Abstract
Root knot nematodes (RKNs) are the world's most damaging plant-parasitic nematodes (PPNs), and they can infect almost all crops. At present, harmful chemical nematicides are applied to control RKNs. Using microbial nematicides has been proposed as a better management strategy than chemical control. In this study, we describe a novel nematicidal bacterium named Alcaligenes faecalis ZD02. A. faecalis ZD02 was isolated from Caenorhabditis elegans cadavers and has nematostatic and nematicidal activity, as confirmed by C. elegans growth assay and life span assay. In addition, A. faecalis ZD02 fermentation broth showed toxicity against C. elegans and Meloidogyne incognita. To identify the nematicidal virulence factor, the genome of strain ZD02 was sequenced. By comparing all of the predicted proteins of strain ZD02 to reported nematicidal virulence factors, we determined that an extracellular serine protease (Esp) has potential to be a nematicidal virulence factor, which was confirmed by bioassay on C. elegans and M. incognita. Using C. elegans as the target model, we found that both A. faecalis ZD02 and the virulence factor Esp can damage the intestines of C. elegans. The discovery that A. faecalis ZD02 has nematicidal activity provides a novel bacterial resource for the control of RKNs.
INTRODUCTION
Plant-parasitic nematodes (PPNs) are an important agricultural pest of many crops, and they cause agricultural losses amounting to an estimated $157 billion worldwide every year (1, 2). Root knot nematodes (RKNs; Meloidogyne spp.) are the most damaging crop PPNs, and they are capable of infecting almost all crops (2, 3). The management of RKNs is more difficult than that of other pests because RKNs are obligate root parasites of thousands of plant species, and most of the life cycle of RKNs is spent as parasites in the host roots (2, 4). The current means of controlling RKNs relies on chemical nematicides, which are often toxic and harmful to humans and the environment (3). In addition, chemical nematicides are generally expensive and cannot provide long-term RKN suppression (5).
Biological control of RKNs is a better management strategy that avoids the potential environmental problems associated with the chemical control of RKNs. Indeed, the utilization of beneficial bacterial nematicides has been proposed as a sustainable alternative for RKN management, and bacterial nematicides are also regarded as economical and ecologically friendly nematicides for the control of RKNs (3, 6). Currently, for the bacterial control of RKNs, many problems remain, one of which is that nematicidal bacteria are relatively scarce. Only a few bacteria have been reported to possess nematicidal activity and to show potential for the biological control of RKNs. The typical nematicidal bacteria are Bacillus, Pseudomonas, and Pasteuria species (4, 7). Identifying novel nematicidal bacteria has become urgent for the bacterial control of RKNs and is of vital practical and economic significance.
However, until now, it has been a challenge to isolate novel nematicidal bacteria using RKNs as the host model because RKNs are obligate parasites of the roots and cannot be artificially cultured (2, 8). The parasitic life cycle of RKNs passes through an embryonic stage, four juvenile stages (J1 to J4), and an adult stage.
The second juvenile stage (J2) is only a short free-living stage in the rhizosphere of host plants, and it is an easily controlled phase in all life cycles for the management of RKNs (1). The J2 of RKNs is similar in function to that of the dauer larva in the animal model Caenorhabditis elegans, which is also an important host model for studying host-pathogen interactions and a simple target model for identifying novel nematicidal bacteria (4, 9). Several bacteria with nematicidal activity against RKNs have been successfully isolated using C. elegans as the target model (9–11), for example, Bacillus thuringiensis (12) and Bacillus nematocida B16 (13). These data suggest that C. elegans can be used as a target model for the screening of RKN-toxic bacteria.
Alcaligenes faecalis was first discovered in feces, and is commonly found in soil, water, and other environments (14–16). Currently, this bacterium has wide applications in the sewage treatment and pharmaceutical industries. Some A. faecalis strains produce enzymes that degrade organic contaminants and can be applicable in the biodegradation of organic pollutants and industrial wastewater in environmental industry (16, 17). Additionally, a number of A. faecalis strains can produce R-(−)-mandelic acid, an important precursor to various drugs (18, 19).
In 1974, an Alcaligenes strain was isolated from a nematode (20); until now, the nematicidal activity of this Alcaligenes strain had not been reported. In this study, A. faecalis ZD02 isolated from C. elegans cadavers was found to have nematicidal activity against C. elegans and Meloidogyne incognita. Genome analysis showed that an extracellular serine protease (Esp) has the potential to be a nematicidal virulence factor, which was confirmed by bioassay on C. elegans and M. incognita. In addition, A. faecalis ZD02 and the virulence factor Esp were confirmed to be capable of damaging the intestines of C. elegans.
MATERIALS AND METHODS
C. elegans strains.
The wild-type strain C. elegans N2 and the transgenic strain C. elegans FT63 (dlg::gfp) used in our study were kindly provided by the Caenorhabditis Genetics Center (University of Minnesota). C. elegans was cultured on nematode growth medium (NGM) agar plates using normal food (Escherichia coli OP50) under standard conditions (21). The root knot nematode M. incognita was propagated on tomato plants (Lycopersicon esculentum Mill cv. 144) in the greenhouse. The A. faecalis strain used in this work, ZD02, was isolated from C. elegans cadavers and was deposited at the China Center for Type Culture Collection (CCTCC) under accession number AB2015392.
Isolation of strain ZD02.
Dead worms were collected, transferred into conical tubes, and ground into tissue homogenate using a sterile homogenizer. The tissue homogenate was diluted and incubated for 1 day at 30°C on Luria-Bertani (LB) agar plates (1% tryptone, 0.5% yeast extract, 0.5% NaCl, 1.5% agar). Single colonies were streaked on separate plates, grown in liquid culture, and preserved by freezing.
Genome sequencing of A. faecalis ZD02.
A. faecalis ZD02 cells in the late-log growth phase were harvested and incubated with lysozyme (10 mg/ml) in 2 ml TE (50 mM Tris base, 10 mM EDTA) at 4°C for 8 h. Total DNA was purified using the genomic DNA purification kit (A1120; Promega). DNA purity was measured by the A260/A280 ratio (NanoDrop 2000 spectrophotometer; Thermo Scientific) and by gel electrophoresis. The A. faecalis ZD02 genome was sequenced using the Illumina HiSeq 2000 and MiSeq sequencing platforms, and it was assembled de novo using ABySS 1.3.4 software (22).
Cloning and expression of the extracellular serine protease gene (esp) from strain ZD02.
The cloning and expression of gene esp were performed with the pET expression system. The genomic DNA of strain ZD02 was extracted using the genomic DNA purification kit (A1120; Promega) and used as a template for PCR. The oligonucleotide primers were 5′GAATTCATGTCGAACAAAAAACGC3′ and 5′CCGCTCGAGTTAGAAACGGACCTTC3′. The PCR cycling conditions were as follows: 5 min at 95°C; 30 cycles of 30 s at 95°C, 30 s at 55°C, and 3 min at 72°C; and 10 min at 72°C. The 2.8-kb PCR product was digested with XhoI and EcoRI and then inserted into the XhoI and EcoRI sites of the expression vector pET28a(+) (Novagen, 69864). The recombinant vector pET28a(+)-Esp was transformed into E. coli BL21(DE3), and then the E. coli BL21(DE3)(pET28a(+)-Esp) bacteria were cultured in 5 ml LB medium with kanamycin, followed by vigorous shaking (220 rpm) overnight at 37°C. Then, 1 ml of the culture medium was transferred into 100 ml LB medium along with an appropriate amount of kanamycin and cultured with shaking to an optical density at 600 nm of 0.6. The expression of the Esp protein was induced for 12 h in the kanamycin-supplemented LB medium by the addition of 0.1 μM isopropyl-β-d-thiogalactopyranoside (IPTG) at 16°C. Soluble protein in the supernatant was collected by centrifugation (12,000 rpm, 10 min, and 4°C) after high-pressure shaking. Finally, the target protein was purified using His-Bind columns (31314; Qiagen), according to the manufacturer's instructions.
Synchronization of C. elegans.
The method for worm synchronization is described in reference 23. Three NGM plates full of gravid adult worms were washed with sterilized water in a 15-ml conical tube and centrifuged at 500 × g for 45 s. The gravid adult worms were lysed using a bleaching solution (0.5 M KOH, 0.8 to 1.2% sodium hypochlorite) for 3 to 10 min. After the eggs were fully released, 10 ml of H2O was quickly added. These eggs were washed three times with sterilized water and resuspended in 1 ml M9 buffer. Synchronized L1-stage worms were acquired by incubating these eggs overnight at room temperature. The synchronized L1-stage worms were placed on E. coli OP50 NGM plates and cultured at 20°C for 44 h, and the L1-stage worms grew into synchronized L4-stage worms.
Growth assay of C. elegans.
The growth assay was performed in a 96-well plate, as described in reference 23. Each well contained 20 to 30 vigorous synchronized L1-stage worms, 10 μl E. coli OP50 or A. faecalis ZD02, and S medium to bring the total volume to 100 μl. At least 60 worms were photographed under a microscope (IX71; Olympus), and the average area was calculated using the software NIH Image J1.33. Each experiment was independently replicated at least three times.
C. elegans mortality assay.
The assay of C. elegans mortality was performed in a 96-well plate. A single well included 30 to 40 vigorous synchronized L4-stage worms, 10 μl Esp protein or fermentation broth of A. faecalis ZD02 (with LB medium as the control), 10 μl E. coli OP50 at a final absorbance at 600 nm of 0.6 and 2.5 μl 8 μM FUdR, and S medium to a total volume of 100 μl. The mortality in each well was scored after incubation at 20°C for 5 days. Whether worms were alive was determined based on the worms' movement. A moving worm was marked as alive. Nonmoving worms were gently touched with a platinum pick to observe their response. Each experiment was independently replicated at least three times.
Life span assay of C. elegans.
The life span assay was performed at 20°C on NGM plates. A. faecalis ZD02 was fully spread on NGM plate and grown to a bacterial lawn to prevent worms avoiding or escaping the bacteria. Approximately 60 L4-stage worms were incubated on an E. coli OP50 NGM plate or an A. faecalis ZD02 NGM plate. The determination of whether worms were alive was as described for the mortality assay of C. elegans. The surviving worms on each plate were counted every 12 h. Statistical significance was assessed by Kaplan-Meier survival analysis followed by a log rank test.
Mortality assay of M. incognita J2.
The mortality assay of M. incognita J2 was set up in a 96-well plate. A single well included 30 to 40 vigorous M. incognita J2s, 10 μl of either Esp protein or fermentation broth of A. faecalis ZD02 (LB medium as a control), 10 μl 10% (wt/vol) resorcinol to increase the stylet pulsing frequency, 10 μl 1 mg/ml nystatin, and 70 μl sterile water to bring the total volume to 100 μl. The 96-well culture plates were placed in a box to maintain humidity. The whole set was incubated at 20°C for 5 days. Whether M. incognita J2s were alive was also determined based on the movement of M. incognita. A visibly moving J2 was marked as alive. A nonmoving J2 was gently touched with a platinum pick to observe the response. If the worm failed to respond after several touches, it was considered dead. The entire assay was performed in triplicate.
Nucleotide sequence accession numbers.
The complete genome sequences of A. faecalis ZD02 have been submitted to GenBank under accession numbers CP013119 and CP013143.
RESULTS
A. faecalis ZD02 isolated from C. elegans cadavers shows nematostatic and nematicidal activity.
In almost 10 years of C. elegans research, we have sometimes observed that almost all worms die on NGM agar plates under standard culture conditions. To find potential reasons, we tried to isolate pathogens from C. elegans cadavers. Strain ZD02 was one of the bacteria isolated from C. elegans cadavers. This bacterium formed milky white, round colonies with a smooth surface on solid LB medium (see Fig. S1A in the supplemental material). Microscopy observations indicated that the bacterium was rod shaped (0.5 to 1.0 μm by 0.5 to 2.6 μm in diameter) (see Fig. S1B in the supplemental material).
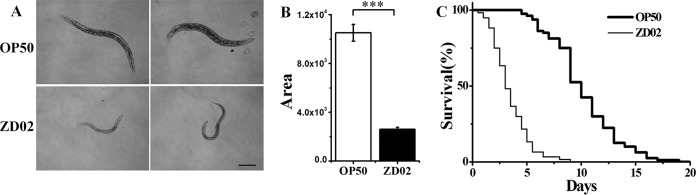
To test whether this strain has nematostatic activity against C. elegans, a growth assay was performed. Synchronized L1-stage worms were fed strain ZD02 or the normal food E. coli OP50. The growth development of worms fed ZD02 and that of worms fed OP50 exhibited large differences. As shown in Fig. 1A, worms fed OP50 developed into adults, whereas worms fed ZD02 remained in the larval stage. The average size of the worms fed ZD02 or OP50 was calculated. The worms fed OP50 were approximately five times larger than the worms fed ZD02 (Fig. 1B). To further determine whether strain ZD02 has toxicity against C. elegans, a life span assay of C. elegans was performed with worms incubated on a ZD02 or OP50 NGM plate at 20°C. As shown in Fig. 1C, more than 50% of worms fed ZD02 were dead after 3 days. Under the same conditions, almost all worms fed OP50 survived. After 7 days, only approximately 3.33% of worms fed ZD02 were alive, and more than 81% of worms fed OP50 were alive. Furthermore, all worms fed ZD02 were dead after 8 days, whereas 75% of worms fed OP50 survived. Until 15 days, approximately 10% of worms fed OP50 were still alive. These data demonstrated that strain ZD02 has nematostatic and nematicidal activity, as it inhibits the growth development of C. elegans and shortens the longevity of C. elegans.
FIG 1.
Growth assay and life span assay when wild-type N2 worms were fed strain ZD02 or the normal food E. coli OP50. (A) Wild-type N2 L1-stage worms were fed strain ZD02 or E. coli OP50 and, after incubation at 20°C for 72 h, were photographed using a light microscope. (B) The average size of the worms fed strain ZD02 or E. coli OP50 was calculated using the software NIH Image J 1.33. (C) A. faecalis ZD02 shortened the longevity of C. elegans. Wild-type L4 worms were washed with M9 buffer, transferred to A. faecalis ZD02 plates or normal-food (E. coli OP50) plates and incubated at 20°C. The survival rate of the worms on each plate was counted at 0.5-day intervals. These results are from three independent experiments. More than 60 worms were used in each well or on each plate. Bar, 0.2 mm. Significance was determined using two-sample t tests: ***, P < 0.001 (a lack of any symbol indicates no significant difference).
To identify the species of strain ZD02, the 16S rRNA gene fragment of this strain was amplified using primers 27F (AGAGTTTGATCCTGGCTCAG) and 1492R (GGTTACCTTGTTACGACTT) and sequenced. This 16S rRNA gene was found to be 100% identical to that of 12 A. faecalis species (see Table S1 in the supplemental material) and closely matched that of A. faecalis NBRC 13111 (the type strain of A. faecalis), with 99% identity (Fig. 2). This finding indicated that strain ZD02 belongs to the species A. faecalis.
FIG 2.
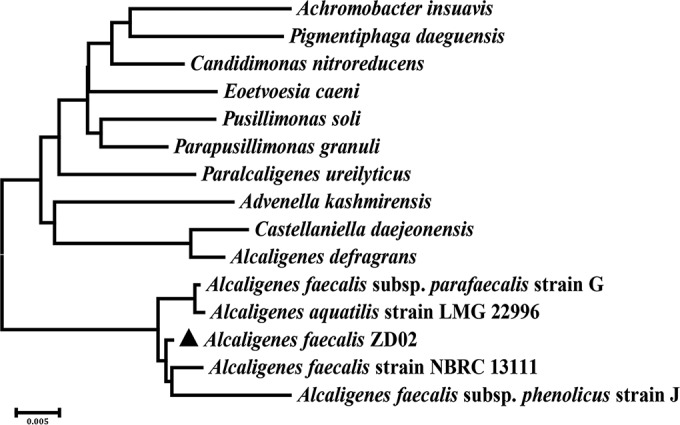
Phylogenetic tree highlighting the position of strain ZD02 relative to other strains within the genus Alcaligenes. A neighbor-joining phylogenetic tree showing the phylogenetic relationship of A. faecalis ZD02 (triangle) was generated using MEGA 6-based 16S rRNA gene sequence alignments.
Fermentation broth of A. faecalis ZD02 has toxicity against C. elegans and M. incognita.
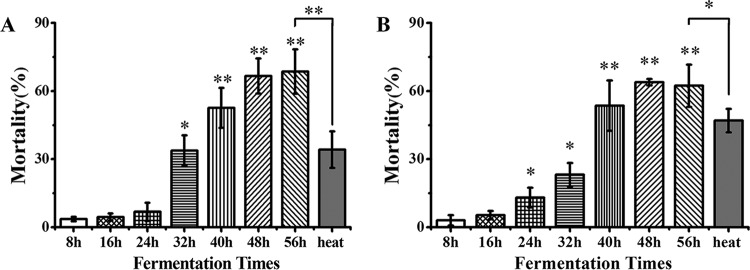
To determine whether A. faecalis ZD02 has potential as a bacterial nematicide in RKN biological management, we collected fermentation broths of A. faecalis ZD02 and then filtered them using a 0.45-μm membrane to remove the bacterial cells. A mortality assay of C. elegans was performed, with synchronized L4-stage worms added to broths in which fermentation had taken place for various durations and with LB medium as the control. As shown in Fig. 3A, the 32-h to 56-h fermentation broths had evident toxicity against C. elegans, with mortality from 33.84% ± 4.73% to 68.62% ± 9.82% (Fig. 3A). When M. incognita J2s were incubated in 8-h to 56-h fermentation broths, significant toxicity was present, with mortality from 13.03% ± 4.33% to 62.36% ± 9.28% (Fig. 3B). These data showed that the fermentation broths of A. faecalis ZD02 have evident toxicity against C. elegans and M. incognita. When the 56-h fermentation broth was treated for 30 min at 80°C, the mortality of C. elegans and M. incognita J2s was reduced to 34.20% ± 8.03% (Fig. 3A) and 46.98% ± 5.17% (Fig. 3B), respectively. Therefore, we speculated that the nematicidal activity of the fermentation broth is attributed to at least two virulence factors, one of which is heat sensitive and the other of which heat resistant.
FIG 3.
A. faecalis ZD02 fermentation broth killed C. elegans (A) and M. incognita J2 (B). Wild-type L4 C. elegans (A) and M. incognita (B) J2s were incubated in A. faecalis ZD02 fermentation broth in 96-well plates at 20°C. The mortality rate of the worms in each well was then counted; LB medium was used as the control. Percent mortality is the corrected mortality, which is the mortality in the fermentation broth minus the mortality in the LB medium. Heat inactivation (“heat”) was performed by heat treating the 56-hour fermentation broth for 30 min at 80°C. More than 60 worms were used in each plate. The results are from three independent experiments. Significance was determined using two-sample t tests: *, P < 0.05; **, P < 0.01 (a lack of any symbol indicates no significant difference).
An extracellular serine protease (Esp) from A. faecalis ZD02 functions as a nematicidal virulence factor.
The above data indicate that A. faecalis ZD02 can produce at least two substances as virulence factors. To further study these virulence factors produced by A. faecalis ZD02, the A. faecalis ZD02 genome was sequenced using the Illumina HiSeq 2000 and MiSeq sequencing platforms, and then the genome was assembled de novo using ABySS 1.3.4 (22). All predicted proteins were compared with the reported nematicidal virulence factors using BLAST, and protein WP_045929589.1 was discovered to be similar to several serine protease virulence factors that had been reported to be toxic to nematodes (24–26) (see Table S2 in the supplemental material).
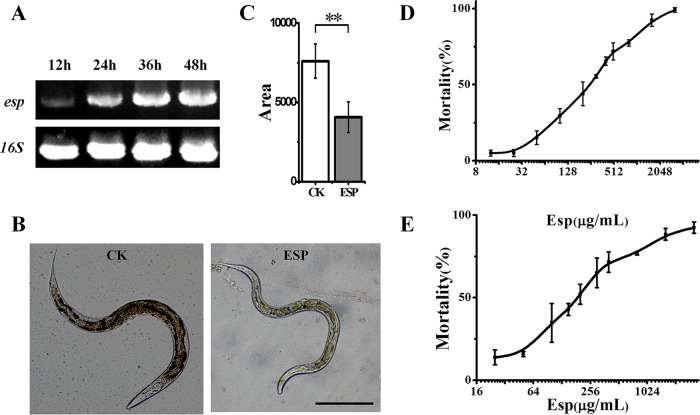
The protein contained the typical serine protease S8 superfamily motif and the signal peptide predicted by SignalP 4.0 (27); therefore, it was named the extracellular serine protease (Esp). Reverse transcription-PCR analysis confirmed that the esp gene is transcribed in A. faecalis ZD02 (Fig. 4A). The Esp protein was expressed in E. coli BL21(DE3) and purified using His-Bind columns (31314; Qiagen). Its serine protease activity was confirmed and measured to be 1,572 U/ml at 37°C and pH 7.5. To determine the nematicidal activity of the Esp protein, a growth assay of C. elegans was performed with the worms fed E. coli ESP [E. coli BL21(DE3) transformed with the recombinant vector pET28a(+)-Esp] or E. coli CK [E. coli BL21(DE3) transformed with the vector pET28a(+)]. As shown in Fig. 4B and C, worms fed E. coli ESP were obviously smaller than the worms fed E. coli CK, which indicated that E. coli-produced Esp protein can inhibit the growth development of C. elegans. To further confirm the nematicidal activity of Esp protein, mortality assays of C. elegans and M. incognita J2s were performed using various concentrations of the purified Esp protein. As shown in Fig. 4D and E, the mortality of C. elegans and M. incognita J2s increased, respectively, with increasing concentration of Esp protein; thus, the Esp protein showed significant toxicity against C. elegans and M. incognita.
FIG 4.
The Esp protein inhibited the growth of C. elegans and had toxicity against the nematode C. elegans and M. incognita. (A) Transcript levels of esp mRNA of A. faecalis ZD02 when cultured for 12 h, 24 h, 36 h, and 48 h in LB medium at 30°C. The 16S rRNA gene was used as a reference. (B) Wild-type N2 L1-stage worms were exposed to E. coli ESP or E. coli CK and, after incubation at 20°C for 72 h, photographed at a magnification of ×100. E. coli ESP is E. coli BL21(DE3) transformed with the recombinant vector pET28a(+)-Esp, and E. coli CK is E. coli BL21(DE3) transformed with empty vector pET28a(+), which was used as the control. (C) The average size of the worms fed E. coli ESP or E. coli CK was calculated using the software NIH Image J 1.33. More than 60 worms were photographed. Bar, 0.2 mm. Significance was determined using two-sample t tests: **, P < 0.01. (D) Mortality assays of C. elegans that were incubated with various concentrations of purified Esp protein. Wild-type L4 worms were washed with M9 buffer, transferred to 48-well plates, and incubated at 20°C with different concentrations of Esp protein, with E. coli OP50 as the food source; the mortality rate of the worms in each well was determined after 7 days. (E) Mortality assays of M. incognita J2s that were incubated with different concentrations of purified Esp protein. These results are from three independent experiments.
The median lethal dose (LC50) of Esp against C. elegans was 260.45 μg/ml (Fig. 4D; also, see Table S3 in the supplemental material), and the LC50 against M. incognita was 211.50 μg/ml (Fig. 4E; also, see Table S3 in the supplemental material). These data indicated that the Esp protein from strain ZD02 functions as a nematicidal virulence factor.
A. faecalis ZD02 and the Esp protein damage the intestines of C. elegans.
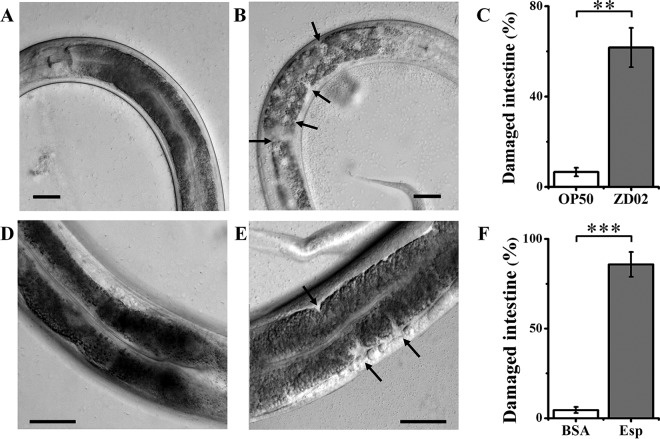
To further study the nematicidal mechanism of A. faecalis ZD02, L4-stage C. elegans worms were fed A. faecalis ZD02 or E. coli OP50, and the intestinal morphology was examined using a differential interference contrast (DIC) microscope (IX83; Olympus); the two groups were found to be different. More than 60% of worms fed strain ZD02 displayed the following symptoms. The anterior intestines were disordered and discontinuous (Fig. 5B and C); under the same conditions, more than 94% of worms fed OP50 retained an intact anterior intestine (Fig. 5A and C). These data showed that A. faecalis ZD02 displayed nematicidal activity via negative effects on host intestines. Whether the virulence factor Esp can damage the intestines was also tested. As shown in Fig. 5E and F, when the worms were incubated with Esp protein for 24 h, the intestines of more than 85% of worms were observed to be disordered; in contrast, almost all worms fed bovine serum albumin (BSA) as the control retained intact intestines (Fig. 5D and F).
FIG 5.
A. faecalis ZD02 and the Esp protein damaged the intestines of C. elegans. Wild-type N2 L4-stage worms were fed the normal food E. coli OP50 (A), A. faecalis ZD02 (B), BSA (as the control) (D), and Esp protein (E) at 20°C for 24 h. The anterior intestines of the nematodes were photographed using a differential interference contrast (DIC) microscope. Arrows indicate intestinal damage by A. faecalis ZD02. (C) Frequency of intestinal damage in worms fed E. coli OP50 or A. faecalis ZD02. (F) Frequency of intestinal damage in worms fed BSA or Esp. Values are the percentage of worms with damaged intestines. More than 60 worms were inspected by microscopy. Bar, 20 μm. Significance was determined using two-sample t tests: **, P < 0.01; ***, P < 0.001.
To allow a different visual observation of the effects of A. faecalis ZD02 on worms' intestines, the transgenic worms FT63 (dlg::gfp) were labeled with green fluorescent protein (GFP) at the intestinal epithelial cell junctions to determine the integrity of the intestine by observing GFP fluorescence (28, 29). When FT63 (dlg::gfp) worms were fed E. coli OP50 or BSA, most of their intestines showed an integrated line of clear green fluorescence with a bamboo-like shape under a fluorescence microscope (IX83; Olympus) (Fig. 6A and C). When FT63 (dlg::gfp) worms were fed A. faecalis ZD02 or Esp protein, more than 70% of worms' intestines exhibited a GFP fluorescence mislocalization phenotype: the intestinal bamboo-like lines were blurred or even absent, and the GFP fluorescence had dispersed to other locations, such as the bottom or vesicular structures (Fig. 6B and D). Here, we conclude that A. faecalis ZD02 and Esp can damage the intestine of C. elegans.
FIG 6.
The anterior intestines were observed in GFP-labeled transgenic FT63 (dlg::gfp) worms by epifluorescence microscopy after feeding with E. coli OP50 (A), A. faecalis ZD02 (B), BSA (C), or Esp protein (D). Twenty to 30 worms were incubated for 24 h at 20°C. More than 60 worms were inspected by microscopy. Bar, 200 μm.
The above data indicated that A. faecalis ZD02 and the virulence factor Esp appeared to exert their nematicidal activity at least in part via damaging the intestines of C. elegans. By comparing the effects of the bacterium with the effects of the putative toxin (Fig. 5B and E and 6B and D), we also found that A. faecalis ZD02 damaged the intestines of C. elegans more quickly and to a greater extent than the putative virulence factor Esp protein.
DISCUSSION
RKNs cause huge economic losses every year (2, 3). The most widely used chemical nematicides, such as ethoprop, fenamiphos, fosthiazate, and oxamyl, are often unsatisfactory and potentially harm human health and the environment (3). The control of RKNs using microbial nematicides has several advantages over chemical control. In our study, A. faecalis ZD02 isolated from C. elegans cadavers was able to kill C. elegans and M. incognita. By comparison with the genome sequence of known nematicidal virulence factors, we hypothesized that an extracellular serine protease, the Esp protein, is a virulence factor against the nematodes C. elegans and M. incognita. Subsequently, it was confirmed that A. faecalis ZD02 and the Esp protein can damage the intestines of C. elegans. To the best of our knowledge, A. faecalis ZD02 is a novel nematicidal Alcaligenes species, and we conclude that it has potential applications in agriculture.
Several types of proteases have been reported to serve as virulence factors in various bacteria, particularly in Bacillus spp. of rhizobacteria (25). An extracellular serine protease from Bacillus nematocida B16 serves as a virulence factor against nematodes (24). Brevibacillus laterosporus G4 has been shown to infect nematodes and to have a high nematicidal activity; the virulence factor was also found to be an extracellular protease (26). Another nematicidal protease that has been shown to act as a virulence factor is the metalloproteinase Bmp1 from B. thuringiensis strain YBT-1518, which not only has toxicity against C. elegans but also can enhance the toxicity of the nematicidal Cry protein Cry5Ba from this B. thuringiensis (30). The nematicidal mechanism of protease virulence factors is primarily that these proteases can hydrolyze several intestinal or cuticle tissues of nematodes (13, 24–26, 30). In this study, the Esp protein from A. faecalis ZD02 was shown to be a virulence factor against the nematodes C. elegans and M. incognita and was able to damage the intestines of C. elegans. Therefore, we speculated that the nematicidal activity of A. faecalis ZD02 is attributable to intestinal damage to C. elegans caused by the virulence factor Esp protein.
However, according to the data in Fig. 3, A. faecalis ZD02 most likely has several virulence factors against nematodes, and the nematicidal activity of strain ZD02 is most likely attributable to other factors in addition to the Esp protein. By further analyzing the genome of strain ZD02, the PhzE-like protein WP_045930653.1 was detected (see Table S2 in the supplemental material). PhzE is an aminodeoxyisochorismate synthase that is necessary for the biosynthesis of pyocyanin and phenazine-1-carboxylate in P. aeruginosa PAO1 (31), and pyocyanin and phenazine-1-carboxylate have been reported to be capable of killing nematodes (32). This finding implied that A. faecalis ZD02 may produce pyocyanin-like or phenazine-1-carboxylate-like compounds that kill nematodes. We also found that the live bacteria were more toxic than dead ones on the NGM plates. The A. faecalis ZD02 organisms grown on enriched nematode growth (ENGM) plates killed more quickly than those on NGM plates (data not shown). These findings indicated that the nematicidal activity of this bacterium is partly attributable to the growth of A. faecalis ZD02. Further research is required to determine whether this or other virulence factors have nematicidal activity and how they target the nematode host to better understand the molecular mechanism of strain ZD02's action against nematodes.
A. faecalis is a Gram-negative, rod-shaped bacterium commonly found in soil, water, and other natural environments (14, 33). This bacterium has wide applications in environmental protection. Some A. faecalis strains can be used to biodegrade organic pollutants and industrial wastewater because they can produce organic contaminant-degrading enzymes (16, 17). For example, A. faecalis subsp. phenolicus, isolated from a gray water bioprocessor, is a phenol-degrading bacterium (16). A. faecalis JBW4, isolated from activated sludge, has been reported to be capable of degrading the organochlorine pesticide endosulfan in soils (17). A. faecalis has also been shown to degrade petroleum hydrocarbons, which makes A. faecalis an excellent candidate for the bioremediation of hydrocarbon pollution (34). A. faecalis no. 4, isolated from sewage sludge, has been shown to have aerobic denitrification abilities and to convert ammonium into denitrification products, which could be used in ammonium removal from wastewater (35). A. faecalis also has the ability to neutralize environments contaminated by arsenite, because it can metabolize arsenite (AsO2−) to the less harmful molecule arsenate (AsO4−) (15, 36, 37). Moreover, some A. faecalis strains can produce a variety of plant growth-hastening hormones that are used as biofertilizers in agriculture (38). Here, we report that the Alcaligenes bacterium A. faecalis ZD02 has toxicity against M. incognita. In comparison with other known nematicidal bacteria, a huge advantage of using A. faecalis as a microbial nematicide is that A. faecalis is capable of colonizing organisms in soil (17). This feature can make the strain a dominant bacterium in rhizosphere soil and can increase the probability of its coming into contact with RKNs. This feature may make A. faecalis an excellent candidate for the development of novel multifunctional microorganism-derived nematicides. This A. faecalis nematicide not only can be used as a nematicide for the biocontrol of RKNs but also can bioremediate soils contaminated with organic substances or arsenite and can even be used as a biofertilizer to hasten the growth of crops.
Although A. faecalis ZD02 has great potential as a microbial nematicide, more research is needed before it can be applied as a microbial nematicide in agriculture. In-depth studies are ongoing, and further research will primarily evaluate the following: the systemic nematicidal activities of A. faecalis ZD02 against RKNs, including lethal activity, the suppression of egg hatching and motility, the control of RKNs by A. faecalis ZD02 in potted soil, the effects of A. faecalis ZD02 on RKN infestation in greenhouses and in fields, and a safety evaluation and risk assessment.
Here, we show that a novel A. faecalis strain, ZD02, has nematicidal activity and that an extracellular serine protease serves as the virulence factor. The present study will provide an important model for understanding the relationship between bacterial pathogens and nematodes. In addition, the discovery that A. faecalis ZD02 can kill M. incognita will provide new nematicidal bacterial resources and promising possibilities for the biocontrol of plant-parasitic nematodes.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grants from the National High Technology Research and Development Program (863) of China (2011AA10A203), the China 948 Program of the Ministry of Agriculture (2011-G25), the College Excellent Youth Science and Technology Innovation Team Project of Hubei Province (T201535), and the National Natural Science Foundation of China (31170047).
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.03444-15.
REFERENCES
- 1.Abad P, Gouzy J, Aury JM, Castagnone-Sereno P, Danchin EG, Deleury E, Perfus-Barbeoch L, Anthouard V, Artiguenave F, Blok VC, Caillaud MC, Coutinho PM, Dasilva C, De Luca F, Deau F, Esquibet M, Flutre T, Goldstone JV, Hamamouch N, Hewezi T, Jaillon O, Jubin C, Leonetti P, Magliano M, Maier TR, Markov GV, McVeigh P, Pesole G, Poulain J, Robinson-Rechavi M, Sallet E, Segurens B, Steinbach D, Tytgat T, Ugarte E, van Ghelder C, Veronico P, Baum TJ, Blaxter M, Bleve-Zacheo T, Davis EL, Ewbank JJ, Favery B, Grenier E, Henrissat B, Jones JT, Laudet V, Maule AG, Quesneville H, Rosso MN, et al. . 2008. Genome sequence of the metazoan plant-parasitic nematode Meloidogyne incognita. Nat Biotechnol 26:909–915. doi: 10.1038/nbt.1482. [DOI] [PubMed] [Google Scholar]
- 2.Jones JT, Haegeman A, Danchin EG, Gaur HS, Helder J, Jones MG, Kikuchi T, Manzanilla-Lopez R, Palomares-Rius JE, Wesemael WM, Perry RN. 2013. Top 10 plant-parasitic nematodes in molecular plant pathology. Mol Plant Pathol 14:946–961. doi: 10.1111/mpp.12057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trudgill DL, Blok VC. 2001. Apomictic, polyphagous root-knot nematodes: exceptionally successful and damaging biotrophic root pathogens. Annu Rev Phytopathol 39:53–77. doi: 10.1146/annurev.phyto.39.1.53. [DOI] [PubMed] [Google Scholar]
- 4.Tian B, Yang J, Zhang KQ. 2007. Bacteria used in the biological control of plant-parasitic nematodes: populations, mechanisms of action, and future prospects. FEMS Microbiol Ecol 61:197–213. doi: 10.1111/j.1574-6941.2007.00349.x. [DOI] [PubMed] [Google Scholar]
- 5.Yu Z, Xiong J, Zhou Q, Luo H, Hu S, Xia L, Sun M, Li L, Yu Z. 2015. The diverse nematicidal properties and biocontrol efficacy of Bacillus thuringiensis Cry6A against the root-knot nematode Meloidogyne hapla. J Invertebr Pathol 125:73–80. doi: 10.1016/j.jip.2014.12.011. [DOI] [PubMed] [Google Scholar]
- 6.Li J, Zou C, Xu J, Ji X, Niu X, Yang J, Huang X, Zhang KQ. 2015. Molecular mechanisms of nematode-nematophagous microbe interactions: basis for biological control of plant-parasitic nematodes. Annu Rev Phytopathol 53:67–95. doi: 10.1146/annurev-phyto-080614-120336. [DOI] [PubMed] [Google Scholar]
- 7.Siddiqui ZA, Mahmood I. 1999. Role of bacteria in the management of plant parasitic nematodes: a review. Bioresour Technol 69:167–179. doi: 10.1016/S0960-8524(98)00122-9. [DOI] [Google Scholar]
- 8.Opperman CH, Bird DM, Williamson VM, Rokhsar DS, Burke M, Cohn J, Cromer J, Diener S, Gajan J, Graham S, Houfek TD, Liu Q, Mitros T, Schaff J, Schaffer R, Scholl E, Sosinski BR, Thomas VP, Windham E. 2008. Sequence and genetic map of Meloidogyne hapla: a compact nematode genome for plant parasitism. Proc Natl Acad Sci U S A 105:14802–14807. doi: 10.1073/pnas.0805946105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tan MW, Shapira M. 2011. Genetic and molecular analysis of nematode-microbe interactions. Cell Microbiol 13:497–507. doi: 10.1111/j.1462-5822.2011.01570.x. [DOI] [PubMed] [Google Scholar]
- 10.Simonsen KT, Gallego SF, Faergeman NJ, Kallipolitis BH. 2012. Strength in numbers: “omics” studies of C. elegans innate immunity. Virulence 3:477–484. doi: 10.4161/viru.21906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hooper LV, Littman DR, Macpherson AJ. 2012. Interactions between the microbiota and the immune system. Science 336:1268–1273. doi: 10.1126/science.1223490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo S, Liu M, Peng D, Ji S, Wang P, Yu Z, Sun M. 2008. New strategy for isolating novel nematicidal crystal protein genes from Bacillus thuringiensis strain YBT-1518. Appl Environ Microbiol 74:6997–7001. doi: 10.1128/AEM.01346-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang XW, Niu QH, Zhou W, Zhang KQ. 2005. Bacillus nematocida sp. nov., a novel bacterial strain with nematotoxic activity isolated from soil in Yunnan, China. Syst Appl Microbiol 28:323–327. doi: 10.1016/j.syapm.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 14.Sarkar JK, Choudhury B, Tribedi BP. 1959. Alcaligenes faecalis; its systematic study. Indian J Med Res 47:1–12. [PubMed] [Google Scholar]
- 15.Phung LT, Trimble WL, Meyer F, Gilbert JA, Silver S. 2012. Draft genome sequence of Alcaligenes faecalis subsp. faecalis NCIB 8687 (CCUG 2071). J Bacteriol 194:5153. doi: 10.1128/JB.01185-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rehfuss M, Urban J. 2005. Alcaligenes faecalis subsp. phenolicus subsp. nov. a phenol-degrading, denitrifying bacterium isolated from a graywater bioprocessor. Syst Appl Microbiol 28:421–429. doi: 10.1016/j.syapm.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 17.Kong L, Zhu S, Zhu L, Xie H, Wei K, Yan T, Wang J, Wang J, Wang F, Sun F. 2014. Colonization of Alcaligenes faecalis strain JBW4 in natural soils and its detoxification of endosulfan. Appl Microbiol Biotechnol 98:1407–1416. doi: 10.1007/s00253-013-5033-4. [DOI] [PubMed] [Google Scholar]
- 18.Xia Z. 2013. Effect of Tween 80 on the production of curdlan by Alcaligenes faecalis ATCC 31749. Carbohydr Polym 98:178–180. doi: 10.1016/j.carbpol.2013.05.073. [DOI] [PubMed] [Google Scholar]
- 19.Yamamoto K, Oishi K, Fujimatsu I, Komatsu K. 1991. Production of R-(-)-mandelic acid from mandelonitrile by Alcaligenes faecalis ATCC 8750. Appl Environ Microbiol 57:3028–3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lysenko O, Weiser J. 1974. Bacteria associated with the nematode Neoplectana carpocapsae and the pathogenicity of this complex for Galleria mellonella larvae. J Invertebr Pathol 24:332–336. doi: 10.1016/0022-2011(74)90140-2. [DOI] [PubMed] [Google Scholar]
- 21.Brenner S. 1974. The genetics of Caenorhabditis elegans. Genetics 77:71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simpson JT, Wong K, Jackman SD, Schein JE, Jones SJ, Birol I. 2009. ABySS: a parallel assembler for short read sequence data. Genome Res 19:1117–1123. doi: 10.1101/gr.089532.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bischof LJ, Huffman DL, Aroian RV. 2006. Assays for toxicity studies in C. elegans with Bt crystal proteins. Methods Mol Biol 351:139–154. [DOI] [PubMed] [Google Scholar]
- 24.Qiuhong N, Xiaowei H, Baoyu T, Jinkui Y, Jiang L, Lin Z, Keqin Z. 2006. Bacillus sp. B16 kills nematodes with a serine protease identified as a pathogenic factor. Appl Microbiol Biotechnol 69:722–730. [DOI] [PubMed] [Google Scholar]
- 25.Lian LH, Tian BY, Xiong R, Zhu MZ, Xu J, Zhang KQ. 2007. Proteases from Bacillus: a new insight into the mechanism of action for rhizobacterial suppression of nematode populations. Lett Appl Microbiol 45:262–269. doi: 10.1111/j.1472-765X.2007.02184.x. [DOI] [PubMed] [Google Scholar]
- 26.Huang X, Tian B, Niu Q, Yang J, Zhang L, Zhang K. 2005. An extracellular protease from Brevibacillus laterosporus G4 without parasporal crystals can serve as a pathogenic factor in infection of nematodes. Res Microbiol 156:719–727. doi: 10.1016/j.resmic.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 27.Petersen TN, Brunak S, von Heijne G, Nielsen H. 2011. SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat Methods 8:785–786. doi: 10.1038/nmeth.1701. [DOI] [PubMed] [Google Scholar]
- 28.Kang J, Shin D, Yu J-R, Lee J. 2009. Lats kinase is involved in the intestinal apical membrane integrity in the nematode Caenorhabditis elegans. Development (Camb) 136:2705–2715. doi: 10.1242/dev.035485. [DOI] [PubMed] [Google Scholar]
- 29.Peng D, Lin J, Huang Q, Zheng W, Liu G, Zheng J, Zhu L, Sun M. 21 December 2015. A novel metalloproteinase virulence factor is involved in Bacillus thuringiensis pathogenesis in nematodes and insects. Environ Microbiol doi: 10.1111/1462-2920.13069. [DOI] [PubMed] [Google Scholar]
- 30.Luo X, Chen L, Huang Q, Zheng J, Zhou W, Peng D, Ruan L, Sun M. 2013. Bacillus thuringiensis metalloproteinase Bmp1 functions as a nematicidal virulence factor. Appl Environ Microbiol 79:460–468. doi: 10.1128/AEM.02551-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Culbertson JE, Toney MD. 2013. Expression and characterization of PhzE from P. aeruginosa PAO1: aminodeoxyisochorismate synthase involved in pyocyanin and phenazine-1-carboxylate production. Biochim Biophys Acta 1834:240–246. doi: 10.1016/j.bbapap.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 32.Cezairliyan B, Vinayavekhin N, Grenfell-Lee D, Yuen GJ, Saghatelian A, Ausubel FM. 2013. Identification of Pseudomonas aeruginosa phenazines that kill Caenorhabditis elegans. PLoS Pathog 9:e1003101. doi: 10.1371/journal.ppat.1003101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bizet J, Bizet C. 1997. Strains of Alcaligenes faecalis from clinical material. J Infect 35:167–169. doi: 10.1016/S0163-4453(97)91710-2. [DOI] [PubMed] [Google Scholar]
- 34.Bharali P, Das S, Konwar BK, Thakur AJ. 2011. Crude biosurfactant from thermophilic Alcaligenes faecalis: feasibility in petro-spill bioremediation. Int Biodeterior Biodegradation 65:682–690. doi: 10.1016/j.ibiod.2011.04.001. [DOI] [Google Scholar]
- 35.Joo HS, Hirai M, Shoda M. 2005. Characteristics of ammonium removal by heterotrophic nitrification-aerobic denitrification by Alcaligenes faecalis no. 4. J Biosci Bioeng 100:184–191. doi: 10.1263/jbb.100.184. [DOI] [PubMed] [Google Scholar]
- 36.Anderson GL, Williams J, Hille R. 1992. The purification and characterization of arsenite oxidase from Alcaligenes faecalis, a molybdenum-containing hydroxylase. J Biol Chem 267:23674–23682. [PubMed] [Google Scholar]
- 37.Philips SE, Taylor ML. 1976. Oxidation of arsenite to arsenate by Alcaligenes faecalis. Appl Environ Microbiol 32:392–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yokoyama S, Adachi Y, Asakura S, Kohyama E. 2013. Characterization of Alcaligenes faecalis strain AD15 indicating biocontrol activity against plant pathogens. J Gen Appl Microbiol 59:89–95. doi: 10.2323/jgam.59.089. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.