Abstract
β -Lapachone and certain of its derivatives directly bind and inhibit topoisomerase I (Topo I) DNA unwinding activity and form DNA-Topo I complexes, which are not resolvable by SDS-K+ assays. We show that β-lapachone can induce apoptosis in certain cells, such as in human promyelocytic leukemia (HL-60) and human prostate cancer (DU-145, PC-3, and LNCaP) cells, as also described by Li et al. (Cancer Res., 55: 0000–0000, 1995). Characteristic 180-200-bp oligonucleosome DNA laddering and fragmented DNA-containing apoptotic cells via flow cytometry and morphological examinations were observed in 4 h in HL-60 cells after a 4-h, ≥0.5 μM β-lapachone exposure. HL-60 cells treated with camptothecin or topotecan resulted in greater apoptotic DNA laddering and apoptotic cell populations than comparable equitoxic concentrations of β-lapachone, although β-lapachone was a more effective Topo I inhibitor. β-Lapachone treatment (4 h, 1–5 μM) resulted in a block at G0/G1,with decreases in S and G2/M phases and increases in apoptotic cell populations over time in HL-60 and three separate human prostate cancer (DU-145, PC-3, and LNCaP) cells. Similar treatments with topotecan or camptothecin (4 h, 1–5 μM) resulted in blockage of cells in S and apoptosis. Thus, β-lapachone causes a block in G0/G1 of the cell cycle and induces apoptosis in cells before, or at early times during, DNA synthesis. These events are p53 independent, since PC-3 and HL-60 cells are null cells, LNCaP are wild-type, and DU-145 contain mutant p53, yet all undergo apoptosis after β-lapachone treatment. Interestingly, β-lapachone treatment of p53 wild type-containing prostate cancer cells (i.e., LNCaP) did not result in the induction of nuclear levels of p53 protein, as did camptothecin-treated cells. Like other Topo I inhibitors, β-lapachone may induce apoptosis by locking Topo I onto DNA, blocking replication fork movement, and inducing apoptosis in a p53-independent fashion. β-Lapachone and its derivatives, as well as other Topo I inhibitors, have potential clinical utility alone against human leukemia and prostate cancers.
Introduction
β-Lapachone (3,4-dihydro-2,2-dimethyl-2H-naphtho[l,2-b] pyran-5,6-dione) is a naturally occurring product that can be easily obtained from lapachol, an abundant quinone present in the lapacho tree (Tabebuia avellanedae) native to South America (1–3). We demonstrated previously that β-lapachone did not alter the activities of eukaryotic Topo II; DNA ligases I, II, or III; or intercalate into DNA (4–7). β-Lapachone did, however, specifically alter the activity of Topo I4 (4). Although our original data indicated that β-lapachone activated Topo I (4), it was later independently shown by Li et al. (8) and Boothman et al. (7) that β-lapachone actually inhibited Topo I by a very different mechanism than other currently used Topo I inhibitors. Like camptothecin, the compound inhibited the unwinding activity of Topo I but did not result in stabilization of DNA-Topo I complexes under standard SDS-K+ assay techniques (7). To our knowledge, β-lapachone is the only known compound that binds directly to Topo I and inhibits its enzymatic activity (7, 8). Thus, the apparent activation of Topo I observed earlier (4) probably resulted from a nicking reaction without stabilization of DNA-Topo I complexes (7). These data with β-lapachone indicated to us that Topo I was involved in DNA repair and may be exploited after radiation therapy in tumors with elevated levels of this enzyme, including melanomas, lung carcinomas, breast cancers, and colon and prostate cancers (6, 9, 10).
Since Topo I inhibitors are known to cause apoptotic reactions (11, 12), we explored β-lapachone-mediated apoptosis in various human cells. We demonstrate that treatment of human promyelocytic leukemia (HL-60) or human prostate (DU-145, PC-3, and LNCaP) cancer cells with β-lapachone specifically resulted in blockage of cell cycle progression at G0/G1 and induced apoptosis. In contrast, treatment of human breast cancer cells with equitoxic concentrations of β-lapachone did not result in visible induction of apoptosis in 24 h.
Materials and Methods
Chemicals
β-Lapachone (mw: 242) was originally obtained from Ciba-Geigy (Zurich, Switzerland). During the course of our investigations, we found that β-lapachone degraded over time and was not stable in air, water, or DMSO. During its degradation in air, a compound of approximate mw:377 was detected by mass spectra analyses using modifications of methods described previously (13). Thus, not only were freshly synthesized and dissolved samples required to observe the effects described below, but the β-lapachone from Ciba-Geigy was found to be one-third impure. As a result, we synthesized β-lapachone using methods described previously (13); therefore, a 100% pure compound was used in the present study. Topotecan (mw: 452.41) was obtained from Smithkline Beecham Pharmaceuticals (King of Prussia, PA). Camptothecin (mw: 348.4) was obtained from Sigma Chemical Co. (St. Louis, MO). All compounds were freshly dissolved in DMSO as a transition solvent and stored in aliquots at −20°C. Control cells were treated with DMSO at a concentration equal to the highest percentage of DMSO used in various experiments described below.
Topo I DNA Unwinding Enzymatic Assays
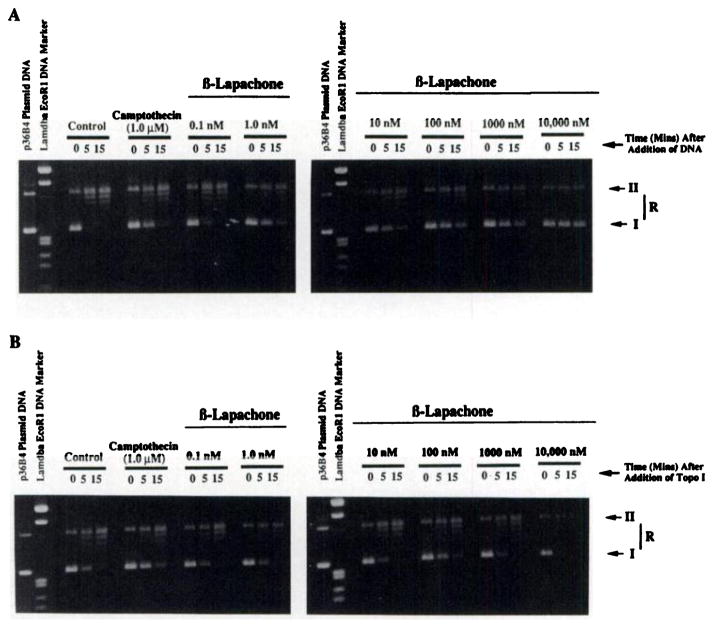
Supercoiled DNA unwinding assays using purified human placenta Topo I (TopoGEN, Inc., Columbus, OH) were performed with or without drug addition as described (4) to assess the inhibitory effects of β-lapachone, camptothecin, and topotecan under various reaction conditions. Enzymatic assays were performed in two basic fashions. In the first reaction sequence (Fig. 1A), Topo I (3.0 units) was incubated with increasing concentrations of β-lapachone, camptothecin (or topotecan), or DMSO for 5 min at 37°C in Topo I reaction buffer (without dATP, as described Refs. 4 and 14). p36B4 supercoiled DNA (1.5 μg) was then added to begin the reactions, and aliquots were taken at various times. In the second reaction sequence (Fig. 1B), p36B4 DNA (1.5 μg) was incubated with various β-lapachone, camptothecin, or topotecan concentrations for 5 min at 37°C, and Topo I (3.0 units) was added at t = 0. Aliquots were removed at various times to follow DNA unwinding reactions and immediately treated with SDS-proteinase K at 65°C and loaded onto 0.7% agarose gels; supercoiled (form I) substrate was separated and quantified from reaction intermediates (R) and open circular (form II) product (4, 15). On most agarose gels, DNA molecular weight markers [λ DNA cut with EcoRI-HindIII (marked “Lambda EcoRI DNA Marker”); Sigma Chemical Co., St. Louis, MO], linearized p36B4 plasmid DNA (cut with PstI), and p36B4 plasmid DNA substrate were concomitantly added. Gels were stained with 50 μg/ml ethidium bromide and destained for 30 min in distilled water; the loss of form I relative to total DNA loaded was quantified by densitometric scans of photographic negatives (type 55; Polaroid, Cambridge, MA) as described (4, 15). Enzyme inhibition was defined as the effects of various drugs on Topo I activity compared to control (DMSO alone) reactions. Topo I activity was defined as described previously (15), and all experiments were performed at least three times.
Fig. 1.
β-Lapachone inhibits the catalytic activity of Topo). A, Topo I (3.0 units) was incubated with camptothecin or various concentrations of β-lapachone for 10 min at 37°C. p36B4 supercoiled plasmid DNA (1.5 μg) was then added to initiate DNA unwinding reactions. Topo I DNA unwinding activity was measured as described (14, 15). B, Topo I DNA unwinding assays were repeated as in (A), except that camptothecin or various concentrations of β-lapachone were incubated with DNA for 5 min at 37°C. Reactions were then initiated by adding 3.0 units of purified human placental Topo I (TopoGEN). β-Lapachone was an effective inhibitor only when incubated with the enzyme prior to adding DNA to initiate the reactions. Form I, supercoiled DNA substrate; Form II, open circular DNA product; R, DNA reaction intermediates.
Cell Lines and Culture Conditions
Human promyelocytic leukemia (HL-60) cells were obtained from the American Type Culture Collection and were grown in RPMI with 10% FBS; all cells were grown in a humidified 10% CO2-90% air atmosphere at 37°C, unless otherwise stated. Human MCF-7:WS-8 breast cancer cells and various cell derivatives (Table 1) were generously provided by Dr. V. Craig Jordan (Northwestern University, Chicago, IL) and were grown in DME containing 10% FBS at 37°C. Human PC-3, DU-145, and LNCaP prostate cancer cells were grown in DME with 5% FBS at 37°C in a humidified 5% CO2-95% air atmosphere as described (16, 17). For all experiments, cancer cells were plated under low density conditions (1.0 × 104 cells/ml), allowed 24 h to initiate log-phase growth, and were then exposed or not to β-lapachone (0.001–10 μM), 5 μM camptothecin, or 5 μM topotecan for continuous treatments or for 4 h. In most experiments, no treatment and DMSO alone (in which cells were treated with up to 1.25% DMSO corresponding to the highest drug treatments) controls were performed in conjunction with Topo I inhibitor treatments. All experiments were repeated at least three times, each in duplicate.
Table 1.
Apoptopic effects and IC50 determinations of β-lapachone on various human cancer cells
| Cell line | p53 statusa | IC50(μM)b
|
Apoptosisc observed | Cell cyclec blockage | |
|---|---|---|---|---|---|
| DNA assay | CF assay | ||||
| Human promyelocytic leukemia cells | |||||
| HL-60 | null | 0.75 | NDd | + | G0/G1 |
| Human prostate cancer cells | |||||
| LNCaP | wt | 0.4 | 0.1 | + | G0/G1 |
| DU-145 | mt | 0.7 | 0.6 | + | G0/G1 |
| PC-3 | null | 0.3 | 0.3 | + | G0/G1 |
| Human breast cancer cells | |||||
| T47D:A18 | wt | 2.0 | ND | − | G0/G1 |
| MCF-7:WS8 | wt | 3.2 | ND | − | G0/G1 |
| MDA:MB:231 | mt | 4.0 | ND | − | G0/G1 |
p53 status was determined by Western analyses, p53 DNA consensus sequence binding using DNA band shift assays, and damage-inducible, nuclear increases in p53 protein amounts via Western analyses. Nuclear p53 levels were not induced by 0.25–5.0 μM β-lapachone exposures to LNCaP or MCF-7:WS8 cells, in contrast to its dramatic induction by 0.25–5.0 μM camptothecin.6 wt, wild-type p53; mt, mutant p53; null, no measurable p53 protein expressed.
Determined by cytotoxicity, DNA assays, and confirmed by colony-forming (CF) assays after 24-h exposures to β-lapachone as described in “Materials and Methods.”
Quantification of apoptotic cells and alterations in cell cycle distribution were determined 24 h after drug treatment (1.0–10 μM;4 h) by flow cytometry as described in “Materials and Methods.”
ND, not done.
Cytotoxicity and Colony-Forming Assays
IC50 calculations for each cell line were determined by DNA amount (18) and anchorage-dependent colony formation (CF) assays as described (4, 18). For the CF assay, cells were seeded at 500 viable cells/well in 6-well plates and incubated overnight, then treated with equal volumes of media containing β-lapachone at final concentrations ranging from 0.005 to 50 μM in half-log increments (controls were treated with 0.25% DMSO, equivalent to the highest dose of β-lapachone used) for 4 h or for continuous 12-h exposures. Plates (3 wells/condition) were stained with crystal violet, and colonies of >50 normal-appearing cells were enumerated (4, 18). IC50 values for various cells were calculated using drug doses with numbers of colonies surrounding 50% of control. For DNA assays, plates were harvested for IC50 determinations 8 days after treatment using a CytoFluor 2350 fluorescence measurement system (Millipore) as described (18). Six-well samplings were included in the calculation of DNA fluor units for each dose. A graph of β-lapachone dose versus percentage control DNA in fluor units was used to calculate each IC50. All experiments were repeated at least twice, each in duplicate.
Flow Cytometry and DNA Laddering Assays
Cells (1–4 × 106/condition) were treated with or without various concentrations of β-lapachone, topotecan, or camptothecin for various times. Trypsinized or pelleted cells were washed with ice-cold Tris/saline solution [10 mM Tris (pH 7.0) and 50 mM NaCl], fixed in 90% ethanol-Tris/saline, and stored at −4°C. Cells were washed with phosphate-citric acid buffer [0.2 M Na2HPO4 and 0.1 M citric acid (pH 7.8)] and stained with a solution containing 0.2% NP40, RNase A (7000 units/ml), and 33 μg/ml propidium iodide at 4°C for 10 min (19). Stained nuclei were then analyzed for DNA-propidium fluorescence using a Becton Dickinson FACScan (San Jose, CA) at a laser setting of 36 mW and an excitation wavelength of 488 nm. Resulting DNA distributions were then analyzed for proportion of cells in apoptosis, G0/G1, S, and G2/M of the cell cycle (19). Data was analyzed by ModFit (Verity Software House, Inc., Topsham, ME). All experiments were repeated at least three times, each in duplicate.
Cells from the above conditions were also analyzed for the formation of 180–200-bp DNA laddering, which can be diagnostic for certain cells under-going apoptosis (20). Treated and control cells were washed twice with PBS containing 1 mM EDTA at ambient temperature and lysed in 10 mM EDTA, 50 mM Tris-HCl (pH 8.0), 0.5% (w/v) sodium lauryl sarkosinate, and 0.5 mg/ml proteinase K. Cell lysates were then treated with 0.5 mg/ml RNase A for at least 1 h at 37°C and then with 1.0 mg/ml proteinase K at 37°C for at least 1 h. Loading buffer [10 mM EDTA, 1% (w/v) low melting point agarose, 0.25% (w/v) bromophenol blue, and 40% (w/v) sucrose] was then added (10% final concentration), and heated (70°C) samples were loaded onto presolidified, 1.8% (w/v) agarose gels containing 0.1 μg/ml ethidium bromide using end-cut Rainin (Woburn, MA) 1-ml pipette tips to avoid DNA shearing. Agarose gels were run at 65 V/cm for 10 min and then at 15 V/cm overnight in 1× TAE [1.0 M Tris-acetate(pH 7.5) and 10 mM EDTA] running buffer. Gels were photographed and analyzed as described above for Topo I activity measurements.
Results
β-Lapachone-inhibited Purified Human Topo I
Fig. 1A is a representative 0.7% agarose gel that demonstrates the inhibitory effects of various concentrations of β-lapachone compared to camptothecin on Topo I DNA unwinding activity (14, 15). In these reactions, β-lapachone or camptothecin was incubated with the enzyme, and p36B4 supercoiled DNA substrate was added to initiate DNA unwinding reactions. β-Lapachone (which we synthesized and purified) caused partial inhibition (i.e., 20%) of Topo I activity at a concentration of 1.0 nM. Topo I inhibition was not observed at lower concentrations of β-lapachone. At concentrations of 10 nM or greater, β-lapachone effectively inhibited the DNA unwinding reaction of Topo I. An estimated Ki of 25 nM for β-lapachone, where 50% inhibition of Topo I activity was observed, was calculated. Treatment of Topo I with concentrations of 100 nM β-lapachone or greater resulted in >95% inhibition of Topo I DNA unwinding activity with respect to the DMSO control. Topo I inhibition caused by β-lapachone did not cause linearization of the substrate DNA, as found previously (4). In comparison, 100 nM camptothecin resulted in only 16% inhibition of Topo I with respect to the DMSO control, and >1 μM camptothecin was required to achieve ≥60% inhibition of the enzyme under the conditions of this reaction. Thus, β-lapachone was a much more effective Topo I inhibitor than camptothecin when the drugs were incubated with Topo I before the addition of DNA.
When drugs were incubated with the DNA first and reactions initiated by Topo I addition, the effectiveness of Topo I inhibition caused by β-lapachone compared to camptothecin was reversed (Fig. 1B). Camptothecin concentrations of 100 nM or more were effective at inhibiting Topo I, as described previously (7). In contrast, inhibition of Topo I activity by β-lapachone under this sequence of addition was much less effective. Concentrations of 1.0 μM or more were required for measurable inhibition of Topo I DNA unwinding activity (Fig. 1B), and at concentrations higher than 1.0 μM, slight activation of Topo I activity was observed. Thus, the inhibition of Topo I caused by β-lapachone was strictly dependent upon the sequence of addition of the reaction. These data indicated that prior incubation of Topo I with β-lapachone was required to allow binding of the drug to the enzyme for observable inhibition of DNA unwinding activity by Topo I. The reverse was true for camptothecin (Fig. 1). β-Lapachone, therefore, inhibits Topo I by an entirely different mechanism than camptothecin and is a true catalytic inhibitor.
β-Lapachone-induced Apoptosis and G0/G1 Checkpoint Delay in HL-60 Cells
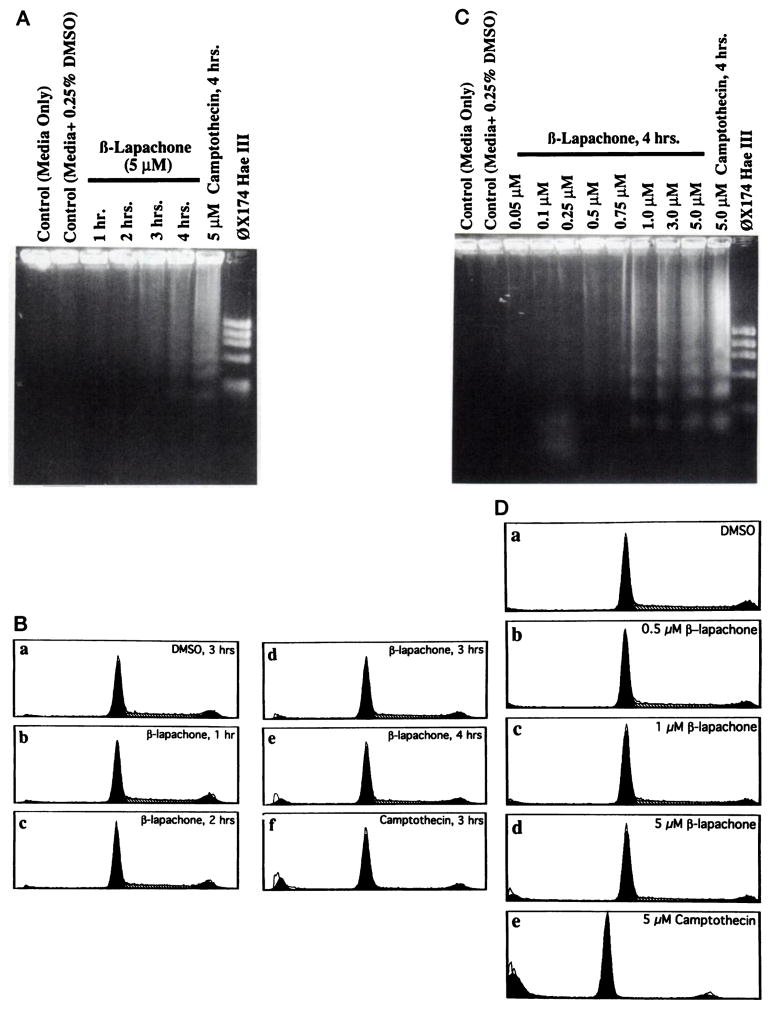
Topo I inhibitors can induce apoptotic responses in certain cells (11, 12). Since β-lapachone inhibited Topo I by a different mechanism than camptothecin or other Topo I inhibitors (Fig. 1; Refs. 4, 7, and 8) and did not result in stabilization of Topo I-DNA complexes observed by SDS-K+ treatments (7, 8), we investigated the ability of β-lapachone to cause apoptosis in human promyelocytic leukemia (HL-60) cells during early times after drug exposure. Log-phase HL-60 cells were exposed to 5 μM β-lapachone for up to 4 h, and cell aliquots were removed at various times. Cells were then analyzed for cell cycle alterations and induction of apoptosis via flow cytometry and agarose gel electrophoresis, respectively (Figs. 2, B and A, respectively). Apoptosis was observed in as little as 2 h, and greater responses were noted in 4 h following a transient 4-h exposure to 5 μM β-lapachone. We, therefore, chose 4-h time points in subsequent experiments as a diagnostic time for the initiation of apoptotic responses. Higher percentages of apoptotic bodies were observed after longer exposure times, and nearly complete responses (i.e., >90% apoptosis) were observed in 24 h (data not shown; Ref. 21). During a 4-h exposure to 5 μM β-lapachone, the population of cells in S and G2/M decreased by 14%, while the population of G0/G1 cells increased by 12%. There was also a noticeable increase in the formation of apoptotic bodies by flow cytometry after a 4-h, 5 μM β-lapachone exposure (by 8%; Fig. 2B). No concomitant increase in apoptotic bodies was observed in DMSO controls (from 0.25% to 1.25%) or after 5 μM lapachol treatment, a compound with a similar structure to β-lapachone but which does not affect Topo I (4). Compared to 5 μM camptothecin (which increased apoptotic bodies by 14% in 4 h), β-lapachone caused much less apoptosis (8.0%), as observed by DNA laddering (Fig. 2A) and flow cytometry (Fig. 2B).
Fig. 2.
Dow-response and temporal kinetics of apoptosis induction in HL-60 cells by β-lapachone. In A and B, log-phase human HL-60 cells were exposed to 5.0 μM β-lapachone or camptothecin and then assayed for apoptotic responses in the first 4 h. A, apoptotic DNA laddering by 1.8% agarose gel electrophoresis; B, cell cycle and apoptotic responses by flow cytometry. Treatment conditions were: a, control; b–e, β-lapachone; or f, camptothecin. In C and D, log-phase HL-60 cells were treated with increasing concentrations of β-lapachone (0.05–5.0 μM) or camptothecin (5.0 μM) for 4 h, and apoptotic responses were noted by 1.8% agarose gel electrophoresis (C) in D and flow cytometry (D) as described in “Materials and Methods.” β-Lapachone induced a G0/G1 block and caused apoptosis in a concentration-dependent fashion. Treatment conditions (4 h at 37°C) in D were: a, 0.25% DMSO-treated control; b, 0.5 μM β-lapachone; c, 1.0 μM β-lapachone; d, 5.0 μM β-lapachone; and e, 5.0 μM camptothecin. In B and D, flow cytometric analyses were generated by computer as described in “Materials and Methods.” Shaded areas, G0/G1 and G2/M cell populations; parallel lines, S-phase cells. Single line tracings are the actual flow cytometric data.
We then tested the effects of various concentrations of β-lapachone on HL-60 cells to find the lowest concentration of drug that induced apoptosis. Exposure of log-phase HL-60 cells to 0.5 μM β-lapachone or higher caused apoptosis (Fig. 2, C and D). At equivalent concentrations (i.e., 5 μM), however, β-lapachone caused much less apoptosis (17%) than camptothecin (31%). Although we noticed some variability in the apoptotic response in 4 h (8–17% with 5 μM β-lapachone), flow cytometric analyses of apoptotic bodies agreed very well with the percentage of cells undergoing the latter steps of apoptosis (i.e., DNA laddering as observed by 1.8% agarose gel electrophoresis; Fig. 2).
β-Lapachone-induced Apoptosis and G0/G1 Checkpoint Delay in Human Prostate Cancer Cells
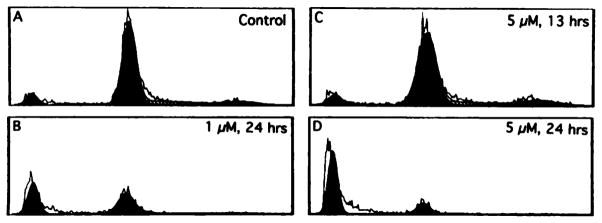
After discovering β-lapachone-mediated induction of apoptosis in HL-60 cells, we investigated β-lapachone-mediated apoptosis in various human prostate and breast cancer cells (Table 1; Fig. 3) following continuous low-dose or transient high-dose exposures. We investigated the cell cycle and apoptotic responses in p53 null, mutant, and wild-type human prostate cancer cells (PC-3, DU-145, and LNCaP, respectively). In each of the human prostate cancer cells examined, apoptosis was observed only after a transient high-dose exposure to β-lapachone. When continuous low doses of β-lapachone were used, very few apoptotic cells were observed by flow cytometry, agarose gel electrophoresis, and Hoescht dye-stained morphological visualization. IC50, values of β-lapachone toxicity on various human breast and prostate cancer cells were calculated (Table 1). Higher β-lapachone concentrations were required to reach IC50, values in human breast cancer cells compared to human prostate cancer cells (Table 1) after continuous low-dose treatments with β-lapachone. In human breast cancer cells, apoptotic responses were not observed in the first 24 h after a transient 4-h 5 μM β-lapachone exposure. A lack of visible apoptosis in human breast cancer cells after β-lapachone exposure may be attributed to longer times required for the induction of apoptosis in these cells or may be due to a protective effect of estrogen (i.e., phenol red was present in our tissue culture medium), as described recently (22). In contrast, very strong apoptotic responses were observed in human prostate cancer cells in 24 h after a 4-h exposure to 5 μM β-lapachone (Fig. 3 shows the results for DU-145). After 24 h, 1.0 μM β-lapachone caused an increase of 38% apoptotic cells compared to the DMSO control. Five μM β-lapachone caused increases of >70% apoptotic cells. However, apoptosis was not observed until 24 h after drug exposure; at 13 h after a 4-h, 5 μM β-lapachone exposure (Fig. 3C), no significant increases in apoptotic cells were noted as compared to the control (Fig. 3A) and the 24-h time point (Fig. 3D). Furthermore, increases in apoptosis appeared to be concentration dependent in human prostate cancer cells, since a 1.0 μM β-lapachone treatment caused less apoptosis than a similar 5.0 μM β-lapachone exposure (Fig. 3, compare B to D). Finally, treatment of human prostate cancer cells with 1–5 μM topotecan caused greater apoptotic responses than equivalent β-lapachone treatments.5 Similar apoptotic responses, in terms of kinetics of apoptosis induction, after β-lapachone or topotecan treatments were observed in all human prostate cancer cells investigated, regardless of p53 status.
Fig. 3.
β-Lapachone causes G0/G1 arrest and subsequent apoptosis in human prostate cancer cells. Log-phase PC-3, DU-145, or LNCaP cells were treated with or without (0.25% DMSO control) 1.0 or 5.0 μM β-lapachone. At various times, cells were analyzed for apoptotic cell populations and for alterations in cell cycle stages by flow cytometry. Shown are DU-145 (mutant p53 prostate cancer) cells, untreated (A) or treated with 1.0 μM(B) or 5.0 μM β-lapachone for 4 h, washed, and examined 13 h (C) or 24 h (D) later. β-Lapachone-treated cells arrested at G0/G1 and underwent apoptosis. Cells treated in a similar fashion with 5.0 μM topotecan (or camptothecin) for 4 h arrested in S but also demonstrated significant apoptotic responses (data not shown). Similar results to that of DU-145 (mutant p53) cells (Fig. 3) were obtained with PC-3 (null p53) and LNCaP (wild-type p53) cells (Table 1).
Discussion
Our results indicate that β-lapachone causes a very strong, specific inhibition of the DNA unwinding enzyme, Topo I. Its inhibitory effects are entirely different than the inhibitory mechanism caused by camptothecin, since the drug is most effective when incubated with the enzyme prior to the addition of supercoiled DNA, the substrate for Topo I. Under this reaction sequence, camptothecin is not very effective as an inhibitor of the enzyme. In contrast, β-lapachone was a much less effective Topo I inhibitor than camptothecin when the reaction sequence was altered by incubating the drugs in the presence of the supercoiled DNA before the addition of Topo I to initiate the reaction. These results are in agreement with our previous results (7) and those of Li et al. (8). They also indicate that β-lapachone inhibits Topo I by direct enzymatic protein binding (i.e., a catalytic inhibitor) in a possible allosteric fashion. We are currently investigating the exact mechanism by which β-lapachone and its derivatives alter Topo I activity.
During our studies, we also discovered that β-lapachone degrades in air, DMSO, and especially in water. We discovered that the β-lapachone available from Ciba-Geigy is roughly two-thirds pure and contains a high molecular weight (mw: 377) degradation byproduct, which has no apparent biological activity. Thus, a severe limitation of β-lapachone is its instability in air and water; the compound is also very water insoluble, which detracts from its clinical utility. On the other hand, the compound is a very active Topo I inhibitor (inhibition observed in vitro by as little as 1.0 nM), which works by a completely different mechanism than camptothecin. The compound does not inhibit Topo I very well unless it is incubated with the enzyme before substrate addition, and β-lapachone does not lead to visible SDS-K+ complexes (7), a fact recently confirmed by Li et al. (8).
Despite differences in the mechanism of Topo I inhibition between β-lapachone and camptothecin, both compounds caused apoptosis in certain human cells. We demonstrated strong apoptotic responses in human HL-60 and in various human prostate cancer cells following transient (4-h) β-lapachone or camptothecin (or topotecan) exposures. Although β-lapachone caused an apparent G0/G1 block in cell cycle progression and camptothecin appeared to freeze cells in S, the overall kinetics of apoptotic responses observed in human cells treated with camptothecin or β-lapachone and the dose-responses for each drug were very similar (Figs. 2 and 3). It is possible that β-lapachone also kills cells entering S (or that are already actively progressing through the cell cycle beyond G0/G1)in a similar fashion to that of camptothecin, giving an apparent G0/G1 block and leading to gradual decreases in the G0/G1 population over time (Fig. 3). It is of interest that in human prostate cancer and HL-60 cells, camptothecin (or topotecan) was a more potent inducer of apoptosis than β-lapachone, although β-lapachone was a much more effective Topo I inhibitor in vitro.
β-Lapachone or topotecan (or camptothecin) did not appear to cause apoptosis in human breast cancer cells in 24 h, as observed by flow cytometry, agarose gel electrophoresis, or morphological analyses.6 We are currently investigating the possibility that human breast cancer cells may undergo apoptosis after longer periods of time, as demonstrated with human epithelial cells (20). Alternatively, the phenol red and/or FBS present in the media during our drug exposures could have played a protective role in preventing apoptosis in human breast cancer cells, as described recently (22). Finally, the apoptotic responses induced by β-lapachone or camptothecin (or topotecan) were not dependent upon p53 status, since null (i.e., PC-3 and HL-60), mutant (DU-145), or wild-type (LNCaP) human cells demonstrated similar apoptotic responses after transient drug exposures. Interestingly, β-lapachone exposure to p53 wild-type cells (LNCaP or MCF-7:WS8) did not result in increases in nuclear p53 protein levels, as observed with similar equitoxic doses of camptothecin.6 We are currently investigating differences in signaling pathways and intracellular protein determinants of the apoptotic responses observed after β-lapachone compared to camptothecin (or topotecan) treatments.
Our data, as well as those of Li et al. (21), indicate that the induction of apoptosis by β-lapachone may be a contributing factor in its ability to act as an enhancer of radiation or chemotherapy (4–7). These data further indicate a very strong antitumor effect against human prostate cancer cells, in general. This dramatic response of human prostate cancers to β-lapachone (or topotecan) may be due to reported elevations of Topo I in these cancer cells (10), which may act to cause enzyme-mediated DNA damage and specific apoptotic responses in a tumor-selective manner.
Acknowledgments
We thank Dr. Thomas W. Davis for his helpful discussions. We are grateful to Drs. V. Craig Jordan and John Pink for providing human breast cancer cells. Mass spectra were carried out at the Bender Chemical Instrumentation Center, University of Wisconsin-Madison.
Footnotes
This work was funded by grants from the Wisconsin Alumni Research Foundation (to D. A. B., D. T. W., and B. F.), the University of Wisconsin Comprehensive Cancer Center (to D. A. B. and B. F.), and the CapCure Foundation (to G. W.).
The abbreviations used are: Topo I, topoisomerase I; FBS, fetal bovine serum.
D. Church, S. M. Planchon, S. Wuerzburger, D. A. Boothman, and G. Wilding, unpublished data.
S. Wuerzberger, S. M. Planchon, and D. A. Boothman, unpublished data.
References
- 1.Thompson RH. Napthoquinones. New York: Academic Press; 1971. Naturally occurring quinones; pp. 198–366. [Google Scholar]
- 2.Docampo R, Lopes JN, Cruz FS, DeSouza W. Trypanosoma cruz: ultrastructural and metabolic alterations of epimastigotes by β-lapachone. Exp Parasitol. 1977;42:142–149. doi: 10.1016/0014-4894(77)90071-6. [DOI] [PubMed] [Google Scholar]
- 3.Docampo R, Cruz FS, Boveris A, Muniz RPA, Esquivel DMS. Lipid peroxide in β-lapachone-treated Trypanosoma cruz epimastigotes. Arch Biochem Biophys. 1978;186:292–297. doi: 10.1016/0003-9861(78)90438-1. [DOI] [PubMed] [Google Scholar]
- 4.Boothman DA, Trask DK, Pardee AB. Inhibition of potentially lethal DNA damage repair in human tumor cells by β-lapachone, an activator of topoisomerase I. Cancer Res. 1989;49:605–612. [PubMed] [Google Scholar]
- 5.Boothman DA, Greer S, Pardee AB. Potentiation of halogenated pyrimidine radiosensitizers in human carcinoma cells by β-lapachone (3,4-dihydro-2,2-dimethyl-2H-naphtho[1,2-b]pyran-5,6-dione), a novel DNA repair inhibitor. Cancer Res. 1987;47:5361–5367. [PubMed] [Google Scholar]
- 6.Boothman DA. Enhanced malignant transformation is accompanied by increased survival recovery following ionizing radiation. Radiat Res. 1994;138:121–125. [PubMed] [Google Scholar]
- 7.Boothman DA, Wang M, Schea R, Burrows HL, Strickfaden S, Owens JK. Posttreatment exposure to camptothecin enhances the lethal effects of X-rays on radioresistant human malignant melanoma cells. Int J Radiat Oncol Biol Phys. 1992;24:939–948. doi: 10.1016/0360-3016(92)90478-z. [DOI] [PubMed] [Google Scholar]
- 8.Li CJ, Averboukh L, Pardee AB. β-Lapachone, a novel topoisomerase I inhibitor with a mode of action different from camptothecin. J Biol Chem. 1993;268:22463–22468. [PubMed] [Google Scholar]
- 9.Crespi MD, Mladovan AG, Baldi A. Increment of DNA topoisomerases in chemically and virally transformed cells. Exp Cell Res. 1988;175:206–215. doi: 10.1016/0014-4827(88)90267-4. [DOI] [PubMed] [Google Scholar]
- 10.Hussain I, Mohler JL, Seigler HF, Besterman JM. Elevation of topoisomerase I messenger RNA, protein, and catalytic activity in human tumors: demonstration of tumor-type specificity and implications for cancer chemotherapy. Cancer Res. 1994;54:539–546. [PubMed] [Google Scholar]
- 11.Solary E, Bertrand R, Kohn KW, Pommier Y. Differential induction of apoptosis in undifferentiated and differentiated HL-60 cells by DNA topoisomerase I and II inhibitors. Blood. 1993;81:1359–1368. [PubMed] [Google Scholar]
- 12.Kamesaki S, Kamesaki H, Jorgensen TJ, Tanizawa A, Pommier Y, Cossman J. bcl-2 protein inhibits etoposide-induced apoptosis through its effects on events subsequent to topoisomerase II-induced DNA strand breaks and their repair. Cancer Res. 1993;53:4251–4256. [PubMed] [Google Scholar]
- 13.Hooker SC. The constitution of lapachic acid (lapachol) and synthesis of β-lapachone. J Chem Soc. 1892:611–650. [Google Scholar]
- 14.Hsiang Y-H, Hertzberg R, Hecht S, Liu LF. Camptothecin induces protein-linked DNA breaks via mammalian DNA topoisomerase I. J Biol Chem. 1985;260:14873–14878. [PubMed] [Google Scholar]
- 15.Boothman DA, Fukunaga N, Wang M. Down-regulation of topoisomerase I in mammalian cells following ionizing radiation. Cancer Res. 1994;54:4618–4626. [PubMed] [Google Scholar]
- 16.Stone KR, Mickey DD, Wunderli H, Mickey GH, Paulson DF. Isolation of a human prostate carcinoma cell line (DU-145) Int J Cancer. 1978;21:274–281. doi: 10.1002/ijc.2910210305. [DOI] [PubMed] [Google Scholar]
- 17.Horoszewisz JS, Leong SS, Kawinski E, Karr JP, Rosenthal H, Chu TM, Mirand EA, Murphy GP. LNCaP model of human prostatic carcinoma. Cancer Res. 1983;43:1809–1818. [PubMed] [Google Scholar]
- 18.Rago R, Mitchen J, Wilding G. DNA fluorometric assay in 96-well tissue culture plates using Hoescht 33258 after cell lysis by freezing in distilled water. Anal Biochem. 1990;191:31–34. doi: 10.1016/0003-2697(90)90382-j. [DOI] [PubMed] [Google Scholar]
- 19.Fried J, Perez AJ, Clarkson BD. Rapid hypotonic method for flow cytofluorometry of monolayer cell cultures: some pitfalls in staining and data analysis. J Histochem Cytochem. 1978;26:921–933. doi: 10.1177/26.11.82573. [DOI] [PubMed] [Google Scholar]
- 20.Smith CA, Williams GT, Kingston R, Jenkinson EJ, Owens JJT. Antibodies to CD 3/T-cell receptor complex induced death by apoptosis in immature T cells in thymic cultures. Nature (Lond) 1989;337:181–184. doi: 10.1038/337181a0. [DOI] [PubMed] [Google Scholar]
- 21.Li CJ, Wang C, Pardee AB. Induction of apoptosis by β-lapachone in human prostate cancer cells. Cancer Res. 1995;55:3712–3715. [PubMed] [Google Scholar]
- 22.Wang TTY, Phang JM. Effects of estrogen on apoptotic pathways in human breast cancer cell line MCF-7. Cancer Res. 1995;55:2487–2489. [PubMed] [Google Scholar]