Abstract
Background: Omega-3 (n–3) fatty acid (FA) consumption is thought to improve depressive symptoms. However, current evidence is limited, and whether this association exists among Puerto Ricans, a population burdened by depression, remains uncertain.
Objectives: We examined the association between ω-3 FA biomarkers and depressive symptoms as well as the potential influence of oxidative stress.
Methods: Baseline and longitudinal analyses were conducted in the Boston Puerto Rican Health Study (n = 787; participants aged 57 ± 0.52 y, 73% women). Urinary 8-hydroxy-2′-deoxyguanosine (8-OHdG) concentration, a measure of oxidative stress, and erythrocyte FA composition were collected at baseline. We calculated the omega-3 index as the sum of eicosapentaenoic and docosahexaenoic acids, expressed as a percentage of total FAs. Baseline and 2-y depressive symptoms were characterized by using the Center for Epidemiological Studies–Depression Scale (CES-D). Statistical analyses included linear and logistic regression.
Results: Urinary 8-OHdG concentration tended to modify the relation between the erythrocyte omega-3 index and baseline CES-D score (P-interaction = 0.10). In stratified analyses, the omega-3 index was inversely associated with CES-D score (β = −1.74, SE = 0.88; P = 0.02) among those in the top quartile of 8-OHdG concentration but not among those in the lower quartiles. The relation between the omega-3 index and CES-D at 2 y was more clearly modified by 8-OHdG concentration (P-interaction = 0.04), where the omega-3 index was inversely associated with CES-D at 2 y, adjusted for baseline (β = −1.66, SE = 0.66; P = 0.02), only among those with elevated 8-OHdG concentrations. Among individuals not taking antidepressant medications and in the top tertile of urinary 8-OHdG concentration, the omega-3 index was associated with significantly lower odds of a CES-D score ≥16 at baseline (OR: 0.72; 95% CI: 0.53, 0.96) but not at 2 y (OR: 0.83; 95% CI: 0.60, 1.15).
Conclusions: An inverse association between the omega-3 index and depressive symptoms was observed among participants with elevated oxidative stress biomarkers. These data suggest that oxidative stress status may identify those who might benefit from ω-3 FA consumption to improve depressive symptoms.
Keywords: omega-3 fatty acids, oxidative stress, depression, APOE, Boston Puerto Rican Health Study
Introduction
Dietary intake of omega-3 FAs has been extensively studied for its potential to reduce cardiovascular risk. Cardiovascular disease and depression frequently co-occur (1), and depression has been shown to be a risk factor for cardiovascular disease (2). This, combined with data that ω-3 FAs are found in high abundance in the brain (3), has sparked interest in the nature of the relation between ω-3 FAs and depressive symptoms and major depressive disorders.
Observational evidence of the relation between self-reported intake of ω-3 FAs and depression has been equivocal (4), in part explained by the use of self-reported intake. Objective dietary biomarkers may reduce the chance of misclassification errors related to self-reported consumption. Most, but not all, examinations of the relation between serum, plasma, or erythrocyte ω-3 FA content and depressive symptoms have reported an inverse association; however, the majority of studies used a cross-sectional design (4–6). In addition, some of these studies found differential relations by type of ω-3 FA, specifically DHA or EPA (7–9).
Oxidative stress—the process by which free radicals cause cellular damage—may adversely affect depressive mood (10). It arises when free radical production exceeds cellular repair capacity and/or when endogenous oxidative defense mechanisms are impaired. The high metabolic activity of the brain makes it susceptible to oxidative stress and associated neuronal damage. Animal and human studies support the role of chronic psychosocial stress in the elevation of oxidative stress (10–12). A recent meta-analysis (13) found that oxidative stress was higher, and antioxidant capacity lower, among individuals with depressive symptoms. In rats, depressive-like behavior induced by chronic stress has been mitigated by ω-3 FA supplementation (14, 15).
More research is needed to understand the relation between ω-3 FA status and depressive symptoms, and little is known about the potential modifying effect of oxidative stress. In addition, there is a dearth of data examining the association between ω-3 FA status and depressive symptoms in Puerto Ricans living on the US mainland, an ethnic group that has a high prevalence of depression, estimated at 38% (in individuals aged 18–74 y) relative to 22.3% among Mexican Americans (16) and to 7.4% and 9.8% of Americans (aged 18–39 y and 40–59 y, respectively) (17). Therefore, our objectives were to examine whether erythrocyte ω-3 FA content is related to depressive symptoms at baseline and at 2-y follow-up in a cohort of US mainland Puerto Rican adults and to test the potential modifying effect of systemic oxidative stress with the use of a urinary biomarker of oxidative DNA damage.
Methods
Participants.
Participants were from the Boston Puerto Rican Health Study, a longitudinal cohort designed to examine sociological, environmental, and genetic risk factors for chronic diseases and quality of life in Puerto Rican adults living in the greater Boston, Massachusetts, area. Baseline data from this ongoing study have been described elsewhere (18). A total of 1500 study participants (aged 45–75 y) were recruited, and data on reasons for nonparticipation were reported previously (18). Information from the 2000 Census identified tracks with at least 25 Puerto Rican adults and, within these, randomly selected census blocks of ≥10 Hispanic adults, which were enumerated by home visit to identify eligible participants. Although most participants were recruited in this manner (18), some were recruited by alternative means, such as random approach at cultural events or personal referral. Recruitment occurred from 2004 to 2009. Participants included those who self-identified as Puerto Rican and who were English or Spanish speaking.
Covariate data were available for 1364 participants. From this sample, we excluded those missing key variables: baseline erythrocyte FA composition (n = 146), baseline (n = 12) and 2-y (n = 209) Center for Epidemiological Studies–Depression Scale (CES-D)8 score, and baseline urinary 8-hydroxy-2′-deoxyguanosine (8-OHdG) concentration [n = 425; this was analyzed in 2006 and was not available for all participants (19)]. A total of 787 individuals had complete data for the current analysis. The protocol for this study was approved by the institutional review boards at Tufts Medical Center, Northeastern University, and the University of Massachusetts Lowell. Written informed consent was obtained from all participants
Erythrocyte membrane FA composition (baseline).
Participants were asked to fast for 12 h preceding the blood draw, which was obtained in-home by a certified phlebotomist, in an evacuated tube containing EDTA. Blood was centrifuged at 3421 g at 4°C for 15 min to obtain plasma and the erythrocyte pellet, and aliquots of each were stored at −70°C for later use (18). As described elsewhere (20), erythrocyte FA composition was ascertained by GC with flame ionization detection (GC2010; Shimadzu Corporation). Individual FAs were expressed as a percentage of total identified FAs, and the omega-3 index was defined as the sum of EPA and DHA. The CVs for EPA, DHA, and the omega-3 index were 6.6%, 4.7%, and 4.6%, respectively.
Depressive symptoms (baseline and 2 y).
Depressive symptoms were assessed with the use of the CES-D score at baseline (CES-Dbaseline) and at the 2-y follow-up (CES-D2y). Median follow-up time was 2.02 y (25th–75th percentile: 1.96–2.15 y). The CES-D scale includes 20 items with questions referring to symptoms during the week before the interview and scores range from 0 to 60 (21). This depression scale has been shown to correlate highly with the Diagnostic and Statistical Manual of Mental Disorders IV categories of depression among Hispanics (22) and has been widely used in studies in Hispanics (16, 22–25). We previously showed that the CES-D was reliable in Puerto Rican adults in Massachusetts (α = 0.90) (26).
Urinary 8-OHdG (baseline).
Urinary 8-OHdG is a by-product of DNA repair resulting from oxidative DNA damage and has been used widely as a biomarker of systemic oxidative stress (27, 28). At baseline, 12-h urine samples were collected from study participants after in-home interviews. Urine samples were kept on dry ice and stored for <2 y at −80°C. Urinary 8-OHdG concentration was estimated at baseline with a monoclonal antibody ELISA kit (Assays Designs) (19). Diluted urine samples (20-fold dilution of 10-μL urine sample) were measured in duplicate in a 96-well microplate format. The inter- and intra-assay CVs were <10%. The total urinary 8-OHdG concentration was calculated by multiplying the measured concentration by total volume of 12-h urine followed by normalizing by urine creatinine concentration.
Other laboratory measures (baseline).
Assays used for LDL and HDL cholesterol, glucose, and insulin have been described previously (18). The HOMA-IR was calculated as follows: [fasting insulin (mU/L) × fasting glucose (mg/dL)]/405 (29). Applied Biosystems’ TaqMan single nucleotide polymorphism genotyping procedures (19, 30) were used to ascertain APOE genotype. This information was available for most participants (n = 756). APOE ɛ2 genotype was defined dichotomously as the presence or absence of the ɛ2 allele. The APOE ɛ4 genotype was characterized in the same fashion.
Other covariates (baseline).
Sociodemographic and medical information were collected by self-reported questionnaire and included age, sex, smoking status, educational attainment, medical history, and medication use. Smoking was categorized as current or not currently smoking at baseline. Education was categorized as high school educated (yes or no) if the participant had received either a high school diploma or GED (General Educational Development). Those presenting with a baseline fasting blood glucose ≥126 mg/dL or taking an antihyperglycemic medication were classified as having diabetes. Participants reporting whether a doctor had ever told them that they have had a heart attack, heart disease (other than a heart attack), or stroke were considered to have cardiovascular disease.
Physical activity level was assessed by a modified Paffenbarger questionnaire from the Harvard Alumni Activity Survey (31, 32), which has been used previously in an elderly Puerto Rican population (33). A physical activity score was calculated as the sum of hours spent on typical 24-h activities (heavy, moderate, light, or sedentary activity and sleeping) multiplied by weighting factors that parallel the rate of oxygen consumption associated with each activity.
Self-reported dietary intake from the previous 12 mo was obtained by trained interviewers using a semiquantitative FFQ that had been modified and validated for use in the Puerto Rican population (18). Nutrient intakes were determined by using the Nutrient Data System for Research software (NDS-R; Nutrition Coordinating Center). As described elsewhere (34), the Healthy Eating Index (HEI) 2005 score was calculated by using procedures consistent with those from the USDA Center for Nutrition Policy and Promotion (35). The HEI 2005 score includes 12 components (e.g., oils, total fruit, total vegetables, whole grains, energy from solid fats, alcoholic beverages, and added sugars). The oils component includes oils from nonhydrogenated vegetable oils and those found in fish, nuts, and seeds (35). To capture variation in dietary quality not due to healthy oils, we adjusted models using a modified HEI 2005 score that excluded the oil component
Statistical analysis.
All of the analyses were conducted with SAS version 9.4 (SAS Institute). A significance level of P ≤ 0.05 was used for associations in all analyses, although stratified analyses were explored when interactions were seen at P ≤ 0.1. A log-transformation was applied to those variables that were severely skewed, including baseline insulin and HOMA-IR. Differences between the study sample and those excluded due to missing data were determined by Student’s t test and chi-square test. Between-group age-adjusted sample characteristic differences were examined by using “proc GLM” in SAS. Pearson correlation coefficients were used to determine the correlation between 8-OHdG concentration and the omega-3 index.
Exposures examined included baseline omega-3 index and DHA and EPA concentrations. Outcome variables were baseline and 2-y CES-D score and CES-D score ≥16. In primary analyses, we examined the association between the omega-3 index and CES-Dbaseline and CES-D2y, along with the potential modifying effect of baseline oxidative stress status. For multivariable linear regression models, baseline omega-3 index, CES-Dbaseline, and CES-D2y were kept on a continuous scale. Models were adjusted for baseline covariates that changed parameter estimates by ≥10% when individually added to linear regression models or for those in which we found evidence of difference by 8-OHdG concentration (P ≤ 0.10). Other covariates considered were baseline diabetes and cardiovascular disease status. Models were adjusted as follows: 1) sex, baseline age, and antidepressant medication use and, in longitudinal analyses, CES-Dbaseline and time between visits; 2) model 1 plus baseline current smoker, physical activity score, high school education, diabetes, and cardiovascular disease; and 3) model 2 plus baseline HEI 2005 score (excluding the oil component). In addition, in separate analyses, we adjusted for baseline erythrocyte arachidonic acid (AA; 20:4n−6) proportion and APOE ɛ2 or ɛ4 genotype. APOE genotype has been associated with depression (36) and oxidative stress (37).
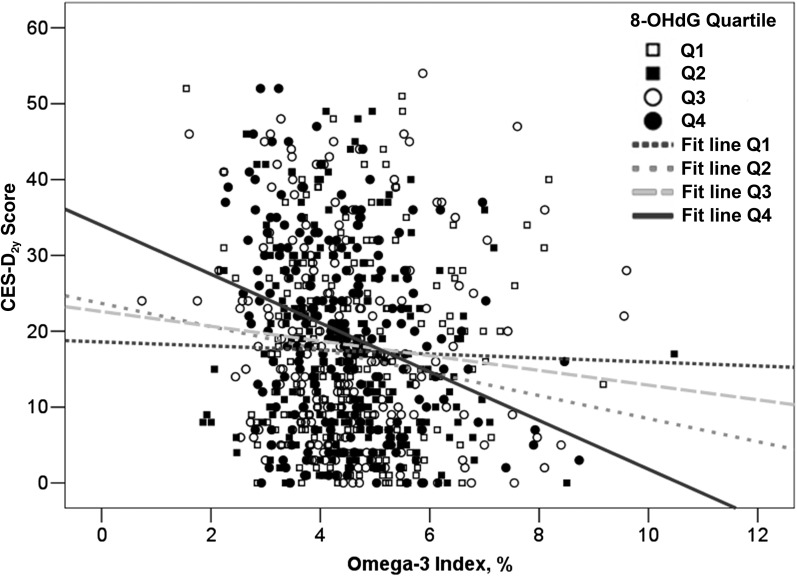
Because of biological plausibility, we tested whether the association between the omega-3 index and CES-Dbaseline or CES-D2y score was modified by baseline 8-OHdG concentration (quartile) (13, 38–40), APOE ɛ2 (yes or no), and ɛ4 (yes or no) genotype (41) or baseline antidepressant use (yes or no) (7). Specifically, in linear regression models, the relation between the omega-3 index (continuous) and either CES-Dbaseline (continuous) or CES-D2y (continuous), interaction terms between the omega-3 index and each of the 4 potential categorical effect modifiers were added to separate models (e.g., omega-3 index by 8-OHdG concentration, omega-3 index by APOE ɛ2 genotype, etc.). In cross-sectional results, urinary 8-OHdG concentration appeared to modify the association between the omega-3 index and CES-Dbaseline score, but this only approached significance (P-interaction = 0.10). In longitudinal models that related the omega-3 index to CES-D2y score, the effect modification was significant (P-interaction = 0.04; Figure 1). Given this consistency of results, we examined stratified as well as unstratified analyses at both time points. It is biologically plausible that ω-3 FAs may have differential effects on depressive symptoms by oxidative stress status, given that oxidative stress is higher in depressed individuals (13), ω-3 FA supplementation lowers depressive-like behaviors (14, 15), and ω-3 FAs have antioxidant properties (38–40).
FIGURE 1.
Relation between the omega-3 index and CES-D2y score stratified by creatinine-adjusted 8-OHdG concentration (quartile) among older Puerto Ricans (n = 196–198/quartile). Data represent CES-D2y scores modeled as a function of the omega-3 index and 8-OHdG quartile and their interaction (P-interaction = 0.04). CES-D, Center for Epidemiological Studies–Depression Scale; Q, quartile; 8-OHdG, 8-hydroxy-2-deoxyguanosine.
It is possible that ω-3 FA status may have changed during the follow-up period for reasons related to depressive symptoms (e.g., a medical provider may have prescribed ω-3 FA supplementation or recommended increased fish consumption to help manage depressive mood or, alternatively, depression may have affected dietary habits). Therefore, we conducted a sensitivity analysis excluding those 45 participants who used ω-3 FA supplements at baseline (n = 19) and/or during follow-up (n = 31).
In addition, multivariable logistic regression was used to determine whether the omega-3 index (continuous) was associated with having a high baseline or 2-y CES-D score, defined as ≥16. A cutoff score of 16 has been recommended to define clinically significant levels of depressive symptoms (21, 42). To minimize classification errors, we excluded those participants who used antidepressant medications at baseline (n = 524; 67%) in cross-sectional analyses and at baseline or during follow-up (n = 453; 67%) in longitudinal analyses. For longitudinal analyses, we did not exclude participants with baseline CES-D scores <16. We stratified 8-OHdG concentration by tertiles rather than quartiles due to the smaller sample size compared with the original sample size. Models were similar to those described for multivariable linear regression analyses but without adjustment for baseline antidepressant medication use or baseline CES-D status in models that predicted a high CES-D2y score. This allows for the examination of the effect of ω-3 FA exposure at baseline on the odds of a CES-D2y score ≥16.
In secondary analyses, we explored whether erythrocyte DHA or EPA—components of the omega-3 index—related to CES-D2y score using multivariable linear regression. The modeling structure previously described for linear regression models was used for these sets of analyses.
Results
Baseline sample characteristics.
We compared selected baseline characteristics between the analytic sample (n = 787) and those 577 participants who were excluded because FA composition, CES-D score, or 8-OHdG concentrations were not available (Supplemental Table 1). Those in the analytic sample were older (57.6 ± 0.27 y compared with 56.4 ± 0.32 y; P = 0.01) and were less likely to be taking ω-3 FA supplements (2.4% and 4.9%, respectively; P = 0.01). Baseline CES-D scores of the analytic sample and those excluded because CES-D2y scores were not available were 19.8 and 21.7, respectively (P = 0.08).
The analytic sample was predominantly female (72.8%) and a higher omega-3 index value was associated with older age (P ≤ 0.0001), better overall diet quality (P ≤ 0.0001), and a lower prevalence of current smokers (P = 0.005) at baseline (Table 1). Those taking antidepressants (compared with not taking antidepressants) had significantly lower omega-3 index values (P = 0.0001), higher 8-OHdG concentrations (P = 0.002), and higher baseline CES-D scores (P ≤ 0.0001) (data not shown).
TABLE 1.
Baseline sample characteristics of older Puerto Rican adults by omega-3 index quartile1
| Omega-3 Index3 |
||||||
| Unstratified2,3 |
Q1 |
Q2 |
Q3 |
Q4 |
||
| 4.5% ± 0.04% (0.7–10.5%) | 3.1% ± 0.02% (0.7–3.7%) | 4.0% ± 0.02% (3.7–4.4%) | 4.7% ± 0.02% (4.4–5.1%) | 6.2% ± 0.07% (5.1–10.5%) | P | |
| n | 787 | 196 | 198 | 197 | 196 | |
| Age,2 y | 57.6 ± 0.27 | 54.9 ± 0.49 | 56.2 ± 0.53 | 59.0 ± 0.52 | 60.2 ± 0.51 | ≤0.0001 |
| Female, % | 72.8 | 76.1 | 70.2 | 76.1 | 68.8 | 0.23 |
| BMI, kg/m2 | 32.1 ± 0.23 | 32.1 | 32.6 | 32.4 | 31.4 | 0.29 |
| High school education or higher, % | 50.4 | 48.0 | 51.4 | 48.9 | 53.5 | 0.68 |
| HEI 2005 score | 71.9 ± 0.33 | 69.7 ± 0.65 | 70.6 ± 0.64 | 72.8 ± 0.64 | 74.5 ± 0.65 | <0.0001 |
| HEI 2005 (excludes oil component) | 62.8 ± 0.32 | 60.8 ± 0.63 | 61.6 ± 0.62 | 63.6 ± 0.62 | 65.4 ± 0.63 | <0.0001 |
| Physical activity score | 31.4 ± 0.16 | 31.0 ± 0.32 | 31.3 ± 0.31 | 31.4 ± 0.32 | 32.1 ± 0.32 | 0.15 |
| Current smoker, % | 22.2 | 27.4 | 27.9 | 17.6 | 16.0 | 0.005 |
| LDL cholesterol,4 mg/dL | 108 ± 1.30 | 109 ± 2.56 | 110 ± 2.51 | 107 ± 2.50 | 107 ± 2.56 | 0.69 |
| HDL cholesterol,4 mg/dL | 46.0 ± 0.45 | 45.9 ± 0.93 | 44.7 ± 0.91 | 45.6 ± 0.92 | 46.0 ± 0.93 | 0.71 |
| Glucose,4 mg/dL | 121 ± 1.80 | 126 ± 3.55 | 118 ± 3.49 | 124 ± 3.49 | 115 ± 3.55 | 0.10 |
| Insulin,4 μIU/mL | 14.0 ± 1.00 | 12.9 ± 1.05 | 13.6 ± 1.05 | 14.1 ± 1.05 | 13.8 ± 1.05 | 0.64 |
| HOMA-IR | 3.60 ± 1.03 | 3.70 ± 1.06 | 3.70 ± 1.06 | 4.02 ± 1.06 | 3.73 ± 1.06 | 0.71 |
| Antidepressant use, % | 33.4 | 36.5 | 35.2 | 34.7 | 27.3 | 0.22 |
| Diabetes, % | 39.6 | 39.1 | 36.4 | 42.6 | 40.5 | 0.64 |
| Cardiovascular disease, % | 21.0 | 18.7 | 22.5 | 20.0 | 24.1 | 0.56 |
| Erythrocyte DHA, % of total FAs | 4.10 ± 0.04 | 2.80 ± 0.03 | 3.68 ± 0.03 | 4.31 ± 0.03 | 5.50 ± 0.03 | <0.0001 |
| Erythrocyte EPA, % of total FAs | 0.44 ± 0.01 | 0.31 ± 0.01 | 0.37 ± 0.01 | 0.43 ± 0.01 | 0.65 ± 0.01 | <0.0001 |
| ω-3 FA supplementation, % | 2.4 | 1.1 | 0.6 | 1.0 | 7.1 | <0.0001 |
| APOE genotype,5 % | ||||||
| ɛ2 | 10.6 | 10.0 | 12.9 | 13.0 | 6.40 | 0.12 |
| ɛ4 | 24.5 | 25.4 | 25.8 | 20.7 | 25.9 | 0.60 |
Values are age-adjusted means ± SEs or proportions unless otherwise noted and were analyzed by using proc GLM in SAS with the omega-3 index treated as an ordinal variable (quartile). HEI, Healthy Eating Index; Q, quartile; 8-OHdG, 8-hydroxy-2-deoxyguanosine.
Data not adjusted for age.
Omega-3 index values are mean ± SE (minimum–maximum) values for unstratified and stratified analyses by omega-3 index quartile.
Biochemical measures were analyzed from fasting blood samples.
APOE status was available for 756 of the 787 participants in the sample.
Erythrocyte omega-3 index and CES-D score: cross-sectional and longitudinal relations.
In cross-sectional analyses, the omega-3 index was not associated with CES-Dbaseline score in minimally (model 1; P = 0.39) or fully adjusted (model 3; P = 0.98) models (Table 2). Longitudinally, baseline omega-3 index and CES-D2y score tended toward an inverse association in the minimally adjusted model, accounting for CES-Dbaseline (model 1; P = 0.06), but this was attenuated after consideration of other potential confounders (model 3; P = 0.20) (Table 2).
TABLE 2.
Associations between the omega-3 index and CES-D score at baseline and 2-y follow-up among older Puerto Rican adults1
| Outcome and model2 | β ± SE | P |
| CES-Dbaseline | ||
| 1 | −0.31 ± 0.36 | 0.39 |
| 2 | −0.05 ± 0.36 | 0.90 |
| 3 | −0.001 ± 0.370 | 0.98 |
| CES-D2y3 | ||
| 1 | −0.55 ± 0.29 | 0.06 |
| 2 | −0.41 ± 0.29 | 0.15 |
| 3 | −0.37 ± 0.30 | 0.20 |
Values are β ± SE estimates; n = 787. Multivariable linear regression was used to determine the relation between the omega-3 index and CES-Dbaseline and CES-D2y scores. CES-D, Center for Epidemiological Studies–Depression Scale.
Model 1 adjusted for antidepressant use, age, and sex; model 2 adjusted as in model 1 plus for current smoker, physical activity score, high school education, diabetes, and cardiovascular disease; and model 3 adjusted as in model 2 plus for Healthy Eating Index 2005 score (excluding oil component).
All of the longitudinal models were additionally adjusted for CES-Dbaseline and time between visits.
Effect modification by urinary 8-OHdG concentration, antidepressant use, and APOE ɛ2 and ɛ4 genotype: cross-sectional and longitudinal relations.
At baseline, urinary 8-OHdG concentration tended to, but did not significantly, modify the cross-sectional relation between the erythrocyte omega-3 index and the CES-Dbaseline score (P-interaction = 0.10). We did not find evidence of effect modification by antidepressant use or APOE ɛ2 or ɛ4 genotype (P-interaction = 0.49–0.80). The longitudinal association between the omega-3 index and CES-D2y score was significantly modified by 8-OHdG concentration (Figure 1; P-interaction = 0.04) but not by antidepressant use or APOE genotype (P-interaction = 0.21–0.65).
Erythrocyte omega-3 index and CES-D score stratified by urinary 8-OHdG concentration: cross-sectional and longitudinal relations.
A higher 8-OHdG concentration was associated with lower physical activity levels (P = 0.03) and greater antidepressant use (P = 0.02) (Supplemental Table 2). The omega-3 index, DHA, and EPA did not vary significantly across 8-OHdG concentration categories at baseline (P = 0.58–0.78). Nonlinear differences were detected for AA concentration (16.8% ± 0.12%, 16.5% ± 0.12%, 16.3% ± 0.12%, and 16.8% ± 0.12% across 8-OHdG quartiles; P = 0.02). With the use of partial Pearson correlations, adjusted for age and sex, we found that 8-OHdG concentration was significantly and positively correlated with CES-Dbaseline (r = 0.08, P = 0.02) and CES-D score2y (r = 0.08, P = 0.03).
Cross-sectionally, the omega-3 index was inversely related to the CES-Dbaseline score among those in the top 8-OHdG quartile but not among those in the lower quartiles in either minimally or fully adjusted models (Table 3). In longitudinal analyses stratified by 8-OHdG concentration, each 1-unit higher omega-3 index value was, on average, related to a 1.66-unit lower CES-D2y score among those in the top 8-OHdG quartile (Table 4; model 3; P = 0.02). We did not detect a significant association between the omega-3 index and 2-y change in depressive symptoms (CES-D2y adjusted for baseline) among those in 8-OHdG concentration quartiles 1, 2, or 3 (P > 0.05 for all). Our findings remained consistent after additional adjustment for erythrocyte AA concentration or APOE ɛ2 or ɛ4 genotype. Furthermore, the exclusion of ω-3 FA supplement users at baseline or follow-up did not appreciably alter our results (data not shown).
TABLE 3.
Cross-sectional relation between the omega-3 index and CES-D score stratified by 8-OHdG concentration among older Puerto Rican adults1
| 8-OHdG concentration3 |
||||
| Q1 |
Q2 |
Q3 |
Q4 |
|
| Model2 | 54.2 ± 1.57 (2.6–86.7) [196] | 108 ± 0.91 (86.8–131) [ 197] | 154 ± 1.01 (131.2–179) [ 197] | 237 ± 4.18 (179–581) [197] |
| 1 | 0.22 ± 0.71 | −0.03 ± 0.71 | −0.06 ± 0.67 | −1.77 ± 0.83* |
| 2 | 0.45 ± 0.72 | 0.12 ± 0.72 | 0.55 ± 0.68 | −1.67 ± 0.84* |
| 3 | 0.44 ± 0.74 | 0.26 ± 0.74 | 0.59 ± 0.7 | −1.74 ± 0.88* |
Values are β ± SE estimates; n = 787. Multivariable linear regression was used to determine the relation between the omega-3 index and CES–Dbaseline score. *P ≤ 0.05. CES-D, Center for Epidemiological Studies–Depression Scale; Q, quartile; 8-OHdG, 8-hydroxy-2-deoxyguanosine.
Model 1 adjusted for antidepressant use, age, and sex; model 2 adjusted as in model 1 plus for current smoker, physical activity score, high school education, diabetes, and cardiovascular disease; and model 3 adjusted as in model 2 plus for Healthy Eating Index 2005 score (excluding oil component).
Unadjusted mean ± SE (minimum–maximum) 8-OHdG concentrations in ng/μg creatinine; n in brackets.
TABLE 4.
Longitudinal relation between the omega-3 index and CES-D score stratified by 8-OHdG concentration among older Puerto Rican adults1
| 8-OHdG concentration3 |
||||
| Q1 |
Q2 |
Q3 |
Q4 |
|
| Model2 | 54.2 ± 1.57 (2.6–86.7) [196] | 108 ± 0.91 (86.8–131) [197] | 154 ± 1.01 (131.2–179) [197] | 237 ± 4.18 (179–581) [197] |
| 1 | −0.053 ± 0.63 | −0.73 ± 0.60 | −0.03 ± 0.50 | −1.64 ± 0.62* |
| 2 | 0.018 ± 0.64 | −0.69 ± 0.60 | 0.18 ± 0.51 | −1.67 ± 0.64* |
| 3 | −0.013 ± 0.66 | −0.73 ± 0.62 | 0.23 ± 0.52 | −1.66 ± 0.66* |
Values are β ± SE estimates; n = 787. Multivariable linear regression was used to determine the relation between the omega-3 index and CES-D2y score stratified by 8-OHdG quartile. *P ≤ 0.05. CES-D, Center for Epidemiological Studies–Depression Scale; Q, quartile; 8-OHdG, 8-hydroxy-2-deoxyguanosine.
Model 1 adjusted for antidepressant use, age, sex, time between visits, and CES-Dbaseline score; model 2 adjusted as in model 1 plus for current smoker, physical activity score, high school education, diabetes, and cardiovascular disease; and model 3 adjusted as in model 2 plus for Healthy Eating Index 2005 score (excluding oil component).
Unadjusted mean ± SE (minimum–maximum) 8-OHdG concentrations in ng/μg creatinine; n in brackets.
With the use of multivariable logistic regression, we investigated the relation between the omega-3 index and baseline and 2-y prevalence of clinically relevant depressive symptoms (CES-D2y scores ≥16) (Table 5). Cross-sectionally, there were reduced odds of CES-Dbaseline scores ≥16 among those in the top 8-OHdG tertile category (model 3 OR: 0.72; 95% CI: 0.53, 0.96) but not among those in lower tertiles. Conversely, the omega-3 index was not significantly related to odds of CES-D2y scores ≥16 in the top tertile (model 3 OR: 0.83; 95% CI: 0.60, 1.15) or in lower tertiles.
TABLE 5.
Relation between omega-3 index and CES-D scores ≥16 at baseline and at 2-y follow-up stratified by 8-OHdG concentration among older Puerto Rican adults1
| 8-OHdG concentration |
||||
| Outcome | Unstratified | Tertile 1 | Tertile 2 | Tertile 3 |
| CES-Dbaseline ≥162 | ||||
| 8-OHdG concentration,3 ng/μg creatinine | 132 ± 3 (6.8–390) | 62.7 ± 1.79 (6.8–96.4) | 124 ± 1.37 (96.6–156) | 209 ± 3.72 (156–390) |
| n | 524 | 174 | 175 | 175 |
| Prevalence, n (%) | 263 (50) | 87 (50) | 85 (49) | 91 (52) |
| Model4 | ||||
| 1 | 0.94 (0.82, 1.08) | 1.02 (0.77, 1.36) | 1.07 (0.84, 1.35) | 0.68 (0.52, 0.90) |
| 2 | 0.98 (0.85, 1.13) | 1.07 (0.79, 1.43) | 1.06 (0.83, 1.35) | 0.73 (0.55, 0.97) |
| 3 | 1.00 (0.86, 1.15) | 1.07 (0.79, 1.43) | 1.08 (0.83, 1.39) | 0.72 (0.53, 0.96) |
| CES-D2y ≥165 | ||||
| 8-OHdG concentration,3 ng/μg creatinine | 133 ± 3.19 (7.7–390) | 63.8 ± 1.96 (7.7–96.6) | 124 ± 1.42 (96.6–155) | 209 ± 3.91 (156–390) |
| n | 453 | 151 | 151 | 151 |
| Prevalence, n (%) | 175 (39) | 62 (41) | 50 (33) | 63 (42) |
| Model4,6 | ||||
| 1 | 0.89 (0.76, 1.04) | 0.81 (0.60, 1.10) | 1.06 (0.83, 1.36) | 0.75 (0.56, 1.01) |
| 2 | 0.89 (0.76, 1.04) | 0.86 (0.62, 1.19) | 1.09 (0.84, 1.40) | 0.82 (0.60, 1.12) |
| 3 | 0.94 (0.80, 1.12) | 0.86 (0.62, 1.20) | 1.01 (0.84, 1.43) | 0.83 (0.60, 1.15) |
Values are ORs (95% CIs) unless otherwise noted. Multivariable logistic regression was used to determine the relation between the omega-3 index and CES-D scores ≥16 at baseline and at 2-y follow-up. CES-D, Center for Epidemiological Studies–Depression Scale; 8-OHdG, 8-hydroxy-2-deoxyguanosine.
Excluded participants reporting antidepressant use at baseline (n = 263).
Values are unadjusted mean ± SE (minimum–maximum) 8-OHdG concentrations.
Model 1 adjusted for age and sex; model 2 adjusted as in model 1 plus for current smoker, physical activity score, high school education, diabetes, and cardiovascular disease; model 3 adjusted as in model 2 plus for Healthy Eating Index 2005 score (excluding oil component).
Excluded those reporting antidepressant use at baseline or follow-up (n = 334).
CES-D2y models were additionally adjusted for time between visits.
Erythrocyte DHA and EPA relations with CES-D score.
To explore whether these associations were due to DHA or EPA, we repeated linear regression analyses with each FA set as the independent variable (Supplemental Table 3). Among those in the top 8-OHdG quartile, erythrocyte DHA concentration was significantly and inversely associated with CES-D2y score (model 3; P = 0.01). Conversely, no significant association was detected between EPA and CES-D2y score (model 3; P = 0.28).
Discussion
In unstratified analyses, the omega-3 index, a blood biomarker of ω-3 FA dietary intake and metabolism, was not significantly associated with depressive symptoms at baseline or after a 2-y follow-up in a sample of participants of the Boston Puerto Rican Health Study. Interestingly, in a subgroup of individuals with high baseline oxidative stress, measured as urinary 8-OHdG concentration, the omega-3 index was significantly associated with lower depressive symptoms in cross-sectional and longitudinal analyses. Inconsistent relations between cross-sectional and longitudinal results were found in examining omega-3 index associations with CES-D scores ≥16.
Earlier observational studies examined whether biological markers of ω-3 FA consumption were related to severity of depressive symptoms by using erythrocytes, serum, and plasma, as reviewed elsewhere (4–6). These were primarily case-control studies that examined clinical populations (e.g., major depressive disorder, multiple sclerosis, acute coronary syndrome). The majority reported an inverse relation between ω-3 FA concentration and the degree of depressive symptoms, whereas some did not detect any association (43, 44). The erythrocyte omega-3 index is a promising biomarker for coronary artery disease (45), and our data suggest that low omega-3 index values may also be a biomarker for risk of depressive symptoms. Consistent with our results, a study in patients with acute coronary syndrome (46) found an inverse cross-sectional relation between the erythrocyte omega-3 index and depressive symptoms (Patient Health Questionnaire). In a large cohort of older women (n = 7086), the omega-3 index was inversely associated with baseline and incident depressive symptomatology (Burnam 8-item scale score ≥0.06) in age-adjusted models, but this was attenuated in multivariable adjusted models (47).
Some observational studies reported that worse depressive mood was related to tissue deficiency of DHA (8, 9, 46) rather than EPA, or vice versa (7). In our study, DHA, but not EPA, exhibited significant protective associations with depressive symptoms. However, this finding must be interpreted cautiously. The variability in erythrocyte DHA (median: 4.0%; 25th–75th percentile: 3.3–4.7%) was larger than that of EPA (0.35%; 0.30–0.50%) in our study population, making it difficult to draw firm conclusions. Moreover, the inverse relation between erythrocyte DHA and depressive symptoms was only seen in a subgroup of individuals with high oxidative stress. In contrast, a meta-analysis of 15 randomized controlled trials, Sublette et al. (48) concluded that oils richer in EPA instead of DHA (≥60%) were more efficacious in lowering depressive symptom scores.
It is not surprising that results vary between previous studies and our findings. There is heterogeneity across studies, including populations of interest (e.g., clinical compared with nonclinical), study design (e.g., cross-sectional compared with longitudinal), biological sample (erythrocyte compared with plasma), and ascertainment of depressive symptoms (e.g., CES-D compared with Burnam 8-item). Moreover, interventional evidence suggests that the protective effect of ω-3 FA supplementation on severity of depressive symptoms may be stronger for individuals with medically diagnosed depression rather than for those without a medical diagnosis (6). In a cross-sectional study conducted among elderly individuals, antidepressant use modified the association between plasma phospholipid EPA composition and CES-D score (7). This differing result from our study may be related to the higher prevalence of antidepressant use in our cohort (18% and 44% among those with low and high CES-D scores, respectively) compared with the other study (8% and 33%, respectively) (7).
The beneficial association between the omega-3 index and depressive symptoms in our cohort was observable only among those with relatively high oxidative stress. In animals, chronic exposure to psychological or physical stressors results in oxidative stress and lipid peroxidation of neuronal cells (10, 11). These findings are consistent with human models of chronic stress (49). For example, Epel et al. (12) found that more years of caregiving were related to higher oxidative stress among biological mothers caring for a chronically ill child. Moreover, under conditions of pervasive stress and ω-3 FA deficiency, rats exhibited greater depressive-like behavior compared with rats exposed to the same stress but fed a diet supplemented with ω-3 FAs (14, 15). Along the same lines, individuals who have suffered from multiple depressive episodes (≥2) presented with higher oxidative stress than did those with a history of only 1 depressive episode (50). In consideration of this evidence, urinary 8-OHdG may be an indicator of chronic stress. Additional longitudinal studies are needed to clarify relations and causal directions.
Our results, along with others, suggest that ω-3 FAs may be protective against oxidative stress–related depression. Oxidative stress is implicated in impaired neuronal signaling, neuroplasticity, and neurogenesis (10), in part through inflammation (51) and mitochondrial dysfunction (52). In vivo and ex vivo studies showed that ω-3 FAs support antioxidant defenses (39), upregulating antioxidant enzyme activity (38). Furthermore, ω-3 FAs are efficient reactive oxygen species scavengers. Richard et al. (40) found that human aortic endothelial cells supplemented with either DHA or EPA resulted in lower reactive oxygen species formation than those supplemented with ω-6 fats. Lipid inflammatory mediators including lipoxins, resolvins, and protectins—products of DHA and or EPA metabolism—are anti-inflammatory and pro-resolving (53), thereby aiding in the reduction in inflammatory burden. ω-3 FAs are predominant structural elements in mitochondrial membranes and can bind to nuclear receptors that activate signaling pathways that modulate mitochondrial function (54)—in concert, these factors support optimal mitochondrial health. Thus, the potential therapeutic value of DHA and EPA on depressive symptoms may partly be related to their effects on oxidative stress.
Our study has several strengths. We used erythrocyte FAs, which are valid markers of fat consumption, particularly for ω-3 FAs, which are dependent on dietary intake (55, 56). Thus, our use of an objective dietary biomarker likely minimized the chance of misclassification errors, although low dietary intakes of ω-3 FAs have been related to depression (57). To our knowledge, our study is one of the largest to investigate the relations between ω-3 FA status and depressive symptoms, which increased our ability to detect effects. Many potential behavioral and metabolic confounders were addressed, including education, smoking status, physical activity level, cardiovascular disease, and diabetes; however, because of the observational nature of this study residual confounding remains a concern. Last, we built upon the current literature by examining the state of oxidative stress in a unique cohort of Puerto Ricans, a group burdened by prevalent depressive symptoms (16).
Some important limitations should be considered. Erythrocyte FA status was available only at baseline and not at follow-up. It is possible that erythrocyte FA concentrations could have changed during the follow-up period, which may be related to changes in depressive symptoms. Depression has been associated with poorer dietary intake and could lead to lower consumption of ω-3 FAs (58). However, the consistency of our findings longitudinally argues against reverse causation. Some randomized controlled trials have shown that ω-3 FA supplementation improved depressive symptoms (6, 48). However, few of our participants used these supplements (2.4% at baseline and 3.9% at follow-up), and our results remained consistent when we repeated analyses without supplement users. Due to the high baseline prevalence of CES-D scores ≥16 (60%) and antidepressant use at baseline (33%) and follow-up (34%), we did not have a sufficient sample size to examine the incidence of CES-D scores ≥16 at 2 y in analyses stratified by 8-OHdG concentration. Future research is needed in cohorts of sufficient size to examine the effect of the omega-3 index on incidence of high depressive symptoms or depression and the influence of oxidative stress on these associations.
Deficiency in neuronal ω-3 FA content is thought to play a role in the occurrence of oxidative stress–induced depressive behavior. Because ω-3 FAs are incorporated into neural tissues more so than others, the use of peripheral indicators of ω-3 FA concentration, as in our study (erythrocytes), may introduce misclassification errors. However, rats fed an ω-3 FA–supplemented diet showed increases in both brain and plasma ω-3 FA content (15), and in humans, the omega-3 index has been related to larger brain volume (59) and better neurocognitive function (60). Moreover, autopsy findings have shown lower brain ω-3 FA concentrations in depressed compared with nondepressed individuals (61). The low mean omega-3 index [4.6% compared with 5.6% in the Framingham Offspring cohort (20)] and relatively narrow range (90% of values between 2.8% and 6.8%) make it difficult to see associations with cardioprotective levels of the index (i.e., >8%) (45). This, along with other factors, may have contributed to the modest effect sizes estimated in our study. Last, although the longitudinal association between the omega-3 index and CES-D score was significantly modified by 8-OHdG concentration (P = 0.04), that of cross-sectional models only approached significance (P = 0.10). This weakens the finding that oxidative stress modifies the relation between ω-3 status and CES-D score at baseline. Studies with larger sample sizes are needed to confirm this result.
In conclusion, an inverse association was observed between objective biomarkers of ω-3 FA consumption and severity of depressive symptoms at baseline and at 2-y follow-up but only among those participants with high urinary 8-OHdG concentrations. Our results suggest that high oxidative stress status may potentially identify those who could benefit most from ω-3 FA consumption for a reduction in depressive symptoms. Additional research is warranted to replicate this observational finding and to test it in a randomized trial with depressed patients who have elevated concentrations of oxidative stress biomarkers.
Acknowledgments
SJB, WSH, and KLT designed the research; LMF, JMO, C-QL, and KLT conducted the research; JMO and C-QL provided essential materials; SJB performed statistical analyses; and SJB and KLT had primary responsibility for final content and wrote the manuscript. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: AA, arachidonic acid; CES-D, Center for Epidemiological Studies–Depression Scale; HEI, Healthy Eating Index; 8-OHdG, 8-hydroxy-2′-deoxyguanosine.
References
- 1.Rumsfeld JS, Ho PM. Depression and cardiovascular disease: a call for recognition. Circulation 2005;111:250–3. [DOI] [PubMed] [Google Scholar]
- 2.Van der Kooy K, van Hout H, Marwijk H, Marten H, Stehouwer C, Beekman A. Depression and the risk for cardiovascular diseases: systematic review and meta analysis. Int J Geriatr Psychiatry 2007;22:613–26. [DOI] [PubMed] [Google Scholar]
- 3.Brenna JT, Diau GY. The influence of dietary docosahexaenoic acid and arachidonic acid on central nervous system polyunsaturated fatty acid composition. Prostaglandins Leukot Essent Fatty Acids 2007;77:247–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grosso G, Galvano F, Marventano S, Malaguarnera M, Bucolo C, Drago F, Caraci F. Omega-3 fatty acids and depression: scientific evidence and biological mechanisms. Oxid Med Cell Longev 2014;2014:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin PY, Huang SY, Su KP. A meta-analytic review of polyunsaturated fatty acid compositions in patients with depression. Biol Psychiatry 2010;68:140–7. [DOI] [PubMed] [Google Scholar]
- 6.Appleton KM, Rogers PJ, Ness AR. Updated systematic review and meta-analysis of the effects of n–3 long-chain polyunsaturated fatty acids on depressed mood. Am J Clin Nutr 2010;91:757–70. [DOI] [PubMed] [Google Scholar]
- 7.Féart C, Peuchant E, Letenneur L, Samieri C, Montagnier D, Fourrier-Reglat A, Barberger-Gateau P. Plasma eicosapentaenoic acid is inversely associated with severity of depressive symptomatology in the elderly: data from the Bordeaux sample of the Three-City Study. Am J Clin Nutr 2008;87:1156–62. [DOI] [PubMed] [Google Scholar]
- 8.Edwards R, Peet M, Shay J, Horrobin D. Omega-3 polyunsaturated fatty acid levels in the diet and in red blood cell membranes of depressed patients. J Affect Disord 1998;48:149–55. [DOI] [PubMed] [Google Scholar]
- 9.Riemer S, Maes M, Christophe A, Rief W. Lowered omega-3 PUFAs are related to major depression, but not to somatization syndrome. J Affect Disord 2010;123:173–80. [DOI] [PubMed] [Google Scholar]
- 10.Hovatta I, Juhila J, Donner J. Oxidative stress in anxiety and comorbid disorders. Neurosci Res 2010;68:261–75. [DOI] [PubMed] [Google Scholar]
- 11.Lucca G, Comim CM, Valvassori SS, Reus GZ, Vuolo F, Petronilho F, Dal-Pizzol F, Gavioli EC, Quevedo J. Effects of chronic mild stress on the oxidative parameters in the rat brain. Neurochem Int 2009;54:358–62. [DOI] [PubMed] [Google Scholar]
- 12.Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, Cawthon RM. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci USA 2004;101:17312–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Palta P, Samuel LJ, Miller ER III, Szanton SL. Depression and oxidative stress: results from a meta-analysis of observational studies. Psychosom Med 2014;76:12–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferraz AC, Delattre AM, Almendra RG, Sonagli M, Borges C, Araujo P, Andersen ML, Tufik S, Lima MM. Chronic omega-3 fatty acids supplementation promotes beneficial effects on anxiety, cognitive and depressive-like behaviors in rats subjected to a restraint stress protocol. Behav Brain Res 2011;219:116–22. [DOI] [PubMed] [Google Scholar]
- 15.Hennebelle M, Balasse L, Latour A, Champeil-Potokar G, Denis S, Lavialle M, Gisquet-Verrier P, Denis I, Vancassel S. Influence of omega-3 fatty acid status on the way rats adapt to chronic restraint stress. PLoS One 2012;7:e42142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wassertheil-Smoller S, Arredondo EM, Cai J, Castaneda SF, Choca JP, Gallo LC, Jung M, LaVange LM, Lee-Rey ET, Mosley T, et al. Depression, anxiety, antidepressant use, and cardiovascular disease among Hispanic men and women of different national backgrounds: results from the Hispanic Community Health Study/Study of Latinos. Ann Epidemiol 2014;24:822–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pratt LA, Brody DJ. Depression in the U.S. household population, 2009–2012. NCHS Data Brief 2014;172:1–8. [PubMed] [Google Scholar]
- 18.Tucker KL, Mattei J, Noel SE, Collado BM, Mendez J, Nelson J, Griffith J, Ordovas JM, Falcon LM. The Boston Puerto Rican Health Study, a longitudinal cohort study on health disparities in Puerto Rican adults: challenges and opportunities. BMC Public Health 2010;10:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gao X, Lai CQ, Scott T, Shen J, Cai T, Ordovas JM, Tucker KL. Urinary 8-hydroxy-2-deoxyguanosine and cognitive function in Puerto Rican adults. Am J Epidemiol 2010;172:271–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harris WS, Pottala JV, Lacey SM, Vasan RS, Larson MG, Robins SJ. Clinical correlates and heritability of erythrocyte eicosapentaenoic and docosahexaenoic acid content in the Framingham Heart Study. Atherosclerosis 2012;225:425–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Radloff L. The CES-D scale: a self report depression scale for research in the general population. Appl Psychol Meas 1977;1:385–401. [Google Scholar]
- 22.Cho MJ, Moscicki EK, Narrow WE, Rae DS, Locke BZ, Regier DA. Concordance between two measures of depression in the Hispanic Health and Nutrition Examination Survey. Soc Psychiatry Psychiatr Epidemiol 1993;28:156–63. [DOI] [PubMed] [Google Scholar]
- 23.Black SA, Markides KS, Miller TQ. Correlates of depressive symptomatology among older community-dwelling Mexican Americans: the Hispanic EPESE. J Gerontol B Psychol Sci Soc Sci 1998;53:S198–208. [DOI] [PubMed] [Google Scholar]
- 24.Mościcki EK, Locke BZ, Rae DS, Boyd JH. Depressive symptoms among Mexican Americans: the Hispanic Health and Nutrition Examination Survey. Am J Epidemiol 1989;130:348–60. [DOI] [PubMed] [Google Scholar]
- 25.Robison J, Gruman C, Gaztambide S, Blank K. Screening for depression in middle-aged and older Puerto Rican primary care patients. J Gerontol A Biol Sci Med Sci 2002;57:M308–14. [DOI] [PubMed] [Google Scholar]
- 26.Falcón LM, Tucker KL. Prevalence and correlates of depressive symptoms among Hispanic elders in Massachusetts. J Gerontol B Psychol Sci Soc Sci 2000;55:S108–16. [DOI] [PubMed] [Google Scholar]
- 27.Black CN, Bot M, Scheffer PG, Cuijpers P, Penninx BW. Is depression associated with increased oxidative stress? A systematic review and meta-analysis. Psychoneuroendocrinology 2015;51:164–75. [DOI] [PubMed] [Google Scholar]
- 28.Evans MD, Dizdaroglu M, Cooke MS. Oxidative DNA damage and disease: induction, repair and significance. Mutat Res 2004;567:1–61. [DOI] [PubMed] [Google Scholar]
- 29.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–9. [DOI] [PubMed] [Google Scholar]
- 30.Livak KJ. Allelic discrimination using fluorogenic probes and the 5′ nuclease assay. Genet Anal 1999;14:143–9. [DOI] [PubMed] [Google Scholar]
- 31.Paffenbarger RS Jr, Wing AL, Hyde RT. Physical activity as an index of heart attack risk in college alumni. Am J Epidemiol 1978;108:161–75. [DOI] [PubMed] [Google Scholar]
- 32.Paffenbarger RS Jr, Hyde RT, Wing AL, Lee IM, Jung DL, Kampert JB. The association of changes in physical-activity level and other lifestyle characteristics with mortality among men. N Engl J Med 1993;328:538–45. [DOI] [PubMed] [Google Scholar]
- 33.Tucker KL, Bermudez OI, Castaneda C. Type 2 diabetes is prevalent and poorly controlled among Hispanic elders of Caribbean origin. Am J Public Health 2000;90:1288–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ye X, Scott T, Gao X, Maras JE, Bakun PJ, Tucker KL. Mediterranean diet, Healthy Eating Index 2005, and cognitive function in middle-aged and older Puerto Rican adults. J Acad Nutr Diet 2013;113:276–81, e1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guenther PM, Reedy J, Krebs-Smith SM. Development of the Healthy Eating Index-2005. J Am Diet Assoc 2008;108:1896–901. [DOI] [PubMed] [Google Scholar]
- 36.Feng F, Lu SS, Hu CY, Gong FF, Qian ZZ, Yang HY, Wu YL, Zhao YY, Bi P, Sun YH. Association between apolipoprotein E gene polymorphism and depression. J Clin Neurosci 2015;22:1232–8. [DOI] [PubMed] [Google Scholar]
- 37.Jofre-Monseny L, Minihane AM, Rimbach G. Impact of apoE genotype on oxidative stress, inflammation and disease risk. Mol Nutr Food Res 2008;52:131–45. [DOI] [PubMed] [Google Scholar]
- 38.Garrel C, Alessandri JM, Guesnet P, Al-Gubory KH. Omega-3 fatty acids enhance mitochondrial superoxide dismutase activity in rat organs during post-natal development. Int J Biochem Cell Biol 2012;44:123–31. [DOI] [PubMed] [Google Scholar]
- 39.Giordano E, Visioli F. Long-chain omega 3 fatty acids: molecular bases of potential antioxidant actions. Prostaglandins Leukot Essent Fatty Acids 2014;90:1–4. [DOI] [PubMed] [Google Scholar]
- 40.Richard D, Kefi K, Barbe U, Bausero P, Visioli F. Polyunsaturated fatty acids as antioxidants. Pharmacol Res 2008;57:451–5. [DOI] [PubMed] [Google Scholar]
- 41.Quinn JF, Raman R, Thomas RG, Yurko-Mauro K, Nelson EB, Van Dyck C, Galvin JE, Emond J, Jack CR Jr, Weiner M, et al. Docosahexaenoic acid supplementation and cognitive decline in Alzheimer disease: a randomized trial. JAMA 2010;304:1903–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weissman MM, Sholomskas D, Pottenger M, Prusoff BA, Locke BZ. Assessing depressive symptoms in five psychiatric populations: a validation study. Am J Epidemiol 1977;106:203–14. [DOI] [PubMed] [Google Scholar]
- 43.Aupperle RL, Denney DR, Lynch SG, Carlson SE, Sullivan DK. Omega-3 fatty acids and multiple sclerosis: relationship to depression. J Behav Med 2008;31:127–35. [DOI] [PubMed] [Google Scholar]
- 44.Maes M, Smith R, Christophe A, Cosyns P, Desnyder R, Meltzer H. Fatty acid composition in major depression: decreased omega 3 fractions in cholesteryl esters and increased C20:4 omega 6/C20:5 omega 3 ratio in cholesteryl esters and phospholipids. J Affect Disord 1996;38:35–46. [DOI] [PubMed] [Google Scholar]
- 45.Harris WS. The omega-3 index as a risk factor for coronary heart disease. Am J Clin Nutr 2008;87(Suppl):1997S–2002S. [DOI] [PubMed] [Google Scholar]
- 46.Amin AA, Menon RA, Reid KJ, Harris WS, Spertus JA. Acute coronary syndrome patients with depression have low blood cell membrane omega-3 fatty acid levels. Psychosom Med 2008;70:856–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Persons JE, Robinson JG, Ammann EM, Coryell WH, Espeland MA, Harris WS, Manson JE, Fiedorowicz JG. Omega-3 fatty acid biomarkers and subsequent depressive symptoms. Int J Geriatr Psychiatry 2014;29:747–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sublette ME, Ellis SP, Geant AL, Mann JJ. Meta-analysis of the effects of eicosapentaenoic acid (EPA) in clinical trials in depression. J Clin Psychiatry 2011;72:1577–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gidron Y, Russ K, Tissarchondou H, Warner J. The relation between psychological factors and DNA-damage: a critical review. Biol Psychol 2006;72:291–304. [DOI] [PubMed] [Google Scholar]
- 50.Stefanescu C, Ciobica A. The relevance of oxidative stress status in first episode and recurrent depression. J Affect Disord 2012;143:34–8. [DOI] [PubMed] [Google Scholar]
- 51.Perry VH, Cunningham C, Holmes C. Systemic infections and inflammation affect chronic neurodegeneration. Nat Rev Immunol 2007;7:161–7. [DOI] [PubMed] [Google Scholar]
- 52.Morava E, Kozicz T. Mitochondria and the economy of stress (mal)adaptation. Neurosci Biobehav Rev 2013;37:668–80. [DOI] [PubMed] [Google Scholar]
- 53.Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol 2008;8:349–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Eckert GP, Lipka U, Muller WE. Omega-3 fatty acids in neurodegenerative diseases: focus on mitochondria. Prostaglandins Leukot Essent Fatty Acids 2013;88:105–14. [DOI] [PubMed] [Google Scholar]
- 55.Harris WS, Von Schacky C. The omega-3 index: a new risk factor for death from coronary heart disease? Prev Med 2004;39:212–20. [DOI] [PubMed] [Google Scholar]
- 56.Block RC, Harris WS, Pottala JV. Determinants of blood cell omega-3 fatty acid content. Open Biomark J 2008;1:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Whited MC, Schneider KL, Appelhans BM, Ma Y, Waring ME, DeBiasse MA, Busch AM, Oleski JL, Merriam PA, Olendzki BC, et al. Severity of depressive symptoms and accuracy of dietary reporting among obese women with major depressive disorder seeking weight loss treatment. PLoS One 2014;9:e90361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jacka FN, Cherbuin N, Anstey KJ, Butterworth P. Does reverse causality explain the relationship between diet and depression? J Affect Disord 2015;175:248–50. [DOI] [PubMed] [Google Scholar]
- 59.Pottala JV, Yaffe K, Robinson JG, Espeland MA, Wallace R, Harris WS. Higher RBC EPA + DHA corresponds with larger total brain and hippocampal volumes: WHIMS-MRI study. Neurology 2014;82:435–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Johnston DT, Deuster PA, Harris WS, Macrae H, Dretsch MN. Red blood cell omega-3 fatty acid levels and neurocognitive performance in deployed U.S. servicemembers. Nutr Neurosci 2013;16:30–8. [DOI] [PubMed] [Google Scholar]
- 61.McNamara RK, Hahn CG, Jandacek R, Rider T, Tso P, Stanford KE, Richtand NM. Selective deficits in the omega-3 fatty acid docosahexaenoic acid in the postmortem orbitofrontal cortex of patients with major depressive disorder. Biol Psychiatry 2007;62:17–24. [DOI] [PubMed] [Google Scholar]