Abstract
Calcium-induced calcium release is the principal mechanism that triggers the cell-wide [Ca2+]i transient that activates muscle contraction during cardiac excitation-contraction coupling (ECC). Here, we characterize this process in mouse cardiac myocytes with a novel mathematical action potential (AP) model that incorporates realistic stochastic gating of voltage-dependent L-type calcium (Ca2+) channels (LCCs) and sarcoplasmic reticulum (SR) Ca2+ release channels (the ryanodine receptors, RyR2s). Depolarization of the sarcolemma during an AP stochastically activates the LCCs elevating subspace [Ca2+] within each of the cell’s 20,000 independent calcium release units (CRUs) to trigger local RyR2 opening and initiate Ca2+ sparks, the fundamental unit of triggered Ca2+ release. Synchronization of Ca2+ sparks during systole depends on the nearly uniform cellular activation of LCCs and the likelihood of local LCC openings triggering local Ca2+ sparks (ECC fidelity). The detailed design and true SR Ca2+ pump/leak balance displayed by our model permits investigation of ECC fidelity and Ca2+ spark fi-delity, the balance between visible (Ca2+ spark) and invisible (Ca2+ quark/sub-spark) SR Ca2+ release events. Excess SR Ca2+ leak is examined as a disease mechanism in the context of “catecholaminergic polymorphic ventricular tachycardia (CPVT)”, a Ca2+-dependent arrhythmia. We find that that RyR2s (and therefore Ca2+ sparks) are relatively insensitive to LCC openings across a wide range of membrane potentials; and that key differences exist between Ca2+ sparks evoked during quiescence, diastole, and systole. The enhanced RyR2 [Ca2+]i sensitivity during CPVT leads to increased Ca2+ spark fidelity resulting in asynchronous systolic Ca2+ spark activity. It also produces increased diastolic SR Ca2+ leak with some prolonged Ca2+ sparks that at times become “metastable” and fail to efficiently terminate. There is a huge margin of safety for stable Ca2+ handling within the cell and this novel mechanistic model provides insight into the molecular signaling characteristics that help maintain overall Ca2+ stability even under the conditions of high SR Ca2+ leak during CPVT. Finally, this model should provide tools for investigators to examine normal and pathological Ca2+ signaling characteristics in the heart.
Keywords: Local-control, Mouse, Computational model, CPVT, Action potential, Calcium leak
1. Introduction
The coupling of electrical stimuli to cardiac contraction requires the synchronous release of calcium (Ca2+) from thousands of Ca2+ release units (CRUs) across each cardiac myocyte. Local control of Ca2+ release at individual CRUs is an important feature of excitation-contraction coupling (ECC) that is required for the “graded” release of sarcoplasmic reticulum (SR) Ca2+ during systole [1,2]. Each CRU is composed of a number of (~6) L-type Ca2+ channels (LCCs) within the sarcolemmal (SL) transverse tubule (TT) membrane or exterior SL membrane that are positioned across a dyadic “subspace” (~15 nm) from a para-crystalline array of (~10–300) type 2 ryanodine receptors (RyR2) within the junctional SR (JSR) membrane [3,4]. During systole, the action potential (AP) depolarization of the sarcolemma (including the TTs) activates the LCC current which elevates local (i.e., subspace) Ca2+ to activate and open the clustered RyR2s in the JSR to produce a “triggered” Ca2+ spark. The Ca2+ spark is the fundamental unit of Ca2+-induced Ca2+-release (CICR) at a given CRU. The signal process is a high-gain positive feedback mechanism, which can operate with great stability due to the local Ca2+ signaling. It is the stochastic recruitment of triggered Ca2+ sparks that translates the electrical AP to the [Ca2+]i transient and the cardiac contraction during ECC. Despite over 20 years of experimental and computational study, questions still abound regarding quantitative details of Ca2+ sparks and CICR dynamics at individual CRUs during systole and diastole.
Many mathematical models have been developed to investigate the mechanisms governing CICR and ECC. However, to our knowledge, a fully stochastic, local control computational model of mouse AP dynamics with a realistic CRU formulation and physiological SR Ca2+ pump/ leak balance has not been produced, until now. We have constrained the biophysical parameters of our model to match those of the mouse ventricular myocyte, as the mouse is by far the most commonly studied animal in biological research. To date, one of the most influential mouse cardiomyocyte models was developed by Bondarenko and colleagues in 2004 [5]. Their model has been adapted by numerous groups to study the molecular regulation of ECC, myosin cross-bridge cycling and contraction, as well as whole-heart function [6–11]. While these modeling efforts have provided exciting new data, they contain two critical features, common in many cardiac Ca2+ signaling models, that limit their mechanistic relevance: 1) They use a single “local [Ca2+]i” level that is identical for all RyR2s and LCCs (i.e., common pool) [5,12–22]. 2) They use non-physiological RyR2 gating schemes [23–25] or terminate Ca2+ release from the SR using a non-physiological [Ca2+]i-dependent inactivation scheme for RyR2 [2,21,26–32]. These two features are utilized in many cardiomyocyte computational models across species to either alleviate computational demand, enable Ca2+ spark termination, or both. We find that under normal conditions, using a physiological description of RyR2 gating, depletion of the [Ca2+]jsr promotes the stochastic termination of Ca2+ sparks in a realistic timespan [33,34]. The model presented here does not contain either of these two non-physiological features; and thus, our mathematical description of cardiac ECC is the most mechanistic to date.
Here, we advance our previous computational model formulation presented in Williams et al., 2011 from a local control, quiescent cardio-myocyte model with physiological RyR2 gating and realistic local Ca2+ signals to a full ECC model with systolic Ca2+ signaling and robust AP generation. We have previously shown how three SR Ca2+ leak pathways (Ca2+ sparks, Ca2+ quarks, and rogue RyR2s) combine to balance the activity of the SR Ca2+-ATPase (SERCA2a) during prolonged periods of diastole, or quiescent conditions [34]. During diastole, spontaneous Ca2+ sparks form the primary pathway for SR Ca2+ leak. Additional Ca2+ release occurs through “non-spark” events that represent the opening of single RyR2s (i.e., Ca2+ quarks) or the opening of only a few RyR2s (i.e., Ca2+ sub-sparks) which fail to induce a full Ca2+ spark and are invisible by conventional confocal imaging modalities [35]. There is also a small population of RyR2s distributed sporadically throughout the network SR, known as “rogue” RyR2s, that form a third route for SR Ca2+ leak [4,36,37]. SR Ca2+ pump/leak balance is a critical feature of cardiomyocyte Ca2+ homeostasis required for normal muscle function and overall survival. Realistic SR Ca2+ leak/pump dynamics gives the model significant relevance when studying pathological conditions related to changes in SR Ca2+ release (i.e., high “leak” conditions) or elevated SR Ca2+ content (i.e. load).
The pathophysiological Ca2+ signaling that arises from “leaky” RyR2s has been linked to diverse cardiac pathologies including catechol-aminergic polymorphic ventricular tachycardia (CPVT), an inherited ar-rhythmogenic disorder characterized by syncope and sudden cardiac death. While specific mutations may confer different pathologies in CPVT (e.g., autosomal dominant RyR2 mutations and autosomal recessive calmodulin, triadin, and calsequestrin (CASQ2) mutations), the development of overly sensitive RyR2s (assuming identical SR Ca2+ load) that display increased PO appears to be a common theme in CPVT [38]. The model presented here is uniquely capable of investigating abnormal Ca2+ leak dynamics such as this because of the mechanistic formulation of Ca2+ sparks and SR Ca2+ leak it contains. Here we conduct a quantitative assessment of quiescent, diastolic, and systolic Ca2+ sparks and “invisible” Ca2+ leak pathways in the environment of enhanced RyR2 PO and reduced JSR buffering, designed to simulate a genetic “knock-in” murine model of CASQ2-R33Q driven CPVT [39]. Our findings support those previously identified experimentally for the basal differences between wild-type (WT) and CASQ2-R33Q mutant cardiomyocytes (i.e., decreased amplitude of global [Ca2+]i transients at slow, 0.2 Hz, pacing and decreased total SR Ca2+ content in CPVT myocytes) [40–43]. Our model identifies new critical differences in local calcium signals that may ultimately serve as the substrate for cellular arrhythmia (Ca2+ waves) and the rare, but deadly arrhythmia, CPVT.
The physiological, mathematical model of ECC presented here provides novel, important findings that change our understanding of Ca2+ signaling at both the local and global (cell-wide) levels. One key achievement in this model is a novel formulation for the LCC that is sensitive to changes in local Ca2+, a critical feature necessary for local control of CICR. Important new findings of this model include the following: 1) Quiescent, diastolic, and systolic Ca2+ sparks differ in a number of ways including frequency and amplitude; 2) Quantitative details describing the relative insensitivity of RyR2s (and, therefore, Ca2+ sparks) to LCC openings; 3) Simulated CPVT mutations lead to prolonged and at times, metastable Ca2+ sparks that increase the probability of activating neighboring CRUs; and 4) Ca2+ spark fidelity is significantly altered during CPVT and results in asynchronous systolic Ca2+ spark activity and increased diastolic SR Ca2+ leak. This model accounts for how local Ca2+ and realistic SR Ca2+ pump/leak balance influences global Ca2+ dynamics during ECC. These features offer a critical step toward development of a realistic whole cardiomyocyte spatial model of ECC.
1.1. Model formulation
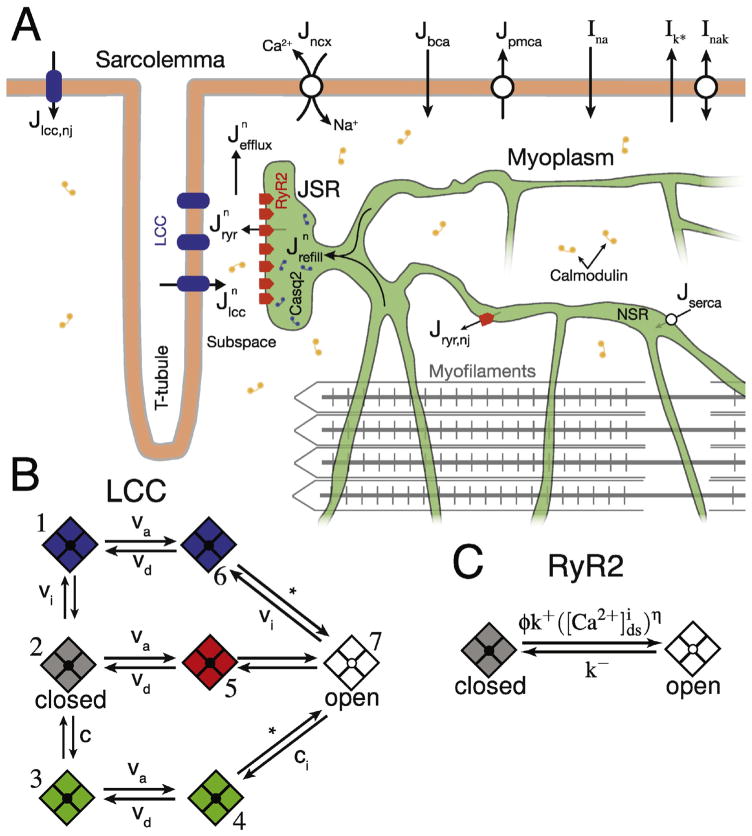
Here, we present a whole-cell local control, Monte Carlo model of ECC in the mouse cardiac ventricular myocyte. The description of CICR from Williams et al. has been modified to include a novel stochastic model for LCCs that completes the physiologic CRU [34]. The stochastic RyR2 formulation has been updated to integrate the most recent biophysical characterization of the channel. Additionally, key SL membrane channels and transporters (see Fig. 1 A) have been incorporated into the model to facilitate robust AP generation. The resulting compartmental model of a mouse ventricular myocyte has numerous independent CRUs (i.e., N = 20,000), each containing multiple stochastically gated LCCs and RyR2s (i.e., NL = 6 and NR = 50, respectively) that are instantaneously coupled (see Eq. (S56)) to a local, dyadic subspace [Ca2+] ([Ca2+]ds) [34]. Each CRU is associated with an individual junctional SR (JSR) compartment that is depleted during its own CICR and is refilled through transport from the network SR (NSR) compartment. Changes in bulk myoplasmic [Ca2+] ([Ca2+]i) are generated via efflux of Ca2+ from each individual CRU subspace compartment (see in Fig. 1 A). The model contains physiological representations of SERCA2a (Eq. (S35)), Na+/Ca2+ exchanger (NCX) (Eq. (S37)), and the plasma membrane Ca2+ ATPase (PMCA) (Eq. (S38)) to facilitate SR Ca2+ re-uptake and myoplasmic Ca2+ extrusion. For this study, we assume no [Ca2+]i buffering by the mitochondria and do not include a compartment for this organelle nor formulations for the associated Ca2+ handling proteins. Recent studies have shown that under physiological conditions there is minimal mitochondrial contribution to direct changes in [Ca2+]i signals [44–47]. The Monte Carlo model presented here consists of 2N + 20 ordinary differential equations (ODEs) representing the time-evolution of membrane voltage (V), various in-tracellular ion concentrations (i.e., [Ca2+], [K+], [Na+]), and membrane current gating variables (see Eqs. (S9)–(S17)). The concentration balance equations, consistent with Fig. 1, can be found in the SI (Eqs. (S2)–(S7)). Each ODE was solved in Matlab using the first-order Euler method with variable time-step designed to ensure stability. A mathematical description of critical model components is presented below and includes: LCC, RyR2, SL membrane potential, and spatial sarcomere model.
Fig. 1.
Diagram of ECC model and Ca2+ channel models. (A) Model compartments, Ca2+ fluxes, and various membrane currents (black arrows). Only one of the cell’s N = 20,000 CRUs is shown for visual clarity and IK+ represents all K+ currents described fully in Supporting Materials. (B) Transition-state diagram for the 7-state continuous-time, discrete-state Markov chain describing a single LCC. Transitions labeled as; va represent voltage-dependent activation transitions, vi represent voltage-dependent inactivation transitions, vd represent voltage-dependent deactivation transitions, and ci represent Ca2+-dependent inactivation transitions (see SI for formulation of these rates). (C) Transition-state diagram for the 2-state continuous-time, discrete-state Markov chain representing a single RyR2 channel. Note, each CRU consists of 6 LCCs and 50 RyR2s.
1.1.1. LCC model
While numerous LCC models exist [14,25,29,48–52], few are capable of generating realistic LCC current in a local control model. Specifically, the dynamics of Ca2+-dependent inactivation must be modified when an LCC is experiencing realistic local Ca2+ concentrations (i.e., [Ca2+]ds). We developed a novel LCC formulation where each voltage activated LCC is represented by a 7-state Markov chain containing six inactivated or closed states and one open state (state 7 represented in white in Fig. 1B). Voltage-dependent inactivation (VDI) is achieved by transitions from either the open state (state 7) or the closed state (state 2) to the VDI states (states 1 and 6) highlighted in blue (see Fig. 1B). Similarly, Ca2+-dependent inactivation (CDI) is modeled by transitions from either the open state or the closed state to the CDI states (states 3 and 4) highlighted in green (see Fig. 1B). State 5 highlighted in red (see Fig. 1B) represents a transient “activated” (but not open) state designed to achieve physiological LCC current rise times. Fig. S2 A shows that the model generates a realistic IV curve peaking between 0 and 10 mV, consistent with experimental results [53]. The LCC dynamics in response to both voltage- and Ca2+-dependent inactivation is displayed in Fig. S2B. While similar in number of states to Mahajan et al. [25], our novel description of transition rates between LCC states facilitates our ability to model local control of ECC by: 1) making the channels sensitive to realistic [Ca2+]ds, and 2) permitting the use of a our vectorized channel gating approach to solving stochastic channels [54]. The second point is critical in order to reduce the computational demand of six 7-state LCCs present in each of our N = 20,000 CRUs. Junctional and non-junctional LCC formulations and the kinetics for LCC transitions can be found in the SI (see Eqs. (S25)–(S34) and Table S3).
1.1.2. RyR2 model
Recent work in lipid-bilayers by Michael Fill and co-workers [39] as well as others [55–57] has shown that under “near-physiological” conditions (e.g., Mg2+ and SR Ca2+) RyR2s are much less sensitive to Ca2+ than previously thought. We have modified our RyR2 model (depicted in Fig. 1C) to account for these updated experimental characterizations of RyR2 Ca2+ sensitivity. Our current RyR2 gating leads to a EC50 of ~33 μM compared to our earlier work ([34]) where RyR2 EC50 was closer to 10 μM. This change in RyR2 [Ca2+]i sensitivity has allowed us to remove the allosteric coupling factors previously required to “quiet” the RyR2 channel gating under quiescent conditions. This assumption does not preclude a physical connection between neighboring RyR2s, but simply does not assert that this physical connection is the basis for propagating conformational changes between RyR2s. Additionally, we have updated our luminal Ca2+ sensitivity function, ϕ (see Eq. (S45) and Walker et al., 2014 [58]), which reflects the most recent biophysical characterizations of RyR2 gating with varied luminal Ca2+ levels. With normal or reduced SR Ca2+ content ϕ has minimal effect on RyR2 PO, however, during SR Ca2+ overload ϕ results in a drastic increase in RyR2 PO, see Fig. S1A. A complete description of the junctional and non-junctional RyR2 formulations can be found in the SI (Eqs. (S43)–(S47)). The Ca2+ spark dynamics (e.g., duration, amplitude, frequency, and fidelity) shown in Fig. 2, when using the modified (ϕ) and without inter-channel coupling remain remarkably similar to those shown in previous work [34]. Taken together, our LCC and RyR2 formulations are able to reproduce the graded nature of RyR2 flux, a hallmark of “local control” of CICR, in relation to membrane potential and high ECC gain (~20 fold at V = 0 mV) as measured by peak RyR2 flux divided by peak LCC flux (see Fig. S2C and D) [59].
Fig. 2.
Quiescent Ca2+ spark and non-spark Ca2+ leak dynamics. Shown are spontaneous Ca2+ sparks (large events), Ca2+ quarks (single RyR2 events), and Ca2+ sub-sparks (multi-RyR2 events). Each color represents the behavior from a unique CRU within the whole-cell model. Only 10% of the cell’s 20,000 CRUs are shown (for clarity). See Fig. S2 for and [Ca2+]jsr dynamics. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
The RyR2 gating parameters for both WT and CPVT conditions are given in Table 1, where k+ is the RyR2 opening rate, k− is the RyR2 closing rate, KM is the half maximal point for RyR2 PO as a function of [Ca2+]i, and βjsr is the total concentration of buffer within the JSR. The RyR2 steady-state open probability in response to changes in [Ca2+]i for WT and CPVT simulations is shown in Fig. S1B. The effect of decreased k− and slight rise in k+ on RyR2 PO is consistent with the ~2 fold reduction in Km caused by CASQ2-R33Q mutation examined in [39]. The 57% reduction in βjsr is designed to simulate the altered CASQ2 expression seen in CASQ-R33Q knock-in mice [41].
Table 1.
RyR2 gating dynamics and JSR buffering under normal and pathological conditions.
| k+ (s−1 μM−η,ryr) | k− (s−1) | RyR2 Km (μM) | βjsr (mM) | |
|---|---|---|---|---|
| WT | 0.2 | 425 | 33 | 30 |
| CPVT | 0.275 | 120 | 16 | 13 |
1.1.3. Sarcolemmal membrane potential
In this study, two forms of electrical stimuli are utilized to trigger LCC opening and CICR, “voltage clamp” and “current clamp”. The “voltage clamp” protocol forces a step change in SL membrane potential while a small inward current, Iapp, is applied in the “current clamp” protocol to elicit an action potential. The SL membrane potential during “current-clamp” mode is governed by a single ODE (Eq. (S1)). The formulation of the K+ currents used here can be found in the SI (see Eqs. (S12)–(S22)) and are primarily based on the currents from the mouse action potential model by Bondarenko and co-workers [5]. The fast Na+ current is adapted from [60] and is also outlined in the SI (see Eqs. (S9)–(S11), (S22), and (S23)).
1.1.4. Spatial sarcomere model
To further elucidate the physiologic consequences of “leaky” RyR2s we developed a 3 dimensional (3D) spatial implementation of our local control model that represents a transverse subsection of a cardio-myocyte (see Eq. (S57)). This spatial model represents a single sarco-mere (M-line to M-line) centered on a Z-line and contains equally distributed CRUs, separated from one another by 600 nm [61]. Linescan images were generated by assessing levels of Ca2+-bound Fluo4 concentration [CaF] (see Eq. (S58)) over time with optical blurring and Gaussian white noise added afterwards, consistent with previous work by Smith et al. [62]. While a whole cell 3D spatial model integrating 20,000 CRUs would be ideal, it is beyond the scope of the current work, however the model presented here could serve as the backbone of such a model in the future.
2. Results
2.1. Quiescent Ca2+ Sparks and SR Ca2+ Leak
Ca2+ leak dynamics, at physiological RyR2 sensitivity, are shown in Fig. 2 for quiescent conditions (prolonged rest/diastole) and include experimentally visible Ca2+ sparks and invisible Ca2+ sub-sparks and Ca2+ quarks. The Ca2+ sparks shown in Fig. 2 are consistent with quiescent Ca2+ sparks observed in small rodents in both frequency (~ 150 cell−1 s−1) and duration (~20 ms). Note that Ca2+ spark duration is measured by the duration of the number of open RyR2s within a CRU (NR,O, see Fig. S3). Prior work has shown that RyR2 currents of this duration yield F/F0 signals consistent with experimental recordings [33,62]. We observe here, and previously (see [34]), that Ca2+ quarks occasionally lead to Ca2+ sparks via the regenerative nature of CICR. Note that these Ca2+ quarks while frequent are very brief (~2–5 ms) and account for a small fraction of SR Ca2+ leak under normal SR Ca2+ load conditions (see [34]). While a Ca2+ quark represents the opening of a single RyR2 we observe that sometimes multiple RyR2s, open but fail to trigger a Ca2+ spark, we term these events, “Ca2+ sub-sparks”. This detailed, molecular mechanism for SR Ca2+ leak gives this model of ECC increased relevance for studying local regulation of Ca2+ signaling as well as pathophysiological modulation of Ca2+ dynamics.
2.1.1. Action potential and global CICR dynamics
The mouse action potential, shown in Fig. 3A, is more effective at recruiting LCC current, shown in Fig. 3B, than a voltage clamp to 0 mV. This surprising result is due to the combination of the rapid upstroke of the AP (activating the voltage-activated states of the LCC) followed by the rapid repolarization (due to the outward K+ currents) which increases the driving force for Ca2+ entry [63]. The time to peak for the [Ca2+]i transients during current clamp is influenced by the rapid recruitment of LCC current during an AP (see Fig. 3B). Despite synchronous and uniform exposure of each LCC within the model to the same membrane potential, not all CRUs activate to trigger a Ca2+ spark during systole. The subsection on ECC fidelity, the probability that an LCC opening will activate CICR and trigger a Ca2+ spark, investigates this phenomenon in greater detail. The membrane dynamics and the underlying membrane currents for our mouse AP formulation are depicted in Fig. S7 and are consistent with Bondarenko et al. [5]. The resulting AP has an APD90 (time to 90% recovery of depolarization) of ~40 ms, which is consistent with whole cell ruptured patch clamp and micro-electrode recordings of mouse APs [64,65]. Additional [Ca2+]i and AP dynamics are shown in Figs. S7, S10, and S11. Global Ca2+ dynamics in response to both current- (Fig. 3C–D) and voltage-clamp (Fig. 3E–F) conditions highlight key differences of these excitation methods. The dashed lines represent the initial [Ca2+]i transient after a prolonged period of quiescence. The SR content is equivalent between the current-and voltage-clamp protocols. While [Ca2+]nsr only depletes moderately (15–25%) during an AP, the fraction of total SR Ca2+ released (~50% during an AP) is greater due to the strong depletion of [Ca2+]jsr (see Fig. S3) and significant buffering (βjsr) present in the JSR compartment (see Table 1). [Ca2+]i and [Ca2+]nsr dynamics shown in Fig. 3C–F are provided for both simulated WT and CPVT conditions, using gating parameters and total JSR buffering, described in Table 1. Consistent with high ECC gain observed experimentally, during a single AP, ECC gain is 12–25 depending on SR Ca2+ content.
Fig. 3.
Global [Ca2+]i dynamics during ECC. Shown is A) membrane potential and B) respective whole-cell LCC currents, where solid black are representative results during current-clamp conditions following an applied current to generate an AP and dashed is representative of a voltage-clamp stimulus to 0 mV. C) [Ca2+]i transients and D) NSR Ca2+ dynamics shown for current-clamp stimulus. E) [Ca2+]i transients and F) NSR Ca2+ dynamics shown for voltage-clamp stimulus. For C–F, blue traces represent WT conditions while red traces depict CPVT. Additionally, solid lines represent [Ca2+]i and [Ca2+]nsr dynamics at steady-state while dashed traces represent the first transient after quiescence. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
2.1.2. Ca2+ sparks and SR Ca2+ leak during ECC
Systolic and diastolic Ca2+ sparks (and Ca2+ quarks) elicited by LCC openings during (and following) an AP are shown in Fig. 4. Each color indicates activity from a different CRU within the 20,000 CRU compartmental model. The bulk cytosol and NSR dynamics displayed in Fig. 3 result from the integration of local Ca2+ signals at the dyadic subspace and JSR level, see Fig. 4, synchronized during the AP interval (indicated by the blue bar between 10 and 50 ms). We show that at steady-state, 1 Hz pacing, ~7000 Ca2+ sparks are recruited during an action potential, or roughly 35% of the CRUs within a cardiomyocyte are activated for a given electrical stimulus. Systolic Ca2+ sparks displayed in Fig. 4 are roughly the same amplitude and duration as quiescent Ca2+ sparks (see Fig. 2), even though they are initiated at a much lower SR Ca2+ content, [Ca2+]nsr ~ 700 μM at 1 Hz pacing vs. [Ca2+]nsr ~ 950 μM during quiescence. Further comparison of systolic Ca2+ release and quiescent Ca2+ leak as functions of [Ca2+]nsr can be found in the subsection, Dependence of spontaneous and triggered SR Ca2+ release on SR Ca2+ content. Interestingly, diastolic Ca2+ sparks, shown in Fig. 4, are less frequent and noticeably smaller (with a peak [Ca2+]ds b 100 μM due to lower SR Ca2+ content, see Fig. 3D) than Ca2+ sparks displayed during quiescent conditions (see Fig. 2). See Restitution of Ca2+ spark fidelity subsection below for analysis of how Ca2+ sparks and Ca2+ quark dynamics “restore” following an AP.
Fig. 4.
Systolic and diastolic Ca2+ spark and non-spark Ca2+ release dynamics during an AP (similar to the solid blue line in Fig. 3A). Each colored line represents the behavior from a different CRU within the whole-cell model. Only 10% of the cell’s 20,000 CRUs are shown (for clarity in the display). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
2.1.3. Implications of leaky RyR2s on CICR
The influence of leaky RyR2s, a hallmark of CPVT [66], on Ca2+ sparks, Ca2+ quarks, SR Ca2+, and systolic [Ca2+]i transients is shown in Fig. 5 and S6. Fig. 5A shows Ca2+ spark and Ca2+ quark dynamics of the model during quiescence under simulated CPVT conditions. These “leaky” RyR2s result in longer Ca2+ sparks (~50 ms). In some cases, Ca2+ sparks fail to fully terminate and even attempt to reactivate (see thick purple line in Fig. 5 A). These “unstable” Ca2+ release events are reminiscent of the “metastable” Ca2+ sparks described by Stern et al., 2013 [67]. While each quiescent Ca2+ spark is smaller in peak [Ca2+]ds (due to lower [Ca2+]nsr) they more than make up for it in both increased frequency (~500 Ca2+ sparks cell−1 s−1) and duration. If only RyR2 PO is altered, see Fig. S4, we again see “unstable” Ca2+ sparks as well as increased Ca2+ spark frequency, while Ca2+ spark frequency and termination are normal with lower JSR buffering alone, see Fig. S5. To better visualize these “metastable” Ca2+ sparks we incorporated our CRU formulation into a 3D model of Ca2+ diffusion within a single sarcomere (centered on z-line). A representative simulated linescan shown in Fig. 6 (see SI for more details) dramatically shows the resulting unstable CICR during simulated CPVT conditions. A representative linescan for normal, WT conditions is shown in Fig. S9.
Fig. 5.
CPVT Ca2+ spark and Ca2+ leak dynamics. Influence of increased RyR2 open probability and reduced JSR buffering on Ca2+ dynamics during (A) quiescent conditions (see Fig. S5 for and [Ca2+]jsr dynamics) and (B) ECC. Each color represents activity from a different CRU within the whole-cell model. For clarity only 10% of the cell’s 20,000 CRUs are shown. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Fig. 6.
Pro-arrhythmic Ca2+ release behavior during CPVT. A) Stochastic RyR2 gating during quiescent, CPVT conditions and B) simulated transverse line-scan of quiescent Ca2+ release dynamics (as F/Fo) during CPVT conditions. Realistic noise and confocal blurring are added after simulation (see SI).
In addition to altered quiescent Ca2+ spark and Ca2+ leak dynamics observed under CPVT conditions (see Fig. 5A), we have examined the influence of CPVT channels on CICR dynamics during ECC (see Fig. 3 and Fig. 5B). After prolonged quiescence, WT [Ca2+]i transient amplitude is greater than CPVT (see Fig. 3C and E), similar to what was shown experimentally at 0.2 Hz pacing frequency of CASQ-R33Q knock-in cardiomyocytes [40]. This is likely due to greater [Ca2+]nsr under WT conditions ([Ca2+]nsr = ~950 μM vs. ~800 μM, WT and CPVT respectively). As expected, the increased sensitivity of RyR2s to [Ca2+]i under CPVT conditions corresponds to more Ca2+ sparks (though smaller in mass) recruited during a 1 s interval of steady-state 1 Hz AP pacing, ~11,000 (see Fig. 5B). There is a clear increase in late systolic and diastolic Ca2+ sparks under CPVT conditions. At steady-state 1 Hz pacing, we show little difference between WT and CPVT [Ca2+]i transient amplitude (see Fig. 3C and E). If only RyR2 PO is altered, the steady-state [Ca2+]i transient is larger than WT with asynchronous late systolic Ca2+ spark activity, while reduced JSR buffering results in a smaller [Ca2+]i transient than WT, see Fig. S8. The rate that Ca2+ sparks occur and the restitution of Ca2+ spark fidelity (the probability that opening of a single RyR2 will trigger a Ca2+ spark) following an AP is discussed below.
2.1.4. Restitution of Ca2+ spark fidelity
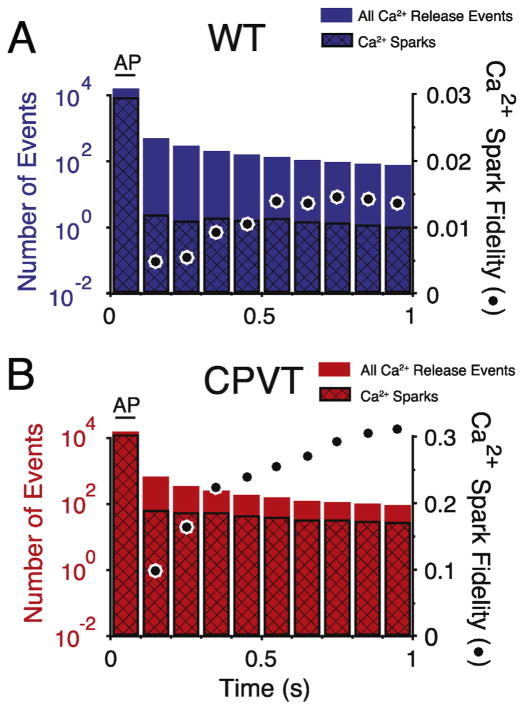
Following the burst of Ca2+ sparks produced by an AP there is an immediate, rapid drop in the overall rate of Ca2+ sparks. Fig. 7A–B shows the average rate of Ca2+ sparks over time following an AP binned into 100 ms (WT and CPVT, respectively). Ca2+ spark fidelity is near 60% during the AP (see Fig. 9H), but falls below steady-state diastolic levels immediately after the AP with Ca2+ quarks and Ca2+ sub-sparks dominating SR Ca2+ leak for WT conditions during diastole (see Fig. 4). Under normal conditions, at steady-state, the probability that a Ca2+ quark will induce a Ca2+ spark is <2% during diastole. There is a slow restitution of Ca2+ spark fidelity that corresponds to the time course for [Ca2+]nsr refilling after ECC (see Fig. 3D). This suggests that [Ca2+]nsr levels are responsible for governing the balance between invisible (Ca2+ quarks) and visible (Ca2+ sparks) leak. This is consistent with prior work that showed that visible Ca2+ leak transitions to invisible leak as SR Ca2+ content declines [34,68]. The decline in the total number of Ca2+ leak events, Ca2+ sparks, Ca2+ sub-sparks, and Ca2+ quarks corresponds well with the decline of the [Ca2+]i transient (see Figs. 3 C and 7) and suggests that [Ca2+]i plays a critical role in governing the overall Ca2+ leak rate. The restitution of Ca2+ spark fidelity under simulated CPVT conditions (see Fig. 7B) is similar to WT but with two critical quantitative differences. 1) The number of Ca2+ sparks following the AP spark burst is much higher and 2) the Ca2+ spark fidelity is significantly greater (about 10-fold in this example) with ~30% of RyR2 openings resulting in a Ca2+ spark (both likely due to the higher RyR2 PO). The increased Ca2+ spark rate and higher RyR2 PO both increase the likelihood of aberrant activity (e.g., early after depolarizations (EADs), Ca2+ waves, etc.).
Fig. 7.
Restitution of Ca2+ spark fidelity following an action potential. For WT (n = 400, blue, A) and CPVT (n = 65, red, B) The total number Ca2+ release events (Ca2+ Sparks and Ca2+ Sub-Sparks) are plotted (left y-axis) with the black cross-hatch showing the number of Ca2+ sparks. Ca2+ spark fidelity is represented by black circles (far right y-axis). Ca2+ release events and Ca2+ spark fidelity during an AP initiated [Ca2+]i transient are binned into 100 ms bins. Note that Ca2+ spark fidelity during the AP bin (0–100 ms) is not shown, but is ~0.6 for WT and ~0.8 for CPVT conditions. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Fig. 9.
Effect of SR Ca2+ load on spontaneous and triggered SR Ca2+ release. A–B) Number of Ca2+ sparks during quiescence and systole (the first 100 ms after an AP), respectively. C–D) Number of Ca2+ quarks and sub-sparks (invisible leak events) during quiescence systole, respectively. E–F) Average Ca2+ spark duration during quiescence and systole, respectively. G–H) Ca2+ spark fidelity during quiescence and systole, respectively For all tests (A–H) WT results are shown in blue and CPVT conditions are shown in red, with steady-state quiescent [Ca2+]nsr marked by * and diastolic [Ca2+]nsr marked by #. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
2.1.5. ECC fidelity
The sensitivity of RyR2s to LCC openings has been a focus of numerous studies since the first experimental measurements by Santana et al. [69]. Note that the likelihood of a LCC opening will trigger a Ca2+ spark, termed “ECC fidelity”, is distinct from “Ca2+ spark fidelity”. The inverse relationship between ECC fidelity (defined as the number of Ca2+ sparks divided by the number of LCC openings) and membrane potential is shown in Fig. 8A is primarily due to declining driving force for Ca2+ entry via the LCC and the brief duration of an LCC opening (~0.5 ms). When the membrane is significantly depolarized (V ≥ 0 mV) numerous LCC openings are required to trigger a single Ca2+ spark. ECC fidelity is lower in normal conditions than during pathological states such as CPVT (see red line), with a pronounced increase in RyR2 sensitivity to brief LCC openings at more negative membrane potentials (−40 to −10 mV), see Fig. 8A. As expected, there are no significant differences in the number of LCC openings between WT and CPVT conditions, see Fig. 8B. However, “leaky” RyR2s result in an increased number of Ca2+ sparks, shown in Fig. 8C, across all membrane potentials. The substantial increase in Ca2+ spark sensitivity to brief LCC openings and resulting increase in Ca2+ spark frequency under CPVT conditions likely creates an environment that could promote instability and arrhythmia.
Fig. 8.
ECC fidelity. A) ECC fidelity (measured as number of Ca2+ sparks divided by the number of LCC openings during a 200 ms voltage pulse to the various test potentials). B) Number of LCC openings per CRU (N−1) during same conditions as (A). C) Number of Ca2+ sparks per N CRUs during same conditions as (A). For all tests (A–C) WT results are shown in blue and simulated CPVT conditions are shown in red. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
2.1.6. Dependence of spontaneous and triggered SR Ca2+ release on SR Ca2+ content
Consistent with our prior work, we show that as SR Ca2+ content declines, Ca2+ quarks and Ca2+ sub-sparks (i.e. “non-spark” based leak), begin to account for the majority of SR Ca2+ leak, see Figs. 9A and C. Under normal conditions, quiescent Ca2+ sparks are infrequent at [Ca2+]nsr < 750 μM and reach ~150 Ca2+ sparks per cell per second at steady-state quiescent [Ca2+]nsr of ~950 μM. Ca2+ spark frequency during CPVT was significantly greater across a broad range of SR Ca2+ content and compares well with experimental results [70,71]. Experimentally invisible Ca2+ leak events, Ca2+ quarks and Ca2+ sub-sparks, quantified in Fig. 9C as non-sparks, were stable across SR Ca2+ load for WT conditions, but decreased with increasing [Ca2+]nsr under CPVT conditions. Average Ca2+ spark duration (ASD) for WT conditions was relatively constant, ~20 ms, regardless of SR Ca2+ load, see Fig. 9E. However in the CPVT state, Ca2+ sparks fail to efficiently terminate above a [Ca2+]nsr of 1 mM resulting in ASD > 100 ms. Near quiescent steady-state SR Ca2+ content, WT Ca2+ spark fidelity is ~0.15 and increases above a [Ca2+]nsr of 1 mM, see Fig. 9G. Leaky RyR2s have greater Ca2+ spark frequency and decreased invisible Ca2+ leak events which contribute to the overall increase in Ca2+ spark fidelity of, ~0.55, at quiescent steady-state SR Ca2+ content of ~800 μM.
Systolic Ca2+ release is also sensitive to SR Ca2+ load, with Fig. 9B showing the increase in number of Ca2+ sparks during the first 100 ms following the start of an AP with increasing [Ca2+]nsr. RyR2s during simulated CPVT promote greater recruitment of Ca2+ sparks during an AP, while “invisible” Ca2+ release events decrease with increasing [Ca2+]nsr under normal and CPVT conditions, see Fig. 9D. Ca2+ spark duration during systole, shown in Fig. 9F, is relatively stable at ~20 ms and ~50–60 ms, for WT and CPVT respectively. The depletion of the [Ca2+]nsr during the [Ca2+]i transient likely underlies the added stability of systolic ASD even under “leaky” RyR2 conditions. While Ca2+ spark fidelity under normal conditions is ~0.5 at [Ca2+]nsr of ~700 μM (steady-state 1 Hz pacing) and can increase to >0.7 at high SR Ca2+ content (Fig. 9H), Ca2+ spark fidelity is much less dynamic during CPVT. At 1 Hz steady-state pacing [Ca2+]nsr is ~650 μM and Ca2+ spark fidelity is already >0.7 and only slightly rises with increasing SR Ca2+ content (Fig. 9 H).
3. Discussion
A novel mathematical model of ECC in mouse heart has been presented. The model builds upon our previous stochastic description of quiescent RyR2 mediated SR Ca2+ leak where Ca2+ spark and “invisible”, Ca2+ sub-spark events combine to balance the activity of SERCA2a [34]. Importantly, it includes a novel, 7-state Markov chain formulation of the LCC where transitions depend on membrane potential and also on [Ca2+]ds thereby allowing for the generation of a realistic LCC current. The model also includes a quantitative description of the electrical behavior of the myocyte with detailed descriptions for the SL current components found in the external sarcolemma and in the transverse and axial tubule system. With LCC and AP membrane current components in place, the model can simulate systolic and diastolic Ca2+ dynamics while capturing key details of individual CRU activity (e.g., LCC and RyR2 openings, [Ca2+]ds, and [Ca2+]jsr). This unique tool allows us to explore characteristic properties of elementary Ca2+ sparks, the summation of local signals during ECC, as well as pathological Ca2+ dynamics derived from conditions associated with “leaky” RyR2s.
3.1. Local Ca2+ release underlies [Ca2+]i transients
The Ca2+ spark is the fundamental unit for both diastolic and systolic SR Ca2+ release in cardiomyocytes. The stochastic opening of SR RyR2 channels mediates “invisible” sub-spark Ca2+ release which has the potential to induce a full Ca2+ spark through CICR. As shown in [34] Ca2+ sparks are the major contributor to SR Ca2+ leak within the resting car-diomyocyte and play an important role in cellular Ca2+ homeostasis by balancing SERCA2a activity. Ca2+ sparks observed after long periods of rest have equivalent characteristics (i.e. frequency, amplitude, duration, and decay constant) whether they occur due to spontaneous RyR2 opening or are evoked experimentally through transient LCC opening during membrane depolarization. We show that Ca2+ sparks elicited through ECC are of similar size and duration to those observed during quiescence. In the diastolic period after a [Ca2+]i transient the cardio-myocyte CRUs are less capable of participating in CICR. The induction of a Ca2+ spark quickly results in depletion of [Ca2+]jsr for that CRU, and when taken across the ~7000 CRUs activated during ECC leads to a large decrease in SR Ca2+ content. This leads to a decrease in diastolic Ca2+ spark amplitude and Ca2+ spark fidelity, as diastolic Ca2+ spark frequency is diminished dramatically and the proportion of “invisible” leak, Ca2+ quarks, rises. Our findings highlight the critical importance of SR Ca2+ content and RyR2 Ca2+ sensitivity in controlling Ca2+ spark frequency and, while not the focus of this study, may help to explain the low quiescent Ca2+ spark rate in other animals including larger mammals.
3.1.1. Insights into Pathological Conditions Related to “Leaky” RyR2s
We observe significant abnormalities affecting Ca2+ pump/leak balance, local Ca2+ sparks, and whole-cell Ca2+ homeostasis in our simulation of CASQ2-R33Q mutant cardiomyocyte. We incorporated two critical features in the pathogenesis of CASQ2-R33Q mediated CPVT, reduced JSR Ca2+ buffering and enhanced RyR2 PO and [Ca2+]i sensitivity. Decreased JSR Ca2+ buffering seemed to limit global [Ca2+]i transient abnormalities, while the reduction in k− and slight increase in k+ that increased RyR2 [Ca2+]i sensitivity and PO under CPVT conditions were the critical alterations that lead to aberrant Ca2+ handling. Under these conditions Ca2+ sparks are not well synchronized by the AP and persist into “late” systole as the RyR2s are much more sensitive to openings of neighboring RyR2s and LCCs. While Ca2+ spark fidelity and frequency decline after ECC under both WT and CPVT conditions, the decline is far less pronounced for the “leaky” RyR2 state. In fact, the Ca2+ spark fidelity for CPVT conditions is always much greater than that of WT. Individual CPVT Ca2+ sparks display all the hallmarks of unstable Ca2+ release such as delayed termination and even reactivation of the CRU. The “metastable” sparks are capable of activating neighboring transverse CRUs which has the potential to induce an arrhythmogenic Ca2+ wave. The dynamic difference between ECC fidelity displayed by CPVT conditions compared to WT at more negative potentials (−40 to −10 mV) might prime the cell for EADs and possibly delayed afterdepolarizations (DADs). Under normal conditions there should be little SR Ca2+ release at these potentials. However, increased release of SR Ca2+ due to “leaky” RyR2s may lead to buildup of [Ca2+]i over time and activate [Ca2+]i-dependent currents, notably Incx. The baseline differences in local and global Ca2+ signals between simulated WT and CPVT mutation cardiomyocytes in the current study may form the molecular basis for cellular Ca2+ waves and possibly the rare but lethal ventricular arrhythmia during CPVT.
3.1.2. Mouse model to human translation
We designed our model to recapitulate the electrophysiology and Ca2+ handling properties of the mouse as it is the most commonly used animal in biomedical research. It should, however, be noted the Ca2+ signaling components in our model respond appropriately to different AP waveforms. While our model was designed to reproduce mouse AP dynamics during ruptured patch conditions, Fig. S11A shows minimal difference in Ca2+ signals after steady-state stimulation with an AP based on those recorded from perforated patch measurements [72]. Additionally, our model can respond to longer duration APs (APD90 of ~250–350 ms) typical of larger mammals such as rabbit, see Fig. S11B [73]. While not the focus of the present study, future work could adapt our model to fit large mammal (human, canine, or rabbit) cardiomyocyte biophysical characteristics (i.e. SL membrane channels, transporters, and detailed Ca2+ handling properties). Importantly, the principles of Ca2+ pump/leak balance, inherent in our model design, are critical for modeling Ca2+ signaling regardless of species. SL Ca2+ fluxes regulate [Ca2+]i, while the balance of SERCA2a [Ca2+]i reuptake and RyR2 based SR Ca2+ leak control [Ca2+]nsr levels. Any change to the delicate balance of SL or SR Ca2+ fluxes will shift steady-state [Ca2+]i and [Ca2+]nsr. When comparing mice and humans, the increased NCX activity in cardiomyocytes of larger mammals promotes extrusion of [Ca2+]i and results in depletion of [Ca2+]nsr of during quiescence. As shown in Williams et al., 2011, the lower resting [Ca2+]nsr of large mammals likely leads to a shift of SR Ca2+ leak from primarily visible (e.g., Ca2+ sparks) toward more invisible leak pathways (e.g., Ca2+ quarks and sub-sparks). Furthermore, the increased Incx may also pre-dispose human cardiomyocytes to EADs and possibly DADs, especially under conditions characterized by “leaky” RyR2s as presented here.
3.2. Future challenges
While the present work provides much insight into cardiac CICR dynamics during both quiescence and ECC under physiological and pathological conditions, it is still just a stepping stone in a sequence of model development. We think a whole-cell spatially resolved model of the entire cardiomyocyte will be necessary to more completely investigate CICR instabilities (e.g., Ca2+ waves, Ca2+ alternans, etc.). Such a model could better investigate the alterations in Ca2+ signaling in an environment that promotes CPVT arrhythmia. A comprehensive whole-cardiomyocyte spatial model with robust β-adrenergic signaling may provide important insight into the conditions that elicit deadly arrhythmia in patients with CPVT and account for the rarity of sudden cardiac death. The physiological Ca2+ spark and Ca2+ leak dynamics presented here which now provide novel insights into normal and pathological cardiac Ca2+ signals in mouse heart, should serve as a solid base for that new work.
Supplementary Material
Acknowledgments
This work was supported by NIH grants, R01 HL106056 (WJL), R01 HL105239 (WJL and MSJ), U01 HL116321 (WJL and MSJ), F32 HL108604 (GSBW), and K25 HL125762 (GSBW). APW has been supported by NIH training grants, 2T32GM092237-06 and 5T32GM008181-28 to the Medical Scientist Training Program and Training Program in Integrative Membrane Biology, respectively.
Abbreviations
- CICR
calcium induced calcium release
- ECC
excitation contraction coupling
- AP
action potential
- APD90
duration to 90% repolarization of action potential
- LCC
L-type calcium channels
- VDI
voltage dependent inactivation
- CDI
calcium dependent inactivation
- RyR2
type 2 ryanodine receptor
- SERCA2a
sarcoplasmic reticulum calcium ATPase
- CASQ2
calsequestrin
- NCX
sodium/calcium exchanger
- PMCA
plasma membrane calcium ATPase
- SR
sarcoplasmic reticulum
- JSR
junctional sarcoplasmic reticulum
- CRU
calcium release unit
- N
number of CRUs
- ASD
average calcium spark duration
- CPVT
catecholaminergic polymorphic ventricular tachycardia
Appendix A. Supplementary data
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.yjmcc.2016.01.024.
Footnotes
Disclosures
None.
References
- 1.Niggli E, Lederer WJ. Voltage-independent calcium release in heart muscle. Science. 1990;250:565–568. doi: 10.1126/science.2173135. [DOI] [PubMed] [Google Scholar]
- 2.Stern MD. Theory of excitation-contraction coupling in cardiac muscle. Biophys J. 1992;63:497–517. doi: 10.1016/S0006-3495(92)81615-6. http://dx.doi.org/10.1016/S0006-3495(92)81615-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Franzini-Armstrong C, Protasi F, Ramesh V. Comparative ultrastructure of Ca2+ release units in skeletal and cardiac muscle. Ann N Y Acad Sci. 1998;853:20–30. doi: 10.1111/j.1749-6632.1998.tb08253.x. [DOI] [PubMed] [Google Scholar]
- 4.Baddeley D, Jayasinghe ID, Lam L, Rossberger S, Cannell MB, Soeller C. Optical single-channel resolution imaging of the ryanodine receptor distribution in rat cardiac myocytes. Proc Natl Acad Sci U S A. 2009;106:22275–22280. doi: 10.1073/pnas.0908971106. http://dx.doi.org/10.1073/pnas.0908971106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bondarenko VE, Szigeti GP, Bett GCL, Kim S-J, Rasmusson RL. Computer model of action potential of mouse ventricular myocytes. Am J Physiol Heart Circ Physiol. 2004;287:H1378–H1403. doi: 10.1152/ajpheart.00185.2003. http://dx.doi.org/10.1152/ajpheart.00185.2003. [DOI] [PubMed] [Google Scholar]
- 6.Koivumäki JT, Korhonen T, Takalo J, Weckström M, Tavi P. Regulation of excitation-contraction coupling in mouse cardiac myocytes: integrative analysis with mathematical modelling. BMC Physiol. 2009;9:16. doi: 10.1186/1472-6793-9-16. http://dx.doi.org/10.1186/1472-6793-9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li L, Niederer SA, Idigo W, Zhang YH, Swietach P, Casadei B, et al. A mathematical model of the murine ventricular myocyte: a data-driven biophysically based approach applied to mice overexpressing the canine NCX isoform. AJP: Heart and Circulatory Physiology. 2010;299:H1045–H1063. doi: 10.1152/ajpheart.00219.2010. http://dx.doi.org/10.1152/ajpheart.00219.2010. [DOI] [PubMed] [Google Scholar]
- 8.Li L, Louch WE, Niederer SA, Andersson KB, Christensen G, Sejersted OM, et al. Calcium dynamics in the ventricular myocytes of SERCA2 knockout mice: a modeling study. Biophys J. 2011;100:322–331. doi: 10.1016/j.bpj.2010.11.048. http://dx.doi.org/10.1016/j.bpj.2010.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petkova-Kirova PS, London B, Salama G, Rasmusson RL, Bondarenko VE. Mathematical modeling mechanisms of arrhythmias in transgenic mouse heart overex-pressing TNF-α. AJP: Heart and Circulatory Physiology. 2012;302:H934–H952. doi: 10.1152/ajpheart.00493.2011. http://dx.doi.org/10.1152/ajpheart.00493.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mullins PD, Bondarenko VE. A mathematical model of the mouse ventricular myocyte contraction. PLoS ONE. 2013;8:e63141. doi: 10.1371/journal.pone.0063141. http://dx.doi.org/10.1371/journal.pone.0063141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Land S, Niederer SA, Aronsen JM, Espe EKS, Zhang L, Louch WE, et al. An analysis of deformation-dependent electromechanical coupling in the mouse heart. J Physiol (Lond) 2012;590:4553–4569. doi: 10.1113/jphysiol.2012.231928. http://dx.doi.org/10.1113/jphysiol.2012.231928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DiFrancesco D, NOBLE D. A model of cardiac electrical activity incorporating ionic pumps and concentration changes. Philos Trans R Soc Lond Ser B Biol Sci. 1985;307:353–398. doi: 10.1098/rstb.1985.0001. [DOI] [PubMed] [Google Scholar]
- 13.Nordin C. Computer model of membrane current and intracellular Ca2+ flux in the isolated guinea pig ventricular myocyte. Am J Physiol. 1993;265:H2117–H2136. doi: 10.1152/ajpheart.1993.265.6.H2117. [DOI] [PubMed] [Google Scholar]
- 14.Luo CH, Rudy Y. A dynamic model of the cardiac ventricular action potential. I. Simulations of ionic currents and concentration changes. Circ Res. 1994;74:1071–1096. doi: 10.1161/01.res.74.6.1071. [DOI] [PubMed] [Google Scholar]
- 15.Luo CH, Rudy Y. A dynamic model of the cardiac ventricular action potential. II. Afterdepolarizations, triggered activity, and potentiation. Circ Res. 1994;74:1097–1113. doi: 10.1161/01.res.74.6.1097. [DOI] [PubMed] [Google Scholar]
- 16.Saucerman JJ, Brunton LL, Michailova AP, McCulloch AD. Modeling beta-adrenergic control of cardiac myocyte contractility in silico. J Biol Chem. 2003;278:47997–48003. doi: 10.1074/jbc.M308362200. http://dx.doi.org/10.1074/jbc.M308362200. [DOI] [PubMed] [Google Scholar]
- 17.Amanfu RK, Saucerman JJ. Modeling the effects of β1-adrenergic receptor blockers and polymorphisms on cardiac myocyte Ca2+ handling. Mol Pharmacol. 2014;86:222–230. doi: 10.1124/mol.113.090951. http://dx.doi.org/10.1124/mol.113.090951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iyer V, Mazhari R, Winslow RL. A computational model of the human left-ventricular epicardial myocyte. Biophys J. 2004;87:1507–1525. doi: 10.1529/biophysj.104.043299. http://dx.doi.org/10.1529/biophysj.104.043299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pandit SV, Clark RB, Giles WR, Demir SS. A mathematical model of action potential heterogeneity in adult rat left ventricular myocytes. Biophys J. 2001;81:3029–3051. doi: 10.1016/S0006-3495(01)75943-7. http://dx.doi.org/10.1016/S0006-3495(01)75943-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Puglisi JL, Wang F, Bers DM. Modeling the isolated cardiac myocyte. Prog Biophys Mol Biol. 2004;85:163–178. doi: 10.1016/j.pbiomolbio.2003.12.003. http://dx.doi.org/10.1016/j.pbiomolbio.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 21.Grandi E, Pandit SV, Voigt N, Workman AJ, Dobrev D, Jalife J, et al. Human atrial action potential and Ca2+ model: sinus rhythm and chronic atrial fibrillation. Circ Res. 2011;109:1055–1066. doi: 10.1161/CIRCRESAHA.111.253955. http://dx.doi.org/10.1161/CIRCRESAHA.111.253955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu ZI, Clancy CE. L-type Ca2+ channel mutations and T-wave alternans: a model study. Am J Physiol Heart Circ Physiol. 2007;293:H3480–H3489. doi: 10.1152/ajpheart.00476.2007. http://dx.doi.org/10.1152/ajpheart.00476.2007. [DOI] [PubMed] [Google Scholar]
- 23.Keizer J, Levine L. Ryanodine receptor adaptation and Ca2+(−)induced Ca2+ release-dependent Ca2+ oscillations. Biophys J. 1996;71:3477–3487. doi: 10.1016/S0006-3495(96)79543-7. http://dx.doi.org/10.1016/S0006-3495(96)79543-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Winslow RL, Rice J, Jafri S, Marbán E, O’Rourke B. Mechanisms of altered excitation-contraction coupling in canine tachycardia-induced heart failure, II: model studies. Circ Res. 1999;84:571–586. doi: 10.1161/01.res.84.5.571. [DOI] [PubMed] [Google Scholar]
- 25.Mahajan A, Shiferaw Y, Sato D, Baher A, Olcese R, Xie LH, et al. A rabbit ventricular action potential model replicating cardiac dynamics at rapid heart rates. Biophys J. 2008;94:392–410. doi: 10.1529/biophysj.106.98160. http://dx.doi.org/10.1529/biophysj.106.98160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greenstein JL, Winslow RL. An integrative model of the cardiac ventricular myocyte incorporating local control of Ca2+ release. Biophys J. 2002;83:2918–2945. doi: 10.1016/S0006-3495(02)75301-0. http://dx.doi.org/10.1016/S0006-3495(02)75301-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rice JJ, Jafri MS, Winslow RL. Modeling gain and gradedness of Ca2+ release in the functional unit of the cardiac diadic space. Biophys J. 1999;77:1871–1884. doi: 10.1016/s0006-3495(99)77030-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greenstein JL, Hinch R, Winslow RL. Mechanisms of excitation-contraction coupling in an integrative model of the cardiac ventricular myocyte. Biophys J. 2006;90:77–91. doi: 10.1529/biophysj.105.065169. http://dx.doi.org/10.1529/biophysj.105.065169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hinch R, Greenstein JL, Tanskanen AJ, Xu L, Winslow RL. A simplified local control model of calcium-induced calcium release in cardiac ventricular myocytes. Biophys J. 2004;87:3723–3736. doi: 10.1529/biophysj.104.049973. http://dx.doi.org/10.1529/biophysj.104.049973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hinch R, Greenstein JL, Winslow RL. Multi-scale models of local control of calcium induced calcium release. Prog Biophys Mol Biol. 2005;90:136–150. doi: 10.1016/j.pbiomolbio.2005.05.014. http://dx.doi.org/10.1016/j.pbiomolbio.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 31.Groff JR, Smith GD. Calcium-dependent inactivation and the dynamics of calcium puffs and sparks. J Theor Biol. 2008;253:483–499. doi: 10.1016/j.jtbi.2008.03.026. http://dx.doi.org/10.1016/j.jtbi.2008.03.026. [DOI] [PubMed] [Google Scholar]
- 32.Shannon TR, Wang F, Puglisi J, Weber C, Bers DM. A mathematical treatment of integrated Ca dynamics within the ventricular myocyte. Biophys J. 2004;87:3351–3371. doi: 10.1529/biophysj.104.047449. http://dx.doi.org/10.1529/biophysj.104.047449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sobie EA, Dilly KW, dos Santos Cruz J, Lederer WJ, Jafri MS. Termination of cardiac Ca(2+) sparks: an investigative mathematical model of calcium-induced calcium release. Biophys J. 2002;83:59–78. doi: 10.1016/s0006-3495(02)75149-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Williams GSB, Chikando AC, Tuan HTM, Sobie EA, Lederer WJ, Jafri MS. Dynamics of calcium sparks and calcium leak in the heart. Biophys J. 2011;101:1287–1296. doi: 10.1016/j.bpj.2011.07.021. http://dx.doi.org/10.1016/j.bpj.2011.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brochet DXP, Xie W, Yang D, Cheng H, Lederer WJ. Quarky calcium release in the heart. Circ Res. 2011;108:210–218. doi: 10.1161/CIRCRESAHA.110.231258. http://dx.doi.org/10.1161/CIRCRESAHA.110.231258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lukyanenko V, Ziman A, Lukyanenko A, Salnikov V, Lederer WJ. Functional groups of ryanodine receptors in rat ventricular cells. J Physiol (Lond) 2007;583:251–269. doi: 10.1113/jphysiol.2007.136549. http://dx.doi.org/10.1113/jphysiol.2007.136549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sobie EA, Song LS, Lederer WJ. Restitution of Ca(2+) release and vulnerability to arrhythmias. J Cardiovasc Electrophysiol. 2006;17(Suppl 1):S64–S70. doi: 10.1111/j.1540-8167.2006.00385.x. http://dx.doi.org/10.1111/j.1540-8167.2006.00385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van der Werf C, Wilde AAM. Catecholaminergic polymorphic ventricular tachycardia: from bench to bedside. Heart. 2013;99:497–504. doi: 10.1136/heartjnl-2012-302033. http://dx.doi.org/10.1136/heartjnl-2012-302033. [DOI] [PubMed] [Google Scholar]
- 39.Chen H, Valle G, Furlan S, Nani A, Györke S, Fill M, et al. Mechanism of calsequestrin regulation of single cardiac ryanodine receptor in normal and pathological conditions. J Gen Physiol. 2013 doi: 10.1085/jgp.201311022. http://dx.doi.org/10.1085/jgp.201311022. [DOI] [PMC free article] [PubMed]
- 40.Liu N, Denegri M, Dun W, Boncompagni S, Lodola F, Protasi F, et al. Abnormal propagation of calcium waves and ultrastructural remodeling in recessive catechol-aminergic polymorphic ventricular tachycardia. Circ Res. 2013 doi: 10.1161/CIRCRESAHA.113.301783. http://dx.doi.org/10.1161/CIRCRESAHA.113.301783. [DOI] [PubMed]
- 41.Rizzi N, Liu N, Napolitano C, Nori A, Turcato F, Colombi B, et al. Unexpected structural and functional consequences of the R33Q homozygous mutation in cardiac calsequestrin: a complex arrhythmogenic cascade in a knock in mouse model. Circ Res. 2008;103:298–306. doi: 10.1161/CIRCRESAHA.108.171660. http://dx.doi.org/10.1161/CIRCRESAHA.108.171660. [DOI] [PubMed] [Google Scholar]
- 42.Brunello L, Slabaugh JL, Radwanski PB, Ho H-T, Belevych AE, Lou Q, et al. Decreased RyR2 refractoriness determines myocardial synchronization of aberrant Ca2+ release in a genetic model of arrhythmia. Proc Natl Acad Sci U S A. 2013 doi: 10.1073/pnas.1300052110. http://dx.doi.org/10.1073/pnas.1300052110. [DOI] [PMC free article] [PubMed]
- 43.Terentyev D, Nori A, Santoro M, Viatchenko-Karpinski S, Kubalova Z, Gyorke I, et al. Abnormal interactions of calsequestrin with the ryanodine receptor calcium release channel complex linked to exercise-induced sudden cardiac death. Circ Res. 2006;98:1151–1158. doi: 10.1161/01.RES.0000220647.93982.08. http://dx.doi.org/10.1161/01.RES.0000220647.93982.08. [DOI] [PubMed] [Google Scholar]
- 44.Bassani JW, Bassani RA, Bers DM. Relaxation in rabbit and rat cardiac cells: species-dependent differences in cellular mechanisms. J Physiol (Lond) 1994;476:279–293. doi: 10.1113/jphysiol.1994.sp020130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Williams GSB, Boyman L, Chikando AC, Khairallah RJ, Lederer WJ. Mitochon-drial calcium uptake. Proc Natl Acad Sci U S A. 2013;110:10479–10486. doi: 10.1073/pnas.1300410110. http://dx.doi.org/10.1073/pnas.1300410110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boyman L, Khairallah RJ, Kettlewell S, Lederer WJ. Calcium movement in cardiac mitochondria. Biophys J. 2014;107:1289–1301. doi: 10.1016/j.bpj.2014.07.045. http://dx.doi.org/10.1016/j.bpj.2014.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lu X, Ginsburg KS, Kettlewell S, Bossuyt J, Smith GL, Bers DM. Measuring local gradients of intramitochondrial [Ca(2+)] in cardiac myocytes during sarcoplasmic reticulum Ca(2+) release. Circ Res. 2013;112:424–431. doi: 10.1161/CIRCRESAHA.111.300501. http://dx.doi.org/10.1161/CIRCRESAHA.111.300501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hashambhoy YL, Greenstein JL, Winslow RL. Role of CaMKII in RyR leak, EC coupling and action potential duration: a computational model. J Mol Cell Cardiol. 2010;49:617–624. doi: 10.1016/j.yjmcc.2010.07.011. http://dx.doi.org/10.1016/j.yjmcc.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sun L, Fan J, Clark J, Palade P. A model of the L-type Ca2+ channel in rat ventricular myocytes: ion selectivity and …. J Physiol (Lond) 2000 doi: 10.1111/j.1469-7793.2000.00139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jafri MS, Rice JJ, Winslow RL. Cardiac Ca2+ dynamics: the roles of ryanodine receptor adaptation and sarcoplasmic reticulum load. Biophys J. 1998;74:1149–1168. doi: 10.1016/S0006-3495(98)77832-4. http://dx.doi.org/10.1016/S0006-3495(98)77832-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Williams GSB, Molinelli EJ, Smith GD. Modeling local and global intracellular calcium responses mediated by diffusely distributed inositol 1,4,5-trisphosphate receptors. J Theor Biol. 2008;253:170–188. doi: 10.1016/j.jtbi.2008.02.040. http://dx.doi.org/10.1016/j.jtbi.2008.02.040. [DOI] [PubMed] [Google Scholar]
- 52.Williams GSB, Huertas MA, Sobie EA, Jafri MS, Smith GD. A probability density approach to modeling local control of calcium-induced calcium release in cardiac myocytes. Biophys J. 2007;92:2311–2328. doi: 10.1529/biophysj.106.099861. http://dx.doi.org/10.1529/biophysj.106.099861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rose WC, Balke CW, Wier WG, Marban E. Macroscopic and unitary properties of physiological ion flux through L-type Ca2+ channels in guinea-pig heart cells. J Physiol (Lond) 1992;456:267–284. doi: 10.1113/jphysiol.1992.sp019336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jafri MS, Hoang-Trong TM, Williams GSB. US9009095. Method and System for Utilizing Mar-kov Chain Monte Carlo Simulations. 2012
- 55.Laver DR, Kong CHT, Imtiaz MS, Cannell MB. Termination of calcium-induced calcium release by induction decay: an emergent property of stochastic channel gating and molecular scale architecture. J Mol Cell Cardiol. 2013;54:98–100. doi: 10.1016/j.yjmcc.2012.10.009. http://dx.doi.org/10.1016/j.yjmcc.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 56.Tencerová B, Zahradníková A, Gaburjakova J, Gaburjakova M. Luminal Ca2+ controls activation of the cardiac ryanodine receptor by ATP. J Gen Physiol. 2012;140:93–108. doi: 10.1085/jgp.201110708. http://dx.doi.org/10.1085/jgp.201110708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cannell MB, Kong CHT, Imtiaz MS, Laver DR. Control of sarcoplasmic reticulum Ca(2+) release by stochastic RyR gating within a 3D model of the cardiac dyad and importance of induction decay for CICR termination. Biophys J. 2013;104:2149–2159. doi: 10.1016/j.bpj.2013.03.058. http://dx.doi.org/10.1016/j.bpj.2013.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Walker MA, Williams GSB, Kohl T, Jafri S, Lehnart SE, Greenstein JL, et al. Super-resolution modeling of calcium release in heart. Biophys J. 2014;106:318a. doi: 10.1016/j.bpj.2014.11.003. http://dx.doi.org/10.1016/j.bpj.2013.11.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fabiato A. Time and calcium dependence of activation and inactivation of calcium-induced release of calcium from the sarcoplasmic reticulum of a skinned canine cardiac Purkinje cell. J Gen Physiol. 1985;85:247–289. doi: 10.1085/jgp.85.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fox JJ, McHarg JL, Gilmour RF. Ionic mechanism of electrical alternans. Am J Physiol Heart Circ Physiol. 2002;282:H516–H530. doi: 10.1152/ajpheart.00612.2001. http://dx.doi.org/10.1152/ajpheart.00612.2001. [DOI] [PubMed] [Google Scholar]
- 61.Chen-Izu Y, McCulle S, Ward C, Soeller C, Allen B, Rabang C, et al. Three-dimensional distribution of ryanodine receptor clusters in cardiac myocytes. Biophys J. 2006;91:1–13. doi: 10.1529/biophysj.105.077180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Smith GD, Keizer JE, Stern MD, Lederer WJ, Cheng H. A simple numerical model of calcium spark formation and detection in cardiac myocytes. Biophys J. 1998;75:15–32. doi: 10.1016/S0006-3495(98)77491-0. http://dx.doi.org/10.1016/S0006-3495(98)77491-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sah R, Ramirez RJ, Oudit GY, Gidrewicz D, Trivieri MG, Zobel C, et al. Regulation of cardiac excitation-contraction coupling by action potential repolarization: role of the transient outward potassium current (I(to)) J Physiol (Lond) 2003;546:5–18. doi: 10.1113/jphysiol.2002.026468. http://dx.doi.org/10.1113/jphysiol.2002.026468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Signore S, Sorrentino A, Ferreira-Martins J, Kannappan R, Shafaie M, Del Ben F, et al. Inositol 1,4,5-triphosphate receptors and human left ventricular myocytes. Circulation. 2013 doi: 10.1161/CIRCULATIONAHA.113.002764. http://dx.doi.org/10.1161/CIRCULATIONAHA.113.002764. [DOI] [PMC free article] [PubMed]
- 65.Anumonwo JM, Tallini YN, Vetter FJ, Jalife J. Action potential characteristics and arrhythmogenic properties of the cardiac conduction system of the murine heart. Circ Res. 2001;89:329–335. doi: 10.1161/hh1601.095894. [DOI] [PubMed] [Google Scholar]
- 66.Lehnart SE, Mongillo M, Bellinger A, Lindegger N, Chen BX, Hsueh W, et al. Leaky Ca2+ release channel/ryanodine receptor 2 causes seizures and sudden cardiac death in mice. J Clin Invest. 2008;118:2230–2245. doi: 10.1172/JCI35346. http://dx.doi.org/10.1172/JCI35346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stern MD, Ríos E, Maltsev VA. Life and death of a cardiac calcium spark. J Gen Physiol. 2013;142:257–274. doi: 10.1085/jgp.201311034. http://dx.doi.org/10.1085/jgp.201311034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zima AV, Bovo E, Bers DM, Blatter LA. Ca2+ spark-dependent and -independent sarcoplasmic reticulum Ca2+ leak in normal and failing rabbit ventricular myocytes. J Physiol (Lond) 2010;588:4743–4757. doi: 10.1113/jphysiol.2010.197913. http://dx.doi.org/10.1113/jphysiol.2010.197913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Santana LF, Cheng H, Gómez AM, Cannell MB, Lederer WJ. Relation between the sarcolemmal Ca2+ current and Ca2+ sparks and local control theories for cardiac excitation-contraction coupling. Circ Res. 1996;78:166–171. doi: 10.1161/01.res.78.1.166. http://dx.doi.org/10.1161/01.RES.78.1.166. [DOI] [PubMed] [Google Scholar]
- 70.Knollmann BC, Chopra N, Hlaing T, Akin B, Yang T, Ettensohn K, et al. Casq2 deletion causes sarcoplasmic reticulum volume increase, premature Ca2+ release, and catecholaminergic polymorphic ventricular tachycardia. J Clin Invest. 2006;116:2510–2520. doi: 10.1172/JCI29128. http://dx.doi.org/10.1172/JCI29128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Uchinoumi H, Yano M, Suetomi T, Ono M, Xu X, Tateishi H, et al. Catecholamin-ergic polymorphic ventricular tachycardia is caused by mutation-linked defective conformational regulation of the ryanodine receptor. Circ Res. 2010;106:1413–1424. doi: 10.1161/CIRCRESAHA.109.209312. http://dx.doi.org/10.1161/CIRCRESAHA.109.209312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Macianskiene R, Bito V, Raeymaekers L, Brandts B, Sipido KR, Mubagwa K. Action potential changes associated with a slowed inactivation of cardiac voltage-gated sodium channels by KB130015. British Journal of Pharmacology. 2003;139:1469–1479. doi: 10.1038/sj.bjp.0705379. http://dx.doi.org/10.1038/sj.bjp.0705379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Puglisi JL, Yuan W, Bassani JW, Bers DM. Ca(2+) influx through Ca(2+) channels in rabbit ventricular myocytes during action potential clamp: influence of temperature. Circ Res. 1999;85:e7–e16. doi: 10.1161/01.res.85.6.e7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.