >30-fold-higher levels than RELMα in the jejunum. This suggests that RELMα expression in the lung and serum may be more biologically relevant, while the function of RELMβ may be more important in the intestine. On the other hand, it is also possible that the active concentrations of both RELM proteins may be different, which could impact the biological significance of the different concentrations of these proteins in different tissues. Even though N. brasiliensis is expelled between day 9 and day 12 postinfection in WT mice, previous studies have shown long-lasting inflammatory changes in infected tissue even following worm clearance (21–23). At day 30 postinfection, we observed increased RELMα levels in serum and all infected tissues, while the RELMβ level was significantly increased in serum, suggesting that N. brasiliensis infection induces persistent changes in RELMα and RELMβ expression levels that may be functionally significant.
FIG 1.
RELMα and RELMβ are expressed systemically and locally following N. brasiliensis infection. C57BL/6 mice were injected with PBS or infected with N. brasiliensis for 3, 7, and 30 days, followed by analysis of RELMα and RELMβ in the serum (A), bronchoalveolar lavage fluid (BALF) (B), lung (C), and jejunum (D) (n = 4 to 5 per group). Experiments were repeated 3 to 5 times. Statistical analysis compared to naive mice was performed. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001.
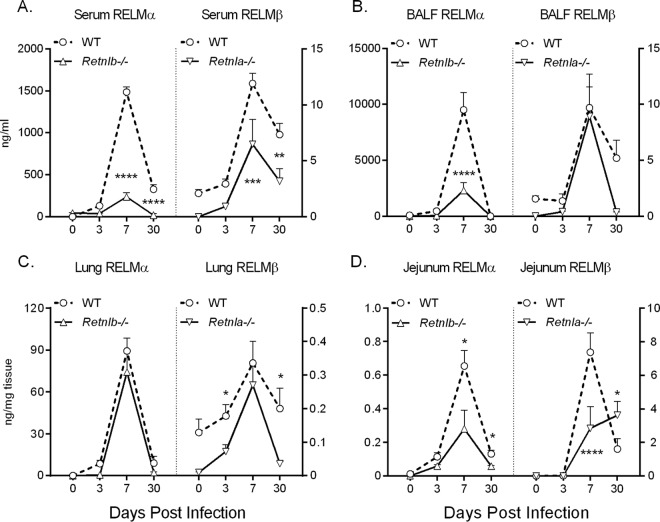
To test the functional significance of RELMα and RELMβ in N. brasiliensis infection, we utilized Retnla−/− and Retnlb−/− mice, which were previously described (12, 17, 24). Following infection with N. brasiliensis, Retnlb−/− mice exhibited a significant impairment in RELMα expression compared to WT mice (Fig. 2A to D, left). Conversely, Retnla−/− mice had significantly reduced infection-induced RELMβ expression levels (Fig. 2A to D, right). Given that the Retnla and Retnlb genes are adjacent in the genome, it is possible that the deletion of one gene removed enhancer elements that influenced the expression of the other gene. This is an important consideration when using these genetically deficient mice.
FIG 2.
Effect of Retnla or Retnlb genetic deletion on expression of RELMβ or RELMα, respectively. C57BL/6 (WT), Retnla−/−, and Retnlb−/− mice were injected with PBS or infected with N. brasiliensis for 3, 7, and 30 days. RELMα and RELMβ expression levels in the serum (A), BAL fluid (B), lung (C), and jejunum (D) were measured. Results are combined data from >3 experiments (n = 3 to 5 for naive mice, and n = 3 to 6 for mice infected at each time point). Statistical analysis was performed for each genotype compared to the WT at the same time point. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001.
RELMα but not RELMβ protects from excessive acute and chronic lung inflammation.
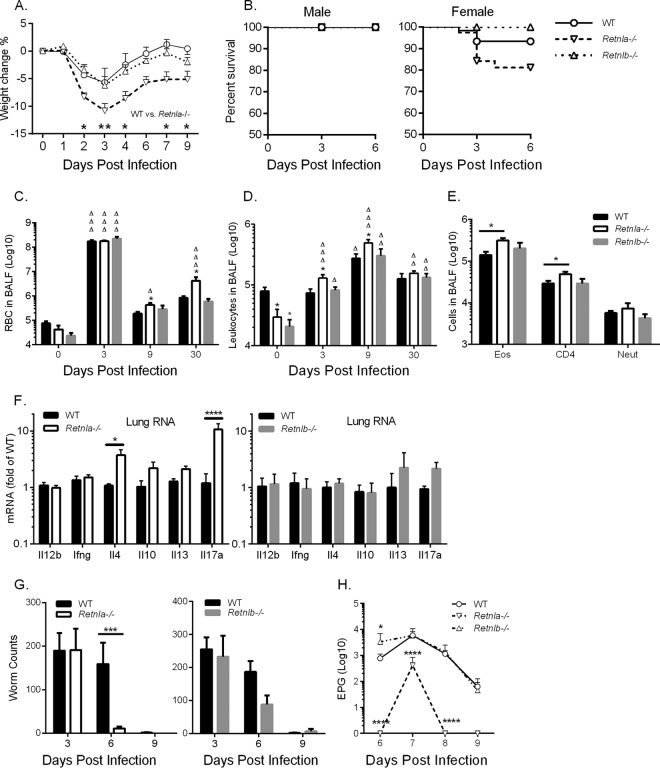
We next examined the functional consequence of RELMα and RELMβ deletion in immunity and inflammation against N. brasiliensis. In response to N. brasiliensis infection, Retnla−/− mice suffered from severe infection-induced inflammation, as evidenced by significantly increased weight loss beginning at day 2 postinfection, when the parasite had invaded the lung tissue (Fig. 3A). We performed a combined analysis of all N. brasiliensis infection experiments to examine the consequences of RELMα and RELMβ deficiency on infection-induced mortality (Fig. 3B). Although all male mice recovered from N. brasiliensis-induced acute inflammation, female WT mice suffered from ∼10% infection-induced mortality, which was more severe in the absence of RELMα (∼20%). In contrast, we did not observe exacerbated infection-induced weight loss or infection-induced mortality in Retnlb−/− mice. We performed BAL washes of the lung and observed infection-induced hemorrhaging at day 3 postinfection in all infected genotypes, as assessed by RBC numbers in the BAL fluid (Fig. 3C). While WT and Retnlb−/− mice recovered from infection-induced hemorrhaging at days 9 and 30 postinfection, Retnla−/− mice had significantly increased RBC numbers at these later time points, implicating a partial defect in the ability to recover from acute inflammation induced by N. brasiliensis. The heightened early inflammatory response in Retnla−/− mice was also reflected by the significantly increased number of BAL fluid leukocytes (Fig. 3D). We characterized the leukocyte populations by flow cytometry and observed significantly increased numbers of eosinophils and CD4+ T cells in the BAL fluid of infected Retnla−/− mice compared to those in WT or Retnlb−/− mice (Fig. 3E). Next, we assessed the cytokine environment in the lungs of infected mice on day 7 by real-time RT-PCR (Fig. 3F). While we observed no significant differences in levels of Th1-associated cytokines, infected Retnla−/− mice exhibited significantly elevated IL-4 and IL-17A expression levels in comparison to WT mice. In contrast, Retnlb−/− mice had no significant differences in lung cytokine expression levels compared to WT mice. Together, the results of these studies demonstrate distinct expression patterns and functions for RELMα and RELMβ in N. brasiliensis infection: RELMα is the dominant RELM protein expressed early in the infected lung, where it plays a critical role in dampening Th2 and Th17 cytokine expression and hookworm-induced lung inflammation.
FIG 3.
RELMα but not RELMβ protects from excessive lung inflammation and impairs parasite expulsion. (A and B) WT, Retnla−/−, and Retnlb−/− mice were infected with N. brasiliensis and monitored for infection-induced weight loss (n = 4 to 9 per group) (A) and mortality (for WT mice, n = 48 males and 52 females; for Retnla−/− mice, n = 24 males and 29 females; and for Retnlb−/− mice, n = 21 males and 15 females) (B). (C and D) Cells recovered from BAL fluid were counted for RBCs (C) and leukocytes (D) (n = 3 for naive mice, and n = 5 to 8 for infected mice). (E) Flow cytometric analysis of cell populations at day 9 postinfection (n = 5 to 8). (F) Real-time PCR analysis of lung mRNA from infected mice on day 7 (n = 4 to 5 per group). (G) Worm counts from the lung (day 3) and small intestine (days 3, 6, and 9) (n = 4 to 9 per group). (H) Fecal egg output over time quantified as eggs per gram of feces (EPG) (n = 5 to 9 per group). For statistical analyses, asterisks indicate comparison to the WT at the same time point, and open triangles indicate comparison to mice of the same genotype at day 0 (naive). For panel H, log transformation was done, followed by two-way ANOVA with Dunnett's multiple-comparison test (compared to the WT at the same time point). *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001. △, P < 0.05; △△, P < 0.01; △△△, P < 0.001.
Retnla−/− mice exhibit equivalent N. brasiliensis burdens in the lung but accelerated expulsion from the intestine compared to WT and Retnlb−/− mice.
To test if the exacerbated early lung inflammation in Retnla−/− mice was due to increased parasite burdens in the lung, we evaluated parasite burdens in the lung and intestine and quantified egg production in feces (Fig. 3G and H). We observed equivalent N. brasiliensis burdens in WT, Retnla−/−, and Retnlb−/− mice at day 3 postinfection; however, Retnla−/− mice exhibited more rapid N. brasiliensis expulsion than did WT mice, with significantly fewer parasites in the intestine at day 7 postinfection. This suggested that the exacerbated inflammatory response in the lungs of Retnla−/− mice could have impaired parasite fitness, leading to more rapid immune-mediated expulsion. Consistent with this hypothesis, parasite egg burdens in the feces were significantly reduced in infected Retnla−/− mice compared to WT mice, while Retnlb−/− mice exhibited significantly higher egg burdens in feces at day 6 postinfection than those of all groups of mice. Combined, these findings suggest that RELMα both downregulates excessive lung inflammation following N. brasiliensis infection and delays parasite expulsion, while RELMβ impairs parasite fecundity.
Generation of Retnla−/− Retnlb−/− mice to evaluate the function of RELMα and RELMβ in N. brasiliensis-induced inflammation and immune-mediated expulsion.
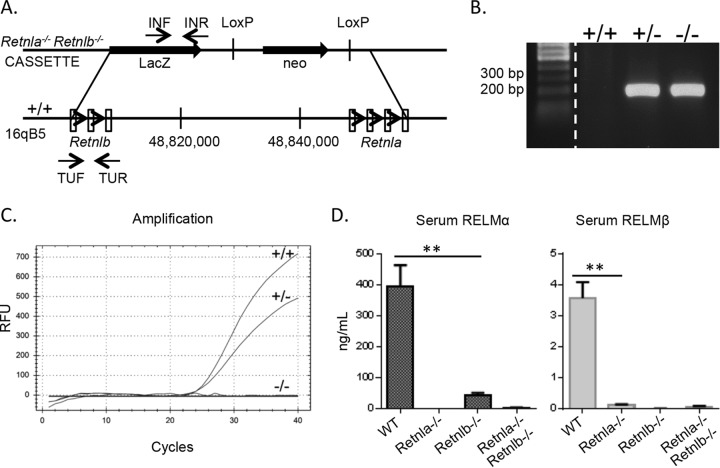
Given the distinct functions of RELMα and RELMβ in response to N. brasiliensis infection, we sought to assess the functional significance of both RELM proteins by generating Retnla−/− Retnlb−/− mice. Since there is only one human RELM protein (human RELMβ) that shares sequence homology with murine RELMα and RELMβ, studies of Retnla−/− Retnlb−/− mice may give more insight into the putative function of human RELMβ. Retnla−/− Retnlb−/− mice could not be generated by breeding since the Retnla and Retnlb genes are adjacent on chromosome 16 and do not segregate. Therefore, Retnla−/− Retnlb−/− mice were generated with VelociGene technology (18), where all exons for RELMα and RELMβ were replaced by a lacZ reporter gene and a neomycin selection cassette (Fig. 4). Gene deletion was confirmed by genotyping PCR, real-time PCR for Retnla and Retnlb, and a serum ELISA for RELMα and RELMβ. Mice were crossed with C57BL/6J mice for 12 generations and validated as fully backcrossed by DartMouse genotyping services. Similar to Retnla−/− and Retnlb−/− mice, naive Retnla−/− Retnlb−/− mice were healthy and fertile and did not exhibit any obvious behavioral changes or impairment in immune cell development or composition of lymphoid compartments (data not shown).
FIG 4.
Generation and characterization of Retnla−/− Retnlb−/− mice. (A) Schematic diagram of Retnla and Retnlb loci. (B to D) The mouse genotype was confirmed by PCR with primers LacInF and LacInR for the KO gene (B), real-time PCR for the WT gene (C), and an ELISA of serum of naive mice (D). RFU, relative fluorescence units. Statistical significance was determined by using an unpaired t test. **, P < 0.01.
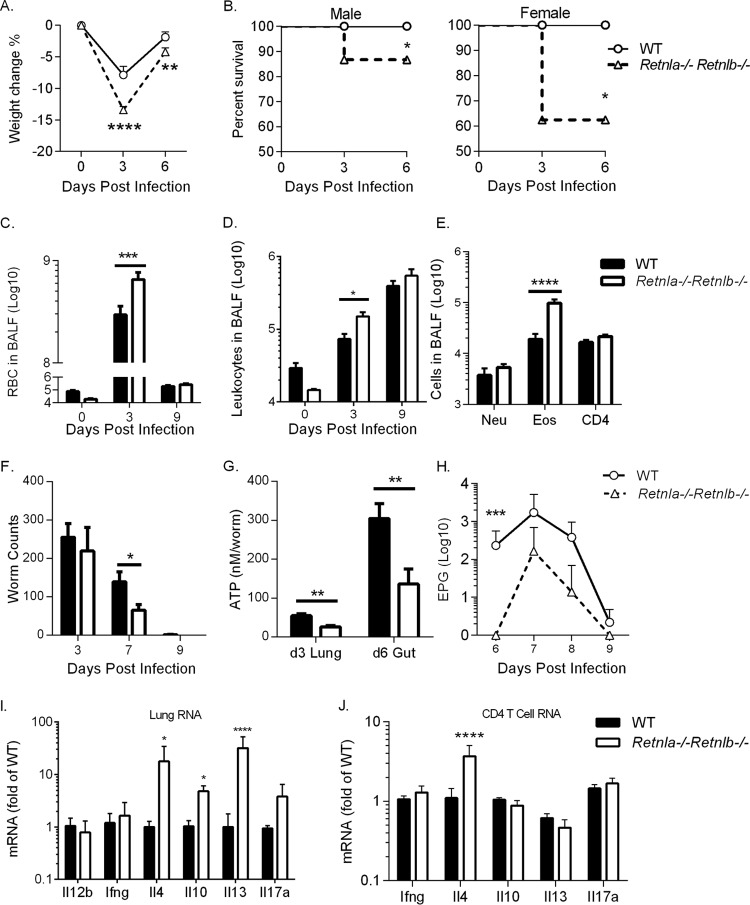
Compared to WT mice, infected Retnla−/− Retnlb−/− mice suffered from significant infection-induced weight loss (Fig. 5A). While WT mice did not succumb to infection, Retnla−/− Retnlb−/− mice exhibited infection-induced mortality by day 3 postinfection, with 15% mortality in males, while females were more susceptible (40% mortality) (Fig. 5B). This infection-induced mortality was more severe than that in single Retnla−/− mice (Fig. 3B). Retnla−/− Retnlb−/− mice also exhibited severe lung inflammation, including increased hemorrhaging (Fig. 5C) and numbers of BAL fluid leukocytes (Fig. 5D) and eosinophils (Fig. 5E). We observed equivalent parasite burdens at day 3 postinfection but reduced intestinal worm and fecal egg burdens at day 7 postinfection (Fig. 5F and H). Additionally, analysis of worm ATP levels as an indicator of worm fitness revealed significantly reduced ATP levels in worms isolated from the lung and small intestine of double-gene KO mice at days 3 and 6 postinfection, respectively (Fig. 5G). We assessed the lung cytokine environment in WT and double-gene KO mice and observed significantly increased IL-4, IL-10, and IL-13 expression levels in the double-gene KO mice (Fig. 5I). This suggested that the impaired worm fitness in the double-gene KO mice might be mediated through an increased Th2 effector immune response. To test if the increased levels of Th2 cytokines were CD4+ T cell derived, we purified lung CD4+ T cells from WT and double-gene KO mice. Although we saw no differences in interferon gamma (IFN-γ), IL-10, IL-13, and IL-17A expression levels, there was a significantly increased expression level of IL-4 by CD4+ T cells isolated from infected double-gene KO mice (Fig. 5J). Therefore, while CD4+ T cells in the Retnla−/− Retnlb−/− mice contributed to the increased IL-4 expression in lung, other lung cell populations contributed to the increased IL-10 and IL-13 expression. These populations may include innate cells such as macrophages, which are known to produce high levels of IL-10, and innate lymphoid cells, natural killer cells, or basophils, which express IL-13. In conclusion, these findings suggest that there are complex interactions between RELMα and RELMβ that positively affect parasite infectivity and viability, potentially through dampening the CD4+ Th2 effector immune response to the worm.
FIG 5.
Function of RELMα and RELMβ in N. brasiliensis-induced inflammation and immune-mediated expulsion. (A and B) C57BL/6 (WT) and Retnla−/− Retnlb−/− mice were infected with N. brasiliensis and monitored daily for infection-induced weight loss (n = 4 to 5 per group) (A) and mortality (for WT mice, n = 31 males and 9 females, and for Retnla−/− Retnlb−/− mice, n = 26 males and 8 females) (B). (C and D) BAL fluid cells were quantified for RBCs (C) and leukocytes (D) (n = 4 to 8 per group). (E) Flow cytometric analysis of cell populations at day 9 postinfection (n = 5 per group). (F and G) Parasites were recovered from the lung and small intestine at day 3 and the small intestine at days 6, 7, and 9 for worm quantification (F) and worm ATP measurements (G) (n = 4 to 8 per group). (H) Fecal egg output over time (n = 5 to 9 per group). (I and J) Real-time PCR analysis of mRNA from whole lung (n = 3 to 5 per group) (I) and purified lung CD4+ T cells (n = 6 to 8 per group) (J) from infected mice on day 7. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001.
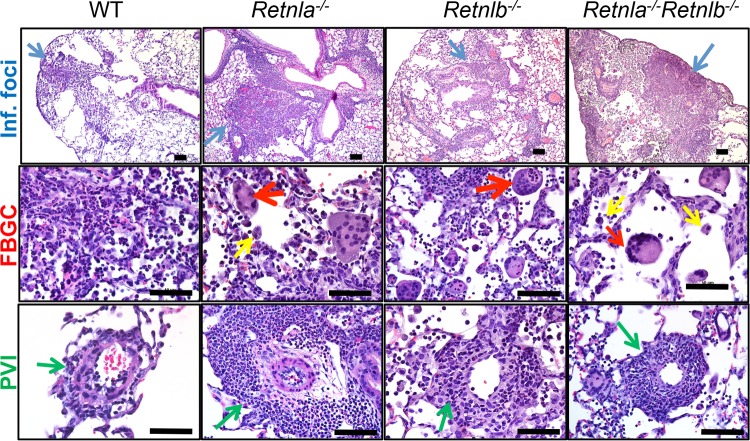
To delineate the effects of RELMα and RELMβ on lung inflammation, histological examination of lung tissue sections from infected WT, single-gene-deficient, and double-gene-deficient mice was performed (Fig. 6). N. brasiliensis infection induced several pathological changes in the lung, including a mixed neutrophil-eosinophil-monocyte infiltrate in the peribronchial connective tissue with variable extension into the alveolar airspaces (Fig. 6, top, blue arrows), a prominence of activated alveolar macrophages (middle, yellow arrows) and foreign-body-type giant cells (FBGCs) (middle, red arrows), and perivascular inflammation (bottom, green arrows). Compared to WT mice, these pathological changes were severe in both single-gene-deficient and double-gene-deficient mice, suggesting that both RELMα and RELMβ dampen excessive lung inflammation. Interestingly, Retnla−/− mice exhibited more severe lung inflammation than did Retnla−/− Retnlb−/− mice. The increased presence of FBGCs is indicative of persistent inflammation and a potential defect in tissue repair and debris clearance in the absence of RELMα.
FIG 6.
Pathological changes induced by N. brasiliensis infection. Lung sections from naive or N. brasiliensis-infected mice were stained with H&E, and pathological changes at day 9 postinfection were independently assessed by a pathologist (J.I.O.). Infection-induced inflammation is depicted by representative pictures. Inflammatory foci (Inf. Foci) (blue) (magnification, ×20; bar, 200 μm), perivascular inflammation (PVI) (green), foreign-body giant cells (FBGC) (red), and activated alveolar macrophages (yellow) (magnification, ×40; bar, 50 μm) are shown.
Generation of Rag1−/− Retnla−/− mice reveals that RELMα-mediated effects are dependent on the adaptive response.
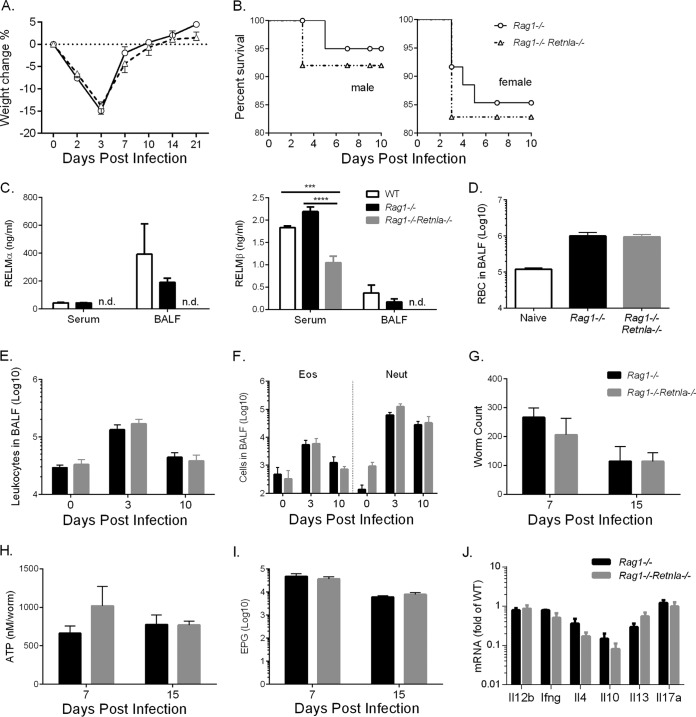
The lung cytokine expression data demonstrated that Retnla−/− and Retnla−/− Retnlb−/− mice exhibited a more Th2-polarized environment than WT mice. Additionally, purification of CD4+ T cells from Retnla−/− Retnlb−/− and WT lungs suggested that CD4+ T cells contribute to the increased Th2 cytokine environment in KO mice. Although the RELMα-mediated effects are observed during acute primary infection with N. brasiliensis, these data implicate the contribution of the adaptive immune response, in particular CD4+ Th2 cells, to this early phenotype. Alternatively, innate cells such as eosinophils and type 2 innate lymphoid cells (ILC2) could contribute to the dysregulated Th2 cytokine environment in Retnla−/− and Retnla−/− Retnlb−/− mice. To definitively test this, we generated Rag1−/− Retnla−/− mice that lack B and T cells and compared these mice to Rag1−/− mice. In contrast to WT mice, which expel N. brasiliensis by day 10 postinfection, Rag1−/− mice remain chronically infected with N. brasiliensis until as long as day 40 postinfection due to the lack of an adaptive immune response. Following infection, Rag1−/− and Rag1−/− Retnla−/− mice suffered equivalent weight loss and exhibited minimal differences in infection-induced mortality (Fig. 7A and B). Quantification of serum and BAL fluid RELMα and RELMβ levels in WT, Rag1−/−, and Rag1−/− Retnla−/− mice (Fig. 7C), revealed equivalent levels of RELMα and RELMβ in infected WT and Rag1−/− mice, suggesting that RELM protein expression following N. brasiliensis infection is not dependent on B or T cells. As expected, given the deficiency in RELMβ expression in Retnla−/− mice, RELMβ expression was also significantly reduced in infected Rag1−/− Retnla−/− mice compared to that in Rag1−/− mice. Evaluation of the lung inflammatory response following N. brasiliensis infection revealed that there were no significant differences in infection-induced hemorrhaging or leukocyte numbers (Fig. 7D to F). Furthermore, parasite burden, viability, and fecundity were equivalent in Rag1−/− and Rag1−/− Retnla−/− mice (Fig. 7G to I). We also monitored egg burdens over time for up to 40 days postinfection and observed no significant differences between Rag1−/− and Rag1−/− Retnla−/− mice (data not shown). Finally, we quantified cytokine RNA levels in the lungs of infected mice and did not observe any significant differences in levels of Th1, Th2, or Th17 cytokines (Fig. 7J). Taken together, these results show that infection-induced RELM protein expression occurs in the absence B and T cells; however, RELMα-mediated downregulation of the Th2 inflammatory responses and delay in parasite expulsion are dependent on the adaptive immune response.
FIG 7.
Generation of Rag1−/− Retnla−/− mice reveals that RELMα-mediated effects are dependent on the adaptive response. (A and B) WT, Rag1−/−, and Rag1−/− Retnla−/− mice were infected with N. brasiliensis and monitored daily for infection-induced weight loss (n = 6 to 8 per group) (A) and mortality (for Rag1−/− mice, n = 23 males and 23 females, and for Rag1−/− Retnla−/− mice, n = 31 males and 29 females) (B). (C) RELMα and RELMβ protein levels in serum and BAL fluid at day 3 postinfection (n = 4 per group). (D and E) Quantification of RBCs (D) and leukocytes (E) at day 10 postinfection (n = 3 to 4 per group). (F) Flow cytometric analysis of BAL fluid cell populations (n = 3 to 4 per group). (G and H) Parasites were recovered from the small intestine for worm quantification (G) and worm ATP measurements (H) (n = 3 to 8 per group). (I) Quantification of fecal egg output (n = 3 to 8 per group). (J) Real-time PCR analysis of lung mRNA from infected mice on day 7. ***, P < 0.001; ****, P < 0.0001.
In conclusion, comparison of single-gene-deficient Retnla−/− and Retnlb−/− mice and double-gene-deficient Retnla−/− Retnlb−/− and Rag1−/− Retnla−/− mice suggests that RELMα is the more functionally important RELM protein in hookworm infection, where it downregulates CD4+ Th2 cell responses and protects the host from potentially fatal lung inflammation at the expense of a moderately impaired antiparasitic effector response.
DISCUSSION
Protective immunity to hookworm infections relies on the balance between effector immune responses that limit parasite burden and regulatory immune responses to control excessive inflammation. In both primary and secondary challenges with N. brasiliensis, immune cells in the lung, including neutrophils, macrophages, and CD4+ T cells, critically affect parasite development and viability, allowing more rapid worm expulsion (25–27). However, if unchecked, this effector immune response can be detrimental to the host, leading to excessive and potentially fatal lung inflammation (27, 28). Here, we examined the immune effector and regulatory functions of the secreted proteins RELMα and RELMβ following N. brasiliensis infection. Previous independent studies showed distinct functions for these proteins, with RELMα acting to suppress Th2 immunity, while RELMβ was reported to be an effector molecule that bound to the parasite (13, 16). To investigate this, we quantified RELMα and RELMβ expression levels following N. brasiliensis infection and systematically characterized the immune response and parasite burdens in single-gene-deficient Retnla−/− mice and Retnlb−/− mice as well as double-gene-deficient Retnla−/− Retnlb−/− mice.
We found that N. brasiliensis infection led to significant increases in RELMα and RELMβ levels systemically and in infected tissue. Functionally, RELMα and RELMβ had distinct effects on the host immune response. While RELMα dampened infection-induced inflammation, leading to an increased parasite burden, RELMβ had modest effects on acute lung inflammation and parasite burden, which was different from a previously reported finding that RELMβ promoted parasite expulsion (16). These differences might be explained by the high virulence of the N. brasiliensis strain used in our studies, which caused excessive lung inflammation and some infection-induced mortality compared to other studies that did not report infection-induced mortality (13, 16). In this context of high virulence, the heightened inflammatory response and high-level RELMα expression in the lung may have overcome the anti-parasitic effects of RELMβ that occurred later in the intestine. RELMα expression may therefore be more functionally relevant for helminths that infect the lung, such as hookworms and Ascaris. In contrast, the effector functions of intestine-derived RELMβ may be more important in helminth infections that are restricted to the gastrointestinal tract.
Analysis of the lung cytokine environment of infected mice revealed that both Retnla−/− and Retnla−/− Retnlb−/− mice, but not Retnlb−/− mice, had increased IL-17A and IL-4 mRNA transcript levels compared to those in WT mice. Given previous studies showing that IL-17A mediates acute N. brasiliensis-induced lung inflammation (28), it is possible that the dysregulated Th17 cytokine environment contributed to acute lung inflammation in Retnla−/− and Retnla−/− Retnlb−/− mice. In that same study, it was shown that IL-4 receptor (IL-4R) deletion led to increased levels of IL-17A associated with increased lung neutrophilia, suggesting that IL-4 signaling inhibits IL-17A. In contrast, we observed that N. brasiliensis-induced lung inflammation in the absence of RELMα was associated with increased levels of both IL-17A and IL-4. Furthermore, characterization of the cell populations recruited to the lung following N. brasiliensis infection revealed increased numbers of CD4+ T cells and eosinophils, but not neutrophils, in Retnla−/− and Retnla−/− Retnlb−/− mice. This suggests that the effects of RELMα deletion are qualitatively different from the effects of deletion of IL-4R or IL-17R signaling. Further studies delineating the effects of IL-4 or IL-17 on Retnla−/− mice may give more insight into which cytokine regulatory pathways are responsible for the downstream RELMα-mediated effects.
Given that eosinophils and CD4+ T cells are important cellular sources of IL-4 (29–32), we postulated that both cell types contributed to the reduced parasite burden observed in Retnla−/− mice through expression of IL-4. However, Rag1−/− Retnla−/− mice exhibited equivalent eosinophil responses, IL-4 expression levels, and parasite burdens compared with those of Rag1−/− mice. These findings suggested that CD4+ T cells and not eosinophils were downstream of the RELMα-mediated effects. Consistent with this, purified lung CD4+ T cells from Retnla−/− Retnlb−/− mice had significantly increased IL-4 mRNA transcript levels compared to those in WT mice. These strategies suggest that the functional effects of RELMα are dependent on the adaptive immune response and are likely mediated through effects on CD4+ Th2 cells. Given that RELMα functional effects are observed in acute primary infection with N. brasiliensis, it is perhaps surprising that the mechanism of action of RELMα is dependent on the adaptive immune response. This suggests that CD4+ Th2 cells may be conditioned by RELMα early or even before the onset of N. brasiliensis-induced inflammation. The existence of innate Th2 cells that function independently of antigen specificity was recently shown in N. brasiliensis infection studies (33). It is possible that RELMα mediates its effects through regulating these innate Th2 cells. Alternatively, given that Rag1−/− mice are deficient in B and CD8+ T cells, it is possible that RELMα functional effects may be dependent on these adaptive immune cells. For instance, B cells are an important cellular source of cytokines (34) and could be regulated by RELMα. However, we previously showed that RELMα preferentially bound CD4+ T cells and not B cells (12), so it is more likely that the RELMα-mediated effects are through CD4+ T cells.
Although intestinal epithelial cells are the main cellular source of murine RELMβ, recent human studies have shown that bronchial epithelial cells in the lung and activated macrophages can express high levels of human RELMβ (35, 36), which fits the expression pattern of mouse RELMα. In our studies, we found that both RELMα and RELMβ were significantly upregulated and maintained in the lung and intestine following N. brasiliensis infection. Delineating the role of both murine RELMα and RELMβ in the context of infection and inflammation may allow a better understanding of human RELMβ function. In previous studies, we have shown the importance of human resistin but not mouse resistin in influencing the immune response to N. brasiliensis (5). Transgenic mice expressing human resistin exhibited delayed parasite expulsion and increased parasite egg burdens, which mirrors our findings here showing that RELMα deficiency leads to improved parasite expulsion and decreased parasite fecundity. Both human resistin and mouse RELMα are expressed by hematopoietic cells, and it is possible that they may share similar immunomodulatory properties. Although both proteins acted to impair the Th2 effector response in N. brasiliensis infection, human resistin promoted a type 1 inflammatory response, including tumor necrosis factor alpha (TNF-α) expression, while murine RELMα inhibited CD4+ Th2 cells. Further studies outlining the similarities and differences in the mechanisms of action of these homologous proteins in helminth infection could provide insight into the immunomodulatory properties of mouse and human RELM proteins.
In conclusion, these studies utilized single- and double-gene-deficient mice for RELMα and RELMβ to delineate the functions of these highly expressed proteins in helminth infection of the lung and intestine. Although early RELMα expression in the lung downregulates Th2 immune effector responses against the parasite, leading to improved viability and fecundity, RELMα critically protects the host from damaging and potentially fatal acute inflammation. In contrast, RELMβ did not affect early lung inflammation but acted to limit parasite fecundity. Finally, experiments with Retnla−/− Retnlb−/− mice confirmed the importance of RELMα in downmodulating lung inflammation but also revealed important functions for both RELMα and RELMβ in mediating lung tissue repair by dampening perivascular inflammation and improving clearance of cell debris. The use of Retnla−/− Retnlb−/− mice therefore provides a valuable tool for biomedical research to understand the function of these two homologous proteins and the significance of the human RELMβ protein.
ACKNOWLEDGMENTS
We thank Michael Hsieh for lung histology examination.
This work was supported by National Institutes of Health grant AI091759 (to M.G.N.).
REFERENCES
- 1.Hotez PJ, Brindley PJ, Bethony JM, King CH, Pearce EJ, Jacobson J. 2008. Helminth infections: the great neglected tropical diseases. J Clin Invest 118:1311–1321. doi: 10.1172/JCI34261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gause WC, Wynn TA, Allen JE. 2013. Type 2 immunity and wound healing: evolutionary refinement of adaptive immunity by helminths. Nat Rev Immunol 13:607–614. doi: 10.1038/nri3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnes MA, Carson MJ, Nair MG. 2015. Non-traditional cytokines: how catecholamines and adipokines influence macrophages in immunity, metabolism and the central nervous system. Cytokine 72:210–219. doi: 10.1016/j.cyto.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steppan CM, Brown EJ, Wright CM, Bhat S, Banerjee RR, Dai CY, Enders GH, Silberg DG, Wen X, Wu GD, Lazar MA. 2001. A family of tissue-specific resistin-like molecules. Proc Natl Acad Sci U S A 98:502–506. doi: 10.1073/pnas.98.2.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jang JC, Chen G, Wang SH, Barnes MA, Chung JI, Camberis M, Le Gros G, Cooper PJ, Steel C, Nutman TB, Lazar MA, Nair MG. 2015. Macrophage-derived human resistin is induced in multiple helminth infections and promotes inflammatory monocytes and increased parasite burden. PLoS Pathog 11:e1004579. doi: 10.1371/journal.ppat.1004579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Babu S, Kumaraswami V, Nutman TB. 2009. Alternatively activated and immunoregulatory monocytes in human filarial infections. J Infect Dis 199:1827–1837. doi: 10.1086/599090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nair MG, Gallagher IJ, Taylor MD, Loke P, Coulson PS, Wilson RA, Maizels RM, Allen JE. 2005. Chitinase and Fizz family members are a generalized feature of nematode infection with selective upregulation of Ym1 and Fizz1 by antigen-presenting cells. Infect Immun 73:385–394. doi: 10.1128/IAI.73.1.385-394.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sandler NG, Mentink-Kane MM, Cheever AW, Wynn TA. 2003. Global gene expression profiles during acute pathogen-induced pulmonary inflammation reveal divergent roles for Th1 and th2 responses in tissue repair. J Immunol 171:3655–3667. doi: 10.4049/jimmunol.171.7.3655. [DOI] [PubMed] [Google Scholar]
- 9.Loke P, Gallagher I, Nair MG, Zang X, Brombacher F, Mohrs M, Allison JP, Allen JE. 2007. Alternative activation is an innate response to injury that requires CD4+ T cells to be sustained during chronic infection. J Immunol 179:3926–3936. doi: 10.4049/jimmunol.179.6.3926. [DOI] [PubMed] [Google Scholar]
- 10.Nair MG, Guild KJ, Du Y, Zaph C, Yancopoulos GD, Valenzuela DM, Murphy A, Stevens S, Karow M, Artis D. 2008. Goblet cell-derived resistin-like molecule beta augments CD4+ T cell production of IFN-gamma and infection-induced intestinal inflammation. J Immunol 181:4709–4715. doi: 10.4049/jimmunol.181.7.4709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Artis D, Wang ML, Keilbaugh SA, He W, Brenes M, Swain GP, Knight PA, Donaldson DD, Lazar MA, Miller HR, Schad GA, Scott P, Wu GD. 2004. RELMbeta/FIZZ2 is a goblet cell-specific immune-effector molecule in the gastrointestinal tract. Proc Natl Acad Sci U S A 101:13596–13600. doi: 10.1073/pnas.0404034101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nair MG, Du Y, Perrigoue JG, Zaph C, Taylor JJ, Goldschmidt M, Swain GP, Yancopoulos GD, Valenzuela DM, Murphy A, Karow M, Stevens S, Pearce EJ, Artis D. 2009. Alternatively activated macrophage-derived RELM-alpha is a negative regulator of type 2 inflammation in the lung. J Exp Med 206:937–952. doi: 10.1084/jem.20082048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pesce JT, Ramalingam TR, Wilson MS, Mentink-Kane MM, Thompson RW, Cheever AW, Urban JF Jr, Wynn TA. 2009. Retnla (relmalpha/fizz1) suppresses helminth-induced Th2-type immunity. PLoS Pathog 5:e1000393. doi: 10.1371/journal.ppat.1000393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee MR, Shim D, Yoon J, Jang HS, Oh SW, Suh SH, Choi JH, Oh GT. 2014. Retnla overexpression attenuates allergic inflammation of the airway. PLoS One 9:e112666. doi: 10.1371/journal.pone.0112666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cook PC, Jones LH, Jenkins SJ, Wynn TA, Allen JE, MacDonald AS. 2012. Alternatively activated dendritic cells regulate CD4+ T-cell polarization in vitro and in vivo. Proc Natl Acad Sci U S A 109:9977–9982. doi: 10.1073/pnas.1121231109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herbert DR, Yang JQ, Hogan SP, Groschwitz K, Khodoun M, Munitz A, Orekov T, Perkins C, Wang Q, Brombacher F, Urban JF Jr, Rothenberg ME, Finkelman FD. 2009. Intestinal epithelial cell secretion of RELM-beta protects against gastrointestinal worm infection. J Exp Med 206:2947–2957. doi: 10.1084/jem.20091268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hogan SP, Seidu L, Blanchard C, Groschwitz K, Mishra A, Karow ML, Ahrens R, Artis D, Murphy AJ, Valenzuela DM, Yancopoulos GD, Rothenberg ME. 2006. Resistin-like molecule beta regulates innate colonic function: barrier integrity and inflammation susceptibility. J Allergy Clin Immunol 118:257–268. doi: 10.1016/j.jaci.2006.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Valenzuela DM, Murphy AJ, Frendewey D, Gale NW, Economides AN, Auerbach W, Poueymirou WT, Adams NC, Rojas J, Yasenchak J, Chernomorsky R, Boucher M, Elsasser AL, Esau L, Zheng J, Griffiths JA, Wang X, Su H, Xue Y, Dominguez MG, Noguera I, Torres R, Macdonald LE, Stewart AF, DeChiara TM, Yancopoulos GD. 2003. High-throughput engineering of the mouse genome coupled with high-resolution expression analysis. Nat Biotechnol 21:652–659. doi: 10.1038/nbt822. [DOI] [PubMed] [Google Scholar]
- 19.National Institutes of Health. 2002. Public Health Service policy on humane care and use of laboratory animals. Office of Laboratory Animal Welfare, National Institutes of Health, Bethesda, MD. [Google Scholar]
- 20.Camberis M, Le Gros G, Urban J Jr. 2003. Animal model of Nippostrongylus brasiliensis and Heligmosomoides polygyrus. Curr Protoc Immunol Chapter 19:Unit 19.12. doi: 10.1002/0471142735.im1912s55. [DOI] [PubMed] [Google Scholar]
- 21.Siracusa MC, Reece JJ, Urban JF Jr, Scott AL. 2008. Dynamics of lung macrophage activation in response to helminth infection. J Leukoc Biol 84:1422–1433. doi: 10.1189/jlb.0308199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reece JJ, Siracusa MC, Southard TL, Brayton CF, Urban JF Jr, Scott AL. 2008. Hookworm-induced persistent changes to the immunological environment of the lung. Infect Immun 76:3511–3524. doi: 10.1128/IAI.00192-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marsland BJ, Kurrer M, Reissmann R, Harris NL, Kopf M. 2008. Nippostrongylus brasiliensis infection leads to the development of emphysema associated with the induction of alternatively activated macrophages. Eur J Immunol 38:479–488. doi: 10.1002/eji.200737827. [DOI] [PubMed] [Google Scholar]
- 24.Munitz A, Waddell A, Seidu L, Cole ET, Ahrens R, Hogan SP, Rothenberg ME. 2008. Resistin-like molecule alpha enhances myeloid cell activation and promotes colitis. J Allergy Clin Immunol 122:1200–1207. doi: 10.1016/j.jaci.2008.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen F, Wu W, Millman A, Craft JF, Chen E, Patel N, Boucher JL, Urban JF Jr, Kim CC, Gause WC. 2014. Neutrophils prime a long-lived effector macrophage phenotype that mediates accelerated helminth expulsion. Nat Immunol 15:938–946. doi: 10.1038/ni.2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harvie M, Camberis M, Tang SC, Delahunt B, Paul W, Le Gros G. 2010. The lung is an important site for priming CD4 T-cell-mediated protective immunity against gastrointestinal helminth parasites. Infect Immun 78:3753–3762. doi: 10.1128/IAI.00502-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sutherland TE, Logan N, Ruckerl D, Humbles AA, Allan SM, Papayannopoulos V, Stockinger B, Maizels RM, Allen JE. 2014. Chitinase-like proteins promote IL-17-mediated neutrophilia in a tradeoff between nematode killing and host damage. Nat Immunol 15:1116–1125. doi: 10.1038/ni.3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen F, Liu Z, Wu W, Rozo C, Bowdridge S, Millman A, Van Rooijen N, Urban JF Jr, Wynn TA, Gause WC. 2012. An essential role for TH2-type responses in limiting acute tissue damage during experimental helminth infection. Nat Med 18:260–266. doi: 10.1038/nm.2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O'Connell AE, Hess JA, Santiago GA, Nolan TJ, Lok JB, Lee JJ, Abraham D. 2011. Major basic protein from eosinophils and myeloperoxidase from neutrophils are required for protective immunity to Strongyloides stercoralis in mice. Infect Immun 79:2770–2778. doi: 10.1128/IAI.00931-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shin EH, Osada Y, Chai JY, Matsumoto N, Takatsu K, Kojima S. 1997. Protective roles of eosinophils in Nippostrongylus brasiliensis infection. Int Arch Allergy Immunol 114(Suppl 1):S45–S50. [DOI] [PubMed] [Google Scholar]
- 31.Shin EH, Osada Y, Sagara H, Takatsu K, Kojima S. 2001. Involvement of complement and fibronectin in eosinophil-mediated damage to Nippostrongylus brasiliensis larvae. Parasite Immunol 23:27–37. doi: 10.1046/j.1365-3024.2001.00352.x. [DOI] [PubMed] [Google Scholar]
- 32.Voehringer D, Shinkai K, Locksley RM. 2004. Type 2 immunity reflects orchestrated recruitment of cells committed to IL-4 production. Immunity 20:267–277. doi: 10.1016/S1074-7613(04)00026-3. [DOI] [PubMed] [Google Scholar]
- 33.Guo L, Huang Y, Chen X, Hu-Li J, Urban JF Jr, Paul WE. 2015. Innate immunological function of TH2 cells in vivo. Nat Immunol 16:1051–1059. doi: 10.1038/ni.3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harris DP, Haynes L, Sayles PC, Duso DK, Eaton SM, Lepak NM, Johnson LL, Swain SL, Lund FE. 2000. Reciprocal regulation of polarized cytokine production by effector B and T cells. Nat Immunol 1:475–482. doi: 10.1038/82717. [DOI] [PubMed] [Google Scholar]
- 35.Grainge C, Dulay V, Ward J, Sammut D, Davies E, Green B, Lau L, Cottey L, Haitchi HM, Davies DE, Howarth PH. 2012. Resistin-like molecule-beta is induced following bronchoconstriction of asthmatic airways. Respirology 17:1094–1100. doi: 10.1111/j.1440-1843.2012.02215.x. [DOI] [PubMed] [Google Scholar]
- 36.Kushiyama A, Sakoda H, Oue N, Okubo M, Nakatsu Y, Ono H, Fukushima T, Kamata H, Nishimura F, Kikuchi T, Fujishiro M, Nishiyama K, Aburatani H, Kushiyama S, Iizuka M, Taki N, Encinas J, Sentani K, Ogonuki N, Ogura A, Kawazu S, Yasui W, Higashi Y, Kurihara H, Katagiri H, Asano T. 2013. Resistin-like molecule beta is abundantly expressed in foam cells and is involved in atherosclerosis development. Arterioscler Thromb Vasc Biol 33:1986–1993. doi: 10.1161/ATVBAHA.113.301546. [DOI] [PubMed] [Google Scholar]