Abstract
The aim of this pilot study was to evaluate the use of advanced proteomics techniques to identify novel protein markers that contribute to the transformation of benign meningiomas to more aggressive and malignant subtypes. Multiplex peptide stable isotope dimethyl labelling and nano-LCMS was used to identify and quantify the differentially expressed proteins in WHO Grade I, II and III meningioma tissues. The proteins identified will help elucidate the process of transformation to malignancy and may contribute to improved diagnosis and treatment of these aggressive tumors
Keywords: Meningioma, Anaplastic meningioma, Atypical meningioma, Proteomics, Biomarkers
Introduction
Meningiomas are the most common benign intracranial tumors and their first-line treatment is surgical removal if the lesion can be largely removed at sufficiently low risk. However, a subset of patients develops more aggressive tumors. According to the World Health Organization (WHO), meningiomas are classified as typical, atypical and anaplastic; up to 20% of patients may have atypical meningiomas and 1-3% may develop anaplastic or malignant subtypes [1]. These aggressive subtypes of tumors typically exhibit more rapid tumor progression, invasiveness and recurrence precluding complete surgical removal and requiring additional therapies of radiosurgery/radiotherapy and chemotherapy [2]. Occasionally, meningiomas have malignant transformation with distant metastases outside the central nervous system (CNS).
Extent of tumor resection has been shown to correlate with recurrence rate. In 1957, Simpson D described a grading system that has been expanded and validated over the decades [3-5]. WHO grade I tumors tend to have a direct inverse correlation between extent of resection and tumor recurrence. MiB (Ki67) level greater than 3%, helps predict recurrence rate in Simpson I-III meningiomas. MiB is not a criterion used for WHO II or WHO III meningiomas. Hence, additional biomarkers are necessary to elucidate the likelihood and mechanisms of tumor recurrence.
Most WHO I tumors harbor a few mutations [6,7] and can be categorized into groups expressing NF2, AKT-1, SMO, TRAF7, KLF4. WHO grade II and III tumors harbor a wider variety of mutations including (hTERT/telomerase, MADH2, MADH4, APM-1, DCC, CDKN2A, p14ARF, CDKN2B, TP53, MEG3, ALPL, Notch, WNT, IGF and NDRG2 [8].
Few genetic and proteomics markers have been studied for meningioma subtypes with various aims [9-11] and their correlation to clinical behaviour and response to therapy is limited. While there is a notable overlap with some biomarkers found in other malignant neoplasms (glioblastoma, adenocarcinoma, squamous cell carcinoma and melanoma), the mechanisms that result in transformation from benignmeningiomastomoreaggressivesubtypesarepoorlyunderstood. This study aims to better define biomarkers of transformation into aggressive tumors in patients with benign meningiomas using and proteomics analysis and may identify targets for future therapies.
Proteomics plays an important role in medical research, because of the link between proteins, genes and diseases [12]. Most current drugs are either proteins or they target specific proteins in the body [13]. Identifying unique protein expression associated with specific tumors is a very important and promising area in the field of clinical proteomics; hence proteomics analysis of brain tissues is an essential part of neuroscience research [14]. Although it faces many challenges, most importantly the difficulty of obtaining sufficient sample for mass spectrometry analysis, and protein purification methods has to be optimized for each type of cell or tissue [14-16]].
Three tumor tissues (typical, atypical and anaplastic), and two controls (fresh cadaveric dura) were used for proteomics analysis. Multiplex peptide stable isotope labelling method was used to label all samples. With this method, all primary amines (the N terminus and the side chain of lysine residues) in a peptide mixture are converted to dimethylamines. The labelled samples are then mixed in equal ratios and analysed by liquid chromatography–mass spectrometry (LC/MS). The mass difference of the dimethyl labels is used to compare the peptide quantity across all samples. The advantages of this labelling method over others, besides allowing the comparison of multiple samples in a single experiment; it uses inexpensive reagents and is applicable to almost any sample (tissue/cell) [17].
Materials and Method
Samples
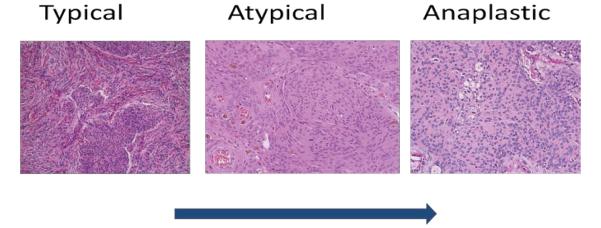
Three meningiomas, typical (I), atypical (II) and anaplastic (III) (Figure 1), that were resected at Providence Saint John’s Health Center by Drs. Barkhoudarian and Kelly, were selected from the John Wayne Cancer Institute brain tumor tissue bank. These tissues had been cryogenically preserved per standard protocol [18]. Dura mater was obtained from two cadaveric specimens, cryogenically preserved, and used as controls.
Figure 1.
Histopathological progression of meningiomas from grade I (typical) to grade II (atypical) to grade III (anaplastic) subtypes. As the tumors become increasingly aggressive the cellularity increases, nuclear atypia formation and loss of cytoarchitecture.
Protein extraction
Tissues homogenization was carried out with12 mM sodium lauryl sarcosine, 0.5% sodium deoxycholate, and 50 mM triethyl ammonium bicarbonate (TEAB). The samples were then centrifuged at 16,000 × g for 5 minutes and the supernatant was collected, heated at 95°C for 5 minutes and placed in a water bath sonicator for 5 minutes.
Protein concentrations
The total protein concentration of the samples was determined using BCA Protein Assay Kit (Pierce, Thermo Fischer Scientific). Bovine serum albumin was used to generate the standard curves.
Reduction, alkylation and trypsin digestion
Protein disulfides were reduced with 5 mM Tris 2-carboxyethyl phosphine, for 30 minutes at room temperature. Ten mM iodoacetamide was then added for alkylation, and incubation in dark for 30 minutes at room temperature. The protein solutions were diluted five-fold with 50 mM TEAB.
Trypsin was prepared in 50 mM TEAB, and added to the samples in (1:100) ratio then incubated for 4hrs at room temperature. This step was repeated twice. The peptide solutions were acidified with a final concentration of 0.5% trifluoroacetic acid (TFA), vortexed for 5 minutes. Detergents were removed by adding 1:1 (vol/vol) of ethyl acetate to the tryptic digests, vortexed for 5 minutes and centrifuge at 12,000 × g for 5 minutes at room temperature, supernatant were discarded. The tryptic peptides lyophilized before dimethy labelling.
Dimethyl labelling
The dimethyl labelling was carried out according to Boersema et al. [17], using in-solution dimethyl labelling protocol. The digested samples were reconstituted in 100 µL of 100 mM TEAB. Four microliters of 4% (vol/vol) formaldehyde isotopes (CH2O, CD2O and 13CD2O) were then added to the samples to be labelled with light, intermediate and heavy dimethyl respectively, samples mixed and spun down. Four microliters of 0.6 M sodium cyanoborohydride (NaBH3CN) isotope was added for light and intermediate labelling and 0.6 M of sodium cyanoborodeuteride (NaBD3CN) isotope for heavy labelling. All samples were then placed on a bench mixer and incubated for 1 hr at room temperature.
The labelling reaction was quenched by adding 16 µL of 1% (vol/vol) ammonia and 8 µL of 5% (vol/vol) formic acid to acidify the samples for mass spectrometry analysis.
The brain tissues were labelled as follows: control 1 = light, control 2 = intermediate, meningioma samples (T1, TII and TIII) = heavy. The samples were grouped in 3 triplex per Table 1 below. The differentially labelled samples were then mixed in 1:1:1 ratios, and analysed by nanoLC-MS.
Table 1.
Triplex samples for analysis. C1 and C2 = controls. S1, S2 and S3 = meningioma samples.
| A | B | C |
|---|---|---|
| C1 + C2 + T1 | C1 + C2 + TII | C1 + C2 + TIII |
Chromatographic separation and nanoLC-MS
C18 and SCX stage tips were prepared in house. The stage tips were conditioned with 20 µL methanol and 20 μL of buffer containing [ammonium acetate (NH4AcO) using gradient elution from 0.2 to 5%, 0.5% acetic acid (AcOH) and 30% of acetonirile (ACN)]. The same buffer was used for SCX fractionation and sample elution. The samples then dried in SpeedVac and reconstituted in acetonirile 3% (ACN) and 0.1 % Formic acid (FA).
Fractionated samples were analysed with an Eksigent 2D nanoLC mass spectrometer attached to a Thermo Orbitrap XL. Peptides were injected onto a laser-pulled nanobore 20 cm × 75 μm C18 column (Acutech Scientific) in buffer A containing (3% acetonitrile with 0.1% formic acid) and resolved using a 3 hour linear gradient from 3-40% buffer B containing (100% acetonitrile with 0.1% formic acid). The Orbitrap XL was operated in data dependent mode with 60,000 resolution and target auto gain control at 5e6 for parent scan. The top 12 ions above +1 charge were subjected to collision induced dissociation set to a value of 35 with target auto gain control of 5000. Dynamic exclusion was set to 30 seconds.
Data Analysis
The MS/MS spectra were analysed using MaxQuant software version 1.5.1.2 (Germany). The different dimethyl isotope labels were set as variable modifications on the peptide N termini and lysine residues. Carbamidomethyl cysteine was set as a fixed modification while oxidized methionine was set as variable modification. Trypsin was set as a proteolytic enzyme, and maximum 2 missed cleavages were allowed, peptide tolerance 10 ppm, fragment ions tolerance 0.5 amu.
Results
Five brain tissues were used for this quantitative proteomic study, grouped per (Table 1) above to study the variability and consistency of protein expressions between; (i) the two controls: (ii) between the controls and tumor samples: (iii) across all three tumor samples [typical (I), atypical (II) and anaplastic (III)]. In total 649 proteins were identified from 15 MS runs. Protein abundances were derived from peptide abundances for multiple peptides. Protein abundances were calculated from the sum of all unique normalised peptide ion abundances for a specific protein on each run. The Supplementary Table 1, includes a list of protein names, their intensity in the controls (C), their intensity in the three phenotypes (I, II and III), the expression ratios of average controls (vs.) phenotypes I, II and III, as well as the expression ratios between all of the three phenotypes (I, II and III).
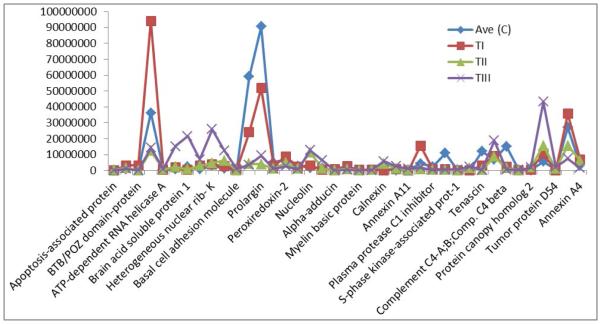
Our analysis and observation was focused on the proteins that showed up or down-regulation in one phenotype compared to the others and compare to the control, as those proteins could potentially be investigated as biomarkers for aggressive tumors, e.g. protein alpha-adducin, was expressed in C, TI and TII only, and it was up-regulated in TI by 3 fold compare to the control, however in TII was down-regulated by 0.25 compare to the control, and wasn’t detected in TIII; hence the expression ratio for TI: TII was 11.6 (Supplementary Table 1). This may suggest that this protein is mainly present in the non-aggressive form of meningioma, or its representing gene (ADD1) may be switched off in the aggressive forms. Other proteins that showed similar pattern to alpha-adducin are summarized in (Table 2 and Figure 2).
Table 2.
Selected protein expressions (intensities), in controls and meningioma tissues.
| Protein name | Ave (C) | TI | TII | TIII |
|---|---|---|---|---|
| Apoptosis-associated protein | 22612 | 0.01 | 391410 | 0.01 |
| Transmembrane protein 109 | 863006 | 3104800 | 1602000 | 1143100 |
| BTB/POZ domain-protein | 326921 | 3116400 | 0.01 | 0.01 |
| Beta-actin-like protein 2 | 36158500 | 94091000 | 12285000 | 14177000 |
| ATP-dependent RNA helicase A | 349960 | 426750 | 629360 | 859420 |
| Protein SET | 731725 | 1848400 | 2145600 | 15151000 |
| Brain acid soluble protein 1 | 2273925 | 259030 | 690070 | 21530000 |
| 40S ribosomal protein S28 | 684885 | 3198000 | 2910100 | 6894400 |
| Heterogeneous nuclear rib- K | 3006850 | 3801700 | 4588700 | 25966000 |
| Activated RNA polymerase II trans p15 | 1338271 | 2809900 | 6207100 | 12637000 |
| Basal cell adhesion molecule | 615190 | 452370 | 234410 | 870010 |
| Lumican | 58945833 | 24028000 | 4039000 | 3530400 |
| Prolargin | 90667333 | 51906000 | 3970600 | 9401300 |
| Malate dehydrogenase, | 4966816 | 2812100 | 1151600 | 1022800 |
| Peroxiredoxin-2 | 6842683 | 8677400 | 4756600 | 2568400 |
| Rab GDP dissociation inhibitor alpha | 2722066 | 2395500 | 1308800 | 672160 |
| Nucleolin | 1565608 | 3120600 | 11362000 | 12647000 |
| Stathmin; Stathmin-2 | 1482545 | 284420 | 1140500 | 6319600 |
| Alpha-adducin | 281103 | 846030 | 72814 | 0.01 |
| Glutathione S-transferase P | 904686 | 2566600 | 933570 | 330930 |
| Myelin basic protein | 177968 | 394030 | 29499 | 0.01 |
| Synaptic vesicle membrane | 596081 | 438400 | 355470 | 184350 |
| Calnexin | 1139366 | 0.01 | 4238500 | 5735800 |
| Serine / arginine-rich splicing F2 | 97133 | 886340 | 1175300 | 2511700 |
| Annexin A11 | 368523 | 0.01 | 321640 | 1155500 |
| Transketolase | 4291416 | 15510000 | 979660 | 1905700 |
| Plasma protease C1 inhibitor | 2377966 | 736090 | 270950 | 327160 |
| Complement factor B | 10816883 | 955230 | 408350 | 466770 |
| S-phase kinase-associated prot-1 | 354538 | 114820 | 149220 | 510400 |
| CD44 antigen | 77701 | 0.01 | 1469000 | 2307700 |
| Tenascin | 12078016 | 3160100 | 238210 | 212320 |
| Cofilin-1 | 6860700 | 9157100 | 8618400 | 18678000 |
| Complement C4-A;B;Comp. C4 beta | 15141850 | 2184300 | 1534700 | 489510 |
| Rho GDP-dissociation inhibitor 2 | 319165 | 0.01 | 225460 | 335440 |
| Protein canopy homolog 2 | 138681 | 567330 | 1771900 | 1879000 |
| Protein disulfide-isomerase A3 | 5554233 | 10539000 | 15571000 | 43184000 |
| Tumor protein D54 | 643386 | 0.01 | 989430 | 1940400 |
| Alpha-enolase | 27090833 | 35751000 | 15517000 | 7497900 |
| Annexin A4 | 3241150 | 6722600 | 6252500 | 1376000 |
Figure 2.
Chart of the expression levels of selected proteins from Table 2. Their intensities in Ave control (C) and meningioma tissues (I, II and III).
Another intriguing observation of this data is the presence of some proteins in one subtype only compare to other subtypes and compare to the control. Twenty three proteins were detected in TIII only (Table 3 and Supplementary Table 1), including tumor protein D52, lysosome membrane protein 2, splicing factor-1 and MUC18. These proteins are of importance in biomarker study of meningiomas due to their unique expression.
Table 3.
Proteins expressed in anaplastic tumor tissues only.
| Protein name |
|---|
| Junctional adhesion molecule B |
| Lysosome membrane protein 2* |
| Eukaryotic translation initiation factor 4B |
| Dihydrolipoyl dehydrogenase, mitochondrial |
| Chromobox protein homolog 1 |
| Amyloid beta A4 protein |
| 26S proteasome non-ATPase regulatory subunit 9 |
| Double-strand break repair protein MRE11A |
| Splicing factor 1* |
| Yorkie homolog* |
| Mitochondrial import inner membrane trans9 |
| Glucose-induced degradation protein 8 homolog |
| PRKC apoptosis WT1 regulator protein* |
| Heme-binding protein 2 |
| Enhancer of rudimentary homolog |
| MARCKS-related protein* |
| Cell surface glycoprotein MUC18 |
| Insulin-like growth factor II |
| Sorting nexin-1 |
| Tumor protein D52* |
| Polyadenylate-binding protein-interacting protein 1 |
| Chromobox protein homolog 1 |
| ADP-sugar pyrophosphatase |
Tumour associated proteins
Conclusion
This data suggests the feasibility of identifying and quantifying the proteins in brain meningioma tissues for comparison studies. Due to rare clinical samples, only five brain tissues were used for this study. Larger numbers of specimen are required to conduct a large scale experiments to significantly obtain novel protein biomarkers that correlate with the aggressive tumors. Concurrent genomic and epigenomic analysis will also be helpful to assess post-transcriptional mechanisms. These biomarkers will be clinically utilized in future management of patients, to better identify aggressive tumors for closer surveillance and application of novel targeted therapies. Ultimately this may potentially reduce the need for major high-risk surgery in this patient population.
Supplementary Material
Acknowledgement
This pilot project was supported by a grant from Meningioma Mommas. Prof Julian Whitelegge, is funded with NIH Grant (P30 DK063491).
Footnotes
Supplementary Information
References
- 1.Commins DL, Atkinson RD, Burnett ME. Review of meningioma histopathology. Neurosurg Focus. 2007;23:E3. doi: 10.3171/FOC-07/10/E3. [DOI] [PubMed] [Google Scholar]
- 2.Doleželová H, Hynková L, Pospíšil P, Kazda T, Slampa P, et al. Therapeutic results of the treatment brain tumors using radiosurgery and stereotactic radiotherapy. Klin Onkol. 2012;25:445–451. [PubMed] [Google Scholar]
- 3.Simpson D. The recurrence of intracranial meningiomas after surgical treatment. J Neurol Neurosurg Psychiatry. 1957;20:22–39. doi: 10.1136/jnnp.20.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oya S, Kawai K, Nakatomi H, Saito N. Significance of Simpson grading system in modern meningioma surgery: integration of the grade with MIB-1 labeling index as a key to predict the recurrence of WHO Grade I meningiomas. J Neurosurg. 2012;117:121–128. doi: 10.3171/2012.3.JNS111945. [DOI] [PubMed] [Google Scholar]
- 5.Heald JB, Carroll TA, Mair RJ. Simpson grade: an opportunity to reassess the need for complete resection of meningiomas. Acta Neurochir (Wien) 2014;156:383–388. doi: 10.1007/s00701-013-1923-6. [DOI] [PubMed] [Google Scholar]
- 6.Clark VE, Erson-Omay EZ, Serin A, Yin J, Cotney J, et al. Genomic analysis of non-NF2 meningiomas reveals mutations in TRAF7, KLF, AKT, and SMO. Science. 2013;339:1077–1080. doi: 10.1126/science.1233009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brastianos PK, Horowitz PM, Santagata S, Jones RT, McKenna A, et al. Genomic sequencing of meningiomas identifies oncogenic SMO and AKT1 mutations. Nat Genet. 2013;45:285–289. doi: 10.1038/ng.2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choy W, Kim W, Nagasawa D, Stramotas S, Yew A, et al. The molecular genetics and tumor pathogenesis of meningiomas and the future directions of meningioma treatments. Neurosurg Focus. 2011;30:E6. doi: 10.3171/2011.2.FOCUS1116. [DOI] [PubMed] [Google Scholar]
- 9.Lusis EA, Chicoine MR, Perry A. High throughput screening of meningioma biomarkers using a tissue microarray. J Neurooncol. 2005;73:219–223. doi: 10.1007/s11060-004-5233-y. [DOI] [PubMed] [Google Scholar]
- 10.Okamoto H, Li J, Vortmeyer AO, Jaffe H, Lee YS, et al. Comparative proteomic profiles of meningioma subtypes. Cancer Res. 2006;66:10199–10204. doi: 10.1158/0008-5472.CAN-06-0955. [DOI] [PubMed] [Google Scholar]
- 11.Sharma S, Ray S, Moiyadi A, Sridhar E, Srivastava S. Quantitative proteomic analysis of meningiomas for the identification of surrogate protein markers. Sci Rep. 2014;4:7140. doi: 10.1038/srep07140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petricoin EF, Zoon KC, Kohn EC, Barrett JC, Liotta LA. Clinical proteomics: translating benchside promise into bedside reality. Nat Rev Drug Discov. 2002;1:683–695. doi: 10.1038/nrd891. [DOI] [PubMed] [Google Scholar]
- 13.Wulfkuhle JD, Liotta LA, Petricoin EF. Proteomic applications for the early detection of cancer. Nat Rev Cancer. 2003;3:267–275. doi: 10.1038/nrc1043. [DOI] [PubMed] [Google Scholar]
- 14.Simonian M, Ogorzalek Loo RR, Loo JA, Stoodley MA, Molloy MP. Proteomics Detection of Endothelial Cell Surface Proteins Following Irradiation as Potential Targets for Brain Arteriovenous Malformations Molecular Therapy. MOJ Proteomics Bioinform. 2014;1:00002. [Google Scholar]
- 15.Simonian M, Molloy MP, Stoodley MA. In Vitro and In Vivo Biotinylation of Endothelial Cell Surface Proteins in the Pursuit of Targets for Vascular Therapies for Brain AVMs. Metabolomics. 2012;S1:007. [Google Scholar]
- 16.Simonian M, Ogorzalek Loo RR, Rannulu N, Loo JA, Molloy MP, et al. Identification of protein targets for brain arteriovenous malformations (AVMs) molecular therapies. J Proteome Research. 2015 doi: 10.1186/s12014-017-9151-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boersema PJ, Raijmakers R, Lemeer S, Mohammed S, Heck AJ. Multiplex peptide stable isotope dimethyl labeling for quantitative proteomics. Nat Protoc. 2009;4:484–494. doi: 10.1038/nprot.2009.21. [DOI] [PubMed] [Google Scholar]
- 18.Chiu CG, Nakamura Y, Chong KK, Huang SK, Kawas NP, et al. Genome-wide characterization of circulating tumor cells identifies novel prognostic genomic alterations in systemic melanoma metastasis. Clinical chemistry. 2014;60:873–885. doi: 10.1373/clinchem.2013.213611. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.