Abstract
Novel decontamination technologies, including cold low-pressure plasma and blue light (400 nm), are promising alternatives to conventional surface decontamination methods. However, the standardization of the assessment of such sterilization processes remains to be accomplished. Bacterial endospores of the genera Bacillus and Geobacillus are frequently used as biological indicators (BIs) of sterility. Ensuring standardized and reproducible BIs for reliable testing procedures is a significant problem in industrial settings. In this study, an electrically driven spray deposition device was developed, allowing fast, reproducible, and homogeneous preparation of Bacillus subtilis 168 spore monolayers on glass surfaces. A detailed description of the structural design as well as the operating principle of the spraying device is given. The reproducible formation of spore monolayers of up to 5 × 107 spores per sample was verified by scanning electron microscopy. Surface inactivation studies revealed that monolayered spores were inactivated by UV-C (254 nm), low-pressure argon plasma (500 W, 10 Pa, 100 standard cubic cm per min), and blue light (400 nm) significantly faster than multilayered spores were. We have thus succeeded in the uniform preparation of reproducible, highly concentrated spore monolayers with the potential to generate BIs for a variety of nonpenetrating surface decontamination techniques.
INTRODUCTION
Microbial contamination on surfaces is a recurring problem within health, pharmaceutical, and food industry sectors (1, 2, 3). Thus, decontamination is a crucial step to ensure the sterility of food processing equipment, minimize spread of pathogens, and prevent the transmission of nosocomial infections (4). Common decontamination and disinfection procedures that are widely used for microbial inactivation include high temperatures, chemicals, or ionizing radiation (reviewed in reference 5). In order to ensure the efficiency and to validate the continuous functionality of a disinfection or sterilization procedure, biological testing standards are required. Bacterial spores are frequently used as a biological indicator (BI) of sterility, primarily because bacterial spores exhibit elevated resistance to chemical and physical methods of sterilization (6–11). Hence, a process that achieves full spore inactivation ensures complete elimination of other contaminating microorganisms.
Variations in the performance of a BI have been reported repeatedly (12, 13). Besides variations in the intrinsic resistance properties of the microorganisms conferred, for instance, by variations in genetic traits or alteration of sporulation conditions (14), extrinsic factors also may affect the performance of BIs and, subsequently, the accurate determination of spore resistance and inactivation. For example, the sterilization results may be altered by poor choices of the carrier material for spore deposition (13, 15) and, in particular, the BI manufacturing procedure (16). The method by which spores are mounted on carriers also is extremely important, as inconsistencies in the procedure affect the homogeneity of spore deposition. In particular, the presence of spore clusters and/or layers is likely to influence the sterilization results, as shielded spores can exhibit increased resistance to some treatments (15, 17). Therefore, adequate control procedures when manufacturing BIs are essential, and key factors that affect the BI manufacturing are the standardized BI design and a reproducible spore deposition technique (10).
Emerging methods for improved surface decontamination of food packaging, medical instruments, and military equipment (1, 2, 18) include cold low-pressure plasma and blue light. Photoinactivation of vegetative cells and spores using visible light, specifically short-wave blue light, has become an area of increasing research interest (19). Advantages of this particular light-based inactivation, in contrast to inactivation by UV-C or ionizing radiation, include improved safety due to lower photon energy and reduced photodegradation of materials (20). Photodynamic inactivation is an oxygen-dependent mechanism based on the photoexcitation of microbial porphyrin molecules which act as endogenous photosensitizers. Excited porphyrin molecules can react with oxygen and transfer energy, resulting in the generation of a variety of cytotoxic oxygen species, predominately singlet oxygen and hydroxyl radicals (21). An accumulation of induced oxidative damage ultimately leads to cell death.
The use of low-pressure plasma discharges is a state-of-the-art procedure that enables the sterilization of innovative heat-sensitive materials, equipment prone to corrosion, and complex electronic instruments. A combination of highly reactive species (ions, free electrons, radicals, neutral/excited atoms, or molecules) with UV and vacuum UV (VUV) photons at different wavelengths leads to rapid microbial inactivation by interacting with essential cell components (22, 23). UV and VUV photons in particular have been shown to have a major role in the reduction of spore survival by plasma (24, 25). However, research to standardize the cold plasma sterilization process in order to eliminate ambiguous sterilization outcomes still is needed (26).
In this report, an electrically operated spray deposition device is described that allows for the reproducible and homogeneous deposition of Bacillus subtilis spore monolayers onto glass surfaces. A detailed description of the structural design of the spray deposition device is provided, including the various components as well as the operating principles. The performance of prepared monolayered spore samples was tested by four decontamination methods, namely, those employing X rays, UV-C radiation at 254 nm, blue light, and low-pressure argon plasma, and compared to the treatment of multilayered spore samples.
MATERIALS AND METHODS
Spore production and purification.
Bacillus subtilis trpC2 strain 168 (DSM 402) was obtained from the German Collection of Microorganisms and Cell Cultures (DSMZ, Braunschweig, Germany) and routinely cultivated on Luria-Bertani (LB) agar plates. Spores were prepared by cultivation in double-strength liquid Schaeffer sporulation medium (27) with vigorous aeration at 37°C for 72 h. Harvested spores were purified by repeated washing steps using sterile water followed by lysozyme and DNase I treatment for the removal of remaining vegetative cells. After a heat inactivation step at 80°C for 10 min to inactivate any remaining vegetative cells or germinated spores, the spores were repeatedly washed with sterile water and checked for purity by phase-contrast microscopy. Spore preparations were free (>99%) of vegetative cells, germinated spores, and cell debris. Spores were stored at 4°C until tested.
Machine setup of the electrically operated spray deposition device.
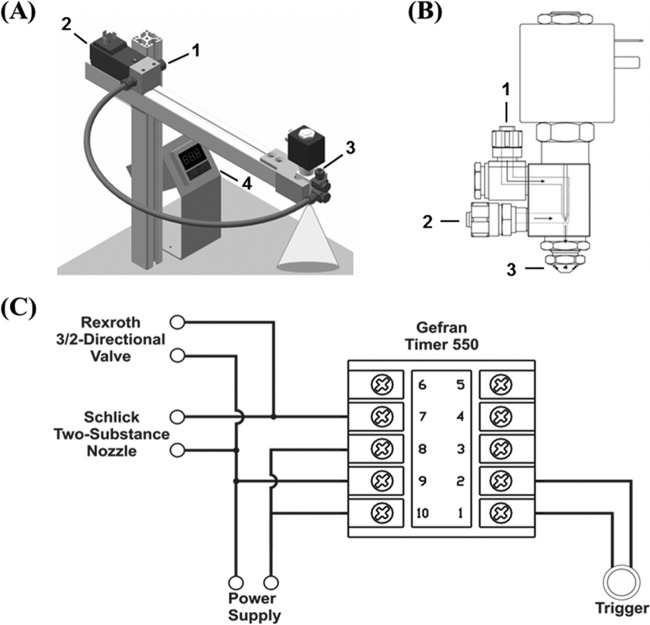
For homogenous aerosol deposition of B. subtilis spores, an electrically operated aerosol deposition unit was developed consisting of a mounting station and three main electric components (Fig. 1A). A high-precision two-substance nozzle with a solenoid valve (230 V, 50 Hz; model 970-8; Schlick, Untersiemau, Germany) (Fig. 1B) equipped with a standard air cap allows for uniform dispersion of B. subtilis spores on sample carriers. The nozzle comprises two inlets, one for the pressurized carrier gas and one for the liquid sample. Oil-free pressurized N2 functions as a propellant gas, preventing unwanted impurities and oxidative reactions. The gas supply is regulated by a pressure gauge and power-driven 3/2 magnetic valve (DO35, 230 A/C; Bosch-Rexroth, Munich, Germany) between the gas supply and the nozzle air inlet. Liquid throughput is regulated by gas pressure, cap position, and synchronized nozzle opening time. The simultaneous opening of the nozzle and the magnetic valve for the N2 supply is determined by a 220-V A/C microprocessor-based quartz counter (550-2-C timer; Gefran, Seligenstadt, Germany). A model circuit diagram is displayed in Fig. 1C. The electrical opening of the magnetic valve controlling N2 flow to the nozzle and the electrical opening of the nozzle liquid inlet are synchronized. The N2 flow allows dispersion of the injected liquid sample supply through the nozzle outlet. A faceplate 3-digit display allows software configuration of instrument type, output function, operation mode, and nozzle opening time. In cyclical double operation mode, timing for opening and closing both valves is adjustable. Time intervals between cyclic operations can be selected and allow fast and serial processing of sample preparation. The continuously variable air cap of the nozzle allows an adjustable spray radius. The nozzle head and the designated sample carrier are enclosed in a particle deposition cabinet to prevent environmental aerosol dispersion.
FIG 1.
(A) Sketch of the device used for the aerosol spray deposition of B. subtilis 168 spores. The spraying device consists of the following components: 1, N2 supply; 2, power-driven 3/2 magnetic valve; 3, two-substance nozzle with solenoid valve and power connector; 4, microprocessor-based quartz counter. (B) Computer-aided-design (CAD) drawing of a two-substance nozzle (provided by Schlick, Untersiemau, Germany) with the following components: 1, inlet of liquid sample; 2, inlet of pressurized N2; 3, variable nozzle air cap for controlling the spray radius. (C) Circuit sketch of the electrical wiring connecting the microprocessor-based quartz counter with the magnetic valve and two-component nozzle for the synchronized opening of the N2 inlet and liquid inlet, respectively. The power button was added between port 1 and port 2. A voltage of 230 V at 50 Hz was applied to ports 9 and 10. Ports 8 and 10 are interconnected.
Preparation of aerosol-deposited spores.
For spray deposition, B. subtilis spores were suspended at variable concentrations in sterile Millipore water (conductivity of 0.056 S/cm). One milliliter was transferred to the filling chamber connected to the nozzle fluid inlet. Commercially available sterilized microscopic slides (VWR, Germany) served as the sample carrier, with each slide representing one experimental sample. The clean sample carrier was placed inside the aerosol-tight dispersion cabinet and positioned in alignment with the nozzle at a distance of 15 cm along surface-engraved grid axes on the removable cabinet unit. The spraying process was initiated. The sprayed spore suspension forms a thin film on the microscopic slide that dries rapidly within seconds. The parameters found to be ideal for aerosol deposition of B. subtilis spores include (i) N2 flow with a pressure of 2.0 × 105 Pa, (ii) a nozzle opening setting of 2 for a spray radius matching the sample carrier size, (iii) a software configuration of the quartz counter to level 0, type 2 (cyclic timer), output function 3 (synchronized relays), and logic for digital input 0 (active in closing), (iv) a nozzle opening time of 0.1 s, and (v) a distance from the nozzle to the sample carrier of 15 cm. The loaded sample carrier was removed from the cabinet and left to air dry completely. The final load of spores on a sample carrier after one spray volume was either 106, 107, or 5 × 107 depending on the initial spore concentration and machine settings. To evaluate whether repeated spray deposition on a sample carrier influences spore distribution, 5 spray volumes with 5 × 107 spores each were applied. For cleaning, the nozzle was rinsed with sterile distilled water, immersed in an ultrasonic bath for 15 min, and then autoclaved. For each experimental sample, 3 replicates were prepared.
Preparation of liquid-deposited spores.
A common method for BI sample preparation is the deposition of B. subtilis spores suspended in water by spot inoculation. To compare the effects of the sample preparation method on spore sensitivity to various decontamination treatments, spores also were applied to carriers by liquid deposition. In this work, 50 μl of a suspension containing 5 × 107 B. subtilis spores in sterile Millipore water was deposited on circular histological glass object slides (VWR, Germany) and dried overnight at room temperature. For each experimental sample 3 replicates were prepared.
SEM analysis.
The distribution of spores applied to carriers as a spray or in liquid was determined using scanning electron microscopy (SEM). Two independent samples each of liquid- or spray-applied spores were used for the analysis. To ensure conductance of spores on glass slides, samples were fixed on substrate holders and then coated with gold with the JEOL JFC-1200 fine coater. The chamber of the fine coater was evacuated below 2 Pa, flushed with Argon up to 80 Pa, and pumped to 7 Pa again to ensure a sufficient clean argon atmosphere before coating the samples for 40 s. After venting, the samples were placed in the SEM chamber and analyzed by SEM (JSM 6510; JEOL, Eching, Germany). Electrons were accelerated with a voltage of 10 keV, and images were recorded at magnifications of 300 and 3,000.
Live-cell imaging.
Spores (107) were applied to a 35-mm-diameter imaging dish with an ibidi standard bottom (ibidi GmbH, Germany) via spray deposition as described above and air dried in ambient air. Spores were covered with a thin (1-mm) layer of 2% LB-agarose (VWR, Germany) and imaged at 37°C in a temperature-controlled incubation system by phase-contrast microscopy (TE2000-E Eclipse, 100× Ph3; LM Nikon). Images were captured with intervals of 5 s for a total of 4 h. To check for an even distribution of the spores sprayed on the plastic surface of ibidi μ-dishes, samples were sputter-coated with 2 nm of gold-palladium and analyzed by SEM (Leo 1530; Carl Zeiss Microscopy, Germany). Images were recorded with the in-lens secondary electron detector at 3 kV and a working distance of about 4 mm.
Assay of spore resistance to germicidal 254-nm UV and ionizing radiation.
Spray- and liquid-applied spores were subjected in triplicate to monochromatic UV-C radiation emitted by a mercury low-pressure lamp with a major emission line at 254 nm (NN 8/15; Heraeus, Germany). The fluence (0.7 J · m−2 · s−1) was measured by a UV-X radiometer fitted with the appropriate calibrated probe at 254 nm (UVP, United Kingdom), and the exposure time for the indicated UV doses was calculated. UV-C irradiation was carried out at room temperature as previously described in reference 28. Spray- and liquid-applied samples were exposed in triplicate to ionizing radiation (200 keV, 15 mA) generated by an X-ray tube (RS225; Gulmay, United Kingdom [now X-Strahl, Surrey, United Kingdom]) at room temperature as described previously in reference 29. The dosimeter UNIDOSwebline with the ionizing chamber TM30013 (PTW, Freiburg, Germany) was used for calculating dose and dose rate. The distance of the samples from the X-ray source was set at 30 cm to provide a constant dose rate of 12.2 Gy/min.
Assay of spore resistance to low-pressure plasma.
Low-pressure plasma treatment of spore samples was carried out at the Institute for Electrical Engineering and Plasma Technology (AEPT), Ruhr University Bochum, Bochum, Germany. A double inductively coupled low-pressure plasma (DICP) source (see Fig. S1 in the supplemental material) (30, 31) was used in this study. The stainless steel cylinder of the DICP vessel is enclosed by two quartz glass plates with enclosed copper coils at the top and bottom, which couple a maximum power of 5 kW at 13.56 MHz to the discharge. A matchbox splits the power equally to both coils. A roots pump achieves a pressure of 2 to 50 Pa with a gas flow of 100 sccm (standard cubic centimeters per minute) argon. Additionally, if the system is not operated, a turbo pump is used for evacuating the system below 5 × 10−4 Pa for maintaining the cleanliness of the system. The plasma discharge is homogeneous over nearly the entire vessel (30). Several flanges in the vessel allow different optical and electrical analytical devices to be coupled to the vessel for plasma diagnostics. Samples were placed in the center of the discharge at the same level as the diagnostic devices and exposed to low-pressure argon plasma discharges (total gas flow of 100 sccm, pressure of 10 Pa, radio frequency power of 500 W). Under these conditions, the total UV flux from 130 to 400 nm amounts to 0.74 J · m−2 · s−1. In addition, argon line emission in the VUV spectra is at 104.8 nm and 106.7 nm. For each of the performed treatments, three replicates of spray- and liquid-applied samples, respectively, were irradiated simultaneously per dose. Nontreated samples served as a control and were handled equally, excluding plasma treatment. The controls of plasma-treated samples were subjected to 90 s of vacuum (10 Pa) to account for any vacuum-induced spore inactivation.
Assay of spore resistance to blue light radiation.
High-intensity blue light was provided by an LED Flood array (Henkel-Loctite, Hemel Hempstead, United Kingdom). This array utilizes 144 reflectorized LEDs, which produce a homogeneous illuminated area of 10 cm by 10 cm. At a distance of 15.5 cm from the target surface, the array produces a uniform light irradiance of 600 J · m−2 · s−1, measured using a PM100D radiant power meter (Thorlabs, Newton, NJ). The fluence rate was calculated accordingly. The emission spectrum of the LED array was determined using a QEB0797 spectrophotometer (Ocean Optics, Dunedin, FL); the emission spectrum peaks at 400 nm, with a full-width half-maximum value of ±8.5 nm. Triplicate spray and liquid-inoculated samples were exposed to high-intensity blue light, while nontreated control samples were placed under the lamp wrapped in aluminum foil to account for the heating effect during the treatment.
Recovery and evaluation of spore survival.
After treatment, spore samples on the carriers were covered with sterile 10% aqueous polyvinyl acetate solution (PVA) and air dried. The dried layer subsequently was removed aseptically, transferred to an Eppendorf tube, and dissolved in 1 ml of sterile water. This procedure leads to >95% recovery of spores and does not affect viability (32). Spore inactivation rates were determined by a standard colony formation assay in which spores were serially diluted in sterile distilled water and plated on nutrient broth agar plates. After overnight incubation at 37°C, CFU numbers were determined as described previously (32, 33).
Numerical and statistical analysis.
Spore survival was determined from the quotient N/N0, where N is the average CFU of treated samples and N0 is the average CFU of untreated controls. Spore inactivation is expressed as the lethal dose at which 90% of the treated spore population is inactivated (LD90) and was plotted as a function of fluence (UV-C and blue light, in joules per square meter), dose (X-ray irradiation, in grays), or time (argon plasma treatment, in seconds). All data are expressed as averages ± standard deviations (n = 3). The slope of semilogarithmic survival curves (IC) was determined for each treatment. Significance differences in the survival rates were calculated by single-factor analysis of variance (ANOVA). Differences with P values of <0.05 were considered statistically significant (33, 34).
RESULTS
Evaluation of the spatial distribution of spores on surfaces.
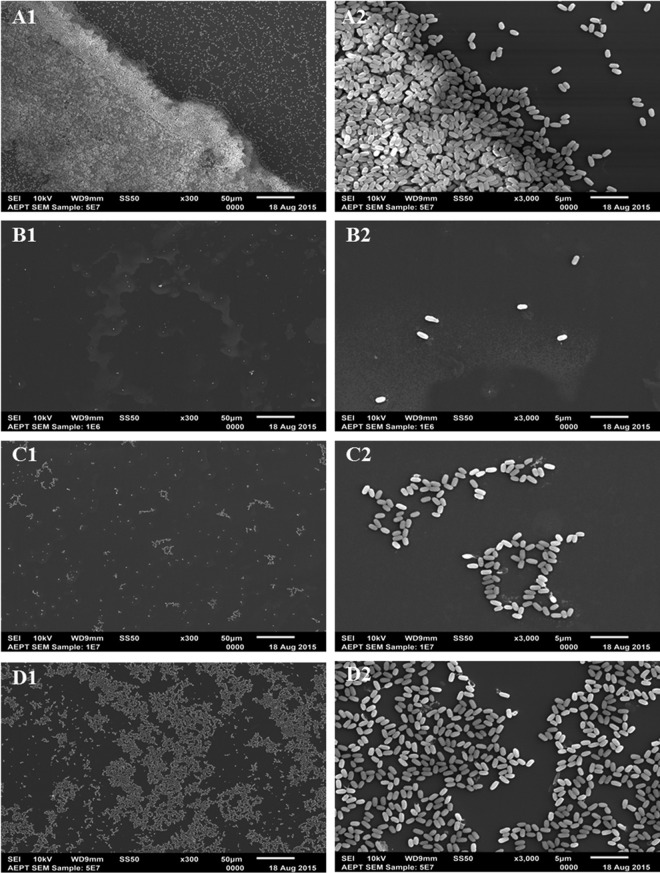
An obvious question that arises when spores are deposited on a surface to prepare a BI is what is the exact distribution of spores on the surface. Are the spores in a dispersed monolayer or in clumps, or are they piled on top of one another in multilayered structures? The latter arrangement is extremely important to identify, as spores in lower layers of a multilayered arrangement likely would be shielded from damaging effects of agents, such as photons of various wavelengths of light. To address this issue, SEM imaging was performed to determine the deposition patterns given by spray and liquid application methods. SEM imaging of samples applied by liquid deposition of 5 × 107 spores revealed an increased abundance of spores at the rim of the deposited liquid droplet leading to significant numbers of overlapping spores (Fig. 2A). In contrast, depositing various concentrations of spores by spray application produced almost exclusively a spore monolayer on a 25- by 75-mm area of a glass slide (Fig. 2B to D). Spray deposition of 106 spores reproducibly showed an even distribution of spores across the sample carrier (Fig. 2B). Spray deposition of 107 spores also exhibited an even dispersal with some small spore clusters, but spores still do not appear stacked (Fig. 2C). SEM imaging of up to 5 × 107 spores revealed a spore monolayer with increased cluster formation (Fig. 2D). In addition, a closer look at the latter cluster formations revealed that even at this high sample load the spores did not overlap but accumulated only in a monolayer. This monolayer of spray-deposited spores was in contrast to the multilayered pattern of liquid-deposited spores.
FIG 2.
Scanning electron microscopy (SEM) imaging of B. subtilis 168 spores deposited on glass slides at 300× (1) and 3,000× (2) magnification. (A) Spores (5 × 107) inoculated by liquid spot deposition form multilayered clusters. Spores inoculated by spray deposition with 106 (B), 107 (C), and 5 × 107 (D) spores on sample carriers form homogeneous monolayers. Scale bars represent 50 μm (1) or 5 μm (2).
In contrast to the results noted above with spores deposited with a single spraying, with repeated spraying of large amounts with ≥108 spores added per sample carrier, the spores did start to overlap due to the increased density (see Fig. S2 in the supplemental material). However, if higher monolayered sample loads are required in specific applications, then larger sample carriers, repeated sprays in close proximity, or subsequent sample pooling may be used to accomplish this with no increase in multilayered spores.
Live-cell imaging.
To evaluate whether B. subtilis spores remain viable after the spraying procedure, spores were deposited on the plastic surface of ibidi μ-dishes and covered with a layer of LB-agarose, which induces germination. As found with spores deposited on glass, SEM imaging of spores deposited by spray deposition on the ibidi μ-dishes indicated an even distribution of spores in monolayers with marginal cluster formation (see Fig. S3 in the supplemental material). Importantly, sprayed monolayered spores germinated uniformly and proceeded to outgrowth and subsequent vegetative growth (see Movie S1).
Spore inactivation by ionizing radiation (X rays), UV-C radiation, low-pressure argon plasma, and blue light radiation.
Given the differences seen between spore deposition on carriers by liquid and spray application, it was of obvious interest to compare the resistance of spores that had been applied by these two procedures. Mono- and multilayered spores deposited on sterile glass slides by spray and liquid deposition, respectively, were exposed to X rays, monochromatic UV-C irradiation at 254 nm, low-pressure argon plasma discharge, and blue light at 400 nm. Spore survival was assessed at various doses (X rays), fluences (UV-C and blue light), or time points (argon plasma) (Fig. 3), and LD90 values were calculated from plotted inactivation curves (Table 1).
FIG 3.
Survival curves of 5 × 107 B. subtilis spores inoculated by spray (∘, open circles) or liquid (•, closed circles) deposition after exposure to X rays (200 mA, 15 keV) (A), 254-nm UV-C radiation (B), low-pressure argon plasma (500 W, 10 Pa, 100 sccm Ar) (C), and 400-nm blue light (D).
TABLE 1.
Inactivation rates of mono- and multilayered B. subtilis spores after treatment with surface decontamination techniques
| Treatment | LD90a |
ICa |
||
|---|---|---|---|---|
| Monolayer | Multilayer | Monolayer | Multilayer | |
| X rays | 140.6 ± 18.4 Gy | 116.2 ± 10.7 Gy | −1.7 × 10−2 ± 1.8 × 10−3 Gy−1 | −1.4 × 10−2 ± 8.1 × 10−4 Gy−1 |
| UV-C | 116.54 ± 4.66 J · m−2* | 695.4 ± 134.20 J · m−2 | −1.6 × 10−2 ± 5.2 × 10−4 J · m−2*** | −3.4 × 10−3 ± 4.5 × 10−4 J · m−2 |
| Blue light | 6.1 × 106 ± 1.7 × 105 J · m−2* | 6.7 × 106 ± 1.1 × 105 J · m−2 | −1.2 × 10−6 ± 1.4 × 10−7 J · m−2 | −1.04 × 10−6 ± 2.3 × 10−8 J · m−2 |
| Argon plasma | 7.4 ± 0.6 s** | 156.1 ± 18.2 s | −1.6 × 10−1 ± 2.8 × 10−3 J · m−2*** | −1.5 × 10−2 ± 1.56 × 10−3 J · m−2 |
Shown is a comparison of LD90 values and IC (i.e., slope of semilogarithmic survival curves) of mono- and multilayered B. subtilis 168 spores prepared by spray and liquid deposition, respectively, after treatment with various surface decontamination techniques. Standard deviations and significance are given as determined by ANOVA (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
Liquid- and spray-deposited spores treated with X rays showed comparable dose-dependent decreases in survival (Fig. 3A). No significant differences in survival rates were found between spore populations prepared by spray deposition and spore populations prepared by liquid deposition (the P value was 0.17). In contrast to the results with X rays, spray-deposited spore monolayers were inactivated ∼4-fold faster by monochromatic UV-C radiation (P < 0.05) than liquid-deposited spores (Fig. 3B). Similar findings were obtained for these spores deposited by these two procedures and exposed to low-pressure argon plasma. Here, monolayered spray-deposited spores were killed ∼8-fold faster (P < 0.01) than multilayered spores deposited in liquid (Fig. 3C). Control samples did not reveal vacuum-induced spore inactivation. Likewise, deposited monolayers of spores were inactivated significantly faster by visible short-wave light in the blue wavelength range (P < 0.05) than multilayered spores (Fig. 3D). Treated samples exposed only to the elevated temperature produced during blue light treatment showed no significant decrease in survival (data not shown).
Clearly, there were significant differences in rates of inactivation of spores deposited by spray and liquid with 3 of the 4 inactivating agents tested (Fig. 3B to D). These results show that the method of spore deposition and the resulting spore distribution pattern have large effects on spore survivability in an experimental setup for evaluating UV-C-, plasma-, and blue light-mediated inactivation. In addition, there were quite notable differences in the standard deviations of the calculated inactivation rates (LD90 and IC) of spray- and liquid-deposited spores after UV-C and plasma treatment (Table 1), indicating that the preparation of samples by spray deposition led to noticeably more consistent and reliable results with lower variance.
DISCUSSION
BIs are routinely employed in the pharmaceutical, food, and medical industries to monitor the efficiency of a variety of sterilization processes. A major problem in decontamination studies assessing spore survival is the lack of reproducible and standardized experimental parameters for BI production and performance (15, 35, 36). Commonly, spore survival is assessed in the dry state, and dry spore samples frequently are prepared by liquid spot inoculation. Spore aggregation results from fluid evaporating from a solid surface leading to a stacked deposition of the spores, especially at the edges of the drop, in a ring-like fashion, sometimes referred to as the coffee ring effect (37). This accumulation of spore multilayers shields the spores in lower layers against nonpenetrating surface disinfectant treatments by the upper spore layers. Other factors hampering the reliability of surface inactivation studies are frequently used agar and filter surfaces containing microscopic irregularities, such as pits and fissures, which may shield deposited spores from the incident treatment and can significantly affect the measured spore viability (15). This leads to an apparent reduction in the efficiency of the treatment and an artificial overestimation of spore resistance. To counteract these issues, we have developed an electrically operated device for reproducible spray deposition of B. subtilis spores that ensures a uniform sample preparation process and produces reproducible, homogeneous samples of highly concentrated spore monolayers.
When subjecting mono- and multilayered B. subtilis spore samples to different surface decontamination methods, striking differences in spore inactivation efficiency were observed, as monolayered spores were inactivated significantly faster by UV-C radiation at 254 nm (P < 0.05), low-pressure argon plasma (P < 0.01), and blue light at 400 nm (P < 0.05) than multilayered spores. As UV-C is a common disinfecting agent with limited penetration depth (38), the reliable and reproducible assessment of the decontamination efficacy is dependent on the use of monolayered samples (15, 17, 36). Indeed, spore clumping and multilayered spore aggregates significantly reduce penetration of UV radiation at 254 nm to underlying spores (15). Thus, with a bacterial spore of 1 μm in diameter that shields an underlying spore, only 61% of the incident beam is transmitted. Assuming two spores shield a third, only 37% of the incident beam would reach the underlying spore (39). Hence, even minor accumulations of spores in multilayers can dramatically affect the decontamination by nonpenetrating agents. In contrast, mono- and multilayered spore populations showed comparable sensitivities when exposed to ionizing radiation, as the penetration capability of X rays with an energy of 200 keV is significantly higher (98.7% of incident intensity is transmitted through 1 mm H2O [40]). Therefore, the greater penetrating power of the X rays makes shielding by one or more spore layers minimal. As UV and VUV are major factors in spore inactivation mediated by low-pressure argon plasma, the impact on spore viability of mono- and multilayered spore populations is similar to that with UV-C radiation alone, and low-pressure argon plasma discharges emit photons mainly at wavelengths of 104.8 and 106.7 nm (41). Additionally, impurities in the chamber for plasma generation (N2, O2, and H2O from samples and residual gas after ventilation of the chamber for sample insertion) radiate in the bactericidal range from 120 nm to 380 nm and produce radicals in the form of atomic oxygen and nitrogen, nitric oxide, and hydroxyl radicals. In the field of cold plasma for decontamination purposes, work is still needed to standardize the sterilization process and develop a suitable BI for sterilization assurance (26), which is an absolute requirement for plasma sterilization devices to be adopted in industrial settings. Relative changes within the concentrations of the active species present in a plasma discharge greatly depend on the gas composition, the device setup, and associated operation settings (such as pressure, power source, or matching conditions). Hence, a highly reproducible and quality-controlled BI for the evaluation of plasma sterilization efficiency is crucial for this process to be widely implemented.
The photodynamic inactivation mechanism of short-wave visible light in the blue wavelength range is thought not to be caused by DNA damage but rather by oxidative lesions due to excited endogenous photosensitizers (19–21). However, the vast majority of this work has been performed on vegetative bacteria, and the detailed mechanism of spore killing remains to be investigated. Based on our results, a defined BI with homogeneous single-spore layers is required for the accurate determination of spore resistance and inactivation by blue light at 400 nm.
In recent years, various methods have been developed in order to generate reproducible spore monolayers (42–44). However, the lack of detail concerning the individual instruments installed in the particular setup or the precise operating principle (42, 43), as well as elaborate and costly technology (44), makes it difficult to recreate the reported spraying devices for sample preparation. In this study, we present a method for easy sample preparation by spray deposition, which was successfully employed for the production of homogeneous B. subtilis spore monolayers. The instrument setup is inexpensive and its operation is simple and requires minimal effort, but at the same time it offers defined operating conditions. The automated but tunable procedure enables the operator to adapt the spraying process to the actual sample condition or carrier size, for example, by increasing or decreasing the spraying radius, deposition time, or pressure. Compared to common sample preparation methods, such as spot deposition, this new instrument offers a more reliable assessment of sterilization efficiency with extensive application to diverse traditional and innovative decontamination methods, with particular focus on nonpenetrating surface agents.
This study also has demonstrated the application of monolayered spores deposited by spray application for studying the cellular dynamics of spore germination and outgrowth with time-lapse microscopy. Thus, the spraying device proved suitable for reproducibly preparing live-cell monolayers, enabling the time-resolved investigation of highly dynamic events in single organisms. As a consequence, the application of this new device may not be limited to bacterial spores but also may include the reproducible and homogeneous distribution of vegetative cells and a broad range of biomolecules and nanoparticles on surfaces for a variety of studies on single cells or particles.
Supplementary Material
ACKNOWLEDGMENTS
We especially thank Andrea Schroeder, Christoph Steger, Birgit Ritter, Karel Marsalek, Tobias Harzen, and Andre Parpart for their excellent technical support. We thank the Schlick company for kindly providing the CAD design. The results of this study will be included in the Ph.D. thesis of Marina Raguse.
This study was supported in part by grants from the German Aerospace Center (DLR-FuE-Projekt ISS-Nutzung in der Biodiagnostik, Programm RF-FuW, Teilprogramm 475, to M.R. and R.M.).
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.03934-15.
REFERENCES
- 1.Abreu AC, Tavares RR, Borges A, Mergulhão F, Simões M. 2013. Current and emergent strategies for disinfection of hospital environments. J Antimicrob Chemother 68:2718–2732. doi: 10.1093/jac/dkt281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gómez-López VM, Ragaert P, Debevere J, Devlieghere F. 2008. Decontamination methods to prolong the shelf-life of minimally processed vegetables, state-of-the-art. Crit Rev Food Sci Nutr 48:487–495. doi: 10.1080/10408390701638878. [DOI] [PubMed] [Google Scholar]
- 3.Horneck G, Klaus DM, Mancinelli RL. 2010. Space microbiology. Microbiol Mol Biol Rev 74:121–156. doi: 10.1128/MMBR.00016-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Williams GJ, Denyer SP, Hosein IK, Hill DW, Maillard JY. 2009. Limitations of the efficacy of surface disinfection in the healthcare setting. Infect Control Hosp Epidemiol 30:570–573. doi: 10.1086/597382. [DOI] [PubMed] [Google Scholar]
- 5.Rutala WA, Weber DJ. 2013. Disinfection and sterilization: an overview. Am J Infect Control 41:S2–S5. doi: 10.1016/j.ajic.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 6.Nicholson W, Munakata N, Horneck G, Melosh HJ, Setlow P. 2000. Resistance of Bacillus endospores to extreme terrestrial and extraterrestrial environments. Microbiol Mol Biol Rev 64:548–572. doi: 10.1128/MMBR.64.3.548-572.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Henriques AO, Moran CP Jr. 2007. Structure, assembly, and function of the spore surface layers. Annu Rev Microbiol 61:555–588. doi: 10.1146/annurev.micro.61.080706.093224. [DOI] [PubMed] [Google Scholar]
- 8.Setlow P. 2006. Spores of Bacillus subtilis: their resistance to and killing by radiation, heat and chemicals. J Appl Microbiol 101:514–525. doi: 10.1111/j.1365-2672.2005.02736.x. [DOI] [PubMed] [Google Scholar]
- 9.Penna TCV, Ishii M, Machoshvili IA, Marques M. 2002. The effect of bioindicator preparation and storage on thermal resistance of Bacillus stearothermophilus spores. Appl Biochem Biotechnol 98:525–538. doi: 10.1385/ABAB:98-100:1-9:525. [DOI] [PubMed] [Google Scholar]
- 10.Humphreys PN. 2011. Testing standards for sporicides. J Hosp Infect 77:193–198. doi: 10.1016/j.jhin.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 11.Haberer K, van Doorne H. 2011. Biological indicators, tools to verify the effect of sterilization processes—position paper prepared on behalf of group 1 (biological methods and statistical analysis). Pharmeur Bio Sci Notes 2:25–39. [PubMed] [Google Scholar]
- 12.Reich RR, Morien LL. 1982. Influence of environmental storage relative humidity on biological indicator resistance, viability, and moisture content. Appl Environ Microbiol 43:609–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sigwarth V, Stärk A. 2003. Effect of carrier materials on the resistance of spores of Bacillus stearothermophilus to gaseous hydrogen peroxide. PDA J Pharm Sci Technol 57:3–11. [PubMed] [Google Scholar]
- 14.Nguyen TMH, Durand A, Loison P, Perrier-Cornet JM. 2011. Effect of sporulation conditions on the resistance of Bacillus subtilis spores to heat and high pressure. Appl Microbiol Biotechnol 90:1409–1417. doi: 10.1007/s00253-011-3183-9. [DOI] [PubMed] [Google Scholar]
- 15.Coohill TP, Sagripanti JL. 2008. Overview of inactivation by 245 nm ultraviolet radiation of bacteria with particular relevance to biodefense. Photochem Photobiol 84:1084–1090. doi: 10.1111/j.1751-1097.2008.00387.x. [DOI] [PubMed] [Google Scholar]
- 16.Shintani H, Akers JE. 2000. On the cause of performance variation of biological indicators used for sterility assurance. PDA J Pharm Sci Technol 54:332–342. [PubMed] [Google Scholar]
- 17.Shintani H. 2014. Important points to attain reproducible sterility assurance. Biochem Physiol 3:135. doi: 10.4172/2168-9652.1000135. [DOI] [Google Scholar]
- 18.Doona JC, Feeherry FE, Kustin K, Olinger GO, Setlow P, Malkin AJ, Leighton T. 2015. Fighting Ebola with novel spore decontamination technologies for the military. Front Microbiol 6:663. doi: 10.3389/fmicb.2015.00663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maclean M, Murdoch LE, MacGregor SJ, Anderson JG. 2013. Sporicidal effects of high-intensity 405 nm visible light on endospore-forming bacteria. Photochem Photobiol 89:120–126. doi: 10.1111/j.1751-1097.2012.01202.x. [DOI] [PubMed] [Google Scholar]
- 20.Maclean M, MacGregor SJ, Anderson JG, Woosley G. 2009. Inactivation of bacterial pathogens following exposure to light from a 405-nanometer light-emitting diode array. Appl Environ Microbiol 75:1932–1937. doi: 10.1128/AEM.01892-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamblin MR, Viveiros J, Yang C, Ahmadi A, Ganz RA, Tolkoff MJ. 2005. Helicobacter pylori accumulates photoactive porphyrins and is killed by visible light. Antimicrob Agents Chemother 49:2822–2827. doi: 10.1128/AAC.49.7.2822-2827.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moisan M, Barbeau J, Moreau S, Pelletier J, Tabrizian M, Yahia L. 2001. Low temperature sterilization using gas plasmas: a review of the experiments and an analysis of the inactivation mechanisms. Int J Pharm 226:1–21. doi: 10.1016/S0378-5173(01)00752-9. [DOI] [PubMed] [Google Scholar]
- 23.Lerouge S, Wertheimer M, Yahia L. 2001. Plasma sterilization: a review of parameters, mechanisms, and limitations. Plasmas Polym 6:175–188. doi: 10.1023/A:1013196629791. [DOI] [Google Scholar]
- 24.Halfmann H, Denis B, Bibinov N, Wunderlich J, Awakowicz P. 2007. Identification of the most efficient VUV/UV radiation for plasma based inactivation of Bacillus atrophaeus spores. J Phys D Appl Phys 40:5907. doi: 10.1088/0022-3727/40/19/019. [DOI] [Google Scholar]
- 25.von Keudell A, Awakowicz P, Benedikt J, Raballand V, Yanguas-Gil A, Opretzka J, Flötgen C, Reuter R, Byelykh L, Halfmann H, Stapelmann K, Denis B, Wunderlich J, Muranyi P, Rossi F, Kylián O, Hasiwa N, Ruiz A, Rauscher H, Sirghi L, Comoy E, Dehen C, Challier L, Deslys JP. 2010. Inactivation of bacteria and biomolecules by low-pressure plasma discharges. Plasma Process Polym 7:327–352. doi: 10.1002/ppap.200900121. [DOI] [Google Scholar]
- 26.Shintani H. 2015. Current mistaken interpretation of microbiological data on gas plasma sterilization. Pharm Regul Aff 4:138. doi: 10.4172/2167-7689.1000138. [DOI] [Google Scholar]
- 27.Schaeffer P, Millet J, Aubert JP. 1965. Catabolic repression of bacterial sporulation. Proc Natl Acad Sci U S A 54:704–711. doi: 10.1073/pnas.54.3.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moeller R, Douki T, Cadet J, Stackebrandt E, Nicholson WL, Rettberg P, Reitz G, Horneck G. 2007. UV-radiation-induced formation of DNA bipyrimidine photoproducts in Bacillus subtilis endospores and their repair during germination. Int Microbiol 10:39–46. [PubMed] [Google Scholar]
- 29.Moeller R, Setlow P, Horneck G, Berger T, Reitz G, Rettberg P, Doherty AJ, Okayasu R, Nicholson WL. 2008. Roles of the major, small, acid-soluble spore proteins and spore-specific and universal DNA repair mechanisms in resistance of Bacillus subtilis spores to ionizing radiation from X rays and high-energy charged-particle bombardment. J Bacteriol 190:1134–1140. doi: 10.1128/JB.01644-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Halfmann H, Bibinov N, Wunderlich J, Awakowicz P. 2007. A double inductively coupled plasma for sterilization of medical devices. J Phys D Appl Phys 40:4145–4154. doi: 10.1088/0022-3727/40/14/008. [DOI] [Google Scholar]
- 31.Messerer P, Boenigk B, Keil G, Scheubert P, Awakowicz P. 2003. Plasma diagnostics in a double inductively coupled source (DICP) for plasma sterilization. Surf Coat Technol 174-175:570–573. doi: 10.1016/S0257-8972(03)00630-3. [DOI] [Google Scholar]
- 32.Horneck G, Rettberg P, Reitz G, Wehner J, Eschweiler U, Strauch K, Panitz C, Starke V, Baumstark-Khan C. 2001. Protection of bacterial spores in space, a contribution to the discussion on Panspermia. Orig Life Evol Biosph 31:527–547. doi: 10.1023/A:1012746130771. [DOI] [PubMed] [Google Scholar]
- 33.Moeller R, Reitz G, Berger T, Okayasu R, Nicholson WL, Horneck G. 2010. Astrobiological aspects of the mutagenesis of cosmic radiation on bacterial spores. Astrobiology 10:509–521. doi: 10.1089/ast.2009.0429. [DOI] [PubMed] [Google Scholar]
- 34.Moeller R, Setlow P, Reitz G, Nicholson WL. 2009. Roles of small, acid-soluble spore proteins and core water content in survival of Bacillus subtilis spores exposed to environmental solar UV radiation. Appl Environ Microbiol 75:5202–5208. doi: 10.1128/AEM.00789-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sandle T. 17 March 2014. Variations in the resistance of biological indicators used to assess sterilization. J Validation Technol http://www.ivtnetwork.com/article/variations-resistance-biological-indicators-used-assess-sterilization.
- 36.Nicholson WL, Galeano B. 2003. UV resistance of Bacillus anthracis spores revisited: validation of Bacillus subtilis spores as UV surrogates for spores of B. anthracis Sterne. Appl Environ Microbiol 69:1327–1330. doi: 10.1128/AEM.69.2.1327-1330.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yunker PJ, Still T, Lohr MA, Yodh AG. 2011. Suppression of the coffee-ring effect by shape-dependent capillary interactions. Nature 476:308–311. doi: 10.1038/nature10344. [DOI] [PubMed] [Google Scholar]
- 38.Bolton JR. 2000. Terms and definitions in ultraviolet disinfection, p 25–40. In Proceedings of Disinfection 2000: disinfection of wastes in the new millennium, 15–18 March 2000, New Orleans, LA Water Environment Federation, Alexandria, VA. [Google Scholar]
- 39.Coohill TP. 1986. Virus-cell interactions as probes for vacuum ultraviolet radiation damage and repair. Photochem Photobiol 44:359–363. doi: 10.1111/j.1751-1097.1986.tb04676.x. [DOI] [PubMed] [Google Scholar]
- 40.Hubbell JH, Seltzer SM. 1996. Tables of X-ray mass attenuation coefficients and mass energy-absorption coefficients from 1 keV to 20 MeV for elements Z = 1 to 92 and 48 additional substances of dosimetric interest. NIST, Gaithersburg, MD. [Google Scholar]
- 41.Mertmann P, Bibinov N, Halfmann H, Awakowciz P. 2009. Determination of argon resonance line emission in an ICP hitting a biological sample. Plasma Sources Sci Technol 7:665–675. [Google Scholar]
- 42.Lee SD, Ryan SP, Synder EG. 2011. Development of an aerosol surface inoculation method for Bacillus spores. Appl Environ Microbiol 77:1638–1645. doi: 10.1128/AEM.02237-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Levy C, Bornard I, Carlin F. 2011. Deposition of Bacillus subtilis spores using an airbrush-spray or spots to study surface decontamination by pulsed light. J Microbiol Methods 84:223–227. doi: 10.1016/j.mimet.2010.11.021. [DOI] [PubMed] [Google Scholar]
- 44.Edmonds JM, Collett P, Valdes ER, Skowronski EW, Pellar GJ, Emanuel PA. 2009. Surface sampling of spores in dry-deposition aerosols. Appl Environ Microbiol 75:39–44. doi: 10.1128/AEM.01563-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.