Abstract
Periodontal disease is associated with changes in the composition of the oral microflora, where health-associated oral streptococci decrease while Gram-negative anaerobes predominate in disease. A key feature of periodontal disease-associated anaerobes is their ability to produce hydrogen sulfide (H2S) abundantly as a by-product of anaerobic metabolism. So far, H2S has been reported to be either cytoprotective or cytotoxic by modulating bacterial antioxidant defense systems. Although oral anaerobes produce large amounts of H2S, the potential effects of H2S on oral streptococci are currently unknown. The aim of this study was to determine the effects of H2S on the survival and biofilm formation of oral streptococci. The growth and biofilm formation of Streptococcus mitis and Streptococcus oralis were inhibited by H2S. However, H2S did not significantly affect the growth of Streptococcus gordonii or Streptococcus sanguinis. The differential susceptibility of oral streptococci to H2S was attributed to differences in the intracellular concentrations of reduced glutathione (GSH). In the absence of GSH, H2S elicited its toxicity through an iron-dependent mechanism. Collectively, our results showed that H2S exerts antimicrobial effects on certain oral streptococci, potentially contributing to the decrease in health-associated plaque microflora.
INTRODUCTION
Periodontal disease affects up to 40% of the adult population (1) and is associated with changes in the composition of the oral microflora. Gram-positive facultative anaerobes predominate during periodontal health, while Gram-negative anaerobic bacteria species dominate in disease. These health-associated bacteria, namely, Streptococcus mitis, Streptococcus oralis, Streptococcus gordonii, and Streptococcus sanguinis, are early colonizers of plaque biofilm and play central roles in initiating dental biofilm formation by providing binding sites for subsequent colonizers of plaque biofilm (2, 3). Increasing amounts of Gram-negative anaerobes, such as Fusobacterium nucleatum, Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia, occur during periodontal disease. A key characteristic of these bacterial species is their ability to produce volatile sulfur compounds (VSCs) (4). VSCs are produced from the metabolism of human serum proteins and sulfur-containing amino acids in the local environment. H2S is the predominant VSC, found in up to 90% of diseased periodontal pockets, with concentrations of up to 2 mM (5–7).
Traditionally, H2S is regarded as a microbial by-product of sulfur metabolism in oral anaerobes, with few studies available regarding its potential effects on non-H2S-producing facultative anaerobes in the oral environment (8). Reports have shown that H2S plays opposite roles in terms of the oxidative stress response. So far, H2S has been shown to protect Bacillus anthracis, Pseudomonas aeruginosa, Staphylococcus aureus, and Escherichia coli from the bactericidal effects of antibiotics by mitigating oxidative stress. This is achieved through upregulating the activity of key antioxidant defense enzymes, namely, superoxide dismutase and catalase, to detoxify reactive oxygen species (ROS) (9). Conversely, a recent report demonstrated that H2S killed microorganisms through inducing oxidative stress by inhibiting antioxidant enzymes (10). In the complex environment of the multispecies oral biofilm, interbacterial interactions can be either competitive or mutualistic in nature. Although periodontal disease-associated anaerobes produce H2S abundantly, it is currently unknown whether H2S elicits protective or detrimental effects on health-associated oral streptococci during the shift toward a pathogenic microbial community. Thus, the aim of this study was to investigate the potential effect of H2S on the survival and biofilm formation of oral streptococci.
MATERIALS AND METHODS
Chemicals.
l-Cysteine, sodium hydrosulfide hydrate (NaHS), methemoglobin (MetHb), N-acetyl-l-cysteine (NAC), Triton X-100, diethyl maleate (DEM), hemin, vitamin K, crystal violet, methanol, acetic acid, zinc acetate, N,N-dimethyl-p-phenylenediamine sulfate salt, iron(III) chloride, deferoxamine (DFO), and reduced l-glutathione (GSH) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Bradford reagent was obtained from Bio-Rad (Hercules, CA, USA).
Strains and culture conditions.
Fusobacterium nucleatum ATCC 25586, Streptococcus mitis ATCC 49456, Streptococcus oralis ATCC 35037, Streptococcus gordonii ATCC 35105, and Streptococcus sanguinis ATCC 10556 strains were purchased from the American Type Culture Collection, Manassas, VA, USA. F. nucleatum was cultured in brain heart infusion (BHI) broth (Acumedia, Lansing, MI, USA) supplemented with yeast extract (Acumedia), 5 μg/ml of hemin (Sigma), and 1 μg/ml of vitamin K (Sigma) and incubated at 37°C anaerobically in a DG250 anaerobic workstation (Don Whitley Scientific, West Yorkshire, United Kingdom) supplemented with 80% N2, 10% H2, and 10% CO2. S. mitis, S. oralis, S. gordonii, and S. sanguinis strains were cultured in BHI broth and incubated at 37°C in an incubator supplied with 5% CO2.
Quantification of H2S.
The amount of H2S was determined using the Clines methylene blue assay (11), with minor modifications. F. nucleatum, S. mitis, S. oralis, S. gordonii, and S. sanguinis (1 × 107 CFU) were inoculated into BHI broth supplemented with l-cysteine (5 mM) and incubated at 37°C in an incubator supplied with 5% CO2 for 24 h. Thereafter, 10 μl of overnight bacterial cultures was transferred to a 96-well plate with 72 μl of 1% zinc acetate (in 3% NaOH), after which 18 μl of a solution of 17.1 mM N,N-dimethyl-p-phenylenediamine sulfate salt and 37.0 mM FeCl3 in 6 M HCl was added. The reaction mixtures were incubated at room temperature for 10 min prior to absorbance being read at an optical density at 670 nm (OD670) using a microplate reader. The limit of detection was determined to be 1 μM H2S. The amount of quantified H2S production was then normalized to the respective bacterial culture density.
Bacterial viability determination.
The MICs of NaHS for oral streptococci were determined using a broth microdilution assay. A 96-well microtiter plate (Nunc, Rochester, NY) containing 100 μl of serially diluted NaHS dissolved in BHI broth was inoculated with 1 × 106 CFU/ml bacteria. Bacterial growth after incubation for 24 h at 37°C with 5% CO2 was determined by taking OD600 measurements using a microplate reader (Biotek, Winooski, VT, USA). Viability of F. nucleatum under aerobic incubation was determined as follows: BHI broth was inoculated with 1 × 108 CFU/ml F. nucleatum in a final volume of 2 ml with and without NaHS (2 mM) or l-cysteine (5 mM) and incubated for 2, 4, 8, 16, and 32 h at 37°C aerobically in 14-ml polystyrene snap-cap tubes (Becton Dickinson, Franklin Lakes, NJ, USA) without shaking. Viability of bacteria was determined by serial dilution and plating on tryptic soy agar (TSA) with 5% sheep blood (Oxoid, Hampshire, United Kingdom). Agar plates were then incubated anaerobically, as described above, and the number of bacterial colonies was enumerated after 48 to 72 h of incubation.
Transwell assay.
F. nucleatum (1 × 107 CFU) was cultured in BHI broth supplemented with l-cysteine in the bottom chamber of a Transwell plate with 0.4-μm pore polycarbonate membrane inserts (Corning, NY, USA). An inoculum size of 1 × 107 CFU each of S. mitis, S. oralis, S. gordonii, and S. sanguinis was cultured in the upper chambers of the Transwell plate systems in BHI broth with or without methemoglobin (50 mg/ml) (H2S scavenger) for 16 h. The setup was incubated at 37°C aerobically with 5% CO2. Viability of the oral streptococci was determined by serial dilution, plating on BHI agar, and incubation at 37°C with 5% CO2.
Biofilm formation.
Whole saliva was obtained from a volunteer in one sitting by expectoration into a polypropylene tube. Saliva was filter sterilized by passing it through a 0.2-μm syringe filter (Sartorius, Goettingen, Germany) and stored in single-use aliquots at −70°C. A 96-well flat-bottom microtiter plate (Nunc) was coated with whole saliva for 24 h. Overnight cultures of oral streptococci (1 × 106 CFU/ml) were inoculated into the saliva-coated plate in BHI broth in the presence or absence of NaHS. After 24 h, planktonic cells were carefully transferred to another microtiter plate for subsequent serial dilution and plating on BHI agar plates to quantify planktonic titer. Biofilm formation was determined by crystal violet assay. The biofilm was washed once with sterile 1× phosphate-buffered saline (PBS) to remove residual planktonic cells. The biofilm was fixed with methanol (Sigma) for 10 min, stained with 1% crystal violet (Sigma) for 30 min, and washed 5 times with distilled H2O to remove unbound crystal violet. Bound crystal violet was dissolved using 33% acetic acid (Sigma). The extracted crystal violet in 33% acetic acid was further diluted 1:2 with distilled H2O, and its absorbance was read at an OD580 by a microplate reader. Viability of bacteria in biofilm was determined by serial dilution, plating on BHI agar, and incubation at 37°C with 5% CO2. To elute biofilm cells from wells, 100 μl of BHI broth was added to the well, and vigorous pipetting of the suspension was sufficient to remove the biofilm from the surface of the wells. The bacterial suspension was collected and vortexed to disperse the biofilm to a homogenous suspension prior to serial dilution and plating.
Determination of intracellular sulfide.
An inoculum size of 1 × 108 CFU of either S. mitis, S. oralis, S. gordonii, or S. sanguinis was inoculated into 1 ml of BHI broth in 1.5-ml microcentrifuge tubes. The cultures were left untreated or were treated with 2 mM NaHS for 2 h. Cells were pelleted, and the resulting cell pellets were washed twice with 1× PBS. The cell pellet was resuspended in 1% Triton X-100 (12) with 0.1-mm glass beads (Scientific Industries, Inc., NY, USA) and lysed through the simultaneous actions of agitation and vortexing for 15 min using a Disruptor Genie 2 (Scientific Industries, Inc.) (13). Efficiency of lysis was determined by plating on BHI agar plates. Cell debris was removed by centrifugation (16,000 × g) for 10 min. The intracellular sulfide concentrations were determined using the Clines methylene blue colorimetric assay, described above.
Determination of GSH and GSSG.
Overnight bacterial cultures (1 × 108 CFU) were harvested by centrifugation (16,000 × g) at room temperature for 10 min. The resulting cell pellets were washed twice with 1× PBS prior to lysis with 1% Triton X-100 (Sigma), as described above. Cell debris was removed by centrifugation (16,000 × g) for 10 min. The concentrations of protein in the lysates were determined using the Bradford reagent (Bio-Rad). The intracellular GSH and oxidized glutathione (GSSG) concentrations were determined from equal amounts of bacterial lysates using the GSH/GSSG-Glo assay kit (Promega). Intracellular GSH in S. gordonii and S. sanguinis (1 × 106 CFU/ml) was depleted with DEM (5 mM) treatment for 16 h and incubated at 37°C with 5% CO2. NAC (5 mM) was added to replenish intracellular glutathione in DEM-treated S. gordonii and S. sanguinis.
Statistical analysis.
Results are presented as means ± standard deviations from 3 independent experiments with triplicates. Statistical significance was determined by one-way analysis of variance, with Tukey post hoc analysis using GraphPad Prism software 6.0 (San Diego, CA, USA). Differences were considered significant if the P value was <0.05.
RESULTS
Differential susceptibility of oral streptococci to H2S.
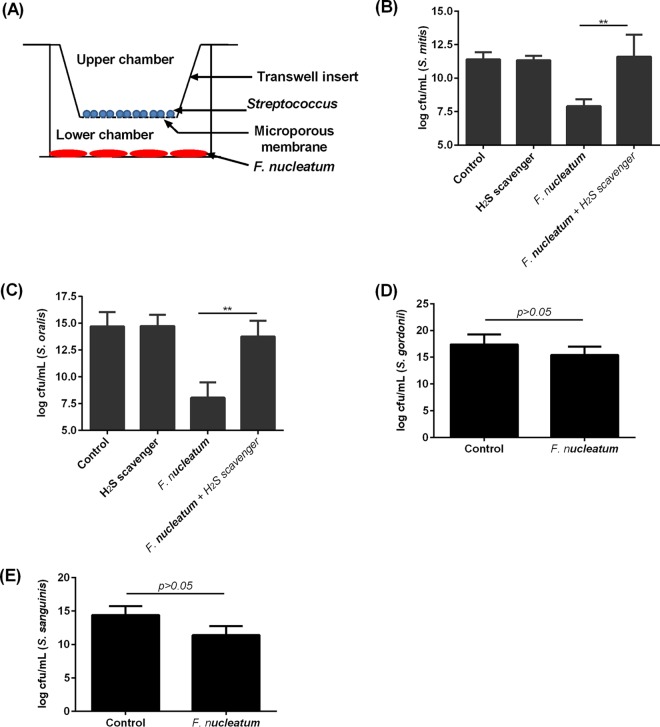
F. nucleatum produces H2S through metabolism of human serum proteins and amino acids, such as l-cysteine (14). Indeed, in the presence of l-cysteine, F. nucleatum produced up to 3 mM H2S (see Fig. S1 in the supplemental material). Under aerobic conditions, in the absence of H2S production, the viability of F. nucleatum was maintained for up to 2 h but was decreased by 40% after 4 h and could not be maintained for >16 h (see Fig. S2 in the supplemental material). However, the reducing condition provided by either NaHS or l-cysteine was sufficient to sustain the viability of F. nucleatum for >32 h. The potential bactericidal effect of H2S from F. nucleatum on non-H2S-producing bacteria was tested. To determine if oral streptococci are susceptible to H2S, four species that did not produce H2S, namely, S. mitis, S. oralis, S. gordonii, and S. sanguinis (data not shown), were treated with a 2-fold serial dilution of NaHS, an H2S donor. The concentration of H2S required to inhibit the growth of S. mitis and S. oralis was 0.25 mM. However, the growth of S. gordonii and S. sanguinis was not affected at concentrations of up to 2 mM H2S, which is the highest concentration so far reported in the plaque of patients with periodontal disease (6). To determine if S. mitis and S. oralis strains are susceptible to H2S derived from F. nucleatum when in coculture, a Transwell setup was employed, where F. nucleatum and the respective oral streptococci were placed in the lower and upper chambers, respectively (Fig. 1A). The presence of F. nucleatum led to approximately 103- and 104-fold decreases in S. mitis and S. oralis viable counts, respectively, while the presence of an H2S scavenger rescued the viability of S. mitis and S. oralis in the presence of F. nucleatum (Fig. 1B and C). In contrast, the viability of S. gordonii and S. sanguinis did not change significantly in the presence of F. nucleatum (Fig. 1D and E).
FIG 1.
H2S inhibited the growth of S. mitis and S. oralis but not S. gordonii and S. sanguinis. (A) The Transwell setup used to investigate the bactericidal properties of H2S-producing F. nucleatum on S. mitis, S oralis, S. gordonii, and S. sanguinis is shown. (B and C) S. mitis (1 × 107 CFU) cultured in BHI broth (control) (B) or S. oralis (1 × 107 CFU) cultured in BHI broth (control) (C) was placed in the upper chamber of the Transwell apparatus in the presence or absence of methemoglobin (H2S scavenger), while H2S-producing F. nucleatum (1 × 107 CFU) was applied to the lower chamber. This setup was incubated aerobically at 37°C in 5% CO2 for 16 h. The viability of S. mitis and S. oralis was determined by serial dilution and plating on BHI agar plates. **, P < 0.01. (D and E) S. gordonii (1 × 107 CFU) cultured in BHI broth (control) (D) or S. sanguinis (1 × 107 CFU) cultured in BHI broth (control) (E) was placed in the upper chamber of the Transwell apparatus, while H2S-producing F. nucleatum (1 × 107 CFU) was added to the lower chamber. This setup was incubated at 37°C in a 5% CO2 incubator for 16 h. The viability of S. gordonii and S. sanguinis was determined by serial dilution and plating on BHI agar plates. The experiments were carried out 3 times, each time in triplicate. Data shown are the mean results obtained from 3 independent experiments.
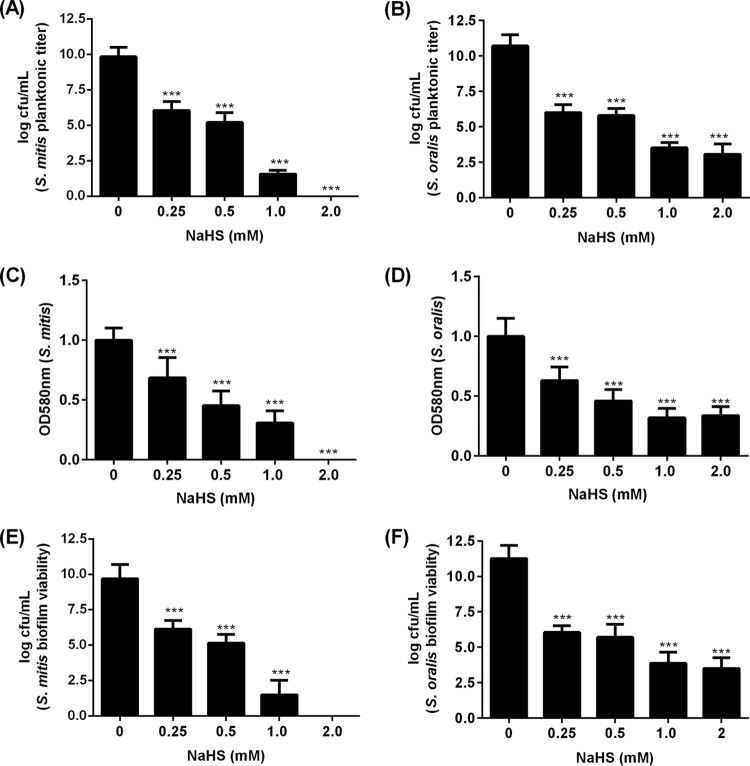
H2S inhibits formation of streptococcal biofilm.
The MIC of H2S for planktonic S. mitis and S. oralis was determined to be 0.25 mM. At the MIC in the biofilm setup, significant amounts of S. mitis and S. oralis remained viable, albeit significantly reduced compared to the bacteria unexposed to H2S (Fig. 2A and B). These surviving bacteria adhered to a saliva-coated surface to form biofilm, as determined by crystal violet assay (Fig. 2C and D). Since the crystal violet assay cannot differentiate between the extracellular polymeric substances (EPS) and bacterial cells (15), the viability of bacteria in biofilm was additionally investigated by plating. Viable counts of S. mitis and S. oralis in biofilms were significantly affected in the presence of H2S (Fig. 2E and F).
FIG 2.
H2S influenced biofilm formation of S. mitis and S. oralis. (A and B) Viability of planktonic titers of S. mitis (A) and S. oralis (B) in the presence and absence of NaHS from a starting inoculum of 1 × 106 CFU/ml after 24 h. (C to F) S. mitis and S. oralis biofilms were formed on a saliva-coated polystyrene well plate in the presence and absence of NaHS from a starting inoculum of 1 × 106 CFU/ml for 24 h. The thickness of S. mitis (C) and S. oralis (D) biofilms was determined by crystal violet assay. The viability of S. mitis (E) and S. oralis (F) bacteria in biofilm was determined by serial dilution and plating on a BHI agar plate. The experiments were carried out 3 times, each time in triplicate. Data shown are the mean results obtained from 3 independent experiments. ***, P < 0.001.
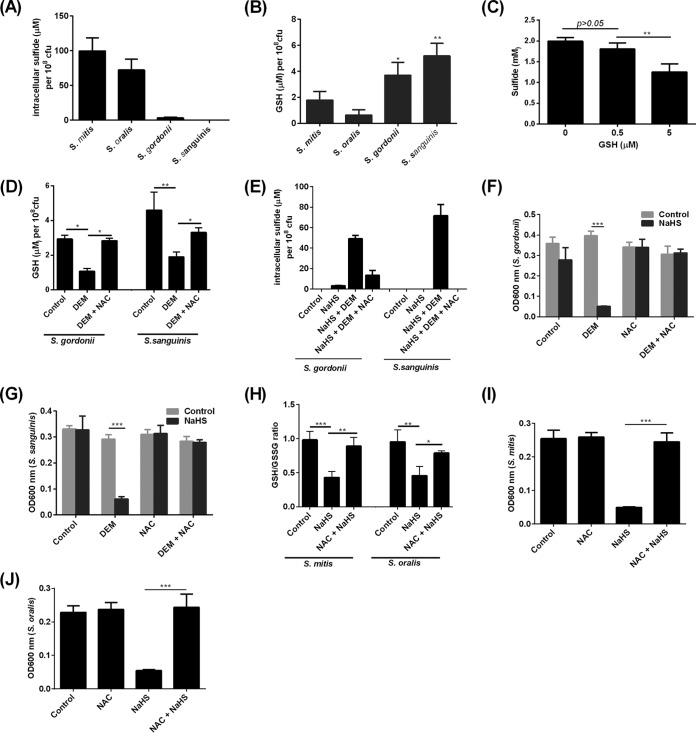
Susceptibility of oral streptococci to H2S is dependent on intracellular glutathione.
Intracellular sulfide levels were not detectable in S. mitis, S. oralis, S. gordonii, or S. sanguinis (data not shown). Following treatment with NaHS, intracellular sulfide concentrations increased by up to 100 μM and 70 μM in S. mitis and S. oralis, respectively (Fig. 3A). In contrast, sulfide did not accumulate to significant amounts in S. gordonii or S. sanguinis (Fig. 3A). Reduced glutathione (GSH) was found to be present in S. mitis, S. oralis, S. gordonii, and S. sanguinis at concentrations ranging from 0.6 μM to 5 μM (Fig. 3B). Notably, S. gordonii and S. sanguinis possessed significantly higher concentrations of GSH than S. mitis and S. oralis (Fig. 3B). This contrasting inverse relationship between intracellular sulfide and GSH concentrations in oral streptococci led us to hypothesize that GSH may be able to directly detoxify H2S. NaHS was added to GSH under aerobic conditions in vitro, and the amount of sulfide was quantified. GSH at 5 μM significantly reduced sulfide levels from NaHS (Fig. 3C). To determine if GSH indeed protected oral streptococci against the bactericidal effects of H2S, intracellular GSH in S. gordonii and S. sanguinis was depleted with diethyl maleate (DEM), a glutathione-depleting agent, at a concentration that did not affect bacterial viability (16). Depletion of intracellular GSH in S. gordonii and S. sanguinis led to accumulation of intracellular sulfide (Fig. 3D and E) and inhibition of bacterial growth (Fig. 3F and G). Conversely, when intracellular GSH was restored with N-acetyl-l-cysteine (NAC), a glutathione precursor, intracellular sulfide failed to accumulate to significant amounts, allowing S. gordonii and S. sanguinis to grow despite H2S treatment (Fig. 3D to G). Exposure of S. mitis and S. oralis to H2S led to a decrease in the GSH/GSSG ratio, an indicator of oxidative stress, which was mitigated in the presence of NAC (Fig. 3H). Correspondingly, the presence of NAC led to the resistance of S. mitis and S. oralis to H2S (Fig. 3I and J).
FIG 3.
Effects of H2S on intracellular sulfide levels and GSH redox in oral streptococci. (A) Intracellular sulfide concentrations in S. mitis, S. oralis, S. gordonii, and S. sanguinis following treatment with NaHS (2 mM) for 2 h. (B) Cellular GSH levels of S. mitis, S. oralis, S. gordonii, and S. sanguinis were determined by luminescent assay. *, P < 0.05; **, P < 0.01 compared to S. mitis group. (C) NaHS (2 mM) was added to GSH at the indicated concentrations, and the remaining sulfide levels were quantified by Clines methylene blue assay. (D) S. gordonii or S. sanguinis in BHI broth (control) or treated with either DEM or DEM plus NAC for 16 h prior to determination of intracellular GSH concentrations. (E) S. gordonii and S. sanguinis strains were left untreated (control), were treated with NaHS (2 mM) for 2 h after overnight treatment with DEM (5 mM), or were cotreated with DEM plus NAC prior to determination of intracellular sulfide levels. (F and G) Culture density of S. gordonii (F) and S. sanguinis (G) in BHI broth without treatment (control), when treated with NaHS (2 mM) for 16 h with or without GSH depletion with DEM, or when cotreated with DEM plus NAC. (H) GSH-to-GSSG ratio in S. mitis and S. oralis in BHI broth when left untreated (control) or when treated with NaHS (2 mM) for 2 h in the presence or absence of NAC. (I and J) Culture density of S. mitis (I) and S. oralis (J) without treatment (control) or when treated with NaHS (2 mM) in the presence of absence of NAC for 16 h in BHI broth. The experiments were carried out 3 times, each time in triplicate. Data shown are the mean results obtained from 3 independent experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
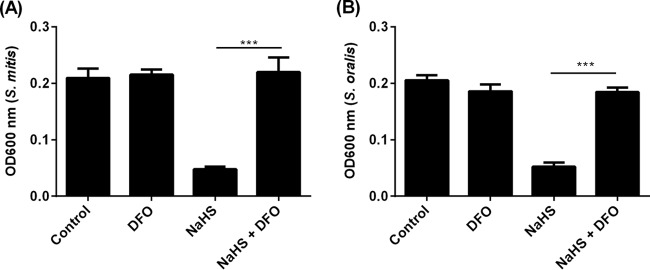
Iron sequestration rescues oral streptococci from H2S.
H2S has been shown to reduce intracellular bound ferric iron to form unbound ferrous iron, causing an increase in cytosolic iron (17). Free cytosolic iron can either act as a substrate for the Fenton reaction to generate highly microbicidal free radicals or directly inhibit bacterial enzymes in oral streptococci (18). To determine if H2S exerts its bactericidal effects through an iron-mediated pathway, S. mitis and S. oralis strains were exposed to NaHS in the presence and absence of deferoxamine (DFO), a ferric chelator, at a concentration (0.5 mM) that did not affect bacterial viability on its own. In the presence of DFO, the bactericidal effect of H2S on S. mitis and S. oralis was obliterated (Fig. 4A and B).
FIG 4.
H2S-mediated bactericidal effect on oral streptococci requires iron. S. mitis (A) and S. oralis (B) in BHI broth were left untreated (control) or treated with DFO (0.5 mM) in the presence or absence of NaHS (2 mM) for 16 h, after which culture density was determined by taking optical density at 600 nm. The experiments were carried out 3 times, each time in triplicate. Data shown are the mean results obtained from 3 independent experiments. ***, P < 0.001.
DISCUSSION
Oral streptococci are the initial colonizers of plaque biofilm. In the process of biofilm maturation, F. nucleatum functions as an important bridging organism for coaggregation with oral streptococci and the late colonizers of plaque biofilm, which are disease-associated anaerobes, such as Porphyromonas gingivalis, Tannerella forsythia, and Treponema denticola (19). It has been shown that oral streptococci coaggregate only with F. nucleatum and not with oral anaerobes, such as Porphyromonas gingivalis and Prevotella nigrescens (20, 21). Intermicrobial interaction between F. nucleatum and oral streptococci occurs during the initial stages of plaque biofilm formation. Thus, F. nucleatum was used as the H2S-producing organism in this study to determine the effects of H2S on oral streptococci.
Oral streptococci produce hydrogen peroxide (H2O2) that can oxidize cellular proteins, nucleic acids, and lipids, causing cellular stress or cell death. The production of H2O2 by oral streptococci has been hypothesized to equip the bacteria with the competitive advantage to colonize oral surfaces (22). However, given the bactericidal property of H2O2, for oral bacteria to successfully coaggregate with oral streptococci in plaque biofilm, bacterial species with mechanisms to overcome the cytotoxic effects of H2O2 should possess a survival advantage in the oral environment (22, 23). In addition, during periods of mechanical disruption of dental plaque, anaerobes associated with periodontal disease may be exposed to oxygen. Although periodontal disease-associated anaerobes lack or express low levels of superoxide dismutase and catalase to detoxify ROS compared to aerobes or facultative anaerobes (24–26), production of H2S can serve as a means to reduce the overall oxygen tension in the local environment to enhance survival during periods of oxidative stress. Indeed, in this study, we showed that the reducing property of H2S sustained the viability of F. nucleatum under aerobic conditions for a prolonged duration.
While H2S is beneficial to the survival of oral anaerobes, it can exert toxic effects on other microorganisms. It has been reported that H2S elicited microbicidal effects by inhibiting the activities of superoxide dismutase and catalase (10, 27). In addition, high levels of sulfide impose survival stress on Desulfovibrio vulgaris by sequestering essential trace metals (28). In this report, we showed that exogenously added H2S, as well as H2S derived from F. nucleatum, inhibited the growth and biofilm formation of S. mitis and S. oralis but not of S. gordonii and S. sanguinis.
The differential susceptibility of oral streptococci species to H2S toxicity was attributed to the differing intracellular GSH levels. GSH is the most abundant intracellular thiol present in eukaryotes. Its presence in prokaryotes has so far been described in cyanobacteria, proteobacteria, and some Gram-positive bacteria, such as Streptococcus pneumoniae (29) and Lactobacillus lactis (30). GSH protects bacteria against various physiological insults, such as ROS, osmotic stress, and toxic metal ions (31, 32). Under these conditions, GSH plays a central role in the maintenance of normal cellular processes through its action as a protein reductant, either directly or via enzyme-mediated action. For instance, GSH protects Escherichia coli from toxic chlorine compounds, such as hypochlorous acid and monochloroamine, directly through its action of scavenging chlorine oxidants (33), while GSH detoxifies xenobiotics (34), electrophiles, and antibiotics (35) via GSH-S-transferases which catalyze a conjugation reaction with GSH to produce less toxic and more hydrophilic products that can be partially metabolized and excreted (31). Here, we showed that GSH could directly detoxify H2S in vitro. Nevertheless, GSH-mediated resistance of S. gordonii and S. sanguinis to sulfide toxicity may also occur via enzymatic means, since enzymes involved in cellular redox regulation, such as GSH reductase, GSH-S-transferase, and GSH peroxidase, are encoded in the genomes of S. gordonii and S. sanguinis (36, 37). Under normal physiological conditions, the intracellular ratio of GSH to GSSG is tightly regulated, and GSH is kept mostly in its reduced form. Cysteine thiol groups have key catalytic functions in enzymes but are readily oxidized by ROS. Under oxidative stress, ROS mediates the formation of intramolecular or intermolecular disulfide bonds between cysteine residues, resulting in aberrant protein functions. In this context, GSH maintains protein thiols in the reduced state by itself being preferentially oxidized to GSSG, so essential cellular proteins are not damaged by oxidation (31). In Escherichia coli mutants lacking GSH biosynthetic enzymes, for instance, 3′-phosphoadenylylsulfate reductase and methionine synthase involved in toxic sulfate catabolism are inactivated in the presence of an oxidizing agent (38, 39). In the case of S. mitis and S. oralis strains, where intracellular GSH levels are insufficient to detoxify H2S, a significant decrease in the GSH/GSSG ratio was observed. Restoring the GSH/GSSG redox with NAC, a precursor of GSH biosynthesis, protected these bacterial species against the deleterious effects of H2S.
H2S-mediated toxicity in S. mitis and S. oralis could be prevented with an iron chelator. One of the main mechanisms of H2S-mediated toxicity is attributed to its ability to elicit the release of iron from ferritin into the cytosol as unbound toxic iron (17). This free cytosolic iron might subsequently promote reactive oxygen radical generation via the Fenton or Haber-Weiss reaction (17), leading to damage of cell membranes, proteins, and nucleic acids. Iron is also capable of inducing cell damage through radical-independent means. For instance, Fe2+ has been shown be lethal to streptococci by inhibiting F-ATPase activity, consequently affecting the ability of the organism to carry out glycolysis (18).
In conclusion, this study showed that H2S exerted toxicity to certain oral streptococci species, potentially contributing to dysbiosis in plaque biofilm through reduction of periodontal-health-associated microflora. Cellular GSH in oral streptococci was important to overcome the toxicity of H2S. Thus, compounds which augment cellular GSH in oral streptococci may be beneficial to enhance the survival of oral streptococci against H2S-producing oral pathogens.
Supplementary Material
ACKNOWLEDGMENTS
We thank Samantha Quah for her input and critical reading of the manuscript.
We declare no conflicts of interest.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.03946-15.
REFERENCES
- 1.Eke P, Dye B, Wei L, Thornton-Evans G, Genco R. 2012. Prevalence of periodontitis in adults in the United States: 2009 and 2010. J Dent Res 91:914–920. doi: 10.1177/0022034512457373. [DOI] [PubMed] [Google Scholar]
- 2.Marsh P. 1994. Microbial ecology of dental plaque and its significance in health and disease. Adv Dent Res 8:263–271. [DOI] [PubMed] [Google Scholar]
- 3.Marsh P. 2003. Are dental diseases examples of ecological catastrophes? Microbiology 149:279–294. doi: 10.1099/mic.0.26082-0. [DOI] [PubMed] [Google Scholar]
- 4.Torresyap G, Haffajee A, Uzel N, Socransky S. 2003. Relationship between periodontal pocket sulfide levels and subgingival species. J Clin Periodontol 30:1003–1010. doi: 10.1034/j.1600-051X.2003.00377.x. [DOI] [PubMed] [Google Scholar]
- 5.Morita M, Wang HL. 2001. Association between oral malodor and adult periodontitis: a review. J Clin Periodontol 28:813–819. doi: 10.1034/j.1600-051x.2001.028009813.x. [DOI] [PubMed] [Google Scholar]
- 6.Persson S. 1992. Hydrogen sulfide and methyl mercaptan in periodontal pockets. Oral Microbiol Immunol 7:378–379. doi: 10.1111/j.1399-302X.1992.tb00641.x. [DOI] [PubMed] [Google Scholar]
- 7.Horowitz A, Folke L. 1973. Hydrogen sulfide production in the periodontal environment. J Periodontol 44:390. doi: 10.1902/jop.1973.44.7.390. [DOI] [PubMed] [Google Scholar]
- 8.Clarke PH. 1953. Hydrogen sulfide production by bacteria. J Gen Microbiol 8:397–407. doi: 10.1099/00221287-8-3-397. [DOI] [PubMed] [Google Scholar]
- 9.Shatalin K, Shatalina E, Mironov A, Nudler E. 2011. H2S: a universal defense against antibiotics in bacteria. Science 334:986–990. doi: 10.1126/science.1209855. [DOI] [PubMed] [Google Scholar]
- 10.Fu L-H, Hu K-D, Hu L-Y, Li Y-H, Hu L-B, Yan H, Liu Y-S, Zhang H. 2014. An antifungal role of hydrogen sulfide on the postharvest pathogens Aspergillus niger and Penicillium italicum. PLoS One 9(8):e104206. doi: 10.1371/journal.pone.0104206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cline JD. 1969. Spectrophotometric determination of hydrogen sulfide in natural waters. Limnol Oceanogr 14:454–458. doi: 10.4319/lo.1969.14.3.0454. [DOI] [Google Scholar]
- 12.Steinmoen H, Knutsen E, Håvarstein LS. 2002. Induction of natural competence in Streptococcus pneumoniae triggers lysis and DNA release from a subfraction of the cell population. Proc Natl Acad Sci U S A 99:7681–7686. doi: 10.1073/pnas.112464599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Uhl JR, Vetter EA, Boldt KL, Johnston BW, Ramin KD, Adams MJ, Ferrieri P, Reischl U, Cockerill FR. 2005. Use of the Roche LightCycler Strep B assay for detection of group B Streptococcus from vaginal and rectal swabs. J Clin Microbiol 43:4046–4051. doi: 10.1128/JCM.43.8.4046-4051.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Claesson R, Edlund MB, Persson S, Carlsson J. 1990. Production of volatile sulfur compounds by various Fusobacterium species. Oral Microbiol Immunol 5:137–142. doi: 10.1111/j.1399-302X.1990.tb00411.x. [DOI] [PubMed] [Google Scholar]
- 15.Welch K, Cai Y, Strømme M. 2012. A method for quantitative determination of biofilm viability. J Funct Biomater 3:418–431. doi: 10.3390/jfb3020418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chasseaud L. 1979. The role of glutathione and glutathione-S-transferases in the metabolism of chemical carcinogens and other electrophilic agents. Adv Cancer Res 29:175–274. doi: 10.1016/S0065-230X(08)60848-9. [DOI] [PubMed] [Google Scholar]
- 17.Truong DH, Eghbal MA, Hindmarsh W, Roth SH, O'Brien PJ. 2006. Molecular mechanisms of hydrogen sulfide toxicity. Drug Metab Rev 38:733–744. doi: 10.1080/03602530600959607. [DOI] [PubMed] [Google Scholar]
- 18.Dunning J, Ma Y, Marquis R. 1998. Anaerobic killing of oral streptococci by reduced, transition metal cations. Appl Environ Microbiol 64:27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kolenbrander PE, Palmer RJ, Periasamy S, Jakubovics NS. 2010. Oral multispecies biofilm development and the key role of cell-cell distance. Nat Rev Microbiol 8:471–480. doi: 10.1038/nrmicro2381. [DOI] [PubMed] [Google Scholar]
- 20.Bradshaw DJ, Marsh PD, Watson GK, Allison C. 1998. Role of Fusobacterium nucleatum and coaggregation in anaerobe survival in planktonic and biofilm oral microbial communities during aeration. Infect Immun 66:4729–4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diaz P, Zilm P, Rogers A. 2002. Fusobacterium nucleatum supports the growth of Porphyromonas gingivalis in oxygenated and carbon-dioxide-depleted environments. Microbiology 148:467–472. doi: 10.1099/00221287-148-2-467. [DOI] [PubMed] [Google Scholar]
- 22.Kreth J, Vu H, Zhang Y, Herzberg MC. 2009. Characterization of hydrogen peroxide-induced DNA release by Streptococcus sanguinis and Streptococcus gordonii. J Bacteriol 191:6281–6291. doi: 10.1128/JB.00906-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu L, Kreth J. 2012. The role of hydrogen peroxide in environmental adaptation of oral microbial communities. Oxid Med Cell Longev 2012:717843. doi: 10.1155/2012/717843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kapatral V, Anderson I, Ivanova N, Reznik G, Los T, Lykidis A, Bhattacharyya A, Bartman A, Gardner W, Grechkin G. 2002. Genome sequence and analysis of the oral bacterium Fusobacterium nucleatum strain ATCC 25586. J Bacteriol 184:2005–2018. doi: 10.1128/JB.184.7.2005-2018.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Henry LG, McKenzie RM, Robles A, Fletcher HM. 2012. Oxidative stress resistance in Porphyromonas gingivalis. Future Microbiol 7:497–512. doi: 10.2217/fmb.12.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Caldwell C, Marquis R. 1999. Oxygen metabolism by Treponema denticola. Oral Microbiol Immunol 14:66–72. doi: 10.1034/j.1399-302X.1999.140109.x. [DOI] [PubMed] [Google Scholar]
- 27.Wu G, Wan F, Fu H, Li N, Gao H. 2015. A matter of timing: contrasting effects of hydrogen sulfide on oxidative stress response in Shewanella oneidensis. J Bacteriol 197:3563–3572. doi: 10.1128/JB.00603-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Caffrey SM, Voordouw G. 2010. Effect of sulfide on growth physiology and gene expression of Desulfovibrio vulgaris Hildenborough. Antonie Van Leeuwenhoek 97:11–20. doi: 10.1007/s10482-009-9383-y. [DOI] [PubMed] [Google Scholar]
- 29.Potter AJ, Trappetti C, Paton JC. 2012. Streptococcus pneumoniae uses glutathione to defend against oxidative stress and metal ion toxicity. J Bacteriol 194:6248–6254. doi: 10.1128/JB.01393-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Y, Hugenholtz J, Abee T, Molenaar D. 2003. Glutathione protects Lactococcus lactis against oxidative stress. Appl Environ Microbiol 69:5739–5745. doi: 10.1128/AEM.69.10.5739-5745.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fahey RC. 2013. Glutathione analogs in prokaryotes. Biochim Biophys Acta 1830:3182–3198. doi: 10.1016/j.bbagen.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 32.Masip L, Veeravalli K, Georgiou G. 2006. The many faces of glutathione in bacteria. Antioxid Redox Signal 8:753–762. doi: 10.1089/ars.2006.8.753. [DOI] [PubMed] [Google Scholar]
- 33.Chesney JA, Eaton JW, Mahoney J. 1996. Bacterial glutathione: a sacrificial defense against chlorine compounds. J Bacteriol 178:2131–2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pegram RA, Andersen ME, Warren SH, Ross TM, Claxton LD. 1997. Glutathione-S-transferase-mediated mutagenicity of trihalomethanes in Salmonella typhimurium: contrasting results with bromodichloromethane and chloroform. Toxicol Appl Pharmacol 144:183–188. doi: 10.1006/taap.1997.8123. [DOI] [PubMed] [Google Scholar]
- 35.Perito B, Allocati N, Casalone E, Masulli M, Dragani B, Polsinelli M, Aceto A, Di Ilio C. 1996. Molecular cloning and overexpression of a glutathione transferase gene from Proteus mirabilis. Biochem J 318:157–162. doi: 10.1042/bj3180157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu P, Alves JM, Kitten T, Brown A, Chen Z, Ozaki LS, Manque P, Ge X, Serrano MG, Puiu D. 2007. Genome of the opportunistic pathogen Streptococcus sanguinis. J Bacteriol 189:3166–3175. doi: 10.1128/JB.01808-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vickerman M, Iobst S, Jesionowski A, Gill S. 2007. Genome-wide transcriptional changes in Streptococcus gordonii in response to competence signaling peptide. J Bacteriol 189:7799–7807. doi: 10.1128/JB.01023-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hondorp ER, Matthews RG. 2004. Oxidative stress inactivates cobalamin-independent methionine synthase (MetE) in Escherichia coli. PLoS Biol 2:e336. doi: 10.1371/journal.pbio.0020336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lillig CH, Potamitou A, Schwenn J-D, Vlamis-Gardikas A, Holmgren A. 2003. Redox regulation of 3′-phosphoadenylylsulfate reductase from Escherichia coli by glutathione and glutaredoxins. J Biol Chem 278:22325–22330. doi: 10.1074/jbc.M302304200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.