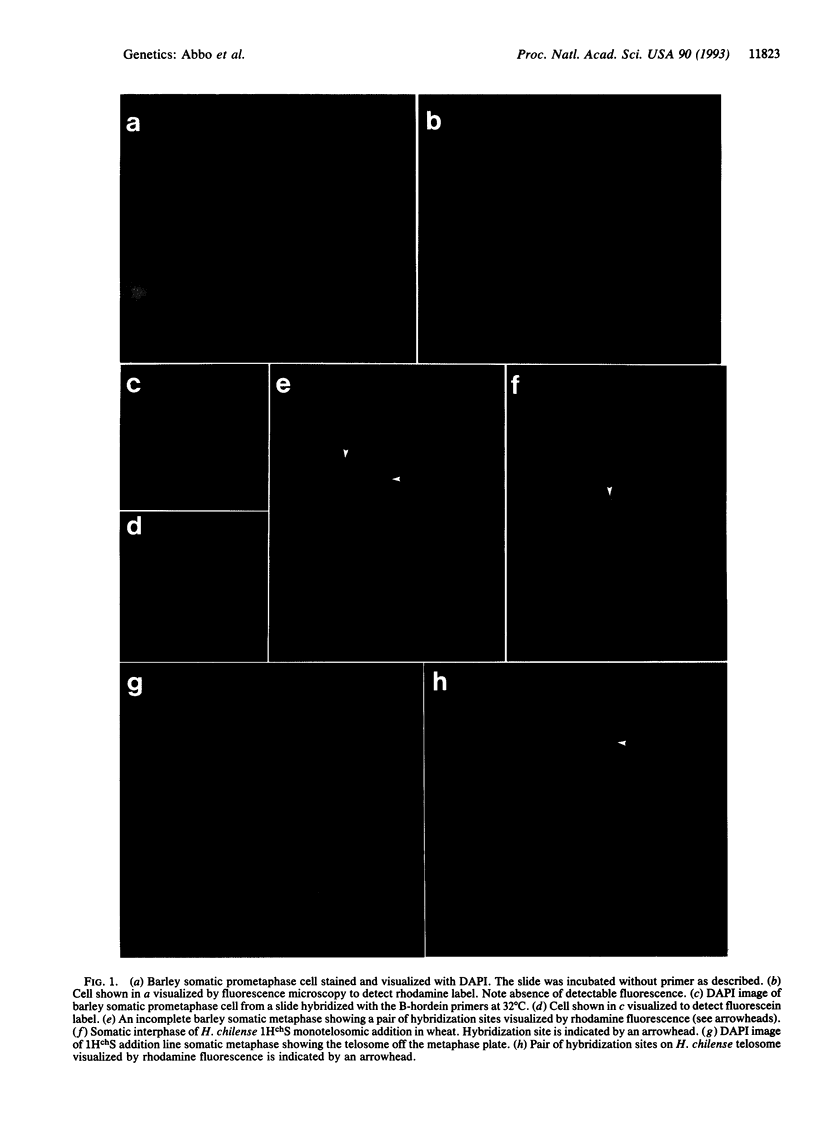
Abstract
In situ hybridization methods allow the detection of specific DNA sequences on whole chromosomes. The technique has been widely used as a diagnostic and research tool by animal cytogeneticists, for whom detection of unique sequences on mammalian chromosomes is routinely achieved. However, detection of unique sequences on plant chromosomes is less reliable. The recently developed primer-induced in situ hybridization (PRINS) technique allows rapid and reliable in situ detection by the hybridization of primers to denatured target DNA, followed by extension with DNA polymerase in the presence of a labeled nucleotide. The use of short oligonucleotide primers could allow improved penetration of debris and highly condensed chromatin common in preparations of plant chromosomes, thus increasing the sensitivity of in situ detection. The feasibility of this approach is demonstrated by the oligonucleotide primer-mediated detection of the B-hordein gene cluster on a barley chromosome. Applications of the PRINS technique for plant cytogeneticists are discussed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cheung W. Y., Chao S., Gale M. D. Long-range physical mapping of the alpha-amylase-1 (alpha-Amy-1) loci on homoeologous group 6 chromosomes of wheat. Mol Gen Genet. 1991 Oct;229(3):373–379. doi: 10.1007/BF00267458. [DOI] [PubMed] [Google Scholar]
- Ferguson-Smith M. A. Putting the genetics back into cytogenetics. Am J Hum Genet. 1991 Feb;48(2):179–182. [PMC free article] [PubMed] [Google Scholar]
- Gustafson J. P., Butler E., McIntyre C. L. Physical mapping of a low-copy DNA sequence in rye (Secale cereale L.). Proc Natl Acad Sci U S A. 1990 Mar;87(5):1899–1902. doi: 10.1073/pnas.87.5.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson J. P., Dillé J. E. Chromosome location of Oryza sativa recombination linkage groups. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8646–8650. doi: 10.1073/pnas.89.18.8646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoovers J. M., Mannens M., John R., Bliek J., van Heyningen V., Porteous D. J., Leschot N. J., Westerveld A., Little P. F. High-resolution localization of 69 potential human zinc finger protein genes: a number are clustered. Genomics. 1992 Feb;12(2):254–263. doi: 10.1016/0888-7543(92)90372-y. [DOI] [PubMed] [Google Scholar]
- Kimball B. P., Mildenberger R. R., Lefkowitz C. A., Ross B. L., Houle S., McLaughlin P. R. Effect of intracoronary nifedipine on coronary bloodflow and myocardial metabolism during pacing-induced ischemia. Can J Cardiol. 1990 Oct;6(8):333–339. [PubMed] [Google Scholar]
- Koch J. E., Kølvraa S., Petersen K. B., Gregersen N., Bolund L. Oligonucleotide-priming methods for the chromosome-specific labelling of alpha satellite DNA in situ. Chromosoma. 1989 Oct;98(4):259–265. doi: 10.1007/BF00327311. [DOI] [PubMed] [Google Scholar]
- Koch J., Mogensen J., Pedersen S., Fischer H., Hindkjaer J., Kølvraa S., Bolund L. Fast one-step procedure for the detection of nucleic acids in situ by primer-induced sequence-specific labeling with fluorescein-12-dUTP. Cytogenet Cell Genet. 1992;60(1):1–3. doi: 10.1159/000133281. [DOI] [PubMed] [Google Scholar]
- Kreis M., Shewry P. R., Forde B. G., Rahman S., Miflin B. J. Molecular analysis of a mutation conferring the high-lysine phenotype on the grain of barley (Hordeum vulgare). Cell. 1983 Aug;34(1):161–167. doi: 10.1016/0092-8674(83)90146-0. [DOI] [PubMed] [Google Scholar]
- Mills K. K., Bauer W. D. Rhizobium attachment to clover roots. J Cell Sci Suppl. 1985;2:333–345. doi: 10.1242/jcs.1985.supplement_2.18. [DOI] [PubMed] [Google Scholar]
- Payne P. I., Holt L. M., Reader S. M., Miller T. E. Chromosomal location of genes coding for endosperm proteins of Hordeum chilense, determined by two-dimensional electrophoresis of wheat-H. chilense chromosome addition lines. Biochem Genet. 1987 Feb;25(1-2):53–65. doi: 10.1007/BF00498951. [DOI] [PubMed] [Google Scholar]
- Trask B. J. Fluorescence in situ hybridization: applications in cytogenetics and gene mapping. Trends Genet. 1991 May;7(5):149–154. doi: 10.1016/0168-9525(91)90378-4. [DOI] [PubMed] [Google Scholar]
- Wang M. L., Leitch A. R., Schwarzacher T., Heslop-Harrison J. S., Moore G. Construction of a chromosome-enriched HpaII library from flow-sorted wheat chromosomes. Nucleic Acids Res. 1992 Apr 25;20(8):1897–1901. doi: 10.1093/nar/20.8.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner J. E., Endo T. R., Gill B. S. Toward a cytogenetically based physical map of the wheat genome. Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11307–11311. doi: 10.1073/pnas.89.23.11307. [DOI] [PMC free article] [PubMed] [Google Scholar]