Abstract
Reexamination of Dermacentor albipictus (Packard, 1869 holdings stored in the United States National Tick Collection revealed several collections of a morphologically distinct Dermacentor species. Comparison of these specimens with other Dermacentor taxa showed that they are identical to an old taxon originally described as Dermacentor variegatus kamshadalus Neumann, 1908. For more than a century, this taxon was known only from the male holotype specimen collected in Russia, and the name was considered a junior synonym of D. albipictus. D. kamshadalus is reinstated here to a full species rank, and its male is redescribed and its female and nymph are described for the first time. Adults of D. kamshadalus can be distinguished from those of D. albipictus by a short spur on trochanters I, shorter spurs on coxae I, shorter dorsal cornua, more numerous perforations on spiracular plates, less numerous and shorter setae on idiosoma, especially around spiracular plates, and considerably paler coloration of the conscutum and scutum. The nymph of D. kamshadalus can be differentiated from that of D. albipictus by shorter spurs on coxae I and the numerous perforations on the spiracular plates. Adults and nymphs of D. kamshadalus are recorded from the United States, Canada, and Russia, where they have been collected from mountain goats, Oreamnos americanus (de Blainville), bighorn sheep, Ovis canadensis Shaw, and sheep, Ovis sp. of which the species was not stated.
Keywords: Dermacentor kamshadalus, Oreamnos americanus, Ovis canadensis
Despite a relatively small number of species, the systematics of the genus Dermacentor is still not completely resolved. Identification of the immature stages and often the adults causes problems especially for nontick experts. As with many other ixodid ticks, marked similarity between the species and considerable geographical and individual variability are the main reasons for identification difficulties. It is generally believed that the Dermacentor species of North America, especially those of the United States, are among the best studied taxonomically and that little can be done to improveonthis. The major contributions to this achievement are the classical work of Cooley (1938), who brilliantly revised all the Dermacentor species of the United States, and the publications of Brinton et al. (1965), who added descriptions of the immature stages, and Yunker et al. (1986), who provided identification keys for the adults of all American species.
Dermacentor albipictus (Packard, 1869) is one of the most common North American Dermacentor species. This species is variable morphologically, and this is reflected in the existence of some nomenclatural issues. Most taxonomical controversy is associated with a taxon designated Dermacentor nigrolineatus (Packard, 1869), which was considered a valid species or its distinct form (“nigrolineatus”) close to D. albipictus. However, Cooley (1938) morphologically, and Ernst and Gladney (1975) genetically via crossbreeding experiments, proved that D. nigrolineatus is identical to D. albipictus and should be treated as a junior synonym of the latter species. Based on molecular data, Crosbie et al. (1998) and Leo et al. (2010) more recently disclosed considerable genetical variations among D. albipictus populations. This variations led Crosbie et al. (1998) to suggest that D. albipictus may be a complex of closely related species.
During the reexamination of D. albipictus holdings stored in the United States National Tick Collection (USNTC), several collections of morphologically distinct Dermacentor species were found. These specimens had been identified as D. albipictus by previous workers. Comparison of these specimens with other Dermacentor taxa revealed that they are identical to an old taxon originally described as Dermacentor variegatus kamshadalus Neumann, 1908, a name that has been treated as a junior synonym of D. albipictus by the majority of recent researchers. The presence of a number of distinctive diagnostic characters on the male, female, and nymph persuaded us to reinstate D. kamshadalus as a separate valid species. This species is closely related to D. albipictus morphologically and apparently also biologically.
The discovery of D. kamshadalus should encourage a more thorough examination of polymorphic D. albipictus from a morphological and molecular perspective.
Materials and Methods
The material examined is summarized in Table 1. Only field-collected ticks were available for study. The specimens that were examined are deposited in the USNTC (Georgia Southern University, Statesboro) and Canadian National Collection of Insects, Arachnida and Nematodes (Agriculture and Agri-Food Canada, Research Center, Ottawa, Canada).
Table 1.
D. kamshadalus, material examined
| No. of ticksa |
Host | Locality | Date | Collectorb | Accession no. | ||
|---|---|---|---|---|---|---|---|
| ♂ | ♀ | N | |||||
| 3 | 4 | 5 | O. canadensis | British Columbia, Canada Churn Creek |
14.III.1953 | CNCI15280 | |
| 11 | 6 | O. canadensis | California Sierra Nevada Mtns, Mt Baxter |
15.I.1915 | EHO | USNMENT00714861 | |
| 11 | 10 | O. americanus | Idaho Lemhi Co. |
12.I.1953 | USNMENT00714360 | ||
| 3 | 2 | O. americanus | Lemhi Co., Corn Creek | 5.IV.1950 | B, FJB, NK, WJ | USNMENT00714418 | |
| 1 | O. canadensis | Lemhi Co., Salmon | II.1941 | ERQ | USNMENT00714211 | ||
| 1 | 3 | O. americanus | Montana Ravalli Co. |
20.II.1935 | USNMENT00714790 | ||
| 2 | 1 | O. americanus | Ravalli Co. | 18.II.1935 | USNMENT00714681 | ||
| 1 | 1 | 1 | O. americanus | Ravalli Co. | 20.II.1935 | USNMENT00714646 | |
| 13 | 22 | 1 | O. americanus | Ravalli Co. | IV.1930 | USNMENT00714889 | |
| 1 | Ravalli Co. | USNMENT00714957 | |||||
| 1 | Ravalli Co. | USNMENT00714890 | |||||
| 1 | Ravalli Co. | USNMENT00714497 | |||||
| 3 | Ravalli Co. | USNMENT00714625 | |||||
| 1 | Clothing | Ravalli Co., Florence | 24.V.1910 | WVK | USNMENT00714898 | ||
| 257 | 90 | O. americanus | Ravalli Co., Blodgett Canyon | 28.II.1933 | CC | USNMENT00714658 | |
| 28 | 21 | O. americanus | Ravalli Co., Lost Horse Canyon | 11.IV.1947 | BT, LH | USNMENT00714674 | |
| 6 | 4 | O. americanus | Ravalli Co., Lost Horse Canyon | 6.V.1933 | NK | USNMENT00714581 | |
| 2 | Ovis canadensis beds | Lincoln Co., Kootenai Ntl Forest | 22.I.1941 | BWB | USNMENT00714445 | ||
| 21 | 7 | 1 | O. canadensis | Lincoln Co., Warland Peak | 5.III.1941 | MB | USNMENT00714514 |
| 5 | 3 | O. canadensis | Washington Yakima Co. |
3.IV.2013 | KM | USNMENT00714132 | |
| 2 | 2 | O. canadensis | Yakima Co. | 3.IV.2013 | KM | USNMENT00714824 | |
| 2 | O. canadensis | Wyoming Fremont Co., Torrey Creek |
17.IV.1954 | W, H | USNMENT00714491 | ||
| 374 | 178 | 8 | Total | ||||
N, nymphs.
EHO, E. H. Ober; B, Brandborg; FJB, F. J. Bell; NK, N. Kramis; WJ, W. Jellison; ERQ, E. R. Quortrup; WVK, W. V. King; CC, C. Carpenter; BT, B. Tibbs; LH, L. Humble; BWB, B. W. Brink; MB, Mr. Brink; KM, K. Mansfield; W, Winter; H, Honess.
The nymphs were mounted on glass slides and examined under a light microscope. Nymphs and the microstructures of adults were also studied by means of a scanning electron microscope, and the macrostructures of males and females under a stereoscopic microscope. Measurements for the male and female are given in millimeters and those for the various features of the nymph in micrometers. The measurements are arranged as follows: minimum–maximum (mean, n = number of specimens measured). All illustrations have been drawn by D. A. Apanaskevich.
Dermacentor kamshadalus Neumann, 1908
Male
(redescription) (Figs. 1A, 2A–D and 3): Conscutum (Figs. 1A and 2A–D): broadly oval, widest posterior to mid-length; distance from scapular apices to posterior margin of conscutum 4.06–7.12 (5.41; n = 50), maximum width 2.44–4.31 (3.22; n = 50), ratio length to width 1.56–1.79 (1.68; n = 50). Coloration: extensive pale ivory-colored ornamentation covering most of conscutum. Light- to dark-brown background arranged as six pairs of lateral patches: patches in cervical fields and in paramedian areas narrow, second–fifth patches elongatedly oval (fourth and fifth patch may be fused); and two central patches: diffused in the center of pseudoscutum and narrow in the posteromedian area; patches often not solid but consisting of numerous smaller patches; pseudoscutum and cervical grooves with numerous small irregular patches giving them a speckled appearance; lateral fields without patches. Some specimens (Fig. 2C and D) have patches on festoons varying from light to distinct and an additional pair of patches lateral to posteromedian patch, which can be fused anteriorly with opposite patch forming an inverted U-shaped single patch. Cervical grooves shallow; a pair of shallow central depressions, and a second posterior pair that correspond to paramedian grooves; very faint lateral grooves, often indistinct or distinguishable only close to first festoon; 11 distinct festoons. Mediumsized brown punctations sparse, very fine brown punctations dense, evenly distributed over scutum. Eyes (Fig. 1A): oval, very slightly convex, at anterior one-fifth of scutal length. Setae relatively sparse and short. Spiracular plates (Fig. 3A): positioned on ventral surface in unengorged specimens, suboval, greatest diameter in anteroposterior plane, length 0.49–0.87 (0.65; n = 31); dorsal prolongation absent, large perforations fairly numerous. Gnathosoma (Figs. 1A and 3B and C): length from palpal apices to cornual apices dorsally 0.67–1.14 (0.95; n = 50), width of basis capituli 0.54–0.84 (0.70; n = 50), ratio length to width 1.21–1.49 (1.36; n = 50). Basis capituli (Figs. 1A and 3B and C): dorsally subrectangular; posterior margin nearly straight; length 0.32–0.56 (0.47; n = 50), ratio width to length 1.33–1.67 (1.47; n = 50); cornua broad, moderately long, equivalent to a proportion of 3.87–5.79 (4.57; n = 50) of length of basis capituli; lateral margins with whitish enameling. Basis capituli ventrally sub-rectangular; posterior margin convex. Palpi (Figs. 1A and 3B and C): short, broad; length dorsally (I–III segments) 0.35–0.60 (0.48; n = 50), width 0.24–0.40 (0.32; n = 50), ratio length to width 1.33–1.75 (1.51; n = 50), length of segments in descending order: 2, 3, 1, 4; segment I well-developed ventrally; segment II narrower at base and thereafter widening; segment III subrectangular with broadly rounded apex; segments II and III with whitish enamelling on dorsal surfaces. Hypostome (Fig. 3C): club-shaped; dental formula 3/3. Legs (Fig. 1A): of medium length, robust; with extensive whitish enameling mostly on dorsal and lateral aspects. Coxae (Fig. 3D and E): coxa I with long triangular closely spaced internal and external spurs with pointed apices; external spur slightly longer than internal; both spurs of coxa I directed posterolaterally; coxae II and III each with larger triangular external spur with tapering apex and smaller internal spur; coxa IV with larger triangular external spur with tapering apex; coxa IV enlarged, ratio length to width 1.11–1.58 (1.34; n = 50). Trochanter I (Fig. 3F) with short, broadly triangular dorsal spur with tapering apex. Genu and Tibia (Fig. 1A) with two rows of short projections ventrally. Genu IV length 0.61–1.25 (0.99; n = 50), width 0.34–0.74 (0.58; n = 50), ratio length to width 1.52–1.91 (1.72; n = 50).
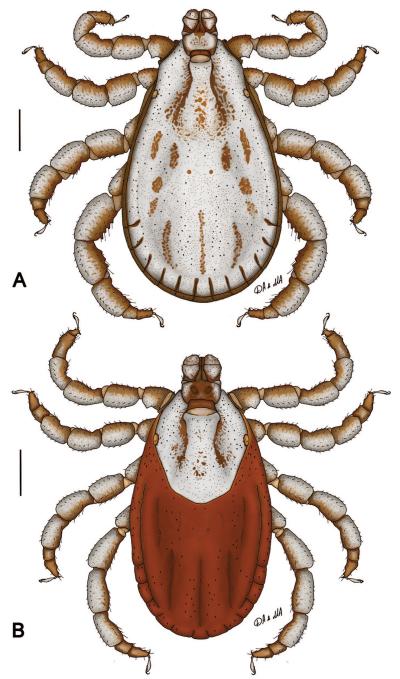
Fig. 1.
Dermacentor kamshadalus, dorsally. (A) Male. (B) Female. (USA, MT, Ravalli Co., Blodgett Canyon, USNMENT00714658) Bar = 1 mm.
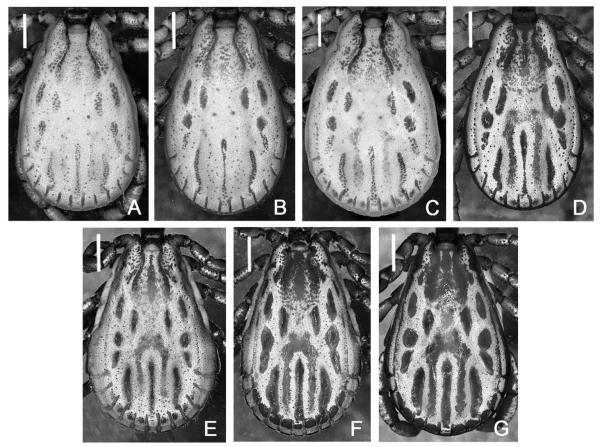
Fig. 2.
Dermacentor kamshadalus and Dermacentor albipictus, variations in the coloration of male conscuta. (A–C) D. kamshadalus (USA, MT, Ravalli Co., Blodgett Canyon, USNMENT00714658). (D) D. kamshadalus (USA, CA, Sierra Nevada Mtns, Mt Baxter, USNMENT00714861). (E and F) D. albipictus (USA, MT, Ravalli Co., East Fork, USNMENT00714253). (G) D. albipictus (USA, MT, Granite Co., Maxville, USNMENT00714194). Bar = 1 mm.
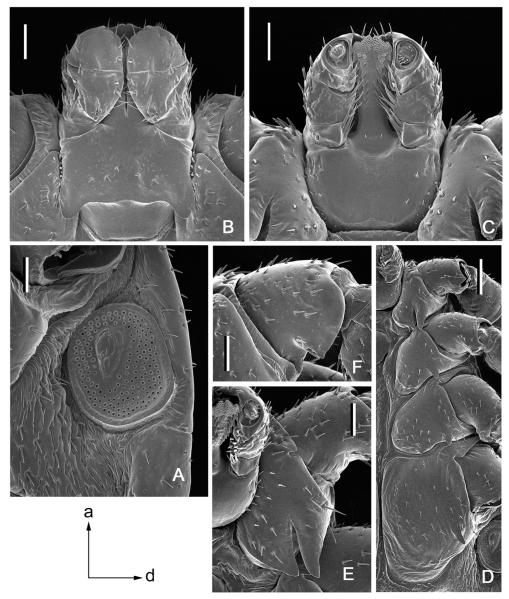
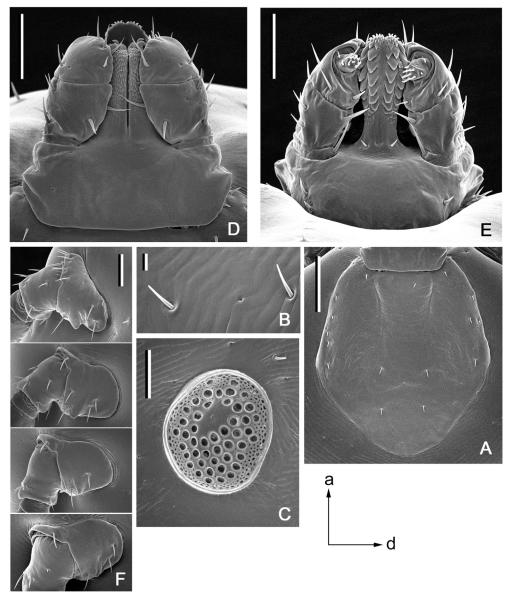
Fig. 3.
Dermacentor kamshadalus, male (USA, MT, Ravalli Co., Blodgett Canyon, USNMENT00714658). (A) Spiracular plate and circumspiracular setae. Bar = 0.2 mm. Arrows show orientation of spiracular plate (a—anterior; d—dorsal). (B) Gnathosoma dorsally. Bar = 0.2 mm. (C) Gnathosoma ventrally. Bar = 0.2 mm. (D) Coxae. Bar = 0.5 mm. (E) Coxa I. Bar = 0.2 mm. (F) Trochanter I. Bar = 0.2 mm.
Female
(description) (Figs. 1B and 4): Idiosoma (Fig. 1B): broadly oval, widest near mid-length. Scutum (Fig. 1B): long, length 1.72–2.50 (2.21; n = 50), width 1.60–2.22 (1.89; n = 50), ratio length to width 1.02–1.31 (1.18; n = 50), margins in anterior half of scutum diverge posteriorly, thereafter gradually converging to narrowly rounded posterior margin, posterolateral angular projections prominent. Coloration: ornamentation extensive, major portion of scutal surface covered with whitish enameling; two narrow, brown, cervical patches extend from cervical pits to posterior quarter of scutal length; central field with fragmented central patch and irregular small brown patches. Cervical grooves distinct, shallow. Mediumsized brown punctations sparse, very fine brown punctations dense, evenly distributed over scutum. Eyes oval, very slightly convex, positioned at mid-length. Setae relatively sparse and short. Alloscutum (Fig. 1B): as illustrated; 11 festoons. Setae of alloscutum relatively short, moderately dense. Venter: setae numerous and relatively short. Genital aperture (Fig. 4A): at level of coxae II, U-shaped; preatrial fold flat or slightly concave. Spiracular plates (Fig. 4B): suboval or subcircular; positioned on ventral surface in unengorged specimens, greatest diameter in anteroposterior plane, length 0.47–0.70 (0.59; n = 24); dorsal prolongation absent, large perforations fairly numerous. Gnathosoma (Figs. 1B and 4C and D): length from palpal apices to posterior margin of basis capituli dorsally 0.82–1.07 (0.97; n = 50), width of basis capituli 0.67–0.90 (0.77; n = 50), ratio length to width 1.13–1.38 (1.26; n = 50). Basis capituli (Figs. 1B and 4C and D): dorsally subrectangular; posterior margin nearly straight; cornua broad, moderately long comprising a 5.00–8.00 (5.99; n = 50) proportion of length of basis capituli; lateral margins with whitish enameling. Porose areas inwardly inclined, deeply sunken with clearly circumscribed borders, separated by space nearly equal to their width. Basis capituli ventrally subrectangular; with convex posterior margin. Palpi (Figs. 1B and 4C and D): short and broad; length dorsally (I–III segments) 0.47–0.62 (0.55; n = 50), width 0.27–0.37 (0.31; n = 50), ratio length to width 1.54–2.04 (1.75; n = 50), length of segments in descending order: 2, 3, 1, 4; segment I well developed ventrally; segment II narrower at base and thereafter parallel-sided; segment III broad, subrectangular with broadly rounded apex; segments II and III with whitish enameling on dorsal surfaces. Hypostome (Fig. 4D): club-shaped; dental formula 3/3. Legs (Fig. 1B): of medium length, robust; with extensive whitish enameling mostly on dorsal and lateral aspects. Coxae (Fig. 4E and F): coxaI with long triangular closely spaced internal and external spurs with pointed apices; external spur slightly longer than internal; both spurs of coxa I directed posterolaterally; coxae II and III each with larger triangular external spur with tapering apex and smaller internal spur; coxa IV with large triangular external spur with tapering apex. Trochanter I (Fig. 4G) with short, broad, triangular dorsal spur with tapering apex. Genu IV length 0.70–1.06 (0.90; n = 50), width 0.32–0.55 (0.44; n = 50), ratio length to width 1.89–2.26 (2.06; n = 50).
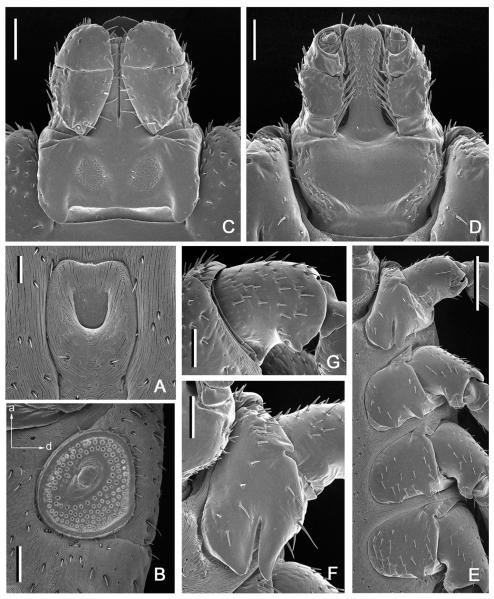
Fig. 4.
Dermacentor kamshadalus, female (USA, MT, Ravalli Co., Blodgett Canyon, USNMENT00714658). (A) Genital aperture. Bar = 0.1 mm. (B) Spiracular plate and circumspiracular setae. Bar = 0.2 mm. Arrows show orientation of spiracular plate (a—anterior; d—dorsal). (C) Gnathosoma dorsally. Bar = 0.2 mm. (D) Gnathosoma ventrally. Bar = 0.2 mm. (E) Coxae. Bar = 0.5 mm. (F) Coxa I. Bar = 0.2 mm. (G) Trochanter I. Bar = 0.2 mm.
Nymph
(description) (Fig. 5): Idiosoma: suboval, widest at mid-length, length of unengorged specimen from apices of scapulae to posterior body margin 1675 (n = 1), width 875 (n = 1), ratio length to width 1.91 (n = 1). Scutum (Fig. 5A): length 590–765 (691; n = 7), width 493–675 (616; n = 7), ratio length to width 0.94–1.14 (1.03; n = 7); pentagonal, posterior margin gradually tapering, with slight posterolateral angles; cervical grooves shallow. Setae ≈10 pairs. Eyes suboval, located on lateral margins of scutum at approximately its mid-length. Setae of alloscutum (Fig. 5B) ≈40 pairs (one unengorged nymph studied), without dentition; setae in anterolateral part length 40–45 (42.5; n = 2), setae in central rows length 26–31 (29; n = 2). Spiracular plates (Fig. 5C): oval, maximal length in anteroposterior plane; large perforations fairly numerous, small perforations numerous along entire perimeter of plate; no dorsal prolongation. Gnathosoma (Fig. 5D and E): length from palpal apices to posterior ventral margin of basis capituli 260–289 (278; n = 7), maximum width of basis capituli 289–340 (317; n = 7); ratio length to width 0.81–0.94 (0.88; n = 7). Basis capituli (Fig. 5D and E): dorsally and ventrally subrectangular, without lateral projections, posterior margin straight or slightly concave; dorsally short and broad. Posthypostomal setae one pair. Palpi (Fig. 5D and E): short, length 167–180 (174; n = 2), maximum width 95–97 (96, n = 2), ratio length to width 1.76–1.85 (1.80; n = 2); segment I short, cylindrical; distinct suture between segments II and III, segment II the longest, sides subparallel, segment III sub-rectangular; segment I with one ventral seta, segment II with four dorsal and three ventral setae, segment III with five dorsal and two ventral setae, segment IV with ≈11 setae. Hypostome (Fig. 5E): length from apex to the level of posthypostomal setae 140–162 (151; n = 2), width at narrowest point 65–66 (66; n = 2), ratio length to width 2.15–2.45 (2.30; n = 2); protruding anteriorly slightly beyond palpal apices, club-shaped, broadly rounded at apex, dental formula 2/2 (first row may be 3/3), ≈6 denticles in files. Coxae (Fig. 5F): coxa I with short, broadly arcuate internal spur and short triangular external spur; coxae II–IV with small triangular or fold-like external spurs only. Genu I: length 202–207 (205; n = 2), maximum width 120 (n = 1), ratio length to width 1.73 (n = 1).
Fig. 5.
Dermacentor kamshadalus, nymph (Canada, British Columbia, Churn Creek, CNCI15280). (A) Scutum. Bar = 200 μm. (B) Setae of alloscutum. Bar = 20 μm. (C) Spiracular plate. Bar = 100 μm. Arrows show orientation of spiracular plate (a—anterior; d—dorsal). (D) Gnathosoma dorsally. Bar = 100 μm. (E) Gnathosoma ventrally. Bar = 100 μm. (F) Coxae. Bar = 100 μm.
Larva
Unknown.
Holotype
Male, from Ovis sp., Kamchatka (precise locality unknown), Russia; whereabouts of depository not known. Neumann (1908) indicated that this specimen is deposited in the Natural History Museum of Leiden (the Netherlands), but it was not found in the Naturalis Biodiversity Center (Leiden) or the Natural History Museum (Paris) during the present investigation. The existence of the holotype in the Neumann collection at the Ecole Nationale Vétérinaire de Toulouse could also not be verified.
D. kamshadalus was described and illustrated by Neumann (1908), and all subsequent authors who referred to this taxon based their descriptions and illustrations on Neumann’s original work. However, nobody actually reexamined the holotype male. Some of the illustrations of D. albipictus adults in Cooley (1938, Plate 23: Figs. 3 and 4 and Plate 29: Figs. 1 and 2) are in fact those of D. kamshadalus.
Synonyms
Dermacentor variegatus kamshadalus Neumann, 1908; Dermacentor variegatus kamschadalus Neumann, 1908 sensu Yakimoff, 1917; Dermacentor variegatus kamtshadalus Neumann, 1908 sensu Olenev, 1928; Dermacentor variegatus kamtschadalus Neumann, 1908 sensu Olenev, 1931; Dermacentor varius kamtschadalus Neumann, 1908 sensu Schulze, 1933; Dermacentor varius kamtshadalus Schulze, 1933 in Doss and Anastos, 1977; Dermacentor variegatus kamchadalus Neumann, 1908 sensu Pomerantzev, 1950 (lapsus); Dermacentor kamtschadalus Neumann, 1908 sensu Serdyukova, 1956; Dermacentor albipictus kamshadalus Neumann, 1908 sensu Arthur, 1960; Dermacentor kamtschadalus Neumann, 1908 sensu Emel’yanova, 1966; Dermacentor albipictus kamtshadalus Neumann, 1908 sensu Arthur, 1960 in Doss and Anastos, 1977; Dermacentor albipictus kamtschadalus Neumann, 1908 sensu Filippova, 1984 in Camicas, Hervy, Adam, and Morel, 1998 (Neumann 1908; Yakimoff 1917; Olenev 1928, 1931; Schulze 1933; Pomerantzev 1950; Serdyukova 1956; Arthur 1960; Emel’yanova 1966; Doss and Anastos 1977; Camicas et al. 1998). The list above contains the first use of combinations and spellings by the various authors cited.
There are several variations in the spelling of the specific name in the literature. Originally Neumann (1908) used “kamshadalus” as did Yakimoff (1922) and Arthur (1960). Later various, and more particularly Soviet authors used variations in spelling such as “kamschadalus,” “kamtschadalus,” “kamtshadalus,” and “kamchadalus” (Olenev 1928, Schulze 1933, Pomerantzev 1950 and others). According to the rules of the International Code of Zoological Nomenclature (Article 32.5.1, 1999), the original spelling of Neumann, that is, “kamshadalus” has to be accepted as the correct one. All subsequent variations in the spelling of the species name were not accompanied by the required statement of intent to correct Neumann’s binomen and shouldthusbe considered as incorrect (ICZN, Article 33). Because of the rarity with which this species name appears in the literature, we do not consider that the subsequent variations in spelling should prevail over the original.
Hosts
The host data for D. kamshadalus are summarized in Table 1. Adults and nymphs have been collected from the mountain goat, Oreamnos americanus (de Blainville), the bighorn sheep, Ovis canadensis Shaw, and unspecified sheep, Ovis sp. One male was found secondarily attached to a person who had collected specimens of this species from the skin of O. americanus. Apparently D. kamshadalus is a one-host tick. All nymphs were collected from hosts on the same occasions as the adults, and two of the nymphs contain fully developed pharate adults. All the adults studied and few, mostly engorged nymphs, were collected on hosts from January till May. This indicates that the season of activity for this species is apparently from autumn through winter to spring as it is for D. albipictus.
There is one lot containing both D. kamshadalus and D. albipictus collected on the same occasion from a single bighorn sheep, and two collections in which it was found together with Dermacentor andersoni Stiles, 1908 on the same mountain goat.
Distribution
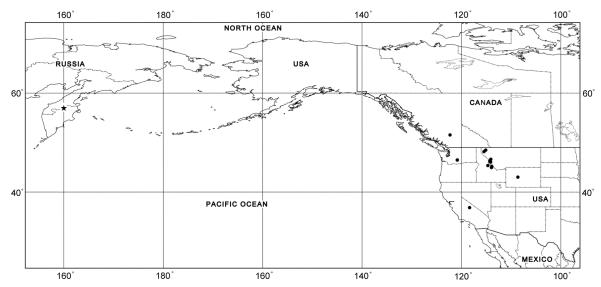
The distribution data for D. kamshadalus are summarized in Table 1 (Fig. 6). The known distribution of this species is confined to the United States (California, Idaho, Montana, Washington, and Wyoming), Canada (British Columbia), and Russia (Kamchatka Peninsula), where the holotype male was collected.
Fig. 6.
Dermacentor kamshadalus, map of geographical distribution. Star shows type locality (precise locality is unknown), filled circles show confirmed localities.
Etymology
The specific name is apparently derived from Kamchatka, a peninsula in eastern Russia, from where the type specimen was collected.
Related Species
Morphologically adults and nymphs of D. kamshadalus are most similar to those of D. albipictus. Adults of both species have a similar pattern of coloration on the conscutum and scutum, and their spiracular plates have large perforations and no dorsal prolongation, while the dorsal aspect of the basis capituli of the nymphs is rectangular in shape.
Both males and females of D. kamshadalus can easily be distinguished from those of D. albipictus by the short and broad dorsal spur on trochanter I (long and narrow in D. albipictus [Fig. 7I and J]), less dense and shorter setae on idiosoma, especially those around the spiracular plates (very dense and longer setae, especially prominent around spiracular plates in D. albipictus [Fig. 7A and B]), generally considerably more ornate conscutum and scutum than D. albipictus (there are some specimens of D. albipictus that are almost as white as D. kamshadalus, but the vast majority of ticks are darker [Fig. 2E–G]), spurs on coxa I are shorter and clearly directed posterolaterally (longer and narrower spurs directed posteriorly in D. albipictus [Fig. 7F and G]), shorter dorsal cornua (longer dorsal cornua in D. albipictus; on avg. a 3.66 proportion of the length of the basis capituli in males and a 4.86 proportion of the length in females [Fig. 7D and E]), more numerous perforations on spiracular plates (fewer in D. albipictus from Western United States [Fig. 7A and B]). Additionally males of D. kamshadalus can be distinguished by very robust (shorter and broader) legs, especially legs IV (slender legs in D. albipictus), while females of D. kamshadalus can be distinguished by shorter and broader spurs on coxae II and III (longer and narrower spurs in D. albipictus).
Fig. 7.
Dermacentor albipictus, male, female (USA, MT, Ravalli Co., East Fork, USNMENT00714253), nymph (USA, MT, Ravalli Co., USNMENT00714103). (A) Male spiracular plate and circumspiracular setae. Bar = 0.2 mm. (B) Female spiracular plate and circumspiracular setae. Bar = 0.2 mm. (C) Nymphal spiracular plate. Bar = 100 μm. (D) Male gnathosoma dorsally. Bar = 0.2 mm. (E) Female gnathosoma dorsally. Bar = 0.2 mm. (F) Male coxa I. Bar = 0.2 mm. (G) Female coxa I. Bar = 0.2 mm. (H) Nymphal coxa I. Bar = 100 μm. (I) Male trochanter I. Bar = 0.1 mm. (J) Female trochanter I. Bar = 0.1 mm.
Our present knowledge of the systematics of the immature stages of Dermacentor does not allow for a comprehensive diagnosis of D. kamshadalus based on the nymphal stage. Nymphs of D. kamshadalus can preliminarily be distinguished from those of D. albipictus in the Western United States by a shorter external spur on coxa I (longer in D. albipictus [Fig. 7H]) and by smaller as well as more numerous larger perforations on spiracular plates (few large perforations and no small perforations in D. albipictus [Fig. 7C]).
Acknowledgments
We express our sincere thanks to F. Beaulieu and K. W. Wu (Canadian National Collection of Insects, Arachnida and Nematodes, Agriculture and Agri-Food Canada, Research Center, Ottawa, Canada) for making their specimens available for examination. We sincerely thank Kristin Mansfield (Washington Department of Fish and Wildlife) for providing us with valuable specimens which we were lucky to include in this manuscript in the very last moment. We are indebted to Karen van Dorp (Naturalis Biodiversity Center, Leiden) and Mark Judson (Natural History Museum, Paris) for checking their collections for the presence of the holotype of D. kamshadalus. We are most grateful to Maria A. Apanaskevich for her kind help in editing and coloring the illustrations. We thank Ivan G. Horak (University of Pretoria, South Africa) for his careful editing of the manuscript. This project was supported by Grant Number R15AI096317 from the National Institute of Allergy and Infectious Diseases. The content is the sole responsibility of the author and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
References Cited
- Arthur DR. On the genera Dermacentor, Anocentor, Cosmiomma, Boophilus and Margaropus. Cambridge at the University Press; Cambridge, United Kingdom: 1960. Ticks. A monograph of the Ixodoidea. Part V. [Google Scholar]
- Brinton EP, Beck DE, Allred DM. Identification of the adults, nymphs and larvae of ticks of the genus Dermacentor Koch (Ixodidae) in the western United States. Vol. 5. Brigham Young University Science Bulletin; Provo, Utah: 1965. pp. 1–44. (Biological Series). [Google Scholar]
- Camicas JL, Hervy JP, Adam F, Morel PC. Nomenclature, described stages, hosts, distribution. Orstom éditions; Paris, France: 1998. The ticks of the world (Acarida, Ixodida) [Google Scholar]
- Cooley RA. The genera Dermacentor and Otocentor (Ixodidae) in the United States, with studies in variation. Natl. Inst. Health Bull. 1938;171:1–89. [Google Scholar]
- Crosbie PR, Boyce WM, Rodwell TC. DNA sequence variation in Dermacentor hunteri and estimated phylogenies of Dermacentor spp. (Acari: Ixodidae) in the New World. J. Med. Entomol. 1998;35:277–288. doi: 10.1093/jmedent/35.3.277. [DOI] [PubMed] [Google Scholar]
- Doss MA, Anastos G. III. Checklist of families, genera, species, and subspecies of ticks. United States Department of Agriculture; Washington, DC: 1977. Index-catalogue of medical and veterinary zoology. Special publication No. 3. Ticks and tickborne diseases. [Google Scholar]
- Emel’yanova ND. Tezisy dokladov, 1 Akarologicheskoe Soveshchaniye. Akademiya Nauk SSSR; Moscow, Leningrad, USSR: 1966. Some results and goals in the study of ixodid ticks in Eastern Siberia and the Far East; pp. 89–90. [Google Scholar]
- Ernst SE, Gladney WJ. Dermacentor albipictus: hybridization of the two forms of the winter tick. Ann. Entomol. Soc. Am. 1975;68:63–67. [Google Scholar]
- International Code of Zoological Nomenclature The International Trust for Zoological Nomenclature. 1999 http://www.nhm.ac.uk/hosted-sites/iczn/code/
- Leo SST, Pybus MJ, Sperling FAH. Deer mitochondrial DNA lineage divergences within Alberta populations of Dermacentor albipictus (Acari: Ixodidae) do not indicate distinct species. J. Med. Entomol. 2010;47:565–574. doi: 10.1093/jmedent/47.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann LG. Notes sur les Ixodidés. VII. Notes Leyden Mus. 1908;30:73–91. [Google Scholar]
- Olenev NO. Determination of ticks of the family Ixodidae Murray of the USSR. Dokl. Akad. Mauk. SSSR. 1928;16:84–96. [Google Scholar]
- Olenev NO. Die Zecken (Ixodoidea) der Fauna Russlands. Z. Parasitenk. 1931;4:126–139. [Google Scholar]
- Pomerantzev BI. Fauna of USSR, Arachnida. 2. Vol. 4. USSR Academy of Sciences; Moscow, Leningrad, USSR: 1950. Ixodid ticks (Ixodidae) [Google Scholar]
- Schulze P. Die arten der zeckengattung Dermacentor s.l. aus Europa, Asien und Neu-Guinea. Z. Parasitenk. 1933;6:416–431. [Google Scholar]
- Serdyukova GV. Opredeliteli po faune SSSR izdavaemye Zoologicheskim Institutom Akademii Nauk SSSR. Vol. 64. USSR Academy of Sciences; Moscow, Leningrad, USSR: 1956. Ixodid ticks of the fauna of USSR. [Google Scholar]
- Yakimoff WL. Les tiques des animaux domestiques du Turkestan russe. Bull. Soc. Path. Exot. 1917;10:298–301. [Google Scholar]
- Yakimoff WL. Contribution à l’étude des Ixodidés de Russie. Bull. Soc. Path. Exot. 1922;15:41–46. [Google Scholar]
- Yunker CE, Keirans JE, Clifford CM, Easton ER. Dermacentor ticks (Acari: Ixodoidea: Ixodidae) of the New World: a scanning electron microscope atlas. Proc Entomol. Soc. Wash. 1986;88:609–627. [Google Scholar]